Hv1 is a voltage-gated proton channel that is composed of two voltage-sensing domains, each of which is permeable to protons. Pathak et al. find that these voltage-sensing domains are mechanosensitive and that membrane stretch results in a long-lived facilitation of Hv1 activation.
Abstract
The voltage-gated proton channel, Hv1, is expressed in tissues throughout the body and plays important roles in pH homeostasis and regulation of NADPH oxidase. Hv1 operates in membrane compartments that experience strong mechanical forces under physiological or pathological conditions. In microglia, for example, Hv1 activity is potentiated by cell swelling and causes an increase in brain damage after stroke. The channel complex consists of two proton-permeable voltage-sensing domains (VSDs) linked by a cytoplasmic coiled-coil domain. Here, we report that these VSDs directly respond to mechanical stimuli. We find that membrane stretch facilitates Hv1 channel opening by increasing the rate of activation and shifting the steady-state activation curve to less depolarized potentials. In the presence of a transmembrane pH gradient, membrane stretch alone opens the channel without the need for strong depolarizations. The effect of membrane stretch persists for several minutes after the mechanical stimulus is turned off, suggesting that the channel switches to a “facilitated” mode in which opening occurs more readily and then slowly reverts to the normal mode observed in the absence of membrane stretch. Conductance simulations with a six-state model recapitulate all the features of the channel’s response to mechanical stimulation. Hv1 mechanosensitivity thus provides a mechanistic link between channel activation in microglia and brain damage after stroke.
Introduction
The Hv1 protein (a.k.a. HVCN1 or VSOP) consists of a proton-conducting voltage-sensing domain (VSD) connected to a coiled-coil domain (CCD) that mediates dimerization (Ramsey et al., 2006; Sasaki et al., 2006; Li et al., 2010; Fujiwara et al., 2012). As a result, the channel has two VSD subunits and two gated proton permeation pathways that open and close cooperatively (Koch et al., 2008; Lee et al., 2008; Tombola et al., 2008, 2010; Gonzalez et al., 2010; Musset et al., 2010b). The Hv1 VSD is similar to the corresponding domain of voltage-gated potassium, sodium, and calcium channels and voltage-sensitive phosphatases (Long et al., 2005; Payandeh et al., 2011; Zhang et al., 2012; Li et al., 2014; Takeshita et al., 2014). The domain is made of four membrane-spanning segments, S1–S4, with the fourth segment providing the major contribution to the gating charge (Gonzalez et al., 2013; Chamberlin et al., 2014).
Because of its roles in human health and disease (DeCoursey, 2013; Pupo and Gonzalez León, 2014; Seredenina et al., 2015), Hv1 is an emerging drug target for cancer and stroke. The channel is found in cellular compartments that experience strong mechanical forces, such as the apical membrane of airway epithelial cells (Iovannisci et al., 2010), where motile cilia beat in coordinated waves, and the flagellar tail of sperm cells (Lishko et al., 2010). In highly metastatic breast cancer cells, Hv1 is involved in the intensely mechanical process of cell migration (Wang et al., 2011, 2012; McGrail et al., 2015). In phagocytic cells, including neutrophils, macrophages, and microglia, Hv1 is present on both the plasma membrane and phagosomes, where it modulates the production of reactive oxygen species (ROS) by NOX enzymes to kill engulfed pathogens (DeCoursey, 2013). The plasma membrane of these cells experiences mechanical forces during spreading (Henry et al., 2015), migration (Houk et al., 2012), and phagocytosis (Masters et al., 2013). The transmembrane protein complex NOX generates reactive superoxide anions by transferring electrons from NADPH to oxygen molecules. The process causes cytoplasmic accumulation of protons and membrane depolarization, both of which inhibit NOX activity (Henderson et al., 1987; Morgan et al., 2005). Hv1 promotes sustained NOX activity by extruding excess cytoplasmic protons and by counteracting membrane depolarization (DeCoursey, 2013).
We wondered whether Hv1 activity could be modulated by mechanical forces. Because voltage-gated potassium and sodium channels were previously reported to be sensitive to membrane stretch (Laitko et al., 2006; Beyder et al., 2010; Schmidt et al., 2012; Morris et al., 2015), we investigated the response of Hv1 to this particular type of mechanical stimulus. We found that membrane stretch strongly affects Hv1 gating and that the channel’s activation rate is the most mechanosensitive parameter. Our findings are consistent with a scenario in which Hv1 switches to a “facilitated” mode upon mechanical stimulation. Once in this mode, the channel can be opened by smaller membrane depolarizations. We also found that membrane stretch affects a form of Hv1 lacking the CCD and previously shown to be monomeric (Koch et al., 2008; Tombola et al., 2008; Fujiwara et al., 2013). A comparison of how dimeric and monomeric forms of Hv1 respond to membrane stretch showed that, although the channel’s VSD is intrinsically mechanosensitive, interactions between the two subunits in the dimeric complex modulate VSD mechanosensitivity. The behavior of monomeric Hv1 was reproduced by a four-state model in which the mechanical stimulus induced transitions increasing the rate of channel opening and the channel maximal conductance. Two additional states were necessary to simulate the long-lasting effects of membrane stretch on dimeric Hv1 activation.
Microglial cells exposed to brain damage experience intracellular acidification (Siesjö et al., 1996; Xiong et al., 2004) and cell swelling (Mongin, 2007; Song and Yu, 2014), which lead to Hv1 hyperactivation (Morihata et al., 2000). Our findings provide a direct mechanism linking cell swelling to Hv1 hyperactivation via membrane stretch. The mechanosensitivity of the Hv1 protein is particularly relevant in the context of ischemic stroke because excessive activity of the microglial channel is known to enhance neuronal death via NOX-mediated ROS production (Wu et al., 2012).
Materials and methods
Plasmid constructions
Human Hv1 constructs were generated as described previously, by subcloning cDNA of the Hv1 channel (provided by D. Clapham [Harvard University, Cambridge, MA; Ramsey et al., 2006] and from IMAGE clone 5577070 [Open Biosystems]) into the pGEMHE vector (Liman et al., 1992). In the Hv1NCVSP chimera, residues 1–96 and 228–273 of Hv1 were replaced by residues 1–113 and 240–576 of Ci-VSP, respectively (Murata et al., 2005), as described previously (Hong et al., 2013).
Channel expression in Xenopus laevis oocytes
Plasmids were linearized with either NheI or SphI (New England Biolabs, Inc.) before in vitro transcription with a T7 mMESSAGE mMACHINE transcription kit (Ambion). RNA quality was confirmed by running a denaturing RNA gel. mRNAs were injected in Xenopus oocytes (50 nl per cell, 0.3–1.5 µg/µl; Ecocytes Biosciences), and electrophysiological measurements were performed 1–3 d later. Cells were maintained at 18°C in ND96 medium (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, 5 mM pyruvate, and 100 µg/ml gentamycin at 18°C, pH 7.2).
Patch clamp recordings
After 1–4 d, the oocytes injected with mRNA were examined under patch clamp configuration. Patch measurements were performed in excised inside-out configuration, using an Axopatch 200B patch clamp amplifier set up on an IX71 microscope (Olympus). Pipettes had 2–4 MΩ access resistance in our solutions. The bath solution was composed of 100 mM Mes buffer, 30 mM TEA methanesulfonate, 5 mM TEA chloride, 5 mM EGTA, adjusted to pH 6.0 with TEA hydroxide. For recordings performed in the absence of pH gradient (pHi and pHo 6.0), the pipette solution had the same composition of the bath solution. Some of the measurements were performed in the presence of a pH gradient (pHi 6.0 and pHo 7.5). In these cases, the extracellular solution contained 100 mM HEPES, 40 mM TEA methanesulfonate, and 5 mM TEA chloride, adjusted to pH 7.5 with TEA hydroxide. Current traces were filtered at 1 kHz, digitized at 5 kHz, and analyzed with Clampfit 10.2 (Molecular Devices) and Origin 8.1 (OriginLab). All measurements were performed at 22 ± 2°C.
