Abstract
Photorespiration, a process that diminishes net photosynthesis by ≈25% in most plants, has been viewed as the unfavorable consequence of plants having evolved when the atmosphere contained much higher levels of carbon dioxide than it does today. Here we used two independent methods to show that exposure of Arabidopsis and wheat shoots to conditions that inhibited photorespiration also strongly inhibited nitrate assimilation. Thus, nitrate assimilation in both dicotyledonous and monocotyledonous species depends on photorespiration. This previously undescribed role for photorespiration (i) explains several responses of plants to rising carbon dioxide concentrations, including the inability of many plants to sustain rapid growth under elevated levels of carbon dioxide; and (ii) raises concerns about genetic manipulations to diminish photorespiration in crops.
Keywords: global climate change, CO2 acclimation, Arabidopsis, wheat
Rubisco, the most prevalent protein in plants, indeed in the biosphere, catalyzes the reaction of ribulose-1,5-bisphosphate with either CO2 or O2 and thereby initiates, respectively, the CO2 assimilatory (C3 reductive) or photorespiratory (C2 oxidative) pathways. The balance between the two reactions depends on the relative concentrations of CO2 and O2 at the site of catalysis. At current atmospheric levels of CO2 (≈360 μmol·mol-1) and O2 (≈209,700 μmol·mol-1), photorespiration in C3 plants dissipates >25% of the carbon fixed during CO2 assimilation (1). Thus, photorespiration has been viewed as a wasteful process, a vestige of the high CO2 atmospheres under which plants evolved (2). At best, according to current thought, photorespiration may mitigate photoinhibition under high light and drought stress (2, 3) or may generate amino acids such as glycine for other metabolic pathways (4). Genetic modification of Rubisco to minimize photorespiration in crop plants has been the goal of many investigations (5).
Atmospheric CO2 concentrations will rise to somewhere between 600 and 1,000 μmol·mol-1 by the end of the 21st century (6). Transferring C3 plants from ambient (≈360 μmol·mol-1) to elevated (≈720 μmol·mol-1) CO2 concentrations decreases photorespiration and initially stimulates net CO2 assimilation and growth by ≈30% (7). With longer exposures to elevated CO2 concentrations (days to weeks), however, net CO2 assimilation and plant growth slow down until they stabilize at rates that average 12% (8) and 8% (9), respectively, above those of plants kept at ambient CO2 concentrations. This phenomenon, known as CO2 acclimation, is often associated with diminished activities of Rubisco and other enzymes in the C3 reductive photosynthetic carbon cycle (10, 11), but the influence of elevated CO2 may not be specific to these enzymes (12). Rather, CO2 acclimation follows a 14% decline in overall shoot nitrogen concentrations (13), a change nearly double what would be expected if a given amount of nitrogen were diluted by the additional biomass that accumulates under elevated CO2 concentrations (9, 12).
We proposed a relatively simple explanation for these responses: elevated CO2 concentrations inhibit the assimilation of nitrate (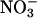 ) in shoots of C3 plants (14-16). Because
) in shoots of C3 plants (14-16). Because 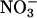 is the prominent form of inorganic nitrogen available to plants from temperate well aerated soils (17), diminished
is the prominent form of inorganic nitrogen available to plants from temperate well aerated soils (17), diminished 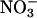 assimilation dramatically alters the nitrogen balance in C3 plants (15). Much of our evidence was based on estimates of shoot
assimilation dramatically alters the nitrogen balance in C3 plants (15). Much of our evidence was based on estimates of shoot 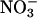 assimilation derived from calculations of the difference in the assimilatory quotient (ΔAQ, ratio of net CO2 consumption to net O2 evolution) between plants that received
assimilation derived from calculations of the difference in the assimilatory quotient (ΔAQ, ratio of net CO2 consumption to net O2 evolution) between plants that received 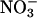 as their sole nitrogen source and those that received ammonium (
as their sole nitrogen source and those that received ammonium (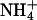 ) as their sole source. Here, we establish ΔAQ as a measure of
) as their sole source. Here, we establish ΔAQ as a measure of 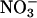 assimilation using genotypes of Arabidopsis in which
assimilation using genotypes of Arabidopsis in which 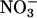 reductase activities are enhanced or deficient. We then use both ΔAQ and an independent measure to demonstrate that
reductase activities are enhanced or deficient. We then use both ΔAQ and an independent measure to demonstrate that 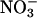 assimilation depends on photorespiration in a dicotyledon (Arabidopsis) and a monocotyledon (wheat). These results offer a different perspective on the importance of photorespiration and on attempts to minimize it.
assimilation depends on photorespiration in a dicotyledon (Arabidopsis) and a monocotyledon (wheat). These results offer a different perspective on the importance of photorespiration and on attempts to minimize it.
Materials and Methods
Materials and Growth Conditions. We used three genotypes of Arabidopsis thaliana cv. Columbia: (i) the wild type, (ii) a transgenic line harboring the chimeric gene Lhch1*3::Nia1*2 that overexpresses one form of 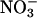 reductase (18), and (iii) a genotype with mutations in both structural genes for
reductase (18), and (iii) a genotype with mutations in both structural genes for 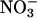 reductase, nia1 nia2 (19). Seeds were germinated on plates filled with a dilute Murashige-Skoog medium (2.3 g·liter-1) in 0.75% Phytagar (GIBCO/BRL). The plates were placed in controlled environment chambers (Conviron, Winnipeg, MB, Canada) at ambient CO2 levels and received 9 h of 350 μmol·m-2·s-1 photosynthetically active radiation and 24°C. After 10 d, seedlings were transferred one at a time to 5 × 40-mm pieces of rock wool (Grodania, Hovedgaden, Denmark). Twenty seedlings were transplanted to an opaque 4-liter polyethylene container, the end of the rock wool opposite the seedling being immersed in an aerated nutrient solution containing 200 μM NH4Cl and 200 μM KNO3 as nitrogen sources (20). This solution was changed every 3 d. The container was placed in the same controlled environment chamber as the plates.
reductase, nia1 nia2 (19). Seeds were germinated on plates filled with a dilute Murashige-Skoog medium (2.3 g·liter-1) in 0.75% Phytagar (GIBCO/BRL). The plates were placed in controlled environment chambers (Conviron, Winnipeg, MB, Canada) at ambient CO2 levels and received 9 h of 350 μmol·m-2·s-1 photosynthetically active radiation and 24°C. After 10 d, seedlings were transferred one at a time to 5 × 40-mm pieces of rock wool (Grodania, Hovedgaden, Denmark). Twenty seedlings were transplanted to an opaque 4-liter polyethylene container, the end of the rock wool opposite the seedling being immersed in an aerated nutrient solution containing 200 μM NH4Cl and 200 μM KNO3 as nitrogen sources (20). This solution was changed every 3 d. The container was placed in the same controlled environment chamber as the plates.
