Abstract
There exist reaction products of nitric oxide (NO) with blood that conserve its bioactivity and transduce an endocrine vasomotor function under certain conditions. Although S-nitrosated albumin has been considered the major species subserving this activity, recent data suggest that additional NO species, such as nitrite, nitrated lipids, N-nitrosamine, and iron-nitrosyl complexes, may contribute. We therefore examined the end products of NO reactions in plasma and blood in vitro and in vivo by using reductive chemiluminescent assays and electron paramagnetic resonance spectroscopy. We found that NO complexes in plasma previously considered to be S-nitrosated albumin were <10 nM after elimination of nitrite and were mercury-stable, consistent with iron-nitrosyl or N-nitrosamine complex. During clinical NO gas inhalation protocols or in vitro NO donor treatment of human plasma, S-nitroso-albumin did not form with NO exposure <2 μM, but plasma methemoglobin was detectable by paramagnetic resonance spectroscopy. Consistent with this formation of methemoglobin, human plasma was found to consume ≈2 μM NO at a rate equivalent to that of hemoglobin. This NO consumption was mediated by the reaction of NO with plasma haptoglobin-hemoglobin complexes and limited slower reaction pathways required for S-nitrosation. These data suggest that high-affinity, metal-based reactions in plasma with the haptoglobin-hemoglobin complex modulate plasmatic NO reaction products and limit S-nitrosation at low NO flux. The studies further suggest that alternative NO reaction end products in plasma, such as nitrite, N-nitrosamines, iron-nitrosyls, and nitrated lipids, should be evaluated in blood NO transport along the vasculature.
Nitric oxide (NO) is a diatomic free radical molecule that plays a principal role in basal blood flow regulation and vascular homeostasis. Although NO is inactivated by dioxygenation reaction with oxyhemoglobin, a perierythrocytic unstirred layer (1, 2), membrane diffusion barrier (3), and cell-free zone in laminar flowing blood (4) reduce NO-hemoglobin reactions ≈600-fold, permitting tonic NO-dependent vasodilation and NO reactions with plasmatic proteins and other gas molecules (recently reviewed in ref. 5). The reaction of NO with plasma has been the subject of intense study after initial reports that human plasma contains 7 μM S-nitroso-albumin (SNO-albumin), effectively creating a major paradigm that S-nitrosothiols serve as a stable circulating storage form of NO (6). Such a model has been supported by observations of the vasoactivity of infusions of SNO-albumin in animal models, distal vasodilation during infusions of NO solutions into the brachial artery, and the in vitro and in vivo formation of SNO-albumin with micromolar NO exposure to plasma (7-16).
However, over the last 10 years the measured levels of plasma S-nitrosothiol and SNO-albumin have varied widely with different analytical methodologies and different species (Table 1 and Supporting Text, which are published as supporting information on the PNAS web site). One year after the initial observation of 7 μM SNO-albumin in plasma (6), the same laboratory published levels of SNO-albumin of 0.7 μM (17). The development of new methods to selectively eliminate nitrite and the use of the reductive cleavage of the S-NO linkage with triiodide solutions suggested that the levels of SNO-albumin were actually <30 nM (9, 12-14, 18-20). Feelisch and colleagues (19) subsequently reported, and we now confirm, that this signal is largely mercury-stable, inconsistent with an S-nitrosothiol. Although the differences in levels reported in the literature may be secondary to methodologies used (photolysis of samples versus chemical reduction), species differences in thiol reactivities, and disease or treatment states (sepsis, renal failure, etc.), it is now clear that, in addition to SNO-albumin, other reaction products form that may be capable of NO storage and delivery along the vasculature. These species may include nitrite (21), N-nitrosamines (19, 22, 23), iron-nitrosyls, (24) and recently identified nitrated lipids (25).
In this study, we characterize, in vitro and in vivo, the reaction of physiological levels of NO (<2 μM NO) with human plasma. We find that, in addition to SNO-albumin, nitrite and mercury-stable NO species form. Importantly, human plasma rapidly consumes ≈1-2 μM NO, and this reaction is mediated by the dioxygenation reaction of NO with plasma haptoglobin-hemoglobin complexes. This metal-based, high-affinity reaction appears to limit formation of plasma S-nitrosothiols during NO gas inhalation or if plasma is exposed to a <2 μM NO donor or solution. Interestingly, nitrite formation partially out-competes hemoglobin-based NO inactivation, suggesting that metal-based, high-affinity NO oxidase pathways might contribute to nitrite formation in vivo.
Materials and Methods
Chemicals and Reagents. All chemicals were purchased from Sigma-Aldrich unless otherwise indicated. (Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA NONOate) was purchased from Cayman Chemical (Ann Arbor, MI). Antibody against human hemoglobin was obtained from Bethyl Laboratories (Montgomery, TX). Antibodies against haptoglobin and albumin were from Sigma. Seize mammalian immunoprecipitation kit was purchased from Pierce. When needed, both human and bovine SNO-albumin were prepared according to methods described in refs. 12 and 13.
Protocol. The study protocol was approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute, and all normal volunteers gave written, informed consent. Blood was processed for these studies by using methodologies to limit artifactual ex vivo hemolysis, and levels of plasma hemoglobin were consistent with previous studies (26, 27). Briefly, blood was collected from artery or vein by using large-bore catheters (18 gauge). The first 3 ml of blood was discarded, and the blood then slowly drawn into heparinized syringes. Blood was then spun at 750 × g for 5 min without braking, and then the plasma was removed. Plasma was then spun at 14,000 × g for 10 min to eliminate residual erythrocytes and platelets. To validate that blood processing and sampling did not lyse erythrocytes, we did serial transfers and centrifugations from tube to tube by using angiocatheters and syringes and found no increase in hemolysis with each transfer. For the measurement of plasma S-nitrosothiol content, blood was collected in heparinized vacutainers containing 0.1 mM diethylenetriaminepentaacetic acid (DTPA) and 8 mM N-ethylmaleimide (NEM) (12, 13).
