Abstract
The structure of a partially deuterated rubredoxin from the hyperthermophilic archaeon Pyrococcus furiosus, an organism that grows optimally at 100°C, was determined by using the neutron single-crystal diffractometer dedicated for biological macromolecules (BIX-3) at the JRR-3M reactor of the Japan Atomic Energy Research Institute. Data were collected at room temperature up to a resolution of 1.5 Å, and the completeness factor of the data set was 81.9%. The model contains 306 H and 50 D atoms. A total of 37 hydration water molecules were identified, with 15 having all three atoms fully located and the remaining D2O molecules partially defined. The model has been refined to final agreement factors of R = 18.6% and Rfree = 21.7%. Several orientations of the O–D bonds of side chains, whose assignments from x-ray data were previously ambiguous, were clearly visible in the neutron structure. Although most backbone N–H bonds had undergone some degree of H/D exchange throughout the rubredoxin molecule, 5 H atom positions still had distinctly negative (H) peaks. The neutron Fourier maps clearly showed the details of an extensive set of H bonds involving the ND3+ terminus that may contribute to the unusual thermostability of this molecule.
It is beyond doubt that H atoms and hydration water molecules of proteins play an important role in biological systems. They are indispensable components in many naturally occurring processes (e.g., hydrolysis, dehydrogenation, etc.). In addition, H atoms are involved in the folding and stabilization of protein structure by means of their participation in H bonding. Neutron crystallography can readily determine H positions. Moreover, neutron diffraction can determine whether H/D exchange has occurred at a particular site because H and D have dramatically different neutron-scattering lengths (negative and positive, respectively). Thus, x-ray and neutron diffraction methods are complementary, and between the two techniques, it is possible in principle to determine all of the atomic positions in a protein (1, 2).
We have developed an elastically bent perfect-Si crystal monochromator (3–5) that serves to focus the reflected neutrons onto the sample position, thus increasing the available intensity. In addition, we have developed a neutron-imaging plate (6–11), which is a key component in our detector system, covering a large area with a high signal-to-noise ratio, high spatial accuracy, and good neutron-detection efficiency. Several studies in which our neutron-imaging plate detectors were used in neutron crystallography have been reported (12–18), including contributions from other groups who have adopted portions of our design (13, 14, 16–18). All of these features have been incorporated in a neutron-imaging-plate-based diffractometer called BIX-3 (19–21) that has been built and installed at the JRR-3M reactor of the Japan Atomic Energy Research Institute. The present study on rubredoxin was performed by using this diffractometer (22, 23), and subsequent studies with sperm whale myoglobin (to a 1.5-Å resolution) (22, 24, 25) and a rubredoxin mutant (to a 1.6-Å resolution) (26, 27) have followed. A preliminary account of the work described in this article appeared in ref. 23.
Rubredoxins occur in several anaerobic bacteria and are small proteins containing an iron atom coordinated by the S atoms of four cysteine residues. They have been proposed to play a role in electron transfer as part of a novel pathway for O detoxification (28). They were among the first proteins to be studied crystallographically at high resolution. Pioneering work by Watenpaugh et al. (29, 30) in 1972 described the structure analysis of rubredoxin from Clostridium pasteurianum. That analysis, carried out at a resolution of 1.5 Å, featured the first report of a least-squares refinement of a protein at a truly atomic level. The structural analysis of rubredoxin from C. pasteurianum was eventually extended to 1.2-Å resolution (31) and 1.1-Å resolution (32), and other rubredoxin structures have been reported over the years from other mesophilic (room temperature) organisms (33–39). The refinement of one of these, from Desulfovibrio vulgaris, has been extended to a 1.0-Å resolution (38), and that data set was used in a study describing the application of direct methods to protein crystallography (39). All of those studies indicated that rubredoxins contain a very small three-stranded antiparallel β-sheet, a hydrophobic core, and several loops (29–39).
Pyrococcus furiosus is an anaerobic S-reducing archaeon exhibiting optimal growth near 100°C. The x-ray (resolution 1.8 Å) and NMR structures of the rubredoxin purified from this organism were originally determined in 1992 (40–43). Subsequently, higher-resolution x-ray diffraction analyses of the WT protein (resolution 0.95 Å) and of two site-directed mutants (resolutions 1.1 and 1.2 Å) were performed to understand the differences in thermostability between the different forms (44). The overall folding of the rubredoxins from P. furiosus turned out to be very similar to those of the mesophilic microorganisms. In the 0.95-Å x-ray study of the WT hyperthermophilic rubredoxin, about half of the H atoms could be clearly seen in the difference maps because of the high resolution of the data (44). However, no unambiguous correlation between the structures of those proteins and their thermostabilities could be found.