We surface-sterilized wheat (Triticum aestivum cv. Veery 10) seeds for 1 min in 2.6% NaClO, washed them thoroughly with water, and germinated them for several days on thick paper toweling saturated with 10 mM CaSO4. Twenty seedlings were transplanted to a 19-liter opaque polyethylene tub filled with an aerated nutrient solution containing 200 μM NH4NO3 (21). The solution was replenished every 3 d. The tubs were placed in a controlled environment chamber (Conviron), providing a photosynthetic photon flux density (PFD) of 650 μmol of quanta m-2·s-1 at plant height and a 16 h/25°C day and 8 h/15°C night. After ≈14 d, we transferred a seedling that had three true leaves into a gas-exchange measurement system.
Nitrate Reductase Activity. To assess 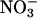 reductase activity in Arabidopsis, 1 g of leaf material was ground with fine glass beads in a cold mortar that contained 4 ml of 0.1 M K-phosphate (pH 7.5), 1 mM EDTA, 3 mM cysteine, and 3% (wt/vol) casein (22). The homogenate was centrifuged at 30,000 × g for 10 min and the supernatant assayed for in vivo and fully activated
reductase activity in Arabidopsis, 1 g of leaf material was ground with fine glass beads in a cold mortar that contained 4 ml of 0.1 M K-phosphate (pH 7.5), 1 mM EDTA, 3 mM cysteine, and 3% (wt/vol) casein (22). The homogenate was centrifuged at 30,000 × g for 10 min and the supernatant assayed for in vivo and fully activated 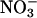 reductase activity according to the procedure of Kaiser et al. (23).
reductase activity according to the procedure of Kaiser et al. (23).
Gas-Exchange Measurements. A plant was sealed by a rubber stopper around its stem into a shoot and root cuvette (24, 25). Leaves in the shoot cuvette were at their normal orientation; thus the angle of incidence was between 0° and 45° for Arabidopsis and 70° and 80° for wheat. Net gas fluxes from the shoot were monitored with the instrumentation described previously (15, 24). In brief, an infrared gas analyzer (Horiba VIA-500R, Kyoto) measured CO2 fluxes, a custom O2 analyzer based on heated zirconium oxide ceramic cells measured O2 fluxes, and relative humidity sensors (Vaisala, Helsinki) measured water vapor fluxes. Mass flow controllers (Tylan, Torrance, CA) prepared the various gas mixtures, and a pressure transducer (Validyne, North Ridge, CA) monitored the gas flows through the shoot cuvette. We also placed wheat leaves in a leaf cuvette (LI-6400-40, Li-Cor, Lincoln, NE) and estimated the gross O2 exchange from chlorophyll fluorescence, but this measure did not respond to nitrogen source or CO2 level (26).
Nitrate Absorption and Accumulation. Wild-type Arabidopsis and wheat were grown as described above, except that 3 d before measurement for Arabidopsis and 2 d for wheat, the plants were shifted from a medium containing 200 μM NH4Cl and 200 μM KNO3 to one devoid of nitrogen. This protocol induced 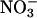 absorption and
absorption and 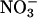 reductase but then depleted the plant tissue of free
reductase but then depleted the plant tissue of free 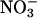 . The night before measurements, five to eight plants were transferred to a multiplant measurement system (27). The next morning, Arabidopsis or wheat plants received, respectively, 500 or 1,000 μmol·m-2·s-1 photosynthetically active radiation at plant height. The plants were exposed to an atmosphere of (i) 360 μmol·mol-1 CO2 and 21% O2, (ii) 720 μmol·mol-1 CO2 and 21% O2, or (iii) 360 μmol·mol-1 CO2 and 2% O2. Then during a measurement period of 1 h for the Arabidopsis and 2 h for wheat, the plants were shifted to an aerated medium containing 0 or 5.5 μmol
. The night before measurements, five to eight plants were transferred to a multiplant measurement system (27). The next morning, Arabidopsis or wheat plants received, respectively, 500 or 1,000 μmol·m-2·s-1 photosynthetically active radiation at plant height. The plants were exposed to an atmosphere of (i) 360 μmol·mol-1 CO2 and 21% O2, (ii) 720 μmol·mol-1 CO2 and 21% O2, or (iii) 360 μmol·mol-1 CO2 and 2% O2. Then during a measurement period of 1 h for the Arabidopsis and 2 h for wheat, the plants were shifted to an aerated medium containing 0 or 5.5 μmol 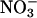 . Absorption was assessed by the amount of
. Absorption was assessed by the amount of 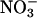 remaining in the medium after the measurement period. After the measurement period, the plants were divided into shoots and roots, oven-dried, and ground to a powder in a ball mill. Water extracts of the powder were analyzed for
remaining in the medium after the measurement period. After the measurement period, the plants were divided into shoots and roots, oven-dried, and ground to a powder in a ball mill. Water extracts of the powder were analyzed for 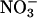 via HPLC (28), and
via HPLC (28), and 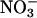 accumulation in the shoots and roots were calculated from the difference in
accumulation in the shoots and roots were calculated from the difference in 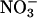 content between the plants that had received
content between the plants that had received 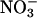 during the measurement period and those that had not. Nitrate assimilation was calculated as the difference in the rates of
during the measurement period and those that had not. Nitrate assimilation was calculated as the difference in the rates of 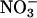 absorption and plant
absorption and plant 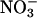 accumulation. The rate of shoot
accumulation. The rate of shoot 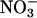 accumulation was the amount of
accumulation was the amount of 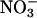 accumulated in the shoots during the measurement period divided by the time.
accumulated in the shoots during the measurement period divided by the time.
Statistics. A repeated-measures analysis of variance was performed by using the mixed procedure in sas (PROC MIXED, SAS Institute, Cary, NC). The PFD was considered to be a repeated factor, because each canopy was measured at all five levels of PFD. Effects of the treatments and their interactions were considered significant when P < 0.05.
