Abstract
Human emotional experience is typically associated with enhanced episodic memory. We have used functional magnetic resonance imaging to demonstrate that successful encoding of emotional, compared to neutral, verbal stimuli evokes increased human amygdala responses. Items that evoke amygdala activation at encoding evoke greater hippocampal responses at retrieval compared to neutral items. Administration of the β-adrenergic antagonist propranolol at encoding abolishes the enhanced amygdala encoding and hippocampal retrieval effects, despite propranolol being no longer present at retrieval. Thus, memory-related amygdala responses at encoding and hippocampal responses at recognition for emotional items depend on β-adrenergic engagement at encoding. Our results suggest that human emotional memory is associated with a β-adrenergic-dependent modulation of amygdala-hippocampal interactions.
The human hippocampus is critical for episodic memory (1). By contrast, enhanced memory for emotional events is amygdala-dependent, as it is abolished by human amygdala lesions (2). Animal data suggest that the amygdala enhances emotional stimulus encoding by modulating responses in other brain regions (3). In the case of episodic memory, engagement of amygdala by emotional stimuli is thought to up-regulate responses in hippocampus, resulting in memory enhancement (3).
The amygdala is thought to exert its modulatory effect on hippocampus by means of a β-adrenergic system. β-adrenergic blockade with the β1β2 antagonist propranolol selectively impairs episodic memory for emotionally arousing material without affecting memory for neutral stimuli (4, 5). This modulation of emotional memory by propranolol is centrally mediated, as peripheral β-adrenergic blockade has no such effect (6). A previous study demonstrated amygdala modulation of hippocampal/parahippocampal function by using path analysis (7). However, studies in humans have yet to provide evidence for an adrenergic-dependent amygdala-mediated modulation of hippocampal function during emotional encoding.
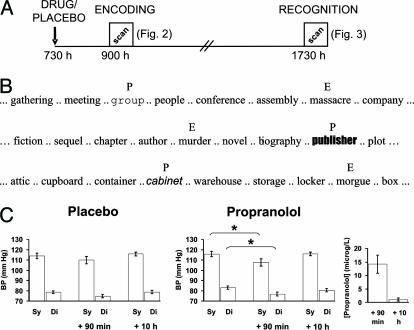
To investigate the roles of amygdala and hippocampus in emotional memory, and their β-adrenergic dependency, we conducted an event-related functional MRI experiment in which 24 subjects received either 40 mg of propranolol or placebo in a double-blind experimental design. There were two distinct scanning sessions corresponding to encoding and retrieval respectively (Fig. 1A). Drug/placebo was administered in the morning, with the encoding scanning session coinciding with propranolol's peak plasma concentration. The retrieval session, scanned 10 h later, was not contaminated by the presence of drug. During encoding, subjects viewed nouns presented serially, every 3 s, in semantically related lists. Each list contained two “oddball” nouns: an emotionally aversive noun (E) and a perceptual oddball (P) presented in a different font (Fig. 1B). Because all other nouns in each list were emotionally neutral, the E nouns constituted emotional oddballs. Perceptual oddballs were presented to control for a nonspecific oddball effect (8).
Fig. 1.
Experimental design, blood pressure data, and drug serum concentrations. (A) Experimental timeline. (B) Examples of presented nouns. (C) Systolic (sy) and diastolic (di) blood pressure (BP; mmHg, 1 mmHg = 133 Pa) for placebo and drug groups as well as drug serum concentrations (propranolol; in μg/liter). E, emotional noun; P, perceptual oddball. *, P < 0.05 on a two-tailed paired t test.