Detection of S-nitrosothiol (Mercury-Labile) and Mercury-Stable Complexes in Plasma. We determined the plasma levels of NO-modified proteins in plasma by using a triiodide/ozone-based chemiluminescence assay. Briefly, blood samples were collected in heparinized vacutainers containing added DTPA (0.1 mM) and NEM (8 mM) then chilled and centrifuged at 750 × g for 5 min. To measure total NO-modified proteins, plasma was treated with a 1/10th volume of 5% acidified sulfanilamide (5% sulfanilamide in 1 M HCl) for 3 min and then injected into a triiodide-containing vessel actively purged with a helium stream in line with an NO chemiluminescence analyzer (Sievers, Boulder, CO) (12). To discriminate S- nitrosothiol (mercury-labile) and iron-nitrosyl/N-nitrosamine (mercury-stable) complexes, plasma was treated with and without HgCl2 (5 mM) for 2 min, followed by a 3-min treatment with acidified sulfanilamide before injection of sample into triiodide solution (19). Extensive assay validation was performed for human and bovine SNO-albumin (Fig. 1 A and B). Reductive decomposition of SNO-albumin was limited by plasma treatment with NEM as previously reported (12, 14) and by collection of plasma in heparin with DTPA (as opposed to EDTA, which produced variable S-nitrosothiol decomposition) (data not shown).
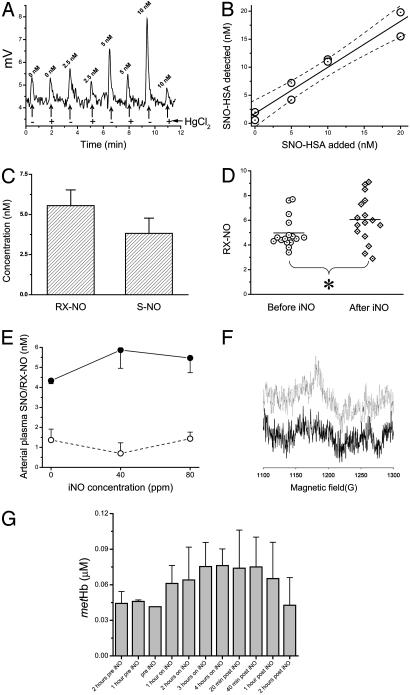
Fig. 1.
Measurement of plasma NO-modified proteins by chemiluminescence and plasma methemoglobin by EPR. (A) Chemiluminescent detection of NO (in mV) released from 400 μl of plasma separated from blood collected in heparin and 8 mM NEM. Human SNO-albumin at 0, 2.5, 5, or 10 nM was added to plasma samples, which were then treated with water (-) or 3 mM HgCl2 (+) and then with 0.5% sulfanilamide and 0.1 M HCl to eliminate background nitrite. (B) Human SNO-albumin (SNO-HSA) detected after 0, 5, 10, and 20 nM human SNO-albumin was added to plasma as described in A.(C) Mean plasma concentrations of iron-nitrosyl or N-nitrosamine species (RX-NO; mercury-stable) or S-nitrosothiols (S-NO; mercury-labile) in plasma separated from venous blood from healthy volunteers collected in 8 mM NEM and heparin. (D) Mean plasma concentrations of mercury-stable species in plasma from arterial blood samples drawn from healthy volunteers (n = 16) before (circles) and after (diamonds) 30 min of NO inhalation (iNO) at 80 ppm. (E) Mean plasma concentrations of mercury-stable species (filled circles) and S-nitrosothiols (open circles) in plasma from arterial blood samples drawn from healthy volunteers (n = 3) after 30 min of NO inhalation at 0, 40, and 80 ppm. (F and G) EPR results show that NO inhalation increased the methemoglobin levels in plasma (g = 6, 9.63 GHz).
NO Consumption Assay. A 50-ml solution of 40 μM DETA NONOate in PBS, pH 7.4, was prepared in a glass vessel actively purged with helium in-line with an NO chemiluminescence analyzer. This solution produced a steady-state NO signal of ≈50-70 mV, which was generated by the decay of DETA-NONOate and the release of NO. When the signal became stable, 50-μl samples of standards or plasma fractions were injected into the NONOate solution. Known concentrations of hemoglobin were used to create standard curves and produced concentration-dependent, linear, and reproducible decreases in the baseline NO signal (NO consumption, mV). Data were transferred to the software program origin Version 6.1 (OriginLab, Northampton, MA) for analysis of the area under the curve of decreasing NO signal over time. The amount of NO consumed is quantified by comparison of the area under the curve with that of NO gas standards (produced from injections of nitrite into triiodide), and this amount of NO consumed stoichimetrically matches the concentration of the oxyhemoglobin standards injected (Fig. 2A Inset). Similar volumes of plasma, albumin solutions, and plasma treated or prepared as described in Materials and Methods and Results were injected into the DETA-NONOate solution to determine the amount and mechanism of NO consumption by plasma.
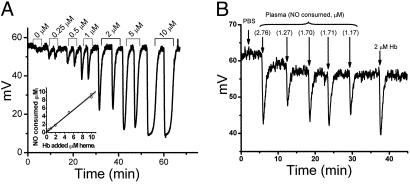
Fig. 2.