Mechanical stimulation of membrane patches
Mechanical stimulation of excised inside-out patches was performed with a high-speed pressure clamp (HSPC-1; ALA Scientific) controlled by pCLAMP 10.2. Suction pulses were administered to the membrane patch through the patch pipette connected to the HSPC-1.
Data analysis
Analysis of data was performed using Clampfit (version 10; Molecular Devices) and Origin (version 8; OriginLab). G-V plots were fitted with the Boltzmann equation G/Gmax = 1/[1 + exp((V1/2 − V)/s)], where V1/2 is the potential of half-maximal activation and s is the slope parameter. Time constants of activation (τa) and deactivation (τd) were obtained by fitting the currents to an exponential function of the form I(t) = b + a * exp(−t/τ). In Figs. 3 and 4, EP = 100 * (IEP − IP0)/IP0, where IEP is the current measured at the end of the pressure pulse and IP0 is the corresponding isochronal current measured in the absence of the pulse. Similarly, ED = 100 * (IED − ID0)/ID0, where IED is the current measured at the end of the depolarization step in the presence of the pressure pulse and ID0 is the corresponding isochronal current measured in the absence of the pulse. Unless otherwise stated, data are represented as mean ± SEM. Effect of pressure stimuli on patches was statistically examined by comparing control conditions and the presence of pressure using the paired t test. P-values <0.05 are considered statistically significant.
Figure 3.
Effects of brief mechanical stimulation on Hv1 activation. (A) Hv1 current was elicited by membrane depolarization as in Fig. 1 B and measured in the absence of mechanical stimulation (black) and in the presence of a brief pulse of negative pressure in the pipette (red). The pulse was delivered when the fraction of open channels generated by voltage-dependent activation was still small. See Materials and methods for details. (B) Enlarged view of current traces (I) enclosed in the blue square in A. Pressure traces recorded during stimulation are also shown (P). (C) Hv1 currents were measured as in A, but with the mechanical stimulus delivered when voltage-dependent activation had produced a larger fraction of open channels. (A and C) Inset bar charts indicate the mean relative increase in current measured at the end of the pulse (EP) and the mean residual increase in current measured at the end of the depolarization step (ED). Error bars are SEM (n = 5). (D) Enlarged view of current traces (I) enclosed in the blue square in C and corresponding pressure traces (P). Black arrowheads in A and C indicate difference in isochronal current at the end of the two traces (ED > 0). Red, blue, and gray arrowheads in B and D indicate different components of the effect of mechanical stimulation on Hv1 activation.
Figure 4.
Effects of brief mechanical stimulation on Hv1NCVSP activation. (A) Hv1NCVSP current was elicited by membrane depolarization as in Fig. 2 B and measured in the absence of mechanical stimulation (black) and in the presence of a brief pulse of negative pressure in the pipette (red). The pulse was delivered when the fraction of open channels generated by voltage-dependent activation was still small. See Materials and methods for details. (B) Enlarged view of current traces (I) enclosed in the blue rectangle in A. Pressure traces recorded during stimulation are also shown (P). (C) Hv1 currents were measured as in A, but with the mechanical stimulus delivered when voltage-dependent activation had produced a larger fraction of open channels. (A and C) Inset bar chart indicates the mean relative increase in current measured at the end of the pulse (EP) and the mean residual increase in current measured at the end of the depolarization step (ED). Error bars are SEM (n = 5). (D) Enlarged view of current traces (I) enclosed in the blue square in C and corresponding pressure traces (P). There was no difference in isochronal current at the end of the two traces in A and C (black arrowheads, ED ≈ 0). Red, blue, and gray arrowheads in B and D indicate different components of the effect of mechanical stimulation on Hv1 activation.
Modeling
The modeling results shown in Fig. 7 were obtained using the two-, four-, and six-state kinetic models illustrated in Fig. S1. The rate constants are given in Tables S1, S2, and S3. For dimeric Hv1, the relative values of the rate constants were determined from the experimentally obtained G-V curves (see Fig. 1 F) and then fitted to obtain the correct rate constants (Tables S1 and S2). For monomeric Hv1, a four-state model based on the dimer model reproduced the data very well once the rate constants were scaled to reflect the much faster kinetics of the monomer and the post-stimulus constants were adjusted to reflect the faster than expected return to the pre-stimulus state (Table S3). The two-, four-, and six-state kinetic models were solved using a python code. The set of first order differential equations were integrated with scipy.integrate.odeint.
Figure 7.
Modeling mechanosensitive responses of Hv1 proton channels via two-, four-, or six-state kinetic schemes. Simulations of time courses of normalized proton conductance (G/Gmax(t)) in response to increase in membrane tension for dimeric (A–C) and monomeric Hv1 (D). The kinetic schemes used for the simulations are shown in Fig. S1. Parameter values are in Tables S1, S2, and S3. Note that dimer kinetics are ∼10 times slower than monomer kinetics. (A and B) Simulation of Hv1 WT at Vm near the foot of its G-V curve (“−20 mV”, see Fig. 6). B shows how the O (blue) and Op (green) states contribute to the total conductance in the four-state model. Insets show the changes in state probabilities at the onset and offset of the pressure pulse. (C) Simulation of Hv1 dimer at Vm near the top of the G/V curve (“+80 mV”; see data traces in Fig. 3). Inset shows an enlarged view of the currents predicted by the three models during the pressure pulse. (D) Simulation of Hv1NCVSP at Vm = 80 mV (see data traces in Fig. 4). Inset in the top panel shows an enlarged view of the currents predicted by the two- and four-state models during the pressure pulse. Instantaneous onset and offset of increased membrane tension (in response, experimentally, to applied pipette aspiration, i.e., “Pressure on”) is assumed. Asterisks in A and B indicate a step to Vhold simultaneous with Pressure off, as in some experiments.
Figure 1.
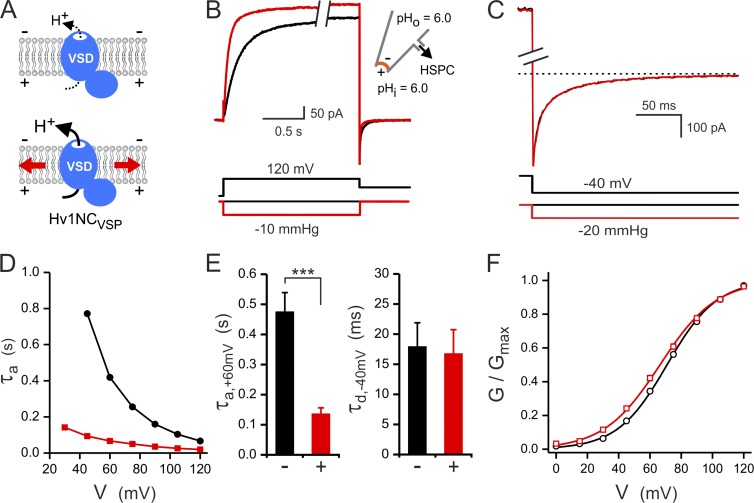
Hv1 response to combined electrical and mechanical stimulation. (A) Schematic representation of dimeric Hv1 stimulated by membrane stretch (red arrows). (B) Effect on Hv1 activation: Proton currents were measured in response to membrane depolarization in the absence (black) and presence (red) of negative pressure in the pipette. Holding potential: −80 mV, holding pressure: 0 mmHg. (C) Effect on Hv1 deactivation: Tail currents were measured at −40 mV after a depolarization pulse at 120 mV in the absence (black) and presence (red) of negative pressure in the pipette. Holding conditions and proton concentrations were as in B. Dotted line corresponds to 0 pA. Break in the current axis corresponds to 200 pA. (D) τa as a function of applied voltage without pressure stimulation (black circles) and in the presence of −10 mmHg pressure in the pipette (red squares). (E, left) Effect of −10-mmHg-pressure stimulation on τa measured at 60 mV; n = 11; ***, P < 0.0001. (right) Effect of −20-mmHg-pressure stimulation on τd measured at −40 mV; n = 3; P > 0.05. (F) Effect on G-V curve of negative pressure in the pipette. G-Vs measured at 0 mmHg and −10 mmHg are shown in black and red, respectively. Boltzmann fit parameters are V1/2 = 60.5 ± 1.5 mV and s = 11.0 ± 0.8 mV for the 0-mmHg condition (n = 10) and V1/2 = 46.1 ± 1.2 mV and s = 7.5 ± 0.4 mV for the −10-mmHg condition (n = 10). P < 10−6 and P < 0.002 for differences in V1/2 and s, respectively. Errors bars are SEM.
