Abstract
Background
Posttraumatic stress disorder (PTSD) has been linked to hypertension, but most research on PTSD and hypertension is cross-sectional, and potential mediators have not been clearly identified. Moreover, PTSD is twice as common in women than in men, but understanding of the PTSD-hypertension relation in women is limited. We examined trauma exposure and PTSD symptoms in relation to incident hypertension over 22 years in 47,514 civilian women in the Nurses’ Health Study II.
Methods
We used proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for new-onset hypertension (N=15,837).
Results
PTSD symptoms assessed with a screen were modestly associated with incident hypertension in a dose-response fashion after adjusting for potential confounders. Compared to women with no trauma exposure, women with 6–7 PTSD symptoms had the highest risk of developing hypertension (HR=1.20 [95% CI, 1.12–1.30]), followed by women with 4–5 symptoms (HR=1.17 [95% CI, 1.10–1.25]), women with 1–3 symptoms (HR=1.12 [95% CI, 1.06–1.18]), and trauma-exposed women with no symptoms (HR=1.04 [95% CI, 1.00–1.09]). Findings were maintained, although attenuated, adjusting for hypertension-relevant medications, medical risk factors, and health behaviors. Higher body mass index and antidepressant use accounted for 30% and 21% of the PTSD symptom-hypertension association.
Conclusions
Screening for hypertension and reducing unhealthy lifestyle factors, particularly obesity, in women with PTSD may hold promise for offsetting cardiovascular risk.
Keywords: Hypertension, posttraumatic stress disorder, trauma, epidemiology, women
Introduction
In the U.S., over 40 million women have diagnosed hypertension, and the prevalence of hypertension in women has been rising in the last three decades (Mozaffarian et al., 2015). Hypertension is a major modifiable risk factor for cardiovascular disease (CVD), the leading cause of death in women (Mozaffarian et al., 2015). Recently, we demonstrated that elevated posttraumatic stress disorder (PTSD) symptoms were associated with increased CVD risk in younger and middle-aged women (Sumner et al., 2015). Better understanding of who is at risk for hypertension could inform CVD prevention efforts.
Exposure to trauma, including childhood abuse (Riley et al., 2010) and combat (Granado et al., 2009), has been associated with increased hypertension risk. PTSD, the sentinel trauma-related disorder, has been tied to hypertension across numerous, and largely cross-sectional, studies (e.g., Abouzeid et al., 2012; Cohen et al., 2009; Davidson et al., 1991; Glaesmer et al., 2011; Kang et al., 2006; Lauterbach et al., 2005; O’Toole & Catts, 2008; Paulus et al., 2013; Pietrzak et al., 2011; Sareen et al., 2007; Stein et al., 2014). Additionally, rates of metabolic syndrome—a constellation of cardiometabolic risk factors that includes hypertension—have been elevated in those with (vs. without) PTSD (e.g., Bartoli et al., 2013). However, although PTSD is twice as common in women than in men (Kessler et al., 1995), studies examining PTSD and hypertension in younger and middle-aged civilian women exposed to a range of traumas are generally lacking. Much research has been in predominantly male veteran samples (e.g., Abouzeid et al., 2012; Kang et al., 2006; O’Toole & Catts, 2008; Paulus et al., 2013; Schnurr et al., 2000; see Cohen et al., 2009, for an exception). Several studies have also suggested that PTSD is linked to increased hypertension risk in civilians (e.g., Davidson et al., 1991; Glaesmer et al., 2011; Lauterbach et al., 2005; Pietrzak et al., 2011; Sareen et al., 2007; Stein et al., 2014). However, relatively few of these civilian investigations have evaluated if the link between PTSD and hypertension is similarly evident in men vs. women; it is not yet clear whether associations hold in civilian women. One exception is the cross-sectional study by Pietrzak et al. (2011), which found that odds of hypertension were higher for men, but not women, with PTSD in the National Epidemiologic Survey on Alcohol and Related Conditions sample. Stein et al. (2014) also tested whether the PTSD-hypertension association was moderated by sex using the cross-sectional World Mental Health Surveys, although they did not present sex-stratified results given the nonsignificant PTSD by sex interaction. Further studies are needed to understand if PTSD symptoms are associated with hypertension risk in women from the general population.
Furthermore, the effects of trauma with and without PTSD have rarely been considered in a single study. In a general population sample of German elderly individuals, trauma exposure without PTSD and current PTSD were cross-sectionally associated with increased hypertension risk compared to no trauma, although the magnitude of elevation in risk was greater for those with (vs. without) PTSD (Glaesmer et al., 2011). O’Toole and Catts (2008) also considered trauma and PTSD separately in their cross-sectional study of male veterans; only PTSD—not trauma alone—was associated with hypertension. Additional research examining effects of trauma and PTSD separately is needed, particularly in younger and middle-aged civilians.
Although research suggests a relation between PTSD and hypertension, the American Heart Association (2014) maintains that “stress is not a confirmed risk factor for high blood pressure (BP).” Virtually all PTSD-hypertension research has been cross-sectional (excepting Schnurr et al., 2000); longitudinal research is needed to demonstrate more convincingly that PTSD increases risk of, rather than is a consequence of, hypertension. Additionally, knowledge of mediating factors is limited. Mediating behavioral risk factors are likely given a sizeable proportion of incident hypertension can be reduced by addressing health behaviors, such as diet and exercise (Forman et al., 2009), and PTSD has been associated with obesity and unhealthy behaviors (e.g., Zen et al., 2012; Kubzansky et al., 2014). Furthermore, antidepressants—frequently prescribed for PTSD (Friedman & Davidson, 2007)—have been linked to hypertension (Licht et al., 2009). Although several studies have adjusted for specific health behaviors (e.g., Schnurr et al., 2000), whether a range of health behaviors and antidepressant use can explain an observed PTSD-hypertension relation is not known.
