Abstract
Distraction can impede our ability to detect and effectively process task-relevant stimuli in our environment. Here we leveraged the high temporal resolution of event-related potentials (ERPs) to study the neural consequences of a global, continuous distractor on signal-detection processes. Healthy, young adults performed the dSAT task, a translational sustained-attention task that has been used across different species and in clinical groups, in the presence and absence of ongoing distracting stimulation. We found the presence of distracting stimuli impaired participants’ ability to behaviorally detect task-relevant signal stimuli and greatly affected the neural cascade of processes underlying signal detection. Specifically, we found distraction reduced an anterior and a posterior early-latency N2 ERP component (~140–220 ms) and modulated long-latency, detection-related P3 subcomponents (P3a: ~200–330 ms, P3b: 300–700 ms), even to correctly detected targets. These data provide evidence that distraction can induce powerful alterations in the neural processes related to signal detection, even when stimuli are behaviorally detected.
Keywords: signal detection, target detection, distraction, attentional control, electroencephalography (EEG), event-related potential (ERP)
1.0 Introduction
The ability to detect task- and goal-relevant stimuli is a critical cognitive function at play almost continuously in daily life. The presence of distracting stimuli can challenge our ability to successfully detect and process relevant stimuli in our environment. Here, we studied how a global, continuous distractor influences the brain’s processing of task-relevant signals.
This work uses a translational sustained attention task, the distractor Sustained Attention Task (dSAT; Demeter, Guthrie, Taylor, Sarter, & Lustig, 2013; Demeter, Sarter, & Lustig, 2008). This task requires participants to report the presence or absence of a brief, variable-duration visual signal stimulus during either a baseline, no-distraction condition (SAT) or in a distractor condition (dSAT) designed to increase the demands on attentional control. Originally developed in rodents, this task has been used to study the role of the cortical cholinergic system in mediating attention (e.g., Gill, Sarter, & Givens, 2000; McGaughy, Kaiser, & Sarter, 1996). Cholinergic projections from the basal forebrain to prefrontal cortex are necessary for attentional functions (see review by Hasselmo & Sarter, 2011). Cholinergic neurotransmission in right prefrontal cortex in particular is critical for signal detection (Gritton et al., 2016; Howe et al., 2013; Martinez & Sarter, 2004; Parikh, Kozak, Martinez, & Sarter, 2007). Additionally, cholinergic neurotransmission in right prefrontal and posterior parietal cortex is theorized to mediate attentional control functions that are engaged when attention is challenged, such as when distraction is present (Broussard, Karelina, Sarter, & Givens, 2009; Gill et al., 2000; Kozak, Bruno, & Sarter, 2006; St Peters, Demeter, Lustig, Bruno, & Sarter, 2011). Converging evidence from human functional magnetic resonance imaging (fMRI) work using the dSAT has shown attentional performance during distraction activates a right-lateralized frontoparietal network. This network includes a region in the right middle frontal gyrus (Brodmann’s Area 9) that is sensitive to both the attentional demands imposed by distraction (Demeter, Hernandez-Garcia, Sarter, & Lustig, 2011) and to endogenous cholinergic capacity (Berry, Blakely, Sarter, & Lustig, 2015).
Several fMRI and electroencephalography (EEG) studies using different attention paradigms have also investigated distraction’s effects on the neural processes mediating attentional control and target detection processes. fMRI work using visual search paradigms and flanker tasks, for example, have identified regions in dorsal frontoparietal cortex and in right middle frontal gyrus in particular as being especially important for responding to the attentional demands of distraction (Leber, 2010; Marini, Demeter, Roberts, Chelazzi, & Woldorff, 2016). Mirroring the right-lateralized frontal activation pattern seen in the fMRI literature, we have recently identified a right-lateralized frontal event-related potential (ERP) activation in response to transient distractor stimuli (Demeter & Woldorff, 2016). Broadly, these frontal activations are often interpreted as reflecting attentional control processes designed to filter or suppress distractor stimuli (Zanto & Rissman, 2015). Beyond this attentional control-related activation in frontal cortex, our recent ERP study also demonstrated that brief distractors presented during the presentation of a task-relevant target stimulus could decrease the amplitude of the central parietal P3b ERP component evoked by that target stimulus (Demeter & Woldorff, 2016). Other ERP studies have found distractor stimuli can influence the amplitude of early-latency (150-250 ms) N2 activity over occipital cortex and subsequent P3a activity over frontocentral cortex (Berti & Schröger, 2001). In addition, it was reported that successfully ignoring salient distractors evokes a lateralized occipital ERP component known as the “PD”, which has been found to be a marker of attentional suppression (Gaspar & McDonald, 2014).
While these earlier studies used discrete distractor stimuli, ongoing distraction can also impinge upon attentional performance. For instance, evidence from cross-modal ERP studies has shown that ongoing, concurrently-presented distractor streams in one modality can influence attentional processing of task-relevant stimuli in another modality (Bendixen et al., 2010; Gherri & Eimer, 2010). Within the visual modality, Müller & Hübner (2002) examined whether spatial selective attention could successfully ignore one of two spatially overlapping, centrally-presented information streams. They found that spatial selective attention could successfully ignore the irrelevant stream, even when that irrelevant stream was presented physically on top of the relevant stream. While this work adds to our understanding about how we can selectively direct our attentional focus, Müller & Hübner did not manipulate the presence versus absence of their irrelevant stimulus streams and did not study how continuous distraction affects the neural cascade of processes related to detecting task-relevant targets.
Here, we examined the behavioral and neural consequences of a global, continuous visual distractor on detecting and processing task-relevant visual target signals. Behaviorally, we predicted distraction would impair participants’ task accuracy, in line with other studies using the dSAT (e.g., Berry et al., 2015; Demeter et al., 2013; Demeter et al., 2011; Demeter et al., 2008). Neurally, we predicted that distraction would disrupt the neural cascade of processes related to detecting signal stimuli. Specifically, in line with previous ERP studies involving target-detection paradigms, we predicted that detected signal stimuli would elicit early sensory responses over visual cortex followed by an N2-P3 complex, a functionally-linked set of ERP subcomponents temporally delineating the neural stages of identifying and classifying task-relevant target stimuli (see review by Patel & Azzam, 2005). Based on our previous investigations with brief distractors (Demeter & Woldorff, 2016), we predicted that distraction would reduce or delay these activations. To preview our results, we did not find any effects of distraction on the earliest sensory processing activity (P1 component). In line with our predictions, we did find distraction reduced and delayed early-latency negative-going activity (the anterior and posterior N2 subcomponents) and later-latency positive-going activity (the P3a subcomponent). However, at longer-latencies the P3b subcomponent was enhanced in amplitude in the presence of distraction, suggesting a late compensatory process. These data thus provide a detailed window into the effects of continuous, within-modality distraction on the neural processes mediating target detection.