NO consumption assay and NO scavenging by normal plasma. (A) Standard curves were generated by injections of 50 μl of hemoglobin (Hb from 0-10 μM heme) diluted in PBS produced concentration-dependent, transient decreases in the baseline NO signal (NO consumption, mV) generated by a 40-μM solution of DETA-NONOate in PBS, which was purged with a helium stream in-line with an NO analyzer. (Inset) A plot of the NO consumed in response to the hemoglobin added. (B) Raw data from NO consumption assay showing that plasma consumes 1.17-2.76 μM NO. Blood was collected by using a rigorous approach to minimize hemolysis.
In this experimental system, the concentration of NO in the purge vessel is proportional to the kinetic relationship between the rate of NO formation from DETA-NONOate and the rate of NO disappearance, which is the sum of purging and the reaction of injected proteins with NO. Because the flow rates of the purging gas and all other parameters (volume of injection, volume in purge vessel, and surface area) are constant, the decrease in signal after a plasma or protein-sample injection reflects the removal of NO at a rate comparable with that of an equivalent area under the curve observed for oxyhemoglobin (in terms of heme concentration).
Purification of NO-Scavenging Protein. Plasma was passed over an Affi-Gel Blue resin (Bio-Rad) to adsorb albumin and then subjected to isoelectric focusing (IEF). IEF fractions were collected, and the pH of each was measured to verify the existence of a pH gradient. Fractions were assayed for NO consumption activity and analyzed by SDS/PAGE and matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF). Active fractions were further purified by chromatography on a Q Sepharose HP and Sephacryl S-200 columns (Amersham Pharmacia). Mass spectrometry (MALDI-TOF) was performed as described in ref. 24. Detailed methodology is published as supporting information.
EPR of Human Plasma Methemoglobin Before and After NO Inhalation. Methemoglobin was measured by EPR by using a Bruker Elexsys spectrometer equipped with a liquid helium cryostat with the following settings: microwave power, 2 mW; scan width, 500 G (1 G = 0.1 mT); sweep time, 42 s, time constant, 21 ms; modulation amplitude, 5 G; temperature, 4.5 K. The observed signals were at the level of detectability, and the signal/noise ratio was too low to allow double integration. For this reason, the spectra were taken under nonsaturating conditions to preserve spectral shape, and quantification was accomplished by correlation of the data with that of a known standard taken under identical conditions. This method allowed quantitative analysis of plasma methemoglobin at concentrations >20 nM. In validation experiments, plasma with immunodepleted hemoglobin had no detectable hemoglobin by liquid-helium EPR, and added methemoglobin standards in plasma from the low nM to 4 μM were readily detected at g = 6 and 9.63 GHz (r2 = 0.992, P < 0.001).
In Vitro Reactions of NO and Plasma. To measure the yields of nitrite and S-nitrosothiols from exogenous NO added to plasma and in PBS, we added various concentrations of the NO donor ProliNONOate (ProliNO, Cayman Chemical, Ann Arbor, MI) to 750 μl of plasma or buffer and allowed the mixture to incubate for 30 min at room temperature. Volumes at 1, 2, 4, 8, 16, and 32 μl of the diluted, ice-cold ProliNO solution were added to 750 μl of sample. After a 30-min incubation, the samples were frozen on dry ice. To measure nitrite concentration, the samples were thawed and directly injected into I3- solution for chemiluminescence detection as described above. To measure S-nitrosothiol formation, the samples were treated with acidified sulfanilamide with and without HgCl2 before injection. In some experiments, before NO donor additions, free thiols in PBS or plasma were blocked by a 10-min room temperature incubation with freshly prepared NEM at a final concentration of 5 mM.
Results
Levels of S-nitrosothiol in Human Plasma and Reaction Products of NO in Plasma During NO Gas Inhalation. SNO-albumin is rapidly reduced or eliminated by transnitrosation in human plasma and must be stabilized by addition of NEM to block free thiols (12, 14). We used the method of Marley et al. (12) for detection of plasma SNO-albumin; however, we hypothesized that the detected signal in triiodide-based reductive chemiluminescence could represent an iron-nitrosyl complex (26) or an N-nitrosamine complex (19) because this assay releases NO from both iron-nitrosyls, N- nitrosamines, and S-nitrosothiols. We drew venous blood from fasting normal human volunteers into heparin-containing vacutainers with 8 mM NEM (final concentration in plasma). Additional NEM was added to plasma, and then plasma was reacted with and without 5 mM HgCl2 (to eliminate S-nitrosothiol) and then in 0.1 M HCl/0.5% sulfanilamide to remove nitrite. The assay was extensively validated by using human and bovine SNO-albumin (Fig. 1 A and B; r2 = 0.99, P = 0.007 for human SNO-albumin and r2 = 0.96, P = 0.004 for bovine SNO-albumin). As shown in Fig. 1 A and B, the assay is sensitive down to ≈1 nM SNO-albumin in plasma (collected in heparin, NEM, and DTPA to stabilize SNO-albumin). By using this assay, the 7.5-10 nM NO signal detected in plasma by triiodide-based reductive chemiluminescence was largely mercury-stable, which is chemically consistent with an iron-nitrosyl or N-nitrosamine complex and confirms experiments recently published by Rassaf et al. (19). The levels of mercury-subtractable signal (i.e., SNO-albumin) were <5 nM (Fig. 1 C and E). The low yields of S-nitrosothiol were not increased by collection of blood in excess NEM, heparin, EDTA, or DTPA (data not shown). All samples for S-nitrosothiol measurement were performed immediately without freeze-thaw.
