Abstract
Background Seeds adjust their germination based on conditions experienced before and after dispersal. Post-dispersal cues are expected to be more accurate predictors of offspring environments, and thus offspring success, than pre-dispersal cues. Therefore, germination responses to conditions experienced during seed maturation may be expected to be superseded by responses to conditions experienced during seed imbibition. In taxa of disturbed habitats, neighbours frequently reduce the performance of germinants. This leads to the hypotheses that a vegetative canopy will reduce germination in such taxa, and that a vegetative canopy experienced during seed imbibition will over-ride germination responses to a canopy experienced during seed maturation, since it is a more proximal cue of immediate competition. These hypotheses were tested here in Arabidopsis thaliana.
Methods Seeds were matured under a simulated canopy (green filter) or white light. Fresh (dormant) seeds were imbibed in the dark, white light or canopy at two temperatures (10 or 22 °C), and germination proportions were recorded. Germination was also recorded in after-ripened (less dormant) seeds that were induced into secondary dormancy and imbibed in the dark at each temperature, either with or without brief exposure to red and far-red light.
Key Results Unexpectedly, a maturation canopy expanded the conditions that elicited germination, even as seeds lost and regained dormancy. In contrast, an imbibition canopy impeded or had no effect on germination. Maturation under a canopy did not modify germination responses to red and far-red light. Seed maturation under a canopy masked genetic variation in germination.
Conclusions The results challenge the hypothesis that offspring will respond more strongly to their own environment than to that of their parents. The observed relaxation of germination requirements caused by a maturation canopy could be maladaptive for offspring by disrupting germination responses to light cues after dispersal. Alternatively, reduced germination requirements could be adaptive by allowing seeds to germinate faster and reduce competition in later stages even though competition is not yet present in the seedling environment. The masking of genetic variation by maturation under a canopy, moreover, could impede evolutionary responses to selection on germination.
Keywords: Seed germination, dormancy, maternal environmental effects, shade, R:FR, Arabidopsis thaliana
INTRODUCTION
Organisms can adjust their phenotypes in response to conditions experienced by their parents (parental environmental effects) and by themselves (Schlichting, 1986; Roach and Wulff, 1987; West-Eberhard, 1989; Sultan, 2000; Snell-Rood, 2013). While parental environmental effects can produce adaptive responses in offspring (Mousseau and Fox, 1998; Herman and Sultan, 2011), cues experienced by offspring themselves are likely to be more reliable predictors of their immediate environments, and in turn performance, than cues in the parental environment (DeWitt et al., 1998). Therefore, it is expected that responses to conditions experienced by an individual in its immediate environment will over-ride responses to cues that were experienced during its maturation in the parental environment. However, parental environments may predict future conditions that are not yet present in an individual’s immediate environment. If so, then plastic responses to parental environments could increase performance and would be expected to over-ride responses to the immediate environment.
Seed germination responds to cues experienced before and after dispersal in a manner that prevents seedlings from emerging under sub-optimal conditions (Gutterman, 2000; Donohue, 2009; Donohue et al., 2010; Baskin and Baskin, 2014). One environmental factor that is likely to influence seedling performance is neighbouring vegetation. Plants detect vegetation using irradiance and the ratio of red to far-red light (R:FR), both of which are reduced when sunlight is filtered through or reflected from photosynthetic tissue (Casal and Sánchez, 1998; Smith, 2000). Phytochromes are plant photoreceptors that sense R and FR light; the inactive conformation (Pr) is converted by R light into the bioactive conformation (Pfr) that stimulates germination and other developmental processes (Casal and Smith, 1989; Casal and Sánchez, 1998). Phytochromes therefore regulate responses to R:FR, which can predict competition even before it occurs (Smith, 2000), but other photoreceptors and resource-sensing mechanisms are involved in responses to total irradiance (Aphalo and Ballaré, 1995; Casal, 2013).
Conditions of low irradiance or R:FR, indicating nearby vegetation, often impede germination in taxa that require gaps for establishment (King, 1975; Deregibus et al., 1994; Gutterman, 2000; Pons, 2000; Dechaine et al., 2009). In such taxa, preventing germination under competitive conditions will increase a seed’s fitness; therefore, germination should be less likely to occur under light cues that indicate competition. The value of preventing germination under vegetation will then depend on the risk of mortality in seeds that do not germinate (Donohue et al., 2010). However, light cues may interact with other seasonal cues to regulate germination. In particular, temperature varies seasonally, and seeds of most species require specific temperatures during imbibition in order to germinate (Baskin and Baskin, 2014). If temperature requirements are not met, seeds will not germinate even when other conditions, such as light, are favourable. Therefore, the hypothesized inhibitory effect of a vegetative canopy is more likely to be observed under temperatures that promote germination.
While light cues experienced during seed maturation can indicate competitive conditions in the maternal environment, vegetation varies through space and time and is unlikely to remain perfectly stable from the time of seed maturation to the time of seed germination. Consequently, vegetation cues experienced by dispersed seeds may be more accurate predictors of seedling competitive conditions and therefore performance than those same cues experienced during maturation. If so, germination responses to vegetation in the seed environment are expected to over-ride germination responses to vegetation in the parental environment.
Seed dormancy will further reduce the ability of vegetation cues in the maternal environment to predict vegetation present in the seedling environment. Dormancy prevents germination under ephemeral conditions that would stimulate germination in non-dormant seeds, thereby synchronizing non-dormancy with the appropriate germination season (Bewley, 1997; Finch-Savage and Leubner-Metzger, 2006; Baskin and Baskin, 2014; Footitt et al., 2014). Seeds that must lose dormancy over time (after-ripen) would require more ‘lag time’ between the perception of cues during maturation and responses to those cues during germination (DeWitt et al., 1998), which could reduce the accuracy of pre-dispersal cues in predicting seedling performance. Consequently, the effects of vegetation cues during maturation on germination are hypothesized to be weaker in species or genotypes with stronger dormancy.
In species with non-deep physiological dormancy, seeds that lose dormancy during after-ripening may be induced into secondary dormancy if germination requirements are not met, which allows seeds to delay germination until the next favourable season (Bewley and Black, 1994; Baskin and Baskin, 2014; Footitt et al., 2014; Auge et al., 2015). Such additional delays of germination through secondary dormancy induction may further reduce the accuracy with which the maturation environment can predict the seedling environment. As a consequence, after-ripened seeds that are induced into secondary dormancy are expected to respond to cues experienced during imbibition, but not to cues in the maternal environment.
For the reasons discussed above, it is hypothesized that a dispersed seed’s environment is a more accurate predictor of its potential to experience competition than the environment of the maternal parent. Therefore, if cues are in conflict between maternal and offspring environments, one would expect that seeds will respond to their own environment more strongly than to that of their maternal parent. However, the maternal environment may provide cues of future competition that are not yet present in a dispersed seed’s immediate environment, in which case germination responses to the maternal environment may supersede responses to the seed’s environment. Further, parent–offspring conflict and selection acting directly on maternal plants for their regulation of offspring phenotypes may cause offspring to produce phenotypes that reduce their individual performance (Marshall and Uller, 2007; Uller, 2008).
In this study, we tested germination responses to light cues in pre- vs. post-dispersal environments in genotypes of Arabidopsis thaliana (Brassicaceae) that differ in dormancy. Specifically, we tested whether: (1) germination proportions are lower in seeds matured or imbibed under a vegetative canopy compared with those matured or imbibed in white light; (2) germination responses to a canopy experienced during imbibition are stronger than responses to a canopy experienced during maturation; (3) seeds with greater dormancy, as determined by genotype or the induction of secondary dormancy, are less likely to respond to a vegetative canopy experienced during seed maturation; and (4) germination responses to vegetative canopies are mediated by the detection of R and FR light.
MATERIALS AND METHODS
Plant material and growth conditions
Arabidopsis thaliana is an annual plant in the Brassicaceae with physiological seed dormancy (Baskin and Baskin, 1983). Dormancy varies among natural populations of A. thaliana and has been shown to be under natural selection and to contribute to local adaptation (Bentsink et al., 2010; Huang et al., 2010; Kronholm et al., 2012; Montesinos-Navarro et al., 2012; Debieu et al., 2013; Postma et al., 2016). Arabidopsis thaliana is usually a winter annual, germinating in the autumn and flowering in the spring, but it can exhibit a spring-annual life history – germinating and flowering in spring – or a rapid-cycling life history – germinating and flowering multiple times per year (Ratcliffe, 1965, 1976; Baskin and Baskin, 1972, 1983; Thompson, 1994; Donohue, 2009). Variation in germination contributes to this life-history variation.
