Abstract
Many load bearing tissues possess structurally and functionally distinct regions, typically accompanied by different cell phenotypes with differential mechanosensing characteristics. Engineering and analysis of these tissue types remain a challenge. Layered hydrogel constructs provide an opportunity for investigating the interactions among multiple cell populations within single constructs. Alginate hydrogels are both biocompatible and allow for easy isolation of cells after experimentation. Here, we describe a method for the development of small sized dual layered alginate hydrogel discs. This process maintains high cell viability of human mesenchymal stem cells during the formation process and these layered discs can withstand unconfined cyclic compression, commonly used for stimulation of hMSCs undergoing chondrogenesis. These layered constructs can potentially be scaled up to include additional levels, and also be used to segregate cell populations initially after layering. This dual layer alginate hydrogel culture platform can be used for many different applications including engineering and analysis of cells of load bearing tissues and co-cultures of other cell types.
Keywords: Bioengineering, Issue 114, Alginate, bi-layered constructs, disc-shaped scaffold, heterogeneous tissue layers, cell-seeded constructs, hydrogels
Introduction
Compressive load bearing tissues such as articular cartilage or intervertebral discs consist of heterogeneous tissue regions that are critical for both biomechanical function and appropriate mechano-transduction in the tissue. Not only is cellular organization and function distinct in different regions, but the extracellular matrices (ECM) are also varied in composition and organization. For example, articular cartilage consists of three primary zones with varying cell morphology, mechanical function, and ECM. Differences in their ECM lead to differential load bearing responsibilities; the superficial layer is primarily involved in tensile response to load, while the middle and deep zones are mainly accountable for response to compression 1. Similarly, in the intervertebral disc, a gel-like nucleus pulposus is surrounded by a lamellar annulus fibrosis and the cells within these two distinct areas experience different types of biophysical stimuli 2. In these types of tissues, cells and the extracellular matrices within the tissue layers interact with each other as the tissue undergoes and responds to mechanical forces.
Recapitulation of such heterogeneous tissue structures remains a challenge in tissue engineering and regenerative medicine, and our understanding of their biological significance is limited. There is a need for culturing platforms for analyzing stratified tissues as well as co-cultures of different populations of cells within one construct. In articular cartilage tissue engineering, scaffold-less layered constructs have been constructed by harnessing the ability of zonal chondrocytes to deposit varied ECM to mimic the different layers of this tissue 3,4. However, layered hydrogel constructs provide an opportunity for investigating the interaction of diverse types of cell populations that lack the ability to form a robust tissue independently. For example, different populations of mesenchymal stem cells can be co-cultured within layered constructs. Such layered scaffolds have been used with both chondrocytes and differentiating mesenchymal stem cells for improved tissue engineering 5. Not only can different cell populations be co-cultured in similar hydrogel layers, but a single cell type can also be cultured within layers that have been manipulated to have varying stiffness or biochemical content to elicit different responses from cells 6,7.
Many different biomaterial hydrogels have been used to layer cell populations for cartilage tissue engineering such as those using polyethylene glycol or poly vinyl alcohol bases 7-9. However, alginate hydrogels are one of the simplest biomaterials from which to create layered scaffolds for studying heterogeneous cell populations in co-culture. While agarose hydrogels are also easily formed, alginate hydrogels have the added benefit of allowing easy isolation of cells from the 3-D construct for analysis of individual cells as has been described previously 10. In previous studies, bi-layered alginate hydrogels have been formed in thin sheets and from these sheets, sections were sliced (e.g., using a biopsy punch) for particular applications such as for analysis of biochemical content or interfacial shear properties 11,12. Another method for forming thin alginate sheets has been described with the potential for stratification into multiple layers, but still would require alteration to the hydrogel for use in mechanical testing 13.
Here, we present a method for reproducibly creating bi-layered alginate hydrogel discs for use in co-culturing different populations of cells. This alginate disc platform possesses several advantages. Primarily, the reproducible shape and small size is conducive for mechanical stimulation of the embedded cells without requiring a biopsy punch or other physical alteration to the hydrogel for many applications. Additionally, cell viability remains high during the layering process, and after gel formation a clear separation of the two cell populations within the gel is visible with no initial overlapping region.