In 16 subjects, 80-ppm inhaled NO was delivered by facemask with 21% oxygen. There was no detectable increase in plasma SNO-albumin (0.59 ± 0.31 nM to 0.67 ± 0.34 nM, P = 0.86), whereas the mercury-stable signal increased ≈0.5 nM (4.98 ± 0.32 nM to 5.52 ± 0.35 nM, P = 0.013; see Fig. 1D). Similar results were obtained in dose-response experiments with 40- and 80-ppm NO gas inhalation (Fig. 1E). Plasma methemoglobin levels measured by liquid-helium EPR (g = 6, 9.63 GHz) rose by 32 nM, from 44 ± 5 nM to 76 ± 14 nM (P = 0.018), consistent with greater reactivity of NO in plasma with a circulating Fe(II) heme complex (Fig. 1 F and G).
Plasma NO-Scavenging Activity. We previously observed that normal human plasma consumes 2 μM NO within the reaction time of an amperometric NO electrode (26). The ability of plasma to extinguish this concentration of NO would have important effects on the disposition of NO in plasma. To measure the NO-consuming activity of plasma, we took advantage of DETA NONOate, which releases NO with a prolonged half-life at room temperature and neutral pH. A solution of PBS and 40 μM DETA NONOate was placed in a glass vessel actively purged with helium in-line with a chemiluminescent NO detector (Seivers). In our experiments, a 50- to 70-mV baseline signal resulted from 40 μM DETA NONOate in solution and served as a steady-state signal of NO production from which NO consumption was determined. Because free hemoglobin is a known NO scavenger, it was used to standardize our system. Injection of free hemoglobin resulted in a rapid, transient reduction of NO signal (Fig. 2A). The area under the curve of this signal was proportional to the amount of NO scavenged.
As shown in Fig. 2A, NO scavenging by oxyhemoglobin was linear when tested at 0-10 μM oxyhemoglobin. In our system, 50 μl of plasma obtained from normal volunteers resulted in the consumption of ≈1.72 ± 0.28 μM of NO (Fig. 2B). This result is similar to our previous observations in normal volunteers, for which we used an NO-electrode-based consumption assay (26). Rafikova et al. (16) recently reported that plasma consumed 1.7 μM NO and hypothesized that this occurred secondary to catalytic NO autooxidation in the hydrophobic core of albumin, suggesting that albumin might account for our observed NO consumption. However, Rafikova et al. (16) demonstrated plasmatic consumption of 1.7 μM NO when 0.4 ml of 50 mM nitrite was added to albumin or rat plasma (0.1 g of dry powder from Sigma) in a 10-ml solution of 0.1 M H2SO4 with 0.1 M KI; these data are likely consistent with S-nitrosothiol formation in presence of acidified nitrite. In our experiments, we find that plasma consumes ≈1.7 μM NO under physiological conditions (undiluted plasma at neutral pH), and, as described below, albumin does not account for this consumption.
Source of NO Consumption by Human Plasma. To further characterize NO scavenging by normal plasma, we approached the problem in several different ways shown in Fig. 3 A and B. First, we determined whether albumin was the NO-scavenging complex in plasma. Albumin is the most abundant protein in plasma and has been considered the major storage pool of NO bioactivity by means of stable S-nitrosation of its free cysteine residue (6, 16). Reduced human and bovine albumins were injected into the NO consumption system and neither albumin solution scavenged NO. Furthermore, there was no observed NO scavenging by albumin whether under helium or 100% oxygen. Second, ultrafiltration revealed that plasma NO scavenging was contained in a high-molecular-mass fraction of plasma, because the retentate from 100-kDa filtration maintained the NO scavenging activity. Third, we demonstrated that plasma NO scavenging was partially sensitive to metal chelation (12-h incubation with DTPA and EDTA) and completely inhibited by oxidation with ferricyanide.
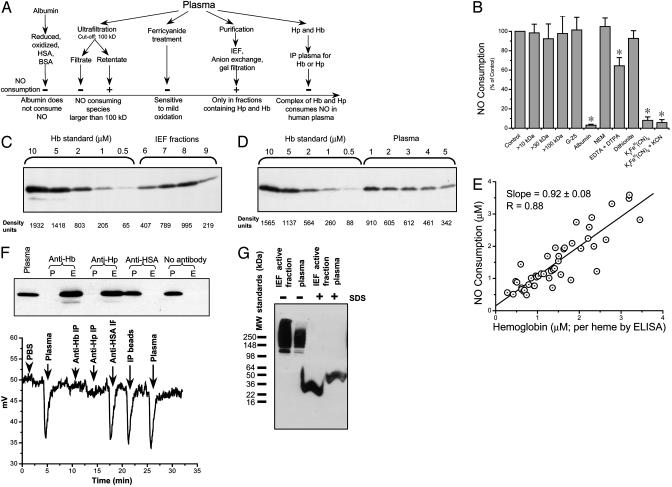
Fig. 3.
Determining the source of NO consumption in human plasma. (A) Scheme of experiments performed to determine the source of NO consumption in normal human plasma. HSA, human serum albumin; Hb, hemoglobin; Hp, haptoglobin; IP, immunoprecipitation. (B) Mean relative (%) NO consumption (n = 2-6) by 50-μl injections of the indicated samples. Error bars indicate SEM. *, P < 0.05. (C) Immunoblot of IEF fractions by using antihemoglobin antibodies. A standard curve of hemoglobin was run together with IEF fractions 6-9. (D) Immunoblot of plasma by using antihemoglobin antibodies. (E) Plot of NO scavenging measured by chemiluminescence versus heme concentration measured by hemoglobin ELISA. (F) Immunoprecipitation with antihemoglobin and antihaptoglobin antibodies eliminated plasma NO consumption. (Upper) Immunoblot of plasma and plasma pass-through from the antibody columns. P, plasma pass-through; E, acid glycine eluate from bead-antibody complex. (Lower) NO consumption of plasma and plasma pass-through from immunoprecipitation experiments. (G) Immunoblot with antihemoglobin antibodies showed that hemoglobin was complexed with haptoglobin and migrated slowly as a large-molecular-mass multimeric complex. MW, molecular mass.
