Abstract
The development of materials and technologies for the assembly of cells and/or vesicles is a key for the next generation of tissue engineering. Since the introduction of the tissue engineering concept in 1993, various types of scaffolds have been developed for the regeneration of connective tissues in vitro and in vivo. Cartilage, bone and skin have been successfully regenerated in vitro, and these regenerated tissues have been applied clinically. However, organs such as the liver and pancreas constitute numerous cell types, contain small amounts of extracellular matrix, and are highly vascularized. Therefore, organ engineering will require the assembly of cells and/or vesicles. In particular, adhesion between cells/vesicles will be required for regeneration of organs in vitro. This review introduces and discusses the key technologies and materials for the assembly of cells/vesicles for organ regeneration.
Keywords: organ engineering, assembly, cell, vesicle, material, technology
Introduction
Regeneration of organs is a major challenge for the next generation of tissue engineering. Since the introduction of the tissue engineering concept in 1993 [1], various types of scaffolds, including hydrogels, polymeric porous materials, bioceramics and composites, have been developed for the regeneration of connective tissues containing large amounts of extracellular matrix (ECM) [2–6]. Cartilage, bone and skin have been the main targets for tissue engineering, and tissues regenerated in vitro have been applied clinically [7–9]. Unlike connective tissues, organs such as liver and pancreas have a large cellular component and complex, highly vascularized structures with little ECM. In addition, there are marked functional differences between two-dimensional and three-dimensional (3D) cellular constructs. Therefore, controlling cell–cell interaction and cell–cell bonding is crucial for the regeneration of organs in vitro [10–14], and materials or technologies serving this purpose will contribute to architect various types of organs using different types of cells. Cellular spheroids, which are small 3D aggregates of cells, are an attractive model for organ regeneration. Some materials and techniques have been used to study the formation of spheroids in vitro; however, problems remain in building organ-like constructs. This review focuses on materials and technologies for the assembly of living cells and/or vesicles in organ engineering.
Technologies for cell/vesicle assembly
Gravity control
Cell assembly is affected by the gravity of the culture condition. The National Aeronautics and Space Administration developed a bioreactor of rotating wall vessels (RWVs) for the evaluation of cell proliferation and function in microgravity conditions [15]. With an RWV bioreactor, hepatocytes [15], bone marrow cells [16–18], stem cells [19], chondrocytes [20] and osteoblasts [21] have been cultured and used for the formation of organoids and tissues. Brown et al [15] cultured hepatocytes in an RWV and reported that the resulting organoids exhibited liver-like functions. These functions resulted from the fact that cells cultured under low-shear conditions undergo enhanced cell–cell interactions. Uemura et al reported that bone marrow cells cultured in an RWV bioreactor regenerated a large, cylindrical, cartilaginous tissue [16, 17]. The resulting organoid showed a high expression of cartilage markers, including aggrecan and collagen type II, compared with chondrocytes in a flat culture. Bone marrow cells were also cultured in an RWV in the presence of bioceramic microspheres [18]. In addition, Okamura et al [19] cultured hepatic stem/progenitor cells in an RWV bioreactor to mimic the condition of microgravity. The resulting 3D tissue showed biologic functions such as albumin secretion and glycogen storage. Chondrocytes have also been cultured in an RWV to produce tissue-like cartilage aggregates for clinical application [20]. Nishikawa et al [22] cultured rat marrow mesenchymal cells (MMCs) in a porous hydroxyapatite scaffold using a 3D clinostat, which is a bioreactor that generates multidirectional g-force. They reported that alkaline phosphatase activity of MMCs was significantly decreased when compared with that of MMCs cultured in a static environment.
Surface patterning/cell adhesion control
In general, cells do not adhere to hydrophilic surfaces, such as hydrophilic polymer-grafted substrates, but they do adhere to hydrophobic surfaces. The optimum water contact angle for cell adhesion is reported to be 70° [23]. Hydrophobic surfaces promote the adsorption of cell adhesion proteins such as fibronectin (FN) and vitronectin. Exploiting this property, Otsuka et al [24] fabricated a patterned, microfabricated, polyethylene glycol (PEG) brush surface on glass. They cultured hepatocytes underlaid with endothelial cells on the patterned surface to obtain hepatocyte heterospheroids. These spheroids secreted albumin, a typical functional marker, for at least 1 month. Collagen/PEG microcontact printing has also been used to obtain hepatocyte spheroids [25,26] (figure 1). The resulting hepatocyte spheroids showed liver-like functions including albumin secretion, expression of urea cycle enzymes and liver-enriched transcriptional factors, and intercellular adhesion. Anada et al [27] reported that spheroids with narrow size distribution were rapidly obtained when cultured on polydimethylsiloxane membrane deformed by decompression. Yamauchi et al [28] reported that spheroids of various sizes can be readily obtained by culturing bovine endometrial stromal cells on nonadherent culture plates.