The present study originally was motivated by the hope that the differences in thermostability might be explained by using the more accurate parameters of H bonds obtained by neutron crystallography. The present neutron diffraction study on WT rubredoxin from P. furiosus (Pf Rd) is the starting point for a structural comparison with its mutants (26, 27), which have different thermostabilities from that of WT protein.
Methods
Crystallization. The construction of the gene encoding Pf Rd, its expression in Escherichia coli, and the isolation of the recombinant rubredoxin are as described in refs. 45 and 46. Crystals of WT protein were grown by the sitting-drop vapor diffusion method in conjunction with microseeding techniques. The rubredoxin solution used in the crystallization experiments contained 40 mg/ml–1 protein, 50 mM Tris/Tris·HCl buffer (pH 8.0), and 0.3 M NaCl. The reservoir contained 3.8 M NaK phosphate (equimolar NaH2PO4 plus K2HPO4) as precipitant. These conditions are similar to those described in ref. 44. To reduce background scattering from H atoms, which have a large incoherent scattering cross section, the rubredoxin solution was subjected to H2O/D2O exchange before crystallization. This method allows D atoms to be substituted for H atoms, not only in the hydration water molecules but also at sites containing “exchangeable” H atoms (mostly H atoms of N–H and O–H bonds exposed to the solvent). To collect high-resolution diffraction data, a single crystal with approximate dimensions of 2.5 × 2.5 × 0.8 mm was mounted in a sealed quartz capillary 3 mm in diameter.
Data Collection and Refinement. Diffraction data were collected at room temperature on the BIX-3 diffractometer (19–21) installed on the 1G-A site of the JRR-3M reactor at the Japan Atomic Energy Research Institute. Data collection was carried out by using the step-scan method, with 0.3° intervals in ϕ and exposure times ranging from 60 to 77 min per frame. The net time required to collect the total of 717 data frames was 35 days. From the rubredoxin crystal, diffraction data up to a resolution of 1.5 Å were recorded with the present experimental arrangement. The spots were integrated, scaled, and merged by using the programs denzo and scalepack (47). The detailed statistics of data collection and reduction are listed in Table 1.
Table 1. Data-collection and refinement statistics.
| Data collection | |
| Space group | P212121 |
| Unit-cell parameters a, b, and c, Å | 34.32, 35.31, 44.23 |
| Maximum resolution, Å | 1.5 |
| Highest-resolution shell, Å | 1.55-1.5 |
| No. observed reflections | 24,462 |
| No. unique reflections | 7,417 |
| Completeness,* % | 81.9 (53.1) |
| Rmerge,* % | 9.6 (23.2) |
| Average I/σ(I)* | 8.5 (3.1) |
| Crystallographic refinement | |
| Resolution range, Å | 30-1.5 |
| R value with F > 2 σF cutoff, % | 18.6 |
| Rfree with F > 2 σF cutoff,† % | 21.7 |
| rms deviation bond lengths, Å | 0.010 |
| rms deviation bond angles, ° | 1.242 |
| No. hydration water molecules | 37 |
| No. identified H atoms | 306 |
| No. identified D atoms | 50 |
Numbers in parentheses are for the highest-resolution shells.
Rfree calculated with 10% of reflections that were not used for refinement.
Refinement of the structure was carried out by using the programs x-plor (48) and cns (49), in which the topology and parameter files were specially modified for neutron crystallography by adding the necessary parameters for H and D atoms. Model building was performed by using the programs turbo-frodo (50) and xtalview (51). At the end of the refinement, the R and Rfree values were 18.6% and 21.7%, respectively. A total of 306 H and 50 D atoms were included in the refinement of the structure, and a total of 37 hydration water molecules were identified. The rms deviations of our neutron structure from an ideal geometry are 0.01 Å for the bond lengths and 1.2° for the bond angles. When the neutron model is compared with the x-ray structure (PDB ID code 1BRF), the overall rms deviations of main-chain and side-chain atoms are 0.19 Å and 0.75 Å, respectively (with the disordered residues Glu-52 and Asp-53 excluded from the calculation). Refinement statistics are also given in Table 1. Coordinate and diffraction data from the present analysis have been deposited in the Protein Data Bank (PDB ID code 1VCX).