We addressed limitations in prior literature by examining associations between trauma exposure, PTSD symptoms, and risk of incident hypertension over 22 years in the Nurses’ Health Study II (NHS II), an ongoing longitudinal cohort study of younger and middle-aged women begun in 1989. A previous study in this cohort found that childhood abuse was associated with increased hypertension risk (Riley et al., 2010). Although PTSD could partly mediate the childhood abuse-hypertension relation, Riley et al. (2010) did not consider the psychological sequelae of early abuse. Here, we build on these findings by examining PTSD symptoms in response to a variety of traumas and incident hypertension. Additionally, we considered the effects of trauma and PTSD symptoms separately. We hypothesized that, compared to no trauma, higher PTSD symptoms would be related to increased risk of developing hypertension, after accounting for a range of potential confounders (determined from previous studies; e.g., Chasan-Taber et al., 1996; Gangwisch et al., 2013). Unlike most research on PTSD and hypertension, we also considered a range of potential mediators. We predicted that health behaviors and antidepressant use would explain some of this association.
Methods
Sample
The NHS II includes 116,430 female nurses in the U.S., aged 25–42 years at enrollment in 1989. Women complete questionnaires biennially; follow-up is ongoing. Supplemental questionnaires with special interests are periodically administered. In 2001, a supplemental questionnaire on violence exposure was mailed to 91,297 women and returned by 68,376 women. In 2008, when participants were aged 44–62 years, 60,804 women who completed the 2001 Violence questionnaire and 2007 biennial questionnaire were mailed a supplemental questionnaire assessing trauma and PTSD (Morgan et al., 2001). After repeated mailings, 54,224 women returned the supplemental questionnaire (89% response rate). This study was approved by the Partners Healthcare Human Research Committee. Return of the questionnaire constituted implied consent. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Trauma and PTSD Symptom Assessment
PTSD symptom severity and age of onset were derived from information on trauma and PTSD symptoms. Trauma exposure was measured with a 16-item modified version of the Brief Trauma Questionnaire (Morgan et al., 2001; Schnurr et al., 1999), a reliable and valid measure of trauma exposure that parallels interview measures (Schnurr et al., 1999). Lifetime exposure to 15 traumatic events (e.g., natural disaster exposure, physical assault), in addition to “a seriously traumatic event not already covered,” was assessed. Respondents reported whether events occurred, which event occurred first, and which was their worst experience, along with their ages at the first and worst events. The Short Screening Scale for DSM-IV PTSD (Breslau et al., 1999) was used to assess whether women ever experienced seven PTSD symptoms in response to their worst trauma. Reliability of self-reported age-of-onset of trauma and PTSD has been excellent in this sample (ICC=.95).
A time-varying variable characterizing women according to their trauma/PTSD symptom status was derived, with PTSD symptom onset dated according to the year women reported their worst trauma. For each study year, participants were classified into five groups based on their dates of trauma exposure and PTSD symptom severity: 1) no trauma exposure, 2) trauma-exposed with no PTSD symptoms, 3) trauma-exposed with 1–3 symptoms, 4) trauma-exposed with 4–5 symptoms, and 5) trauma-exposed with 6–7 symptoms. Before their first trauma (if reported), women were categorized as having no trauma exposure. After their first trauma, women were classified as being trauma-exposed with no symptoms. Following their worst trauma, women were classified as being trauma-exposed with the number of PTSD symptoms reported subsequent to the worst event. If women reported only one event, the years of the first and worst traumas were identical. In other words, if no trauma was ever reported, women were classified as having no trauma at all follow-up waves. Prior to reporting trauma, women were classified as having no trauma at all waves until trauma or trauma and PTSD symptoms were reported. Once trauma/PTSD symptom onset was reported, the severity of trauma/PTSD symptoms at onset was carried through all subsequent waves.
Trauma/PTSD symptom categorizations were selected based on previous research with the Short Screening Scale for DSM-IV PTSD recommending scores of 4+ (Breslau et al., 1999) and 6+ (Kimerling et al., 2006) as clinical cutoffs for PTSD. Following this rubric resulted in one group of women with trauma and subclinical PTSD symptoms and two groups of women with trauma and PTSD symptom counts above the recommended clinical cutoff on this screening questionnaire, with the latter two grouped according to increasing symptom severity. With this nuanced classification rubric, we could examine hypertension onset in women with no trauma, trauma and no symptoms, and trauma and varying symptom severity.
Hypertension Assessment
Women reported lifetime physician-diagnosed hypertension at baseline in 1989. At each subsequent biennial questionnaire, participants indicated whether they had physician-diagnosed hypertension in the past 2 years. Self-reported hypertension has been validated in this cohort. In randomly-chosen NHS II participants self-reporting hypertension, 94% had confirmed hypertension (Forman et al., 2008). Moreover, BP was measured in an age-stratified sample of 194 NHS II participants to examine unreported hypertension. Of the 161 women not reporting hypertension, only 11 (7%) had a BP>140/90mmHg. None had a BP>160/95mmHg (Chasan-Taber et al., 1996). For this study, women who reported ever having hypertension at the baseline assessment were excluded. We considered incident hypertension reported on the 1991–2011 questionnaires.
Covariates
Potential confounders included age at baseline, race/ethnicity (African American, Latina, Asian, Caucasian, other), maximum parental education at the participant’s birth (high school or less, some college, 4+ years of college), maternal and paternal history of hypertension, and age 5 somatotype (to estimate childhood adiposity). Time-varying indicators for hypertension-relevant medical risk factors and medications were included as biomedical covariates; all were assessed biennially unless otherwise indicated (see Figure 1 for study timeline). These included: oral contraceptive use (never used, current user, former user; Chasan-Taber et al., 1996); menopausal status and hormone therapy (HT) use (pre-menopausal, post-menopausal/never HT, post-menopausal/past HT, post-menopausal/current HT, post-menopausal/missing HT, unknown menopausal status; Gangwisch et al., 2013); current aspirin use, acetaminophen use, and other nonsteroidal anti-inflammatory drug (NSAID) use (these were assessed with each biennial questionnaire except in 1991; Gangwisch et al., 2013); physician-diagnosed hypercholesterolemia.
Figure 1.
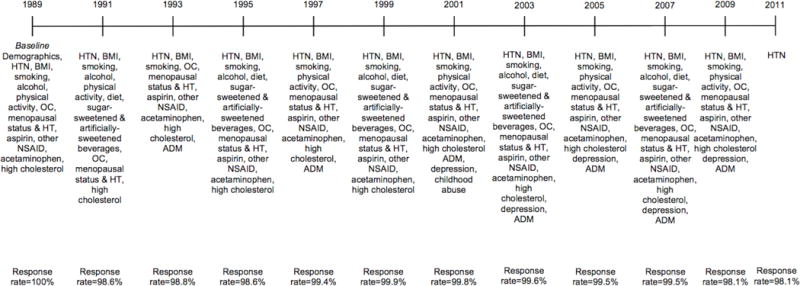
Timeline of variables collected at biennial assessments. HTN=hypertension. BMI=body mass index. OC=oral contraceptive use. HT=hormone therapy use. NSAID=nonsteroidal anti-inflammatory drug. ADM=antidepressant medication. Response rates for the analytic sample are listed for each time point. Trauma/PTSD symptom status was assigned for each year of the study and updated based on reported age of worst trauma (assessed with a supplemental questionnaire in 2008).