To test the hypothesis that germination responses to a canopy experienced during maturation and imbibition depend on seed dormancy, we used three genotypes of A. thaliana that differ in dormancy and represent a sub-set of the natural variation in germination behaviour in this species (for a schematic overview of our experimental design, see Supplementary Data Fig. S1). Our most dormant genotype was the standard accession Landsberg erecta (Ler). We also used a near isogenic line (‘NIL’) that has a quantitative trait locus (QTL) associated with germination on chromosome 5 from the Cape Verde Islands (Cvi) ecotype introgressed onto the Ler background (Alonso-Blanco et al., 1998). The NIL has lower dormancy than Ler, manifest as a wider range of temperatures that elicit germination in non-after-ripened seeds (Chiang et al., 2009). Finally, we used the Columbia (Col) accession to compare germination responses to vegetative canopies in the two standard lab strains of A. thaliana (Ler and Col) that are known to differ in germination behaviour. Col is less dormant than Ler under most conditions (Chiang et al., 2009; Burghardt et al., 2016).
To test whether a canopy during seed maturation alters germination, we matured seeds of the three genotypes under unfiltered white light (‘white light’ maturation) and a green filter (‘canopy’ maturation; LEE colour effect filters, #089 ‘moss green’; LEE Filters, Andover, Hampshire, UK). The green filter reduced R:FR from 1·4 to 0·3 and total irradiance by 50 %, simulating dense vegetation. While the green filter cannot distinguish the effects of total irradiance, R:FR and changes in other spectral qualities such as blue light and UVA/B, the filter realistically mimics the effect of a vegetative canopy by reducing both R:FR and total irradiance.
Seeds of the three genotypes were induced to germinate in pots filled with Metromix 360 soil (Scotts Sierra, Maysville, OH, USA) and vernalized for 4 weeks at 4–5 °C to initiate and synchronize flowering. Maternal plants were then transferred to GCW-30 growth chambers (Environmental Growth Chambers, Chagrin Falls, OH, USA) at 22 °C under a 12 h light cycle. After bolting, maternal plants were transferred to 15 °C in 8 h light into the two maturation environments (white light and canopy) in the same chambers, experiencing different light treatments only after bolting. The temperature and daylength during seed maturation were chosen to differentiate the dormancy levels of the three genotypes – specifically to increase dormancy in the less dormant genotypes (Col and the NIL) without inducing deep dormancy in the more dormant genotype (Ler). Six plants per genotype were matured in each treatment, with three plants each in two chambers, and fertilized every 2 weeks with a 300 ppm nitrogen solution of Blossom Booster Fertilizer (JR Peters, Allentown, PA, USA). Mature seeds were synchronously harvested and stored dry at room temperature prior to germination assays.
Germination assays
Fresh seeds (primary dormancy).
We performed germination assays using fresh seeds (<1 week of after-ripening) to assess the effects of seed-maturation light on the temperature and light requirements for germination immediately after seeds are shed. We used three imbibition light treatments – white light (12 h photoperiod), green filter (12 h photoperiod, ‘canopy’ hereafter) and dark – and two imbibition temperatures – 10 and 22 °C. Comparisons between the white light and dark treatments test whether light is required for germination, while comparisons between the white light and canopy treatments test for germination responses to a canopy during imbibition. Comparisons between temperatures (10 and 22 °C) test whether the effects of genotype, maturation light and imbibition light are more likely to occur when seeds experience temperatures that promote germination (10 °C) as opposed to temperatures that are less conducive to germination (Auge et al., 2015; Burghardt et al., 2016).
Seeds from six maternal plants (biological replicates) of each genotype in each maturation-light treatment were used for germination assays, for a total of six sowing replicates per combination of genotype, maturation light and imbibition. Twenty seeds per replicate were sown in Petri plates on 0·7 % (w/v) agar. Plates were wrapped with parafilm to prevent desiccation and immediately transferred to their treatments in GC-82 growth chambers (Environmental Growth Chambers). Total irradiance [photosynthetically active radiation (PAR)] was 240 μmol m–2 s–1 in 10 °C and 160 μmol m–2 s–1 in 22 °C. For the dark treatment, plates were placed in cardboard boxes wrapped in two layers of aluminium foil. For the white light and canopy treatments, plates were randomly arranged on plastic trays with transparent lids. For the canopy treatment, the same green filters used for the maturation treatment were fitted under each tray lid. We censused plates in the white light and canopy imbibition treatments for germination every 2–5 d until day 14, at which point germination had plateaued. Exposure of plates in these treatments to light in the laboratory during censuses was minimal. Plates in the dark treatment were censused on day 14. The criterion for germination was the emergence of the radicle from the seed coat. Germination proportion was scored as the number of germinated seeds per total number of viable seeds in each plate; thus, the plate is our unit of analysis. Seeds were considered viable if they were firm after germination had plateaued.
Hot-stratified, after-ripened seeds (secondary dormancy).
We performed a second germination assay to test whether the effects of a seed-maturation canopy persist after seeds are induced into secondary dormancy. In addition, to examine potential physiological mechanisms of germination responses to a maturation canopy, we exposed after-ripened seeds that were induced into secondary dormancy to light pulses of different wavelengths (R and FR) during imbibition.
Seeds were first after-ripened to allow them to lose primary dormancy, then re-induced into secondary dormancy using hot stratification (Auge et al., 2015). Specifically, seeds were stored dry at room temperature for 6 weeks to allow them to after-ripen. Then, after-ripened seeds were sown into Petri plates filled with 0·7 % (w/v) agar, wrapped with parafilm, placed in aluminium-wrapped cardboard boxes and imbibed in the dark at 35 °C for 2 d. Although we did not test germination of after-ripened seeds to confirm that they were non-dormant before stratification, these genotypes lose most dormancy after 6 weeks of after-ripening under the conditions of this experiment. Previous studies have shown that seeds of the NIL and Col that were matured under a similar temperature (14 °C) and after-ripened for a comparable period (7 weeks) germinate to 100 % at 8 °C and to 75 % at 22 °C, while Ler germinated to 80 % at 8 °C and to 60 % at 22 °C (Burghardt et al., 2016). Thus, some residual primary dormancy may have remained in Ler at the time of hot stratification. Regardless, differences in germination between the seed-maturation treatments in our experiment would indicate that the effect of a maternal canopy persists as seeds lose and re-gain dormancy.
Following hot stratification, seeds were imbibed in the dark or given pulses of FR and/or R light using light-emitting diode chambers (Percival Scientific, Perry, IA, USA) then transferred to darkness. Active phytochrome (Pfr) is involved in light-stimulated germination (Whitelam and Devlin, 1997; Heschel et al., 2007). FR light converts phytochrome from its active (Pfr) to inactive (Pr) form, and R light converts Pr to bioactive Pfr. Seeds given the FR and R treatments were first exposed to a 30 min 10 μmol m–2 s–1 FR pulse to convert Pfr to inactive Pr, then either moved immediately to darkness (FR treatment) or exposed to a 30 min 10 μmol m–2s–1 R pulse (to convert Pr to active Pfr) and then moved to darkness (R treatment). Following the light pulses, plates in all three treatments (dark, R and FR) were placed in cardboard boxes double-wrapped in aluminium foil and then imbibed in the dark at either 10 or 22 °C for 14 d. After 14 d, plates were assayed for germination as the number of germinated seeds out of the total number of viable seeds per plate, as in the first experiment.
