Abstract
Sporamin is a protein without glycans that accumulates in large quantities in the vacuoles of the tuberous root of the sweet potato. It is synthesized as a prepro precursor with an N-terminal extension composed of a 21-amino-acid signal peptide and a 16-amino-acid propeptide. A total of 48 base pairs, corresponding to the nucleotide sequence that encodes the propeptide, was deleted from a cDNA clone for sporamin. This delta pro mutant sequence, as well as the sequence of the wild-type sporamin cDNA, was placed downstream from the promoter of the 35S transcript from cauliflower mosaic virus and introduced into the genome of suspension-cultured tobacco cells by Agrobacterium-mediated transformation. In contrast to the vacuolar localization of sporamin in cells that expressed the wild-type precursor, sporamin was secreted into the culture medium from cells in which the delta pro precursor was expressed. The secreted form of sporamin was shorter by two amino acids at its N terminus than authentic sporamin; it migrated anomalously during electrophoresis on SDS/polyacrylamide gel as a result of the presence of intramolecular disulfide bridges, as does authentic sporamin. The kinetics of secretion of sporamin from the cell were similar to those of proteins normally secreted by the host tobacco cells. These results indicate that the propeptide of the precursor to sporamin is required for correct targeting of sporamin to the vacuole and that proteins can be secreted from plant cells by a bulk-flow default pathway in the absence of a functional sorting signal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Clercq A., Vandewiele M., De Rycke R., Van Damme J., Van Montagu M., Krebbers E., Vandekerckhove J. Expression and Processing of an Arabidopsis 2S Albumin in Transgenic Tobacco. Plant Physiol. 1990 Apr;92(4):899–907. doi: 10.1104/pp.92.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J., Botterman J., Deblaere R. Protein secretion in plant cells can occur via a default pathway. Plant Cell. 1990 Jan;2(1):51–59. doi: 10.1105/tpc.2.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorel C., Voelker T. A., Herman E. M., Chrispeels M. J. Transport of proteins to the plant vacuole is not by bulk flow through the secretory system, and requires positive sorting information. J Cell Biol. 1989 Feb;108(2):327–337. doi: 10.1083/jcb.108.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood J. S., Chrispeels M. J. Correct targeting of the bean storage protein phaseolin in the seeds of transformed tobacco. Plant Physiol. 1985 Sep;79(1):65–71. doi: 10.1104/pp.79.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Ichihara S., Nakamura K. Processing of a plant vacuolar protein precursor in vitro. Eur J Biochem. 1987 Aug 3;166(3):533–538. doi: 10.1111/j.1432-1033.1987.tb13546.x. [DOI] [PubMed] [Google Scholar]
- Hattori T., Yoshida N., Nakamura K. Structural relationship among the members of a multigene family coding for the sweet potato tuberous root storage protein. Plant Mol Biol. 1989 Nov;13(5):563–572. doi: 10.1007/BF00027316. [DOI] [PubMed] [Google Scholar]
- Hood E. E., Helmer G. L., Fraley R. T., Chilton M. D. The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J Bacteriol. 1986 Dec;168(3):1291–1301. doi: 10.1128/jb.168.3.1291-1301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga G., Jefferson R. A., Bevan M. W. Endoplasmic reticulum targeting and glycosylation of hybrid proteins in transgenic tobacco. Plant Cell. 1989 Mar;1(3):381–390. doi: 10.1105/tpc.1.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. M., Bankaitis V. A., Emr S. D. Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell. 1987 Mar 13;48(5):875–885. doi: 10.1016/0092-8674(87)90084-5. [DOI] [PubMed] [Google Scholar]