Online supplemental material
Fig. S1 shows kinetic schemes used to simulate Hv1 mechanosensitive gating. Fig. S2 shows the proposed vicious cycle connecting brain damage to Hv1 hyperactivation in the microglia. Fig. S3 shows a schematic representation of two processes proposed to be involved in the facilitation of Hv1 activation by membrane stretch. Fig. S4 shows long-lasting facilitation observed in dimeric Hv1, which is proposed to be linked to changes in the dimer interface. Tables S1 and S2 show modeling of the application of tension at −20 mV and 80 mV, respectively, to the Hv1 dimer. Table S3 shows modeling of the application of tension at 80 mV to the Hv1 monomer.
Results
Hv1 voltage-dependent activation is facilitated by membrane stretch
We recorded proton currents in inside-out membrane patches from Xenopus oocytes expressing wild-type human Hv1 in response to membrane depolarization and tested the effect of membrane stretch on the channel’s activation and deactivation kinetics and voltage dependence of activation. To minimize changes in local proton concentration induced by Hv1 activity, we used a high concentration of pH buffer (100 mM) in both pipette and bath solutions and optimized channel density to have proton currents no bigger than 1 nA. The major charged species in all media were buffer ions, TEA, and methanesulfonate (see Materials and methods). Under these conditions, we did not observe activity of endogenous mechanosensitive channels. Changes in membrane stretch were achieved via HSPC connected to the patch pipette (Fig. 1, A and B). Currents were measured soon after membrane excision under no-pressure conditions (Fig. 1 B, black trace) and then again under a negative pressure step (Fig. 1 B, red trace). The magnitudes of the pressure pulses were in the range 10–25 mmHg, typically used to stimulate the opening of bona fide stretch-activated cation channels (Patel et al., 1998; Maingret et al., 1999; Maroto et al., 2005; Coste et al., 2010; Pathak et al., 2014). Negative pressure in the pipette in inside-out configuration corresponds to positive pressure inside the cell (i.e., pressure that would cause cell swelling). To quantify the effect of negative pressure stimulation, we performed monoexponential fits of the current traces (DeCoursey and Cherny, 1995) and used the single time constant (τa) as an approximate kinetic parameter. The effect on the deactivation kinetics parameter (τd) was quantified by monoexponential fitting of tail currents measured after a depolarization step (Fig. 1 C). Whereas activation kinetics showed strong acceleration (reduction in τa) in the presence of negative pressure (more than fourfold change at 60 mV; Fig. 1, B, D, and E), the effect on channel deactivation (τd) was not statistically significant (Fig. 1, C and E). The voltage dependence of channel activation was derived from tail currents as previously described (Tombola et al., 2010). Comparison of G-V curves measured in the absence and in the presence of negative pressure (Fig. 1 F) shows that membrane stretch produced a shift in the voltage of half-activation (V1/2) of −14.4 ± 2.7 mV (activation requires less depolarized potentials) and a 32 ± 8% increase in the steepness of the curve (measured as a reduction in the slope factor s).
We wondered whether the observed effects of membrane stretch required a homodimerically assembled channel complex or could be attributed to mechanosensitive properties of the individual VSD subunit. To address this question, we examined the response to negative pressure stimulation of the Hv1NCVSP channel (Fig. 2 A), in which the Hv1 cytoplasmic N- and C-terminal domains are replaced by the corresponding parts of the voltage-sensitive phosphatase CiVSP (Murata et al., 2005; Kohout et al., 2008). Hv1NCVSP was previously found to be monomeric (Tombola et al., 2008). Hv1 constructs lacking the C-terminal CCD have faster gating kinetics and open at more depolarized voltages than the dimeric wild-type channel (Koch et al., 2008; Tombola et al., 2008). Activation in these constructs is well represented by a monoexponential curve (Fujiwara et al., 2013).
Figure 2.
Hv1NCVSP response to combined electrical and mechanical stimulation. (A) Schematic representation of monomeric Hv1NCVSP stimulated by membrane stretch (red arrows). (B) Effect on Hv1NCVSP activation: Proton currents were measured in response to membrane depolarization in the absence (black) and presence (red) of negative pressure in the pipette. Holding potential: −80 mV, holding pressure: 0 mmHg. Break in the time axis corresponds to 1.5 s. (C) Effect on Hv1NCVSP deactivation: Tail currents were measured at −40 mV after a depolarization pulse at 120 mV in the absence (black) and presence (red) of negative pressure in the pipette. Holding conditions and proton concentrations were as in B. Dotted line corresponds to 0 pA. Break in the current axis corresponds to 650 pA. (D) τa values measured as a function of applied voltage without pressure stimulation (black circles) and in the presence of −10 mmHg pressure in the pipette (red squares). (E, left) Effect of −10-mmHg-pressure stimulation on τa measured at 60 mV; n = 14; ***, P < 0.0001. (right) Effect of −20-mmHg-pressure stimulation on τd measured at −40 mV; n = 5; P > 0.05. (F) Effect on G-V curve of negative pressure in the pipette. G-Vs measured at 0 mmHg and −10 mmHg are shown in black and red, respectively. Boltzmann fit parameters are V1/2 = 70.9 ± 0.7 mV and s = 16.1 ± 0.4 mV for the 0-mmHg condition (n = 26) and V1/2 = 66.4 ± 1.0 mV and s = 18.1 ± 0.4 mV for the −10-mmHg condition (n = 26). P < 0.001 and P < 0.002 for differences in V1/2 and s, respectively. Errors bars are SEM. In F, error bars are smaller than symbols.
Hv1NCVSP activation was accelerated (τa decreased) by negative pressure stimulation (Fig. 2, A, B, D, and E) to an extent similar to what was observed with dimeric Hv1, whereas the kinetics of deactivation were not sensitive to the stimulus (Fig. 2, C and E). Negative pressure in the pipette shifted the voltage dependence of activation of Hv1NCVSP to less depolarized potentials (Fig. 2 F), as observed with dimeric Hv1, but to a smaller extent: ΔV1/2 = −4.5 ± 1.7 mV. The steepness of the G-V curve did not increase with the pressure stimulus, but rather decreased 11 ± 4%.
Thus, Hv1 activation is facilitated by electrical and mechanical co-stimulation, and the most mechanosensitive parameter both in the monomeric and dimeric channel is the rate constant of channel activation (or its inverse, τa). For the rest of the study, we use this parameter as a readout of the channel’s response to membrane stretch. The different effects of mechanical stimulation on the G-V curves of monomeric and dimeric Hv1 indicate that allosteric interactions between subunits can influence the intrinsic mechanosensitivity of the Hv1 VSD.