Seeds imbibed in the dark without any light pulse have Pfr levels established during maturation. Dark imbibition therefore assesses whether seeds can germinate without light, and a difference in germination in the dark between the maturation treatments would indicate that the effect of a maturation canopy persists as seeds lose and re-gain dormancy. The comparison between dark and FR treatments tests whether Pfr levels established during maturation are adequate to induce germination in seeds with secondary dormancy, compared with a treatment in which most Pfr was abolished by exposure to FR light. Thus, the comparison of dark vs. FR across maturation light treatments reveals whether a maturation canopy influences germination via differences in Pfr established during maturation. The R treatment increases bioactive Pfr during imbibition; therefore, the comparison between FR and R treatments quantifies the degree to which germination increases in response to increased de novo Pfr. A comparison of R vs. FR across maturation light treatments, moreover, would test whether seed maturation under a canopy affects germination responses to de novo Pfr induced by exposure to R light during imbibition.
Statistical analysis
All analyses were performed using R v. 3.1.3 (R Core Team, 2015). P-values were adjusted for multiple comparisons using the Holm procedure in ‘p.adjust’. Final germination proportions were analysed using generalized linear models (glms) fit with a logit link function using ‘glm’ in the ‘stats’ package, and likelihood ratio (LR) tests were performed using ‘lrtest’ in ‘lmtest’ (Zeileis and Hothorn, 2002). When treatments had little germination and data separation prevented glms from converging, we instead used bias-reduced logistic regression (‘brglm’ package; Kosmidis, 2013) followed by LR tests. The magnitudes of effects were determined using coefficients from bias-reduced logistic regression models. In cases where no germination occurred in any of the treatments being compared, we did not perform statistical tests. For both experiments, maturation light (‘Maturation’), genotype (‘Genotype’) and imbibition temperature (‘Temperature’) were treated as fixed factors, and reference levels were white light for maturation, Ler for genotype and 10 °C for temperature. For the experiment with fresh seeds, imbibition light (‘Imbibition’) was treated as a fixed factor and white light was used as the reference level. For the experiment with hot-stratified seeds induced into secondary dormancy, imbibition pulse (‘Imbibition Pulse’) was treated as a fixed factor and FR was used as the reference level.
In fresh seeds, we first tested the effects of Maturation, Genotype, Imbibition and Temperature on germination probability. Temperature frequently interacted with other factors (Supplementary Data Table S1), so we subsequently analysed each temperature separately to interpret these interactions. To interpret Maturation × Imbibition, we tested whether Maturation altered light requirements for germination (Maturation × Imbibition) in dark vs. white light imbibition for each genotype. We also tested Maturation × Imbibition in white light vs. canopy imbibition to test whether Maturation altered germination responses to a canopy during imbibition. To interpret Maturation × Genotype, we tested for differences between genotypes (Ler vs. NIL and Ler vs. Col) in their sensitivity to Maturation (Maturation × Genotype) within each combination of Imbibition and Temperature. To interpret Maturation × Imbibition and Maturation × Genotype further, we tested Maturation within each combination of Imbibition, Genotype and Temperature. We estimated the magnitudes of Maturation effects in each combination of Imbibition, Genotype and Temperature, and of Imbibition effects in each combination of Maturation, Genotype and Temperature. Finally, we tested Genotype in each combination of Maturation, Imbibition and Temperature.
To examine effects on dormancy induction in after-ripened seeds that were hot stratified and induced into secondary dormancy, we first tested the effects of Maturation, Genotype and Temperature on the depth of dormancy induction measured as germination proportion of hot-stratified, dark-imbibed seeds only. To interpret Maturation × Temperature × Genotype interactions, we next tested Maturation in each Genotype and Temperature separately, and estimated the magnitude of the Maturation effect on dark germination.
To determine whether the effect of canopy maturation on germination after secondary dormancy induction may be mediated by phytochromes, we tested the effects of Maturation, Genotype and Temperature on germination responses to R and FR light during imbibition. First, we tested whether Pfr established during seed maturation (dark imbibition) induced more germination than in seeds without Pfr (FR treatment), and whether those effects depended on genotype and temperature (Imbibition Pulse interactions with Maturation, Genotype and Temperature in dark vs. FR treatments). To interpret these interactions, we next tested Maturation on germination responses to dark vs. FR imbibition (Maturation × Imbibition Pulse) in each combination of Genotype and Temperature. Finally, we determined the magnitude of germination responses to FR light compared with the dark treatment for each combination of Maturation, Genotype and Temperature.
To test whether a maturation canopy alters Pfr requirements (i.e. R light requirements) for germination in seeds with secondary dormancy, we tested the effects of maturation and genotype on germination responses to FR vs. R imbibition in each temperature (Imbibition Pulse interactions with Maturation, Genotype and Temperature). We interpreted these interactions by testing the effect of a maturation canopy on germination responses to FR vs. R treatments (Maturation × Imbibition Pulse) in each combination of Genotype and Temperature. Finally, we quantified the magnitude of germination responses to R light compared with FR.
RESULTS
Vegetative canopies experienced during seed maturation and seed imbibition have opposing effects on germination of fresh seeds
Seed maturation under a canopy almost always increased germination proportions, and those germination proportions were usually greater at 10 °C than at 22 °C (Figs 1 and 2; Supplementary Data Table S2). At both temperatures, a maturation canopy increased germination more in some imbibition light treatments and genotypes than others (Genotype × Maturation × Imbibition in Table 1).
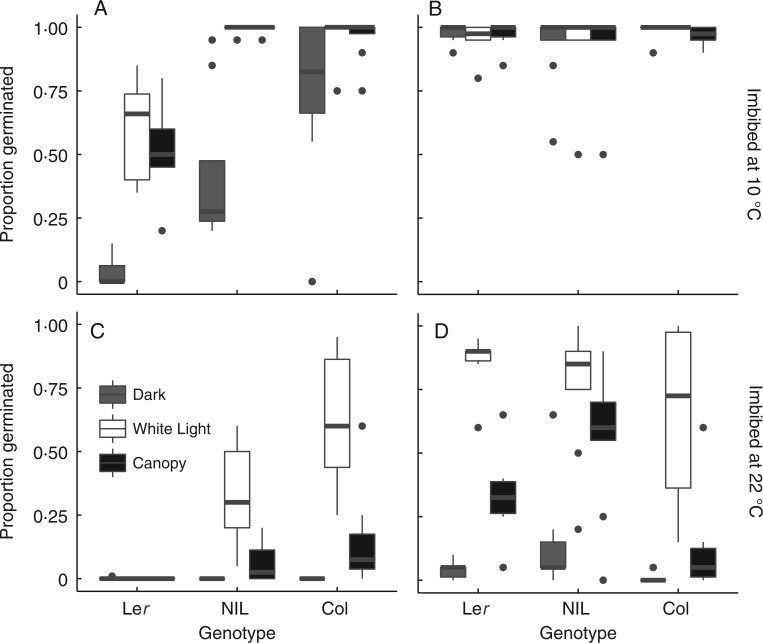
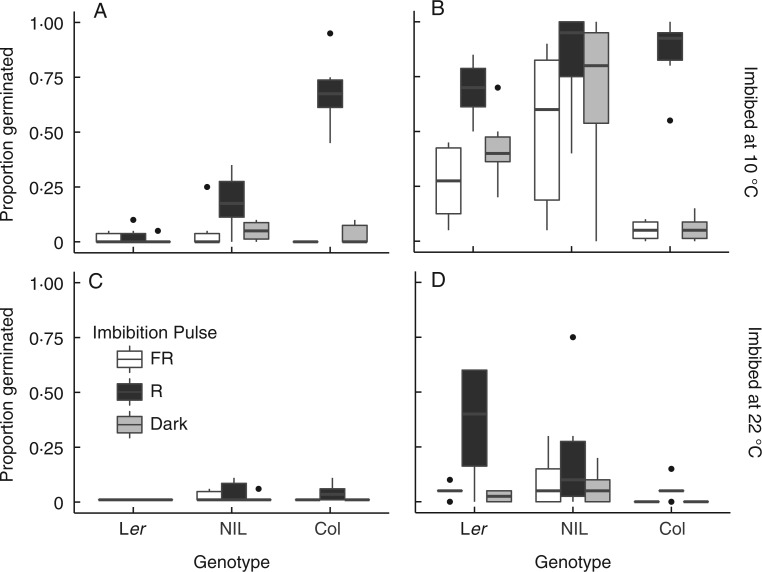
Fig. 1.
