Summary
The mosquito midgut stages of malaria parasites are crucial for establishing an infection in the insect vector and to thus ensure further spread of the pathogen. Parasite development in the midgut starts with the activation of the intraerythrocytic gametocytes immediately after take‐up and ends with traversal of the midgut epithelium by the invasive ookinetes less than 24 h later. During this time period, the plasmodia undergo two processes of stage conversion, from gametocytes to gametes and from zygotes to ookinetes, both accompanied by dramatic morphological changes. Further, gamete formation requires parasite egress from the enveloping erythrocytes, rendering them vulnerable to the aggressive factors of the insect gut, like components of the human blood meal. The mosquito midgut stages of malaria parasites are unprecedented objects to study a variety of cell biological aspects, including signal perception, cell conversion, parasite/host co‐adaptation and immune evasion. This review highlights recent insights into the molecules involved in gametocyte activation and gamete formation as well as in zygote‐to‐ookinete conversion and ookinete midgut exit; it further discusses factors that can harm the extracellular midgut stages as well as the measures of the parasites to protect themselves from any damage.
Introduction
Malaria, a vector‐borne blood disease caused by protozoan parasites of the genus Plasmodium, results in 214 million infections and claims 438 000 deaths every year. Children are particularly susceptible to malaria, and in 2015, an estimated 306 000 children under 5 years of age were killed, mostly in the African region (WHO World Malaria Report, 2015). Once transmitted to the human by a blood‐feeding female Anopheles mosquito, the parasites initially multiply in the human liver, before they progress to the pathologic blood stages. These blood infections can last for months, and only once sexual precursor cells, the gametocytes, have matured, the malaria parasites are able to leave the human host and to continue the life‐cycle in the insect vector.
In the mosquito midgut, the parasites are able to differentiate into their sexual forms, the female macrogametes and male microgametes, and to then undergo sexual reproduction in order to newly combine their chromosomal sets. The midgut phase lasts for approximately 20 h and includes two phases of stage conversion, the rapid conversion of gametocytes into fertile gametes upon activation and the conversion of zygotes into the motile and invasive ookinetes that once formed, immediately exit the gut lumen by traversing the midgut epithelial cell layer. Subsequently, the ookinetes settle down at the basal site of the midgut epithelium and convert to sessile oocysts, in which sporogonic replication takes place. This replication phase requires roughly 2 weeks and results in the formation of infective sporozoites that migrate to the salivary glands to be released into the human dermis with the next bite of the mosquito, wherewith the life‐cycle of Plasmodium is completed (reviewed in Aly et al., 2009; Ghosh and Jacobs‐Lorena, 2009; Kuehn and Pradel, 2010; Ménard et al., 2013).
The plasmodial midgut stages are attractive objects to study a variety of cell biological aspects, including cell conversion, signal perception, parasite/host co‐adaptation and immune evasion. Boosted by technical advances in parasite genetics and live‐cell imaging and complemented with data gained by transcriptomics and proteomics, in recent years, novel insights into the mosquito midgut phase of the malaria parasite were obtained. Two models were used in the majority of these studies, the rodent malaria parasite Plasmodium berghei and the in vitro cultivable human parasite Plasmodium falciparum.
This review summarizes the recent findings on the mosquito midgut stages of Plasmodium. In this context, three consequent steps are considered, the signalling cascade leading to gametogenesis, the formation of gametes and the zygote‐to‐ookinete conversion. A fourth aspect deals with the interplay between parasites and midgut factors, including the gut microbiota and epithelium, components of the human blood meal and insect immune molecules.
The initiation of gametogenesis
Sexual precursor cells, the intraerythrocytic gametocytes, develop in the human blood in response to stress factors (reviewed in Pradel, 2007; Kuehn and Pradel, 2010). A time period of about 10 days is required for gametocyte development in P. falciparum, during which they pass five morphological stages, termed stages I–V. Once the mature stage V gametocytes are ingested with the blood meal of an Anopheles mosquito, they are activated in the mosquito midgut by environmental stimuli, and gametogenesis is initiated (Fig. 1). Signals inducing gamete formation include a drop of temperature by approximately 5 °C, which is mandatory for gametocyte activation, and the presence of the mosquito‐derived molecule xanthurenic acid (XA), a metabolic intermediate of the tryptophan catabolism. An additional trigger of gametogenesis is the increase of extracellular pH from 7.2 to about 8 (Kawamoto et al., 1991; Billker et al., 1997, 1998; Garcia et al., 1998; Sologub et al., 2011).
Figure 1.
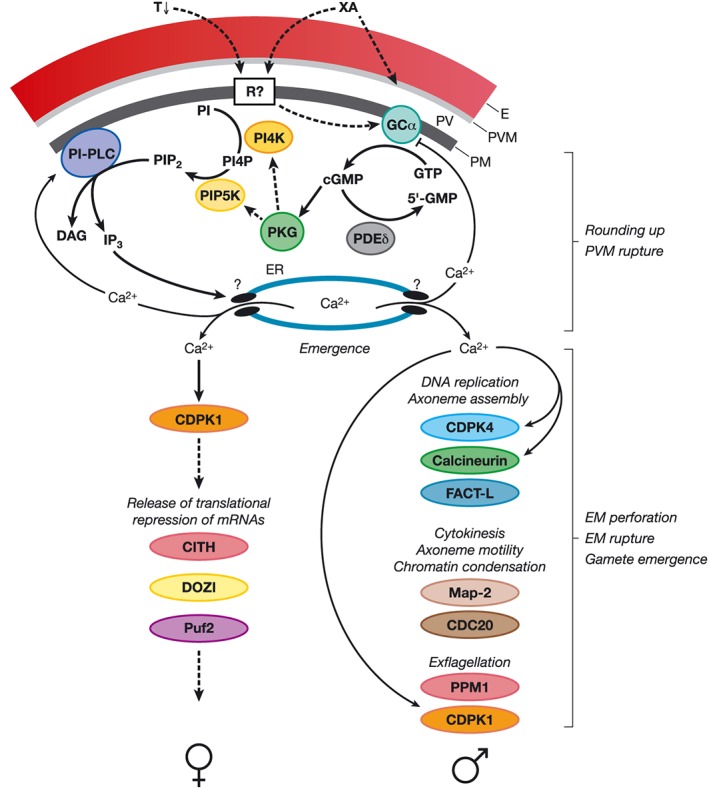
The signalling pathways involved in gametogenesis. Ca2+, calcium ion; CDC20, cell division cycle protein 20; CDPK, calcium‐dependent protein kinase; cGMP, cyclic guanosine monophosphate; CITH, homolog of worm CAR‐I and fly Trailer Hitch; DAG, diacylglycerol; DNA, deoxyribonucleic acid; DOZI, development of zygote inhibited; E, erythrocyte; EM, erythrocyte membrane; ER endoplasmic reticulum; FACT‐L, facilitates chromatin transcription protein L; GC, guanylyl cyclase; 5ʹ‐GMP, guanosine monophosphate; GTP, guanosine triphosphate; IP3, inositol triphosphate; Map‐2, Mitogen‐activated protein kinase 2; mRNA, messenger ribonucleic acid; PDE, phosphodiesterase; PI, phosphatidyl‐1D‐myo‐inositol; PI4K, phosphatidylinositol 4‐kinase; PI4P, phosphatidylinositol 4‐phosphate; PIP2, phosphatidylinositol‐4,5‐bisphosphate; PIP5K, phosphatidylinositol 4‐phosphate 5‐kinase; PI‐PLC, phosphoinositide‐specific phospholipase C; PKG, cGMP‐dependent protein kinase; PPM1, metallo‐dependent protein phosphatase 1; Puf2, Pumilio/FBF (fem‐3 binding factor) family protein 2; PV, parasitophorous vacuole; PVM, parasitophorous vacuole membrane; R, receptor; T, temperature; XA, xanthurenic acid.
Up to now, a plasmodial receptor that binds XA has not been identified. XA, however, was shown to trigger guanylyl cyclase activity in gametocyte membranes (Muhia et al., 2001). In P. falciparum, two integral guanylyl cyclases have been identified, GCα and GCβ (Carucci et al., 2000). While GCα was refractory to deletion in both P. falciparum and P. berghei, pointing to an essential role during erythrocytic replication, the gene coding for GCβ was successfully disrupted in both species. Parasites lacking GCβ were not impaired in gametogenesis, while in P. berghei revealed a severe defect in ookinete motility (Hirai et al., 2006; Taylor et al., 2008), indicating that GCα, not GCβ, is important for gametocyte activation.
Guanylyl cyclase activation leads to the synthesis of the second messenger cyclic GMP (cGMP), which was found to be dependent on the presence of either Mg2+ or Mn2+. Ca2+, on the other hand, was shown to inhibit guanylyl cyclase activity (Muhia et al., 2001). In eukaryotic cells, the intracellular cGMP concentrations are generally regulated by cGMP‐synthetizing guanylyl cyclases and cGMP‐hydrolyzing phosphodiesterases (PDE). The genome of P. falciparum encodes for four putative cyclic nucleotide PDEs, PDEα‐δ (Young et al., 2005). Chemical inhibition of PDE activity stimulates rounding up of mature gametocytes in the absence of XA, thereby verifying that increased cGMP‐levels trigger gametogenesis (McRobert et al., 2008) (Fig. 1). Interestingly, deletion of the gene coding for PDEδ leads to an impaired ability of P. falciparum to undergo gametogenesis, indicating that the tight regulation of intracellular cGMP concentrations is crucial for gametocyte activation and that premature, abnormally high cGMP levels have a deleterious effect on this process (Taylor et al., 2008).
Downstream signalling of gametogenesis involves activation of the cGMP‐dependent protein kinase (PKG) by cGMP. Chemical inhibition of PKG‐activity arrests gametogenesis of P. falciparum in a dose‐dependent manner, whereas mutant strains expressing an inhibitor‐insensitive PKG are not affected by inhibitor treatment (McRobert et al., 2008). Because rounding‐up of gametocytes is a Ca2+‐independent step and cannot be inhibited by chelators, PKG acts prior to Ca2+ increase in the activated gametocytes (Fig. 1).