Results and Discussion
H and D Atoms of the OH Groups. Usually, the H/D atoms in a protein structure (especially those of C–H and N–H bonds) can be located at predictable positions, based on the coordinates of the known C, N, and O atoms. However, for Ser and Thr residues, there is a threefold ambiguity in the orientation of the O–D bonds, and for Tyr residues, there are two possibilities in the O–D bond orientation. Although these orientations usually cannot be determined by the x-ray technique, they can be readily established by using neutron crystallography.
For example, the O–D bond of Tyr-12 is clearly coplanar with the aromatic ring (Fig. 1a) and is oriented toward an O atom of a neighboring residue (data not shown). In addition, because the resolution of the present data set is quite high (1.5 Å), the nuclear density for the phenyl ring in Fig. 1a shows a clear hole at its center, a feature usually not evident in 2.0-Å-resolution maps.
Fig. 1.
H and D atoms of the OH groups obtained from the neutron analysis. (a) Fo – Fc omit map of Tyr-12; blue contours are positive at 3.5σ, and red contours are negative at –3.5σ. Note that the O–H bond has been deuterated but the C–H bonds have not. Also note the hole in the middle of the aromatic ring, indicating the high resolution of this neutron data set. (b) Fo – Fc omit map of the side chain of Ser-24 and neighboring two waters (blue contours at 3.5σ; red contours at –3.0σ). This diagram shows how the O–D bond of serine (whose orientation often cannot be determined in an x-ray study) is clearly visible and is in a trans configuration relative to one of the hydrogens of the adjacent CH2 group. Orange dotted lines indicate H bonds.
In the case of Ser-24, it can clearly be seen from Fig. 1b that the O–D hydroxyl group is in a staggered conformation around the C–O bond and is in a trans orientation with respect to one of the C–H bonds of the neighboring CH2 group. Furthermore, it can be seen that the O(γ) atom of Ser-24 is serving as a H-bond donor to water #1 and is a H-bond acceptor to water #2, conclusions that would not have been obtainable from the x-ray data. The important point is that the high resolution of the neutron data enables an unprecedented level of detail to be revealed in this protein structure.
H/D Exchange. The rubredoxin solution used in this experiment was subjected to H2O/D2O exchange before the growth of crystals. Of a total of 74 H/D atoms at potentially exchangeable sites, 24 atoms do not have significant positive (D) peaks at the expected positions. Of those, 11 atoms are bonded to N atoms of the main chain, implying that those positions are not fully accessible to the H/D exchange process. In particular, five have prominent negative (H) peaks. Fig. 2a shows a Fo – Fc omit map around some of those H atoms, calculated without contributions from any H and D atoms bonded to the main-chain N atoms. Of those five, Val-4 H, Cys-5 H, and Tyr-12 H are located at the β-sheet (Fig. 2a), whereas Cys-38 H and Ala-43 H (as well as Val-4 H and Cys-5 H) are near the region of the iron site (data not shown). These all form H bonds with neighboring O atoms of the main chain. In Fig. 2a, negative densities (red contour lines) can be clearly seen at those H atom positions, whereas the D positions have positive densities (blue contour lines). Those five H atoms (with red contours) remained, even after exposure to D2O, presumably because of their poor solvent accessibility.
Fig. 2.
H/D exchange. (a) Fo – Fc omit map in the β-sheet region (blue contours at 4.0σ; red contours at –3.5σ). The red (negative) and blue (positive) contours correspond to H and D atoms bonded to the main-chain N atoms in this region. The title rubredoxin molecule had been subjected to H/D exchange before crystallization, resulting in most of the backbone amide bonds being deuterated. This plot shows three of the five H atoms that have not experienced significant H/D exchange. (b) The values of the neutron H/D exchange ratios (Top) and B factors (Middle) of main chain H/D atoms. Also shown are the H/D exchange rates derived from the NMR results (Bottom) (data taken from ref. 53). (Top) The red dots correspond to atoms that have resisted H/D exchange (i.e., atoms that are still mostly H), according to the neutron results.
To obtain quantitative information about the distribution of H/D populations in this rubredoxin molecule, the occupancies of the H and D atoms bonded to the main-chain N atoms were refined. The result is shown in Fig. 2b (Top). The five atoms mentioned above have values close to zero for the H/D exchange ratio (i.e., they have remained largely H). Compared with the distribution of B factors of the main-chain atoms, it is seen that these five H/D atoms having small H/D exchange ratios also have small B factor values (Fig. 2b Middle). The distribution of accessible surface areas of the main-chain atoms (data not shown) also indicates the same trends. These results suggest that the regions around those atoms are relatively inaccessible to solvent.