2.0 Methods
2.1 Participants
Thirty-seven young adults were recruited from Duke University and the surrounding community. Participants were screened for medications and neurological or psychiatric conditions known to affect cognition, vision, ore hearing, and for minimal levels of English proficiency. Nine participants were excluded due to having excessive artifacts in the EEG data (>40% of epochs excluded for conditions of interest) or inability to follow task instructions (including falling asleep, failure to respond at the appropriate time in the task, etc). This left a final dataset of 28 healthy, young adults (17 males, 24 right-handed, aged M: 24.36 yr, SD: 5.00 yr). Participants were compensated at a $15.00 hourly rate. Prior to initiating any experimental procedures, all participants provided written informed consent as approved by and in accordance with Duke University’s Institutional Review Board policies.
2.2 SAT/dSAT paradigm
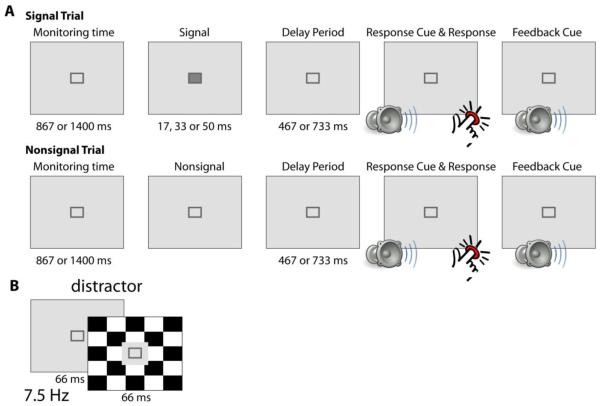
The SAT/dSAT paradigm (Demeter et al., 2008) was modified in order to make it amenable to EEG data collection and analysis (Fig. 1). For each trial of the SAT condition without distraction, participants fixated on a dark gray central square on a light gray background. After an initial monitoring period (867 or 1400 ms duration, randomly), participants were presented with either a signal event (the fixation square filled in with a dark gray square, durations of 17, 33 or 50 ms, all durations with equal probability) or a nonsignal event (the fixation square remained unfilled). The signal and nonsignal events occurred with equal likelihood and in a randomized order across trials. After a delay period (467 or 733 ms, randomly), participants were presented with an auditory response cue. Participants then had up to 1000 ms to make a buttonpress response to indicate whether the signal was or was not presented on that trial (left and right index fingers used indicating presence versus not; button assignment counterbalanced across participants). Participants received auditory feedback on their response accuracy (tones assigned to correct and incorrect responses counterbalanced across participants).
Figure 1. SAT/dSAT design.
A) On each trial of the SAT condition, participants were asked to fixate on a small, centrally presented fixation stimulus (dark gray outline of a square on a light gray background). After a variable amount of time, a signal event (square filled in with dark gray for 17, 33 or 50 ms, with equal probability) or nonsignal event (square remained unfilled) was presented. Signal and nonsignal events were equally presented in a pseudorandom order. After a short delay, participants heard an auditory tone cueing them to buttonpress to indicate whether a signal was (one index finger) or was not (the other index finger) presented on that trial. Participants received auditory-tone feedback based on their accuracy. B) In the dSAT condition, participants performed the same task, now in the presence of ongoing distraction. The distractor stimulus consisted of the background screen alternating between a light gray background (66 ms) and a black-and-white checkerboard background (66 ms). The area immediately surrounding the fixation stimulus remained a constant light gray in this condition.
In the dSAT condition with distraction, participants performed the exact same task, but now with a black-and-white checkerboard flashing in the background. The flashing checkerboard background was generated by alternating between a standard light-gray background and a black-and-white checkerboard background screen at a frequency of 15 Hz. The black-and-white regions of the checkerboard flipped in color with each checkerboard presentation. The screen area immediately adjacent to the position of signal or non-signal presentation stayed light-gray and did not display the flashing checkerboard distractor. Signal and non-signal events were jittered relative to the flashing background screens. That is, signal and non-signal event onsets were randomly distributed in time throughout the period encompassed by one cycle of the flashing distractor screens. Identical presentation timings for signal and non-signal events were also used for the SAT condition where background screens were all a constant light gray.
Participants completed thirty-two 2.5 min blocks of SAT or dSAT (block order pseudo-randomized) while scalp EEG was recorded. Block order was constrained so that there were no more than 3 blocks in a row of the same task condition and so that equal numbers of SAT and dSAT blocks were presented in the first and second half of the experimental session. Participants completed 49 trials per block.
2.3 Data acquisition and analysis
EEG data were acquired using a 64-channel active-electrode system (Brain Vision BrainAmp MR Plus with actiCAP Control Box, Brain Products, Gilching Süd, Germany), mounted on a customized electrode cap (Duke64 layout, EASYCAP, Herrsching). These caps were designed to have an extended coverage of the head from just above the eyebrows to below the inion posteriorly and to have electrodes that are equally spaced across the cap. An electrode was placed below each eye in order to monitor vertical eye movements, and two electrodes just lateral to the left and right outer canthi were used for monitoring lateral eye movements. The scalp sites of our equidistant-electrode custom cap are reported in terms of the closest location in the standard 10–10 system if within a couple of millimeters; in those cases where our electrode varies more than a few millimeters from the related 10-20 or 10-10 location, the electrodes are denoted with an “a”, “p”, “i”, or “s” to indicate they are slightly anterior, posterior, inferior, or superior to the 10-20 or 10-10 location, respectively. Relevant electrodes are also specifically identified on schematic head figures in the Results section. Recordings were referenced online to the right mastoid electrode, band-pass filtered between 0.01-250 Hz and digitized with a 500 Hz sampling rate. Electrode impedances were maintained below 5 kΩ for the ground and reference and below 15 kΩ for all other channels.