Protocol
1. Preparation for Formation of Alginate Discs
Prepare a 4% (w/v) alginate solution in 1x Dulbecco's Phosphate Buffered Saline without added calcium chloride or magnesium chloride and place in a 37 °C water bath. Concentrations of the alginate solution can vary, but 1 - 4% (w/v) alginate solutions are recommended.
Mix the alginate solution at a ratio of 1:1 with warm cell culture media base (e.g., Dulbecco's Modified Eagle Medium) for the desired cell type. The alginate concentration is now half of the original concentration, i.e., 2% (w/v). Sterilize alginate/media solution using sterile 0.2 µm nylon syringe filters.
Prepare a bath of sterile 102 mM calcium chloride dihydrate in sterile water with enough solution to submerge molds (~ 200 - 300 ml).
- Collect the sterile "gel formation mold" (6 mm diameter x 3 mm tall cylindrical wells in a 3 in x 3 in aluminum plate, see Figure 1) and endplates (Bottom: one 3 in x 3 in aluminum plate, Top: one 1.5 in x 3 in aluminum plate) and prepare molds as follows:
- Cut thick filter paper (blotter filter paper) and the cell microsieve membrane (10 µm pore size) to the sizes of the top and bottom endplates and place them in the calcium chloride bath until saturated, approximately 1 min. If desired, sterilize the thick filter paper and the microsieve membrane via autoclave or ultraviolet light for 30 min prior to use.
- Set-up one half (the bottom half) of the mold construct.
- Place the following items atop one another in the following order and smoothen using a sterile spatula: large endplate (3 in x 3 in), thick filter paper, and then the microsieve membrane.
- Invert and press the mold onto a stack of paper towels to ensure loss of excess calcium chloride solution.
- Place the "gel formation mold" with the cylindrical wells on top of the cell microsieve membrane and gently fasten the mold together on two sides using binder clips on the left and right sides. Make sure to leave enough room for the top endplate (1.5 in x 3 in) to cover the wells completely later.
- Set-up the second half (the top half) of the mold construct.
- Place the following items atop one another in the following order and smoothen: small endplate, thick filter paper, microsieve membrane.
- Invert and press this half of the mold onto a stack of paper towels to ensure loss of excess calcium chloride solution. Do not fasten this half of the mold using binder clips at this time.
- Culture cells as recommended per manufacturer's instructions. Harvest the desired cells to embed in the layered alginate hydrogels. The recommended cell density is 1 - 2 x 106 cells/ml, but this can be varied depending on desired experiments. Note: When using this method for making layers with different cell types, make sure to culture two cell types in parallel for layering. Mesenchymal stem cells (MSCs) seeded at a cell density of 1 x 106 cells/ml will be used as an example for the following protocol. Cell number and disc number can be scaled for experiments as needed.
- One to two weeks prior to embedding, seed mesenchymal stem cells at a cell density of 3,000 - 5,000 cells/cm2 in 10 ml of basal growth media (Dulbecco's Modified Eagle Medium, 10% Fetal Bovine Serum, 2% L-Glutamine, 1% Non-essential Amino Acids, 1% Penicillin/Streptomycin) in T-75 flasks.
- Remove spent media every 2 - 3 days and replace with 10 ml of basal growth media until the cells are 80 - 90% confluent.
- To harvest cells, remove spent media, and pipette 3 ml of 1x Dulbecco's Phosphate Buffered Saline without added calcium chloride or magnesium chloride to rinse cells. Remove this solution, pipette 3 ml of 0.25% Trypsin-EDTA onto cells growing in T-75 flasks, and incubate for 5 min at 37 °C. After cells have lifted from the adherent surface, add 6 - 9 ml of basal growth media.
- Centrifuge MSCs at 600 x g for 5 min at room temperature, aspirate the supernatant, and re-suspend in 1 - 5 ml of basal growth media. Count the cells using a hemocytometer using device instructions.
- Remove 1 x 106 cells from the total cell resuspension, centrifuge using the same conditions, and aspirate the supernatant.