Figure 1.
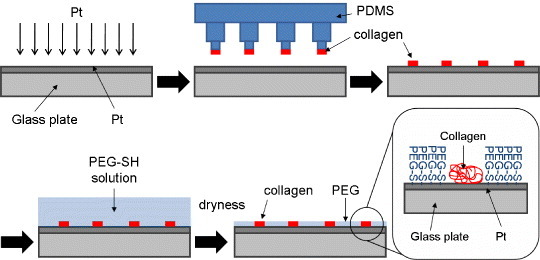
Fabrication of surface patterned with collagen/polyethylene glycol (PEG) using microcontact printing. Here PDMS stands for polydimethylsiloxane and SH for thiol group.
Cell printing and molding
Cell printing and injection molding techniques are an easy way to create organ-like constructs of desired shape. Cells with or without polymers have been used for the fabrication of various 3D structures. Xu et al [29] printed cells as a kind of ‘ink’ onto several ‘biopapers’ made from soy agar and collagen gel. They succeeded in computer-aided inkjet printing of mammalian cells with high viability. Nakamura et al [30] also established a technique for seeding living cells with an electrostatically driven inkjet system which generates little heat. With this system, they ejected a suspension of bovine vascular endothelial cells onto cell culture disks. Matsunaga et al [31] attempted to construct a macroscopic 3D structure by molding cell beads (figure 2). They cultured cells on collagen-based beads and molded the resulting cell beads to form tissues. Millimeter-sized tissue was obtained with this technology. Chang et al [32] reported injection molding of chondrocyte-alginate constructs of desired shape. The resulting cartilage-like constructs expressed glycosaminoglycan and showed excellent mechanical properties. Biocompatible polymers, including fibrin gel [33], poly(acrylamide) gel [34] and Pluronic F-127 [35], have been used with cells such as neurons [33] and stem cells [34] for cell printing and molding.
Figure 2.
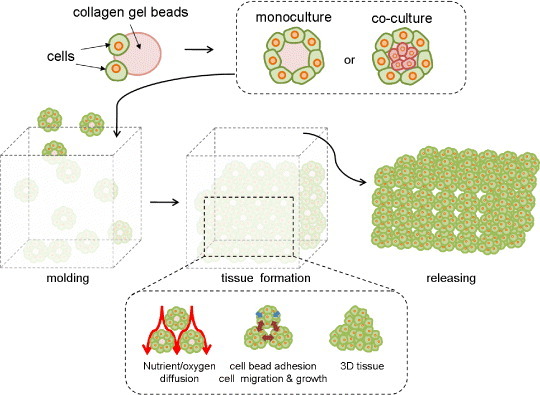
Tissue formation using collagen gel beads.
Layer-by-layer technique
The layer-by-layer (LbL) technique involves the placement of nanometer-sized films on various solid surfaces via alternate immersion in interactive polymer solutions [36] (figure 3). With the use of the LbL technique, various materials have been modified to control biological properties of solid surfaces [37] or gel matrices [38]. Matsusaki et al [39] fabricated a multilayer cellular structure using fibronectin and gelatin as binding reagents for fibroblastic cells (figure 4). They successfully prepared multilayered cellular structures of more than 3 layers in vitro. Liposomes have also been assembled on the surface of a quartz crystal microbalance using the membrane protein bacteriorhodopsin as a binding reagent [40].
Figure 3.
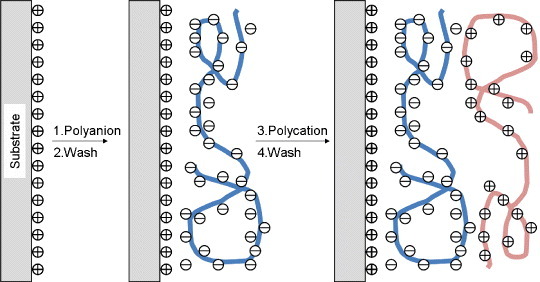
Layer-by-layer technique for the assembly of interactive polymers on a solid substrate.
Figure 4.
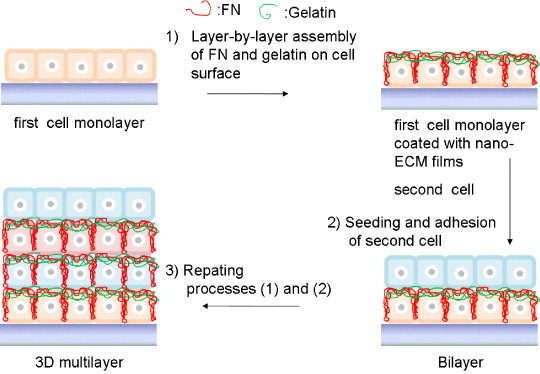
Assembly of two kinds of cells using the layer-by-layer technique.