Offline, the EEG data were low-pass filtered to 30 Hz (half amplitude cutoff) and re-referenced to the algebraic average of the right and left mastoids. Epochs with eye movements, blinks or muscle movements were excluded from analyses. The SAT and dSAT conditions had comparable numbers of trials accepted for analysis (~85 trials per condition per subject accepted). Our ERP analyses focused on signal trials. ERPs were created by time-lock averaging the epoched EEG data to the onset of signal events for each of the 3 signal durations, separately for the SAT and the dSAT conditions. The selectively averaged ERPs were baseline corrected by subtracting the mean amplitude of the baseline period (−200 to 0 ms) from the ERP for each time-locked stimulus. The ERP data were subjected to a notch filter to mitigate the residual 15 Hz noise arising from the flashing checkerboard distractor; this filter was applied to ERP data from all task conditions.
2.4 Statistical analysis
In order to assess the effects of signal duration and distraction on behavioral performance, the proportion of hits to signal trials were subjected to a repeated-measures ANOVA with the factors of Signal Duration (17, 33, 50) and Distraction (SAT, dSAT). The effect of distraction on false alarms and omissions was tested with paired t-tests. Hit and false alarm data were also used to derive SAT scores for each signal duration, in line with previous studies using the dSAT (e.g., Demeter et al., 2011). As is typical, the SAT scores were calculated for each signal duration and task condition using the proportion of hits (h) and false alarms (fa) according the formula: SAT score = (h−fa) / (2(h+fa)−(h+fa)2). SAT scores vary from −1 to +1, with −1 indicating all responses were misses or false alarms and +1 indicating all responses were hits or correct rejections. SAT score data were subjected to repeated-measures ANOVAs with the factors of Distraction (SAT, dSAT) and Signal Duration (17, 33, 50). For all behavioral analyses, proportions were calculated for each subject using only the trials that were free of any EEG artifacts in order to maintain a match between the behavioral and neural data.
For the ERP data, analyses focused on epochs time-locked to the onset of detected signal stimuli. ANOVAs on the mean amplitudes over specified time ranges and on activity latencies were conducted in order to test statistical differences between neural responses to different conditions, described in further detail in the relevant Results sections. Which channels to analyze and the latency window for mean amplitude analyses were selected based on examination of a grand ERP trace produced by averaging across task conditions. For consistency, our more widespread midline effects were interrogated by creating a region of interest (ROI) based on data from four channels, while a channel from each hemisphere was used to interrogate the more focalized lateralized effects. For all analyses, the Huyhn–Feldt sphericity correction was applied as needed. Corrected F and p values are reported, with degrees of freedom rounded to integers for ease of reading.
3.0 Results
3.1 Impaired attentional performance with distraction and shorter target signal durations
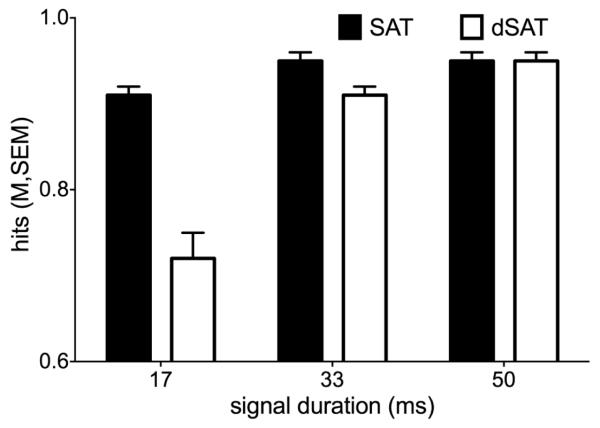
Behavioral results generally replicated previous behavioral and fMRI studies using the dSAT task (e.g., (Demeter et al., 2013; Demeter et al., 2011; Demeter et al., 2008)). For the hit data, the Distraction and Signal-Duration factors interacted to produce the lowest hit rate for 17 ms signals during the dSAT condition (F(2,54) = 51.55, p < 0.0001, ηp2 = 0.66, Figure 2). Distraction also reduced the hits overall (main effect of Distraction: F(1,27) = 39.44, p < 0.0001, ηp2 = 0.59), as did decreasing signal duration (main effect of Duration: F(1,27) = 63.63, p < 0.0001, ηp2 = 0.70). False alarms numerically increased with distraction, but this effect was not statistically significant (t(27) = −1.68, p = 0.10, Cohen’s d = 0.32, Table 1). Omissions did not differ as a function of distraction (t(27) = −0.30, p = 0.77, Cohen’s d = 0.06).
Figure 2. Distraction decreases hit accuracy.
Bars represent the mean proportion of hits for each signal duration for the no-distraction SAT condition (black bars) and for the dSAT condition with distraction (white bars). Error bars represent the between-subjects standard error around the mean. Hit accuracy on signal trials decreased as a function of decreasing signal duration and in the presence of distraction.
Table 1.
Data are the means (standard error) of the proportion of false alarms, the proportion of omissions, and the SAT scores for each signal duration. These data were calculated for the SAT condition without distraction and for the dSAT condition with distraction.
| SAT condition | ||||
|---|---|---|---|---|
|
| ||||
| False Alarms | Omissions | SAT score 17 ms |
SAT score 33 ms |
SAT score 50 ms |
| 0.05(0.01) | 0.01(0.00) | 0.87(0.02) | 0.90(0.02) | 0.90(0.02) |
|
| ||||
| dSAT condition | ||||
| False Alarms | Omissions |
SAT score
17 ms |
SAT score
33 ms |
SAT score
50 ms |
|
| ||||
| 0.06(0.01) | 0.01(0.00) | 0.69(0.04) | 0.85(0.02) | 0.89(0.02) |
Analysis of the SAT score data revealed similar patterns to those seen in the hit data. Signal Duration and Distraction interacted (F(2,54) = 52.62, p < 0.0001, ηp2 = 0.66), producing the lowest attentional performance at the shortest signal duration during distraction (see Table 1 for means). Performance declined both as a function of shortening signal duration (F(1,27) = 69.26, p < 0.0001, ηp2 = 0.72) and with distraction (F(1,27) = 22.55, p < 0.0001, ηp2 = 0.50).