The conclusion from these H/D exchange ratio measurements is roughly comparable with the results from NMR studies (52, 53), which indicate that there are two broad regions in the protein that display slow NMR H/D exchange behavior, one centered around the Cys-5/Cys-8 region and the other around Cys-38/Cys-41 (Fig. 2b Bottom). In fact, the set of backbone N–H bonds that indicate the slowest NMR exchange behavior (Trp-3, Val-4, Cys-5, Ile-7, Cys-8, Tyr-10, Tyr-12, Cys-38, Ile-40, Cys-41, and Ala-43) include the five resistant N–H bonds that we have identified from our neutron data. Thus, there is very rough agreement between the NMR and neutron results, even though, strictly speaking, the two techniques are not directly comparable: NMR measures the dynamic behavior of a protein in solution, whereas neutron diffraction results reflect a static situation (i.e., a snapshot “frozen in time”).
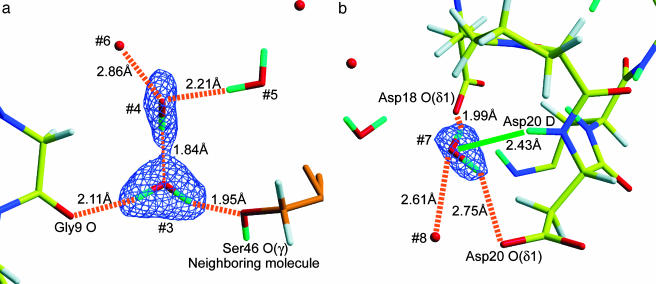
Hydration Water Molecules. A total of 37 hydration water molecules were found in the rubredoxin structure. Of those, 15 could be oriented unambiguously because the densities for those water molecules have a triangular shape, which allows all three atomic positions in each molecule (D, O, D) to be assigned. These represent the best-ordered solvent molecules, whereas the shapes of the other solvent peaks revealed additional information about the degree of order of each hydration water molecule. We have discussed the topic of shapes of water molecules derived from neutron data in great detail in a recent article (54).
Fig. 3a shows some examples of these shapes: a triangular contour that is characteristic of a well ordered D2O molecule (peak #3) and a stick-shaped (ellipsoidal) contour (peak #4) that is characteristic of a partially ordered water molecule. In this case, it can be concluded that the D–O portion of water #4 is ordered, whereas the third atom (D) is rotationally disordered and unobserved (Fig. 3a). The two ends of this O–D fragment (cyan/red stick) can be assigned by an inspection of its neighbors: the portion close (1.84 Å) to a neighboring O atom must be D (cyan portion of peak #4), whereas the other end, which is close (2.21 Å) to another D atom (from water #5), can be assigned as O (red part of peak #4).
Fig. 3.
Examples of the hydration water molecules. (a) Fo – Fc omit map (at 4.0σ) of the hydration water molecules near the residues Gly-9 and Ser-46 of a neighboring molecule. Note the presence of two types of water molecules. Water #3 has a triangular shape and is fully ordered, whereas water #4 has an ellipsoidal shape and is only partially ordered with only one D atom (cyan) and one O atom (red) visible. The third atom (D) of water #4 is rotationally disordered and is not observable in this map. (b) Fo – Fc omit map at 4.0σ level of the hydration water molecule (#7) near Asp-18 and Asp-20. The green line corresponds to a previously suspected H bond whose existence has been disproved on the basis of neutron data (see text). The dotted lines indicate H bonds.
Finally, in Fig. 3b, which shows the region around the residues Asp-18 and Asp-20, we have an interesting case in which the neutron diffraction results can be used to refute an assignment based on x-ray data. Without prior information about H/D positions, it originally appeared (from the x-ray data) that the O atom of the water molecule labeled #7 is within H-bonding distance of three atoms: Asp-18 O(δ1), Asp-20 O(δ1), and Asp-20 D (Fig. 3b). The neutron map confirms the first two assignments (orange dotted lines), but the third assignment is now in doubt: the orientation of the triangular D2O peak makes it clear that the proposed N–D... O hydrogen bond to water #7 (green line in Fig. 3b) would result in a D(Asp-20)... O(#7)–D(#7) angle that would be too acute to be reasonable. Instead, the O atom of water #7 was found to be a H-bond acceptor to water #8 (Fig. 3b).
Other investigators have found that around surface regions of a protein that contain hydrophobic residues, or “hydrophobic patches,” the water molecules generally avoid those protein surfaces and instead make extensive H bonds between themselves (55). We have searched for these characteristic features around Pf Rd without success: Our protein molecule is apparently too small to contain large “patches” of hydrophobic residues.