Short- and long-lasting effects of membrane stretch on Hv1 activation
To gain further insight on Hv1 mechanosensitivity, we examined the changes in proton current induced by pulses of negative pressure of short duration applied during channel activation by membrane depolarization (Fig. 3, A and C). If the only effect of mechanical stimulation was to reversibly increase the channel’s activation rate, the proton current should increase more rapidly during the pressure pulse, and the excess of open channels thus generated should gradually decrease after termination of the pressure pulse to satisfy the steady-state conditions for the applied membrane potential at zero pressure. These features were evident in the proton currents during and after the pressure pulses (indicated by red and gray arrowheads in Fig. 3 [B and D], respectively), but two additional features revealed that the effect of membrane stretch on Hv1 had multiple components. One of the additional features was a fast (<10-ms time scale) increase in proton current at the onset of the pressure pulse, followed by a fast decrease in current coinciding with the termination of the pulse (Fig. 3, B and D, blue arrowheads). The extent of this fast component was small when the mechanical stimulus was applied at the beginning of the depolarization step (Fig. 3, A and B) and became larger when the mechanical stimulus was applied later during the depolarization step (Fig. 3, C and D). Because the fraction of open channels increases over time during depolarization before reaching steady state, one possible explanation for the observed fast component is a stretch-induced conformational change that increases the conductance of the open channels. The other additional feature was the excess current remaining after termination of the mechanical stimulus (Fig. 3, A and C, black arrowheads). The current after the pressure pulse was consistently larger than the isochronal current expected at the end of the voltage step in the absence of the pressure pulse, suggesting that the effect of membrane stretch has a long-lasting component.
We then measured proton currents from Hv1NCVSP in response to pressure pulses of short duration (Fig. 4). We found that the fast components of the change in current (Fig. 4, B and D, blue arrowheads) were similar to those observed in the wild-type channel. The slower components (Fig. 4, B and D, red and gray arrowheads) were also similar. However, with Hv1NCVSP, there was no excess current remaining after termination of the mechanical stimulus (Fig. 4, A and C, black arrowheads). The current after the pressure pulse was the same as the isochronal current expected at the end of the voltage step in the absence of the pressure pulse. This finding suggests that the long-lasting alteration induced by membrane stretch in wild-type Hv1 is either absent in Hv1NCVSP or quickly reversible.
We wondered whether the long-lasting alteration in wild-type Hv1 responsible for the excess current after mechanical stimulation could be revealed by consecutive membrane depolarizations and asked whether this alteration could occur when membrane stretch and membrane depolarization are not delivered simultaneously. We interrogated Hv1 with a protocol consisting of two consecutive voltage steps separated by a fixed interval during which a pressure pulse was applied with or without a simultaneous voltage step (Fig. 5, A–D). To quantify changes in activation kinetics between the “T” depolarization (test) and the “R” depolarization (reference), we calculated the ratio τa,T/τa,R. We found that this ratio significantly decreased when a pressure pulse was applied between the two voltage steps (Fig. 5 E, P < 0.0001), indicating that membrane stretch causes an acceleration of channel activation that persists in the absence of the pressure pulse. In addition, we found that electrical co-stimulation is not required to achieve membrane stretch–induced facilitation of channel activation (Fig. 5 E).
Figure 5.
Long-lasting effect of membrane stretch on Hv1 activation does not require membrane depolarization and is slowly reversible. (A) Hv1 proton current elicited by consecutive depolarization steps R (reference) and T (test) in the absence of mechanical stimulation (pHi and pHo 6.0). Changes in kinetics of activation between the first and second depolarization are quantified by the ratio τa,T/τa,R reported in E. (B) Hv1 current measured as in A with mechanical stimulation (negative pressure) delivered at holding membrane potential between T and R pulses. (C) Hv1 current measured as in A with an additional depolarization step (P) between the reference and test pulses. (D) Hv1 current measured as in C with mechanical stimulation delivered during the depolarization step (P) between T and R pulses. (E) Box plot comparing τa,T/τa,R values measured under the voltage and pressure protocols shown in A–D. τa,T/τa,R < 1 indicates accelerated activation kinetics during the T pulse compared with the R pulse. Boxes indicate median ± SD. Whiskers show 5th and 95th percentiles. Asterisks indicate statistically significant difference: ***, P < 0.0001. (F) Recovery from membrane stretch–induced acceleration of Hv1 activation. Hv1 was first activated with a combined voltage/pressure protocol as in D. (left) τa,R, τa,P, and τa,T parameters were measured and normalized, using τa,R as reference. After the time interval Δt, the combined voltage/pressure protocol was applied again, and the normalized τa values were recalculated. The protocol was applied only once per patch. Light gray circles (Δt = 1 s): n = 4. Gray squares (Δt = 60 s): n = 5. Black diamonds (Δt = 300 s): n = 7. Error bars are SEM. (right) Bar chart showing the recovery of τa,R over time after mechanical stimulation (black columns). The reference values corresponding to full recovery are shown as purple columns. Error bars are SEM. Asterisks indicate statistically significant difference: *, P < 0.01. The normalized τa,P and τa,T values in the left panel corresponding to a particular Δt (τa,P(Δt)/τa,R(Δt), and τa,T(Δt)/τa,R(Δt)) can be renormalized relative to τa,R(control) using the corresponding τa,R(Δt)/τa,R(control) ratios from the right panel as multiplication factors.
When Hv1 was stimulated with three consecutive depolarizations (R, P, and T), as shown in Fig. 5 (D and F), the pressure pulse delivered with the second depolarization induced faster channel activation during the third depolarization as compared with the first depolarization (τa,T < τa,R, P < 0.0001). Remarkably though, channel activation elicited by the third pulse (T) was also faster than the activation elicited by combined electrical and mechanical stimulation (second pulse [P]; τa,T < τa,P < τa,R; Fig. 5 F, purple open diamonds; P < 0.001). The simplest explanation for this hysteretic behavior is that when the channel closes at the end of the second depolarization, it does not go back to the original closed state, but to a nonconducting facilitated state from which it is easier to transition to the open state.
We asked whether the facilitated state would slowly revert to the normal closed state in the absence of mechanical stimulation. We applied the double three-pulse protocol shown in Fig. 5 F in which the first set of pulses (control) is used to induce facilitation, and the second set is used to assess the recovery from the facilitated state after a time interval Δt. Facilitation was estimated as an acceleration of channel activation during the second and third depolarizations compared with the first depolarization (reduction in normalized τa within each set of pulses). With the shortest interval (Δt = 1 s; Fig. 5 F, left, light gray filled circles), the recovery of facilitation was partial, as mechanical stimulation could still accelerate activation in the second pulse (τa,P < τa,R, P < 0.001), but no further acceleration was observed in the third pulse (τa,T ≈ τa,P, P > 0.05). Similar results were obtained with a Δt of 60 s (Fig. 5 F, left, gray filled squares). To observe full recovery of facilitation, an extension of Δt to 300 s was required (Fig. 5 F, left, black filled diamonds and control purple open diamonds almost superimposable).
The finding that facilitation within individual sets of pulses can be completely recovered after 300 s suggests that the effect of mechanical stimulation on channel activity is fully reversible. If this were the case, we would also expect τa,R to completely recover within 300 s to its original value (τa,R(Δt = 300 s)/τa,R(Control) = 1). So we measured the ratio τa,R(Δt)/τa,R(Control) at Δt = 1, 60, and 300 s (Fig. 5 F, right, black columns) and compared it with the value expected for complete recovery (Fig. 5 F, right, purple columns). At the shorter Δts, channel activation was still faster than the control (τa,R(Δt)/τa,R(Control) < 1, P < 0.01), indicating that the accelerating effect of the mechanical stimulation had not yet reversed. In contrast, at Δt = 300 s, channel activation was no longer accelerated (τa,R(Δt = 300 s)/τa,R(Control) ≥ 1; Fig. 5 F, right). Although the τa,R(Δt = 300 s)/τa,R(Control) ratio was not significantly higher than 1 (P > 0.05), we cannot rule out the possibility of an additional process that slows down channel activation after the accelerating effect of mechanical stimulation has subsided. Nonetheless, based on these results and the complete recovery from facilitation within individual set of pulses reported above, it is reasonable to conclude that the effect of membrane stretch on Hv1 activation is reversible.
Together, these measurements show that the mechanical stimulus causes dimeric Hv1 to accelerate its voltage-dependent activation and to switch to a facilitated state from which the channel can open more easily. Switching to the facilitated state does not require membrane depolarization, and the channel can stay facilitated for minutes after the mechanical stimulus is terminated. The mechanical stimulus causes similar effects in monomeric Hv1 (Figs. 2 and 4). But, in the monomeric form, the channel can return to the nonfacilitated state more rapidly, as indicated by the lack of poststimulation current enhancement in Fig. 4 (A and C).