Increase in cytosolic Ca2+ occurs approximately 10 s after XA‐mediated activation, as shown for P. berghei gametocytes (Billker et al., 2004), resulting in male gametogenesis. Ca2+ mobilization is linked to the phosphoinositide‐specific phospholipase C (PI‐PLC). Stimulation of PI‐PLC upon gametocyte activation by XA leads to the hydrolysis of phosphatidylinositol‐(4,5)‐bisphosphate (PIP2) into diacylglycerol (DAG) and inositol‐(1,4,5)‐trisphosphate (IP3) (Martin et al., 1994; Raabe et al., 2011) (Fig. 1). IP3 is suggested to be responsible for the opening of Ca2+‐channels in the endoplasmic reticulum, although no orthologue of an IP3 receptor channel has been identified in Plasmodium yet. PI‐PLC activity itself appears to be regulated by Ca2+, because it can be impaired by Ca2+ ionophores, pointing to a Ca2+‐regulated feedback mechanism.
Evidence suggests that the Ca2+ levels are further regulated by PKG. The enzyme controls the synthesis of phosphatidylinositol 4‐phosphate (PI4P) from phosphatidyl‐1D‐myo‐inositol (PI) and the following conversion to PIP2 via phosphorylation of the phosphoinositol kinases involved in these steps, phosphatidylinositol 4‐kinase (PI4K) and phosphatidylinositol 4‐phosphate 5‐kinase (PIP5K) (Brochet et al., 2014). PIP5K activity is further controlled by a small G protein of the ADP‐ribosylation factor (ARF1) family (Leber et al., 2009).
The increase of intracellular Ca2+ during induction of gametogenesis is sensed by specific Ca2+‐dependent protein kinases, the CDPKs. In female macrogametocytes, CDPK1 initiates the release of the translational repression of messenger RNAs (mRNAs) (Sebastian et al., 2012). Translational repression in macrogametocytes has been linked to components of regulatory ribonucleoprotein complexes. In P. berghei, a total of 731 mRNAs associate with the RNA helicase DOZI (development of zygote inhibited) and the Sm‐like factor CITH (homolog of worm CAR‐I and fly Trailer Hitch), which function in translational transcript repression, including mRNAs encoding for the GPI‐anchored female‐specific protein P25 and the glideosome‐associated proteins GAP45 and 50 (Mair et al., 2006, 2010; Guerreiro et al., 2014). For P. falciparum, translational repression of mRNAs was shown to involve the Pumilio/FBF (Puf) family RNA‐binding protein Puf2. Puf2 deletion leads to increased mRNA and protein levels in gametocytes, whereas overexpression of Puf2 further reduces their mRNA and protein levels (Miao et al., 2013). The repressed mRNAs mostly encode for proteins important for zygote‐to‐ookinete conversion (Table 1).
Table 1.
Summary of published data on proteins involved in malaria parasite development in the mosquito midgut
| Protein | Function | Species | Gender* | Reference |
|---|---|---|---|---|
| Actin 2 | Male gametogenesis; ookinete formation | P. berghei | M | Deligianni et al., 2011; Andreadaki et al., 2014 |
| Calcineurin | Male gametogenesis; fertilization | P. berghei | M | Philip and Waters, 2015 |
| CDC20 | Chromatin condensation; axoneme motility; cytokinesis (M) | P. berghei | Guttery et al., 2012a | |
| CDPK1 | Essential in ABS; P. berghei: ookinete development; release of translational repression of mRNAs (F); exflagellation (M) | P. falciparum | Sebastian et al., 2012 | |
| P. berghei | ||||
| CDPK3 | Ookinete motility; mosquito midgut invasion | P. berghei | Ishino et al., 2006; Siden‐Kiamos et al., 2006 | |
| CDPK4 | P. falciparum: microgamete egress; P. berghei: DNA synthesis; transmission to the mosquito | P. falciparum | M | Billker et al., 2004; Ojo et al., 2012, 2014 |
| P. berghei | ||||
| CelTOS | Ookinete midgut traversal | P. berghei | Kariu et al., 2006 | |
| Chitinase | Hydrolysis of peritrophic membrane during ookinete midgut traversal | P. falciparum | Vinetz et al., 1999, 2000; Langer et al., 2000; Dessens et al., 2001; Tsai et al., 2001 | |
| P. berghei | ||||
| P. gallinaceum | ||||
| CITH | Translational repression of mRNAs | P. berghei | F | Mair et al., 2006, 2009 |
| CTRP | Ookinete motility | P. falciparum | Trottein et al., 1995; Dessens et al., 1999; Yuda et al., 1999; Templeton et al., 2000; Li et al., 2004 | |
| P. berghei | ||||
| DHHC2 | Lipidation modification during zygote‐to‐ookinete conversion | P. berghei | Santos et al., 2015 | |
| DOZI | Translational repression of mRNAs | P. berghei | F | Mair et al., 2006, 2010 |
| FACT‐L | Essential in ABS; DNA replication (M) | P. berghei | Laurentino et al., 2011 | |
| GAK | Phosphate group transfer during ookinete formation | P. berghei | Tewari et al., 2010 | |
| GAP45 | Ookinete development | P. berghei | Sebastian et al., 2012 | |
| GAP50 | Part of the IMC; relocalization to the plasmalemma during gametogenesis; receptor of factor H in gametes | P. falciparum | Simon et al., 2013 | |
| GCα | Essential in ABS; signalling during GC activation; cGMP synthesis | P. falciparum | Carucci et al., 2000; Muhia et al., 2001 | |
| P. berghei | ||||
| GCβ | Ookinete motility | P. berghei | Hirai et al., 2006 | |
| GCS1 (HAP2) | Gamete fusion | P. berghei | Hirai et al., 2008; Liu et al., 2008 | |
| GEST | Osmiophilic body protein; GC egress; PV rupture | P. berghei | Talman et al., 2011 | |
| IMC1a, b, h | Alveolins important for mechanical strength of ookinete | P. berghei | Volkmann et al., 2012 | |
| ISP1 and 3 | Association with apical complex in retorts | P. berghei | Poulin et al., 2013 | |
| LCCL‐domain proteins | Microgamete‐macrogamete attachment | P. falciparum | F | Pradel et al., 2004; Simon et al., 2009, 2016 |
| P. berghei | ||||
| Map‐2 | Chromatin condensation; axoneme motility; cytokinesis | P. berghei | M | Rangarajan et al., 2005; Tewari et al., 2005 |
| MDV‐1/Peg3 | Osmiophilic body protein; GC egress; PV rupture | P. falciparum | Furuya et al., 2005; Silvestrini et al., 2005; Lanfrancotti et al., 2007; Ponzi et al., 2009; Olivieri et al., 2015 | |
| P. berghei | ||||
| Nek‐2 and Nek‐4 | Genome replication | P. falciparum | Nek‐4: F | Reininger et al., 2005, 2009 |
| P. berghei | ||||
| P25 | Ookinete surface protein; ookinete survival in the mosquito midgut, traversal of the epithelium; ookinete–oocyst transformation | P. berghei | F | Tomas et al., 2001 |
| P28 | Ookinete surface protein; ookinete survival in the mosquito midgut, traversal of the epithelium; ookinete–oocyst transformation | P. berghei | F | Tomas et al., 2001 |
| PDEδ | Hydrolysis of cGMP; rounding up of activated GC | P. falciparum | McRobert et al., 2008 | |
| PF16 | Axoneme motility | P. berghei | Straschil et al., 2010 | |
| Pg377 | Osmiophilic body protein; GC egress | P. falciparum | F (P. f.) | Alano et al., 1995; Severini et al., 1999; Olivieri et al., 2015 |
| P. berghei | ||||
| PI4K | Essential in ABS; phosphorylation of PI during GC activation | P. berghei | Brochet et al., 2014 | |
| PIP5K | P. berghei: essential in ABS; phosphorylation of PI4P during GC activation | P. falciparum | Leber et al., 2009; Brochet et al., 2014 | |
| P. berghei | ||||
| PI‐PLC | PIP2 degradation into DAG and IP3 during GC activation | P. falciparum | Martin et al., 1994; Raabe et al., 2011 | |
| P. berghei | ||||
| PK7 | Phosphate group transfer during ookinete formation | P. berghei | Tewari et al., 2010 | |
| PKG | Gametogenesis; regulation of Ca2+ levels; P. berghei: ookinete gliding | P. falciparum | McRobert et al., 2008; Moon et al., 2009; Brochet et al., 2014 | |
| P. berghei | ||||
| POS1‐10 | Ookinete surface protein | P. berghei | Kaneko et al., 2015 | |
| PPKL | Ookinete development and motility | P. berghei | F | Guttery et al., 2012b |
| PPLP2 | GC egress; EM perforation | P. falciparum | Deligianni et al., 2013; Wirth et al., 2014 | |
| P. berghei | ||||
| PPLP3‐5 | Ookinete midgut traversal | P. falciparum | PPLP4: F | Kadota et al., 2004; Ecker et al., 2007, 2008; Kaneko et al., 2015; Wirth et al., 2015 |
| P. berghei | ||||
| PPM1 | Exflagellation | P. berghei | Guttery et al., 2014 | |