Health behaviors were examined as potential mediators of the PTSD symptom-hypertension relation. These were time-varying covariates, assessed at baseline via self-report and updated biennially, unless otherwise noted (see Figure 1). Adult body mass index (BMI) in kg/m2 was computed from self-reported height and weight (validated in NHS research; Rimm et al., 1990), and categorized as <18.5,18.5–<21,21–<23,23–<25,25–<27,27–<30, and 30+ (Munger et al., 2009). Participants were classified as nonsmokers, former smokers, or current smokers of 1–14,15–24, or 25+ cigarettes/day. Alcohol consumption (0,1–<5,5–<10,10–<20,20+ grams/day) was assessed in 1989, 1991, 1995, 1999, 2003, and 2007. Physical activity (<3,3–<9,9–<18,18–<27,27+ metabolic equivalent hours/week) was measured in 1989, 1991, 1997, 2001, 2005, and 2009. Diet quality based on the Alternative Healthy Eating Index (Chiuve et al., 2012) was assessed every 4 years beginning in 1991. Diet quality was divided into quintiles; the highest quintile represented the healthiest diet. Sugar-sweetened and artificially-sweetened beverage consumption were each categorized as <1/month, 1–4/month, 2–6/week, and 1+/day (Cohen et al., 2012). Lifetime antidepressant use was assessed in 1993, and regular past 2-year antidepressant use was assessed in 1997, 2001, 2003, 2005, 2007, and 2009. Women endorsing lifetime use in 1993 were coded as using antidepressants from 1989–1993; use was updated as available.
Sensitivity Analyses
Childhood abuse has been associated with hypertension in the NHS II (Riley et al., 2010), and meta-analytic evidence has linked depression (often comorbid with PTSD) with incident hypertension (Meng et al., 2012). We examined potential confounding by childhood abuse and lifetime depression. Sexual abuse before age 18 was measured in 2001 with four questions regarding unwanted sexual touching or forced/coerced sexual contact by an adult or older child (Moore et al., 1995), and categorized as no sexual abuse, sexual touch only, or forced sex (Sumner et al., 2015). Childhood physical abuse was assessed in 2001 with the revised Conflict Tactics Scale (Straus & Gelles, 1990), and classified as none, mild, moderate, or severe (Sumner et al., 2015). Lifetime depression (experiencing depressive symptoms for 2+ weeks) was assessed in 2001, and women reported experiencing depressive symptoms for 2+ weeks or receiving a depression diagnosis in 2003, 2005, 2007, and 2009. Lifetime depression was assigned if women reported ever having depressive symptoms for 2+ weeks or receiving a depression diagnosis.
Exclusions
Figure 2 shows exclusions for deriving the analytic sample (N=47,514). Compared to those in the analytic sample, women who returned the trauma/PTSD questionnaire but were excluded from analyses were slightly older at baseline (35.6 vs. 34.7 years), had higher rates of maternal (37.6% vs. 29.8%) and paternal (36.7% vs. 30.1%) hypertension and childhood adiposity (highest somatotype: 8.9% vs. 6.6%), and had parents with lower educational attainment (maximum parental education of high school or less: 50.3% vs. 47.1%). Cumulative incidence of hypertension (1989–2011) was similar for those included (33.5%) vs. excluded (34.0%).
Figure 2.
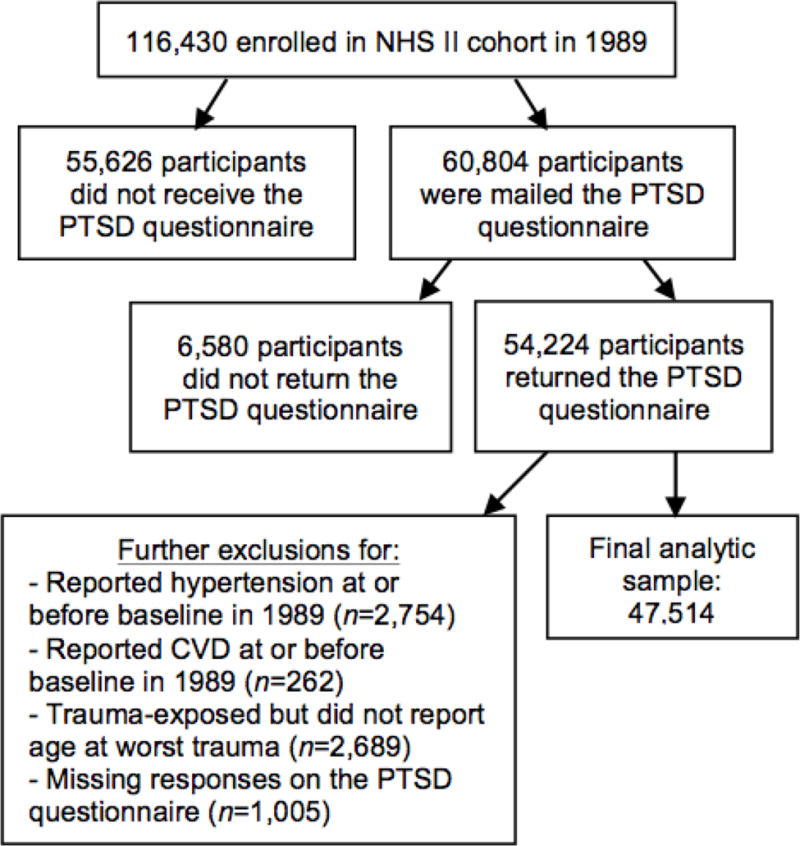
Exclusions for deriving the analytic sample.