In previous studies, we have shown that memory for emotionally aversive nouns is enhanced, relative to neutral items, when retrieval is tested by free recall, an enhancement abolished by propranolol and amygdala lesions (5). The encoding session in the current experiment therefore comprised the same stimulus set, encoding task, and general procedure as that used previously. However, subsequent memory at retrieval in the current experiment was assessed by an online recognition test. Recall advantage for emotional words does not usually extend to tests of recognition memory (9-11). This has an advantage in the context of neuroimaging in that retrieval-evoked responses measured during recognition are not biased by performance differences. Hence, having established enhanced recall of E nouns (5), our emphasis in this study is on neurophysiological measures evoked at encoding and retrieval by these emotional items, and the modulation of these responses by propranolol. Our prediction, based on previous observations (12-17), was that successful encoding of E nouns would evoke enhanced activation in the amygdala. As propranolol abolishes memory enhancement for emotional stimuli (4, 5), we predicted attenuation of emotional memory-evoked amygdala responses by drug.
Materials and Methods
Subjects. Twenty-four right-handed, native English speaking subjects [12 male (age range, 20-39 years; mean age, 29.2 years); 12 female (age range, 20-29 years; mean age, 24.7 years)] partook in this double-blind placebo-controlled study. Twelve subjects were administered a 40-mg dose of propranolol, and 12 received a vitamin E placebo. Drug allocation was balanced for gender i.e., six males and six females received propranolol. All subjects gave informed consent and were free of neurological or psychiatric history. The study had full ethics approval.
Task. Scanning involved two separate sessions (Fig. 1A). Subjects were administered propranolol or placebo at 0730 hours. In view of the kinetics of propranolol's peak plasma concentration (1-2 h), the memory task started 90 min after drug administration. Retrieval-related neurophysiological responses were assessed by means of a recognition task. Given propranolol's half-life (≈4 h), the recognition test was scanned 10 h after administration (≈2.5 propranolol half-lives). Blood pressure (BP) was measured at the time of drug/placebo administration, and blood samples and BP were taken immediately before the encoding and recognition scanning sessions (all BP measurements were performed after patients reclined for 20 min at 45°).
Encoding. At encoding, subjects were scanned while viewing nouns presented visually in lowercase at a rate of one every 3 s (stimulus duration, 1 s). Subjects were presented with 38 lists of 14 nouns, identical to our previous experiments (5), with each list separated by presentation of the words “New List.” For each list, 12 nouns were of the same semantic category, emotionally neutral, and presented in the same font (neutral nouns). The first five nouns in each list were always neutral nouns. A perceptual oddball was presented in a different font but was emotionally neutral and of the same semantic category as the neutral nouns. The emotional oddball was aversive in content but of the same category and perceptually identical to neutral nouns. Nouns were presented in Times font (48 point; 4-10° of horizontal visual angle) except for perceptual oddballs, which appeared in 19 different fonts (Fig. 1B). The 38 lists were normalized for semantic relatedness and emotional valence by a separate group of 12 subjects [five male (age range, 24-37 years; mean age, 28.2 years) and seven female (age range, 23-37 years; mean age, 27.9 years)].
Subjects indicated with a push-button whether or not the first letter in the noun had an enclosed space (shallow encoding) (18). Encoding instructions were provided visually at the start of each session. Performance at recognition (see below) is expressed relative to two randomly selected neutral nouns (one for each oddball type; Ce, Cp) presented at encoding. These control nouns, like oddballs, could not occur within the first five nouns of each list and could not immediately follow an oddball or another chosen control noun. Different control nouns were chosen for emotional and perceptual oddballs to facilitate analyses of evoked neuronal responses common to both oddball types (not reported here). Behavioral and imaging results reported are identical if the two controls are combined.
Recognition. During scanning, subjects made a push-button response to indicate whether they had seen the word in the encoding session (old vs. new). The stimuli presented at recognition comprised the following (old) stimuli from encoding: 38 emotional oddballs (E) and 38 perceptual oddballs (P), the 38 neutral words that appeared before and after each oddball type (not discussed here), and the 38 control words for the two oddball types (Ce, Cp), giving a total of 304 old words (57% old). New stimuli (43%) comprised 190 neutral foils (semantically related to encoding nouns) and 38 emotional foils (also semantically related to encoding nouns). All nouns (including previously perceptual oddballs) were presented in Times font (48 point; 4-10° of horizontal visual angle).