| PPM2 | Ookinete development | P. berghei | Guttery et al., 2014 | |
| Ps230 | Rosetting; microgamete‐macrogamete attachment | P. falciparum | Eksi et al., 2006 | |
| Ps47 | Microgamete‐macrogamete attachment; evasion of TEP1‐related killing in the mosquito midgut | P. falciparum | F | van Schaijk et al., 2006; Molina‐Cruz et al., 2013 |
| P. berghei | ||||
| Ps48/45 | Microgamete–macrogamete attachment | P. falciparum | van Dijk et al., 2001 | |
| P. berghei | ||||
| PSOP1, 2, 6, 7, 12 | Ookinete‐secreted proteins | P. berghei | Kaneko et al., 2015 | |
| Puf2 | Translational repression of mRNAs | P. falciparum | Miao et al., 2013 | |
| SAS‐6 | Flagellum formation | P. berghei | M | Marques et al., 2015 |
| SHLP1 | Ookinete development | P. berghei | F | Patzewitz et al., 2013 |
| SOAP | Ookinete midgut traversal | P. berghei | Dessens et al., 2003 | |
| WARP | Ookinete midgut traversal | P. berghei | Yuda et al., 2001; Li et al., 2004 |
Proteins specifically expressed in male (M) or female (F) sexual stages. ABS, asexual blood stages; Ca2+, calcium ion; CDC20, cell division cycle protein 20; CDPK, calcium‐dependent protein kinase; CelTOS, cell‐traversal protein for ookinetes and sporozoites; cGMP, cyclic guanosine monophosphate; CITH, homolog of worm CAR‐I and fly Trailer Hitch; CTRP, circumsporozoite and thrombospondin‐related adhesive protein‐related protein; DAG, diacylglycerol; DHHC2, palmitoyl‐S‐acyl‐transferase; DOZI, development of zygote inhibited; EM, erythrocyte membrane; FACT‐L, facilitates chromatin transcription protein L; GAK, cyclin G‐associated kinase; GAP, glideosome‐associated protein; GC, gametocyte; GCα/β, guanylyl cyclase; GCS1/HAP2, generative cell specific 1; GEST, gamete egress and sporozoite traversal; IMC, inner membrane complex; IP3, inositol triphosphate; ISP, IMC sub‐compartment proteins; LCCL, Limulus coagulation factor C; Map‐2, Mitogen‐activated protein kinase 2; MDV‐1/Peg3, male development‐1/protein of early gametocyte 3; Nek, NIMA‐related kinase; PDE, phosphodiesterase; P. f., Plasmodium falciparum; PI, phosphatidyl‐1D‐myo‐inositol; PI4K, phosphatidylinositol 4‐kinase; PI4P, phosphatidylinositol 4‐phosphate; PIP2, phosphatidylinositol‐4,5‐bisphosphate; PIP5K, phosphatidylinositol 4‐phosphate 5‐kinase; PI‐PLC, phosphoinositide‐specific phospholipase C; PK7, protein kinase 7; PKG, cGMP‐dependent protein kinase; POS, putative ookinete surface‐associated protein; PPKL, kelch‐like domain‐containing phosphatase; PPLP, plasmodial perforin‐like protein; PPM, metallo‐dependent protein phosphatase; PSOP, putative secreted ookinete protein; Puf2, Pumilio/FBF (fem‐3 binding factor) family protein 2; PV, parasitophorous vacuole; SAS‐6; centriole/basal body protein; SHLP1; Shewanella‐like protein phosphatase 1; SOAP, secreted ookinete adhesive protein; TEP1, thioesther‐containing protein 1; WARP, von Willebrand factor A domain‐related protein.
Ca2+ is also important for microgametogenesis, because CDPK1 knock down leads to a delayed exflagellation in P. berghei (Sebastian et al., 2012). Another Ca2+ effector in male parasites is CDPK4, and P. berghei parasites lacking CDPK4 are not able to undergo DNA synthesis, which is a prerequisite for the following three mitotic divisions that result in the formation of eight male gametes (Billker et al., 2004). Chemical inhibition of CDPK4 was shown to inhibit egress of P. falciparum microgametes in vitro and transmission of P. berghei to the mosquito in vivo (Ojo et al., 2012, 2014) (Table 1).
Further, a plasmodial FACT (facilitates chromatin transcription) protein (termed FACT‐L) is linked to DNA replication in the males (Laurentino et al., 2011). Downstream of these events, the loss of mitogen‐activated protein kinase Map‐2 and cell division cycle protein CDC20 interferes with chromatin condensation, axoneme motility and cytokinesis during the formation of the male flagellar microgametes (Rangarajan et al., 2005; Tewari et al., 2005; Guttery et al., 2012a). Also, depletion of the metallo‐dependent protein phosphatase PPM1 in P. berghei results in impaired exflagellation (Guttery et al., 2014), while depletion of the Ca2+‐dependent phosphatase Calcineurin affects male gametogenesis and subsequent fertilization in the rodent malaria parasite (Philip and Waters, 2015) (Fig. 1).
The formation of gametes
The activation of gametocytes results in a first rapid step of stage conversion, during which the activated gametocytes round up, egress from the enveloping red blood cells and transform into fertile female macrogametes and male microgametes (Fig. 2). Gametocytes egress from the erythrocyte via an inside‐out mode, during which the membrane of the PV (PVM) ruptures prior to the opening of the erythrocyte membrane (EM). PVM rupture occurs at multiple sites and is a Ca2+‐independent event that takes less than 2 min following parasite uptake by the mosquito (Sologub et al., 2011). Electron‐dense vesicles, the osmiophilic bodies, can be observed accumulating underneath the rupture sites, which disappear simultaneously with the disintegration of the PVM, while the activated gametocytes are rounding‐up (Sologub et al., 2011; reviewed in Wirth and Pradel, 2012).
Figure 2.
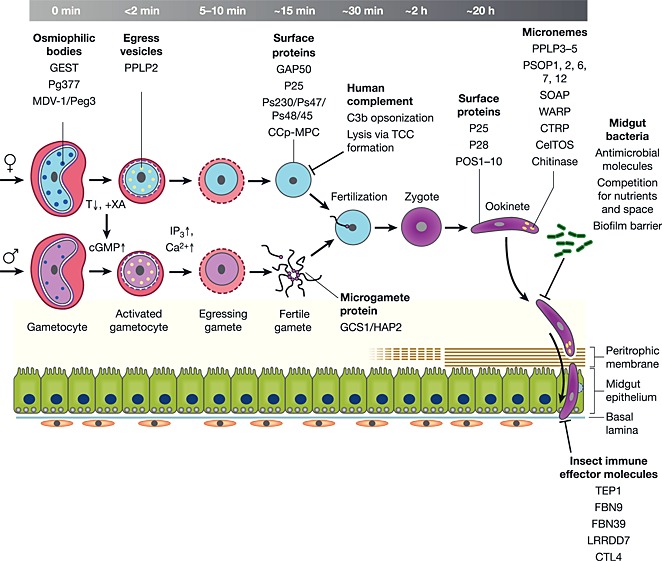
Molecules involved in malaria parasite development in the mosquito midgut. Ca2+, calcium ion; CCp‐MCP, CCp‐based multiprotein complex; CelTOS, cell‐traversal protein for ookinetes and sporozoites; CTL4, C‐type lectin 4; CTRP, circumsporozoite and thrombospondin‐related adhesive protein‐related protein; DAG, diacylglycerol; EM, erythrocyte membrane; FBN9/39, fibrinogen immunolectin 9/39; GAP50, glideosome‐associated protein 50; GCS1/HAP2, generative cell specific 1; GEST, gamete egress and sporozoite traversal; IP3, inositol triphosphate; LRRDD7, leucine‐rich‐repeat domain‐containing protein 7; MDV‐1, male development‐1; Peg3/4, protein of early gametocyte 3/4; PIP2, phosphatidylinositol‐4,5‐bisphosphate; PPLP2/3/4/5, Plasmodium perforin‐like protein 2/3/4/5; POS1‐10, putative ookinete surface‐associated protein 1‐10; PSOP1/2/6/7/12, putative secreted ookinete protein 1/2/6/7/12; PV, parasitophorous vacuole; SOAP, secreted ookinete adhesive protein; TCC, terminal complement complex; TEP1, thioesther‐containing protein 1; WARP, von Willebrand factor A domain‐related protein; XA, xanthurenic acid.
Osmiophilic bodies have originally been assigned to female gametocytes (Sinden, 1982; Aikawa et al., 1984); in P. berghei, they were meanwhile also reported in the males. However, they appear to be smaller and less abundant than female osmiophilic bodies (Olivieri et al., 2015) (Fig. 2). Female osmiophilic bodies contain gametocyte‐specific proteins like Pg377 (Alano et al., 1995; Severini et al., 1999; Olivieri et al., 2015), MDV‐1/Peg3 (Furuya et al., 2005; Silvestrini et al., 2005; Lanfrancotti et al., 2007; Ponzi et al., 2009) and GEST (gamete egress and sporozoite traversal; Talman et al., 2011), which are discharged into the PV following gametocyte activation. Gametocytes lacking MDV‐1/Peg3 or GEST are impaired in egress and stuck within the PV (Ponzi et al., 2009; Talman et al., 2011). Pg377, on the other hand, appears to play a role in egress of P. berghei but not of P. falciparum gametocytes (Suaréz‐Cortés et al., 2014; Olivieri et al., 2015) (Table 1).
In a second, Ca2+‐dependent step, a second type of vesicles is released into the cytoplasm of the host erythrocyte. These egress vesicles contain the plasmodial pore‐forming perforin PPLP2, which is able to perforate the EM. This event occurs approximately 6 min post‐activation of the gametocyte and results in the release of the erythrocyte cytoplasm; hence, the gamete is then contained by the EM only (Deligianni et al., 2013; Wirth et al., 2014). Approximately 15 min after uptake by the blood‐feeding mosquito, the EM opens and releases the fertile gamete (Sologub et al., 2011; Wirth et al., 2014; reviewed in Wirth and Pradel, 2012).