3.2 Effects of distraction and signal duration on early latency ERP components
In order to investigate how distraction and signal duration affect the neural cascade of processes related to signal detection, ERPs time-locked to the onset of detected signal stimuli were derived and analyzed. For these ERP data, there was an early positivity starting around 100 ms over midline posterior channels (a sensory-related P1 component). This positivity was most evident in the SAT condition for 50 ms duration signals. To statistically analyze this activity, a grand average ERP was created that collapsed across all Distraction and Duration conditions, across all subjects. This grand average showed this P1 component was strongest over midline posterior channels (channels Pz, POz, PO1, and PO2) from 100-160 ms post-signal onset. Mean amplitudes from a region of interest (ROI) consisting of these four posterior channels were extracted from 100-160 ms and subjected to a repeated-measures ANOVA. This analysis revealed a main effect of Duration (F(2,54) = 5.19, p = 0.01), with 50 ms signals generating the largest-amplitude P1 effects (paired t-tests versus 33 ms and 17 ms P1 mean amplitudes, both p < 0.02). However, there was no interaction between Duration and Distraction, and no main effect of Distraction (both F < 1.52, p > 0.23).
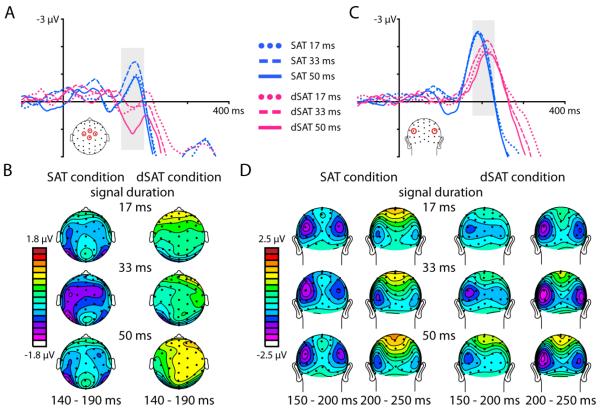
Following this early sensory-related P1 activity, detected signals elicited an anterior and a posterior negative-going activation, starting around 140 ms and 150 ms, respectively, reflecting the first neural stage of identifying the signal stimulus as a task-relevant target. While some reports have referred to activity in this latency range as a visual N1 component (Vogel & Luck, 2000), we have labeled these activations as an anterior N2 and a posterior N2 component because these activations were tightly linked with the subsequent P3a and P3b activations. Detected signals in the SAT condition without distraction elicited an anterior fronto-central negativity starting around 140 ms (anterior N2 component; Figure 3 A and B). In the dSAT condition with distraction, however, this anterior N2 component was not apparent at this time latency. To statistically analyze this anterior N2 effect, mean amplitudes from 140-190 ms were extracted from a fronto-central ROI consisting of channels FCz, Cz, C1a and C2a and subjected to a repeated-measures ANOVA. These channels and this latency range were selected based on a grand average across all Distraction and Duration conditions, across all subjects. This analysis revealed a main effect of Distraction (F(1,27) = 25.24, p < 0.0001, ηp2 = 0.48). There was also a main effect of Signal Duration (F(2,54) = 6.06, p < 0.01, ηp2 = 0.18), driven by a difference between the 17 ms and 50 ms durations (p < 0.05) and the 33 ms and 50 ms durations (p < 0.01). There was no interaction between Distraction and Duration on this anterior N2 component. We note for completeness that while anterior N2 activity was not observed in the latency range determined by the grand average ERP collapsed across all task conditions, based on visual inspection of the ERP data it appears possible that the anterior N2 could be substantially delayed in the dSAT condition (peaking at ~200 ms). Such a delay during the distraction condition would push this negative-going activity into the latency range of the later positive-going components, making the absolute voltage positive. In this situation, distraction would still have a pronounced effect on the latency of the anterior N2 component.
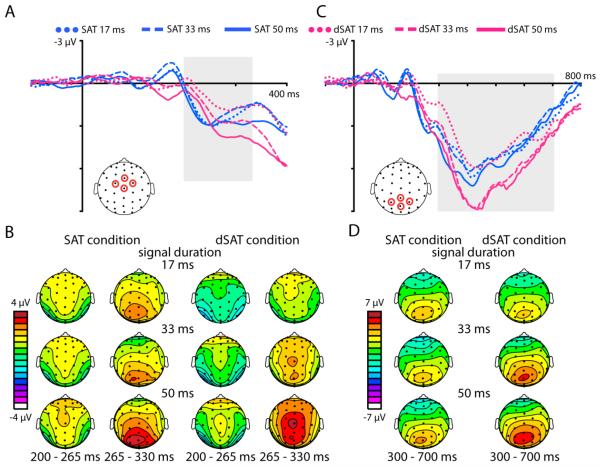
Figure 3. Distraction disrupts early-latency signal detection processes.
Distraction severely disrupted an early anterior negativity (anterior N2 component, latency 140-190 ms). A) ERP traces from channels FCz, Cz, C1a, and C2a (see head schematic) time-locked to the onset of detected signals (durations 17, 33, or 50 ms) in the SAT (blue traces) and dSAT (pink traces) conditions. B) Topographies of the mean amplitude (from 140 to 190 ms) of the response to detected signals, for each duration and task condition. C) Detected signals also evoked a posterior negativity over bilateral occipital cortex (posterior N2 component starting ~150 ms). ERP traces averaged from channels P3i and P4i (see head schematic) and time-locked to the onset of detected signals (durations 17, 33, or 50 ms) in the SAT (blue traces) and dSAT (pink traces) conditions show this posterior N2 was smaller in amplitude and delayed in latency during distraction (dSAT). D) Topographies of the mean amplitude of the response to detected signals show the posterior N2 activity over bilateral occipital cortex. Two time windows (150 to 200 ms and 200 to 250 ms) are included to illustrate the delayed latency of the posterior N2 during distraction.
Posteriorly, detected signal stimuli elicited a bilateral negativity over occipital channels starting around 150 ms and peaking around 200 ms post-signal onset (posterior N2 component; Figure 3 C and D). In contrast to the effects seen anteriorly, this posterior N2 component was evident in both the SAT and dSAT conditions, although it appeared reduced in amplitude and delayed in latency in the dSAT condition relative to the SAT. In order to analyze these effects, mean amplitudes from 180-220 ms were extracted from channels P3i and P4i, the channels with the largest posterior N2 amplitudes in the grand ERP trace created based on the average of all task conditions (see Figure 3C for diagram). The data from these two channels were averaged together as there were no significant differences in mean amplitude between the left and right hemisphere channels. The latency range was determined based on a grand average collapsed across all task conditions. Mean amplitude data were analyzed with a repeated-measures ANOVA with the factors of Distraction (SAT, dSAT) and Duration (17, 33, 50). This analysis revealed a significant effect of Distraction (F(1,27) = 8.23, p = 0.01, ηp2 = 0.23), with mean amplitudes smaller for the dSAT condition than the SAT condition. There were no other significant amplitude effects.