Statistical Analyses
We examined whether trauma and PTSD symptoms were associated with incident hypertension with Cox proportional hazards models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Trauma/PTSD symptom status was considered a time-varying variable, and trauma/PTSD symptom status for each year of follow-up was determined based on the year women reported experiencing their worst trauma. SAS PROC PHREG was used for analyses (SAS Institute, Cary, NC). The Andersen-Gill data structure was utilized because it can accommodate updating time-varying exposures and covariates, left-censored data, and potentially discontinuous intervals of risk (Therneau, 1997). With the Andersen-Gill data structure, a new data record was created for every questionnaire cycle when a participant was at risk, with covariates set to their values at the time the questionnaire was returned. This method accounts for the nested structure of our data; it allows explicitly for correlated data, multiple time scales, and, if needed, multiple events per subject (Therneau, 1997). Participants contributed person-time (in months) from baseline in 1989 until hypertension onset, death, end of follow-up in 2011, or their last questionnaire (if they did not return the 2011 questionnaire). Individuals who developed CVD (myocardial infarction or stroke) during the study (n=242) were censored at CVD onset.
We tested a series of models that first adjusted for demographic confounders, further adjusted for hypertension-relevant biomedical covariates, and further adjusted for potential mediators. Model 1 adjusted for demographics, family history, and childhood adiposity. Model 2 further adjusted for hypertension-relevant medical risk factors and medications. Model 3 additionally adjusted for health behaviors. Model 4 further adjusted for antidepressant use. Missing data in covariates were handled by treating missing as a separate category. Variables added in Models 2–4 (i.e., hypertension-relevant medical risk factors, medications, health behaviors) were time-varying and updated every 2, 4, or 6 years as available. For each period of prediction, variables added in Models 2–4 were lagged by one period, and trauma/PTSD symptom status was lagged to represent the year prior to the period for the Model 2–4 covariates. For example, for hypertension status on the 1993 questionnaire, covariates added in Models 2–4 (including proposed mediators) were derived from the 1991 questionnaire, and trauma/PTSD symptoms reflected status in 1990. The outcome for the final period of prediction was hypertension status on the 2011 questionnaire, with trauma/PTSD symptoms characterized/updated only through 2008 (when the trauma/PTSD questionnaire was administered). To assess which trauma/PTSD symptom groups differed significantly from one another, we varied the reference group in further analyses using the same modeling strategy described above.
Health behaviors (e.g., smoking, diet), BMI, and antidepressant use were examined as potential mediators. We first considered their separate associations with trauma/PTSD symptoms using generalized estimating equations (trauma/PTSD symptoms and potential mediators were time-varying variables that were updated over the course of the study period). We also considered separate associations of potential mediators with hypertension risk using Cox proportional hazards models. We then tested for mediation by adding each variable individually to Model 2. Attenuation ≥10% in trauma/PTSD symptom parameter estimates after including the hypothesized mediator was used as a cutoff considered sufficient to be consistent with mediation (Gangwisch et al., 2013). We examined the percent of the PTSD symptom-hypertension relation accounted for by potential mediators using the SAS Mediate macro (2009), which calculates point estimates and 95% CIs of the percent of exposure explained by each intermediate variable.
We conducted three sensitivity analyses. We tested for potential confounding by childhood abuse and depression by re-estimating Model 1 adjusting for each relevant indicator. Additionally, because many hypertension events occurred before assessing trauma/PTSD symptoms in 2008, we considered the possible impact of recall bias by analyzing prospectively detected new-onset hypertension events reported on the 2009 and 2011 questionnaires (N=35,187; 3,554 hypertension events).
Results
Table 1 presents participant characteristics by trauma and PTSD symptom status at baseline. Many women (69%) reported trauma by 1989. Of trauma-exposed participants at baseline, 73% reported no PTSD symptoms, 14% reported 1–3 symptoms, 8% reported 4–5 symptoms, and 5% reported 6–7 symptoms. Over the study, 5,144 additional women reported trauma exposure.
Table 1.
Baseline participant characteristics as a function of trauma exposure and PTSD symptoms at the NHS II 1989 assessment (N=47,514)
| No trauma (n=14,720) | Trauma-exposed (n=32,794)
|
||||
|---|---|---|---|---|---|
| No symptoms (n=23,872) | 1–3 symptoms (n=4,489) | 4–5 symptoms (n=2,664) | 6–7 symptoms (n=1,769) | ||
|
| |||||
| Mean (SD) or % (n) | Mean (SD) or % (n) | Mean (SD) or % (n) | Mean (SD) or % (n) | Mean (SD) or % (n) | |
| Age, years | 34 (5) | 35 (5) | 35 (4) | 35 (4) | 35 (4) |
| Parents’ education at birth, ≥college, % | 23.8 (3,503) | 22.3 (5,334) | 22.6 (1,014) | 22.3 (594) | 24.6 (436) |
| Maternal history of hypertension, % | 29.0 (4,270) | 30.3 (7,233) | 29.1 (1,308) | 30.2 (804) | 29.5 (521) |