Gametocytes possess an inner membrane complex (IMC) underneath their plasmalemma, which consists of flat membranous sacs, the alveoli, and supporting structural elements and which is thought to be required for the stability of the crescent‐shaped parasite stages (Dearnley et al., 2012; Kono et al., 2012; Simon et al., 2013; reviewed in Harding and Meissner, 2014; Boucher and Bosch, 2015). During gametogenesis, the IMC disintegrates, resulting in gametes solely confined by the plasmalemma. Hitherto, the fate of the alveoli during breakdown is unknown; noteworthy, the isochronal formation of nanotubes in the developing gametes was previously reported. Nanotubes are long membranous tubules that originate from the gamete surface and represent long‐distance cell‐to‐cell connections proposed to facilitate contact between gametes as a pre‐requisite for mating (Rupp et al., 2011). While the question of the origin of the membranous tubules remains, one explanation might be that the alveolar membranes are reused for nanotube formation.
The activated microgametocyte replicates its genome three times, progressing from haploid to octaploid (Janse et al., 1986, 1988) and mitotically produces eight flagellar microgametes in a process termed exflagellation (Fig. 2). Flagellum formation requires axonemal assembly from basal bodies, which involves the centriole/basal body protein SAS‐6 (Marques et al., 2015). Axoneme motility, on the other hand, is regulated by the conserved Armadillo‐repeat protein PF16, and P. berghei parasites lacking this protein show abnormal flagellar movements (Straschil et al., 2010). Exflagellating microgametes typically adhere avidly to neighbouring erythrocytes and are hidden within such rosettes. In P. falciparum, rosetting involves among others the abundantly expressed surface‐associated six‐cys motif protein Ps230 (Eksi et al., 2006), as well as sialic acid and glycophorins of the erythrocyte membrane (Templeton et al., 1998).
During exflagellation, the microgamete detaches from the residual body and is freely motile in search of a macrogamete. Several proteins have been linked to microgamete attachment to macrogametes, including the LCCL‐domain proteins (termed CCp proteins in P. falciparum) and the six‐cys motif proteins Ps47, Ps48/45 and Ps230 (van Dijk et al., 2001; Pradel et al., 2004; van Schaijk et al., 2006; Simon et al., 2009, 2016). The latter two are considered as promising candidates for transmission blocking vaccines (reviewed in Pradel, 2007). It has recently been shown that in P. falciparum Ps230, Ps48/45 and the six CCp proteins assemble to multi‐protein complexes, which are linked via P25 to the macrogamete surface (Simon et al., 2009, 2016; reviewed in Kuehn et al., 2010).
Fertilization begins by the fusion of the two gamete plasma membranes, and the axoneme and attached male nucleus enter the female cytoplasm. While the exact proteins involved in initial binding of male and female gametes are yet unknown, gamete fusion is mediated by the microgamete protein GCS1 (generative cell specific 1; also termed HAP2), and disruption of the respective gene in P. berghei resulted in male sterility and blocked fertilization (Hirai et al., 2008; Liu et al., 2008). Following gamete fusion, nuclear fusion ensues, and over the next 3 h, meiosis occurs, and the zygote becomes tetraploid (Janse et al., 1986), a process involving the NIMA‐related kinases Nek‐2 and Nek‐4 (Reininger et al., 2005, 2009). Parasite tetraploidy persists throughout the ookinete stage until sporozoite budding in the oocyst restores the haploid state (Janse et al., 1986) (Table 1).
The zygote‐to‐ookinete conversion
Development of zygotes and their transformations into ookinetes is accompanied by strict regulations of transcript expression. A de novo synthesis study in zygotes identified 91 proteins synthesized only after fertilization, most of which are involved in motility and invasion (Sebastian et al., 2012). A recent cross‐fertilization study further showed that the zygote/ookinete stage exhibits a maternal phenotype either from maternal mRNA inheritance or transcription of the maternal alleles, while the respective paternal alleles are silenced in these stages (Ukegbu et al., 2015). Gene expression is further regulated by the apicomplexan transcription factor AP‐O, which associates with more than 500 genes important for ookinete development, motility, midgut penetration and protection against mosquito immunity (Yuda et al., 2009; Kaneko et al., 2015). Among the genes identified are the ones encoding for IMC components like GAP40, 45 and 50, micronemal secretory proteins like the perforins PPLP3‐5, the putative ookinete‐secreted proteins PSOP1, 2, 6, 7 and 12, the secreted ookinete adhesive protein SOAP, the von Willebrand factor A domain‐related protein WARP and for ookinete surface‐associated proteins like POS1‐10 or P25 and P28 (Kaneko et al., 2015). P25 and P28 are targets of highly effective transmission‐blocking antibodies, which interfere with oocyst development. The proteins exhibit multiple and partially redundant functions, for example, they play a role in ookinete survival in the mosquito midgut, traversal of the epithelium and ookinete–oocyst transformation (Tomas et al., 2001; reviewed in Pradel, 2007).
During the transformation of zygotes into the invasive ookinetes, intermediate stages, the retorts, develop, and here, the apical complex as well as the IMC is newly formed. When the ubiquitously expressed kinase CDPK1 is downregulated in the midgut stages of P. berghei, these arrest in the zygote stage and lack IMC components and microneme proteins like SOAP and the thrombospondin‐related adhesive protein‐related protein CTRP. Similarly, downregulation of GAP45 expression in the midgut stages resulted in zygotes unable to transform into ookinetes (Sebastian et al., 2012). Two IMC sub‐compartment proteins, ISP1 and 3, associate with the forming apical complex in P. berghei retorts, which are N‐myristoylated, phosphorylated and membrane‐bound (Poulin et al., 2013). Indeed, lipidation modifications appear to be important in general during zygote‐to‐ookinete conversion, and a palmitoyl‐S‐acyl‐transferase DHHC2 is crucial for this process (Santos et al., 2015). Further, the sexual stage‐specific actin isoform actin 2, which was originally reported to play a role in male gametogenesis of P. berghei, is additionally important for ookinete formation (Deligianni et al., 2011; Andreadaki et al., 2014). Also, deletion of three ookinete‐specific alveolins, IMC1a, b and h, resulted in reduced mechanical strength of this midgut stage (Volkmann et al., 2012) (Table 1).
Besides lipidations, post‐transcriptional modifications via phosphate group transfer are important for ookinete formation. P. berghei parasites lacking the protein kinase PK7 or the cyclin G‐associated kinase GAK show severely reduced ookinete numbers (Tewari et al., 2010). Further, deletion of the Shewanella‐like protein phosphatase (SHLP1), PPM2 or the kelch‐like domain‐containing phosphatase PPKL results in impaired P. berghei ookinete development, and phenotypes include impaired IMC and microneme formation (Guttery et al., 2012b, 2014; Philip et al., 2012; Patzewitz et al., 2013) (Table 1).
The maturation of ookinetes is completed between 19 and 36 h post‐blood meal, and the ookinetes then quickly exit the midgut lumen (Aikawa et al., 1984; Sinden et al., 1985; Vlachou et al., 2004). Ookinete motility appears to be regulated by cGMP levels, because disruption of the gene encoding GCβ in P. berghei impaired ookinete gliding (Hirai et al., 2006; Moon et al., 2009). A similar phenotype was observed, when an inhibitor of the cGMP‐dependent protein kinase PKG was added to the midgut stages; hence, an essential role of this kinase in ookinete motility is suggested (Moon et al., 2009). Also, the activity of CDPK3 is required for P. berghei ookinete motility and engagement with the mosquito midgut epithelium (Ishino et al., 2006; Siden‐Kiamos et al., 2006), suggesting that besides cGMP, Ca2+ is important for ookinete motility. In accord with these findings, quantitative phosphoproteomics comparing P. berghei ookinetes sensitive and resistant to PKG inhibitors demonstrated that the kinase is involved among others in phosphorylation of IMC components like GAP45 and IMC1b as well as of enzymes involved in the inositol phospholipid metabolism, like phosphoinositol kinases, which in consequence results in the maintenance of high cytosolic Ca2+ levels (Brochet et al., 2014) (Table 1).
An impressive number of micronemal proteins important for midgut traversal were identified in the past (Fig. 2). CTRP, once secreted, inserts into the ookinete surface to form a molecular link between the epithelium and the actin/myosin‐motor and hence to mediate motility (Trottein et al., 1995; Dessens et al., 1999; Yuda et al., 1999; Templeton et al., 2000; Li et al., 2004). Furthermore, three of the five plasmodial perforins were assigned to mediating midgut traversal via breaching of the epithelial membranes, that is, PPLP3‐5. Other microneme proteins assigned to midgut traversal of P. berghei ookinetes include SOAP, WARP and CelTOS (cell‐traversal protein for ookinetes and sporozoites) (Yuda et al., 2001; Dessens et al., 2003; Kadota et al., 2004; Li et al., 2004; Kariu et al., 2006; Ecker et al., 2007, 2008; Kaneko et al., 2015; Wirth et al., 2015) (Table 1).
One last challenge that the ookinete has to master before exiting the midgut lumen is the peritrophic membrane. This structure, secreted by the midgut epithelium, forms within 1–2 days post‐blood meal. It consists of chitin and cross‐linked proteins and functions in protecting the midgut from food particles as well as microbial infections (reviewed in Lehane, 1997). In order to breach through the peritrophic membrane before it has fully matured, the ookinetes secrete a chitinase able to hydrolyse the polysaccharide (Vinetz et al., 1999, 2000; Langer et al., 2000) (Table 1). Lack of this enzyme leads to reduced ookinete midgut infection (Dessens et al., 2001; Tsai et al., 2001).
The effect of midgut factors on parasite development
The mosquito midgut represents a major bottleneck during the life‐cycle of Plasmodium. Here, the midgut stages have to persevere for more than 20 h outside a protective host cell and have to defend themselves against factors of the blood meal like components of the human immune system, the natural midgut microbial flora and the innate immune system of the mosquito (Fig. 2). This exposure leads to an approximate 300‐fold loss of parasite abundance during the transmission to the mosquito (Vaughan et al., 1994).