Decellularization
Decellularization is a powerful tool of designing ideal scaffolds for organ and tissue regeneration [41, 42]. Various technologies have been reported that use detergents, enzymes, supercritical carbon dioxide, or high hydrostatic pressure for decellularization [43–49]. The main components of the resulting decellularized constructs are ECM proteins such as collagen, proteoglycans, elastin and cell adhesion proteins. The ECM proteins in these decellularized constructs are highly organized. Therefore, the mechanical properties of the constructs are superior to those of artificial scaffolds made from biopolymers [50]. In addition, decellularized constructs show enhanced biological functions compared with collagen or other scaffolds because these constructs retain endogenous cytokines [51]. Heart valves [43, 50], blood vessels [44, 45, 52], total heart [47] and cornea [46, 48] have been decellularized. Uygun et al [53] reported a transplantable, recellularized liver graft using decellularized liver matrix. They applied hepatocytes to recellularize the liver matrix, and the resulting liver graft showed liver-specific functions such as albumin secretion, urea synthesis and cytochrome P450 expression. Lung has also been decellularized [54], and because of its limited regenerative capacity, this methodology holds great promise for lung regeneration.
Materials for cell/vesicle assembly
Thermoresponsive polymer-grafted surfaces
Poly(N-isopropylacrylamide) (PNIPAAm) is a thermoresponsive polymer that is water soluble below its lower critical solution temperature (LCST) and water insoluble above the LCST. Exploiting this property, PNIPAAm has been grafted to the surface of polystyrene culture dishes for the generation of cell sheets [55, 56] (figure 5). After cells become confluent on the dishes, cell sheets are readily obtained by decreasing the temperature below the LCST as the adhesion properties of the surface of the dish change from hydrophobic to hydrophilic. This temperature-responsive cell culture dish can be applied to the fabrication of cell sheets made from various kinds of cells including cardiac myocytes [57], hepatocytes [58, 59], corneal cells [60, 61], and periodontal ligament cells [62]. The coculture of hepatocytes and fibroblasts on a patterned PNIPAAm-grafted surface has also been studied [63], and cocultured cell sheets were obtained with a patterned, dual-thermoresponsive surface [64]. The resulting cell sheets are interconnected by cell adhesion molecules including cadherin [65].
Figure 5.
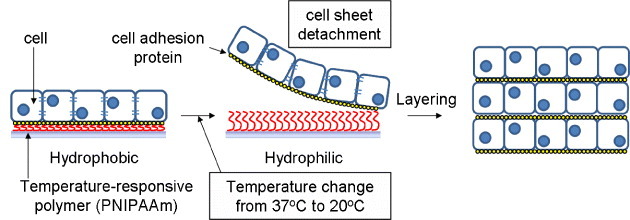
Concept of cell sheet engineering using temperature-responsive polymer.
Intermolecular interaction-induced cells/vesicle assembly
Figure 6 summarizes the assembly of cells/vesicles using intermolecular interaction. Materials or molecules that can bond cells/vesicles have been developed as discussed below.
Figure 6.
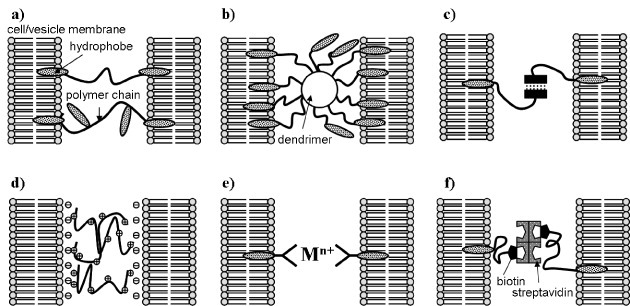
Assembly of cells/vesicles by intermolecular interaction: (a) hydrophobic interaction of hydrophobically modified hydrophilic polymer, (b) hydrophobic interaction of hydrophobically modified dendrimer, (c) hydrogen bonding, (d) electrostatic interaction, (e) coordinate bond formation and (f) molecular recognition.