For recognition memory performance, reaction time (RT) was taken as an index of confidence (19). A median split on RTs divided stimuli into confident (fastest responses) and unconfident correct hits, confident and unconfident correct rejections, incorrect hits (false alarms) and incorrect rejections (misses). Data from one propranolol subject was discarded because of equivalent serum propranolol concentration at encoding and recognition.
Data Acquisition. A 2T Siemens VISION system (Siemens, Erlangen, Germany) was used to acquire both T1-weighted anatomical images and gradient-echo echo-planar T2*-weighted MRI image volumes with blood oxygenation level-dependent contrast. During the encoding session, a total of 690 volumes were acquired per subject, and during recognition, a total of 645 volumes were acquired per subject. For both sessions, volumes were acquired continuously every 2,506 ms. Each volume comprised 33 3.3-mm axial slices, with an in-plane resolution of 3 × 3 mm, positioned to cover the cerebrum. The medial temporal lobe, particularly its anterior extent, which includes the amygdala, is subject to functional MRI susceptibility artifacts and signal drop-out (20), yielding less signal to noise in anterior medial temporal structures compared to most other cortical regions. Hence, each subject's mean functional image was first inspected to ensure adequate signal in bilateral amygdala. Each imaging time series was then realigned to correct for interscan movement, slice-time corrected, normalized into a standard anatomical space (Talairach and Tournoux), and smoothed with a Gaussian kernel of 6 mm full width half-maximum (21).
Data Analysis. Imaging data for both encoding and recognition sessions were analyzed by using statistical parametric mapping (SPM99) employing an event-related model with a two-stage random effects procedure.
Encoding. To test for subsequent memory effects, we specified eight effects of interest: the events at encoding corresponding to the E and P nouns and the two randomly selected control nouns (Ce, Cp) separated according to whether they were later confidently recognized or forgotten. Trial-specific responses were modeled by convolving a delta function (or “stick” function) that indicated each event onset with a synthetic, canonical hemodynamic response function (HRF) to create regressors of interest. The encoding events corresponding to unconfident responses at recognition were modeled separately. Encoding events for which there were absent or incorrect responses at encoding or an absent response at recognition, as well as events corresponding to all other words and the presentation of the “New List” marker, were modeled as regressors of no interest. Movement parameters and low-frequency drifts in signal (cutoff, 120 s) were modeled as nuisance covariates. For the drug group, data from one subject was not included in this analysis because of poor image quality.
Recognition. The effects of interest were confident hits and misses for the different classes of old stimuli, as well as correct rejections and false alarms to emotional and neutral foils. Unconfident responses, absent responses at recognition, and stimuli for which encoding responses were incorrect or absent were modeled separately. It should be noted that, before scanning, the presentation of new stimuli and the different classes of old stimuli had been randomly ordered with the constraint that, once the events had been convolved with the HRF, the correlation coefficient between one event type and any other type was <0.1.
Random Effects. From the encoding and recognition first level analyses described above, session-specific parameter estimates of the magnitude of the hemodynamic response for each stimulus type were calculated for each voxel in the brain (22). A contrast of parameter estimates modeling each comparison of interest (e.g., remembered vs. forgotten emotional vs. control nouns) was calculated in a voxel-wise manner to produce, for each subject, one contrast image for that particular effect. For the random effects analysis, each subject's contrast image was entered into a one-sample t test across the 12 placebo subjects. To compare activations between groups, contrast images for each comparison of interest were entered into a two-sample t test.
The whole-brain statistical parametric maps that ensued from the one- and two-sample t tests were thresholded at P < 0.001 uncorrected and examined for evidence of medial temporal activation. We carried out a small volume correction (SVC) to the P values of the ensuing medial temporal maxima on all reported regions. We report medial temporal responses at a threshold of P < 0.05, corrected for the search volume of amygdala (a sphere of 12-mm diameter centered on x, y, z coordinates -26, 2, -26) and of hippocampus (a sphere of 12-mm diameter centered on -24, -22, -18). Uncorrected P values are also provided for effects not surviving this correction if the same region reaches corrected significance in another comparison (see Fig. 3). The term hippocampus is used here to refer to dentate gyrus, CA subfields, and subiculum.