Germination of fresh seeds of each genotype matured under white light (A, C) or a canopy (B, D) and then imbibed in the dark, white light or canopy at either 10 °C (A, B) or 22 °C (C, D). Black horizontal lines within boxes represent median germination proportion. Box hinges indicate 75th and 25th percentiles. Whiskers span 1·5 times the interquartile range, and black points are observations that fall outside these values.
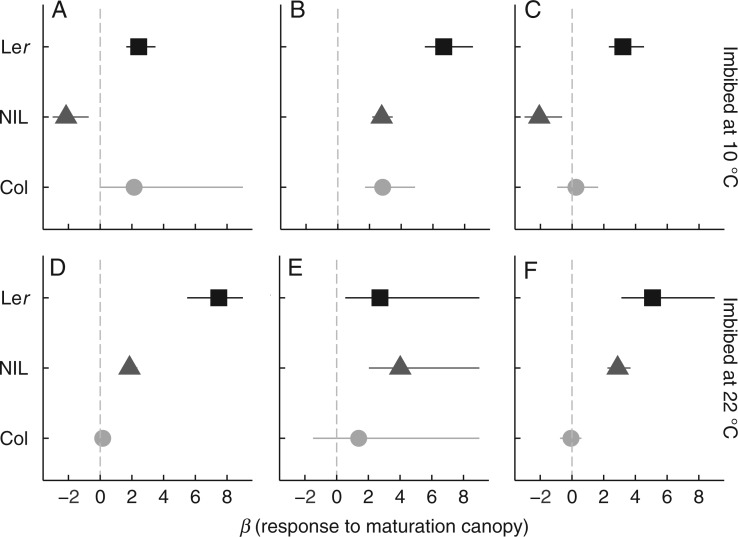
Fig. 2.
Direction and strength of the effect of seed maturation under a canopy on germination of fresh seeds imbibed in white light (A, D), darkness (B, E) or canopy (C, F) at either 10 °C (A, B, C) or 22 °C (D, E, F). Colours and shapes indicate the genotype. Each estimate (β) is the change in log odds with 95 % confidence intervals of germination caused by canopy maturation: positive values indicate an increase in germination compared with white light maturation. Confidence intervals crossing zero (vertical grey line) indicate that there was no effect of canopy maturation.
Table 1.
Analysis of germination proportions of fresh seeds at 10 and 22 °C
| Imbibition at 10 °C | Imbibition at 22 °C | ||||
|---|---|---|---|---|---|
| Source | d.f. | LR χ2 | P-value | LR χ2 | P-value |
| Genotype | 2 | 123·39 | <0·001 | 52·09 | <0·001 |
| Maturation | 1 | 50·09 | <0·001 | 269·30 | <0·001 |
| Imbibition | 2 | 153·49 | <0·001 | 618·20 | <0·001 |
| Genotype × Maturation | 2 | 42·39 | <0·001 | 200·20 | <0·001 |
| Genotype × Imbibition | 4 | 23·64 | <0·001 | 23·48 | <0·001 |
| Maturation × Imbibition | 2 | 33·57 | <0·001 | 7·79 | 0·020 |
| Genotype × Maturation × Imbibition | 4 | 10·54 | 0·032 | 1·80 | 0·773 |
Effects of genotype, seed maturation treatment (‘Maturation’), seed imbibition light (‘Imbibition’) and their interactions on germination proportions, based on logit-linked generalized linear models. Likelihood ratios (LRs) were tested based on χ2. Reference levels were white light for maturation, Ler for genotype and white light for imbibition light. The residual d.f. in each imbibition temperature = 117. To aid in interpretation of significant effects, see Tables 2 and 3; Tables S2–S4.
At 10 °C, the temperature that elicited greater germination, seed maturation under a canopy relaxed the light requirement for germination by allowing seeds to germinate in the dark (Fig. 3A; Table 2; Supplementary Data Table S3). In Col, seeds did not have a strong light requirement for germination even when matured under white light, so the effect of canopy maturation was not significant (Table 2).
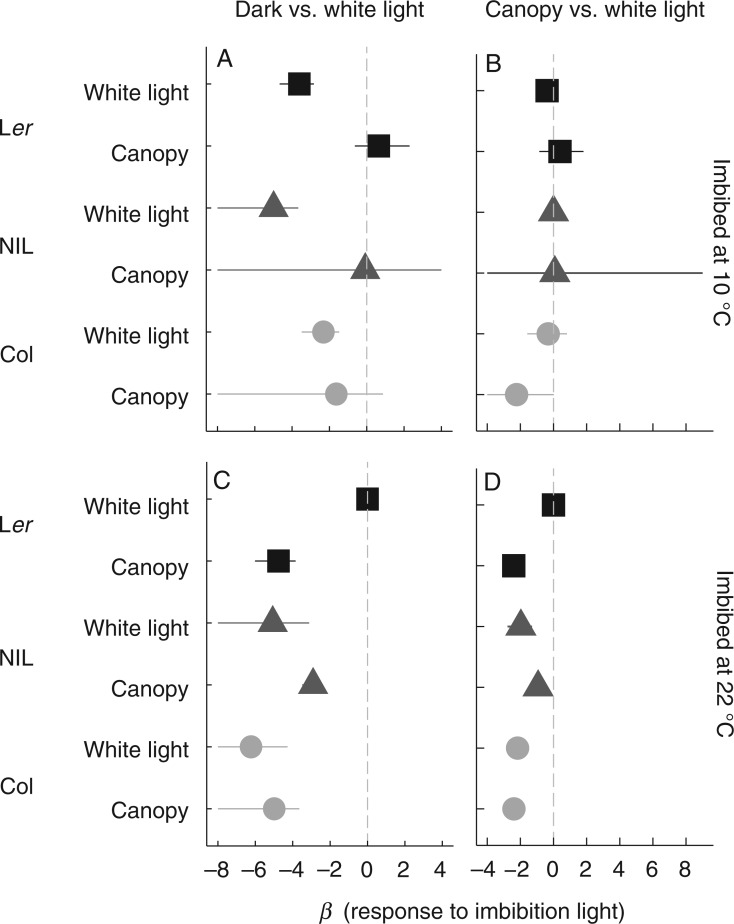
Fig. 3.
Direction and strength of the effect of imbibition light on germination of fresh seeds matured under white light or canopy conditions (left axis) and imbibed at either 10 °C (A, B) or 22 °C (C, D). Colours and shapes indicate the genotype. A light requirement for germination was assessed as the difference in germination between dark and white light imbibition (A, C); negative values indicate seeds germinated less in white light. Germination response to an imbibition canopy was assessed as the difference in germination between white light and canopy imbibition (B, D); negative values indicate a decrease in germination compared with white light imbibition. Each estimate (β) is the change in log odds with 95 % confidence intervals of germination caused by dark (A, C) or canopy imbibition (B, D): negative values indicate a decrease in germination compared with white light imbibition. Confidence intervals crossing zero (vertical grey line) indicate that there was no effect of imbibition light.
Table 2.
The effect of seed-maturation light on germination responses to imbibition light in fresh seeds of each genotype imbibed at 10 and 22 °C
| Geno-type | Type of imbibition response | Imbibition at 10 °C | Imbibition at 22 °C | ||||
|---|---|---|---|---|---|---|---|
| LR χ2 | Effect | P-value | LR χ2 | Effect | P-value | ||
| Ler | White light vs. dark | 27·78 | ↓ | <0·001 | – | – | – |
| White light vs. canopy | 1·40 | – | 0·237 | – | – | – | |
| NIL | White light vs. dark | 52·54 | ↓ | <0·001 | 7·01 | ↑ | 0·008 |
| White light vs. canopy | 0·01 | – | 0·954 | 6·59 | ↓ | 0·020 | |
| Col | White light vs. dark | 0·35 | – | 0·557 | 1·50 | – | 0·220 |
| White light vs canopy | 3·58 | – | 0·118 | 0·29 | – | 0·591 | |
We tested whether maturation light modified two types of germination responses to imbibition light (Type of imbibition response): a light requirement for germination (White light vs. dark imbibition light) and germination response to an imbibition canopy (White light vs. canopy imbibition light). Germination proportions were analysed with logit-linked generalized linear models, and likelihood ratio (LR) tests were used to compare full models with reduced models that lacked the interaction between Maturation and Imbibition. Reference levels were white light for maturation and white light for imbibition. For each test, d.f. = 1 with 24 residual d.f. Arrows in the Effect column indicate that a maturation canopy significantly increased or decreased germination responses to imbibition light, as determined by the LR tests. Data separation prevented tests for Ler seeds at 22 °C, but these seeds responded to imbibition light treatments only if they were canopy matured (see Table S2).