| Paternal history of hypertension, % | 29.0 (4,269) | 30.7 (7,340) | 29.9 (1,343) | 31.2 (831) | 28.1 (497) |
| Highest somatotype, age 5, % | 6.0 (887) | 6.7 (1,604) | 6.5 (293) | 7.4 (196) | 8.1 (143) |
| Caucasian race, % | 93.5 (13,762) | 93.9 (22,407) | 94.3 (4,235) | 94.9 (2,528) | 94.6 (1,674) |
| Body mass index, kg/m2 | 23.3 (4.4) | 23.7 (4.6) | 23.8 (4.7) | 23.9 (4.8) | 23.9 (5.0) |
| Cigarette smoking, % | |||||
| Never | 72.3 (10,637) | 65.9 (15,729) | 61.8 (2,775) | 61.3 (1,632) | 55.1 (975) |
| Former smoker | 18.2 (2,671) | 22.3 (5,330) | 25.0 (1,124) | 26.5 (706) | 27.8 (491) |
| Current smoker | 9.3 (1,367) | 11.5 (2,737) | 12.7 (571) | 11.8 (314) | 16.7 (296) |
| Alcohol intake, grams/day | 2.9 (4.9) | 3.0 (5.6) | 3.3 (6.0) | 3.0 (5.5) | 3.1 (5.6) |
| Physical activity, MET hrs/wk* | 24.4 (36.1) | 24.7 (35.8) | 23.6 (34.0) | 24.5 (35.2) | 24.1 (31.4) |
| Worst diet (1st quintile) on the Alternative Healthy Eating Index,+ % | 20.2 (2,970) | 18.1 (4,309) | 16.7 (751) | 16.7 (444) | 15.4 (272) |
| 1+ sugar-sweetened beverages/day,+ % | 14.5 (2,134) | 13.8 (3,301) | 13.7 (613) | 14.5 (385) | 14.3 (253) |
| 1+ artificially-sweetened beverages/day,+ % | 33.2 (4,887) | 32.8 (7,823) | 33.2 (1,488) | 32.5 (866) | 31.8 (562) |
| Aspirin user, % | 9.8 (1,435) | 11.5 (2,754) | 11.6 (521) | 11.8 (315) | 13.5 (239) |
| Acetaminophen user, % | 18.9 (2,775) | 20.2 (4,810) | 20.2 (906) | 21.6 (576) | 24.9 (441) |
| Other nonsteroidal anti-inflammatory drug user, % | 15.8 (2,328) | 19.0 (4,529) | 20.0 (896) | 23.1 (615) | 26.5 (469) |
| Oral contraceptive use, % | |||||
| Never | 18.7 (2,759) | 15.3 (3,649) | 14.2 (636) | 13.4 (356) | 13.1 (231) |
| Former user | 65.9 (9,696) | 73.0 (17,427) | 75.1 (3,370) | 76.4 (2,035) | 77.5 (1,371) |
| Current user | 15.3 (2,247) | 11.6 (2,773) | 10.7 (478) | 10.1 (269) | 9.4 (167) |
| Pre-menopausal status, % | 97.8 (14,401) | 97.3 (23,215) | 96.7 (4,342) | 96.3 (2,565) | 95.8 (1,694) |
| Hypercholesterolemia, % | 8.9 (1,309) | 10.0 (2,388) | 9.6 (430) | 11.5 (306) | 12.1 (214) |
| Antidepressant use,‡ % | 6.3 (921) | 10.0 (2,389) | 13.6 (609) | 20.9 (556) | 32.4 (573) |
MET hrs/wk=metabolic equivalent hours/week.
First assessed in 1991.
First assessed in 1993.
Over the 22-year study period, 15,837 women developed hypertension. Time-updated PTSD symptoms were modestly associated with increased risk of incident hypertension in a dose-response fashion. Women with 6–7 PTSD symptoms had a 20% higher rate of developing hypertension compared to women without trauma, after adjusting for demographics, family history, and childhood adiposity (Table 2, Model 1; see Figure 3 for survival curves). Analyses considering different reference groups indicated that the HRs for the no trauma and trauma/no symptoms groups were lower than those for all PTSD symptom groups; however, the PTSD symptom groups did not differ significantly from each other (Supplementary Table 1). Findings remained significant, although attenuated, after adjusting for time-varying hypertension-relevant medical risk factors and medications (Table 2, Model 2; Supplementary Table 2 includes HRs for hypertension risk associated with Model 2 covariates). Despite additional attenuation after adjusting for time-updated health behaviors, PTSD symptoms remained associated with significantly elevated hypertension risk (Table 2, Model 3). Covarying time-varying antidepressant use attenuated associations further, particularly for women with 6–7 symptoms (Table 2, Model 4).
Table 2.
Adjusted hazard ratios (95% confidence intervals) for the association of trauma exposure and PTSD symptoms with risk of incident hypertension, 1989–2011
| No trauma | Trauma-exposed
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No symptoms | 1–3 symptoms | 4–5 symptoms | 6–7 symptoms | ||||||
|
| |||||||||
| Cases, n (person-y) | 3,558 (243,699) | 7,741 (480,677) | 2,211 (119,662) | 1,412 (73,763) | 915 (46,575) | ||||
| Hazard Ratio (95% Confidence Interval) | |||||||||
|
| |||||||||
| P | P | P | P | ||||||
|
Model 1: Minimally adjusted model* |
1 (ref) | 1.04 (1.002–1.09) | .038 | 1.12 (1.06–1.18) | <.0001 | 1.17 (1.10–1.25) | <.0001 | 1.20 (1.12–1.30) | <.0001 |
|
Model 2: Model adjusted for biomedical covariates+ |
1 (ref) | 1.02 (0.98–1.06) | .276 | 1.09 (1.03–1.15) | .002 | 1.13 (1.06–1.20) | .0002 | 1.13 (1.05–1.21) | .001 |
|
Model 3: Pathways model 1† |
1 (ref) | 1.00 (0.96–1.04) | .919 | 1.07 (1.01–1.13) | .018 | 1.09 (1.03–1.16) | .006 | 1.08 (1.002–1.16) | .043 |
|
Model 4: Pathways model 2§ |
1 (ref) | 1.00 (0.96–1.04) | .979 | 1.06 (1.005–1.12) | .034 | 1.08 (1.01–1.15) | .020 | 1.06 (0.98–1.14) | .142 |
Adjusted for age at baseline, race/ethnicity, parental education, maternal and paternal history of hypertension, and age 5 somatotype.
Additionally adjusted for use of oral contraceptives, acetaminophen, aspirin, and other nonsteroidal anti-inflammatory drugs, menopausal status and hormone therapy use, and hypercholesterolemia.
Additionally adjusted for body mass index, physical activity, diet quality, sugar-sweetened beverage consumption, artificially-sweetened beverage consumption, cigarette smoking, and alcohol consumption.
Additionally adjusted for antidepressant use.
Figure 3.
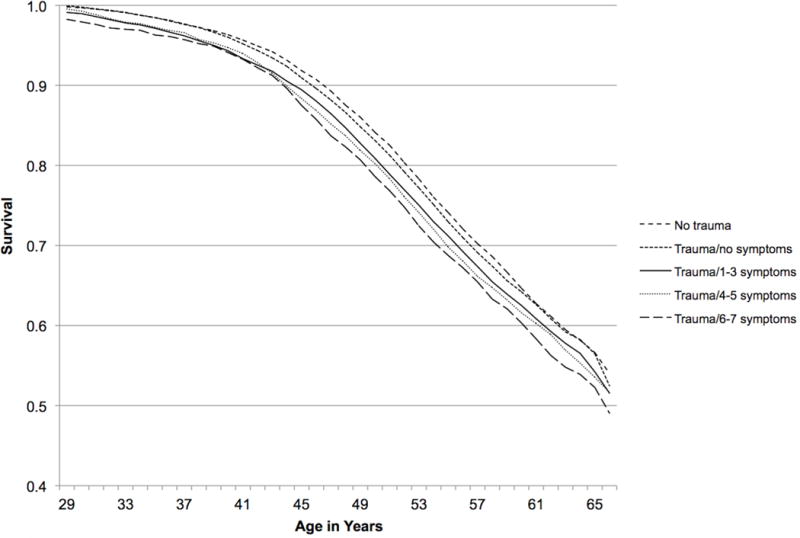
Survival by trauma/PTSD symptom status, 1989–2011. Model adjusted for age at baseline, race/ethnicity, parental education, maternal and paternal history of hypertension, and somatotype at age 5.