Hydrophobic interaction
The plasma membrane is composed of a phospholipid bilayer; where the phospholipids self-assemble by hydrophobic interaction. Cells and vesicles have been assembled by exploiting this hydrophobic interaction and used in biomedical applications [66–68]. Meier et al [66] reported that a PEG derivative capped with cholesteryl groups can function as a physical crosslinker of vesicles and cells. It was also reported that the crosslinking of liposomes with this PEG derivative enables rapid recovery, even after 1000% strain [69]. Taguchi and co-worker [70] prepared dioleoyl PEG, which can physically crosslink pancreatic beta cells by a hydrophobic interaction. The resulting spheroids showed enhanced insulin secretion compared with that of pancreatic beta cells cultured in the absence of dioleoyl PEG. They also showed increased mRNA expression of a typical cell adhesion molecule, E-cadherin, with increasing concentrations of dioleoyl PEG. On the other hand, Mo et al [71] developed a 16-arm polypropylenimine hexadecaamine dendrimer conjugated to a PEG derivative capped with hydrophobic oleoyl groups to accomplish rapid construction of multicellular structures of hepatic cells (figure 7). Lee et al [72] reported on hydrophobically modified chitosan (hm-chitosan) and its biomedical applications [73]. They applied hm-chitosan as a hemostatic agent and found that it coagulated blood even in the presence of heparin. They also reported loss of blood coagulation by the addition of α-cyclodextrin. Nagamune and co-workers [74, 75] described a biocompatible anchor for membrane (BAM) for the immobilization of nonadherent cells (figure 8). They prepared oleoyl groups bearing bovine serum albumin, coated it on glass, and successfully cultured nonadherent cells on BAM-coated glass. The stability of hydrophobically modified synthetic polymers immobilized at the plasma membrane has also been evaluated. Teramura et al [76] synthesized polyvinyl alcohol carrying alkyl side chains (PVA-alkyl) and PEG-conjugated phospholipid (PEG-lipid) and exposed living cells to these polymers. They concluded that these hydrophobically modified synthetic polymers could be immobilized at the plasma membrane; however, most of the polymers were excluded within 24 h.
Figure 7.
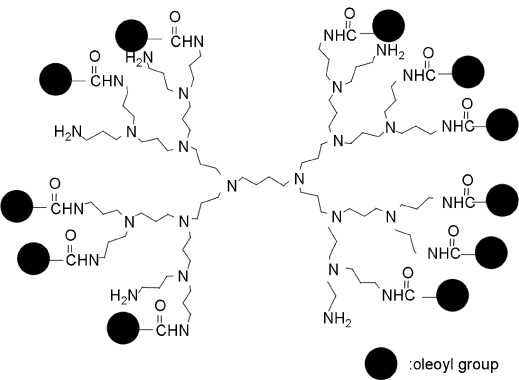
Structure of a 16-arm polypropylenimine hexadecaamine dendrimer conjugated to a polyethylene glycol derivative with an oleoyl group.
Figure 8.
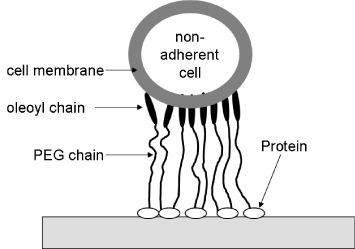
Immobilization of nonadherent cells on a solid substrate using polyethylene glycol terminated with an oleoyl group.
Hydrogen bonding
It is known that 2-ureido-4[1H]-pyrimidinone (Upy) units dimerize via 4 strong hydrogen bonds. Menger and Zhang [77] synthesized cholesteryl groups modified with Upy for adhesion among vesicles. Results of light scattering, light microscopy and low-temperature high-resolution scanning electron microscopy showed self-aggregation of vesicles with a Upy-covered surface.
Electrostatic interaction
Electrostatic interaction has been used to fabricate interactive polymer-coated materials [36, 37]. Antunes et al [78] mixed anionic vesicles and cationic polymers to form a gel-like matrix. An elastic gel could then be formed with this highly charged polymer. This phenomenon was explained by both the charge density and the surfactant crystallization in the vesicles. Zhu et al [79] reported that surfactant-vesicles and liposomes can be codeposited with stimuli-responsive amino polysaccharide chitosan. Thin films were formed on the surface of islets of Langerhans by electrostatic interaction and used for immunosuppressive therapy. Miura et al [80] formed an ultrathin polyion complex membrane on islets of Langerhans with sodium alginate, poly(L-lysine), and a PEG-lipid conjugate terminated with amino groups without affecting cell viability.