At 10 °C, germination did not respond to imbibition under a canopy due to high germination proportions in both white light and canopy imbibition treatments (Figs 1 and 3B; Table S3). Germination of canopy-matured seeds of Col was lower when imbibed under a canopy than when imbibed in white light, but this weak response is unlikely to be biologically significant (Fig. 1B).
At 22 °C, the temperature that was less conducive to germination, seed maturation under a canopy did not allow seeds to germinate in the dark as it did at 10 °C (Figs 1C, D and 3C; Table 2; Table S3). Seeds from the most dormant genotype (Ler) could only germinate if matured under a canopy, and then did so in white light but not in the dark. Thus, when imbibition temperature restricted germination, seeds were able to respond to imbibition light if they had been matured under a canopy, even though a seed-maturation canopy did not allow germination in the dark.
At 22 °C, imbibition under a canopy reduced germination compared with white light in the less dormant genotypes (Col and the NIL), although in the NIL the effect was weaker if seeds had been matured under a canopy (Figs 1 and 3D; Table 2; Table S3). In contrast, seeds of the most dormant genotype (Ler) were able to germinate only if they were matured under a canopy, and then germinated less when imbibed in the canopy treatment than when imbibed in white light.
Differences among genotypes depended on the combination of seed maturation and imbibition canopy treatments. In both temperatures, the most dormant genotype (Ler; see Figs 1 and 2) was also the most sensitive to maturation under a canopy. Because maturation under a canopy increased germination in the most dormant genotype, genetic differences in germination responses to imbibition treatments depended on the maturation-light environment (Fig. 1; Table 3; Supplementary Data Table S4). When matured under a canopy as opposed to white light, differences between Ler and the NIL were reduced or completely masked in all imbibition conditions, except when imbibed at 22 °C in the dark or under a canopy; in these cases, maturation under a canopy slightly increased the difference between the two genotypes (Fig. 1D). Similarly, seed maturation under a canopy modified differences in germination between Ler and Col in nearly all the imbibition conditions, but the effect depended on those conditions. Maturation under a canopy reduced germination differences in these genotypes if seeds were imbibed at 10 °C, but reversed the rank order of the genotypes if seeds were imbibed at 22 °C in white light or under a canopy (Fig. 1; Table S4).
Table 3.
The effect of seed-maturation light on genotype differences in germination of fresh seeds, within each combination of imbibition light and temperature
| Imbibition at 10 °C | Imbibition at 22 °C | |||||
|---|---|---|---|---|---|---|
| Imbibition light | LR χ2 | Effect | P-value | LR χ2 | Effect | P-value |
| Ler vs. NIL | ||||||
| Dark | 37·93 | ↓ | <0·001 | – | – | – |
| White light | 40·41 | ↓ | <0·001 | 69·90 | ↓ | <0·001 |
| Canopy | 49·69 | ↓ | <0·001 | 6·54 | ↑ | 0·011 |
| Ler vs. Col | ||||||
| Dark | 9·73 | ↓ | 0·002 | – | – | – |
| White light | 0·596 | – | 0·440 | 143·78 | ↓ | <0·001 |
| Canopy | 11·81 | ↓ | <0·001 | 47·65 | ↓ | <0·001 |
Germination proportions were analysed with logit-linked generalized linear models, and likelihood ratio (LR) tests were used to compare full models with reduced models that lacked the interaction between Maturation and Genotype. Reference levels were Ler for genotype and white light for maturation. For each test d.f. = 1 with 24 residual d.f. Arrows in the Effect column indicate that a maturation canopy significantly increased or decreased differences between genotypes, based on LR tests. Data separation prevented tests in the dark at 22 °C. However, germination of Ler and the NIL differed in these imbibition conditions only if canopy matured (see Table S4).
In summary, a vegetative canopy increased germination of fresh seeds if experienced during seed maturation, but decreased or had no effect on germination if experienced during imbibition. The magnitude of these effects depended on the temperature of imbibition. In particular, when seeds were matured under a canopy, responses to imbibition canopy were only apparent at the temperature that was less conducive to germination (22 °C). Moreover, maturation under a canopy eliminated light requirements for germination when the imbibition temperature was in the range under which germination could proceed (10 °C), but allowed seeds to respond to imbibition light at the temperature which restricted germination (22 °C). The effects of canopy maturation were strongest in the most dormant genotype, which led to changes in the expression of genotypic differences in germination across most of the imbibition conditions we tested.
Maternal canopy effects on germination persist into secondary dormancy
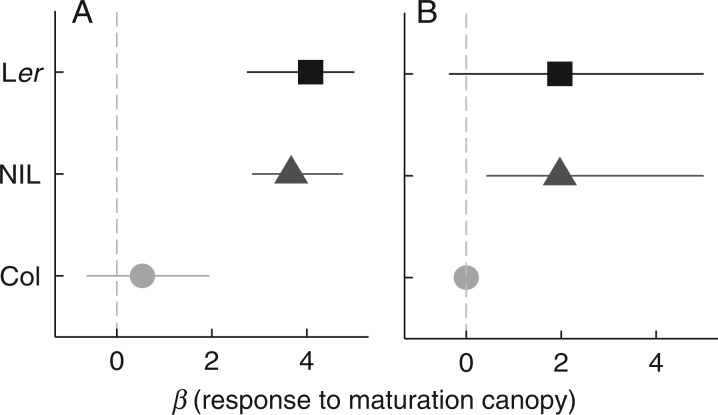
Maturation under a canopy increased the germination of seeds that were induced into secondary dormancy in Ler and the NIL (Figs 4 and 5). Although the effect of seed maturation under a canopy did not differ statistically between imbibition temperatures (Maturation × Temperature in Table 4), it tended to be stronger and to increase germination in more genotypes at 10 °C than at 22 °C.
Fig. 5.
Direction and strength of the effect of seed maturation under a canopy on germination in the dark at 10 °C (A) or 22 °C (B) following after-ripening and hot stratification to induce dormancy. Colours and shapes indicate the genotype. Each estimate (β) is the change in log odds with associated 95 % confidence intervals of germination caused by canopy maturation: positive values indicate that canopy maturation increases germination compared with white light maturation. Confidence intervals crossing zero (vertical grey line) indicate that there was no effect of canopy maturation.
Table 4.
Effects of genotype, maturation light and temperature on germination in the dark, germination responses to a Pfr-reducing treatment (interactions with ‘Imbibition Pulse’) and germination responses to a Pfr-increasing treatment (interactions with ‘Imbibition Pulse’) for seeds that had been hot stratified to induce secondary dormancy
| Source | Dark imbibition | Dark vs. FR treatment | R vs. FR treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LR χ2 | d.f. | P-value | LR χ2 | d.f. | P-value | LR χ2 | d.f. | P-value | |
| Genotype | 114·39 | 2 | <0·001 | 218·94 | 2 | <0·001 | 21·63 | 2 | <0·001 |
| Maturation | 188·35 | 1 | <0·001 | 325·40 | 1 | <0·001 | 375·65 | 1 | <0·001 |
| Imbibition Pulse | 5·78 | 1 | 0·016 | 352·48 | 1 | <0·001 | |||
| Temperature | 167·02 | 1 | <0·001 | 240·09 | 1 | <0·001 | 458·03 | 1 | <0·001 |
| Genotype × Maturation | 15·15 | 2 | <0·001 | 9·96 | 2 | 0·007 | 45·90 | 2 | <0·001 |
| Genotype × Imbibition Pulse | 0·04 | 2 | 0·978 | 87·72 | 2 | <0·001 | |||
| Maturation × Imbibition Pulse | 0·17 | 1 | 0·682 | 0·80 | 1 | 0·798 | |||
| Genotype × Temperature | 0·25 | 2 | 0·885 | 1·71 | 2 | 0·426 | 51·95 | 2 | <0·001 |
| Maturation × Temperature | 0·66 | 1 | 0·416 | 1·69 | 1 | 0·194 | 1·71 | 1 | 0·191 |
| Imbibition Pulse × Temperature | 6·07 | 1 | 0·014 | 0·02 | 1 | 0·883 | |||
| Genotype × Maturation × Imbibition Pulse | 4·55 | 2 | 0·103 | 4·00 | 2 | 0·136 | |||
| Genotype × Maturation × Temperature | 1·56 | 2 | 0·459 | 0·67 | 2 | 0·714 | 2·47 | 2 | 0·291 |
| Genotype × Imbibition Pulse × Temperature | 0·98 | 2 | 0·611 | 0·89 | 2 | 0·643 | |||
| Maturation × Imbibition Pulse × Temperature | 0·37 | 1 | 0·542 | 0·30 | 1 | 0·587 | |||
The four-way interaction did not improve the model (LR χ2 = 3·23, d.f. = 4, P = 0·521) and was dropped to increase power. Germination proportions were analysed with logit-linked generalized linear models, and likelihood ratios (LRs) were tested based on χ2. Residual d.f. = 59 for Dark imbibition and 122 for Dark vs. FR treatment and Dark vs. FR treatment. Reference levels were white light for maturation, Ler for genotype and 10 °C for temperature. Because the effects of Imbibition Pulse are not tested in Dark imbibition, the rows for associated terms are left blank. For models that included the effects of imbibition pulses, reference levels were dark (dark vs. FR) or FR (R vs. FR). To aid in interpretation of significant effects, see Tables S5 and S6.