As all potential mediators were significantly associated with trauma/PTSD symptoms and hypertension risk (Supplementary Table 3), all were included as described above in pathway models. However, as only two potential mediators—BMI and antidepressant use—resulted in ≥10% attenuation in trauma/PTSD symptom parameter estimates in relation to hypertension risk, we present only findings for these variables. Results of generalized estimating equations indicated higher PTSD symptoms were associated with higher BMI and greater likelihood of antidepressant use (Supplementary Table 3). BMI and antidepressant use were significantly associated with hypertension risk, after adjusting for demographics, family history, and childhood adiposity. Increasing BMI was monotonically related to elevated hypertension risk, with the largest increases in risk observed for BMI of 27+ (Supplementary Table 3). Antidepressant use (vs. non-use) was also associated with increased risk of hypertension (Supplementary Table 3). In models adjusting for all other covariates, BMI accounted for an estimated 30% (95% CI, 12–57%) and antidepressant use accounted for an estimated 21% (95% CI, 9–43%) of the PTSD symptom-hypertension association.
After including childhood abuse in Model 1, PTSD symptoms remained associated with elevated hypertension risk (trauma/no symptoms: HR=1.02 [95% CI, 0.98–1.06]; trauma/1–3 symptoms: HR=1.09 [95% CI, 1.03–1.15]; trauma/4–5 symptoms: HR=1.12 [95% CI, 1.05–1.19]; trauma/6–7 symptoms: HR=1.13 [95% CI, 1.05–1.22]). When including lifetime depression with Model 1 covariates, PTSD symptoms remained significantly associated with hypertension risk, although HRs were attenuated (trauma/no symptoms: HR=1.03 [95% CI, 0.99–1.07]; trauma/1–3 symptoms: HR=1.08 [95% CI, 1.02–1.14]; trauma/4–5 symptoms: HR=1.10 [95% CI, 1.03–1.17]; trauma/6–7 symptoms: HR=1.11 [95% CI, 1.03–1.20]).
In the analysis predicting 3,554 prospectively detected hypertension events after 2008 (adjusted for Model 1 covariates), higher PTSD symptoms were associated with increased hypertension risk (trauma/no symptoms: HR=0.98 [95% CI, 0.90–1.07]; trauma/1–3 symptoms: HR=1.05 [95% CI, 0.94–1.17]; trauma/4–5 symptoms: HR=1.11 [95% CI, 0.98–1.26]; trauma/6–7 symptoms: HR=1.10 [95% CI, 0.95–1.28]). Findings did not reach statistical significance, likely due to limiting to cases that onset when women were older.
Discussion
We present the first study of PTSD symptoms and incident hypertension in women that utilizes a longitudinal population-based cohort and examines potential mediators. PTSD symptoms were modestly associated with increased risk of developing hypertension over 22 years. Effect sizes were largest for the highest PTSD symptom levels, although confidence intervals for the different PTSD symptom groups were overlapping. BMI and antidepressant use emerged as mediators, accounting for an estimated 30% and 21% of the PTSD symptom-hypertension relation. Elevated PTSD symptoms have been associated with myocardial infarction and stroke incidence in women (Sumner et al., 2015); increasing hypertension risk may be one mechanism by which PTSD contributes to these diseases.
Cross-sectional studies have documented associations between PTSD and hypertension but they have not been able to address whether PTSD precedes hypertension (e.g., Abouzeid et al., 2012; Pietrzak et al., 2011). Our investigation expands this literature by examining PTSD symptoms in relation to hypertension onset—assessed longitudinally—over 22 years. PTSD symptoms were significantly associated with developing hypertension, even when adjusting for factors related to PTSD that have been linked to hypertension, including childhood abuse and lifetime depression (Riley et al., 2010; Meng et al., 2012). Our findings are consistent with research suggesting that the PTSD-hypertension relation does not solely reflect risk associated with comorbid depression (Kibler et al., 2009). Furthermore, our study extends previous research by 1) examining the association between PTSD symptoms and incident hypertension in younger and middle-aged civilian women, 2) considering effects of trauma and PTSD symptoms separately, 3) utilizing a longitudinal design, and 4) considering a range of potential mediators.
PTSD symptoms remained associated with hypertension risk when predicting prospectively detected new-onset hypertension, although findings were not statistically significant. This analysis predicted cases that onset when women were older (44–66 years), which may explain the lack of statistical significance. Hypertension becomes more common as women age (Mozaffarian et al., 2015). With increasing age, more hypertension risk factors are at play, making it harder to disentangle differential risk and detect relative effects. We believe it is suggestive that we detected any elevated risk in this subsample, particularly because we had a shorter timeframe in which to examine the PTSD symptom-hypertension relation.
We identified two behavior-related risk factors as potential mediators of the PTSD symptom-hypertension association: BMI and antidepressant use. Even modest weight gain has been associated with increased hypertension risk in women (Huang et al., 1998), and women with PTSD symptoms are at risk of becoming overweight and obese (Kubzansky et al., 2014). Weight loss reduces hypertension risk (Huang et al., 1998); thus it may be important to consider benefits of weight loss among women with PTSD who are overweight or obese. Additionally, our finding that antidepressant use accounted for a portion of the PTSD symptom-hypertension relation is consistent with research suggesting antidepressant use may partly explain the link between PTSD symptoms and chronic disease (e.g., Type 2 diabetes; Roberts et al., 2015). Antidepressant use may indicate greater PTSD severity rather than be a mediator. In our sample, higher PTSD symptoms were associated with greater likelihood of antidepressant use. Additionally, antidepressants have been found to influence BP, in part, by affecting vagal control (Licht et al., 2009).