Coordinate bonding
Constable et al [81] reported metal ion-induced vesicle aggregation. They incorporated 2,2″:6′2″-terpyridine (terpy)-functionalized phospholipids into lecithin vesicles and observed the formation of vesicle aggregates in response to the addition of iron (II). They proposed that this aggregation could be readily deformed by the addition of the chelator ethylenediamine tetraacetic acid; therefore, vesicle aggregation/deformation could be controlled. Mart et al [82] developed cell adhesion molecule (CAM) mimics and reported the induction of vesicle aggregation. The CAM mimics were Cu (iminodiacetate)-capped, lipid-incorporating, perfluoroalkyl-pyrene motifs that can anchor to the phospholipid bilayer. As these molecules can interact with copper, they induced vesicle aggregates with diameters between 20 and 80 μm within 2 min. These authors concluded that phase separation of the CAM mimics in the bilayer is a key for vesicle aggregation. Boonyarattanakalin et al [83] reported an artificial cell-surface receptor using metal-dependent, cell-penetrating peptides. A synthetic cell surface receptor with an oligohistidine binding motif and an N-alkyl-3beta-cholesterylamine motif was prepared for the delivery of His-tagged proteins into mammalian cells by receptor-mediated endocytosis.
Molecular recognition
Meier [84] reported reversible cell aggregation via specific ligand–receptor coupling. He employed asymmetrically substituted PEGs carrying at one end a hydrophobic cholesteryl group and at the other end a hydrophilic biotin group. These polymers can anchor in the lipid membrane of cells via the hydrophobic cholesteryl groups; therefore, biotin groups could be readily immobilized at the cell membrane. Using the intermolecular interaction between streptavidin and biotin, cell aggregation/dispersion could be controlled by the addition of streptavidin or biotin. Wang et al [85] evaluated the effect of induced cell aggregation on the signaling process among cells. They incorporated artificial lipid-anchored streptavidin conjugates into the cells, induced intercellular interaction by the addition of biotin, and found that the activation of signaling processes was caused by clustering of cell membrane components.
Conclusions and outlook
This article reviewed recent research on materials and technologies for cell/vesicle assembly and organ regeneration. Such materials and technologies will enable architecting various types of organs like liver and pancreas. As shown in figure 9, the adhesive materials and technologies will work as ‘glues’ for assembling various kinds of cells. The adhesive materials should be degraded when cells themselves biosynthesize cell adhesion molecules just like E-cadherin. Although integration of newly developed materials and technologies will be required for the regeneration of organs in vitro, this will ultimately lead to the creation of three-dimensionally engineered organs with functions similar to those of natural organs.
Figure 9.
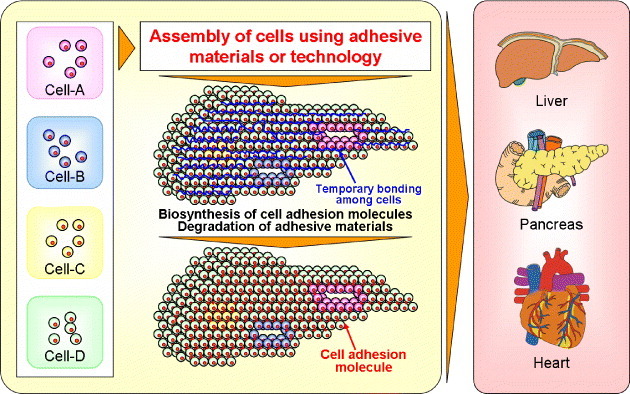
Strategy of organ engineering using cell-assembling adhesive materials or technology.
Acknowledgments
The author thanks Ms T Kojima of the Biofunctional Materials Unit, Nano-Bio Field, Materials Nanoarchitectonics (MANA), National Institute for Materials Science, for technical support. This study was supported in part by a Grant-in-Aid from the National Institute of Biomedical Innovation of Japan and by the World Premier International Research Center (WPI) Initiative on Materials Nanoarchitectonics, MEXT, Japan.