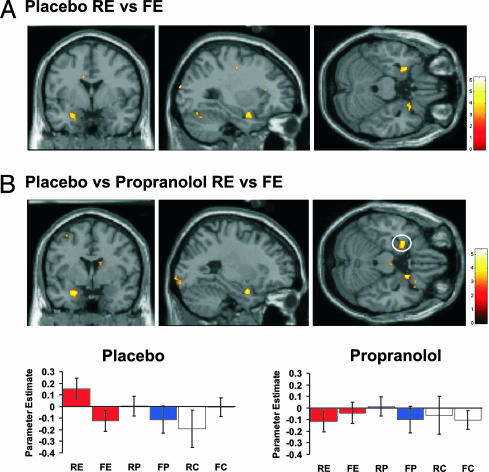
Fig. 3.
Recognition-related neuronal responses during successful retrieval of emotional nouns. In the placebo group, left hippocampal body (-24, -22, -18; Z = 3.68) is more active for confident emotional hits vs. correct rejections of emotional foils relative to confident control hits vs. correct neutral rejections. The activation is overlaid on coronal (y = -22) and sagittal (x = -18) sections of the T1 image (Upper) and a single-subject mean functional T2* image (Lower; color contrast inverted for illustration). Activation in this region (-26, -22, -18; Z = 2.48; P < 0.01 uncorrected) was present in the three-way interaction of (placebo vs. drug) × (correct hit vs. correct rejection) × (emotion vs. neutral). Hippocampal response estimates for these event types, as well as to confidently recognized perceptual oddballs, are plotted at right for placebo and drug groups. H, confident correct hits; CR, confident correct rejections; E, emotional noun/emotional foils; C, control noun (mean of Ce and Cp)/neutral foils; P, perceptual oddball.
Results
Behavior. Recognition performance (Pr) is calculated as probability(confident hit) - probability(confident false alarm) and expressed as a percentage. As shown in Table 1, discrimination of old vs. new neutral nouns (C Pr) was poor, most likely reflecting the use of a shallow encoding task (18) and the 10-h delay between study and test. Furthermore, because encoding lists were semantically related, the foils in the recognition test were semantically related to old nouns, which accounts for a high false alarm rate (23). A group (placebo, drug) × oddball type (emotional, perceptual) 2 × 2 ANOVA did not reveal a significant main effect nor interaction. This absence of an effect of emotion at recognition is in line with previous studies (9-11).
Table 1. Recognition memory performance.
| Neutral nouns
|
Emotional nouns
|
Oddball effect
|
||||||
|---|---|---|---|---|---|---|---|---|
| C P(Hit) | C P(fa) | C Pr | E P(Hit) | E P(fa) | E Pr | E (E Pr - C Pr) | P (P Pr - C Pr) | |
| Placebo | 20.2 (2.1) | 17.3 (2.3) | 2.9 (1.4) | 29.0 (3.2) | 19.6 (3.5) | 9.4 (4.3) | 6.5 (3.5) | 6.1 (2.7) |
| Drug | 22.5 (1.9) | 19.3 (1.9) | 3.2 (1.6) | 24.7 (2.7) | 14.7 (2.6) | 9.9 (2.1) | 6.7 (2.1) | 5.2 (3.4) |
Numbers represent percentages. Numbers in parentheses indicate SE. E, emotional noun; P, perceptual oddball; C, neutral control noun; P(Hit/Fa), probability of confident hit/false alarm; Pr, P(Hit) - P(fa).
