Key Clinical Message
Sarcoidosis is a diagnosis that should be considered in patients receiving interferon therapy, who present with anemia and multiorgan dysfunction regardless of the duration of their treatment. When sarcoidosis is suspected, bone marrow biopsy should be considered especially for cases predominant by extrapulmonary features.
Keywords: Anemia, bone marrow, granuloma, interferon, sarcoidosis
Introduction
Sarcoidosis is a rare condition with a prevalence of 4.4–6.3 patients per 100,000 person‐years in Australia, a figure similar to that of the United State 1, 2. It is a multisystem granulomatous disorder of unknown etiology predominantly affecting young and middle‐aged adults 3, 4. Diagnostic criteria are based on a compatible clinical and radiological features supported by the histological evidence of noncaseating granulomas in the affected tissues, and the exclusion of other known causes of granulomatous inflammation 3, 4. Pulmonary infiltration and hilar lymphadenopathy are the most common findings in more than 90% of cases, and transbronchial lung biopsy has been the recommended diagnostic procedure 3, 4. Other organs’ involvement includes skin, eyes, liver, spleen, heart, musculoskeletal system, gastrointestinal tract, bone marrow, and the central nervous system. Anemia has been reported in cases of sarcoidosis with the frequency ranging from 3.4% to 31% with a variety of explanations. These include hypersplenism, burden of chronic disease, autoimmunity, and bone marrow infiltration 5. Histological evaluation of extrapulmonary sarcoidosis depends on the site of involvement, but its diagnosis is rarely made via bone marrow biopsy 6. Features which may suggest bone marrow infiltration in sarcoidosis include extrapulmonary involvement and varying degree of cytopenia 7. The characteristic granuloma in sarcoidosis is a noncaseating focal aggregation of macrophages or epithelioid cells, with or without multinucleated giant cells. Asteroid bodies are crisscrossing bundles of collagen fibers sometimes found within the giant cells. Its presence confirmed the diagnosis of a non‐necrotizing granuloma, in particular sarcoidosis 8, 9.
Case Presentation
A 40‐year‐old Caucasian woman presented with a three‐month history of polyuria, malaise, and weight loss. She had been receiving interferon (IFN) β‐1α for multiple sclerosis for the previous 12 years. Pertinent examination findings were conjunctival pallor and splenomegaly. Investigations revealed moderate normocytic anemia, hypercalcemia, hyperglobulinemia, and acute kidney injury (Table 1). B‐cell monoclonality was not evident on myeloma screen. Her initial chest radiograph was normal, but further investigation with computed tomography showed diffuse lymphadenopathy in her chest and abdomen. There was also splenomegaly with hypodense lesions. Skeletal survey was normal. A bone marrow trephine biopsy demonstrated noncaseating granulomas (Figs 1 and 2) and asteroid bodies (black arrows in Fig. 1), confirming the diagnosis of sarcoidosis. Ziehl‐Neelsen staining did not reveal acid fast bacilli. She received intravenous normal saline therapy for her hypercalcemia. Her IFN‐β treatment was discontinued, and she was commenced on prednisolone (60 mg daily) with a slow tapering regimen over 6 months. Her renal function and hypercalcemia recovered promptly, and her hemoglobin normalized at 12th week. Her overall well‐being improved.
Table 1.
Summary of laboratory investigation results
| Tests [SI units] | Results | Reference interval |
|---|---|---|
| Hemoglobin [g/L] | 86 | 115–155 |
| MCV [fL] | 83 | 80.0–98.0 |
| White cell count [×109/L] | 7.70 | 4.00–11.0 |
| Platelets [×109/L] | 384 | 150–450 |
| Creatinine [μmol/L] | 241 | 50–100 |
| eGFR [mL/min/1.37 m2] | 21 | >60 mL/min/1.37 m2 |
| Calcium [mmol/L] | 3.60 | 2.10–2.55 |
| Ionized calcium [mmol/L] | 1.84 | 1.10–1.30 |
| Phosphate [mmol/L] | 1.41 | 0.65–1.45 |
| Total protein [g/L] | 88 | 65–85 |
| Albumin [g/L] | 35 | 34–48 |
| Globulin [g/L] | 42 | 21–41 |
| Paraprotein [g/L] | 0 | 0 |
| K/L ratio | 1.11 | 0.26–1.65 |
| PTH [pmol/L] | 1.1 | 0.8–5.5 |
| ACE [U/L] | 69 | 8–52 |
| 1,25‐dihydroxyvitamin D [pmol/L] | 344 | 50–160 |
| 25‐hydroxyvitamin D [nmol/L] | 61 | 60–160 |
| Urine calcium [mmol/24 h] | 9.0 | 2.5–7.5 |
eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone; K/L, Kappa/Lambda light chain; ACE, angiotensin converting enzyme; SI, Système International. Bold = values not within normal range.
Figure 1.