- Kay R., Chan A., Daly M., McPherson J. Duplication of CaMV 35S Promoter Sequences Creates a Strong Enhancer for Plant Genes. Science. 1987 Jun 5;236(4806):1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- Kimura T., Takeda S., Asahi T., Nakamura K. Primary structure of a precursor for the delta-subunit of sweet potato mitochondrial F1-ATPase deduced from full length cDNA. J Biol Chem. 1990 Apr 15;265(11):6079–6085. [PubMed] [Google Scholar]
- Klionsky D. J., Banta L. M., Emr S. D. Intracellular sorting and processing of a yeast vacuolar hydrolase: proteinase A propeptide contains vacuolar targeting information. Mol Cell Biol. 1988 May;8(5):2105–2116. doi: 10.1128/mcb.8.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lund P., Lee R. Y., Dunsmuir P. Bacterial chitinase is modified and secreted in transgenic tobacco. Plant Physiol. 1989 Sep;91(1):130–135. doi: 10.1104/pp.91.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Rothman J. H., Yamashiro C. T., Kane P. M., Stevens T. H. Protein targeting to the yeast vacuole. Trends Biochem Sci. 1989 Aug;14(8):347–350. doi: 10.1016/0968-0004(89)90170-9. [DOI] [PubMed] [Google Scholar]
- Sevarino K. A., Stork P., Ventimiglia R., Mandel G., Goodman R. H. Amino-terminal sequences of prosomatostatin direct intracellular targeting but not processing specificity. Cell. 1989 Apr 7;57(1):11–19. doi: 10.1016/0092-8674(89)90167-0. [DOI] [PubMed] [Google Scholar]
- Sonnewald U., von Schaewen A., Willmitzer L. Expression of mutant patatin protein in transgenic tobacco plants: role of glycans and intracellular location. Plant Cell. 1990 Apr;2(4):345–355. doi: 10.1105/tpc.2.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tague B. W., Dickinson C. D., Chrispeels M. J. A short domain of the plant vacuolar protein phytohemagglutinin targets invertase to the yeast vacuole. Plant Cell. 1990 Jun;2(6):533–546. doi: 10.1105/tpc.2.6.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls L. A., Hunter C. P., Rothman J. H., Stevens T. H. Protein sorting in yeast: the localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell. 1987 Mar 13;48(5):887–897. doi: 10.1016/0092-8674(87)90085-7. [DOI] [PubMed] [Google Scholar]
- Valls L. A., Winther J. R., Stevens T. H. Yeast carboxypeptidase Y vacuolar targeting signal is defined by four propeptide amino acids. J Cell Biol. 1990 Aug;111(2):361–368. doi: 10.1083/jcb.111.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker T. A., Herman E. M., Chrispeels M. J. In vitro mutated phytohemagglutinin genes expressed in tobacco seeds: role of glycans in protein targeting and stability. Plant Cell. 1989 Jan;1(1):95–104. doi: 10.1105/tpc.1.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
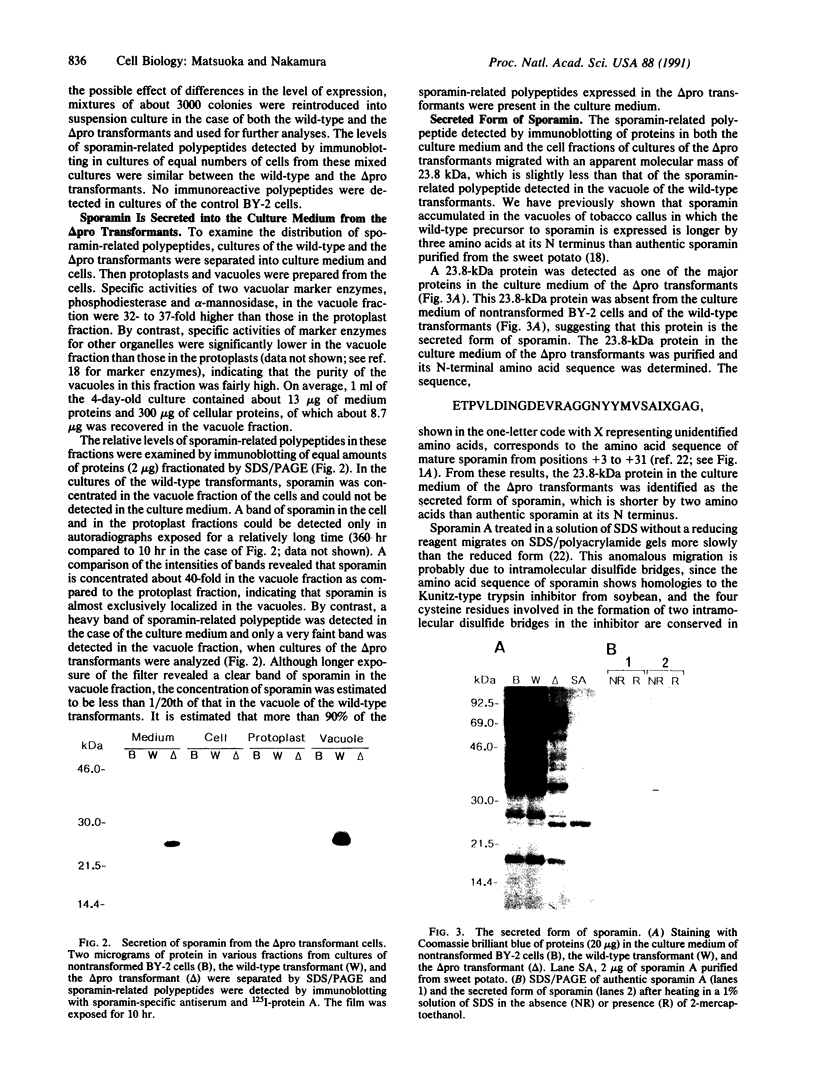
- Wilkins T. A., Bednarek S. Y., Raikhel N. V. Role of propeptide glycan in post-translational processing and transport of barley lectin to vacuoles in transgenic tobacco. Plant Cell. 1990 Apr;2(4):301–313. doi: 10.1105/tpc.2.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford C. A., Daniels L. B., Park F. J., Jones E. W., Van Arsdell J. N., Innis M. A. The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation of Saccharomyces cerevisiae vacuolar hydrolases. Mol Cell Biol. 1986 Jul;6(7):2500–2510. doi: 10.1128/mcb.6.7.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. L., Ohta Y., Jordan F., Inouye M. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature. 1989 Jun 8;339(6224):483–484. doi: 10.1038/339483a0. [DOI] [PubMed] [Google Scholar]