The fast on and off kinetics of the proton current in response to brief pressure pulses and the minimal delays between the changes in pressure and the changes in current (Figs. 3 and 4) indicate that the slack in the system is negligible. We cannot completely exclude that the long-lasting facilitation observed in dimeric Hv1 is caused by residual membrane tension persisting after the termination of the pressure pulse. However, the fact that we do not observe poststimulation current enhancement in monomeric Hv1 (Fig. 4) is more consistent with facilitation being a property of the channel rather than the membrane.
Hv1 activation by membrane stretch in the presence of a transmembrane pH gradient
Hv1 requires strong and sustained depolarizations to open in the absence of a transmembrane pH gradient. The V1/2 of the activation curve has been reported to be between 50 and 65 mV when ΔpHm = pHo − pHi = 0 (Ramsey et al., 2006; Sasaki et al., 2006; Tombola et al., 2008). However, the channel is known to open at less depolarized potentials when the intracellular pH is lower than the extracellular pH (ΔpHm > 0; Cherny et al., 1995; Ramsey et al., 2006; Sasaki et al., 2006; Villalba-Galea, 2014). Because we found that membrane stretch can facilitate Hv1 activation even in the absence of membrane depolarization, we asked whether mechanical stimulation alone could result in channel opening in the presence of a favorable ΔpHm. To test for this possibility, we recorded Hv1 currents from inside-out patches exposed to intracellular and extracellular solutions of different pH (pHi 6.0 and pHo 7.5; Fig. 6). The membrane potential was maintained to a negative value (−30 to −20 mV), close to the channel activation threshold. Under these conditions, the channel was closed (Fig. 6 B, black trace), but opened upon stimulation with a pressure pulse (Fig. 6, B [red trace] and C). The complete absence of stretch-induced currents in noninjected cells (Fig. 6 C, NI) indicates that measured currents are from Hv1 and not from endogenous stretch-activated channels.
Figure 6.
Hv1 response to mechanical stimulation in the presence of a pH gradient. (A) Schematic representation of dimeric Hv1 opened by membrane stretch (red arrows) when ΔpHm = pHo − pHi > 0. (B) Proton currents were recorded at a membrane potential sufficiently negative to prevent voltage-dependent channel activation in the absence of mechanical stimulation (between −30 and −20 mV; black). The current was then recorded again at the same voltage but in the presence of negative pressure in the pipette (red). At the end of the pressure step, the voltage was changed to −80 mV. (C) Quantification of the proton current elicited by negative pressure stimulation under conditions described in B. Membrane patches from noninjected cells (NI) displayed no stretch-induced currents. Error bars are SEM (n = 5 for NI, n = 11 for Hv1). Asterisks indicate statistically significant difference: ***, P < 0.0001.
Models of Hv1 mechanosensitivity
We then set out to determine the simplest kinetic model that could reproduce the main features of Hv1 mechanosensitive activation. We started with a basic two-state scheme (C ↔ O; Fig. S1) in which the forward and backward rates are modulated by membrane tension such that their g(V) curve would hyperpolarize with elevated membrane tension, as per Fig. 1 F. This aspect is essentially as for Kv channels with a mechanosensitive V-dependent transition (Morris et al., 2015). The model was able to mimic the increase in Hv1-mediated proton conductance elicited by pulses of negative pressure delivered at membrane potentials close to the activation threshold (Figs. 6 and 7 A) but could not reproduce the more complex features observed when Hv1 was exposed to short mechanical stimuli in the presence of stronger membrane depolarization (Figs. 3 and 7 C). Thus, we added a second tension-modulated V-dependent closed-open pair (Cp ↔ Op) to the basic scheme (Fig. S1). In the resulting four-state model, the mechanical stimulus affects the C ↔ O transition as in the two-state model, but also facilitates access to conformations Cp and Op. The conductance of the Op state is ∼25% larger than the conductance of the O state to allow for the sharp increase and decrease in total proton conductance when the mechanical stimulus is turned on and off, respectively (Fig. 3, B and D, blue arrowheads; and Fig. 7 C). For simplicity, the rates of the Cp ↔ Op transition were set to be the same as for the C ↔ O transition, and the mechanical stimulus was assumed to affect the two transitions to the same extent. In addition, the forward and backward rates in the fast C ↔ Cp and O ↔ Op transitions were chosen to be equal, explaining why O and Op rise together in Fig. 7 B. The four-state model was able to replicate all of the short-lasting effects of mechanical stimulation on dimeric and monomeric Hv1 (Figs. 3, 4, and 7, C and D; and Tables S1 and S3).
The long-lasting increase in proton conductance observed after mechanical stimulation of Hv1 wild type (Fig. 3, A and C, black arrowheads) could not be properly simulated with the four-state model (Fig. 7 C, black curve). Hence, we added a third tension-modulated V-dependent closed-open pair (Cpp ↔ Opp), increasing the total number of states to six (unitary conductance of Opp same as Op). The mechanical stimulus was assumed to increase the Cpp → Opp rate twice as much as the C → O and Cp → Op rates. The transitions connecting Cpp to Cp and Opp to Op were set to become very slow (rates ≈ 0) in the absence of the mechanical stimulus. This restraint, in combination with a poststimulus deceleration of the Cp → C and Op → O transitions (Table S2) led to an accurate simulation of the long-lasting Hv1 facilitation induced by membrane tension (Fig. 7 C, purple curve).
This approach of stepwise increasing modeling complexity allows us to conclude that the four- and six-state models are the simplest models that accurately simulate the observed effects of mechanical stimulation on monomeric and dimeric Hv1, respectively. A possible structural interpretation for the requirement of two extra states for the dimer is reported in the Discussion.
Discussion
Hv1 is expressed in cells that do not normally change their membrane potential to the extent required to fully activate the whole channel population, especially in the absence of a favorable transmembrane pH gradient. Although Hv1 can experience favorable ΔpHm in phagocytes during the respiratory burst (DeCoursey, 2013), similarly favorable conditions are unlikely to occur in other cells, where homeostatic mechanisms prevent substantial intracellular acidification, e.g., B lymphocytes (Marches et al., 2001; Capasso et al., 2010) and basophils (Musset et al., 2008), or where the intracellular pH can be higher than the extracellular pH, e.g., osteoclasts (Nordström et al., 1995; Mori et al., 2003) and sperm cells (Lishko et al., 2010; O’Hanlon et al., 2013). As a result, it is unlikely that the channel’s full activation can be produced by membrane depolarization alone under physiological conditions.
Besides ΔpHm, little is known about the physical or chemical factors that modulate Hv1 gating. Changes in temperature are known to affect Hv1 activation (DeCoursey and Cherny, 1998; Kuno et al., 2009; Fujiwara and Okamura, 2014), and protein modifications, such as phosphorylation (Morgan et al., 2007; Musset et al., 2010a), or alternative protein initiation (Hondares et al., 2014) can produce Hv1 channels with faster activation kinetics. However, these stimuli operate on a time scale different than voltage gating and are not specific to membrane compartments exposed to mechanical forces.
Facilitation of Hv1 activation by membrane stretch occurs within the time scale of voltage gating and allows the channel to rapidly open under the moderate depolarization conditions typically found in cellular compartments physiologically exposed to mechanical forces, such as the apical membrane of airway epithelial cells and the tail of sperm cells. In cases where Hv1 activation is already primed by other stimuli (e.g., ΔpHm), additional stimulation by membrane stretch can render the channel hyperactive. Proton currents measured in microglial cells under conditions mimicking neuronal damage were previously found to be potentiated by cell swelling induced by intracellular acidosis or hypotonic stress (Morihata et al., 2000). Stroke-induced brain damage is associated with cell swelling (Mongin, 2007; Song and Yu, 2014) and acidosis (Siesjö et al., 1996; Xiong et al., 2004), and proton channel activation in the microglia was found to increase neuronal death in a mouse model of ischemic stroke (Wu et al., 2012). When microglial cells are exposed to acidosis, the ensuing cell swelling may cause the plasma membrane to stretch, at least transiently, triggering Hv1 transition to the facilitated gating mode. Microglia cells could use this mechanism to increase proton extrusion and counteract intracellular acidification (Morihata et al., 2000). However, Hv1 hyperactivation would also increase the production of toxic ROS (Wu et al., 2012), as a result of the electrochemical coupling between the channel and NOX, ultimately leading to enhanced neuronal death (Fig. S2).