In order to examine the effect of distraction and signal duration on the latency of this posterior N2, an analysis of onset latency was performed. More specifically, the latency at which the negativity achieved 50% of its local maximum was determined for each participant, again using data from channels P3i and P4i. The results were submitted to a repeated-measures ANOVA with the factors of Distraction (SAT, dSAT) and Duration (17, 33, 50). This analysis revealed distraction delayed the onset of the posterior N2 by an average of approximately 17 ms (main effect of Distraction, F(1,27) = 39.27, p < 0.0001, ηp2 = 0.59). There was also a main effect of Signal Duration (F(2,54) = 4.14, p = 0.02, ηp2 = 0.13), with the shortest signal duration condition reaching the 50% of maximum peak about 6 ms earlier than the longest signal duration condition. There were no significant interactions for the latency data.
3.3 Posterior N2 amplitude during distraction correlates with attentional performance
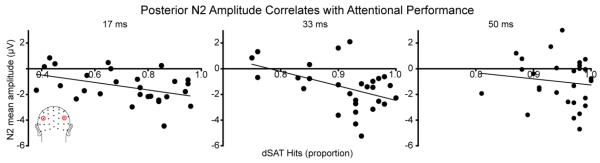
A brain-behavior correlational analysis showed that the posterior N2 mean amplitude in the dSAT condition correlated across participants with their hit rate on signal trials during distraction (Figure 4). The mean amplitude data used for these analyses were the same as those used in section 3.2. For the 17 ms and for the 33 ms signal durations during the dSAT condition, the mean posterior N2 amplitude correlated across participants with their hits during the dSAT condition, such that participants with larger amplitude (more negative) posterior N2’s also had better behavioral performance (dSAT 17 ms condition: r = −0.43, p = 0.02; dSAT 33 ms condition: r = −0.46, p = 0.01). There was no significant correlation for the 50 ms signal duration during the dSAT condition (r = −0.12, p = 0.55), nor were there any significant correlations between the mean amplitudes of the posterior N2 during the SAT condition and performance during that condition (all p > 0.08).
Figure 4. During distraction, the mean amplitude of the posterior N2 correlates across participants with their hits to target signals.
For the dSAT condition, participants’ proportion of hits correlated with the mean amplitude of the posterior N2 component (latency 180-220 ms) for the 17 and the 33 ms signal durations. For these two signal durations, the larger (i.e., more negative) the N2 mean amplitude was, the better the participant’s hit rate on signal trials was. This suggests participants who were better able to enhance this posterior N2 activity to amplitudes qualitatively similar to what was observed in the absence of distraction were also able to maintain better attentional performance during distraction. There was no significant relationship between behavior and the N2 mean amplitude for the 50 ms condition, but hits were generally quite high in this condition. See section 3.3. for statistical details.
3.4 Effects of distraction and signal duration on longer-latency ERP components
As hypothesized, following the early-latency effects described above, detected signals elicited a large, fronto-central positive-polarity wave starting around 200 ms (Figure 5 A and B), followed by a large, more parietally distributed positive-polarity response starting around 300 ms (Figure 5 C and D), corresponding respectively to the classic P3a and P3b components. For the P3a component, mean amplitudes were extracted from a fronto-central ROI consisting of channels FCz, Cz, C1a and C2a (see diagram in Figure 5A) from 200 to 330 ms. As before, these data were subjected to a repeated-measures ANOVA with the factors of Distraction (SAT, dSAT) and Duration (17, 33, 50). This analysis revealed a significant Distraction by Duration interaction (F(2,54) = 5.26, p = 0.01, ηp2 = 0.16) and a main effect of Duration (F(2,54) = 13.84, p < 0.0001, ηp2 = 0.34). For the SAT condition, the P3a mean amplitudes did not differ as a function of signal duration (paired t-tests between all signal durations all p > 0.15). However, in the dSAT condition with distraction, P3a mean amplitudes did differ as a function of signal duration, with the shortest signal generating the smallest amplitude and the longest signal the largest (paired t-tests between all durations all p < 0.01). Looking between the SAT and dSAT conditions, distraction significantly reduced the P3a amplitude for the shortest signal-duration trials (paired t-test, t(27) = 3.34, p = 0.002, Cohen’s d = 0.63), but there were no significant differences between the SAT and dSAT P3a amplitudes for the other durations (both p > 0.47).
Figure 5. Distraction modulates longer-latency activity reflecting signal detection processes.
Detected signals evoked a fronto-central P3a starting at ~200 ms post-signal onset, followed by a large P3b component over parietal cortex starting at ~300 ms. A) ERP traces are time-locked to the onset of detected signals, for each signal duration (17, 33, and 50 ms). The blue traces represent the no-distraction SAT condition and the pink traces represent the dSAT condition with distraction. Traces are averaged from 4 frontocentral channels (see diagram for locations). Distraction delayed the onset of the P3a component, and reduced the P3a amplitude for the 17 ms signal duration. B) Topographies showing the mean amplitude of activity from 200 to 265 ms and 265 to 330 ms post-signal onset for each signal duration and the SAT and dSAT conditions. Two time windows are shown in order to illustrate the distraction-related delay in the P3a latency. In the SAT, the fronto-central P3a is visible starting at 200 ms post-signal onset, while the more parietal activity of the subsequent P3b is evident in the plots from 265-330 ms. In the dSAT, the P3a activity was significantly delayed in time. C) Following the fronto-central P3a, a large-amplitude P3b was observed over parietal cortex. The ERP traces are plotted from channels CPz, Pz, P1, and P2 for each signal duration for the both the SAT (blue traces) and dSAT condition (pink traces). Distraction enhanced the P3b amplitude for the 33 and 50 ms durations. d) Topographies of the mean amplitude from 300 to 700 ms post-signal onset, for each signal duration for the SAT and dSAT conditions, showing the distribution of the parietal P3 activity.
In addition to the effects of distraction and signal duration on the mean amplitude of the P3a, these factors also influenced the latency of this component. To investigate latency effects, an analysis of when each condition reached 50% of its peak amplitude was performed. Distraction significantly delayed the P3a by approximately 17 ms (main effect of Distraction, F(1,27) = 27.63, p < 0.0001, ηp2 = 0.51). There was no effect of Duration on this latency measure, and no interaction between the two factors (both F < .80, p > .44, ηp2 < 0.03).