After the blood meal, human complement is active in the mosquito midgut for 1 h and during this period represents a severe threat for the emerging gametes (Simon et al., 2013). The gametes, though, are able to evade human complement by binding the complement regulatory protein factor H from the blood meal, in consequence inactivating complement factor C3b and thus preventing complement‐induced lysis. Interestingly, a new study showed that factor H is also captured by the Anopheles midgut epithelium to inactivate the human complement cascade that would be able to harm the mosquito (Khattab et al., 2015).
Interestingly, the glideosome‐associated protein GAP50 was identified as a factor H‐binding receptor of gametes. GAP50, a transmembrane protein, is originally present in the outer alveolar membrane of gametocytes but relocates to the plasmalemma during gametogenesis. During relocation, the N‐terminal part of the protein, which originally extends into the alveolar lumen, becomes extracellularly exposed and is then able to highjack factor H from the blood meal (Simon et al., 2013). It is noticeable that after EM perforation by PPLP2, the forming gametes remain sheltered by the EM for about 10 min before they eventually egress from the host cell, and this prolonged stay might represent a strategy of the parasite to transform into fertile gametes unscathed.
Other midgut factors leading to parasite killing are mosquito‐derived immune response molecules targeting the ingested Plasmodium parasites. Their activation is triggered by the recognition of pathogen‐associated molecular patterns (PAMPs) present on the pathogen surfaces like the ones of midgut bacteria or malaria midgut stages via pattern recognition receptors (PRRs). This process leads to the upregulation of different effector molecules from diverse gene families, among others encoding for leucine rich‐repeat (LRR) domain‐containing proteins, fibrinogen‐related proteins (FREPs) and C‐type lectins (CTLs) as identified by microarray analyses and RNAi‐based studies (Christophides et al., 2002; Dimopoulos et al., 2002; Osta et al., 2004; reviewed in Cirimotich et al., 2010).
Microarray analyses identified several mosquito‐derived molecules like the thioester‐containing protein 1 (TEP1), the fibrinogen immunolectin 9 (FBN9), the fibrinogen immunolectin 39 (FBN39), the Leucine rich‐repeat domain‐containing protein 7 (LRRDD7) and the C‐type lectin 4 (CTL4) that exhibit anti‐plasmodial and anti‐bacterial activity (Dong et al., 2006). This suggests that the mosquito uses besides the direct anti‐plasmodial immune response also the anti‐bacterial immune response, initiated through increased multiplication of bacteria after the blood meal, to fight against Plasmodium infection, which makes the bacterial midgut flora an important regulator of the permissiveness of Anopheles to Plasmodium parasites (Dong et al., 2006, 2009).
Several studies showed that the malaria vector Anopheles gambiae defends itself against invading Plasmodium parasites by activating a complement‐like pathway with the complement C3‐like protein TEP1, which is responsible for the killing and clearance through lysis and melanization of Plasmodium ookinetes that traverse the midgut epithelium (Blandin et al., 2004; Fraiture et al., 2009; Povelones et al., 2011). TEP1 is secreted into the hemolymph by the phagocytic hemocytes and knockdown of TEP1 leads to a fivefold increase of developing oocysts. For the binding of TEP1 to the parasite surface, two members of the LRR protein family, LRIM1 and APL1C, are required, which form a disulfide‐bonded complex (Fraiture et al., 2009; Povelones et al., 2011). This complex helps to stabilize and promote the binding of a proteolytically cleaved mature TEP1 (Povelones et al., 2011). Noteworthy, P. falciparum is able to evade this TEP1‐related killing with the help of the surface‐associated Ps47 via suppression of nitration processes involved in the activation of the complement‐like pathway in the mosquito (Molina‐Cruz et al., 2013).
Because the parasite midgut stages are directly exposed to midgut bacteria, the microbiome composition is of large interest. 16S rRNA analyses using field‐caught mosquitoes identified mainly members of the class Proteobacteria with species of the genus Enterobacter, Serratia and Pantoea (Cirimotich et al., 2011a; Boissière et al., 2012). Furthermore, in lab‐reared A. gambiae and Anopheles stephensi mosquitoes Gram‐negative bacteria belonging to the genus Elizabethkingia were identified as the most prominent species, and the prevalence of Elizabethkingia is accompanied with the decrease of the microbial diversity during larvae‐to‐adult conversion (Boissière et al., 2012; Ngwa et al., 2013). A negative correlation between the amount of gut bacteria and P. falciparum infection rates was previously shown and removal of the natural midgut flora via antibiotic treatment leads to a higher susceptibility of A. gambiae to P. falciparum (Pumpuni et al., 1993; Dong et al., 2009; Meister et al., 2009; Cirimotich et al., 2011b). For example, the bacterium Serratia marcescens reduces infection by rodent Plasmodium ookinetes in field‐caught A. gambiae (Bando et al., 2013). While no differences in ookinete numbers between mosquitoes harbouring S. marcescens and aseptic mosquitoes were observed in the midgut lumen, there were approximately eightfold more ookinetes counted within the midgut epithelium of aseptic mosquitoes, indicating that the bacterium influences ookinete invasion of the epithelium.
In some cases, the molecular mechanisms of bacterial defeat of plasmodial intruders were investigated in more detail. The Enterobacter isolate Esp_Z inhibits plasmodial development in the mosquito midgut by producing reactive oxygen species both in vivo and vitro (Cirimotich et al., 2011b). Further, the midgut bacterium Elisabethkingia meningoseptica, isolated from lab‐reared A. stephensi mosquitoes, was demonstrated to produce an anti‐microbial compound active against Plasmodium parasites. Ethyl acetate extracts of this bacterium exhibit anti‐plasmodial effects against the asexual blood stages as well as against gametocyte and mosquito midgut stages of P. falciparum (Ngwa et al., 2013).
Conclusions
The mosquito midgut stages of malaria parasites mark the interface between human host and insect vector. Due to this unique role, they represent optimal objects to study a broad range of biological processes, including stage conversion and parasite/host co‐adaptation. As bottleneck stages of the life‐cycle, they are also prime targets for transmission‐blocking intervention strategies aimed to inhibit spread of the disease by the mosquito. In the past, several players of gametogenesis and zygote‐to‐ookinete conversion have been identified, some of which represent targets for transmission‐blocking vaccines or drugs. However, so far, the identified players represent isolated jigsaw pieces and more work is needed to piece together the puzzle in order to get the big picture on malaria parasite development in the mosquito midgut.
Acknowledgements
The authors thank Christian Flueck and David Baker (London School of Hygiene and Tropical Medicine) for helpful discussions. We further acknowledge funding by the Deutsche Forschungsgemeinschaft (to G. P.).
Bennink, S. , Kiesow, M. J. , and Pradel, G. (2016) The development of malaria parasites in the mosquito midgut. Cellular Microbiology, 18: 905–918. doi: 10.1111/cmi.12604.
References
- Aikawa, M. , Carter, R. , Ito, Y. , and Nijhout, M.M. (1984) New observations on gametogenesis, fertilization, and zygote transformation in Plasmodium gallinaceum . J Protozool 31: 403–13. [DOI] [PubMed] [Google Scholar]
- Alano, P. , Read, D. , Bruce, M. , Aikawa, M. , Kaido, T. , Tegoshi, T. , et al. (1995) COS cell expression cloning of Pfg377, a Plasmodium falciparum gametocyte antigen associated with osmiophilic bodies. Mol Biochem Parasitol 74: 143–56. [DOI] [PubMed] [Google Scholar]
- Aly, A.S.I. , Vaughan, A.M. , and Kappe, S.H.I. (2009) Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol 63: 195–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadaki, M. , Morgan, R.N. , Deligianni, E. , Kooij, T.W.A. , Santos, J.M. , Spanos, L. , et al. (2014) Genetic crosses and complementation reveal essential functions for the Plasmodium stage‐specific actin2 in sporogonic development. Cell Microbiol 16: 751–67. [DOI] [PubMed] [Google Scholar]
- Bando, H. , Okado, K. , Guelbeogo, W.M. , Badolo, A. , Aonuma, H. , Nelson, B. , et al. (2013) Intra‐specific diversity of Serratia marcescens in Anopheles mosquito midgut defines Plasmodium transmission capacity. Sci Rep 3: 1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker, O. , Shaw, M.K. , Margos, G. , and Sinden, R.E. (1997) The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro . Parasitology 115: 1–7. [DOI] [PubMed] [Google Scholar]
- Billker, O. , Lindo, V. , Panico, M. , Etienne, A.E. , Paxton, T. , Dell, A. , et al. (1998) Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392: 289–292. [DOI] [PubMed] [Google Scholar]
- Billker, O. , Dechamps, S. , Tewari, R. , Wenig, G. , Franke‐Fayard, B. , and Brinkmann, V. (2004) Calcium and a calcium‐dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117: 503–514. [DOI] [PubMed] [Google Scholar]
- Blandin, S. , Shiao, S.‐H. , Moita, L.F. , Janse, C.J. , Waters, A.P. , Kafatos, F.C. , and Levashina, E.A. (2004) Complement‐like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae . Cell 116: 661–70. [DOI] [PubMed] [Google Scholar]