References
- Langer R. and Vacanti J P. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lutolf M P. and Hubbell J A. Nat. Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Griffith L G. and Naughton G. Science. 2002;295:1009. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- Drury J L. and Mooney D J. Biomaterials. 2003;24:4337. doi: 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Chen G P, Ushida T. and Tateishi T. Macromol. Biosci. 2002;2:67. doi: 10.1002/(ISSN)1616-5195. [DOI] [Google Scholar]
- Rezwan K, Chen Q Z, Blaker J J. and Boccaccini A R. Biomaterials. 2006;27:3413. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Kuroyanagi Y, Kenmochi M, Ishihara S, Takeda A, Shiraishi A, Ootake N, Uchinuma E, Torikai K. and Shioya N. Ann. Plast. Surg. 1993;31:340. doi: 10.1097/00000637-199310000-00011. [DOI] [PubMed] [Google Scholar]
- Ochi M, Uchio Y, Kawasaki K, Wakitani S. and Iwasa J. J. Bone Joint Surg. Br. 2002;84B:571. doi: 10.1302/0301-620X.84B4.11947. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Oda Y. and Ohgushi H. Sci. Technol. Adv. Mater. 2010;11:1. doi: 10.1088/1468-6996/11/1/014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott A. Nature. 2003;424:870. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- Service R F. Science. 2003;302:46. doi: 10.1126/science.302.5642.46. [DOI] [PubMed] [Google Scholar]
- Seo S J, Kim I Y, Choi Y J, Akaike T. and Cho C S. Biomaterials. 2006;27:1487. doi: 10.1016/j.biomaterials.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Echigo Y, Shinohara S, Gu Y, Miyazaki J, Inoue K. and Imamura M. Cell Transplant. 2001;10:473. [PubMed] [Google Scholar]
- Nagaki M, Shidoji Y, Yamada Y, Sugiyama A, Tanaka M, Akaike T, Ohnishi H, Moriwaki H. and Muto Y. Biochem. Biophys. Res. Commun. 1995;210:38. doi: 10.1006/bbrc.1995.1624. [DOI] [PubMed] [Google Scholar]
- Brown L A, Arterburn L M, Miller A P, Cowger N L, Hartley S M, Andrews A, Silber P M. and Li A P. In Vitro Cell Dev. Biol. Anim. 2003;39:13. doi: 10.1290/1543-706X(2003)039<0013:MOLFIR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ohyabu Y, Kida N, Kojima H, Taguchi T, Tanaka J. and Uemura T. Biotech. Bioeng. 2006;95:1003. doi: 10.1002/(ISSN)1097-0290. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Mishima H, Ohyabu Y, Sakai S, Akaogi H, Ishii T, Kojima H, Tanaka J, Ochiai N. and Uemura T. J. Orthopaed. Res. 2007;25:1291. doi: 10.1002/jor.v25:10. [DOI] [PubMed] [Google Scholar]
- Qiu Q Q, Ducheyne P. and Ayyaswamy P S. Biomaterials. 1999;20:989. doi: 10.1016/S0142-9612(98)00183-5. [DOI] [PubMed] [Google Scholar]
- Okamura A, Zheng Y W, Hirochika R, Tanaka J. and Taniguchi H. J. Nanosci. Nanotechnol. 2007;7:721. doi: 10.1166/jnn.2007.508. [DOI] [PubMed] [Google Scholar]
- Duke P J, Daane E L. and Montufarsolis D. J. Cell. Biochem. 1993;51:274. doi: 10.1002/(ISSN)1097-4644. [DOI] [PubMed] [Google Scholar]
- Rucci N, Migliaccio S, Zani B M, Taranta A. and Teti A. J. Cell Biochem. 2002;85:167. doi: 10.1002/(ISSN)1097-4644. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Ohgushi H, Tamai N, Osuga K, Uemura M, Yoshikawa H. and Myoui A. Cell Transplant. 2005;14:829. doi: 10.3727/000000005783982477. [DOI] [PubMed] [Google Scholar]
- Tamada Y. and Ikada Y. J. Biomed. Mater. Res. 1994;28:783. doi: 10.1002/(ISSN)1097-4636. [DOI] [PubMed] [Google Scholar]
- Otsuka H, Hirano A, Nagasaki Y, Okano T, Horiike Y. and Kataoka K. Chem. Bio. Chem. 2004;5:850. doi: 10.1002/cbic.200300822. [DOI] [PubMed] [Google Scholar]
- Fukuda J, Sakai Y. and Nakazawa K. Biomaterials. 2006;27:1061. doi: 10.1016/j.biomaterials.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Tamura T, Sakai Y. and Nakazawa K. J. Mater. Sci.: Mater. Med. 2008;19:2071. doi: 10.1007/s10856-007-3305-1. [DOI] [PubMed] [Google Scholar]
- Anada T, Masuda T, Honda Y, Fukuda J, Arai F, Fukuda T. and Suzuki O. Sensors Actuators. 2010;147(B):376. doi: 10.1016/j.snb.2010.01.065. [DOI] [Google Scholar]