2. Formation of Cell Seeded Alginate Discs
Re-suspend the cell pellet with 1 ml of the sterile alginate/media solution to achieve a cell density of 1 x 106 cells/ml. The cell-alginate mixture will be homogenously cloudy in appearance when cells are mixed in appropriately.
Pipette 130 µl of the cell-alginate mixture into six 6 mm diameter x 3 mm tall wells in the bottom half of the mold construct dropwise, so as not to create any bubbles. A slight convex meniscus should be visible above the edge of each well.
Carefully smoothen the top half of the mold construct using a sterile spatula and turn it over, so that the cell microsieve membrane is on top of the wells. Place the mold construct on top of the wells, making sure to cover the wells containing the cell and alginate mixture completely.
Lift the loaded mold construct and, while pressing down firmly on the center, binder clip the remaining two sides (top and bottom) to fasten the top and bottom halves of the mold construct. The cell-alginate solutions should be securely nestled in the wells at this time.
Immerse the fastened mold construct in the 102 mM calcium chloride bath, making sure that the entire construct is submerged. Incubate in the cell culture hood at room temperature for 90 min.
At the end of the 90 min, remove the mold construct from the 102 mM calcium chloride bath and place on a stack of paper towels in the cell culture hood. Remove all four binder clips and separate the two halves of the mold construct. Hydrogels formed in the wells should not have any bubbles and should fill the wells completely.
Using a spatula, carefully trace the edge of the wells containing the hydrogels to carefully loosen and wedge out the hydrogels. After removing the hydrogels from the mold construct, drop the hydrogels directly into a bath of 1x Dulbecco's Phosphate Buffered Saline with calcium chloride and magnesium chloride. Cover the gels completely to wash off the excess calcium chloride solution for 1 - 5 min.
Transfer the hydrogels into basal growth media solution in desired dish (e.g., 6 well plates) that completely covers the hydrogels. Incubate for at least 1 hr in the cell culture incubator at 37 °C and 5% CO2 before continuing to the layering step. Note: Hydrogels containing cells can be kept in the cell culture incubator indefinitely at this time until layering of gels with another cell population is desired, provided that media changes are completed every 2 - 3 days.
3. Layering of Alginate Discs
- During the last half hour of the 1 hr incubation, collect and prepare sterile molds and solution for annealing the gels.
- Prepare the "cutting mold" by fastening a mold that has wells half of the height of the "gel formation mold" (6 mm diameter x 1.5 mm tall wells in a 3 in x 3 in aluminum plate) to an endplate (3 in x 3 in aluminum plate) using binder clips on the left and right sides.
- Prepare the "annealing mold" by fastening a mold that is 3 mm larger in diameter than the "gel formation mold" (9 mm diameter x 3 mm tall wells in a 3 in x 3 in aluminum plate) to an endplate (3 in x 3 in aluminum plate) using binder clips on the left and right sides.
- Prepare a solution of 100 mM sodium citrate/30 mM EDTA (sodium citrate dihydrate, ethylenediaminetetraacetic acid tetrasodium salt dihydrate) in sterile water.
Remove gels of desired cell populations (in this example, two discs with hMSCs) from the media in the dish, and place into the "cutting mold" wells. Each gel should fit snugly into the wells with half of the gel protruding above the mold.
Using a scalpel, slice the gel along the surface of the mold (this will cut the hydrogel in half). Flip the top half of the gel and place it into an open mold well. The half of the gel should also fit snugly into the mold well, but now both half gels should be the height of the mold with the cut inner surface visible. Repeat with second gel. Note: Warning: Only the inner cut surfaces will form layered discs. Using the outer surfaces will result in the halves separating. It is suspected that texture from the microsieve membrane transferred onto the gel surface prevents success of the annealing process.
Place a piece of dry cell microsieve membrane on top of the wells, making sure that the microsieve membrane is in contact with all of the gel halves to be annealed and covering them entirely. Place thick filter paper on top of the microsieve membrane, making sure to cover the gels completely.
Pipette a solution of 100 mM sodium citrate/30 mM EDTA (Sodium citrate dihydrate, Ethylenediaminetetraacetic acid tetrasodium salt dihydrate) onto the thick filter paper until it is saturated. Approximately 750 µl is sufficient for four wells.