Finally, detailed purification of the plasma NO-scavenging component was carried out. Albumin was removed by passing plasma through an Affi-Gel Blue column (Bio-Rad). This albumin-depleted plasma maintained NO consumption activity and thus was further fractionated by IEF (Bio-Rad Rotofor system). The active fraction after IEF was further purified by passage through Q Sepharose and Sephacryl S-200 columns. At all steps, the fractions were monitored for NO scavenging ability and analyzed by SDS gel for protein determination. Analysis of active IEF fractions by MALDI-TOF indicated the presence of a number of proteins, including apolipoproteins A, B, and E, as well as transferrin, β2-macroglobulin, haptoglobin, ceruloplasmin, hemopexin, hemoglobin, and complement factor B. After ion exchange chromatography, transferrin was absent from the active fractions.
Immunoblot with anti-human hemoglobin antibody revealed that hemoglobin was present in all active fractions after IEF (Fig. 3C) and in plasma from normal subjects (Fig. 3D). Interestingly, the amount of hemoglobin detected by both immunoblot analysis and by hemoglobin ELISA correlated with NO-scavenging activity (Fig. 3E). These results suggested that hemoglobin may be responsible for NO scavenging; however, this result was unanticipated because the hemoglobin tetramer is <100 kDa. We therefore considered that hemoglobin may complex with other macromolecules in plasma to contribute to the NO-scavenging effect. This hypothesis was consistent with the codetection of hemoglobin and haptoglobin from active IEF/Q Sepharose and Sephacryl S-200 columns by MALDI-TOF. Immunoprecipitation of hemoglobin or haptoglobin from plasma demonstrated that removing these complexes eliminated the plasma NO-scavenging activity (Fig. 3F). Anti-human albumin antibody and beads without coupling of any antibodies were used as controls and did not eliminate NO scavenging. Furthermore, MALDI-TOF demonstrated that both hemoglobin and haptoglobin were present in eluate after plasma immunoprecipitation with anti-hemoglobin and anti-haptoglobin antibodies (data not shown).
We further studied the complex of hemoglobin and haptoglobin by treating the active IEF fraction and plasma with and without 1% SDS and separation by PAGE under native conditions. Immunoblot with anti-hemoglobin showed that hemoglobin was complexed with haptoglobin and migrated slowly as a large-molecular-mass multimeric complex (Fig. 3G).
Reduction of Fe(III) Hemoglobin to Fe(II) Hemoglobin by Plasma. To assess the ability of plasma to reduce Fe(III) hemoglobin to Fe(II) hemoglobin, we incubated 15 μM methemoglobin in 500 μl of freshly obtained human plasma and observed a steady increase in NO consumption over 120 min. A <10-kDa filtrate of human plasma reduced methemoglobin at a comparable rate. Methemoglobin was also reduced by ascorbate and urate (plasma reduced methemoglobin at the same rate as 0.1 and 0.5 mM ascorbate). Consistent with this in vitro effect, after discontinuation of NO inhalation in five normal volunteers, the methemoglobin signal measured by EPR decreased over 1 h, consistent with an in vivo reduction of Fe(III) to Fe(II) plasma hemoglobin (Fig. 1 F and G). These results oppose the widely held view that hemoglobin in plasma is rapidly oxidized to methemoglobin and are consistent with previously published studies showing that plasma hemoglobin in patients with sickle cell disease is 84% Fe(II)-state (26).
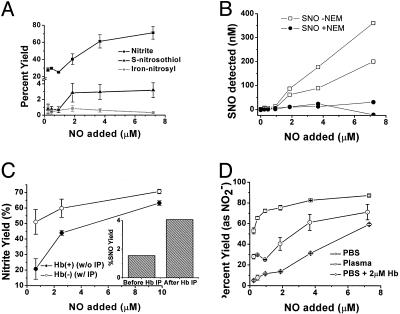
Plasma NO-Scavenging Activity Limits SNO-Albumin Formation. To determine whether the presence of haptoglobin-hemoglobin complexes in plasma modulates NO reaction products in plasma, we examined such product yields at NO concentrations below and above 2 μM. Plasma was incubated with ProliNONOate at concentrations indicated for 30 min and then analyzed for nitrite, S-nitrosothiol, and mercury-stable NO-complexes. Consistent with high-affinity scavenging of NO by haptoglobin-hemoglobin complexes, S-nitrosation chemistry was limited at NO concentrations <2 μM (Fig. 4A). After saturation of the haptoglobin-hemoglobin with NO exposure >2 μM, the levels of S-nitrosothiol and nitrite increased to 3% and 70% of the total NO added, respectively (Fig. 4A). Similar product yields of micromolar NO additions in plasma have been reported by Rassaf and colleagues (19). As an additional assay control, plasma was pretreated with NEM to confirm S-nitrosothiol specificity of assay (Fig. 4B). Immunoprecipitation of hemoglobin from plasma resulted in an increased nitrite (Fig. 4C) and S-nitrosothiol yield (Fig. 4C Inset), supporting a role for the haptoglobin-hemoglobin complex in modulating NO reactions in plasma. Interestingly, although nitrite did not form with the addition of a <2 μM NO donor to PBS with 2 μM hemoglobin, in plasma, 30% of added NO (at concentrations <2 μM NO) formed nitrite even in the presence of haptoglobin-hemoglobin complexes (Fig. 4D). This finding suggests that there exists fast NO oxidase reaction pathways, likely metal-based, for nitrite formation that can compete with the NO-hemoglobin reaction. A similar NO oxidase activity has been observed in cardiomyosytes and ascribed to cytochrome c oxidase (28).
Fig. 4.