Fe–S Core and N–H ... S Hydrogen Bonds. Like the rubredoxins from mesophilic bacteria, the Fe atom of Pf Rd has a tetrahedral array of four cysteinyl S atoms as ligands (41, 44). The Fe–S bond distances in this part of Pf Rd have been already reported in the x-ray analysis, and several N–H...S hydrogen bonds involving backbone N–H groups also have been assigned, according to the distances between N and S atoms (41, 44). From the x-ray data, N...Sdistances in the range 3.4–3.8 Å were used as a criterion to identify the probable existence of N–H...S hydrogen bonds. Nine of these were identified (Table 2). However, from the neutron results, only five of them could be confirmed (labeled with asterisks in Table 2 and with dotted lines in Fig. 4): two to Cys-5 S(γ) from Ile-7 D and Cys-8 D; two to Cys-38 S(γ) from Ile-40 D and Cys-41 D; and one to Cys-41 S(γ) from Ala-43 H. Three proposed (44) N–D...S hydrogen bonds could be refuted: although the N...S distances between Lys-6 N and Cys-5 S(γ), between Gly-9 N and Cys-8 S(γ), and between Gly-42 N and Cys-41 S(γ) are reasonable, the corresponding D...S distances are so large that those pairs cannot be assigned as H bonds (Table 2). Fig. 4 shows a Fo – Fc omit map around the Fe–S core, calculated without contributions from the D(H) and S atoms, which participate in the N–D(H)...S hydrogen bonds. Finally, one assignment was inconclusive: neither negative nor positive peaks could be found at the amide H/D atom position of Tyr-10 (Fig. 4, bottom center), probably because cancellation of the scattering amplitudes occurs by a superposition of the H and D atom positions (this phenomenon happens whenever the ratio of H:D occupancies is about 2:1). Thus, the potential H bond between Tyr-10 D/H and Cys-8 S(γ) could neither be confirmed nor refuted in this study.
Table 2. Distances near the FeS4 core (Å) and potential N-H... S hydrogen bonds.
| Lys-6 | N | 3.81 | Cys-5 | S(γ) |
| Lys-6 | D | 3.87 | Cys-5 | S(γ) |
| Ile-7 | N | 3.75 | Cys-5 | S(γ) |
| Ile-7 | D | 2.94* | Cys-5 | S(γ) |
| Cys-8 | N | 3.75 | Cys-5 | S(γ) |
| Cys-8 | D | 2.86* | Cys-5 | S(γ) |
| Gly-9 | N | 3.58 | Cys-8 | S(γ) |
| Gly-9 | D | 3.72 | Cys-8 | S(γ) |
| Tyr-10 | N | 3.51 | Cys-8 | S(γ) |
| Tyr-10 | H/D | — | Cys-8 | S(γ) |
| Ile-40 | N | 3.59 | Cys-38 | S(γ) |
| Ile-40 | D | 2.76* | Cys-38 | S(γ) |
| Cys-41 | N | 3.57 | Cys-38 | S(γ) |
| Cys-41 | D | 2.62* | Cys-38 | S(γ) |
| Gly-42 | N | 3.54 | Cys-41 | S(γ) |
| Gly-42 | D | 3.67 | Cys-41 | S(γ) |
| Ala-43 | N | 3.46 | Cys-41 | S(γ) |
| Ala-43 | H | 2.52* | Cys-41 | S(γ) |
H-bonding distance as confirmed by the present neutron analysis.
Fig. 4.
Fo – Fc omit map around the Fe–S core (blue contours at 3.5σ; red contours at –3.0σ), calculated without contributions from the S and D atoms of the neighboring backbone N–D bonds. The orange dotted lines indicate the presence of N–D(H)...S hydrogen bonds, as revealed by the neutron analysis. Note that there is no evidence that the S atoms are protonated (or deuterated) and that the S atoms actually appear smaller than the D atoms because of the weaker neutron-scattering amplitude of S.
The possible involvement of N–H... S hydrogen bonds in influencing the redox potentials of Fe–S proteins has been actively debated in the literature (56, 57). This study has determined the precise geometries of such bonds at high resolution in a neutron diffraction investigation. In addition, from the neutron study we could find no evidence that any of the four S atoms were protonated.