Additional behavioral and biological mediating risk factors not captured by the health behaviors examined likely also underlie the PTSD symptom-hypertension relation and merit investigation. For example, sleep disturbances are associated with hypertension in women (Cappuccio et al., 2007), and are commonly reported by individuals with PTSD (Maher et al., 2006). PTSD is also characterized by dysregulation of the autonomic nervous system, hypothalamic-pituitary-adrenal axis, and inflammatory response (Zoladz & Diamond, 2013), which could increase hypertension risk. For example, sympathetic nervous system hyperactivity (e.g., as indicated by elevated norepinephrine levels; Zoladz & Diamond, 2013) has been observed in PTSD. Sympathetic overdrive has been implicated in elevated BP onset and the progression and increased severity of hypertension over time (Mancia & Grassi, 2014).
Methodological limitations of this study include the retrospective assessment of trauma and lifetime PTSD symptoms via screener and the derivation of date of symptom onset based on the date of the worst trauma (to which symptoms were anchored). Nevertheless, we were able to use this information as a time-varying variable in our models, with trauma/PTSD symptom status updated only through 2008. Dating of trauma has been reliable in this sample, but precision of reports may be somewhat lower than contemporaneous report. To avoid concerns about potential reverse causality, we lagged the trauma/PTSD symptom exposure so measures of potential mediators occurred one period after exposure date and hypertension status was evaluated two periods after exposure date. This approach could lead to underestimated results if disease-related effects of trauma/PTSD symptoms appear a few years after trauma/PTSD onset. Furthermore, our study does not address how remission of PTSD symptoms might impact risk of hypertension. If hypertension risk declines once PTSD symptoms remit, our findings may underestimate the strength of the PTSD symptom-hypertension association. Further research is needed to explore this topic. Another limitation is the self-reported hypertension outcome, although it has been validated in this cohort (Chasan-Taber et al., 1996; Forman et al., 2008). Survivor bias is potentially a concern because women needed to remain in the study until 2008 to provide trauma/PTSD data. However, study retention is high (>90% biennial questionnaire response rate), and only 1.6% of the cohort (n=1,826) was deceased by trauma/PTSD symptom assessment. Generalizability of findings may be limited as the NHS II cohort is predominantly white and highly educated. However, findings are consistent with cross-sectional studies in more diverse samples (Davidson et al., 1991; Pietrzak et al., 2011). Despite these limitations, this study is characterized by several unique strengths. It is the largest study of PTSD symptoms and hypertension in women to date and uses longitudinal data, thereby reducing concerns about reverse causality. We are the first study to our knowledge to evaluate the PTSD symptom-hypertension relation in civilian women exposed to various traumas after accounting for a substantial set of potential confounders. Further, we tested a broad range of previously hypothesized mediators.
Conclusions
Approximately 1 in 10 women develop PTSD in their lifetime. Our study suggests that PTSD symptoms not only affect women’s mental health but also their cardiovascular health. These findings, along with results linking PTSD symptoms to myocardial infarction and stroke (Sumner et al., 2015), indicate that women with PTSD symptoms may be particularly susceptible to cardiovascular dysfunction, and this may suggest the value of targeted surveillance of individuals with PTSD to improve early detection of cardiovascular risk. A key goal of future research is to determine whether successful PTSD treatment reduces hypertension. Ultimately, it is clear that the impact of PTSD does not end with the mind but extends to the heart.
Supplementary Material
Acknowledgments
We acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School for managing the NHS II.
Financial Support: This study was supported by the National Institutes of Health grants R01MH078928, R01MH101269, K01HL130650, T32MH017119, and UM1CA176726; and the Yerby Postdoctoral Fellowship.
Footnotes
The authors have no personal or financial disclosures to report.
References
- Abouzeid M, Kelsall HL, Forbes AB, Sim MR, Creamer MC. Posttraumatic stress disorder and hypertension in Australian veterans of the 1991 Gulf War. Journal of Psychosomatic Research. 2012;72:33–38. doi: 10.1016/j.jpsychores.2011.08.002. [DOI] [PubMed] [Google Scholar]
- American Heart Association. Stress and blood pressure. 2014 ( http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/PreventionTreatmentofHighBloodPressure/Stress-and-Blood-Pressure_UCM_301883_Article.jsp). Accessed June 4, 2015.
- Bartoli F, Carrà G, Crocamo C, Carretta D, Clerici M. Metabolic syndrome in people suffering from posttraumatic stress disorder: a systematic review and meta-analysis. Metabolic Syndrome and Related Disorders. 2013;11:301–308. doi: 10.1089/met.2013.0010. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, Kessler RC, Schultz LR. Short screening scale for DSM-IV posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:908–911. doi: 10.1176/ajp.156.6.908. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasan-Taber L, Willett WC, Manson JE, Spiegelman D, Hunter DJ, Curhan G, Colditz GA, Stampfer MJ. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation. 1996;94:483–489. doi: 10.1161/01.cir.94.3.483. [DOI] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. Journal of Nutrition. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Curhan G, Forman J. Association of sweetened beverage intake with incident hypertension. Journal of General Internal Medicine. 2012;27:1127–1134. doi: 10.1007/s11606-012-2069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. Journal of the American Medical Association. 2009;302:489–492. doi: 10.1001/jama.2009.1084. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Hughes D, Blazer DG, George LK. Post-traumatic stress disorder in the community: an epidemiological study. Psychological Medicine. 1991;21:713–721. doi: 10.1017/s0033291700022352. [DOI] [PubMed] [Google Scholar]
- Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. Journal of the American Medical Association. 2009;302:401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, Davidson JR. Pharmacotherapy for PTSD. In: Friedman MJ, Keane T, Resick PA, editors. Handbook of PTSD: Science and Practice. Guilford Press; New York: 2007. pp. 376–405. [Google Scholar]