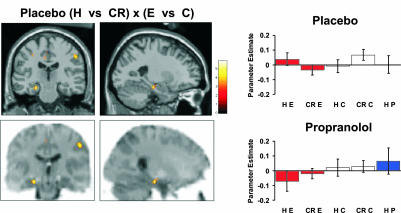
Functional Imaging. Encoding. The specific aim of our experiment was to demonstrate neurophysiological responses that differentiate between subsequently remembered E items at encoding and recognition under placebo and drug. Our experimental design enabled us to measure emotional memory-specific responses by comparing emotional encoding- and retrieval-evoked responses to neutral nouns. Furthermore, comparison of E noun memory-related responses to perceptual oddballs (P) controlled for nonspecific oddball-related memory effects. To index emotion-specific encoding-related responses, we report a contrast of neuronal responses for subsequently recognized (R) compared to forgotten (F) emotional nouns as well as a comparison of this effect under placebo vs. drug (i.e., the group × condition interaction).
Fig. 2 shows the “subsequent memory effect” encoding-related neuronal responses. In the placebo group, successful encoding of emotional oddballs engaged the left amygdala relative to forgotten ones (Z = 3.34; P < 0.05 corrected), in line with previous observations for nonverbal stimuli (12, 14, 15), and the right uncus (Fig. 2A). These effects were greater than those for subsequently remembered vs. forgotten control nouns (Z = 2.75; P < 0.01 uncorrected). Remarkably, an identical pattern of activation was evident in the interaction of subsequently remembered vs. forgotten emotional nouns for placebo vs. propranolol groups (Z = 3.61; P < 0.05 corrected), as shown in plots of response estimates in left amygdala for the two groups (Fig. 2B). Thus, in the presence of propranolol, amygdala activation no longer predicts subsequent memory for emotional nouns (Fig. 2B), thus confirming predicted differences in neurophysiological responses. This region was also significant in the three-way interactions of subsequently remembered vs. forgotten emotional nouns vs. the same comparison for either control nouns (Z = 3.23; P < 0.05 corrected) or perceptual oddballs (Z = 3.12; P < 0.05 corrected) in the placebo vs. drug group. The latter comparison indicates that adrenergic-dependent amygdala responses do not simply reflect oddball encoding. This observation supports our previous finding (5) that human amygdala lesions do not abolish enhanced memory for perceptual oddballs. The activation in left amygdala in the placebo group most likely reflects the verbal nature of the stimuli, although we also observe a right-sided activation adjacent to amygdala in right uncus. Recognition. Retrieval-related effects for emotional words are reported in terms of physiological responses that are greater for recognized emotional nouns vs. correct rejections of emotional foils. We also report the group (placebo versus propranolol) × condition interaction for these effects. Again, we compared these responses with retrieval-evoked responses to neutral nouns and to perceptual oddballs (P). The placebo group demonstrated enhanced activation in left hippocampal body for correct confident hits (H) of emotional nouns vs. correct rejections (CR) of emotional foils (Z = 2.47; P < 0.01 uncorrected). Although this only reached uncorrected significance, this effect was significantly greater than confident H vs. CR for nonemotional control nouns (Z = 3.68; P < 0.05 corrected). Notably, this pattern of left hippocampal body activation for confident recognition was absent in the drug group (Fig. 3), i.e., there was a group × effect interaction (Z = 2.48; P < 0.01 uncorrected). This region was also present in the three-way interaction of group (placebo vs. drug) × response (correct hit vs. correct rejection) × oddball (emotion vs. perceptual) (Z = 2.73; P < 0.01 uncorrected), indicating that the hippocampal emotional recognition response does not simply reflect oddball recognition. Importantly, the amygdala is not engaged by H vs. CR of emotional nouns (Fig. 3).
Fig. 2.