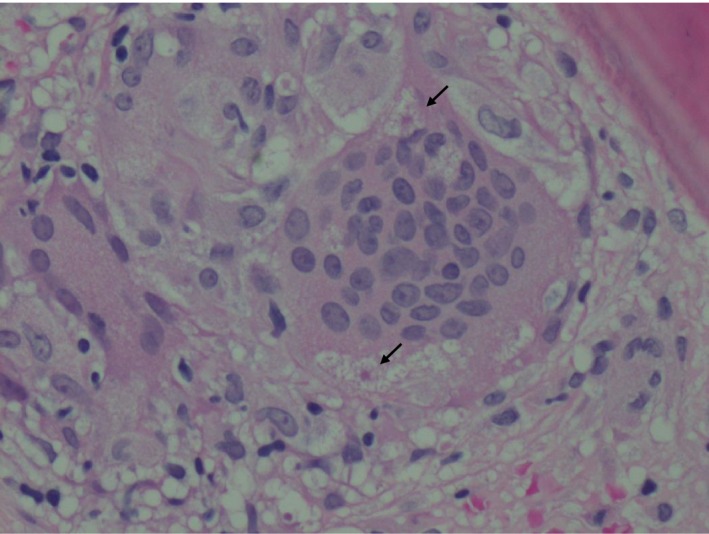
Histopathological section of a trephine bone marrow biopsy revealing a noncaseating granuloma composed of aggregation of multinucleated giant cells containing asteroid bodies (arrowed). Hematoxylin and eosin stain (x40).
Figure 2.
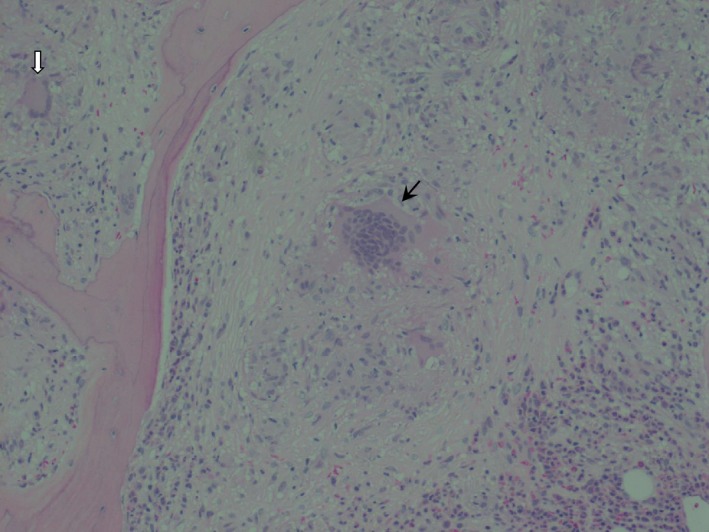
The noncaseating granuloma in the center (black arrow) is surrounded by pale pink amorphous material with scattered mononuclear cells interspersed throughout with some giant cells. There is also a surrounding cuff composed of reactive cells such as eosinophils and neutrophils. A second granuloma is visible in the periphery containing a Langhans giant cell (white arrow). Hematoxylin and eosin stain (x20).
Discussion
The exact etiology of sarcoidosis remains unknown. A cardinal feature of sarcoidosis is the presence of CD+ T cells which display unregulated interaction with the antigen‐presenting cells to initiate the formation and maintenance of noncaseating granulomas 10. Oligoclonal αβ T‐cell repertoire observed in sarcoidosis suggests that the triggering antigens favor the progressive accumulation and activation of selective CD4 T‐cell clones 11. This in turn leads to the preferential differentiation of the Th1 helper cells which predominantly secretes interleukins (IL)‐2 and IL‐12 12. These ILs have been found in higher quantity in the bronchoalveolar lavage fluid of patients with sarcoidosis and subsequently stimulate increased production of IFN‐γ and macrophage activation. IFN‐γ belongs to type II IFN family. It has a different structure and binds to a different receptor than the type I IFN (α and β), and its gene is located on a different chromosome 12. Although endogenous IFN‐γ has been implicated in the pathogenesis of sarcoidosis, there is little evidence for other types of IFNs 11. However, exogenously administered IFN‐α and IFN‐β activate macrophages in vitro 10.
The incidence of sarcoidosis is increasing with the use of type I IFN (IFN‐α, IFN‐β) for various conditions such as hepatitis B, hepatitis C, lymphoproliferative malignancy, and multiple sclerosis 13, 14, 15. To date, 60 cases of sarcoidosis have been reported in the English literature in association with the use of IFN‐α, in comparison with only six cases which were associated with the use of IFN‐β 13, 14, 15, 16, 17, 18, 19, 20. The characteristic of these six cases revealed a predilection for middle age women. The mean onset of development of sarcoidosis was 11.4 months after initiation of IFN therapy (ranged 1–60 months), which is much earlier than our patient 13. The pattern of system involvement is similar to that of the idiopathic form of sarcoidosis. Only one case of the six is proven to have bone marrow involvement with a bone marrow biopsy 16. IFN‐induced sarcoidosis exhibits a relatively mild disease course that usually resolves with cessation of IFN treatment. A small proportion of patients required systemic corticosteroid 13, 14, 15, 16, 17, 18, 19, 20.