Voltage-gated potassium and sodium channels are known to be sensitive to mechanical stimulation, as their activation by membrane depolarization is modulated by membrane stretch (Laitko et al., 2006; Beyder et al., 2010; Schmidt et al., 2012; Hao et al., 2013). However, whether the effect of membrane stretch is mediated by their four VSDs or their pore domain is a subject of debate (Tabarean and Morris, 2002; Schmidt et al., 2012; Morris et al., 2015). Because the Hv1 channel lacks the pore domain and has a dimeric, rather than tetrameric, structural organization, it represents a simpler model to study how mechanical forces affect VSDs. Here, we find that the Hv1 VSD is intrinsically sensitive to membrane stretch, and that the allosteric coupling between the two VSD subunits modulates the effect of the mechanical stimulus on voltage-dependent activation. We also find that the effect has multiple components, some of which revert quickly, whereas others revert slowly and possibly not completely. As a result, the rate of channel activation by membrane depolarization depends on whether the channel has been previously exposed to membrane stretch, a hallmark of mechanical hysteresis.
A previous study on bacterial stretch-activated ion channels proposed that wetting and dewetting of a hydrophobic region in the permeation pathway can play a major role in defining the thermodynamics and kinetics of mechanosensitive channel gating and can be the origin of hysteretic behavior (Anishkin et al., 2010). The hydration/wetting of the core of the Hv1 VSD appears to be an important requirement for proton conduction (Freites et al., 2006; Ramsey et al., 2010; Wood et al., 2012), and water penetration into the core of the channel’s transmembrane module is believed to change considerably upon channel opening (Chamberlin et al., 2014, 2015; Hong et al., 2014), especially in the proximity of the VSD charge transfer center or hydrophobic plug (Tao et al., 2010; Lacroix et al., 2014; Takeshita et al., 2014). So one possible origin for Hv1 mechanosensitivity could reside in changes of VSD wettability induced by membrane stretch, which would result in changes in the thermodynamic and kinetic properties of voltage-dependent gating (Fig. S3 A). Voltage-dependent opening in Hv1 has been proposed to involve conformational changes in the intracellular portion of the VSD, leading to an expansion of the intracellular vestibule (Hong et al., 2013; Mony et al., 2015). Thus, another possible source of mechanosensitivity could derive from alterations in protein–lipid interactions in the proximity of the intracellular vestibule. Membrane stretch could cause a stabilization of the expanded vestibule resulting in a facilitation of channel opening (Fig. S3 B).
The difference in long-lasting facilitation observed in dimeric versus monomeric channels can be explained by a distinct tension-dependent transition in the dimer complex, corresponding to the Cpp ↔ Opp transition in our model (Fig. S1). The simplest interpretation for this additional transition is a tension-dependent alteration of the dimer interface that cannot occur in the monomer (Fig. S4). The two Hv1 subunits are held together at the cytoplasmic CCD (Koch et al., 2008; Lee et al., 2008; Tombola et al., 2008; Li et al., 2010; Fujiwara et al., 2012), but they also interact inside the membrane (Li et al., 2015). The transmembrane VSD–VSD interface is more likely to be sensitive to membrane stretch than the CCD. The S4 helices of the two subunits were previously found to be part of the VSD–VSD interface and to form a continuous structural unit with the CCD (Fujiwara et al., 2014). The outer ends of the S1 segments were also found to participate in the dimer interface (Lee et al., 2008; Qiu et al., 2013; Hong et al., 2015), suggesting that the S4–S4 unit partially unwinds toward the extracellular side of the membrane (Hong et al., 2015). Membrane stretch could change the extent of this unwinding, consequently affecting the S4 movement associated with gating. Further studies will be necessary to test this possibility and other tension-induced changes in dimer interface. Nonetheless, the kinetic characteristics of the Cpp and Opp states (Table S2) suggest that, in the absence of a mechanical stimulus, the dimer interfaces interconvert at a rate that is more than one order of magnitude slower than voltage gating (Fig. S4).
In conclusion, our finding that mechanical stimulation significantly enhances Hv1 activity by acting on the channel’s VSDs provides a simple explanation for the phenomenon of proton current potentiation observed in the microglia after cell swelling induced by intracellular acidosis or hypotonic stress (Morihata et al., 2000). We thus propose that membrane stretch is one of the co-stimuli required to open Hv1 under the moderate depolarization conditions typically found in nonexcitable cells.
Supplementary Material
Acknowledgments
We thank Stephen White for comments on the manuscript.
This work was supported by National Institutes of Health grants GM098973 and NS085628 and University of California, Irvine’s Council on Research Computing and Libraries (CORCL) grant (to F. Tombola). B. Joós acknowledges support from the Natural Sciences and Engineering Research Council of Canada and C.E. Morris from the Ottawa Hospital Research Institute. T. Tran was supported in part by the Undergraduate Research Opportunities Program of the University of California, Irvine.
The authors declare no competing financial interests.
Richard W. Aldrich served as editor.
Footnotes
Abbreviations used:
- CCD
- coiled-coil domain
- HSPC
- high-speed pressure clamp
- ROS
- reactive oxygen species
- VSD
- voltage-sensing domain
References
- Anishkin A., Akitake B., Kamaraju K., Chiang C.S., and Sukharev S.. 2010. Hydration properties of mechanosensitive channel pores define the energetics of gating. J. Phys. Condens. Matter. 22:454120 10.1088/0953-8984/22/45/454120 [DOI] [PubMed] [Google Scholar]
- Beyder A., Rae J.L., Bernard C., Strege P.R., Sachs F., and Farrugia G.. 2010. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J. Physiol. 588:4969–4985. 10.1113/jphysiol.2010.199034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso M., Bhamrah M.K., Henley T., Boyd R.S., Langlais C., Cain K., Dinsdale D., Pulford K., Khan M., Musset B., et al. 2010. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat. Immunol. 11:265–272. 10.1038/ni.1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin A., Qiu F., Rebolledo S., Wang Y., Noskov S.Y., and Larsson H.P.. 2014. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 111:E273–E282. 10.1073/pnas.1318018111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin A., Qiu F., Wang Y., Noskov S.Y., and Larsson H.P.. 2015. Mapping the gating and permeation pathways in the voltage-gated proton channel Hv1. J. Mol. Biol. 427:131–145. 10.1016/j.jmb.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny V.V., Markin V.S., and DeCoursey T.E.. 1995. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 105:861–896. 10.1085/jgp.105.6.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B., Mathur J., Schmidt M., Earley T.J., Ranade S., Petrus M.J., Dubin A.E., and Patapoutian A.. 2010. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 330:55–60. 10.1126/science.1193270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E. 2013. Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the HV family. Physiol. Rev. 93:599–652. 10.1152/physrev.00011.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E., and Cherny V.V.. 1995. Voltage-activated proton currents in membrane patches of rat alveolar epithelial cells. J. Physiol. 489:299–307. 10.1113/jphysiol.1995.sp021051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E., and Cherny V.V.. 1998. Temperature dependence of voltage-gated H+ currents in human neutrophils, rat alveolar epithelial cells, and mammalian phagocytes. J. Gen. Physiol. 112:503–522. 10.1085/jgp.112.4.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freites J.A., Tobias D.J., and White S.H.. 2006. A voltage-sensor water pore. Biophys. J. 91:L90–L92. 