Incubate the gels for 1 min at room temperature. Then, remove the cell microsieve membrane and thick filter paper and discard them. Remove the binder clips and open the mold.
For each annealed gel, transfer one sodium citrate/EDTA-treated half of the hydrogel to the prepared "annealing mold" with the cut surface facing upwards. This will be one half of the annealed construct.
Using a spatula, lift and place a second sodium citrate/EDTA-treated half-gel (may contain a different cell type) onto the gel already in the "annealing mold", flipping this second half-gel so that the cut surface is in contact with the cut surface of the gel already in the "annealing mold".
Press gently down on the two gels using a spatula to remove any bubbles between to the two gels. Also, reposition the gels as needed to make sure that they are directly on top of one another.
Gently lift the "annealing mold" and submerge it in the 102 mM calcium chloride bath for 30 min. Do not cover the wells with an endplate.
After the 30min incubation, remove the "annealing mold" and place it onto paper towels. Remove the binder clips and separate the mold from the endplate.
Using a spatula, collect the annealed gels and place them into a 1x Dulbecco's Phosphate Buffered Saline with calcium chloride and magnesium chloride bath to wash the gels. Subsequently, transfer annealed hydrogels to cell culture media in a desired dish (e.g., 6-well plate) for culture.
Representative Results
Figure 1 depicts the formation and layering of the alginate hydrogels. Completed bi-layered gels exhibit a complete initial separation of cell populations as shown in Figure 2. Cell viability of human mesenchymal stem cells) embedded within these hydrogels and layered remains high and comparable to the bulk hydrogels as shown in Figure 3. Viability was assessed after annealing, slicing the gels vertically to access the center and then staining the live cells in the annealed gels with a live cell tracker, CMFDA (green) and the dead cells (red) with Ethidium homodimer-1 using the manufacturer's instructions. Confocal Z-stacks were taken of the cut surface using a fluorescence microscope approximately 100 µm into the gel. These images were projected into one 2D image. Live and dead cells were then quantified using the particle analyzer feature in the ImageJ software.
We wanted to verify that these layered hydrogels, lacking cells, would be able to withstand cyclic compression, similar to that needed to induce a chondrogenic response. We found that the hydrogels do remain intact after seven days in culture and subsequent unconfined cyclic compression for 4 hr at 1 Hz from 0 - 10% strain. However, after isolating the peak stress from each cycle and analyzing the trends over the four-hour stimulation, the trends indicate that the layered hydrogels have a different response to the cyclic compression compared to the bulk, non-layered, hydrogels. Cyclic compression over four hours resulted in significantly different (p = 0.03) peak stress trends between layered and non-layered gels (Figure 4). Statistics were completed using the Student's T-test (alpha = 0.05).
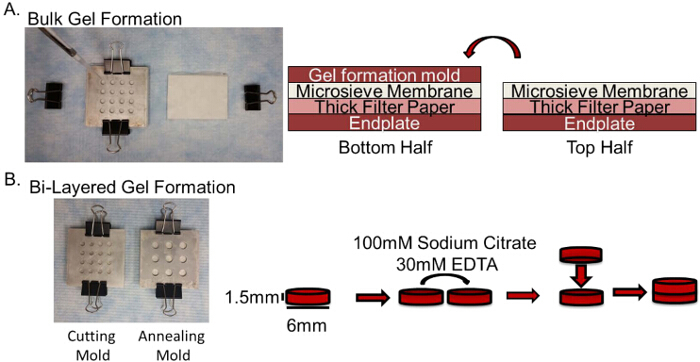 Figure 1:Schematic of Layered Hydrogel Formation. A. Image of the stacked mold for the addition of 2% alginate + cell mixture with a depiction of the stacking order for the bottom and top mold halves. B. Schematic depicting procedure for layering of the gel. Please click here to view a larger version of this figure.
Figure 1:Schematic of Layered Hydrogel Formation. A. Image of the stacked mold for the addition of 2% alginate + cell mixture with a depiction of the stacking order for the bottom and top mold halves. B. Schematic depicting procedure for layering of the gel. Please click here to view a larger version of this figure.