Results
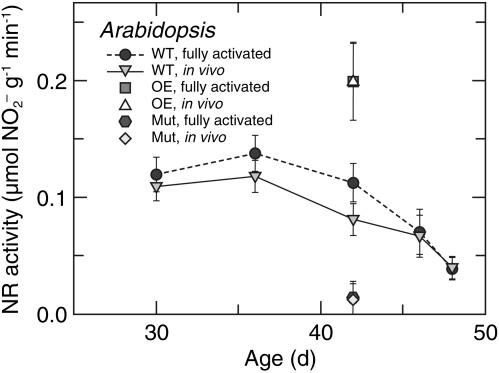
Nitrate Reductase Activities. In Arabidopsis, 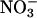 reductase in the shoot was nearly fully activated (Fig. 1). In 36-d-old wild-type plants, the fully activated rates of reduction in μmol of
reductase in the shoot was nearly fully activated (Fig. 1). In 36-d-old wild-type plants, the fully activated rates of reduction in μmol of 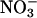 per g of fresh mass per min (mean ± SE, n = 10) were 0.13 ± 0.02 in the shoots (Fig. 1) and 0.030 ± 0.001 in the roots at ambient CO2 concentrations. The short-day regime under which the Arabidopsis plants were grown prevented them from flowering, but as the wild-type plants aged from 36 to 48 d,
per g of fresh mass per min (mean ± SE, n = 10) were 0.13 ± 0.02 in the shoots (Fig. 1) and 0.030 ± 0.001 in the roots at ambient CO2 concentrations. The short-day regime under which the Arabidopsis plants were grown prevented them from flowering, but as the wild-type plants aged from 36 to 48 d, 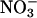 reductase activity in the shoots diminished markedly (Fig. 1). A transgenic line that harbored the chimeric gene Lhch1*3::Nia1*2 (29) had twice the
reductase activity in the shoots diminished markedly (Fig. 1). A transgenic line that harbored the chimeric gene Lhch1*3::Nia1*2 (29) had twice the 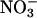 reductase activity of the wild type, whereas a genotype with mutations in both structural genes for
reductase activity of the wild type, whereas a genotype with mutations in both structural genes for 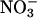 reductase, nia1 nia2 (19), had no significant activity (Fig. 1). In wheat, the fully activated rates of
reductase, nia1 nia2 (19), had no significant activity (Fig. 1). In wheat, the fully activated rates of 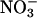 reductase activity in μmol of
reductase activity in μmol of 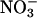 per g of fresh mass per min (mean ± SE, n = 6) were 0.58 ± 0.03 and 0.021 ± 0.003 in the shoots and roots, respectively, at ambient CO2 concentrations and 0.46 ± 0.06 and 0.023 ± 0.002 in the shoots and roots, respectively, at elevated CO2 concentrations (15).
per g of fresh mass per min (mean ± SE, n = 6) were 0.58 ± 0.03 and 0.021 ± 0.003 in the shoots and roots, respectively, at ambient CO2 concentrations and 0.46 ± 0.06 and 0.023 ± 0.002 in the shoots and roots, respectively, at elevated CO2 concentrations (15).
Fig. 1.
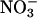 reductase activity (μmol of
reductase activity (μmol of 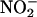 generated per g of fresh mass per min) as a function of plant age (d) in leaves of a wild-type A. thaliana cv. Columbia (WT), a transgenic line harboring the chimeric gene Lhch1*3::Nia1*2 (OE), and a genotype (nia1 nia2) with mutations in both structural genes for
generated per g of fresh mass per min) as a function of plant age (d) in leaves of a wild-type A. thaliana cv. Columbia (WT), a transgenic line harboring the chimeric gene Lhch1*3::Nia1*2 (OE), and a genotype (nia1 nia2) with mutations in both structural genes for 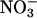 reductase (Mut). Because
reductase (Mut). Because 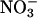 reductase is regulated through phosphorylation, leaf tissue was assayed under conditions that either dephosphorylated the enzyme (fully activated) or did not change its phosphorylation (in vivo). Shown are the mean ± SE (n = 5-8 plants).
reductase is regulated through phosphorylation, leaf tissue was assayed under conditions that either dephosphorylated the enzyme (fully activated) or did not change its phosphorylation (in vivo). Shown are the mean ± SE (n = 5-8 plants).
Shoot Gas Fluxes. We simultaneously monitored net CO2 and O2 fluxes from shoots of intact Arabidopsis and wheat plants as a function of light level. There were six treatments: plants received either 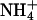 or
or 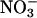 as a nitrogen source and an atmospheric gas composition of either (i) 360 μmol·mol-1 CO2 and 21% O2 (ambient CO2 and O2), (ii) 700 or 720 μmol·mol-1 CO2 and 21% O2 (elevated CO2), or (iii) 360 μmol·mol-1 CO2 and 2% O2 (low O2). Net CO2 consumption was stimulated under elevated CO2 or low O2 concentrations but was similar for both nitrogen treatments (Figs. 5 and 6, which are published as supporting information on the PNAS web site), a response typical for C3 plants that have received ample amounts of nitrogen (30). Net O2 evolution differed most between
as a nitrogen source and an atmospheric gas composition of either (i) 360 μmol·mol-1 CO2 and 21% O2 (ambient CO2 and O2), (ii) 700 or 720 μmol·mol-1 CO2 and 21% O2 (elevated CO2), or (iii) 360 μmol·mol-1 CO2 and 2% O2 (low O2). Net CO2 consumption was stimulated under elevated CO2 or low O2 concentrations but was similar for both nitrogen treatments (Figs. 5 and 6, which are published as supporting information on the PNAS web site), a response typical for C3 plants that have received ample amounts of nitrogen (30). Net O2 evolution differed most between 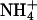 and
and 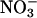 nutrition under ambient CO2 and O2 atmospheres (Figs. 5 and 6).
nutrition under ambient CO2 and O2 atmospheres (Figs. 5 and 6).