Neuronal responses during successful encoding of emotional nouns. (A) Activation in left amygdala (-26, 2, -20; Z = 3.34) was greater for subsequently recognized vs. forgotten emotional nouns in the placebo group. The activation is overlaid on coronal (y = 2), sagittal (x = -26), and transverse (z = -20) sections of the MNI T1 image (SPM threshold for illustration, here and in Fig. 3, P < 0.005 uncorrected). Colored bar indicates the T statistic of the activation. (B) The same left amygdala region (-26, 2, -22; Z = 3.61) was present when successful encoding activation for emotional oddballs was compared between placebo and drug groups. The parameter estimates (arbitrary units) for these event types, as well as to remembered and forgotten control nouns and perceptual oddballs, are plotted (± SE of the mean across all subjects) for left amygdala activation (circled) for both groups below. R and F, confidently subsequently recognized and forgotten, respectively; E, emotional noun; P, perceptual oddball; C, control noun (mean of Ce and Cp). Note that RP vs. FP in the placebo group does not yield significant amygdala activation, nor does FC vs. RC. The transverse sections also demonstrate a right-lateralized activation located anterior to the amygdala in the uncus.
Before the recognition session, blood pressure had returned to pre-drug-administration values, and blood sample data demonstrated absence of serum propranolol (Fig. 1C; see Materials and Methods), indicating that retrieval-related responses were not contaminated by the presence of drug. Recognition-related activation in hippocampal body was therefore specific to emotional items and selectively abolished by propranolol administration at encoding. This activation is not caused by a difference in confidence for emotional vs. neutral stimuli or an RT difference between groups. There were no significant between-group differences in mean RTs and, specifically, there were no significant main effects or interactions in a 2 × 2 × 2 group (placebo, drug) × emotional/neutral × hits/correct rejections RT ANOVA. The absence of within- and between-group memory differences in recognition performance is advantageous in that differences in neurophysiological responses to distinct classes of items cannot be a function of a behavioral confound. Note also that confident hits in this recognition analysis constitute the subsequently remembered nouns in the encoding analysis. Hence, during encoding of emotional stimuli in the placebo group, greater amygdala activation is associated with greater hippocampal engagement for the same items at subsequent retrieval.
Discussion
Our data demonstrate distinct functional roles for medial temporal structures in episodic memory encoding and retrieval of emotional stimuli. Successful encoding of emotional nouns engages left amygdala, a response abolished by administration of the β1β2-adrenergic antagonist propranolol. Recognition of these same emotional items evokes activation in left hippocampus. This hippocampal response was not present if propranolol was administered at encoding.
Amygdala activation that predicts subsequent memory has been shown for emotional pictures (12-15, 17). Our findings support recent evidence for amygdala activation indexing successful encoding of emotional verbal stimuli (24). The novel finding here is from the drug group, which indicates that successful encoding-evoked activation of the amygdala depends on β-adrenergic activation within this structure. This finding is in accordance with animal data demonstrating that inhibitory avoidance training increases norepinephrine/noradrenergic (NE) levels in the amygdala, and the levels assessed in individual animals correlate highly with later retention performance (25). Our data therefore indicate that amygdala activation evoked by successful encoding of emotional stimuli depends on a rise in amygdala NE.
The amygdala does not show enhanced activity during recognition of E nouns, suggesting that memory for emotional items is not stored in amygdala at the time of test. This finding is in accordance with a memory-modulating role of the amygdala during encoding (3). Thus, the fact that emotional stimuli activate the amygdala during encoding and the hippocampus on subsequent presentation is consistent with a role for the amygdala in consolidating plastic changes in the hippocampus (3), an effect revealed on subsequent presentation of the stimuli.
Anatomical studies in animals demonstrate that hippocampus is reciprocally connected with amygdala (26). Electrophysiological studies show that stimulation of the amygdala enhances synaptic transmission, plasticity, and long-term potentiation in the dentate gyrus (DG) of the hippocampus (27, 28). Furthermore, recent evidence demonstrated synchronization of amygdala and hippocampal theta rhythm during retrieval of conditioned fear (29). There is also evidence for an NE-induced enhancement of cellular excitability, synaptic transmission, and plasticity in vivo in rat DG (30). This finding supports the proposal that, during emotional stimulus processing, NE release in amygdala or hippocampus modulates encoding efficiency (25). The amygdala is itself under NE control, and it may be the case that this is mediated by reciprocal connections between amygdala and locus coeruleus, the origin of NE projections (31).