10.1529/biophysj.106.096065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y., and Okamura Y.. 2014. Temperature-sensitive gating of voltage-gated proton channels. Curr. Top. Membr. 74:259–292. 10.1016/B978-0-12-800181-3.00010-5 [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Kurokawa T., Takeshita K., Kobayashi M., Okochi Y., Nakagawa A., and Okamura Y.. 2012. The cytoplasmic coiled-coil mediates cooperative gating temperature sensitivity in the voltage-gated H+ channel Hv1. Nat. Commun. 3:816 10.1038/ncomms1823 [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Kurokawa T., Takeshita K., Nakagawa A., Larsson H.P., and Okamura Y.. 2013. Gating of the designed trimeric/tetrameric voltage-gated H+ channel. J. Physiol. 591:627–640. 10.1113/jphysiol.2012.243006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y., Kurokawa T., and Okamura Y.. 2014. Long α helices projecting from the membrane as the dimer interface in the voltage-gated H+ channel. J. Gen. Physiol. 143:377–386. 10.1085/jgp.201311082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Koch H.P., Drum B.M., and Larsson H.P.. 2010. Strong cooperativity between subunits in voltage-gated proton channels. Nat. Struct. Mol. Biol. 17:51–56. 10.1038/nsmb.1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Rebolledo S., Perez M.E., and Larsson H.P.. 2013. Molecular mechanism of voltage sensing in voltage-gated proton channels. J. Gen. Physiol. 141:275–285. 10.1085/jgp.201210857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J., Padilla F., Dandonneau M., Lavebratt C., Lesage F., Noël J., and Delmas P.. 2013. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 77:899–914. 10.1016/j.neuron.2012.12.035 [DOI] [PubMed] [Google Scholar]
- Henderson L.M., Chappell J.B., and Jones O.T.. 1987. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246:325–329. 10.1042/bj2460325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S.J., Chen C.S., Crocker J.C., and Hammer D.A.. 2015. Protrusive and contractile forces of spreading human neutrophils. Biophys. J. 109:699–709. 10.1016/j.bpj.2015.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E., Brown M.A., Musset B., Morgan D., Cherny V.V., Taubert C., Bhamrah M.K., Coe D., Marelli-Berg F., Gribben J.G., et al. 2014. Enhanced activation of an amino-terminally truncated isoform of the voltage-gated proton channel HVCN1 enriched in malignant B cells. Proc. Natl. Acad. Sci. USA. 111:18078–18083. 10.1073/pnas.1411390111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L., Pathak M.M., Kim I.H., Ta D., and Tombola F.. 2013. Voltage-sensing domain of voltage-gated proton channel Hv1 shares mechanism of block with pore domains. Neuron. 77:274–287. 10.1016/j.neuron.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L., Kim I.H., and Tombola F.. 2014. Molecular determinants of Hv1 proton channel inhibition by guanidine derivatives. Proc. Natl. Acad. Sci. USA. 111:9971–9976. 10.1073/pnas.1324012111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L., Singh V., Wulff H., and Tombola F.. 2015. Interrogation of the intersubunit interface of the open Hv1 proton channel with a probe of allosteric coupling. Sci. Rep. 5:14077 10.1038/srep14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk A.R., Jilkine A., Mejean C.O., Boltyanskiy R., Dufresne E.R., Angenent S.B., Altschuler S.J., Wu L.F., and Weiner O.D.. 2012. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 148:175–188. 10.1016/j.cell.2011.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovannisci D., Illek B., and Fischer H.. 2010. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J. Gen. Physiol. 136:35–46. 10.1085/jgp.200910379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H.P., Kurokawa T., Okochi Y., Sasaki M., Okamura Y., and Larsson H.P.. 2008. Multimeric nature of voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 105:9111–9116. 10.1073/pnas.0801553105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout S.C., Ulbrich M.H., Bell S.C., and Isacoff E.Y.. 2008. Subunit organization and functional transitions in Ci-VSP. Nat. Struct. Mol. Biol. 15:106–108. 10.1038/nsmb1320 [DOI] [PubMed] [Google Scholar]
- Kuno M., Ando H., Morihata H., Sakai H., Mori H., Sawada M., and Oiki S.. 2009. Temperature dependence of proton permeation through a voltage-gated proton channel. J. Gen. Physiol. 134:191–205. 10.1085/jgp.200910213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J.J., Hyde H.C., Campos F.V., and Bezanilla F.. 2014. Moving gating charges through the gating pore in a Kv channel voltage sensor. Proc. Natl. Acad. Sci. USA. 111:E1950–E1959. 10.1073/pnas.1406161111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitko U., Juranka P.F., and Morris C.E.. 2006. Membrane stretch slows the concerted step prior to opening in a Kv channel. J. Gen. Physiol. 127:687–701. 10.1085/jgp.200509394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Letts J.A., and Mackinnon R.. 2008. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl. Acad. Sci. USA. 105:7692–7695. 10.1073/pnas.0803277105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wanderling S., Paduch M., Medovoy D., Singharoy A., McGreevy R., Villalba-Galea C.A., Hulse R.E., Roux B., Schulten K., et al. 2014. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 21:244–252. 10.1038/nsmb.2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Shen R., Treger J.S., Wanderling S.S., Milewski W., Siwowska K., Bezanilla F., and Perozo E.. 2015. Resting state of the human proton channel dimer in a lipid bilayer. Proc. Natl. Acad. Sci. USA. 112:E5926–E5935. 10.1073/pnas.1515043112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.J., Zhao Q., Zhou Q., Unno H., Zhai Y., and Sun F.. 2010. The role and structure of the carboxyl-terminal domain of the human voltage-gated proton channel Hv1. J. Biol. Chem. 285:12047–12054. 10.1074/jbc.M109.040360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E.R., Tytgat J., and Hess P.. 1992. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 9:861–871. 10.1016/0896-6273(92)90239-A [DOI] [PubMed] [Google Scholar]
- Lishko P.V., Botchkina I.L., Fedorenko A., and Kirichok Y.. 2010. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 140:327–337. 10.1016/j.cell.2009.12.053 [DOI] [PubMed] [Google Scholar]
- Long S.B., Campbell E.B., and Mackinnon R.. 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 309:897–903. 10.1126/science.1116269 [DOI] [PubMed] [Google Scholar]
- Maingret F., Fosset M., Lesage F., Lazdunski M., and Honoré E.. 1999. TRAAK is a mammalian neuronal mechano-gated K+ channel. J. Biol. Chem. 274:1381–1387. 10.1074/jbc.274.3.1381 [DOI] [PubMed] [Google Scholar]
- Marches R., Vitetta E.S., and Uhr J.W.. 2001. A role for intracellular pH in membrane IgM-mediated cell death of human B lymphomas. Proc. Natl. Acad. Sci. USA. 98:3434–3439. 10.1073/pnas.061028998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R., Raso A., Wood T.G., Kurosky A., Martinac B., and Hamill O.P.. 2005. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell Biol. 7:179–185. 10.1038/ncb1218 [DOI] [PubMed] [Google Scholar]
- Masters T.A., Pontes B., Viasnoff V., Li Y., and Gauthier N.C.. 2013. Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc. Natl. Acad. Sci. USA. 110:11875–11880. 10.1073/pnas.1301766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail D.J., Kieu Q.M., Iandoli J.A., and Dawson M.R.. 2015. Actomyosin tension as a determinant of metastatic cancer mechanical tropism. Phys. Biol. 12:026001 10.1088/1478-3975/12/2/026001 [DOI] [PubMed] [Google Scholar]