- Boissière, A. , Tchioffo, M.T. , Bachar, D. , Abate, L. , Marie, A. , Nsango, S.E. , et al. (2012) Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog 8 e1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher, L.E. , and Bosch, J. (2015) The apicomplexan glideosome and adhesins – structures and function. J Struct Biol 190: 93–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet, M. , Collins, M.O. , Smith, T.K. , Thompson, E. , Sebastian, S. , Volkmann, K. , et al. (2014) Phosphoinositide metabolism links cGMP‐dependent protein kinase G to essential Ca2+ signals at key decision points in the life cycle of malaria parasites. PLoS Biol 12 e1001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carucci, D.J. , Witney, A.A. , Muhia, D.K. , Warhurst, D.C. , Schaap, P. , Meima, M. , et al. (2000) Guanylyl cyclase activity associated with putative bifunctional integral membrane proteins in Plasmodium falciparum . J Biol Chem 275: 22147–56. [DOI] [PubMed] [Google Scholar]
- Christophides, G.K. , Zdobnov, E. , Barillas‐Mury, C. , Birney, E. , Blandin, S. , Blass, C. , et al. (2002) Immunity‐related genes and gene families in Anopheles gambiae . Science 298: 159–65. [DOI] [PubMed] [Google Scholar]
- Cirimotich, C.M. , Dong, Y. , Garver, L.S. , Sim, S. , and Dimopoulos, G. (2010) Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol 34: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich, C.M. , Ramirez, J.L. , and Dimopoulos, G. (2011a) Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 10: 307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich, C.M. , Dong, Y. , Clayton, A.M. , Sandiford, S.L. , Souza‐Neto, J.A. , Mulenga, M. , and Dimopoulos, G. (2011b) Natural microbe‐mediated refractoriness to Plasmodium infection in Anopheles gambiae . Science 332: 855–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnley, M.K. , Yeoman, J.A. , Hanssen, E. , Kenny, S. , Turnbull, L. , Whitchurch, C.B. , et al. (2012) Origin, composition, organization and function of the inner membrane complex of Plasmodium falciparum gametocytes. J Cell Sci 125: 2053–63. [DOI] [PubMed] [Google Scholar]
- Deligianni, E. , Morgan, R.N. , Bertuccini, L. , Kooij, T.W.A. , Laforge, A. , Nahar, C. , et al. (2011) Critical role for a stage‐specific actin in male exflagellation of the malaria parasite. Cell Microbiol 13: 1714–30. [DOI] [PubMed] [Google Scholar]
- Deligianni, E. , Morgan, R.N. , Bertuccini, L. , Wirth, C.C. , Silmon de Monerri, N.C. , Spanos, L. , et al. (2013) A perforin‐like protein mediates disruption of the erythrocyte membrane during egress of Plasmodium berghei male gametocytes. Cell Microbiol 15: 1438–55. [DOI] [PubMed] [Google Scholar]
- Dessens, J.T. , Beetsma, A.L. , Dimopoulos, G. , Wengelnik, K. , Crisanti, A. , Kafatos, F.C. , and Sinden, R.E. (1999) CTRP is essential for mosquito infection by malaria ookinetes. EMBO J 18: 6221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens, J.T. , Mendoza, J. , Claudianos, C. , Vinetz, J.M. , Khater, E. , Hassard, S. , et al. (2001) Knockout of the rodent malaria parasite chitinase pbCHT1 reduces infectivity to mosquitoes. Infect Immun 69: 4041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens, J.T. , Sidén‐Kiamos, I. , Mendoza, J. , Mahairaki, V. , Khater, E. , Vlachou, D. , et al. (2003) SOAP, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development. Mol Microbiol 49: 319–29. [DOI] [PubMed] [Google Scholar]
- Dijk, M.R., van , Janse, C.J. , Thompson, J. , Waters, A.P. , Braks, J.A. , Dodemont, H.J. , et al. (2001) A central role for P48/45 in malaria parasite male gamete fertility. Cell 104: 153–64. [DOI] [PubMed] [Google Scholar]
- Dimopoulos, G. , Christophides, G.K. , Meister, S. , Schultz, J. , White, K.P. , Barillas‐Mury, C. , and Kafatos, F.C. (2002) Genome expression analysis of Anopheles gambiae: responses to injury, bacterial challenge, and malaria infection. Proc Natl Acad Sci U S A 99: 8814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , Aguilar, R. , Xi, Z. , Warr, E. , Mongin, E. , and Dimopoulos, G. (2006) Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog 2 e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , Manfredini, F. , and Dimopoulos, G. (2009) Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5 e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker, A. , Pinto, S.B. , Baker, K.W. , Kafatos, F.C. , and Sinden, R.E. (2007) Plasmodium berghei: Plasmodium perforin‐like protein 5 is required for mosquito midgut invasion in Anopheles stephensi . Exp Parasitol 116: 504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker, A. , Bushell, E.S.C. , Tewari, R. , and Sinden, R.E. (2008) Reverse genetics screen identifies six proteins important for malaria development in the mosquito. Mol Microbiol 70: 209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksi, S. , Czesny, B. , Gemert, G.‐J., van , Sauerwein, R.W. , Eling, W. , and Williamson, K.C. (2006) Malaria transmission‐blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol 61: 991–8. [DOI] [PubMed] [Google Scholar]
- Fraiture, M. , Baxter, R.H.G. , Steinert, S. , Chelliah, Y. , Frolet, C. , Quispe‐Tintaya, W. , et al. (2009) Two mosquito LRR proteins function as complement control factors in the TEP1‐mediated killing of Plasmodium. Cell Host Microbe 5: 273–84. [DOI] [PubMed] [Google Scholar]
- Furuya, T. , Mu, J. , Hayton, K. , Liu, A. , Duan, J. , Nkrumah, L. , et al. (2005) Disruption of a Plasmodium falciparum gene linked to male sexual development causes early arrest in gametocytogenesis. Proc Natl Acad Sci U S A 102: 16813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, G.E. , Wirtz, R.A. , Barr, J.R. , Woolfitt, A. , and Rosenberg, R. (1998) Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. J Biol Chem 273: 12003–12005. [DOI] [PubMed] [Google Scholar]
- Ghosh, A.K. , and Jacobs‐Lorena, M. (2009) Plasmodium sporozoite invasion of the mosquito salivary gland. Curr Opin Microbiol 12: 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro, A. , Deligianni, E. , Santos, J.M. , Silva, P.A.G.C. , Louis, C. , Pain, A. , et al. (2014) Genome‐wide RIP‐Chip analysis of translational repressor‐bound mRNAs in the Plasmodium gametocyte. Genome Biol 15: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery, D.S. , Ferguson, D.J.P. , Poulin, B. , Xu, Z. , Straschil, U. , Klop, O. , et al. (2012a) A putative homologue of CDC20/CDH1 in the malaria parasite is essential for male gamete development. PLoS Pathog 8 e1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery, D.S. , Poulin, B. , Ferguson, D.J.P. , Szöőr, B. , Wickstead, B. , Carroll, P.L. , et al. (2012b) A unique protein phosphatase with kelch‐like domains (PPKL) in Plasmodium modulates ookinete differentiation, motility and invasion. PLoS Pathog 8 e1002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery, D.S. , Poulin, B. , Ramaprasad, A. , Wall, R.J. , Ferguson, D.J.P. , Brady, D. , et al. (2014) Genome‐wide functional analysis of Plasmodium protein phosphatases reveals key regulators of parasite development and differentiation. Cell Host Microbe 16: 128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, C.R. , and Meissner, M. (2014) The inner membrane complex through development of Toxoplasma gondii and Plasmodium . Cell Microbiol 16: 632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai, M. , Arai, M. , Kawai, S. , and Matsuoka, H. (2006) PbGCbeta is essential for Plasmodium ookinete motility to invade midgut cell and for successful completion of parasite life cycle in mosquitoes. J Biochem 140: 747–57. [DOI] [PubMed] [Google Scholar]
- Hirai, M. , Arai, M. , Mori, T. , Miyagishima, S.‐Y. , Kawai, S. , Kita, K. , et al. (2008) Male fertility of malaria parasites is determined by GCS1, a plant‐type reproduction factor. Curr Biol 18: 607–13. [DOI] [PubMed] [Google Scholar]
- Ishino, T. , Orito, Y. , Chinzei, Y. , and Yuda, M. (2006) A calcium‐dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol 59: 1175–84. [DOI] [PubMed] [Google Scholar]
- Janse, C.J. , Klooster, P.F., van der , Kaay, H.J., van der , Ploeg, M., van der , and Overdulve, J.P. (1986) DNA synthesis in Plasmodium berghei during asexual and sexual development. Mol Biochem Parasitol 20: 173–82. [DOI] [PubMed] [Google Scholar]
- Janse, C.J. , Ponnudurai, T. , Lensen, A.H. , Meuwissen, J.H. , Ramesar, J. , Ploeg, M.V., der , and Overdulve, J.P. (1988) DNA synthesis in gametocytes of Plasmodium falciparum . Parasitology 96: 1–7. [DOI] [PubMed] [Google Scholar]
- Kadota, K. , Ishino, T. , Matsuyama, T. , Chinzei, Y. , and Yuda, M. (2004) Essential role of membrane‐attack protein in malarial transmission to mosquito host. Proc Natl Acad Sci U S A 101: 16310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko, I. , Iwanaga, S. , Kato, T. , Kobayashi, I. , and Yuda, M. (2015) Genome‐wide identification of the target genes of AP2‐O, a Plasmodium AP2‐family transcription factor. PLoS Pathog 11 e1004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariu, T. , Ishino, T. , Yano, K. , Chinzei, Y. , and Yuda, M. (2006) CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol 59: 1369–79. [DOI] [PubMed] [Google Scholar]
- Kawamoto, F. , Alejo‐Blanco, R. , Fleck, S.L. , and Sinden, R.E. (1991) Plasmodium berghei: ionic regulation and the induction of gametogenesis. Exp Parasitol 72: 33–42. [DOI] [PubMed] [Google Scholar]