Over central parietal channels, detected signals generated robust P3b’s starting around 300 ms. To investigate the effects of distraction and duration on the amplitude of this large target-detection-related, longer-latency component, the mean amplitude from 300 – 700 ms was calculated for each condition from channels CPz, Pz, P1, and P2 (see head diagram Figure 5 C) and analyzed with a repeated-measures ANOVA as before. As with all the analyses, the channels of interest and time window of interest were selected based on a grand average ERP collapsed across all task conditions. This analysis revealed a significant Distraction by Duration interaction (F(2,54) = 23.51, p < 0.0001, ηp2 = 0.47). Unlike the pattern seen in the mean amplitude analyses of the N2 and P3a components, where the presence of distraction generally reduced the amplitude of these components, distraction significantly increased the P3b amplitude for the 33 and 50 ms signal duration conditions (paired t-tests, both t > 5.11, p < 0.0001, Cohen’s d > 0.97). For the 17 ms condition, the mean P3b amplitude was numerically smaller during distraction than without distraction, but this effect was not significant (t(27) = 0.71, p = 0.48, Cohen’s d = 0.13). Overall, distraction and increasing signal duration each served to increase the mean amplitude of the P3b (main effect of Distraction F(1,27) = 16.97, p < 0.0001, ηp2 = 0.39; main effect of Duration, F(2,54) = 16.15, p < 0.0001, ηp2 = 0.37).
The latency at which the P3b reached 50% of its peak amplitude for each condition was calculated and analyzed with a repeated-measures ANOVA. This analysis revealed a significant effect of Duration (F(2,54) = 12.17, p < 0.0001, ηp2 = 0.31), with increasing signal duration resulting in faster latencies. There was no significant effect of Distraction or interaction between Distraction and Duration on P3b latencies (both F < 2.21, p > 0.12, ηp2 < 0.08).
4.0 Discussion
In this study, we investigated the effects of an ongoing, global distractor on signal detection processes using a translational attentional control paradigm, the dSAT. Behaviorally, we found distraction and signal duration interacted, producing the largest distraction-related declines in hits at the shortest signal duration. Neurally, early feed-forward processing at the stage of the visual extrastriate P1 component (e.g., Hillyard & Anllo-Vento, 1998) ~100 ms post-signal onset was unaffected by distraction. Subsequently, however, distraction had robust effects on a cascade of neural processes related to higher-level detection processes. Distraction severely disrupted an early anterior N2 component. In the SAT condition, this activity was evident from ~140 to 190 ms post-signal onset. In the dSAT condition, negative activity was not observed within this latency range. Looking posteriorly, distraction delayed and reduced in amplitude a posterior N2 that started around 150 ms. For the short and middle signal durations, the mean amplitude of the posterior N2 activity during distraction correlated with participants’ behavioral performance. Participants with larger-amplitude (more negative) N2’s performed more accurately during distraction than participants with smaller N2’s.
Following these early effects, distraction interacted with signal duration to reduce the amplitude of a frontocentral P3a (starting around 200 ms post-signal onset) for the shortest signal duration. The P3a was also delayed in latency with distraction. Over parietal channels, detected signals produced a large P3b starting around 300 ms post-signal onset. Here, distraction and signal duration interacted, producing larger-amplitude P3b’s for the middle and long signal duration during distraction. Longer signal durations also resulted in faster latencies for the P3b, but the latency of this component was not affected by distraction.
The SAT and dSAT paradigm has been used extensively in animal model studies on the neurobiological mechanisms underlying attentional functions (e.g., Gritton et al., 2016; Kozak et al., 2006; McGaughy et al., 1996; St Peters, Cherian, Bradshaw, & Sarter, 2011; St Peters, Demeter, et al., 2011). The task has also been used in a few human fMRI studies on attentional control and target detection (Berry et al., 2015; Demeter et al., 2011; Howe et al., 2013). However, this is the first study with this task where the temporal resolution of ERPs has been leveraged to examine the effects of distraction on the cascade of neural processes related to target detection. Many of the previous investigations with the dSAT have focused on the role of right frontal cortex in mediating attentional functions. In the present data, we did not observe any right-lateralized patterns to our effects. While it is possible that some of the neural mechanisms mediating signal detection processes are localized to right, but not left hemisphere (e.g., see Parikh, St Peters, Blakely & Sarter (2013)), we did not find specific evidence for a right-lateralized pattern to the neural processing that occurs after the presentation of a target signal stimulus. In the fMRI studies using the dSAT (Berry et al., 2015; Demeter et al., 2011; Howe et al., 2013), the right frontal activations observed could reflect attentional control processes evoked to process or filter out the distractor (see also Demeter & Woldorff, 2016, Marini et al., 2016). In our experiment, our signal stimulus onset was jittered in time relative to the background distractor stimuli, enabling us to average out activity related to the distractor stimuli and isolate activity related to processing the signal stimulus. Thus, we may not have observed right frontal activations specific to processing or suppressing distractors in our analyses.
This dSAT is rather unique in its use of an ongoing, global distractor to challenge attentional performance. In contrast, previous ERP investigations into behavioral distraction have used novel, infrequent distractors (e.g., Berti & Schröger, 2001; Escera, Alho, Winkler, & Naatanen, 1998), salient distractors embedded into visual search arrays (e.g., Gaspar & McDonald, 2014), or discrete distractor items presented during an ongoing task stream (e.g., Demeter & Woldorff, 2016; Pincham & Szucs, 2014). The continuous demands on attentional effort imposed by the ongoing distractor in the dSAT condition are perhaps somewhat more akin to cross-modal studies of distraction that have used continous auditory or visual distractor streams while participants are attending to a task in the other modality. For example, Gherri & Eimer (2010) found active listening to a narrated text passage produced impairments in visual search and visual target detection. These authors found active listening slowed participants’ reaction times to detecting task-relevant visual targets. They also found active listening reduced the amplitude of the N2pc, an ERP component reflecting the shift of spatial attention to visual targets, and reduced the amplitude of the P3b to visual targets.
The present results focused on analyses of the responses to detected signals. Previously, we have demonstrated that missed targets in a rapid serial visual presentation (RSVP) task showed a reduction in P3b amplitudes relative to detected trials, and that brief, transient distractors nearby in time were capable of further reducing the P3b amplitude for missed targets but not for detected targets (Demeter & Woldorff, 2016). While there were significant declines in attentional performance during distraction in the current experiment, participants’ accuracy was still relatively high, especially in the SAT condition without distraction. Thus, there were relatively few missed signal trials available for analysis in the present work.