The temporal profile of the proposed amygdala-dependent modulation of hippocampus is unknown. Our data suggest that up-regulation of hippocampal responses does not occur at the time the E noun is encoded, because there is no hippocampal activation for the successful emotional encoding comparison (Fig. 2). Previous studies of the subsequent memory effect for emotional visual stimuli have also demonstrated amygdala, and not hippocampal, responses (12-15, 17). Our data support a prevailing view that the effects of amygdala activation on consolidation in other brain regions (in this case the hippocampus), occur after initial encoding (32). It is thought that this consolidation operates over weeks (33), but we have recently demonstrated that propranolol abolishes enhanced recall of E nouns after a 30-s delay (5). The current data suggest that a modulation of hippocampal responses occurs within 10 h of encoding. An alternative explanation is that the neurophysiological processes that lead to hippocampal up-regulation occur at the time of encoding but do not yield a detectable blood oxygenation level-dependent response.
From a behavioral perspective, the emotional items presented in the current experiment enjoy enhanced memory if assessed by free recall (5). This memory enhancement may depend on the same amygdala/hippocampal mechanisms described here. Thus, our experiments speak to the neurobiological basis for the enhancing effects of emotion on human episodic memory. Enhanced memory for emotional stimuli may result from augmented amygdala activation modulating hippocampal processing. Both processes are β-adrenergic-dependent at the time of encoding. Thus, enhanced recall associated with emotional stimuli may reflect amygdala-hippocampal interactions that are orchestrated by the β-adrenergic system.
Acknowledgments
We thank P. Patsalos and A. Richardson for storing blood samples and K. Friston and M. Rugg for advice and comments. This work was supported by a program grant from the Wellcome Trust (to R.J.D.).
Abbreviations: RT, reaction time; E, emotionally aversive noun; BP, blood pressure; NE, norepinephrine/noradrenaline.
References
- 1.Scoville, W. B. & Milner, B. (1957) J. Neurosurg. Psychiat. 20, 11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahill, L., Babinsky, R., Markowitsch, H. J. & McGaugh, J. L. (1995) Nature 377, 295-296. [DOI] [PubMed] [Google Scholar]
- 3.McGaugh, J. L., Cahill, L. & Roozendaal, B. (1996) Proc. Natl. Acad. Sci. USA 93, 13508-13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill, L., Prins, B., Weber, M. & McGaugh, J. L. (1994) Nature 371, 702-704. [DOI] [PubMed] [Google Scholar]
- 5.Strange, B. A., Hurlemann, R. & Dolan, R. J. (2003) Proc. Natl. Acad. Sci. USA 100, 13626-13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Stegeren, A. H., Everaerd, W., Cahill, L., McGaugh, J. L. & Gooren, L. J. (1998) Psychopharmacology (Berlin) 138, 305-310. [DOI] [PubMed] [Google Scholar]
- 7.Kilpatrick, L. & Cahill, L. (2003) NeuroImage 20, 2091-2099. [DOI] [PubMed] [Google Scholar]
- 8.von Restorff, H. (1933) Psychol. Forsch. 18, 299-342. [Google Scholar]
- 9.Leiphart, J., Rosenfeld, J. P. & Gabrieli, J. D. (1993) Int. J. Psychophysiol. 15, 197-206. [DOI] [PubMed] [Google Scholar]