In two of the genotypes (Ler and the NIL), some germination occurred even in dark-imbibed seeds when they had matured under a canopy, indicating that seed maturation under a canopy can weaken light requirements for germination in seeds with secondary dormancy. The effect of seed maturation under a canopy was most pronounced for Ler and the NIL (Genotype × Maturation in Table 4), as no effect was observed in Col seeds (Fig. 4).
Fig. 4.
Germination of seeds following after-ripening and hot stratification to induce secondary dormancy. Seeds were matured under white light (A, C) or a canopy (B, D) then imbibed at either 10 °C (A, B) or 22 °C (C, D). Box colours indicate whether seeds were imbibed directly into the dark, pre-treated with an FR pulse prior to imbibition in the dark or pre-treated with FR and R pulses prior to imbibition in the dark. Black horizontal lines within boxes represent median germination proportion. Box hinges indicate 75th and 25th percentiles. Whiskers span 1·5 times the interquartile range, and black points are observations that fall outside these values.
In summary, the effects of maturation under a canopy persisted as seeds were induced into secondary dormancy, and they weakened light requirements for germination.
Maternal canopy effects do not appear to be mediated by Pfr levels
The comparison of seeds imbibed in the dark vs. the FR treatment showed that Pfr acquired during seed maturation did enhance germination slightly in some treatments, but that its effect did not differ between seed-maturation treatments. Although the FR treatment (white bars in Fig. 4) slightly reduced germination compared with dark imbibition, maturation under a canopy did not modify this effect in either temperature, except in Col (Maturation × Imbibition in Table 4; Fig. 4; Supplementary Data Table S5; Fig. S2). Canopy-matured seeds of Col did not respond to FR, but the effect of canopy maturation on this response was extremely small.
The comparison of seeds imbibed in the R treatment vs. the FR treatment indicated that Pfr increases germination, but seed maturation under a canopy did not significantly affect these responses. The R treatment (red bars in Fig. 4) increased germination compared with the FR treatment, indicating that Pfr promotes germination, but was independent of seed-maturation light (Fig. 4; Supplementary Data Fig. S2B, D; Imbibition in Table 4; Maturation × Imbibition in Supplementary Data Table S6).
To summarize, the canopy during seed maturation influenced the germination of seeds even with secondary dormancy, but the effect of a seed-maturation canopy does not appear to be the consequence of increasing Pfr levels in seeds during maturation. Further, maturation under a canopy did not alter the ability of seeds with secondary dormancy to respond positively to the specific wavelength of light (R) that promotes germination.
DISCUSSION
Reduced germination in response to vegetation or vegetation cues has been documented in many taxa, and is interpreted as a mechanism for avoiding competition (King, 1975; Deregibus et al., 1994; Gutterman, 2000; Pons, 2000; Dechaine et al., 2009). Negative germination responses to a vegetative canopy were generally absent in our study, except in a few cases in which seeds were exposed to a canopy during imbibition (post-dispersal). Surprisingly, we observed strong, positive germination responses to a vegetative canopy in the maturation (pre-dispersal) environment that prevented responses to offspring environments and persisted as seeds were induced into secondary dormancy.
Potential ecological and evolutionary consequences of maternal and offspring canopy effects
A canopy in the maternal environment increased germination in nearly all combinations of imbibition light and temperature, consequently reducing the ability of seeds to respond to cues in their immediate environments. This over-riding effect of the maternal environment seems unlikely to be favourable for seedlings, since offspring environments are likely to be better predictors of performance, and germination cueing is crucial to seedling survival (DeWitt et al., 1998; Donohue et al., 2010; Baskin and Baskin, 2014). One way that this over-riding maternal effect may increase offspring performance, however, is if it predicts competition in later stages of the life cycle, even though dispersed seeds do not yet detect competition. The effects of neighbours on plant performance are known to change over the course of an individual’s lifetime (Goldberg et al., 2001; Miriti, 2006; Wright et al., 2014). In such cases, expanding the conditions that permit germination could enable seeds to germinate more quickly after dispersal. Earlier germination could then allow emergence before competitive conditions develop or intensify, and provide an advantage to seedlings over their future competitors (Geber and Griffen, 2003; Mercer et al., 2011; Weis et al., 2015). Further, if neighbours increase seedling fitness, as documented in some studies of A. thaliana (Thompson, 1994; Callahan and Pigliucci, 2002; L. D. Leverett and K. Donohue, unpubl. res.), increased germination following canopy maturation could be beneficial for offspring.
An increase in germination following maturation under a canopy could be favoured by selection at the lineage level, even if that maternal effect comes at a cost of reduced offspring fitness (Kirkpatrick and Lande, 1989; Marshall and Uller, 2007; Uller, 2008). Specifically, if maternal plants benefit from increased germination of offspring, for example because of potential costs of inducing dormancy (Cohen, 1966; Baskin and Baskin, 2014), and that benefit outweighs the risk of offspring emerging under competitive or other sub-optimal conditions, the increase in germination following maturation under a canopy we observed would be advantageous. Field studies that manipulate germination time and neighbour presence at seedling and adult stages and that measure fitness at the maternal and offspring levels could test these adaptive hypotheses concerning the observed responses to maternal and offspring canopy.
Because seeds matured under a canopy had greater germination propensities, genotypes with higher dormancy exhibited stronger germination responses to a seed-maturation canopy. The difference between Ler and the NIL in dormancy and in turn the effect of a maturation canopy could be due to FLOWERING LOCUS C (FLC), which is located in the introgressed chromosomal segment in the NIL. Previous work on Ler and the NIL demonstrated that FLC promotes germination in white light (Chiang et al., 2009). Increased FLC expression may also have led to the weaker maturation-canopy effect that we observed in the NIL by reducing dormancy. However, because Ler and the NIL may differ at other causative loci within the introgressed region, we cannot directly implicate FLC. Regardless, our results do imply that allelic variation in dormancy is associated with differences in the effects of a canopy during maturation, and that the expression of allelic variation in germination can be reduced by maturation under a canopy.
By reducing germination cueing to post-dispersal environments as well as differences among genotypes in such cueing, seed maturation under a canopy could alter the action and outcome of natural selection on germination. First, increased germination in response to vegetation in the maternal environment may lead to a more rapid depletion of below-ground populations. Given the importance of seed banks as temporal sources of gene flow in A. thaliana (Falahati-Anbaran et al., 2014), changes in the size of below-ground populations could influence effective population size and in turn the efficacy of natural selection. Secondly, the reduction or complete masking of differences in germination cueing among genotypes by a maturation canopy could increase the synchrony of germination among genotypes and preclude responses to selection on germination (Falconer and Mackay, 1996).