- Yamauchi N, Yamada O, Takahashi T. and Hashizume K. J. Reprod. Dev. 2001;47:165. doi: 10.1262/jrd.47.165. [DOI] [Google Scholar]
- Xu T, Jin J, Gregory C, Hickman J J. and Boland T. Biomaterials. 2005;26:93. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kobayashi A, Takagi F, Watanabe A, Hiruma Y, Ohuchi K, Iwasaki Y, Horie M, Morita I. and Takatani S. Tissue Eng. 2005;11:1658. doi: 10.1089/ten.2005.11.1658. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y T, Morimoto Y. and Takeuchi S. Adv. Mater. 2011;23:H90. doi: 10.1002/adma.201004375. [DOI] [PubMed] [Google Scholar]
- Chang S C N, Rowley J A, Tobias G, Genes N G, Roy A K, Mooney D J, Vacanti C A. and Bonassar L J. J. Biomed. Mater. Res. 2001;55:503. doi: 10.1002/(ISSN)1097-4636. [DOI] [PubMed] [Google Scholar]
- Xu T, Gregorya C A, Molnara P, Cuia X, Jalotab S, Bhadurib S B. and Bolanda T. Biomaterials. 2006;27:3580. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Ilkhanizadeh S, Teixeira A I. and Hermanson O. Biomaterials. 2007;28:3936. doi: 10.1016/j.biomaterials.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Biase M D, Saunders R E, Tirelliab N. and Derby B. Soft Matter. 2011;7:2639. doi: 10.1039/c0sm00996b. [DOI] [Google Scholar]
- Decher G. Science. 1997;277:1232. doi: 10.1126/science.277.5330.1232. [DOI] [Google Scholar]
- Serizawa T, Yamaguchi M, Matsuyama T. and Akashi M. Biomacromolecules. 2000;1:306. doi: 10.1021/bm000006g. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Kishida A. and Akashi M. Chem. Lett. 1998;8:711. doi: 10.1246/cl.1998.711. [DOI] [Google Scholar]
- Matsusaki M, Kadowaki K, Nakahara Y. and Akashi M. Angew. Chem., Int. Ed. Engl. 2007;46:4689. doi: 10.1002/(ISSN)1521-3773. [DOI] [PubMed] [Google Scholar]
- Nakane Y. and Kubo I. Thin Solid Films. 2009;518:678. doi: 10.1016/j.tsf.2009.07.067. [DOI] [Google Scholar]
- Schmidt C E. and Baier J M. Biomaterials. 2000;21:2215. doi: 10.1016/S0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- Gilbert T W, Sellaro T L. and Badylak S F. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Rieder E, Kasimir M T, Silberhumer G, Seebacher G, Wolner E, Simon P. and Weigel G. J. Thorac. Cardiovasc. Surg. 2004;127:399. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- McFetridge P S, Daniel J W, Bodamyali T, Horrocks M. and Chaudhuri J B. J. Biomed. Mater. Res. 2004;70(A):224. doi: 10.1002/(ISSN)1097-4636. [DOI] [PubMed] [Google Scholar]
- Ketchedjian A, Jones A L, Krueger P, Robinson E, Crouch K, Wolfinbarger L. and Hopkins R. Ann. Thorac. Surg. 2005;79:888. doi: 10.1016/j.athoracsur.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Sawada K, Terada D, Yamaoka T, Kitamura S. and Fujisato T. J. Chem. Technol. Biotechnol. 2008;83:94. doi: 10.1002/(ISSN)1097-4660. [DOI] [Google Scholar]
- Weymann A. et al Circ. J. 2011;75:852. doi: 10.1253/circj.CJ-10-0717. [DOI] [PubMed] [Google Scholar]
- Pang K, Du L. and Wu X. Biomaterials. 2010;31:72573. doi: 10.1016/j.biomaterials.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Funamoto S, Hashimoto Y, Kimura T, Honda T, Hattori S, Kobayashi H, Kishida A. and Mochizuki M. Mol. Vis. 2009;15:216. [PMC free article] [PubMed] [Google Scholar]
- Tedder M E, Liao J, Weed B, Stabler C, Zhang H, Simionescu A. and Simionescu D T. Tissue Eng. 2009;15(A):1257. doi: 10.1089/ten.tea.2008.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Hoshiba T, Kawazoe N. and Chen G. Biomaterials. 2011;32:2489. doi: 10.1016/j.biomaterials.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Funamoto S, Nam K, Kimura T, Murakoshi A, Hashimoto Y, Niwaya K, Kitamura S, Fujisato T. and Kishida A. Biomaterials. 2010;31:3590. doi: 10.1016/j.biomaterials.2010.01.073. [DOI] [PubMed] [Google Scholar]
- Uygun B E. et al Nat. Med. 2010;16:814. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen T H. et al Science. 2010;329:5991. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Yamada N, Sakai H. and Sakurai Y. J. Biomed. Mater. Res. 1993;27:1243. doi: 10.1002/(ISSN)1097-4636. [DOI] [PubMed] [Google Scholar]