- Gangwisch JE, Feskanich D, Malaspina D, Shen S, Forman JP. Sleep duration and risk for hypertension in women: results from the Nurses’ Health Study. American Journal of Hypertension. 2013;26:903–911. doi: 10.1093/ajh/hpt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaesmer H, Brähler E, Gündel H, Riedel-Heller SG. The association of traumatic experiences and posttraumatic stress disorder with physical morbidity in old age: a German population-based study. Psychosomatic Medicine. 2011;73:401–406. doi: 10.1097/PSY.0b013e31821b47e8. [DOI] [PubMed] [Google Scholar]
- Granado NS, Smith TC, Swanson GM, Harris RB, Shahar E, Smith B, Boyko EJ, Wells TS, Ryan MA, Millennium Cohort Study Team Newly reported hypertension after military combat deployment in a large population-based study. Hypertension. 2009;54:966–973. doi: 10.1161/HYPERTENSIONAHA.109.132555. [DOI] [PubMed] [Google Scholar]
- Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Annals of Internal Medicine. 1998;128:81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- Kang HK, Bullman TA, Taylor JW. Risk of selected cardiovascular diseases and posttraumatic stress disorder among former World War II prisoners of war. Annals of Epidemiology. 2006;16:381–386. doi: 10.1016/j.annepidem.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kibler JL, Joshi K, Ma M. Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behavioral Medicine. 2009;34:125–132. doi: 10.3200/BMED.34.4.125-132. [DOI] [PubMed] [Google Scholar]
- Kimerling R, Ouimette P, Prins A, Nisco P, Lawler C, Cronkite R, Moos RH. Brief report: utility of a short screening scale for DSM-IV PTSD in primary care. Journal of General Internal Medicine. 2006;21:65–67. doi: 10.1111/j.1525-1497.2005.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Bordelois P, Jun HJ, Roberts AL, Cerda M, Bluestone N, Koenen KC. The weight of traumatic stress: a prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry. 2014;71:44–51. doi: 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach D, Vora R, Rakow M. The relationship between posttraumatic stress disorder and self-reported health problems. Psychosomatic Medicine. 2005;67:939–947. doi: 10.1097/01.psy.0000188572.91553.a5. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, Seldenrijk A, van Hout HP, Zitman FG, van Dyck R, Penninx BW. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53:631–638. doi: 10.1161/HYPERTENSIONAHA.108.126698. [DOI] [PubMed] [Google Scholar]
- Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20:567–590. doi: 10.2165/00023210-200620070-00003. [DOI] [PubMed] [Google Scholar]
- Mancia G, Grassi G. The autonomic nervous system and hypertension. Circulation Research. 2014;114:1804–1814. doi: 10.1161/CIRCRESAHA.114.302524. [DOI] [PubMed] [Google Scholar]
- Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. Journal of Hypertension. 2012;30:842–851. doi: 10.1097/HJH.0b013e32835080b7. [DOI] [PubMed] [Google Scholar]
- Moore DW, Gallup GH, Schussel R. Disciplining Children in America: A Gallup Poll Report. Gallup Organization; Princeton: 1995. [Google Scholar]
- Morgan CA, Hazlett G, Wang S, Richardson EG, Jr, Schnurr P, Southwick SM. Symptoms of dissociation in humans experiencing acute, uncontrollable stress: a prospective investigation. American Journal of Psychiatry. 2001;158:1239–1247. doi: 10.1176/appi.ajp.158.8.1239. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology. 2009;73:1543–1550. doi: 10.1212/WNL.0b013e3181c0d6e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole B, Catts SV. Trauma, PTSD, and physical health: an epidemiological study of Australian Vietnam veterans. Journal of Psychosomatic Research. 2008;64:33–40. doi: 10.1016/j.jpsychores.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Paulus EJ, Argo TR, Egge JA. The impact of posttraumatic stress disorder on blood pressure and heart rate in a veteran population. Journal of Traumatic Stress. 2013;26:169–172. doi: 10.1002/jts.21785. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosomatic Medicine. 2011;73:697–707. doi: 10.1097/PSY.0b013e3182303775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. Journal of Epidemiology & Community Health. 2010;64:413–418. doi: 10.1136/jech.2009.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Agnew-Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, Hu FB, Rich-Edwards JW, Koenen KC. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry. 2015;72:203–210. doi: 10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Stein MB, Afifi TO, Fleet C, Asmundson GJ. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosomatic Medicine. 2007;69:242–248. doi: 10.1097/PSY.0b013e31803146d8. [DOI] [PubMed] [Google Scholar]
- The SAS Mediate Macro [computer program] Brigham and Women’s Hospital Channing Laboratory; Boston: 2009. [Google Scholar]
- Schnurr PP, Spiro A, 3rd, Paris AH. Physician-diagnosed medical disorders in relation to PTSD symptoms in older male military veterans. Health Psychology. 2000;19:91–97. doi: 10.1037//0278-6133.19.1.91. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Vieilhauer MJ, Weathers F, Findler M. The Brief Trauma Questionnaire. National Center for PTSD; White River Junction: 1999. [Google Scholar]
- Stein DJ, Aguilar-Gaxiola S, Alonso J, Bruffaerts R, de Jonge P, Liu Z, Miguel Caldas-de-Almeida J, O’Neill S, Viana MC, Al-Hamzawi AO, Angermeyer MC, Benjet C, de Graaf R, Ferry F, Kovess-Masfety V, Levinson D, de Girolamo G, Florescu S, Hu C, Kawakami N, Maria Haro J, Piazza M, Posada-Villa J, Wojtyniak BJ, Xavier M, Lim CC, Kessler RC, Scott KM. Associations between mental disorders and subsequent onset of hypertension. General Hospital Psychiatry. 2014;36:142–149. doi: 10.1016/j.genhosppsych.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus MA, Gelles RJ. Physical Violence in American Families: Risk Factors and Adaptations to Violence in 8,145 Families. Transaction Publishers; New Brunswick: 1990. [Google Scholar]
- Sumner JA, Kubzansky LD, Elkind MSV, Roberts AL, Agnew-Blais J, Chen Q, Cerdá M, Rexrode KM, Rich-Edwards JW, Spiegelman D, Suglia SF, Rimm EB, Koenen KC. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–259. doi: 10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM. Extending the Cox model. In: Lin DY, Fleming TR, editors. Proceedings of the First Seattle Symposium in Biostatistics: Survival Analysis. Spring Verlag; New York: 1997. pp. 51–84. [Google Scholar]
- Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the Heart and Soul Study. Health Psychology. 2012;31:94–201. doi: 10.1037/a0025989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neuroscience & Biobehavioral Reviews. 2013;37:860–895. doi: 10.1016/j.neubiorev.2013.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