Germination responses to canopies in the maternal and offspring environments, and in turn the ecological and evolutionary consequences of these environmental effects, are likely to vary among dispersal cohorts. Reduced germination in response to a canopy in the seed environment was only observed in the warmer imbibition temperature (22 °C). Thus, any competitive advantage of avoiding germination under neighbouring vegetation may only be realized in cohorts that are dispersed into warm conditions, such as those associated with late spring or early autumn. Further, because a canopy in the maternal environment did not mask genotypic differences at the warmer imbibition temperature, cohorts that are dispersed in late spring or early autumn may have a greater capacity to respond to natural selection.
Mechanisms of canopy maturation effects on germination
Germination in A. thaliana is known to respond to the ratio of R to FR light during seed maturation. Seeds matured in light enriched in R have been shown to germinate more in the dark than those matured in light enriched in FR (McCullough and Shropshire, 1970; Hayes and Klein, 1974). Similarly, seeds matured under reduced R:FR had stronger light requirements than those matured under higher R:FR, although this effect varied among accessions (Dechaine et al., 2009). The results of these studies are in contrast to our finding that maturation under a canopy increased germination, even in the dark. However, our maturation conditions differed from these other studies in that we manipulated not only wavelength, but also irradiance. Additionally, seeds in the other studies were stored or otherwise treated to release dormancy prior to germination, whereas our seeds had some dormancy. Thus, the disparity between this and previous studies may be due to differences in dormancy and total irradiance.
The canopy effects we observed do not appear to be mediated by phytochromes. Phytochromes were demonstrated to regulate germination responses to R:FR during maturation in the Ler background (Dechaine et al., 2009). However, in that study, germination was tested only under neutral shade, so it is unclear whether phytochromes mediated germination through light requirements in seeds. In our study, seed maturation under a canopy did not alter germination responses to FR light in seeds that had been induced into secondary dormancy through hot stratification; thus, Pfr levels set during maturation cannot fully explain the maternal effects we observed in these seeds. However, it is possible that Pfr reverted to Pr after 2 d in the dark at 35 °C while seeds underwent hot stratification (Heschel et al., 2007; Casal, 2013). If so, we would not expect differences between dark and FR treatments. Although exposing hot-stratified seeds to a pulse of R light increased germination, this did not depend on maturation treatment. Thus, Pfr set during seed maturation and photoconversion by light during imbibition do not appear to mediate the effects of a canopy on germination that we observed.
The effects of a maturation canopy observed here are probably due to light properties other than R and FR. Our experimental design reduces both R:FR and irradiance, as in natural vegetative canopies. Total irradiance under the canopy was lower than under white light, and low irradiance during seed maturation has been shown to increase germination in other species (Schmitt et al., 1992; Galloway, 2001). Further, reduced irradiance combined with a short photoperiod may have simulated autumn conditions, in which case the increased germination of seeds matured under a canopy could be related to seasonal cueing. Our green filter also reduced transmission of blue and UV light, which could have been detected by cryptochromes and UVA or UVB photoreceptors, respectively. In barley, blue light increases dormancy (Gubler et al., 2008), an effect that is consistent with our observation that maturation under a canopy (with reduced blue light) reduced dormancy in fresh seeds.
Finally, the strong maternal effect we observed could be due to limited light during seed development. In Polygonum persicaria (Polygonaceae), seeds matured under severe light limitation (8 % of total light) had thinner seed coats and greater germination than those matured under full light (Sultan, 1996). In our study, canopy-matured seeds experienced 50 % less PAR, which may have reduced resources available during seed development and maturation. If so, the thinner coats of canopy-matured seeds could increase the risk of mortality, in turn lowering the potential longevity of seeds in the seed bank (reviewed in Long et al., 2015). Future experiments could manipulate different wavelengths of light as well as total irradiance to determine the exact mechanisms of canopy effects that we detected.
Conclusion
A maturation canopy increased germination, but an imbibition canopy either had no effect or reduced germination. This stronger effect of the maternal environment prevented offspring from responding to temperature and light cues in their immediate environments. Further, the effects of a maturation canopy on germination persisted as seeds were induced into secondary dormancy. Such trans-generational effects could influence germination behaviour, maternal and offspring fitness, and patterns of natural selection in annual plants.
SUPPLEMENTARY DATA
Supplementary data are available online at www.oxfordjournals.org and consist of the following. Table S1: analysis of germination proportions of fresh seeds. Effects of genotype, maturation treatment, imbibition light and temperature, and their interactions on germination proportions, based on logit-linked generalized linear models. Table S2: effect of seed-maturation light on germination proportions of fresh seeds of each genotype in each combination of imbibition light and temperature. Table S3: effect of light treatments during imbibition on germination proportions of fresh seeds, in each combination of seed-maturation light and imbibition temperature. Table S4: genotype differences in germination proportions of fresh seeds in each combination of maturation light, imbibition light and temperature. Table S5: effect of seed-maturation light on Pfr-mediated germination of after-ripened and hot-stratified seeds at each imbibition temperature. Table S6: effect of seed-maturation light on enhancement of germination of after-ripened and hot-stratified seeds by de novo Pfr in each imbibition temperature. Figure S1: overview of the experimental design for this study. Figure S2: direction and strength of the effect of imbibition pulse treatment on germination of after-ripened, hot-stratified seeds matured under white light or canopy conditions and imbibed at either 10 or 22 °C.
ACKNOWLEDGEMENTS
We thank M. Chen and members of the Chen lab, Department of Biology at Duke University and Department of Botany and Plant Sciences at UC-Riverside, for the use of diode chambers and crucial feedback on this project. We also thank L. K. Blair, L. T. Burghardt, M. D’Aguillo, B. Edwards, T. Imaizumi and other members of the Donohue lab for helpful suggestions on this manuscript and for technical assistance. This manuscript was greatly improved by comments from two anonymous reviewers. S. Harris at Harris-Chewning, Inc. was instrumental in helping us acquire the filters needed for this project. This work was supported by NSF grants IOS-1146383 and DEB-1501710, the Vertical Integration Partners (VIP) program sponsored by the Howard Hughes Medical Institute, and the University Program in Ecology at Duke University.