N-Terminal Region/Thermostability of Pf Rd. Many structural investigations about the high thermostability of Pf Rd have been conducted by using NMR and crystallographic methods (40–44). By comparison with other rubredoxins from mesophilic species, in those studies it is often concluded that the interactions near the N-terminal residues of the protein contributes significantly to the rubredoxin hyperthermostability (40–42). This region (Fig. 5) contains a fairly extensive network of H bonds starting from (i) the backbone N–H bond of Phe-29 and the heterocyclic N–H bond of Trp 3, proceeding through (ii) the O(ε2) and O(ε1) carboxylate oxygens of Glu-14, to (iii) two of the D atoms of the terminal ND3+ group, to (iv) the O–D atoms of a bridging D2O molecule, and finally to (v) the O(δ1) atom of Asp-13. Almost all of the D atoms involved in this elaborate chain of H bonds can be found in Fig. 5, which again shows the remarkable level of detail that can be displayed in a high-resolution neutron Fourier map. It has been speculated that the presence of the Glu-14 residue, which is not present in mesophilic rubredoxins, may partially account for the high thermostability of Pf Rd by H bonding to (and thus “tying down”) the ND3+ terminus (40). If this hypothesis is true, then Fig. 5 provides a clear and detailed picture of this region.
Fig. 5.
Fo – Fc omit map showing the “arc” of H bonds around the N-terminal region of WT rubredoxin. Contouring level is 3.0σ, and H bonds are shown as dotted lines. Note that the high resolution of this map allows all three D atoms of this group to be resolved.
Conclusion
From the neutron crystallography by using the single-crystal diffractometer BIX-3 at the JRR-3M reactor of the Japan Atomic Energy Research Institute, the high-resolution structure of WT Pf Rd was obtained, including most of the H/D positions. The actual information from these H/D positions gave additional details about the H bonding in the molecules. We have shown in this study that neutron diffraction is able to resolve some ambiguous assignments of H bonds from an earlier x-ray investigation and that the identification of H bond donors and acceptors has become possible in many cases. In addition, the details of the H-bonding interactions, especially in the N-terminal region (as well as those in the Fe–S4 and backbone regions), have revealed additional information that could contribute to the hyperthermostability of this unusual and interesting protein.
Acknowledgments
We thank Dr. Yoshiaki Minezaki for extensive contributions to this investigation and Dr. Andreas Ostermann for suggestions and discussions about the protein crystallography. This work was supported in part by an Organized Research Combination System grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan; National Science Foundation Grant CHE-98-16294 (to R.B. and N.M.); American Chemical Society Grant PRF-40715-AC3 (to R.B. and N.M.); Department of Energy Grant FG05-95ER20175 (to M.W.W.A. and F.E.J.); and National Institutes of Health Grant GM-60329 (to M.W.W.A. and F.E.J.).
Abbreviation: Pf Rd, rubredoxin from Pyrococcus furiosus.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 1VCX).
References
- 1.Niimura, N. (1999) Curr. Opin. Struct. Biol. 9, 602–608. [DOI] [PubMed] [Google Scholar]
- 2.Tsyba, I. & Bau, R. (2002) Chemtracts 15, 233–257. [Google Scholar]
- 3.Niimura, N., Tanaka, I., Karasawa, Y. & Minakawa, N. (1995) Physica B 213–214, 929–931. [Google Scholar]
- 4.Tanaka, I., Minezaki, Y., Harada, K. & Niimura, N. (1997) Physica B 241–243, 227–230. [Google Scholar]
- 5.Tanaka, I., Niimura, N. & Mikula, P. (1999) J. Appl. Crystallogr. 32, 525–529. [Google Scholar]