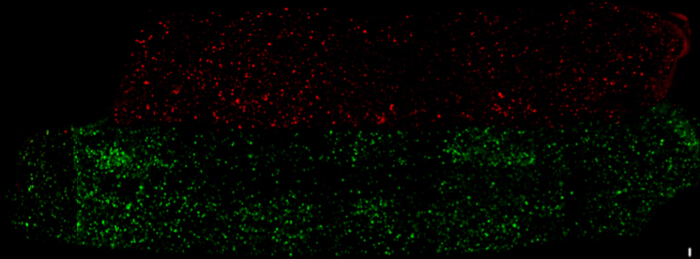 Figure 2: Representative Separation of Cell Populations in Layered Hydrogel. Model cell line 293FT HEK cells were either stained with live cell tracker CMFDA (green) or CMTPX (red). Each of these cell groups were embedded in 2% (w/v) alginate discs, and then halves of these discs were layered together. A piece was cut from the CMTPX side to identify it during imaging. Scale bar = 100 µm. Please click here to view a larger version of this figure.
Figure 2: Representative Separation of Cell Populations in Layered Hydrogel. Model cell line 293FT HEK cells were either stained with live cell tracker CMFDA (green) or CMTPX (red). Each of these cell groups were embedded in 2% (w/v) alginate discs, and then halves of these discs were layered together. A piece was cut from the CMTPX side to identify it during imaging. Scale bar = 100 µm. Please click here to view a larger version of this figure.
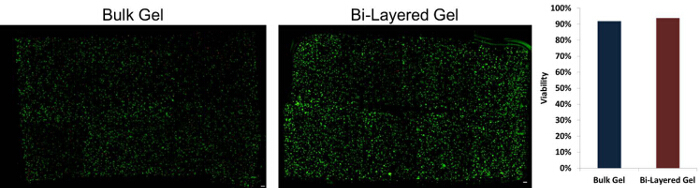 Figure 3:High Cell Viability within Layered Hydrogel after Layering Process is Complete. Human mesenchymal stem cells embedded in bulk and bi-layered hydrogels were stained with live cell (green) tracker CMFDA and dead cells (red) were stained with Ethidium homodimer-1. Viability remained high for both hydrogel groups after the layering process. Scale bar = 100 µm. Please click here to view a larger version of this figure.
Figure 3:High Cell Viability within Layered Hydrogel after Layering Process is Complete. Human mesenchymal stem cells embedded in bulk and bi-layered hydrogels were stained with live cell (green) tracker CMFDA and dead cells (red) were stained with Ethidium homodimer-1. Viability remained high for both hydrogel groups after the layering process. Scale bar = 100 µm. Please click here to view a larger version of this figure.
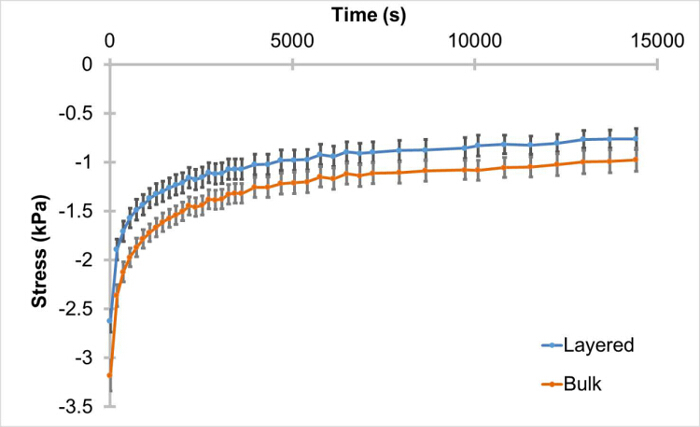 Figure 4:Significantly Different Trends of Layered Hydrogel Cyclic Compression Response to Cyclic Compression. Hydrogels were incubated in a cell culture incubator for seven days and subsequently subjected to 0 - 10% unconfined cyclic compression for four hours at 1 Hz. Peak stresses (± SEM) for each cycle were isolated and the trends over the loading period analyzed. Trends under unconfined cyclic compression from 0 - 10% strain for layered (n = 9) and bulk (n = 8) gels are significantly different (p = 0.03). Please click here to view a larger version of this figure.