- Mongin A.A. 2007. Disruption of ionic and cell volume homeostasis in cerebral ischemia: The perfect storm. Pathophysiology. 14:183–193. 10.1016/j.pathophys.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mony L., Berger T.K., and Isacoff E.Y.. 2015. A specialized molecular motion opens the Hv1 voltage-gated proton channel. Nat. Struct. Mol. Biol. 22:283–290. 10.1038/nsmb.2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., Cherny V.V., Murphy R., Katz B.Z., and DeCoursey T.E.. 2005. The pH dependence of NADPH oxidase in human eosinophils. J. Physiol. 569:419–431. 10.1113/jphysiol.2005.094748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., Cherny V.V., Finnegan A., Bollinger J., Gelb M.H., and DeCoursey T.E.. 2007. Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2α activity. J. Physiol. 579:327–344. 10.1113/jphysiol.2006.124248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Sakai H., Morihata H., Kawawaki J., Amano H., Yamano T., and Kuno M.. 2003. Regulatory mechanisms and physiological relevance of a voltage-gated H+ channel in murine osteoclasts: phorbol myristate acetate induces cell acidosis and the channel activation. J. Bone Miner. Res. 18:2069–2076. 10.1359/jbmr.2003.18.11.2069 [DOI] [PubMed] [Google Scholar]
- Morihata H., Nakamura F., Tsutada T., and Kuno M.. 2000. Potentiation of a voltage-gated proton current in acidosis-induced swelling of rat microglia. J. Neurosci. 20:7220–7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.E., Prikryl E.A., and Joós B.. 2015. Mechanosensitive gating of Kv channels. PLoS One. 10:e0118335 10.1371/journal.pone.0118335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Iwasaki H., Sasaki M., Inaba K., and Okamura Y.. 2005. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 435:1239–1243. 10.1038/nature03650 [DOI] [PubMed] [Google Scholar]
- Musset B., Morgan D., Cherny V.V., MacGlashan D.W. Jr., Thomas L.L., Ríos E., and DeCoursey T.E.. 2008. A pH-stabilizing role of voltage-gated proton channels in IgE-mediated activation of human basophils. Proc. Natl. Acad. Sci. USA. 105:11020–11025. 10.1073/pnas.0800886105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B., Capasso M., Cherny V.V., Morgan D., Bhamrah M., Dyer M.J., and DeCoursey T.E.. 2010a Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes. J. Biol. Chem. 285:5117–5121. 10.1074/jbc.C109.082727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B., Smith S.M., Rajan S., Cherny V.V., Sujai S., Morgan D., and DeCoursey T.E.. 2010b Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J. Physiol. 588:1435–1449. 10.1113/jphysiol.2010.188318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström T., Rotstein O.D., Romanek R., Asotra S., Heersche J.N., Manolson M.F., Brisseau G.F., and Grinstein S.. 1995. Regulation of cytoplasmic pH in osteoclasts. Contribution of proton pumps and a proton-selective conductance. J. Biol. Chem. 270:2203–2212. 10.1074/jbc.270.5.2203 [DOI] [PubMed] [Google Scholar]
- O’Hanlon D.E., Moench T.R., and Cone R.A.. 2013. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One. 8:e80074 10.1371/journal.pone.0080074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.J., Honoré E., Maingret F., Lesage F., Fink M., Duprat F., and Lazdunski M.. 1998. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 17:4283–4290. 10.1093/emboj/17.15.4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak M.M., Nourse J.L., Tran T., Hwe J., Arulmoli J., Le D.T., Bernardis E., Flanagan L.A., and Tombola F.. 2014. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA. 111:16148–16153. 10.1073/pnas.1409802111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J., Scheuer T., Zheng N., and Catterall W.A.. 2011. The crystal structure of a voltage-gated sodium channel. Nature. 475:353–358. 10.1038/nature10238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupo A., and Gonzalez León C.. 2014. In pursuit of an inhibitory drug for the proton channel. Proc. Natl. Acad. Sci. USA. 111:9673–9674. 10.1073/pnas.1408808111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F., Rebolledo S., Gonzalez C., and Larsson H.P.. 2013. Subunit interactions during cooperative opening of voltage-gated proton channels. Neuron. 77:288–298. 10.1016/j.neuron.2012.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey I.S., Moran M.M., Chong J.A., and Clapham D.E.. 2006. A voltage-gated proton-selective channel lacking the pore domain. Nature. 440:1213–1216. 10.1038/nature04700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey I.S., Mokrab Y., Carvacho I., Sands Z.A., Sansom M.S., and Clapham D.E.. 2010. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 17:869–875. 10.1038/nsmb.1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Takagi M., and Okamura Y.. 2006. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 312:589–592. 10.1126/science.1122352 [DOI] [PubMed] [Google Scholar]
- Schmidt D., del Mármol J., and MacKinnon R.. 2012. Mechanistic basis for low threshold mechanosensitivity in voltage-dependent K+ channels. Proc. Natl. Acad. Sci. USA. 109:10352–10357. 10.1073/pnas.1204700109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredenina T., Demaurex N., and Krause K.H.. 2015. Voltage-gated proton channels as novel drug targets: From NADPH oxidase regulation to sperm biology. Antioxid. Redox Signal. 23:490–513. 10.1089/ars.2013.5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö B.K., Katsura K., and Kristián T.. 1996. Acidosis-related damage. Adv. Neurol. 71:209–233. [PubMed] [Google Scholar]
- Song M., and Yu S.P.. 2014. Ionic regulation of cell volume changes and cell death after ischemic stroke. Transl. Stroke Res. 5:17–27. 10.1007/s12975-013-0314-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean I.V., and Morris C.E.. 2002. Membrane stretch accelerates activation and slow inactivation in Shaker channels with S3-S4 linker deletions. Biophys. J. 82:2982–2994. 10.1016/S0006-3495(02)75639-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K., Sakata S., Yamashita E., Fujiwara Y., Kawanabe A., Kurokawa T., Okochi Y., Matsuda M., Narita H., Okamura Y., and Nakagawa A.. 2014. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 21:352–357. 10.1038/nsmb.2783 [DOI] [PubMed] [Google Scholar]
- Tao X., Lee A., Limapichat W., Dougherty D.A., and MacKinnon R.. 2010. A gating charge transfer center in voltage sensors. Science. 328:67–73. 10.1126/science.1185954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F., Ulbrich M.H., and Isacoff E.Y.. 2008. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 58:546–556. 10.1016/j.neuron.2008.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F., Ulbrich M.H., Kohout S.C., and Isacoff E.Y.. 2010. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat. Struct. Mol. Biol. 17:44–50. 10.1038/nsmb.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C.A. 2014. Hv1 proton channel opening is preceded by a voltage-independent transition. Biophys. J. 107:1564–1572. (published erratum appears in Biophys. J. 2014. 107:2477) 10.1016/j.bpj.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li S.J., Pan J., Che Y., Yin J., and Zhao Q.. 2011. Specific expression of the human voltage-gated proton channel Hv1 in highly metastatic breast cancer cells, promotes tumor progression and metastasis. Biochem. Biophys. Res. Commun. 412:353–359. 10.1016/j.bbrc.2011.07.102 [DOI] [PubMed] [Google Scholar]
- Wang Y., Li S.J., Wu X., Che Y., and Li Q.. 2012. Clinicopathological and biological significance of human voltage-gated proton channel Hv1 protein overexpression in breast cancer. J. Biol. Chem. 287:13877–13888. 10.1074/jbc.M112.345280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M.L., Schow E.V., Freites J.A., White S.H., Tombola F., and Tobias D.J.. 2012. Water wires in atomistic models of the Hv1 proton channel. Biochim. Biophys. Acta. 1818:286–293. 10.1016/j.bbamem.2011.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.J., Wu G., Akhavan Sharif M.R., Baker A., Jia Y., Fahey F.H., Luo H.R., Feener E.P., and Clapham D.E.. 2012. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat. Neurosci. 15:565–573. 10.1038/nn.3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z.G., Zhu X.M., Chu X.P., Minami M., Hey J., Wei W.L., MacDonald J.F., Wemmie J.A., Price M.P., Welsh M.J., and Simon R.P.. 2004. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 118:687–698. 10.1016/j.cell.2004.08.026 [DOI] [PubMed] [Google Scholar]
- Zhang X., Ren W., DeCaen P., Yan C., Tao X., Tang L., Wang J., Hasegawa K., Kumasaka T., He J., et al. 2012. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 486:130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.