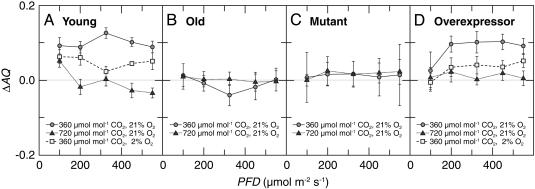
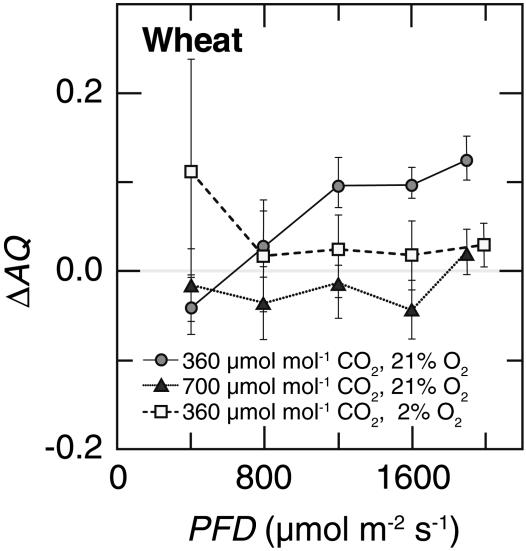
The ΔAQ, the change in the AQ (the ratio of net CO2 consumption to net O2 evolution) with a shift from 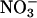 to
to 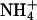 nutrition, highlights these differences (Figs. 2 and 3). Under ambient CO2 and O2 atmospheres, ΔAQ was positive in plants having significant
nutrition, highlights these differences (Figs. 2 and 3). Under ambient CO2 and O2 atmospheres, ΔAQ was positive in plants having significant 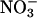 activities (36-d-old wild-type Arabidopsis, Fig. 2A; transgenic Arabidopsis overexpressing
activities (36-d-old wild-type Arabidopsis, Fig. 2A; transgenic Arabidopsis overexpressing 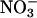 reductase, Fig. 2D; and wheat, Fig. 3), but did not deviate from zero in plants with diminished
reductase, Fig. 2D; and wheat, Fig. 3), but did not deviate from zero in plants with diminished 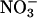 reductase activities (48-d-old wild-type Arabidopsis, Fig. 2B; and the Arabidopsis knockout mutants, Fig. 2C). In Arabidopsis and wheat plants having significant
reductase activities (48-d-old wild-type Arabidopsis, Fig. 2B; and the Arabidopsis knockout mutants, Fig. 2C). In Arabidopsis and wheat plants having significant 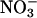 activities, ΔAQ decreased at low O2 concentrations and became negligible at elevated CO2 concentrations (Figs. 2 A and D and 3).
activities, ΔAQ decreased at low O2 concentrations and became negligible at elevated CO2 concentrations (Figs. 2 A and D and 3).
Fig. 2.
Changes in assimilatory quotient with the shift from 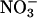 to
to 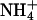 (ΔAQ) as a function of photosynthetic PFD in shoots of A. thaliana cv. Columbia. Thirty-six-day-old wild-type plants (A), 48-d-old wild-type plants (B), a genotype with mutations in the two structural genes for
(ΔAQ) as a function of photosynthetic PFD in shoots of A. thaliana cv. Columbia. Thirty-six-day-old wild-type plants (A), 48-d-old wild-type plants (B), a genotype with mutations in the two structural genes for 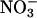 reductase (nia1 nia2) (C), and a transgenic line harboring the chimeric gene Lhch1*3::Nia1*2 (D). The plants were grown under ambient CO2 (360 μmol·mol-1) and measured under ambient CO2 and O2 (360 μmol·mol-1 CO2 and 21% O2; circles), elevated CO2 (720 μmol·mol-1 CO2 and 21% O2; triangles), or low O2 (360 μmol·mol-1 CO2 and 2% O2; squares). Shown are the mean ± SE, n = 5-8 plants.
reductase (nia1 nia2) (C), and a transgenic line harboring the chimeric gene Lhch1*3::Nia1*2 (D). The plants were grown under ambient CO2 (360 μmol·mol-1) and measured under ambient CO2 and O2 (360 μmol·mol-1 CO2 and 21% O2; circles), elevated CO2 (720 μmol·mol-1 CO2 and 21% O2; triangles), or low O2 (360 μmol·mol-1 CO2 and 2% O2; squares). Shown are the mean ± SE, n = 5-8 plants.
Fig. 3.
Changes in assimilatory quotient with the shift from 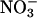 to
to 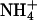 (ΔAQ) as a function of photosynthetic PFD in shoots of wheat (T. aestivum cv. Veery 10). The plants were grown under ambient CO2 (360 μmol·mol-1) and measured under ambient CO2 and O2 (360 μmol·mol-1 CO2 and 21% O2; circles), elevated CO2 (700 μmol·mol-1 CO2 and 21% O2; triangles), or low O2 (360 μmol·mol-1 CO2 and 2% O2; squares). Shown are the mean ± SE, n = 5-8 plants. The data for ambient CO2 and O2 and elevated CO2 and ambient O2 have been published (15).
(ΔAQ) as a function of photosynthetic PFD in shoots of wheat (T. aestivum cv. Veery 10). The plants were grown under ambient CO2 (360 μmol·mol-1) and measured under ambient CO2 and O2 (360 μmol·mol-1 CO2 and 21% O2; circles), elevated CO2 (700 μmol·mol-1 CO2 and 21% O2; triangles), or low O2 (360 μmol·mol-1 CO2 and 2% O2; squares). Shown are the mean ± SE, n = 5-8 plants. The data for ambient CO2 and O2 and elevated CO2 and ambient O2 have been published (15).
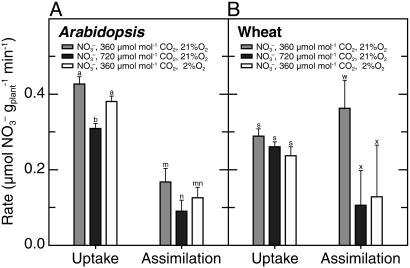
Nitrate Accumulation. Another measure of 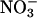 assimilation is the difference between the amount of
assimilation is the difference between the amount of 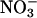 that a plant absorbs and that it accumulates in its tissues. According to this measure, both elevated CO2 and low O2 concentrations inhibited plant
that a plant absorbs and that it accumulates in its tissues. According to this measure, both elevated CO2 and low O2 concentrations inhibited plant 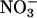 assimilation in Arabidopsis and wheat (Fig. 4), although the influence of low O2 concentrations was significant only at P < 0.2 in Arabidopsis. Absorption of
assimilation in Arabidopsis and wheat (Fig. 4), although the influence of low O2 concentrations was significant only at P < 0.2 in Arabidopsis. Absorption of 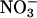 also declined at elevated CO2 and low O2 concentrations but to a lesser extent than
also declined at elevated CO2 and low O2 concentrations but to a lesser extent than 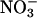 assimilation (Fig. 4). Moreover, the rates at which
assimilation (Fig. 4). Moreover, the rates at which 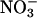 accumulated in the shoots of either species did not differ significantly among treatments (data not shown).
accumulated in the shoots of either species did not differ significantly among treatments (data not shown).