- Khattab, A. , Barroso, M. , Miettinen, T. , and Meri, S. (2015) Anopheles midgut epithelium evades human complement activity by capturing factor H from the blood meal. PLoS Negl Trop Dis 9 e0003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono, M. , Herrmann, S. , Loughran, N.B. , Cabrera, A. , Engelberg, K. , Lehmann, C. , et al. (2012) Evolution and architecture of the inner membrane complex in asexual and sexual stages of the malaria parasite. Mol Biol Evol 29: 2113–32. [DOI] [PubMed] [Google Scholar]
- Kuehn, A. , and Pradel, G. (2010) The coming‐out of malaria gametocytes. J Biomed Biotechnol 2010: 976827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn, A. , Simon, N. , and Pradel, G. (2010) Family members stick together: multi‐protein complexes of malaria parasites. Med Microbiol Immunol 199: 209–26. [DOI] [PubMed] [Google Scholar]
- Lanfrancotti, A. , Bertuccini, L. , Silvestrini, F. , and Alano, P. (2007) Plasmodium falciparum: mRNA co‐expression and protein co‐localisation of two gene products upregulated in early gametocytes. Exp Parasitol 116: 497–503. [DOI] [PubMed] [Google Scholar]
- Langer, R.C. , Hayward, R.E. , Tsuboi, T. , Tachibana, M. , Torii, M. , and Vinetz, J.M. (2000) Micronemal transport of Plasmodium ookinete chitinases to the electron‐dense area of the apical complex for extracellular secretion. Infect Immun 68: 6461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurentino, E.C. , Taylor, S. , Mair, G.R. , Lasonder, E. , Bartfai, R. , Stunnenberg, H.G. , et al. (2011) Experimentally controlled downregulation of the histone chaperone FACT in Plasmodium berghei reveals that it is critical to male gamete fertility. Cell Microbiol 13: 1956–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber, W. , Skippen, A. , Fivelman, Q.L. , Bowyer, P.W. , Cockcroft, S. , and Baker, D.A. (2009) A unique phosphatidylinositol 4‐phosphate 5‐kinase is activated by ADP‐ribosylation factor in Plasmodium falciparum . Int J Parasitol 39: 645–653. [DOI] [PubMed] [Google Scholar]
- Lehane, M.J. (1997) Peritrophic matrix structure and function. Annu Rev Entomol 42: 525–50. [DOI] [PubMed] [Google Scholar]
- Li, F. , Templeton, T.J. , Popov, V. , Comer, J.E. , Tsuboi, T. , Torii, M. , and Vinetz, J.M. (2004) Plasmodium ookinete‐secreted proteins secreted through a common micronemal pathway are targets of blocking malaria transmission. J Biol Chem 279: 26635–44. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Tewari, R. , Ning, J. , Blagborough, A.M. , Garbom, S. , Pei, J. , et al. (2008) The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22: 1051–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair, G.R. , Braks, J.A.M. , Garver, L.S. , Wiegant, J.C.A.G. , Hall, N. , Dirks, R.W. , et al. (2006) Regulation of sexual development of Plasmodium by translational repression. Science 313: 667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair, G.R. , Lasonder, E. , Garver, L.S. , Franke‐Fayard, B.M.D. , Carret, C.K. , Wiegant, J.C.A.G. , et al. (2010) Universal features of post‐transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog 6 e1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, S.R. , Ramakrishnan, C. , Carzaniga, R. , Blagborough, A.M. , Delves, M.J. , Talman, A.M. , and Sinden, R.E. (2015) An essential role of the basal body protein SAS‐6 in Plasmodium male gamete development and malaria transmission. Cell Microbiol 17: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S.K. , Jett, M. , and Schneider, I. (1994) Correlation of phosphoinositide hydrolysis with exflagellation in the malaria microgametocyte. J Parasitol 80: 371–378. [PubMed] [Google Scholar]
- McRobert, L. , Taylor, C.J. , Deng, W. , Fivelman, Q.L. , Cummings, R.M. , Polley, S.D. , et al. (2008) Gametogenesis in malaria parasites is mediated by the cGMP‐dependent protein kinase. PLoS Biol 6 e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister, S. , Agianian, B. , Turlure, F. , Relógio, A. , Morlais, I. , Kafatos, F.C. , and Christophides, G.K. (2009) Anopheles gambiae PGRPLC‐mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog 5 e1000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard, R. , Tavares, J. , Cockburn, I. , Markus, M. , Zavala, F. , and Amino, R. (2013) Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol 11: 701–12. [DOI] [PubMed] [Google Scholar]
- Miao, J. , Fan, Q. , Parker, D. , Li, X. , Li, J. , and Cui, L. (2013) Puf mediates translation repression of transmission‐blocking vaccine candidates in malaria parasites. PLoS Pathog 9 e1003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina‐Cruz, A. , Garver, L.S. , Alabaster, A. , Bangiolo, L. , Haile, A. , Winikor, J. , et al. (2013) The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340: 984–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, R.W. , Taylor, C.J. , Bex, C. , Schepers, R. , Goulding, D. , Janse, C.J. , et al. (2009) A cyclic GMP signalling module that regulates gliding motility in a malaria parasite. PLoS Pathog 5 e1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhia, D.K. , Swales, C.A. , Deng, W. , Kelly, J.M. , and Baker, D.A. (2001) The gametocyte‐activating factor xanthurenic acid stimulates an increase in membrane‐associated guanylyl cyclase activity in the human malaria parasite Plasmodium falciparum . Mol Microbiol 42: 553–60. [DOI] [PubMed] [Google Scholar]
- Ngwa, C.J. , Glöckner, V. , Abdelmohsen, U.R. , Scheuermayer, M. , Fischer, R. , Hentschel, U. , and Pradel, G. (2013) 16S rRNA gene‐based identification of Elizabethkingia meningoseptica (Flavobacteriales: Flavobacteriaceae) as a dominant midgut bacterium of the Asian malaria vector Anopheles stephensi (Dipteria: Culicidae) with antimicrobial activities. J Med Entomol 50: 404–14. [DOI] [PubMed] [Google Scholar]
- Ojo, K.K. , Pfander, C. , Mueller, N.R. , Burstroem, C. , Larson, E.T. , Bryan, C.M. , et al. (2012) Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J Clin Invest 122: 2301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo, K.K. , Eastman, R.T. , Vidadala, R. , Zhang, Z. , Rivas, K.L. , Choi, R. , et al. (2014) A specific inhibitor of PfCDPK4 blocks malaria transmission: chemical‐genetic validation. J Infect Dis 209: 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri, A. , Bertuccini, L. , Deligianni, E. , Franke‐Fayard, B. , Currà, C. , Siden‐Kiamos, I. , et al. (2015) Distinct properties of the egress‐related osmiophilic bodies in male and female gametocytes of the rodent malaria parasite Plasmodium berghei . Cell Microbiol 17: 355–68. [DOI] [PubMed] [Google Scholar]
- Osta, M.A. , Christophides, G.K. , and Kafatos, F.C. (2004) Effects of mosquito genes on Plasmodium development. Science 303: 2030–2. [DOI] [PubMed] [Google Scholar]
- Patzewitz, E.‐M. , Guttery, D.S. , Poulin, B. , Ramakrishnan, C. , Ferguson, D.J.P. , Wall, R.J. , et al. (2013) An ancient protein phosphatase, SHLP1, is critical to microneme development in Plasmodium ookinetes and parasite transmission. Cell Rep 3: 622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, N. , Vaikkinen, H.J. , Tetley, L. , and Waters, A.P. (2012) A unique Kelch domain phosphatase in Plasmodium regulates ookinete morphology, motility and invasion. PLoS One 7: e44617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, N. , and Waters, A.P. (2015) Conditional degradation of Plasmodium calcineurin reveals functions in parasite colonization of both host and vector. Cell Host Microbe 18: 122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzi, M. , Sidén‐Kiamos, I. , Bertuccini, L. , Currà, C. , Kroeze, H. , Camarda, G. , et al. (2009) Egress of Plasmodium berghei gametes from their host erythrocyte is mediated by the MDV‐1/PEG3 protein. Cell Microbiol 11: 1272–88. [DOI] [PubMed] [Google Scholar]
- Poulin, B. , Patzewitz, E.‐M. , Brady, D. , Silvie, O. , Wright, M.H. , Ferguson, D.J.P. , et al. (2013) Unique apicomplexan IMC sub‐compartment proteins are early markers for apical polarity in the malaria parasite. Biol Open 2: 1160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones, M. , Upton, L.M. , Sala, K.A. , and Christophides, G.K. (2011) Structure‐function analysis of the Anopheles gambiae LRIM1/APL1C complex and its interaction with complement C3‐like protein TEP1. PLoS Pathog 7 e1002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel, G. , Hayton, K. , Aravind, L. , Iyer, L.M. , Abrahamsen, M.S. , Bonawitz, A. , et al. (2004) A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. J Exp Med 199: 1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel, G. (2007) Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology 134: 1911–29. [DOI] [PubMed] [Google Scholar]
- Pumpuni, C.B. , Beier, M.S. , Nataro, J.P. , Guers, L.D. , and Davis, J.R. (1993) Plasmodium falciparum: inhibition of sporogonic development in Anopheles stephensi by Gram‐negative bacteria. Exp Parasitol 77: 195–9. [DOI] [PubMed] [Google Scholar]
- Raabe, A.C. , Wengelnik, K. , Billker, O. , and Vial, H.J. (2011) Multiple roles for Plasmodium berghei phosphoinositide‐specific phospholipase C in regulating gametocyte activation and differentiation. Cell Microbiol 13: 955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan, R. , Bei, A.K. , Jethwaney, D. , Maldonado, P. , Dorin, D. , Sultan, A.A. , and Doerig, C. (2005) A mitogen‐activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei . EMBO Rep 6: 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininger, L. , Billker, O. , Tewari, R. , Mukhopadhyay, A. , Fennell, C. , Dorin‐Semblat, D. , et al. (2005) A NIMA‐related protein kinase is essential for completion of the sexual cycle of malaria parasites. J Biol Chem 280: 31957–64. [DOI] [PubMed] [Google Scholar]