- Kwon O H, Kikuchi A, Yamato M, Sakurai Y. and Okano T. J. Biomed. Mater. Res. 2000;50:82. doi: 10.1002/(ISSN)1097-4636. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamoto M, Kikuchi A. and Okano T. Tissue Eng. 2001;7:141. doi: 10.1089/107632701300062732. [DOI] [PubMed] [Google Scholar]
- Okano T, Yamada N, Okuhara M, Sakai H. and Sakurai Y. Biomaterials. 1995;16:297. doi: 10.1016/0142-9612(95)93257-E. [DOI] [PubMed] [Google Scholar]
- Ohashi K. et al Nat. Med. 2007;13:880. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- Nishida K. et al Transplantation. 2004;77:379. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- Nishida K. et al New Engl. J. Med. 2004;351:1187. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Yamato M, Kikuchi A, Okano T. and Ishikawa I. Tissue Eng. 2005;11:469. doi: 10.1089/ten.2005.11.469. [DOI] [PubMed] [Google Scholar]
- Yamato M, Konno C, Utsumi M, Kikuchi A. and Okano T. Biomaterials. 2002;23:561. doi: 10.1016/S0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Kikuchi A, Yamato M, Nakao A, Sakurai Y, Umezu M. and Okano T. Biomaterials. 2005;26:1885. doi: 10.1016/j.biomaterials.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A. and Okano T. Tissue Eng. 2001;7:473. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- Meier W, Hotz J. and Günther-Ausborn S. Langmuir. 1996;12:5028. doi: 10.1021/la960512k. [DOI] [Google Scholar]
- Zhu C, Lee J H, Raghavan S R. and Payne G F. Langmuir. 2006;22:2951. doi: 10.1021/la053475i. [DOI] [PubMed] [Google Scholar]
- Regev O, Marques E F. and Khan A. Langmuir. 1999;15:642. doi: 10.1021/la9803474. [DOI] [Google Scholar]
- Rao Z, Inoue M, Matsuda M. and Taguchi T. Colloids Surf. 2011;82(B):196. doi: 10.1016/j.colsurfb.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Ito M. and Taguchi T. Acta Biomater. 2009;5:2945. doi: 10.1016/j.actbio.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Mo X. et al Biomaterials. 2010;31:7455. doi: 10.1016/j.biomaterials.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Lee J H, Gustin J P, Chen T, Payne G F. and Raghavan S R. Langmuir. 2005;21:26. doi: 10.1021/la048194+. [DOI] [PubMed] [Google Scholar]
- Dowling M B, Kumar R, Keibler M A, Hess J R, Bochicchio G V. and Raghavan S R. Biomaterials. 2011;32:3351. doi: 10.1016/j.biomaterials.2010.12.033. [DOI] [PubMed] [Google Scholar]
- Kato K, Umezawa K, Funeriu D P, Miyake M, Miyake J. and Nagamune T. Biotechniques. 2003;35:1014. doi: 10.2144/03355rr01. [DOI] [PubMed] [Google Scholar]
- Kato K, Itoh C, Yasukouchi T. and Nagamune T. Biotechnol. Prog. 2004;20:897. doi: 10.1021/bp0342093. [DOI] [PubMed] [Google Scholar]
- Teramura Y, Kaneda Y, Totani T. and Iwata H. Biomaterials. 2008;29:1345. doi: 10.1016/j.biomaterials.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Menger F M. and Zhang H. J. Am. Chem. Soc. 2006;128:1414. doi: 10.1021/ja057151s. [DOI] [PubMed] [Google Scholar]
- Antunes F E, Marques E F, Gomes R, Thuresson K, Lindman B. and Miguel M G. Langmuir. 2004;20:4647. doi: 10.1021/la049783i. [DOI] [PubMed] [Google Scholar]
- Zhu C, Wu L Q, Wang X, Lee J H, English D S, Ghodssi R, Raghavan S R. and Payne G F. Langmuir. 2007;23:286. doi: 10.1021/la061421i. [DOI] [PubMed] [Google Scholar]
- Miura S, Teramur Y. and Iwata H. Biomaterials. 2006;27:5828. doi: 10.1016/j.biomaterials.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Constable E C, Meier W, Nardin C. and Mundwiler S. Chem. Commun. 1999;1999:1483. doi: 10.1039/a904665h. [DOI] [Google Scholar]
- Mart R J, Liem K P, Wang X. and Webb S J. J. Am. Chem. Soc. 2006;128:14462. doi: 10.1021/ja0657612. [DOI] [PubMed] [Google Scholar]
- Boonyarattanakalin S, Athavankar S, Sun Q. and Peterson B R. J. Am. Chem. Soc. 2006;128:386. doi: 10.1021/ja056126j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier W. Langmuir. 2000;16:1457. doi: 10.1021/la990915v. [DOI] [Google Scholar]
- Wang T, Leventis R. and Silvius J R. J. Biol. Chem. 2005;280:22839. doi: 10.1074/jbc.M502920200. [DOI] [PubMed] [Google Scholar]