Fig. 4.
In wild-type Arabidopsis and wheat, 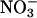 uptake as the amount of
uptake as the amount of 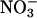 depleted from a medium and
depleted from a medium and 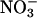 assimilation as the difference between the rates of net
assimilation as the difference between the rates of net 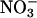 uptake and net accumulation of free
uptake and net accumulation of free 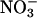 in plant tissues. Thirty-six-d-old Arabidopsis plants (A) or 10-d-old wheat (B) were exposed to either 360 μmol·mol-1 CO2 and 21% O2 (gray), 720 μmol·mol-1 CO2 and 21% O2 (black), or 360 μmol·mol-1 CO2 and 2% O2 (white). Shown are the mean ± SE (n = 13-16). Treatments labeled with different letters differ significantly (P ≤ 0.05). The light levels were 500 and 1,000 μmol·m-2·s-1 PAR for Arabidopsis and wheat, respectively.
in plant tissues. Thirty-six-d-old Arabidopsis plants (A) or 10-d-old wheat (B) were exposed to either 360 μmol·mol-1 CO2 and 21% O2 (gray), 720 μmol·mol-1 CO2 and 21% O2 (black), or 360 μmol·mol-1 CO2 and 2% O2 (white). Shown are the mean ± SE (n = 13-16). Treatments labeled with different letters differ significantly (P ≤ 0.05). The light levels were 500 and 1,000 μmol·m-2·s-1 PAR for Arabidopsis and wheat, respectively.
Discussion
Two independent methods indicated that 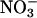 assimilation in Arabidopsis and wheat decreased under both elevated CO2 and low O2 atmospheres.
assimilation in Arabidopsis and wheat decreased under both elevated CO2 and low O2 atmospheres.
The first method was a real-time continuous measure involving AQ, the ratio of net CO2 consumption to net O2 evolution. The AQ decreases as 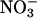 assimilation increases: additional electrons generated from the light-dependent reactions of photosynthesis are transferred to
assimilation increases: additional electrons generated from the light-dependent reactions of photosynthesis are transferred to 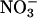 and hence to
and hence to 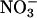 , stimulating net O2 evolution while having little effect on CO2 consumption (15, 24, 31, 32). We present ΔAQ, the change in AQ under
, stimulating net O2 evolution while having little effect on CO2 consumption (15, 24, 31, 32). We present ΔAQ, the change in AQ under 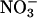 versus
versus 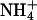 nutrition rather than AQ, because several other biochemical processes such as lipid metabolism can influence AQ, but these processes do not change rapidly with nitrogen source, so ΔAQ should predominantly reflect
nutrition rather than AQ, because several other biochemical processes such as lipid metabolism can influence AQ, but these processes do not change rapidly with nitrogen source, so ΔAQ should predominantly reflect 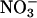 assimilation (32). The ΔAQ also has appropriate scaling, because it should be zero when
assimilation (32). The ΔAQ also has appropriate scaling, because it should be zero when 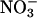 assimilation is negligible and should increase as nitrate assimilation increases. Here (Figs. 2 and 3), ΔAQ differed from zero only in plants with relatively high
assimilation is negligible and should increase as nitrate assimilation increases. Here (Figs. 2 and 3), ΔAQ differed from zero only in plants with relatively high 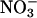 reductase activities, affirming its relationship with
reductase activities, affirming its relationship with 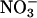 assimilation.
assimilation.
The second method for assessing 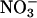 assimilation was a traditional one based on the difference between the total amount of
assimilation was a traditional one based on the difference between the total amount of 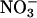 absorbed and that which accumulated in plant tissues (e.g., refs. 33-38). This method has several difficulties.
absorbed and that which accumulated in plant tissues (e.g., refs. 33-38). This method has several difficulties.
It estimates
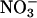 assimilation in the whole plant, not just in the shoots. Nonetheless, the observed changes in total
assimilation in the whole plant, not just in the shoots. Nonetheless, the observed changes in total 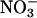 assimilation with CO2 levels (Fig. 4) probably reflected mostly the responses of the shoots, because
assimilation with CO2 levels (Fig. 4) probably reflected mostly the responses of the shoots, because 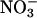 assimilation in the roots usually comprises only a minor percentage of the total during the day (39) and is relatively insensitive to CO2 levels (15). For example,
assimilation in the roots usually comprises only a minor percentage of the total during the day (39) and is relatively insensitive to CO2 levels (15). For example, 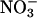 reductase activity was 27 times greater in wheat shoots than roots and 4.3 times greater in 36-d-old wild-type Arabidopsis shoots than roots.
reductase activity was 27 times greater in wheat shoots than roots and 4.3 times greater in 36-d-old wild-type Arabidopsis shoots than roots.This method requires destructive tissue analysis after the uptake measurement and thus cannot be conducted in real time.
Although the plants were deprived of nitrogen for 3 d, free
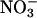 in the tissues of the controls (those that did not receive
in the tissues of the controls (those that did not receive 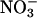 during the uptake measurements) spanned a broad range, causing variation in the estimates of
during the uptake measurements) spanned a broad range, causing variation in the estimates of 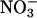 accumulation.
accumulation.Uptake measurements were conducted during the transition from nitrogen deprivation to nitrogen sufficiency. The rates at which
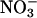 accumulated in the shoots, however, were similar in all treatments (data not shown), indicating that
accumulated in the shoots, however, were similar in all treatments (data not shown), indicating that 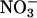 availability in the shoots did not limit assimilation at elevated CO2 concentrations.
availability in the shoots did not limit assimilation at elevated CO2 concentrations.
Despite these difficulties, the decline in 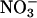 assimilation rates under elevated CO2 or low O2 concentrations determined by this method (Fig. 4) paralleled the results based on the ΔAQ (Figs. 2 and 3).
assimilation rates under elevated CO2 or low O2 concentrations determined by this method (Fig. 4) paralleled the results based on the ΔAQ (Figs. 2 and 3).