Plasma NO-scavenging activity limits SNO-albumin formation. (A) The percent yield of nitrite in plasma compared with the percent yield of S-nitrosothiols and iron-nitrosyl/N-nitrosamine (mercury-stable). (B) NEM treatment (filled circles) completely eliminated formation of S-nitrosothiols (open squares) from NO added to plasma. (C) Percent yield of nitrite from NO in plasma before (filled circles) and after (open circles) immunoprecipitation (IP) with antibodies against hemoglobin (Hb). (Inset) S-nitrosothiol yields below 2 μM NO exposure with and without immunoprecipitation with antibodies against hemoglobin. (D) The percent yield of nitrite from NO in PBS (squares), plasma with 2 μM oxyhemoglobin (in heme; circles) and PBS plus 2 μM oxyhemoglobin (diamonds). Some error bars appear within the symbols.
Discussion
In this study, we evaluate the reaction pathways for NO in human plasma in vivo and in vitro. Consistent with the recent work of Rassaf and colleagues (19), our data suggest that plasma NO complexes, measured by triiodide-based reductive chemiluminescence and previously considered to be SNO-albumin, are largely mercury-stable and thus represent iron-nitrosyl- or N-nitrosamine complexes. In our studies, SNO-albumin does not form during NO gas inhalation in human volunteers, and in vitro we observe limited SNO-albumin formation with NO exposure <2 μM. These studies further suggest that the limited formation of S-nitrosothiol in vitro and in vivo occurs secondary to the rapid, high-affinity dioxygenation reaction of NO with plasma haptoglobin-hemoglobin complexes in addition to the reactions of NO with intraerthrocytic hemoglobin. As opposed to SNO-albumin, nitrite forms at all NO concentrations with approximate 30% yield below 2 μM NO flux and 70% yield above 2 μM NO flux. These data require a reappraisal of current models of NO storage and transport in plasma, suggesting that other NO-derived species may contribute and that heme-NO reactions participate in plasmatic NO homeostasis and modulate reaction pathways. We suggest that these data support an expanded examination of the potential plasma NO storage species, including nitrite (21), N-nitrosamines (19, 22, 23), iron-nitrosyls (24), and recently identified nitrated lipids (25).
The levels of S-nitrosothiol in plasma observed here are consistent with the results of a number of research groups that used reductive chemiluminescent assays and are strikingly lower than previously published values obtained from photolysis-based chemiluminescence. We recognize that these differences in levels reported in the literature may be secondary to methodologies used (photolysis of samples versus chemical reduction), species differences in thiol reactivities, and disease or treatment states (e.g., sepsis, renal failure, etc.). However, the methodology used in this study is highly sensitive and specific for SNO-albumin, SNO-hemoglobin, and S-nitrosated glutathione, cysteine, and penicillamine, and has been independently confirmed by multiple research groups (19, 20, 29-35). Many of the discrepancies in published values are between values measured by using photolysis-based ligand reduction. Although these methods are extremely sensitive, Dejam and colleagues (36) have demonstrated that photolysis-based assays in the presence of thiols may convert nitrate to NO, thus leading to high measured levels of S-nitrosothiol in biological mediums. On the other hand, investigators using such methodologies have been careful to remove contaminating nitrate in many of these studies. Continued studies comparing both methodologies are required to fully resolve these discrepancies.
Regardless of the actual levels of plasma SNO-albumin observed in this study, we observed a striking ability of plasma to rapidly consume NO; this fast consumption reaction appeared to greatly modulate NO product yields both in vitro and in vivo. The identification of the haptoglobin-hemoglobin complex as the source of this NO scavenging is consistent with a role for the haptoglobin-hemoglobin complex in regulating vascular homeostasis and NO bioavailability. It has been reported that (37, 38) complexes of hemoglobin-haptoglobin inhibit endothelium-dependent relaxation. Our results demonstrate that this complex holds hemoglobin dimer in the Fe(II) oxidation state and reacts with NO in a diffusion-limited dioxygenation reaction to scavenge and inactivate NO. These observations potentially provide a mechanism for the observed inhibition of endothelium-dependent relaxation (37) as well as the associations of haptoglobin phenotype with cardiovascular disease risk (39-44). Interestingly, the ability of this complex to inhibit acetylcholine-induced, endothelium-dependent relaxation is less than that of free hemoglobin, suggesting that the formation of high-molecular-mass haptoglobin-hemoglobin multimers serves to limit the NO-scavenging effects on blood vessels. This molecular-mass-associated reduction in vascular NO scavenging has also been observed with the high-molecular-mass, “decorated,” synthetic hemoglobin preparations (45).
The consistent levels of plasma haptoglobin-hemoglobin in human plasma observed in our study and others, the maintenance of the hemoglobin in a reduced form by plasmatic reductants, and the formation of high-molecular-mass multimers suggest a physiologically regulated system that participates in NO homeostasis. NO is capable of diffusion to great distances at physiological oxygen tensions in tissue (46). As such, endothelially produced NO would be capable of distant endocrine vasodilation, resulting in a loss of local metabolic and flow-regulated vasocontrol. We speculate that a teleologically maintained balance of erythrocytic red blood cell mass (with limitation of NO reactivity by perierythrocytic diffusional barriers) and plasma haptoglobin-hemoglobin complexes (with limitation of NO reactivity by formation of high-molecular-mass multimers) limits NO largely as a locally produced and regulated paracrine vasodilator (5, 47). During hypoxic or metabolic stress, intravascular NO-stabilizing species, such as SNO-albumin, nitrite (21), N-nitrosamines (19, 22, 23), iron-nitrosyls (20), and recently identified nitrated lipids (25), may participate in NO homeostasis. Future studies evaluating the role of haptoglobin multimer formation, haptoglobin haplotypes, and haptoglobin polymorphisms will be required to test this thesis.