- 6.Niimura, N., Karasawa, Y., Tanaka, I., Miyahara, J., Akahashi, K., Saito, H., Koizumi, S. & Hidaka, M. (1994) Nucl. Instr. Methods Phys. Res. A 349, 521–525. [Google Scholar]
- 7.Karasawa, Y., Niimura, N., Tanaka, I., Miyahara, J., Takahashi, K., Saito, H., Tsuruno, A. & Matsubayashi, M. (1995) Physica B 213–214, 978–981. [Google Scholar]
- 8.Takahashi, K., Tazaki, S., Miyahara, J., Karasawa, Y. & Niimura, N. (1996) Nucl. Instr. Methods Phys. Res. A 377, 119–122. [Google Scholar]
- 9.Karasawa, Y., Kumazawa, S. & Niimura, N. (1997) Physica B 241–243, 139–141. [Google Scholar]
- 10.Tazaki, S., Neriishi, K., Takahashi, K., Etoh, M., Karasawa, Y., Kumazawa, S. & Niimura, N. (1999) Nucl. Instr. Methods Phys. Res. A 424, 20–25. [Google Scholar]
- 11.Haga, Y. K., Kumazawa, S. & Niimura, N. (1999) J. Phys. Chem. Solids 60, 1619–1621. [Google Scholar]
- 12.Niimura, N., Minezaki, Y., Nonaka, T., Castagna, J. C., Cipriani, F., Hoghoj, P., Lehmann, M. S. & Wilkinson, C. (1997) Nat. Struct. Biol. 4, 909–914. [DOI] [PubMed] [Google Scholar]
- 13.Langan, P., Lehmann, M., Wilkinson, C., Jogl, G. & Kratky, C. (1999) Acta Crystallogr. D 55, 51–59. [DOI] [PubMed] [Google Scholar]
- 14.Bon, C., Lehmann, M. S. & Wilkinson, C. (1999) Acta Crystallogr. D 55, 978–987. [DOI] [PubMed] [Google Scholar]
- 15.Minezaki, Y., Nonaka, T. & Niimura, N. (1999) J. Phys. Chem. Solids 60, 1387–1391. [Google Scholar]
- 16.Cooper, J. B. & Myles, D. A. (2000) Acta Crystallogr. D 56, 246–248. [DOI] [PubMed] [Google Scholar]
- 17.Habash, J., Raftery, J., Nuttall, R., Price, H. J., Wilkinson, C., Kalb, A. J. & Helliwell, J. R. (2000) Acta Crystallogr. D 56, 541–550. [DOI] [PubMed] [Google Scholar]
- 18.Coates, L., Erskine, P. T., Wood, S. P., Myles, D. A. & Cooper, J. B. (2001) Biochemistry 40, 13149–13157. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka, I., Kurihara, K., Haga, Y., Minezaki, Y., Fujiwara, S., Kumazawa, S. & Niimura, N. (1999) J. Phys. Chem. Solids 60, 1623–1626. [Google Scholar]
- 20.Tanaka, I., Kurihara, K., Chatake, T. & Niimura, N. (2001) J. Phys. Soc. Jpn. Suppl. A 70, 459–461. [Google Scholar]
- 21.Tanaka, I., Kurihara, K., Chatake, T. & Niimura, N. (2002) J. Appl. Crystallogr. 35, 34–40. [Google Scholar]
- 22.Niimura, N. (2001) J. Phys. Soc. Jpn. Suppl. A 70, 396–399. [Google Scholar]
- 23.Kurihara, K., Tanaka, I., Adams, M. W. W., Jenney, F. E., Jr., Moiseeva, N., Bau, R. & Niimura, N. (2001) J. Phys. Soc. Jpn. Suppl. A 70, 400–402. [Google Scholar]
- 24.Ostermann, A., Tanaka, I., Engler, N., Niimura, N. & Parak, F. G. (2002) Biophys. Chem. 95, 183–193. [DOI] [PubMed] [Google Scholar]
- 25.Engler, N., Ostermann, A., Niimura, N. & Parak, F. G. (2003) Proc. Natl. Acad. Sci. USA 100, 10243–10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatake, T., Kurihara, K., Tanaka, I., Adams, M. W. W., Jenney, F. E., Jr., Tsyba, I., Bau, R. & Niimura, N. (2002) Appl. Phys. 74, Suppl., S1280–S1282. [Google Scholar]
- 27.Chatake, T., Kurihara, K., Tanaka, I., Tsyba, I., Bau, R. Jenney, F. E., Jr., Adams, M. W. W. & Niimura, N. (2003) Acta Crystallogr. D, in press. [DOI] [PubMed]
- 28.Jenney, F. E., Jr., Verhagen, M. F. J. M., Cui, X. & Adams, M. W. W. (1999) Science 286, 306–309. [DOI] [PubMed] [Google Scholar]
- 29.Watenpaugh, K. D., Sieker, L. C., Herriott, J. R. & Jensen, L. H. (1972) Cold Spring Harbor Symp. Quant. Biol. 36, 359–367. [DOI] [PubMed] [Google Scholar]
- 30.Watenpaugh, K. D., Sieker, L. C., Herriott, J. R. & Jensen, L. H. (1973) Acta Crystallogr. B 29, 943–956. [Google Scholar]
- 31.Watenpaugh, K. D., Sieker, L. C. & Jensen, L. H. (1979) J. Mol. Biol. 131, 509–522. [DOI] [PubMed] [Google Scholar]