A physiological response common to elevated CO2 and low O2 is diminished photorespiration (40). The observed shifts in ΔAQ under elevated CO2 or low O2 concentrations did not result directly from photorespiration. Photorespiration releases CO2 and consumes O2 in equal amounts (41); therefore, if only the photorespiratory pathway were involved, ΔAQ would shift in the opposite direction to the one we observed. For example, the 36-d-old wild-type Arabidopsis under ambient CO2 and O2 had an AQ of 0.94 ± 0.01 under 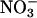 and 1.04 ± 0.01 under
and 1.04 ± 0.01 under 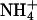 (mean ± SE for the five light levels); equal fluxes of CO2 and O2 from photorespiration would bring the AQ values for these treatments closer together as photorespiration increases and further apart as it decreases. A straightforward interpretation for the decline in ΔAQ at elevated CO2 or low O2 is that
(mean ± SE for the five light levels); equal fluxes of CO2 and O2 from photorespiration would bring the AQ values for these treatments closer together as photorespiration increases and further apart as it decreases. A straightforward interpretation for the decline in ΔAQ at elevated CO2 or low O2 is that 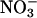 assimilation depends on photorespiration. Our results with the second method for assessing
assimilation depends on photorespiration. Our results with the second method for assessing 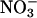 assimilation (Fig. 4) affirm this interpretation.
assimilation (Fig. 4) affirm this interpretation.
Possible Mechanisms. One part of the photorespiratory pathway is the export of malate from the chloroplast through the cytoplasm and into the peroxisome, where it generates NADH, which reduces hydroxypyruvate. This malate “valve” or “shuttle” increases the NADH/NAD ratio in the cytoplasm (42) and thereby may provide NADH instrumental in the reduction of 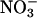 to
to 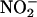 . Malate also serves as a counterion that prevents alkalinization when
. Malate also serves as a counterion that prevents alkalinization when 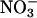 , an anion, becomes incorporated into a neutral amino acid (43). Such processes could explain the observations that
, an anion, becomes incorporated into a neutral amino acid (43). Such processes could explain the observations that 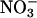 assimilation was fastest in Arabidopsis and wheat under ambient CO2 and O2 concentrations (Figs. 2, 3, 4), the treatment under which photorespiration was highest.
assimilation was fastest in Arabidopsis and wheat under ambient CO2 and O2 concentrations (Figs. 2, 3, 4), the treatment under which photorespiration was highest.
The influence of elevated CO2 concentrations on 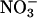 assimilation was more pronounced than that of low concentrations of O2 (Figs. 2 A and D, 3, and 4). Two additional mechanisms contribute to the inhibitory effect of elevated CO2 concentrations on
assimilation was more pronounced than that of low concentrations of O2 (Figs. 2 A and D, 3, and 4). Two additional mechanisms contribute to the inhibitory effect of elevated CO2 concentrations on 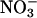 assimilation. (i) Transport of
assimilation. (i) Transport of 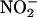 from the cytosol into the chloroplast involves the net diffusion of HNO2 or cotransport of protons and
from the cytosol into the chloroplast involves the net diffusion of HNO2 or cotransport of protons and 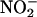 across the chloroplast membrane. This requires the stroma to be more alkaline than the cytosol (44, 45). Elevated concentrations of CO2 can dissipate some of this pH gradient, because additional CO2 movement into the chloroplast acidifies the stroma. As a result, elevated CO2 concentrations inhibited
across the chloroplast membrane. This requires the stroma to be more alkaline than the cytosol (44, 45). Elevated concentrations of CO2 can dissipate some of this pH gradient, because additional CO2 movement into the chloroplast acidifies the stroma. As a result, elevated CO2 concentrations inhibited 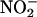 transport into the chloroplast (15). (ii) Several competing processes, the C3 reductive photosynthetic carbon cycle, the reduction of
transport into the chloroplast (15). (ii) Several competing processes, the C3 reductive photosynthetic carbon cycle, the reduction of 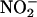 to
to 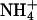 , and the incorporation of
, and the incorporation of 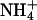 into amino acids, occur in the chloroplast stroma (46) and require reduced ferredoxin generated by photosynthetic electron transport (47). Key enzymes in these processes have different affinities for reduced ferredoxin: ferredoxin-NADP reductase has a Km of 0.1 μM, nitrite reductase has a Km of 0.6 μM, and glutamate synthase has a Km of 60 μM (48). As a result,
into amino acids, occur in the chloroplast stroma (46) and require reduced ferredoxin generated by photosynthetic electron transport (47). Key enzymes in these processes have different affinities for reduced ferredoxin: ferredoxin-NADP reductase has a Km of 0.1 μM, nitrite reductase has a Km of 0.6 μM, and glutamate synthase has a Km of 60 μM (48). As a result, 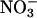 assimilation proceeds only if the availability of reduced ferredoxin exceeds that needed for NADPH formation (49, 50). For wheat (Fig. 3) and tomato (16), this occurred only at high light intensities under ambient CO2 and O2 concentrations, conditions under which CO2 availability limited C3 photosynthesis.
assimilation proceeds only if the availability of reduced ferredoxin exceeds that needed for NADPH formation (49, 50). For wheat (Fig. 3) and tomato (16), this occurred only at high light intensities under ambient CO2 and O2 concentrations, conditions under which CO2 availability limited C3 photosynthesis.
The responses of CO2 and O2 fluxes to the various treatments were similar in the wild-type Arabidopsis and the transgenic that overexpresses 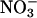 reductase (Fig. 2 A and D). This similarity supports the contention that
reductase (Fig. 2 A and D). This similarity supports the contention that 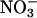 reductase activity by itself limits neither
reductase activity by itself limits neither 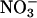 assimilation (23) nor plant performance (51).
assimilation (23) nor plant performance (51).