Supplementary Material
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SNO-albumin, S-nitroso-albumin; DETA NONOate, (Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate; DTPA, diethylenetriaminepentaacetic acid; NEM, N-ethylmaleimide; EPR, electron paramagnetic resonance; IEF, isoelectric focusing; MALDI-TOF, matrix-assisted laser desorption ionization-time-of-flight.
References
- 1.Liu, X., Miller, M. J., Joshi, M. S., Sadowska-Krowicka, H., Clark, D. A. & Lancaster, J. R., Jr. (1998) J. Biol. Chem. 273, 18709-18713. [DOI] [PubMed] [Google Scholar]
- 2.Coin, J. T. & Olson, J. S. (1979) J. Biol. Chem. 254, 1178-1190. [PubMed] [Google Scholar]
- 3.Huang, K. T., Han, T. H., Hyduke, D. R., Vaughn, M. W., Van Herle, H., Hein, T. W., Zhang, C., Kuo, L. & Liao, J. C. (2001) Proc. Natl. Acad. Sci. USA 98, 11771-11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao, J. C., Hein, T. W., Vaughn, M. W., Huang, K. T. & Kuo, L. (1999) Proc. Natl. Acad. Sci. USA 96, 8757-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladwin, M. T., Lancaster, J. R., Freeman, B. A. & Schechter, A. N. (2003) Nat. Med. 9, 496-500. [DOI] [PubMed] [Google Scholar]
- 6.Stamler, J. S., Jaraki, O., Osborne, J., Simon, D. I., Keaney, J., Vita, J., Singel, D., Valeri, C. R. & Loscalzo, J. (1992) Proc. Natl. Acad. Sci. USA 89, 7674-7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamler, J. S., Simon, D. I., Osborne, J. A., Mullins, M. E., Jaraki, O., Michel, T., Singel, D. J. & Loscalzo, J. (1992) Proc. Natl. Acad. Sci. USA 89, 444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minamiyama, Y., Takemura, S. & Inoue, M. (1996) Biochem. Biophys. Res. Commun. 225, 112-115. [DOI] [PubMed] [Google Scholar]
- 9.Rassaf, T., Preik, M., Kleinbongard, P., Lauer, T., Heiss, C., Strauer, B. E., Feelisch, M. & Kelm, M. (2002) J. Clin. Invest. 109, 1241-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scharfstein, J. S., Keaney, J. F., Jr., Slivka, A., Welch, G. N., Vita, J. A., Stamler, J. S. & Loscalzo, J. (1994) J. Clin. Invest. 94, 1432-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nedospasov, A., Rafikov, R., Beda, N. & Nudler, E. (2000) Proc. Natl. Acad. Sci. USA 97, 13543-13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marley, R., Feelisch, M., Holt, S. & Moore, K. (2000) Free Radical Res. 32, 1-9. [DOI] [PubMed] [Google Scholar]
- 13.Marley, R., Patel, R. P., Orie, N., Ceaser, E., Darley-Usmar, V. & Moore, K. (2001) Free Radical Biol. Med. 31, 688-696. [DOI] [PubMed] [Google Scholar]
- 14.Jourd'heuil, D., Hallen, K., Feelisch, M. & Grisham, M. B. (2000) Free Radical Biol. Med. 28, 409-417. [DOI] [PubMed] [Google Scholar]
- 15.Jourd'heuil, D., Gray, L. & Grisham, M. B. (2000) Biochem. Biophys. Res. Commun. 273, 22-26. [DOI] [PubMed] [Google Scholar]
- 16.Rafikova, O., Rafikov, R. & Nudler, E. (2002) Proc. Natl. Acad. Sci. USA 99, 5913-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keaney, J. F., Jr., Simon, D. I., Stamler, J. S., Jaraki, O., Scharfstein, J., Vita, J. A. & Loscalzo, J. (1993) J. Clin. Invest. 91, 1582-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi, R., Giustarini, D., Milzani, A., Colombo, R., Dalle-Donne, I. & Di Simplicio, P. (2001) Circ. Res. 89, E47-E47. [PubMed] [Google Scholar]
- 19.Rassaf, T., Bryan, N. S., Kelm, M. & Feelisch, M. (2002) Free Radical Biol. Med. 33, 1590-1596. [DOI] [PubMed] [Google Scholar]
- 20.Gladwin, M. T., Ognibene, F. P., Pannell, L. K., Nichols, J. S., Pease-Fye, M. E., Shelhamer, J. H. & Schechter, A. N. (2000) Proc. Natl. Acad. Sci. USA 97, 9943-9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosby, K., Partovi, K. S., Crawford, J. H., Patel, R. P., Reiter, C. D., Martyr, S., Yang, B. K., Waclawiw, M. A., Zalos, G., Xu, X., et al. (2003) Nat. Med. 9, 1498-1505. [DOI] [PubMed] [Google Scholar]
- 22.Lippton, H. L., Gruetter, C. A., Ignarro, L. J., Meyer, R. L. & Kadowitz, P. J. (1982) Can. J. Physiol. Pharmacol. 60, 68-75. [DOI] [PubMed] [Google Scholar]
- 23.Gruetter, C. A., Barry, B. K., McNamara, D. B., Kadowitz, P. J. & Ignarro, L. J. (1980) J. Pharmacol. Exp. Ther. 214, 9-15. [PubMed] [Google Scholar]