- 32.Dauter, Z., Wilson, K. S., Sieker, L. C., Moulis, J. M. & Meyer, J. (1996) Proc. Natl. Acad. Sci. USA 93, 8836–8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frey, M., Sieker, L., Payan, F., Haser, R., Bruschi, M., Pepe, G. & LeGall, J. (1987) J. Mol. Biol. 197, 525–541. [DOI] [PubMed] [Google Scholar]
- 34.Sieker, L. C., Stenkamp, R. E., Jensen, L. H., Prickril, B. & LeGall, J. (1986) FEBS Lett. 208, 73–76. [DOI] [PubMed] [Google Scholar]
- 35.Stenkamp, R. E., Sieker, L. C. & Jensen, L. H. (1990) Proteins Struct. Funct. Genet. 8, 252–264. [DOI] [PubMed] [Google Scholar]
- 36.Adman, E. T., Sieker, L. C., Jensen, L. H., Bruschi, M. & LeGall, J. (1977) J. Mol. Biol. 112, 113–120. [DOI] [PubMed] [Google Scholar]
- 37.Adman, E. T., Sieker, L. C. & Jensen, L. H. (1991) J. Mol. Biol. 217, 337–352. [DOI] [PubMed] [Google Scholar]
- 38.Dauter, Z., Sieker, L. C. & Wilson, K. S. (1992) Acta Crystallogr. B 48, 42–59. [DOI] [PubMed] [Google Scholar]
- 39.Sheldrick, G. M., Dauter, Z., Wilson, K. S., Hope, H. & Sieker, L. C. (1993) Acta Crystallogr. D 49, 18–23. [DOI] [PubMed] [Google Scholar]
- 40.Blake, P. R., Park, J. B., Bryant, F. O., Aono, S., Magnuson, J. K., Eccleston, E., Howard, J. B., Summers, M. F. & Adams, M. W. W. (1991) Biochemistry 30, 10885–10895. [DOI] [PubMed] [Google Scholar]
- 41.Day, M. W., Hsu, B. T., Joshua-Tor, L., Park, J. B., Zhou, Z. H., Adams, M. W. W. & Rees, D. C. (1992) Protein Sci. 1, 1494–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blake, P. R., Park, J. B., Zhou, Z. H., Hare, D. R., Adams, M. W. W. & Summers, M. F. (1992) Protein Sci. 1, 1508–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blake, P. R., Day, M. W., Hsu, B. T., Joshua-Tor, L., Park, J. B., Hare, D. R., Adams, M. W. W., Rees, D. C. & Summers, M. F. (1992) Protein Sci. 1, 1522–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bau, R., Rees, D. C., Kurtz, D. M., Jr., Scott, R. A., Huang, H., Adams, M. W. W. & Eidsness, M. K. (1998) J. Biol. Inorg. Chem. 3, 484–493. [Google Scholar]
- 45.Eidsness, M. K., Richie, K. A., Burden, A. E., Kurtz, D. M., Jr., & Scott, R. A. (1997) Biochemistry 36, 10406–10413. [DOI] [PubMed] [Google Scholar]
- 46.Jenney, F. E., Jr., & Adams, M. W. W. (2001) Methods Enzymol. 334, 45–55. [DOI] [PubMed] [Google Scholar]
- 47.Otwinowski, Z. & Minor, W. (1997) Methods Enzymol. 276, 307–326. [DOI] [PubMed] [Google Scholar]
- 48.Brünger, A.T. (1992) x-plor (Yale Univ. Press, New Haven, CT), Version 3.1.
- 49.Brünger, A. T., Adams, P. D., Clore, G. M., Delano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905–921. [DOI] [PubMed] [Google Scholar]
- 50.Roussel, A. & Cambillau, C. (1991) in Silicon Graphics Geometry Partners Directory (Silicon Graphics, Mountain View, CA), p. 86.
- 51.McRee, D. E. (1999) J. Struct. Biol. 125, 156–165. [DOI] [PubMed] [Google Scholar]
- 52.Hiller, R., Zhou, Z. H., Adams, M. W. W. & Englander, S. W. (1997) Proc. Natl. Acad. Sci. USA 94, 11329–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez, G, Jenney, F. E., Jr., Adams, M. W. & LeMaster, D. M. (2000) Proc. Natl. Acad. Sci. USA 97, 3166–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatake, T., Ostermann, A., Kurihara, K., Parak, F. G. & Niimura, N. (2003) Proteins 50, 516–523. [DOI] [PubMed] [Google Scholar]
- 55.Cheng, X. D. & Schoenborn, B. P. (1990) Acta Crystallogr. B 46, 195–199. [Google Scholar]
- 56.Adman, E. T., Waterpaugh, K. D. & Jensen, L. H. (1975) Proc. Natl. Acad. Sci. USA 72, 4854–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephens, P. J., Jollie, D. R. & Warshel, A. (1996) Chem. Rev. 96, 2491–2513. [DOI] [PubMed] [Google Scholar]