- 10.Maratos, E. J., Allan, K. & Rugg, M. D. (2000) Neuropsychologia 38, 1452-1465. [DOI] [PubMed] [Google Scholar]
- 11.Windmann, S. & Kutas, M. (2001) J. Cognit. Neurosci. 13, 577-592. [DOI] [PubMed] [Google Scholar]
- 12.Cahill, L., Haier, R. J., Fallon, J., Alkire, M. T., Tang, C., Keator, D., Wu, J. & McGaugh J. L. (1996) Proc. Natl. Acad. Sci. USA 93, 8016-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cahill, L., Haier, R. J., White, N. S., Fallon, J., Kilpatrick, L., Lawrence, C., Potkin, S. G. & Alkire, M. T. (2001) Neurobiol. Learn. Mem. 75, 1-9. [DOI] [PubMed] [Google Scholar]
- 14.Hamann, S. B., Ely, T. D., Grafton, S. T. & Kilts, C. D. (1999) Nat. Neurosci. 2, 289-293. [DOI] [PubMed] [Google Scholar]
- 15.Canli, T., Zhao, Z., Brewer, J., Gabrieli, J. D. & Cahill, L. (2000) J. Neurosci. 20, RC99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strange, B. A., Henson, R. N., Friston, K. J. & Dolan, R. J. (2000) NeuroImage 12, 425-433. [DOI] [PubMed] [Google Scholar]
- 17.Canli, T., Desmond, J. E., Zhao, Z. & Gabrieli, J. D. (2002) Proc. Natl. Acad. Sci. USA 99, 10789-10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craik, F. I. M. & Lockhart, R. S. (1972) J. Verbal Learn. Verbal Behav. 11, 671-684. [Google Scholar]
- 19.Henson, R. N. A., Rugg, M. D., Shallice, T. & Dolan, R. J. (2000) J. Cognit. Neurosci. 12, 913-923. [DOI] [PubMed] [Google Scholar]
- 20.Ojemann, J. G., Akbudak, E., Snyder, A., McKinstry, R., Raichle, M. & Conyuro, T. (1997) NeuroImage 6, 156-167. [DOI] [PubMed] [Google Scholar]
- 21.Friston, K. J., Ashburner, J., Frith, C. D., Poline, J.-B., Heather, J. D. & Frackowiak, R. S. J. (1995) Hum. Brain Mapp. 2, 165-189. [Google Scholar]
- 22.Friston, K. J., Holmes, A. P., Worsely, K. J., Poline, J.-B., Frith, C. D. & Frackowiak, R. S. J. (1995) Hum. Brain Mapp. 2, 189-210. [DOI] [PubMed] [Google Scholar]
- 23.Roediger, H. L., III, & McDermott, K. B. (1995) J. Exp. Psychol. Learn. Mem. Cogn. 21, 803-814. [DOI] [PubMed] [Google Scholar]
- 24.Kensinger, E. A. & Corkin, S. (2004) Proc. Natl. Acad. Sci. USA 101, 3310-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGaugh, J. L. & Roozendaal, B. (2002) Curr. Opin. Neurobiol. 12, 205-210. [DOI] [PubMed] [Google Scholar]
- 26.Pitkanen, A., Pikkarainen, M., Nurminen, N. & Ylinen, A. (2000) Ann. N.Y. Acad. Sci. 911, 369-391. [DOI] [PubMed] [Google Scholar]
- 27.Ikegaya, Y., Saito, H. & Abe, K. (1996) Eur. J. Neurosci. 8, 1833-1839. [DOI] [PubMed] [Google Scholar]
- 28.Akirav, I. & Richter-Levin, G. (1999) J. Neurosci. 19, 10530-10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seidenbecher, T., Laxmi, T. R., Stork, O. & Pape, H. C. (2003) Science 301, 846-850. [DOI] [PubMed] [Google Scholar]
- 30.Harley, C. (1991) Prog. Brain Res. 88, 307-321. [DOI] [PubMed] [Google Scholar]
- 31.Bouret, S., Duvel, A., Onat, S. & Sara, S. J. (2003) J. Neurosci. 23, 3491-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGaugh, J. L. (2000) Science 287, 248-251. [DOI] [PubMed] [Google Scholar]
- 33.Quevedo, J., Sant' Anna, M. K., Madruga, M., Lovato, I., de-Paris, F., Kapczinski, F., Izquierdo, I. & Cahill, L. (2003) Neurobiol. Learn. Mem. 79, 132-135. [DOI] [PubMed] [Google Scholar]