The electrophysiological data, however, provided important insight into the effects distraction has on the cascade of neural processing of detected target signal stimuli. We found distraction did not affect early feed-forward processing at the level of the visual extrastriate P1 component (~100 ms post-target onset). This suggests the continuous visual distractor stimulus did not serve as a visual mask for the signal stimulus, as a reduced-amplitude P1 may have suggested. It also suggests the presence of continuous distraction does not lead to an overall attentional enhancement of neural activity specific to target stimuli, as an enhanced P1 may have indicated.
In contrast to the early sensory results, continuous distraction greatly affected the later stages of processing the signal stimulus as a task-relevant target. Distraction degraded the early anterior and posterior N2 subcomponents, activity reflecting conscious identification of the task-relevant target stimulus (~140 ms). While the N2 component is generally linked to target identification and discrimination, many studies have supported the idea of separable N2 subcomponents that can be functionally and spatially dissociated (see Folstein & Van Petten (2008) for a review). Anterior N2 activity is often elicited by conscious attention to stimuli that are novel or that deviate from a perceptual template (Hoffman, 1990; Naatanen & Picton, 1986; Sams, Alho, & Naatanen, 1983). In our data, the anterior negativity elicited by detected signals could reflect attentional alerting to and a conscious detection of the target stimulus that deviated from the perceptual template of the fixation stimulus. Posterior N2 activity is often observed in response to target stimuli selectively in visual oddball tasks (e.g., Ritter, Simson, & Vaughan, 1983; Ritter, Simson, Vaughan, & Macht, 1982), as well as in response to pop-out targets in visual search paradigms (e.g., Luck & Hillyard, 1994). In our task, signal stimuli were brief events within the scheme of the total trial time and signal trials were randomly intermixed with nonsignal trials, although signal stimuli did appear on 50% of trials.
Interestingly, our data also suggest the anterior and posterior N2 subcomponents are differentially affected by distraction. For the anterior negativity, these waves were clearly apparent for each signal duration in the SAT condition without distraction, but were essentially gone in the dSAT condition with distraction – at least within the time window analyzed. In contrast, the posterior negativity was clearly observed in both the SAT and dSAT conditions, but with distraction serving to decrease the amplitude and delay the latency of this activity. We also found that the posterior N2 amplitude positively correlated across participants with their attentional performance. We did examine whether other ERP components showed correlations with the behavioral data, but did not find any other systematic relationships. It could be that the visual selective attention processes reflected by the posterior N2 activity most closely track with correct detection of visual target stimuli (hits to targets), whereas the activity reflected by other components perhaps reflects more general attentional alerting, orienting, and target classification processes.
At longer latencies, distraction reduced in amplitude and delayed in latency fronto-central P3a activity (~200 ms), thought to reflect attentional orienting to novel or task-relevant stimuli. Finally, we found that the late-latency centroparietal P3b activity (~300 ms) was significantly enhanced during distraction. P3b activity is often elicited in response to target stimuli – regardless of the modality being tested. It is theorized to reflect the final stage of target classification and evaluation, including the updating of target stimulus representations in working memory (Polich, 2007).
While work by our group and by others has found distraction can reduce the P3b amplitude (Demeter & Woldorff, 2016; Pincham & Szucs, 2014), here we found the P3b amplitude was larger during distraction for the middle and long signal durations. We speculate this increase in P3b amplitude elicited at longer-latencies on distraction trials could reflect some type of compensatory activity for the distraction-related degradation and alteration of activity observed at earlier latencies.. While we did not observe any significant correlations between attentional performance and P3b activity, we note that attentional performance for the middle and longer signal durations was relatively spared during distraction compared to the short signal duration, perhaps a result of such compensatory activity.
To explore this compensation hypothesis further, we examined how P3b mean amplitudes changed over the course of the experimental session. We found that P3b mean amplitudes decreased from the first half to the second half of the experimental session, but that this decrease was much more substantial for the SAT condition than it was for the dSAT condition (percent change from 1st half of experimental session to 2nd half of experimental session, SAT: −28.5 ± 6.0%, dSAT: −7.7 ± 7.2%, main effect of Distraction, F(1,27) = 7.13, p = 0.01, ηp2 = 0.21). In the SAT condition without distraction, it is possible that, as participants became better practiced at the task, they needed to devote fewer neural resources to this final target processing and evaluation stage in order to maintain the same level of behavioral performance. In contrast, in the dSAT condition the demands imposed by distraction may have necessitated higher levels of activity be maintained for this final stage of target detection in order to compensate for distraction’s negative impact on the earlier stages of processing the target signal stimuli. Supporting this interpretation, the difference in P3b mean amplitude between the dSAT and SAT conditions was correlated across participants with the difference in the anterior N2 mean amplitude between the two distraction conditions (mean amplitude in dSAT condition minus amplitude in SAT condition for each signal duration, all r > 0.37, p < 0.05). Similarly, there was also a correlation across participants for the difference in mean amplitude for the dSAT and SAT conditions for the P3b and the posterior N2 (50 ms signal duration, r = 0.45, p = 0.02, 33 and 17 ms durations both n.s.).
The present ERP results expand what is known about brain activity that is elicited during the dSAT, a translational attentional control paradigm that has been applied to both humans (Berry et al., 2015; Demeter et al., 2011; Demeter et al., 2008) and animals (Gritton et al., 2016; Himmelheber, Sarter, & Bruno, 2000; McGaughy & Sarter, 1995; St Peters, Cherian, et al., 2011). Previous work has also demonstrated this task is sensitive to the attentional control deficits seen in patients with schizophrenia (Demeter et al., 2013) and in individuals with an inherent reduced capacity for cholinergic function (Berry et al., 2015). Here, we found distraction had robust effects on the neural processes related to signal detection in our sample of healthy, young adults. Future studies on how these processes are further altered in patient populations could help provide insights into how the neural mechanisms underlying target detection processes are impaired in these clinical populations.
Highlights.
Continuous distraction did not affect earliest sensory processing of target stimuli.
Distraction did affect target identification and classification processes.
Distraction reduced activity in the N2 latency (~140-220 ms post-target onset)
Distraction reduced and delayed P3a activity (~200-330 ms post-target).
Distraction enhanced final evaluation processing of targets (P3b: 300-700 ms).