Implications. Our finding that CO2 inhibits 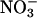 assimilation in shoots of Arabidopsis and wheat is consistent with previous studies on barley (24), tomato (16), and wheat (14, 15). If CO2 inhibition of shoot
assimilation in shoots of Arabidopsis and wheat is consistent with previous studies on barley (24), tomato (16), and wheat (14, 15). If CO2 inhibition of shoot 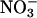 assimilation were common among C3 species, it could account for several responses of plants to elevated CO2, including the decline in shoot protein and the diminished activities of photosynthetic enzymes. Nitrogen availability determines plant responses to elevated CO2 concentrations more than any other environmental factor (52, 53), but ecosystems show a broad range of responses to elevated CO2 concentrations, possibly as a result of the seasonal and spatial fluctuations in the relative availabilities of
assimilation were common among C3 species, it could account for several responses of plants to elevated CO2, including the decline in shoot protein and the diminished activities of photosynthetic enzymes. Nitrogen availability determines plant responses to elevated CO2 concentrations more than any other environmental factor (52, 53), but ecosystems show a broad range of responses to elevated CO2 concentrations, possibly as a result of the seasonal and spatial fluctuations in the relative availabilities of 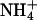 and
and 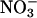 . For instance, ecosystems in which
. For instance, ecosystems in which 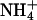 is the dominant nitrogen form, such as pine forests (54) or wetlands (55), show a relatively large increase (≈25%) in net primary productivity under CO2 enrichment, whereas ecosystems in which
is the dominant nitrogen form, such as pine forests (54) or wetlands (55), show a relatively large increase (≈25%) in net primary productivity under CO2 enrichment, whereas ecosystems in which 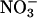 is dominant, such as grasslands (56) or wheat fields, at standard fertilizer levels (low fertilizer treatment at Maricopa, AZ; ref. 57) show declines in net primary productivity under CO2 enrichment.
is dominant, such as grasslands (56) or wheat fields, at standard fertilizer levels (low fertilizer treatment at Maricopa, AZ; ref. 57) show declines in net primary productivity under CO2 enrichment.
Plants vary in their relative dependence on 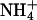 and
and 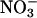 as nitrogen sources and in their balance between shoot and root
as nitrogen sources and in their balance between shoot and root 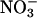 assimilation (17). Our results suggest that rising atmospheric CO2 levels will favor taxa that prefer
assimilation (17). Our results suggest that rising atmospheric CO2 levels will favor taxa that prefer 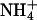 as a nitrogen source or assimilate
as a nitrogen source or assimilate 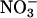 primarily in their roots.
primarily in their roots.
Extensive efforts to increase the specificity of Rubisco for CO2 relative to O2 and thereby increase the productivity of C3 crops have proved unsuccessful (5). Our results indicate that such efforts might have hitherto unforeseen consequences: in agricultural systems where 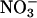 is the dominant form of inorganic nitrogen, minimizing photorespiration may be associated with nitrogen deprivation.
is the dominant form of inorganic nitrogen, minimizing photorespiration may be associated with nitrogen deprivation.
Supplementary Material
Acknowledgments
We thank Y. M. Heimer (Albert Katz Center for Desert Agrobiology, J. Blaustein Institute for Desert Research) for providing seed of the transgenic Arabidopsis that overexpresses 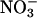 reductase and Y. M. Heimer, Aaron Kaplan, and Alan Stemler for comments on the manuscript. Alan Tan and Chang Tun-Hsiang provided technical assistance. This research was funded in part by National Science Foundation Grants IBN-99-74927 and IBN-03-43127 and by U.S. Department of Agriculture National Research Initiative Competitive Grants Program Grant 2000-00647 (to A.J.B.) and an Israel Binational Agricultural Research and Development Fund Fellowship (to S.R.).
reductase and Y. M. Heimer, Aaron Kaplan, and Alan Stemler for comments on the manuscript. Alan Tan and Chang Tun-Hsiang provided technical assistance. This research was funded in part by National Science Foundation Grants IBN-99-74927 and IBN-03-43127 and by U.S. Department of Agriculture National Research Initiative Competitive Grants Program Grant 2000-00647 (to A.J.B.) and an Israel Binational Agricultural Research and Development Fund Fellowship (to S.R.).
Abbreviations: PFD, photon flux density; ΔAQ, the difference in the assimilatory quotient.
References
- 1.Sharkey, T. D. (1988) Physiol. Plant 73, 147-152. [Google Scholar]
- 2.Wingler, A., Lea, P. J., Quick, W. P. & Leegood, R. C. (2000) Philos. Trans. R. Soc. London B 355, 1517-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozaki, A. & Takeba, G. (1996) Nature 384, 557-560. [Google Scholar]
- 4.Noctor, G., Arisi A.-C., M., Jouanin, L. & Foyer, C. H. (1999) J. Exp. Bot. 50, 1157-1167. [Google Scholar]
- 5.Parry, M. A. J., Andralojc, P. J., Mitchell, R. A. C., Madgwick, P. J. & Keys, A. J. (2003) J. Exp. Bot. 54, 1321-1333. [DOI] [PubMed] [Google Scholar]
- 6.Cox, P. M., Betts, R. A., Jones, C. D., Spall, S. A. & Totterdell, I. J. (2000) Nature 408, 184-187. [DOI] [PubMed] [Google Scholar]
- 7.Woodward, F. I. (2002) Curr. Opin. Plant Biol. 5, 207-211. [DOI] [PubMed] [Google Scholar]
- 8.Curtis, P. S. (1996) Plant Cell Environ. 19, 127-137. [Google Scholar]
- 9.Poorter, H. & Navas, M. L. (2003) New Phytol. 157, 175-198. [DOI] [PubMed] [Google Scholar]
- 10.Bowes, G. (1993) Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 309-332. [Google Scholar]
- 11.Moore, B. D., Cheng, S. H., Rice, J. & Seemann, J. R. (1998) Plant Cell Environ. 21, 905-915. [Google Scholar]
- 12.Makino, A. & Mae, T. (1999) Plant Cell Physiol. 40, 999-1006. [Google Scholar]
- 13.Cotrufo, M. F., Ineson, P. & Scott, A. (1998) Global Change Biol. 4, 43-54. [Google Scholar]
- 14.Smart, D. R., Ritchie, K., Bloom, A. J. & Bugbee, B. B. (1998) Plant Cell Environ. 21, 753-764. [DOI] [PubMed] [Google Scholar]
- 15.Bloom, A. J., Smart, D. R., Nguyen, D. T. & Searles, P. S. (2002) Proc. Natl. Acad. Sci. USA 99, 1730-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Searles, P. S. & Bloom, A. J. (2003) Plant Cell Environ. 26, 1247-1255. [Google Scholar]
- 17.Bloom, A. J. (1997) in Ecology in Agriculture, ed. Jackson, L. E. (Academic, San Diego), pp. 145-172.
- 18.Heimer, Y. M., Brusslan, J. A., Kenigsbuch, D. & Tobin, E. M. (1995) Plant Mol. Biol. 27, 129-136. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson, J. Q. & Crawford, N. M. (1993) Mol. Gen. Genet. 239, 289-297. [DOI] [PubMed] [Google Scholar]
- 20.Gibeaut, D. M., Hulett, J., Cramer, G. R. & Seemann, J. R. (1997) Plant Physiol. 115, 317-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein, E. & Bloom, A. J. (2004) Mineral Nutrition of Plants: Principles and Perspectives (Sinauer, Sunderland, MA), 2nd Ed., in press.