- Reininger, L. , Tewari, R. , Fennell, C. , Holland, Z. , Goldring, D. , Ranford‐Cartwright, L. , et al. (2009) An essential role for the Plasmodium Nek‐2 Nima‐related protein kinase in the sexual development of malaria parasites. J Biol Chem 284: 20858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp, I. , Sologub, L. , Williamson, K.C. , Scheuermayer, M. , Reininger, L. , Doerig, C. , et al. (2011) Malaria parasites form filamentous cell‐to‐cell connections during reproduction in the mosquito midgut. Cell Res 21: 683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, J.M. , Kehrer, J. , Franke‐Fayard, B. , Frischknecht, F. , Janse, C.J. , and Mair, G.R. (2015) The Plasmodium palmitoyl‐S‐acyl‐transferase DHHC2 is essential for ookinete morphogenesis and malaria transmission. Sci Rep 5: 16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaijk, B.C.L., van , Dijk, M.R., van , Vegte‐Bolmer, M., van de , Gemert, G.‐J., van , Dooren, M.W., van , Eksi, S. , et al. (2006) Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum . Mol Biochem Parasitol 149: 216–22. [DOI] [PubMed] [Google Scholar]
- Sebastian, S. , Brochet, M. , Collins, M.O. , Schwach, F. , Jones, M.L. , Goulding, D. , et al. (2012) A Plasmodium calcium‐dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe 12: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severini, C. , Silvestrini, F. , Sannella, A. , Barca, S. , Gradoni, L. , and Alano, P. (1999) The production of the osmiophilic body protein Pfg377 is associated with stage of maturation and sex in Plasmodium falciparum gametocytes. Mol Biochem Parasitol 100: 247–52. [DOI] [PubMed] [Google Scholar]
- Siden‐Kiamos, I. , Ecker, A. , Nybäck, S. , Louis, C. , Sinden, R.E. , and Billker, O. (2006) Plasmodium berghei calcium‐dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Mol Microbiol 60: 1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini, F. , Bozdech, Z. , Lanfrancotti, A. , Giulio, E.D. , Bultrini, E. , Picci, L. , et al. (2005) Genome‐wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum . Mol Biochem Parasitol 143: 100–10. [DOI] [PubMed] [Google Scholar]
- Simon, N. , Scholz, S.M. , Moreira, C.K. , Templeton, T.J. , Kuehn, A. , Dude, M.‐A. , and Pradel, G. (2009) Sexual stage adhesion proteins form multi‐protein complexes in the malaria parasite Plasmodium falciparum . J Biol Chem 284: 14537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, N. , Lasonder, E. , Scheuermayer, M. , Kuehn, A. , Tews, S. , Fischer, R. , et al. (2013) Malaria parasites co‐opt human factor H to prevent complement‐mediated lysis in the mosquito midgut. Cell Host Microbe 13: 29–41. [DOI] [PubMed] [Google Scholar]
- Simon, N. , Kuehn, A. , Williamson, K.C. , and Pradel, G. (2016) Adhesion protein complexes of malaria gametocytes assemble following parasite transmission to the mosquito. Parasitol Int 65: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden, R.E. (1982) Gametocytogenesis of Plasmodium falciparum in vitro: an electron microscopic study. Parasitology 84: 1–11. [DOI] [PubMed] [Google Scholar]
- Sinden, R.E. , Hartley, R.H. , and Winger, L. (1985) The development of Plasmodium ookinetes in vitro: an ultrastructural study including a description of meiotic division. Parasitology 91: 227–44. [DOI] [PubMed] [Google Scholar]
- Sologub, L. , Kuehn, A. , Kern, S. , Przyborski, J. , Schillig, R. , and Pradel, G. (2011) Malaria proteases mediate inside‐out egress of gametocytes from red blood cells following parasite transmission to the mosquito. Cell Microbiol 13: 897–912.21501358 [Google Scholar]
- Straschil, U. , Talman, A.M. , Ferguson, D.J.P. , Bunting, K.A. , Xu, Z. , Bailes, E. , et al. (2010) The Armadillo repeat protein PF16 is essential for flagellar structure and function in Plasmodium male gametes. PLoS One 5 e12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaréz‐Cortés, P. , Silvestrini, F. , and Alano, P. (2014) A fast, non‐invasive, quantitative staining protocol provides insights in Plasmodium falciparum gamete egress and in the role of osmiophilic bodies. Malar J 13: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman, A.M. , Lacroix, C. , Marques, S.R. , Blagborough, A.M. , Carzaniga, R. , Ménard, R. , and Sinden, R.E. (2011) PbGEST mediates malaria transmission to both mosquito and vertebrate host. Mol Microbiol 82: 462–74. [DOI] [PubMed] [Google Scholar]
- Taylor, C.J. , McRobert, L. , and Baker, D.A. (2008) Disruption of a Plasmodium falciparum cyclic nucleotide phosphodiesterase gene causes aberrant gametogenesis. Mol Microbiol 69: 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton, T.J. , Keister, D.B. , Muratova, O. , Procter, J.L. , and Kaslow, D.C. (1998) Adherence of erythrocytes during exflagellation of Plasmodium falciparum microgametes is dependent on erythrocyte surface sialic acid and glycophorins. J Exp Med 187: 1599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton, T.J. , Kaslow, D.C. , and Fidock, D.A. (2000) Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol Microbiol 36: 1–9. [DOI] [PubMed] [Google Scholar]
- Tewari, R. , Dorin, D. , Moon, R. , Doerig, C. , and Billker, O. (2005) An atypical mitogen‐activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol 58: 1253–63. [DOI] [PubMed] [Google Scholar]
- Tewari, R. , Straschil, U. , Bateman, A. , Böhme, U. , Cherevach, I. , Gong, P. , et al. (2010) The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 8: 377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas, A.M. , Margos, G. , Dimopoulos, G. , Lin, L.H., van , Koning‐Ward, T.F., de , Sinha, R. , et al. (2001) P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J 20: 3975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottein, F. , Triglia, T. , and Cowman, A.F. (1995) Molecular cloning of a gene from Plasmodium falciparum that codes for a protein sharing motifs found in adhesive molecules from mammals and plasmodia. Mol Biochem Parasitol 74: 129–41. [DOI] [PubMed] [Google Scholar]
- Tsai, Y.L. , Hayward, R.E. , Langer, R.C. , Fidock, D.A. , and Vinetz, J.M. (2001) Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect Immun 69: 4048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukegbu, C.V. , Cho, J.‐S. , Christophides, G.K. , and Vlachou, D. (2015) Transcriptional silencing and activation of paternal DNA during Plasmodium berghei zygotic development and transformation to oocyst. Cell Microbiol 17: 1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, J.A. , Noden, B.H. , and Beier, J.C. (1994) Sporogonic development of cultured Plasmodium falciparum in six species of laboratory‐reared Anopheles mosquitoes. Am J Trop Med Hyg 51: 233–43. [DOI] [PubMed] [Google Scholar]
- Vinetz, J.M. , Dave, S.K. , Specht, C.A. , Brameld, K.A. , Xu, B. , Hayward, R. , and Fidock, D.A. (1999) The chitinase PfCHT1 from the human malaria parasite Plasmodium falciparum lacks proenzyme and chitin‐binding domains and displays unique substrate preferences. Proc Natl Acad Sci U S A 96: 14061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinetz, J.M. , Valenzuela, J.G. , Specht, C.A. , Aravind, L. , Langer, R.C. , Ribeiro, J.M. , and Kaslow, D.C. (2000) Chitinases of the avian malaria parasite Plasmodium gallinaceum, a class of enzymes necessary for parasite invasion of the mosquito midgut. J Biol Chem 275: 10331–41. [DOI] [PubMed] [Google Scholar]
- Vlachou, D. , Zimmermann, T. , Cantera, R. , Janse, C.J. , Waters, A.P. , and Kafatos, F.C. (2004) Real‐time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cell Microbiol 6: 671–85. [DOI] [PubMed] [Google Scholar]
- Volkmann, K. , Pfander, C. , Burstroem, C. , Ahras, M. , Goulding, D. , Rayner, J.C. , et al. (2012) The alveolin IMC1h is required for normal ookinete and sporozoite motility behaviour and host colonisation in Plasmodium berghei . PLoS One 7: e41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth, C.C. , and Pradel, G. (2012) Molecular mechanisms of host cell egress by malaria parasites. Int J Med Microbiol 302: 172–178. [DOI] [PubMed] [Google Scholar]
- Wirth, C.C. , Glushakova, S. , Scheuermayer, M. , Repnik, U. , Garg, S. , Schaack, D. , et al. (2014) Perforin‐like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell Microbiol 16: 709–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth, C.C. , Bennink, S. , Scheuermayer, M. , Fischer, R. , and Pradel, G. (2015) Perforin‐like protein PPLP4 is crucial for mosquito midgut infection by Plasmodium falciparum . Mol Biochem Parasitol 201: 90–99. [DOI] [PubMed] [Google Scholar]
- World Malaria Report 2015 (2015) http://www.who.int/malaria/publications/world‐malaria‐report‐2015/report/en/ .
- Young, J.A. , Fivelman, Q.L. , Blair, P.L. , de la Vega, P. , Le Roch, K.G. , Zhou, Y. , et al. (2005) The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology‐based pattern identification. Mol Biochem Parasitol 143: 67–79. [DOI] [PubMed] [Google Scholar]
- Yuda, M. , Sakaida, H. , and Chinzei, Y. (1999) Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J Exp Med 190: 1711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuda, M. , Yano, K. , Tsuboi, T. , Torii, M. , and Chinzei, Y. (2001) von Willebrand factor A domain‐related protein, a novel microneme protein of the malaria ookinete highly conserved throughout Plasmodium parasites. Mol Biochem Parasitol 116: 65–72. [DOI] [PubMed] [Google Scholar]
- Yuda, M. , Iwanaga, S. , Shigenobu, S. , Mair, G.R. , Janse, C.J. , Waters, A.P. , et al. (2009) Identification of a transcription factor in the mosquito‐invasive stage of malaria parasites. Mol Microbiol 71: 1402–14. [DOI] [PubMed] [Google Scholar]