- 24.Gladwin, M. T., Shelhamer, J. H., Schechter, A. N., Pease-Fye, M. E., Waclawiw, M. A., Panza, J. A., Ognibene, F. P. & Cannon, R. O., III (2000) Proc. Natl. Acad. Sci. USA 97, 11482-11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim, D. G., Sweeney, S., Bloodsworth, A., White, C. R., Chumley, P. H., Krishna, N. R., Schopfer, F., O'Donnell, V. B., Eiserich, J. P. & Freeman, B. A. (2002) Proc. Natl. Acad. Sci. USA 99, 15941-15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter, C. D., Wang, X., Tanus-Santos, J. E., Hogg, N., Cannon, R. O., Schechter, A. N. & Gladwin, M. T. (2002) Nat. Med. 8, 1383-1389. [DOI] [PubMed] [Google Scholar]
- 27.Telford, R. D., Sly, G. J., Hahn, A. G., Cunningham, R. B., Bryant, C. & Smith, J. A. (2003) J. Appl. Physiol. 94, 38-42. [DOI] [PubMed] [Google Scholar]
- 28.Pearce, L. L., Kanai, A. J., Birder, L. A., Pitt, B. R. & Peterson, J. (2002) J. Biol. Chem. 277, 13556-13562. [DOI] [PubMed] [Google Scholar]
- 29.Samouilov, A. & Zweier, J. L. (1998) Anal. Biochem. 258, 322-330. [DOI] [PubMed] [Google Scholar]
- 30.Han, T. H., Hyduke, D. R., Vaughn, M. W., Fukuto, J. M. & Liao, J. C. (2002) Proc. Natl. Acad. Sci. USA 99, 7763-7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi, M. S., Ferguson, T. B., Jr., Han, T. H., Hyduke, D. R., Liao, J. C., Rassaf, T., Bryan, N., Feelisch, M. & Lancaster, J. R., Jr. (2002) Proc. Natl. Acad. Sci. USA 17, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rassaf, T., Bryan, N. S., Maloney, R. E., Specian, V., Kelm, M., Kalyanaraman, B., Rodriguez, J. & Feelisch, M. (2003) Nat. Med. 9, 481-483. [DOI] [PubMed] [Google Scholar]
- 33.Gladwin, M. T., Wang, X., Reiter, C. D., Yang, B. K., Vivas, E. X., Bonaventura, C. & Schechter, A. N. (2002) J. Biol. Chem. 21, 21. [DOI] [PubMed] [Google Scholar]
- 34.Xu, X., Cho, M., Spencer, N. Y., Patel, N., Huang, Z., Shields, H., King, S. B., Gladwin, M. T., Hogg, N. & Kim-Shapiro, D. B. (2003) Proc. Natl. Acad. Sci. USA 100, 11303-11308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi, M. S., Ferguson, T. B., Jr., Han, T. H., Hyduke, D. R., Liao, J. C., Rassaf, T., Bryan, N., Feelisch, M. & Lancaster, J. R., Jr. (2002) Proc. Natl. Acad. Sci. USA 17, 10341-10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dejam, A., Kleinbongard, P., Rassaf, T., Hamada, S., Gharini, P., Rodriguez, J., Feelisch, M. & Kelm, M. (2003) Free Radical Biol. Med. 35, 1551-1559. [DOI] [PubMed] [Google Scholar]
- 37.Edwards, D. H., Griffith, T. M., Ryley, H. C. & Henderson, A. H. (1986) Cardiovasc. Res. 20, 549-556. [DOI] [PubMed] [Google Scholar]
- 38.Nakai, K., Ohta, T., Sakuma, I., Akama, K., Kobayashi, Y., Tokuyama, S., Kitabatake, A., Nakazato, Y., Takahashi, T. A. & Sadayoshi, S. (1996) J. Cardiovasc. Pharmacol. 28, 115-123. [DOI] [PubMed] [Google Scholar]
- 39.Asleh, R., Marsh, S., Shilkrut, M., Binah, O., Guetta, J., Lejbkowicz, F., Enav, B., Shehadeh, N., Kanter, Y., Lache, O., et al. (2003) Circ. Res. 92, 1193-1200. [DOI] [PubMed] [Google Scholar]
- 40.Chapelle, J. P., Albert, A., Smeets, J. P., Heusghem, C. & Kulbertus, H. E. (1982) N. Engl. J. Med. 307, 457-463. [DOI] [PubMed] [Google Scholar]
- 41.Levy, A. P., Roguin, A., Hochberg, I., Herer, P., Marsh, S., Nakhoul, F. M. & Skorecki, K. (2000) N. Engl. J. Med. 343, 969-970. [DOI] [PubMed] [Google Scholar]
- 42.Levy, A. P., Hochberg, I., Jablonski, K., Resnick, H. E., Lee, E. T., Best, L. & Howard, B. V. (2002) J. Am. Coll. Cardiol. 40, 1984-1990. [DOI] [PubMed] [Google Scholar]
- 43.Nakhoul, F. M., Marsh, S., Hochberg, I., Leibu, R., Miller, B. P. & Levy, A. P. (2000) J. Am. Med. Assoc. 284, 1244-1245. [DOI] [PubMed] [Google Scholar]
- 44.Nakhoul, F. M., Zoabi, R., Kanter, Y., Zoabi, M., Skorecki, K., Hochberg, I., Leibu, R., Miller, B. & Levy, A. P. (2001) Diabetologia 44, 602-604. [DOI] [PubMed] [Google Scholar]
- 45.Dou, Y., Maillett, D. H., Eich, R. F. & Olson, J. S. (2002) Biophys. Chem. 98, 127-148. [DOI] [PubMed] [Google Scholar]
- 46.Thomas, D. D., Liu, X., Kantrow, S. P. & Lancaster, J. R., Jr. (2001) Proc. Natl. Acad. Sci. USA 98, 355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schechter, A. N. & Gladwin, M. T. (2003) N. Engl. J. Med. 348, 1483-1485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.