Figure 4:Significantly Different Trends of Layered Hydrogel Cyclic Compression Response to Cyclic Compression. Hydrogels were incubated in a cell culture incubator for seven days and subsequently subjected to 0 - 10% unconfined cyclic compression for four hours at 1 Hz. Peak stresses (± SEM) for each cycle were isolated and the trends over the loading period analyzed. Trends under unconfined cyclic compression from 0 - 10% strain for layered (n = 9) and bulk (n = 8) gels are significantly different (p = 0.03). Please click here to view a larger version of this figure.
Discussion
Here, we describe a protocol for the formation of layered alginate hydrogel discs for studying co-cultures of multiple cell populations, such as those in physiologically layered tissues, e.g., cartilage. Layered structures, such as the described culture platform, can be used to examine the interplay between two distinct cell populations subjected to the same culture environment or under load.
Alginate is an anionic linear polysaccharide that has been found to be biocompatible and has been successfully used for cartilage biology and tissue engineering research 12,14. Alginate hydrogels are formed using divalent cations for crosslinking, e.g., calcium ions. These crosslinks can be undone by removing the cations using a chelator, such as sodium citrate or EDTA, and this process has been used previously to isolate chondrocytes that have been cultured in the gels 3,4,12. Similarly, the same principle has also been successfully applied for adhering alginate sheets in bilayers or even clusters of alginate beads together 11,12,15. The platform described here relies on this same process, but is used to form small disc-shaped layered hydrogels. This is a two-step process in which first, alginate discs are formed using molds submerged in a calcium-rich bath. These are then sliced in half and treated with a calcium chelating solution to locally release the calcium ions from the crosslinked alginate. By placing these treated surfaces together and re-immersing the discs in a calcium-rich solution, crosslinks are reformed, making bi-layered constructs. These small layered gels eliminate the need for biopsy punches to generate easy-to-culture samples compatible with assays and experiments such as mechanical testing, as shown in Figure 4, thereby minimizing waste of oftentimes precious cells as well as fabrication materials.
As shown in Figure 3, this process of forming and layering the hydrogels resulted in similarly high viability as the control or bulk hydrogels indicating that this procedure is not overly taxing to the cell population despite the length and absence of replenishing nutrients. The lack of an initial overlapping region in the gel allows physical separation during initial co-culture and the ability to observe changes to that interface over time. Studies have shown that over long culture periods this interface may lose definition due to cellular cross-infiltration and extracellular matrix deposition 11. While the initial layered disc does not contain any adhesion molecules, as extracellular matrix is deposited, there is also a potential for investigating the merging of cell populations and the changing interface between the layers.
These bi-layered discs can be used easily for dynamic compression stimulation. We were able to confirm that these hydrogels withstand dynamic compression without separation of the halves. However, during this process, peak load exhibited by the layered hydrogels during the four-hour cyclic compression were found to be significantly different from the non-layered, bulk, hydrogels. This result suggests complex, non-linear strain transfer between the layers and reveals a need for a physically layered control gel, e.g., bi-layered hydrogel with the same cell population/condition in each layer, an important detail to note when planning the experimental design for studies involving mechanical loading. Better understanding of the observed differences could be achieved through computational modeling of spatial mechanics within these gels. Not only would such analyses help clarify the strain distribution and transfer between the layers, but it would also be instrumental for interpreting cellular behavior in these layered gels, especially with layers of varying mechanical properties.
This is a versatile culture system that can be used for physical separation of cell populations during co-culture as described. Additionally, it can also be used as a base structure for further development. Changes to the existing structure could include increasing the number of layers, changing the sizes or the relative stiffness of the layers, and incorporating additional extracellular matrix components into one or more of the layers. However, these expansions will require careful evaluation. In addition to changes in the distribution of forces throughout the discs, increasing the size and number of layers could lead to decreases in cell viability in the center of the gels and physical instability during mechanical stimulation. Further, large additions of extracellular matrix and stimulating proteins or alterations to the alginate to provide adhesion moieties may interfere with gel formation and annealing processes.