LITERATURE CITED
- Alonso-Blanco C, El-Assal SE, Coupland G, Koornneef M. 1998. Analysis of natural allelic variation at flowering time loci in the Landsberg erecta and Cape Verde Islands ecotypes of Arabidopsis thaliana. Genetics 149: 749–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aphalo PJ, Ballaré CL. 1995. On the importance of information-acquiring systems in plant–plant interactions. Functional Ecology 9: 5–14. [Google Scholar]
- Auge GA, Blair LK, Burghardt LT, et al. 2015. Secondary dormancy dynamics depends on primary dormancy status in Arabidopsis thaliana. Seed Science Research 25: 230–246. [Google Scholar]
- Baskin JM, Baskin CC. 1972. Ecological life cycle and physiological ecology of seed germination of Arabidopsis thaliana. Canadian Journal of Botany 50: 353–360. [Google Scholar]
- Baskin J, Baskin C. 1983. Seasonal changes in the germination responses of buried seeds of Arabidopsis thaliana and ecological interpretation. Botanical Gazette 144: 540–543. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press. [Google Scholar]
- Bentsink L, Hanson J, Hanhart CJ, et al. 2010. Natural variation for seed dormancy in Arabidopsis is regulated by additive genetic and molecular pathways. Proceedings of the National Academy of Sciences, USA 107: 4264–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley J. 1997. Seed germination and dormancy. The Plant Cell 9: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley J, Black M. 1994. Seeds: physiology of development and germination. New York: Plenum Press. [Google Scholar]
- Burghardt LT, Edwards BR, Donohue K. 2016. Multiple paths to similar germination behavior in Arabidopsis thaliana. New Phytologist 209: 1301–1312. [DOI] [PubMed] [Google Scholar]
- Callahan H, Pigliucci M. 2002. Shade-induced plasticity and its ecological significance in wild populations of Arabidopsis thaliana. Ecology 83: 1965–1980. [Google Scholar]
- Casal JJ. 2013. Photoreceptor signaling networks in plant responses to shade. Annual Review of Plant Biology 64: 403–427. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Sánchez RA. 1998. Phytochromes and seed germination. Seed Science Research 8: 317–329. [Google Scholar]
- Casal JJ, Smith H. 1989. The function, action and adaptive significance of phytochrome in light-grown plants. Plant, Cell & Environment 12: 855–862. [Google Scholar]
- Chiang GCK, Barua D, Kramer EM, Amasino RM, Donohue K. 2009. Major flowering time gene, flowering locus C, regulates seed germination in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 106: 11661–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. 1966. Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology 12: 119–129. [DOI] [PubMed] [Google Scholar]
- Debieu M, Tang C, Stich B, et al. 2013. Co-variation between seed dormancy, growth rate and flowering time changes with latitude in Arabidopsis thaliana. PLoS One 8: e61075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechaine JM, Gardner G, Weinig C. 2009. Phytochromes differentially regulate seed germination responses to light quality and temperature cues during seed maturation. Plant, Cell & Environment 32: 1297–309. [DOI] [PubMed] [Google Scholar]
- Deregibus VA, Casal JJ, Jacobo EJ, Gibson D, Kauffman M, Rodriguez AM. 1994. Evidence that heavy grazing may promote the germination of Lolium multiflorum seeds via phytochrome-mediated perception of high red/far-red ratios. Functional Ecology 8: 536–542. [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends in Ecology and Evolution 13: 77–81. [DOI] [PubMed] [Google Scholar]
- Donohue K. 2009. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society B: Biological Sciences 364: 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue K, Rubio de Casas R, Burghardt L, Kovach K, Willis CG. 2010. Germination, postgermination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics 41: 293–319. [Google Scholar]
- Falahati-Anbaran M, Lundemo S, Stenøien HK. 2014. Seed dispersal in time can counteract the effect of gene flow between natural populations of Arabidopsis thaliana. New Phytologist 202: 1043–1054. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics. Essex: Longman. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Footitt S, Clay H, Dent K, Finch, Savage W. 2014. Environment sensing in spring‐dispersed seeds of a winter annual Arabidopsis influences the regulation of dormancy to align germination potential with seasonal. New Phytologist 202: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway LF. 2001. The effect of maternal and paternal environments on seed characters in the herbaceous plant Campanula americana (Campanulaceae). American Journal of Botany 88: 832–840. [PubMed] [Google Scholar]
- Geber M, Griffen L. 2003. Inheritance and natural selection on functional traits. International Journal of Plant Sciences 164 (3 Suppl.): S21–S42. [Google Scholar]
- Goldberg D, Turkington R, Olsvig-Whittaker L, Dyer A. 2001. Density dependence in an annual plant community: variation among life history stages. Ecological Monographs 71: 423–446. [Google Scholar]
- Gubler F, Hughes T, Waterhouse P, Jacobsen J. 2008. Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiology 147: 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman Y. 2000. Maternal effects on seeds during development In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities. Wallingford, UK: CAB International, 59–84. [Google Scholar]
- Hayes RG, Klein WH. 1974. Spectral quality influence of light during development of Arabidopsis thaliana plants in regulating seed germination. Plant and Cell Physiology 653: 643–653. [Google Scholar]
- Herman JJ, Sultan SE. 2011. Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Frontiers in Plant Science 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heschel MS, Selby J, Butler C, Whitelam GC, Sharrock RA, Donohue K. 2007. A new role for phytochromes in temperature-dependent germination. New Phytologist 174: 735–741. [DOI] [PubMed] [Google Scholar]
- Huang X, Schmitt J, Dorn L, et al. 2010. The earliest stages of adaptation in an experimental plant population: strong selection on QTLs for seed dormancy. Molecular Ecology 19: 1335–1351. [DOI] [PubMed] [Google Scholar]
- King TJ. 1975. Inhibition of seed germination under leaf canopies in Arenaria serpyllifolia, Veronica arvensis and Cerastium holosteoides. New Phytologist 75: 87–90. [Google Scholar]
- Kirkpatrick M, Lande R. 1989. The evolution of maternal characters. Evolution 43: 485–503. [DOI] [PubMed] [Google Scholar]
- Kosmidis I. 2013. brglm: bias reduction in binomial-response generalized linear models http://www.ucl.ac.uk/∼ucakiko/software.html.
- Kronholm I, Pic FX, Alonso-Blanco C, Goudet J, de Meaux J. 2012. Genetic basic of adaptation in Arabidopsis thaliana: local adaptation at the seed dormancy QTL DOG1. Evolution 66: 2287–2302. [DOI] [PubMed] [Google Scholar]
- Long RL, Gorecki MJ, Renton M, et al. 2015. The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews of the Cambridge Philosophical Society 90: 31–59. [DOI] [PubMed] [Google Scholar]
- Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116: 1957–1963. [Google Scholar]
- McCullough JM, Shropshire W., Jr. 1970. Physiological predetermination of germination responses in Arabidopsis thaliana (L.). Plant and Cell Physiology 11: 139–148. [Google Scholar]
- Mercer KL, Alexander HM, Snow AA. 2011. Selection on seedling emergence timing and size in an annual plant, Helianthus annuus (common sunflower, Asteraceae). American Journal of Botany 98: 975–985. [DOI] [PubMed] [Google Scholar]
- Miriti MN. 2006. Ontogenetic shift from facilitation to competition in a desert shrub. Journal of Ecology 94: 973–979. [Google Scholar]
- Montesinos-Navarro A, Picó F, Tonsor S. 2012. Clinal variation in seed traits influencing life cycle timing in Arabidopsis thaliana. Evolution 66: 3417–3431. [DOI] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends in Ecology and Evolution 13: 403–407. [DOI] [PubMed] [Google Scholar]
- Pons TL. 2000. Seed responses to light In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities . Wallingford, UK: CAB International, 237–260. [Google Scholar]
- Postma FM, Lundemo S, Ågren J. 2016. Seed dormancy cycling and mortality differ between two locally adapted populations of Arabidopsis thaliana. Annals of Botany 117: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- Ratcliffe D. 1965. The geographical and ecological distribution of Arabidopsis and comments on physiological variation. Arabidopsis Information Service 1S.
- Ratcliffe D. 1976. Germination characteristics and their inter- and intra-population variability in Arabidopsis. Arabidopsis Information Service 13.
- Roach DA, Wulff RD. 1987. Maternal effects in plants. Annual Review of Ecology and Systematics 18: 209–235. [Google Scholar]
- Schlichting CD. 1986. The evolution of phenotypic plasticity in plants. Annual Review of Ecology and Systematics 17: 667–693. [Google Scholar]
- Schmitt J, Niles J, Wulff RD. 1992. Norms of reaction of seed traits to maternal environments in Plantago lanceolata. American Naturalist 139: 451. [Google Scholar]
- Smith H. 2000. Phytochromes and light signal perception by plants – an emerging synthesis. Nature 407: 585–591. [DOI] [PubMed] [Google Scholar]
- Snell-Rood EC. 2013. An overview of the evolutionary causes and consequences of behavioural plasticity. Animal Behaviour 85: 1004–1011. [Google Scholar]
- Sultan SE. 1996. Phenotypic plasticity for offspring traits in Polygonum persicaria. Ecology 77: 1791–1807. [Google Scholar]
- Sultan SE. 2000. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science 5: 537–42. [DOI] [PubMed] [Google Scholar]
- Thompson L. 1994. The spatiotemporal effects of nitrogen and litter on the population dynamics of Arabidopsis thaliana. Journal of Ecology 82: 63–68. [Google Scholar]
- Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends in Ecology and Evolution 23: 432–438. [DOI] [PubMed] [Google Scholar]
- Weis AE, Turner KM, Petro B, Austen EJ, Wadgymar SM. 2015. Hard and soft selection on phenology through seasonal shifts in the general and social environments: a study on plant emergence time. Evolution 69: 1361–1374. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. 1989. Phenotypic plasticity and the origins of diversity. Annual Review of Ecology and Systematics 20: 249–278. [Google Scholar]
- Whitelam GC, Devlin PF. 1997. Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant, Cell & Environment 20: 752–758. [Google Scholar]
- Wright A, Schnitzer S, Reich P. 2014. Living close to your neighbors – the importance of both competition and facilitation in plant communities. Ecology 95: 2213–2223. [DOI] [PubMed] [Google Scholar]
- Zeileis A, Hothorn T. 2002. Diagnostic checking in regression relationships. R News 2: 7–10. http://CRAN.R–project.org/doc/Rnews/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.