- 22.Aslam, M., Rosichan, J. L. & Huffaker, R. C. (1987) Plant Physiol. 83, 579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser, W. M., Kandlbinder, A., Stoimenova, M. & Glaab, J. (2000) Planta 210, 801-807. [DOI] [PubMed] [Google Scholar]
- 24.Bloom, A. J., Caldwell, R. M., Finazzo, J., Warner, R. L. & Weissbart, J. (1989) Plant Physiol. 91, 352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloom, A. J. (1989) in Application of Continuous and Steady State Methods to Root Biology, eds. Torrey, J. G. & Winship, L. J. (Kluwer, Dordrecht, The Netherlands), pp. 147-163.
- 26.Cousins, A. B. & Bloom, A. J. (2004) Plant Cell Environ., in press.
- 27.Kosola, K. R. & Bloom, A. J. (1994) Plant Physiol. 104, 435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thayer, J. R. & Huffaker, R. C. (1980) Anal. Biochem. 102, 110-119. [DOI] [PubMed] [Google Scholar]
- 29.Nejidat, A., Zhang, G. F., Grinberg, M. & Heimer, Y. M. (1997) Plant Sci. 130, 41-49. [Google Scholar]
- 30.Sage, R. F. (1994) Photosynth. Res. 39, 351-368. [DOI] [PubMed] [Google Scholar]
- 31.Myers, J. (1949) in Photosynthesis in Plants, eds. Franck, J. & Loomis, W. E. (Iowa State College Press, Ames), pp. 349-364.
- 32.Cen, Y.-P., Turpin, D. H. & Layzell, D. B. (2001) Plant Physiol. 126, 1555-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrader, L. E., Domska, D., Jung, P. E., Jr., & Peterson, L. A. (1972) Agron. J. 64, 690-695. [Google Scholar]
- 34.Huffaker, R. C. & Rains, D. W. (1978) in Nitrogen in the Environment, eds. Nielson, D. R. & MacDonald, J. G. (Academic, New York), pp. 1-43.
- 35.Rufty, T. W., Jr., Jackson, W. A. & Raper, C. D., Jr. (1982) J. Exp. Bot. 33, 1122-1137. [Google Scholar]
- 36.Jackson, W. A., Pan, W. L., Moll, R. H. & Kamprath, E. J. (1986) in Biochemical Basis of Plant Breeding, ed. Neyra, A. (CRC, Boca Raton, FL), Vol. II, pp. 73-108. [Google Scholar]
- 37.MacKown, C. T. (1987) J. Exp. Bot. 38, 1079-1090. [Google Scholar]
- 38.Bloom, A. J., Sukrapanna, S. S. & Warner, R. L. (1992) Plant Physiol. 99, 1294-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews, M. (1986) Plant Cell Environ. 9, 511-519. [Google Scholar]
- 40.Stitt, M. (1991) Plant Cell Environ. 14, 741-762. [Google Scholar]
- 41.Tolbert, N. E. (1994) in Regulation of CO2 and O2 by Photosynthetic Carbon Metabolism, eds. Tolbert, N. E. & Preiss, J. (Oxford Univ. Press, New York), pp. 8-33.
- 42.Backhausen, J. E., Kitzmann, C. & Sheibe, R. (1994) Photosynth. Res. 42, 75-86. [DOI] [PubMed] [Google Scholar]
- 43.Stitt, M., Muller, C., Matt, P., Gibon, Y., Carillo, P., Morcuende, R., Scheible, W. R. & Krapp, A. (2002) J. Exp. Bot. 53, 959-970. [DOI] [PubMed] [Google Scholar]
- 44.Purczeld, P., Chon, C. J., Portis, A. R., Heldt, H. W. & Heber, U. (1978) Biochim. Biophys. Acta 501, 488-498. [DOI] [PubMed] [Google Scholar]
- 45.Shingles, R., Roh, M. H. & McCarty, R. E. (1996) Plant Physiol. 112, 1375-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suess, K. H., Prokhorenko, I. & Adler, K. (1995) Plant Physiol. 107, 1387-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sivasankar, S. & Oaks, A. (1996) Plant Physiol. Biochem. 34, 609-620. [Google Scholar]
- 48.Knaff, D. B. (1996) in Oxygenic Photosynthesis: The Light Reactions, eds. Ort, D. R. & Yocum, C. F. (Kluwer, Dordrecht, The Netherlands), Vol. 4, pp. 333-361. [Google Scholar]
- 49.Baysdorfer, C. & Robinson, M. J. (1985) Plant Physiol. 77, 318-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peirson, D. R. & Elliott, J. R. (1988) J. Plant Physiol. 133, 425-429. [Google Scholar]
- 51.Eichelberger, K. D., Lambert, R. J., Below, F. E. & Hageman, R. H. (1989) Crop Sci. 29, 1398-1402. [Google Scholar]
- 52.Poorter, H. & Perez-Soba, M. (2001) Oecologia 129, 1-20. [DOI] [PubMed] [Google Scholar]
- 53.Dormann, C. F. & Woodin, S. J. (2002) Funct. Ecol. 16, 4-17. [Google Scholar]
- 54.Finzi, A. C., DeLucia, E. H., Hamilton, J. G., Richter, D. D. & Schlesinger, W. H. (2002) Oecologia 132, 567-578. [DOI] [PubMed] [Google Scholar]
- 55.Drake, B. G., Muehe, M. S., Peresta, G., Gonzalez-Meler, M. A. & Matamala, R. (1996) Plant Soil 187, 111-118. [Google Scholar]
- 56.Shaw, M. R., Zavaleta, E. S., Chiariello, N. R., Cleland, E. E., Mooney, H. A. & Field, C. B. (2002) Science 298, 1987-1990. [DOI] [PubMed] [Google Scholar]
- 57.Kimball, B. A., Morris, C. F., Pinter, P. J., Wall, G. W., Hunsaker, D. J., Adamsen, F. J., LaMorte, R. L., Leavitt, S. W., Thompson, T. L., Matthias, A. D., et al. (2001) New Phytol. 150, 295-303. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.