This culture platform was developed for research applications for investigating the relationships between different cell populations in 3D culture. Thus, it is limited by its lack of scalability for clinical applications. Alternative methods for creating layered hydrogels, such as 3D printing, may ultimately be more clinically relevant due to anticipated future scalability advances, control over microstructure in the disc interior, and customizability of macroscale geometric features. For research applications, as presented here, the alginate discs do not independently provide adhesion moieties for cells, which could limit its application to other cells types. The comparable alternative to alginate when considering ease of use is agarose, which suffers from similar limitations. Further, it requires the use of enzymes to release cells for downstream analysis, while alginate crosslinks can be safely removed using calcium chelating agents. Primarily, this culture platform will help with improving the understanding of relationships between cell populations in hydrogels and potentially the effects of mechanical stimulation of these co-cultures. This understanding will then inform the development of therapies for heterogeneous tissues, especially loading bearing tissues such as articular cartilage or intervertebral discs.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was funded by the National Science Foundation (CBET 0845754, AHH).
References
- Sophia Fox AJ, Bedi A, Rodeo SA. The Basic Science of Articular Cartilage. Sports Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidlinger-Wilke C, et al. Mechanical loading of the intervertebral disc: from the macroscopic to the cellular level. Eur. Spine J. 2013;23(3):333–343. doi: 10.1007/s00586-013-2855-9. [DOI] [PubMed] [Google Scholar]
- Klein TJ, et al. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarth. Cartilage. 2003;11(8):595–602. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- Han E, et al. Scaffold-free Grafts for Articular Cartilage Defects. Clin. Orthop. Relat. R. 2008;466(8):1912–1920. doi: 10.1007/s11999-008-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KW, Ateshian GA, Hung CT. Zonal chondrocytes seeded in a layered agarose hydrogel create engineered cartilage with depth-dependent cellular and mechanical inhomogeneity. Tissue Eng. A. 2009;15(9):2315–2324. doi: 10.1089/ten.tea.2008.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KW, et al. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J. Orthop. Res. 2005;23(1):134–141. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Nguyen LH, Kudva AK, Saxena NS, Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32(29):6946–6952. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Leone G, et al. Continuous multilayered composite hydrogel as osteochondral substitute. J. Biomed. Mater. Res. A. 2015;103(8):2521–2530. doi: 10.1002/jbm.a.35389. [DOI] [PubMed] [Google Scholar]
- Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci. Rep. 2013;3 doi: 10.1038/srep03553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigfúsdòttir ÁT, Pasrija C, Thakore PI, Schmidt RB, Hsieh AH. Role of Pericellular Matrix in Mesenchymal Stem Cell Deformation during Chondrogenic Differentiation. Cell. Mol. Bioeng. 2010;3(4):387–397. [Google Scholar]
- Lee CSD, Gleghorn JP, Won Choi N, Cabodi M, Stroock AD, Bonassar LJ. Integration of layered chondrocyte-seeded alginate hydrogel scaffolds. Biomaterials. 2007;28(19):2987–2993. doi: 10.1016/j.biomaterials.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Gleghorn JP, Lee CSD, Cabodi M, Stroock AD, Bonassar LJ. Adhesive properties of laminated alginate gels for tissue engineering of layered structures. J. Biomed. Mater. Res. A. 2008;85(3):611–618. doi: 10.1002/jbm.a.31565. [DOI] [PubMed] [Google Scholar]
- Gharravi AM, Orazizadeh M, Ansari-Asl K, Banoni S, Izadi S, Hashemitabar M. Design and Fabrication of Anatomical Bioreactor Systems Containing Alginate Scaffolds for Cartilage Tissue Engineering. Avicenna J. Med. Biotechnol. 2012;4(2):65–74. [PMC free article] [PubMed] [Google Scholar]
- Xu J, et al. Chondrogenic Differentiation of Human Mesenchymal Stem Cells in Three-Dimensional Alginate Gels. Tissue Eng. A. 2008;14(5):667–680. doi: 10.1089/tea.2007.0272. [DOI] [PubMed] [Google Scholar]
- Yeatts AB, Gordon CN, Fisher JP. Formation of an aggregated alginate construct in a tubular perfusion system. Tissue Eng. C, Methods. 2011;17(12):1171–1178. doi: 10.1089/ten.tec.2011.0263. [DOI] [PubMed] [Google Scholar]