Acknowledgments
This study was supported by a National Service Research Award (T32 NS 51156-10) to E.D. and by NINDS R01-NS051048 to M.G.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bendixen A, Grimm S, Deouell LY, Wetzel N, Madebach A, Schröger E. The time-course of auditory and visual distraction effects in a new crossmodal paradigm. Neuropsychologia. 2010;48(7):2130–2139. doi: 10.1016/j.neuropsychologia.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Berry AS, Blakely RD, Sarter M, Lustig C. Cholinergic capacity mediates prefrontal engagement during challenges to attention: evidence from imaging genetics. Neuroimage. 2015;108:386–395. doi: 10.1016/j.neuroimage.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti S, Schröger E. A comparison of auditory and visual distraction effects: behavioral and event-related indices. Cognitive Brain Research. 2001;10(3):265–273. doi: 10.1016/s0926-6410(00)00044-6. [DOI] [PubMed] [Google Scholar]
- Broussard JI, Karelina K, Sarter M, Givens B. Cholinergic optimization of cue-evoked parietal activity during challenged attentional performance. Eur J Neurosci. 2009;29(8):1711–1722. doi: 10.1111/j.1460-9568.2009.06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Guthrie SK, Taylor SF, Sarter M, Lustig C. Increased distractor vulnerability but preserved vigilance in patients with schizophrenia: evidence from a translational Sustained Attention Task. Schizophr Res. 2013;144(1-3):136–141. doi: 10.1016/j.schres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Demeter E, Hernandez-Garcia L, Sarter M, Lustig C. Challenges to attention: a continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. Neuroimage. 2011;54(2):1518–1529. doi: 10.1016/j.neuroimage.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22(6):787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Woldorff MG. Transient distraction and attentional control during a sustained selective attention task. Journal of Cognitive Neuroscience. 2016 doi: 10.1162/jocn_a_00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escera C, Alho K, Winkler I, Naatanen R. Neural mechanisms of involuntary attention to acoustic novelty and change. Journal of Cognitive Neuroscience. 1998;10(5):590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar JM, McDonald JJ. Suppression of salient objects prevents distraction in visual search. J Neurosci. 2014;34(16):5658–5666. doi: 10.1523/JNEUROSCI.4161-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherri E, Eimer M. Active Listening Impairs Visual Perception and Selectivity: An ERP Study of Auditory Dual-task Costs on Visual Attention. Journal of Cognitive Neuroscience. 2010;23(4):832–844. doi: 10.1162/jocn.2010.21468. [DOI] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20(12):4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. doi:20/12/4745 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M. Cortical cholinergic signaling controls the detection of cues. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1516134113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36(1):52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95(3):781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res Cogn Brain Res. 2000;9(3):313–325. doi: 10.1016/s0926-6410(00)00012-4. doi:S0926641000000124. [DOI] [PubMed] [Google Scholar]
- Hoffman JE. Event-related potentials and automatic and controlled processes. In: Rohrbaugh JW, Parasuraman R, Johnston RJ, editors. Event Related Brain Potentials. Oxford Univerisity Press; New York: 1990. pp. 145–157. [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Sarter M. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. J Neurosci. 2013;33(20):8742–8752. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16(1):9–17. doi: 10.1093/cercor/bhi079. doi:bhi079 [pii] [DOI] [PubMed] [Google Scholar]
- Leber AB. Neural predictors of within-subject fluctuations in attentional control. J Neurosci. 2010;30(34):11458–11465. doi: 10.1523/JNEUROSCI.0809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31(3):291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Marini F, Demeter E, Roberts KC, Chelazzi L, Woldorff MG. Orchestrating Proactive and Reactive Mechanisms for Filtering Distracting Information: Brain-Behavior Relationships Revealed by a Mixed-Design fMRI Study. J Neurosci. 2016;36(3):988–1000. doi: 10.1523/JNEUROSCI.2966-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Sarter M. Lateralized attentional functions of cortical cholinergic inputs. Behav Neurosci. 2004;118(5):984–991. doi: 10.1037/0735-7044.118.5.984. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110(2):247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl.) 1995;117(3):340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- Müller MM, Hübner R. Can the spotlight of attention be shaped like a doughnut? Psychological Science. 2002;13(3):119–124. doi: 10.1111/1467-9280.00422. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton TW. N2 and automatic versus controlled processes. Electroencephalography and Clinical Neurophysiology. 1986;38:169–186. [PubMed] [Google Scholar]
- Parikh V, St Peters M, Blakely RD, Sarter M. The presynaptic choline transporter imposes limits on sustained cortical acetylcholine release and attention. J Neurosci. 2013;33(6):2326–2337. doi: 10.1523/JNEUROSCI.4993-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56(1):141–154. doi: 10.1016/j.neuron.2007.08.025. doi:S0896-6273(07)00674-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SH, Azzam PN. Characterization of N200 and P300: Selected Studies of the Event-Related Potential. Int. J. Med. Sci. 2005;2:147–154. doi: 10.7150/ijms.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincham HL, Szucs D. Disruption reduces accuracy and P3b amplitudes in the attentional blink. Neurosci Lett. 2014;581:26–31. doi: 10.1016/j.neulet.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter W, Simson R, Vaughan HGJ. Event-related potential correlates of two stages of information processing in physical and semantic discrimination tasks. Psychophysiology. 1983;20:168–179. doi: 10.1111/j.1469-8986.1983.tb03283.x. [DOI] [PubMed] [Google Scholar]
- Ritter W, Simson R, Vaughan HGJ, Macht M. Manipulation of event-related potential manifestations of information processing stages. Science. 1982;218:909–911. doi: 10.1126/science.7134983. [DOI] [PubMed] [Google Scholar]
- Sams M, Alho K, Naatanen R. Sequential effects on the ERP in discriminating two stimuli. Biol Psychol. 1983;17:41–58. doi: 10.1016/0301-0511(83)90065-0. [DOI] [PubMed] [Google Scholar]
- St Peters M, Cherian AK, Bradshaw M, Sarter M. Sustained attention in mice: expanding the translational utility of the SAT by incorporating the Michigan Controlled Access Response Port (MICARP) Behav. Brain Res. 2011;225(2):574–583. doi: 10.1016/j.bbr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci. 2011;31(26):9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a distrimination process. Psychophysiology. 2000;37(2):190–203. [PubMed] [Google Scholar]
- Zanto TP, Rissman JA. Top-down suppression. Brain Mapping: An Encyclopedic Reference. 2015;3:261–267. [Google Scholar]