Conclusion
Sarcoidosis is a disease with vast clinical heterogeneity. It is a diagnosis that should be considered in patients receiving IFN therapy of any type who present with anemia and multiorgan dysfunction. The overall pattern of organs’ involvement in sarcoidosis associated with IFN is similar to that of the idiopathic form. Bone marrow biopsy should be considered for cases predominant by extrapulmonary features.
Conflict of Interest
None declared.
Acknowledgment
We thank Dr J P Boey (Advanced Trainee, Haematology Department of Flinders Medical Centre) who provided the histology specimen for this publication.
References
- 1. Gillman, A. , and Steinfort C.. 2007. Sarcoidosis in Australia. Intern. Med. J. 37:356–359. [DOI] [PubMed] [Google Scholar]
- 2. Henke, C. E. , Henke G., Elveback L. R., Beard C. M., and Ballard D.. 1986. The epidemiology of sarcoidosis in Rochester Minnesota: a population‐based study of incidence and survival. Am. J. Epidemiol. 123:840–845. [DOI] [PubMed] [Google Scholar]
- 3. Joint statement of the American Thoracic Society . 1999. European Respiratory Society and World Association of Sarcoidosis and other Granulomatous Disorders. Statement on sarcoidosis. Am. J. Respir. Crit. Care Med. 160:736–755. [DOI] [PubMed] [Google Scholar]
- 4. Iannuzzi, M. , Rybicki B., and Teirstein A.. 2007. Medical progress: sarcoidosis. N. Engl. J. Med. 357:2153–2165. [DOI] [PubMed] [Google Scholar]
- 5. Johns, C. J. , and Michele T. M.. 1999. The clinical management of sarcoidosis. A 50‐year experience at Johns Hopkins Hospital. Medicine 78:65–111. [DOI] [PubMed] [Google Scholar]
- 6. Eskenasy, A. 1987. Histodiagnosis of pulmonary sarcoidosis on different organ biopsies. Morphol. Embryol. 33:111–121. [PubMed] [Google Scholar]
- 7. Yanardağ, H. , Pamuk G. E., and Karayel T.. 2002. Bone marrow involvement in sarcoidosis: an analysis of 50 bone marrow samples. Haematologia 32:419–425. [PubMed] [Google Scholar]
- 8. Azar, H. A. , and Lunardelli C.. 1969. Collagen nature of asteroid bodies of giant cells in sarcoidosis. Am. J. Pathol. 57:81. [PMC free article] [PubMed] [Google Scholar]
- 9. Gadde, P. S. , and Moscovic E. A.. 1994. Asteroid bodies: products of unusual microtubule dynamics in monocyte‐derived giant cells. An immunohistochemical study. Histol. Histopathol. 9:633–642. [PubMed] [Google Scholar]
- 10. Agosttini, C. , Trentin L., and Perin A.. 1999. Regulation of alveolar macrophage‐T cell interactions during Th1‐type sarcoid inflammatory process. Am. J. Physiol. 277:L240–L250. [DOI] [PubMed] [Google Scholar]
- 11. Silver, R. , Crystal R., and Moller D.. 1996. Limited heterogeneity of biased T‐cell receptor V beta gene usage in lung but not blood T cells in active pulmonary sarcoidosis. Immunology 88:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shigehara, K. , Shijubo N., Ohmichi M., and Takahashi R.. 2001. IL‐12 and IL‐18 are increased and stimulate IFN‐gamma production in sarcoid lungs. J. Immunol. 166:642–649. [DOI] [PubMed] [Google Scholar]
- 13. Goldberg, H. , Fiedler D., and Webb A.. 2006. Sarcoidosis after treatment with interferon‐alpha: a case series and review of the literature. Respir. Med. 100:2063–2068. [DOI] [PubMed] [Google Scholar]
- 14. Kikawada, W. , Ichinose Y., and Kunisawa A.. 1998. Sarcoidosis induced by interferon therapy for chronic myelogenous leukaemia. Respiratory 3:41–44. [DOI] [PubMed] [Google Scholar]
- 15. Chakravarty, S. , Schreiner A., and Crow M.. 2012. Sarcoidosis triggered by interferon‐beta treatment of multiple sclerosis: a case report and focused literature review. Semin. Arthritis Rheum. 42:206–212. [DOI] [PubMed] [Google Scholar]
- 16. Bobbio‐Pallavicini, E. , Valsecchi C., Tacconi F., and Moroni M.. 1995. Sarcoidosis following beta‐interferon therapy for multiple myeloma. Sarcoidosis 12:140–142. [PubMed] [Google Scholar]
- 17. Mehta, C. , Tyler R., and Cripps D.. 1998. Granulomatous dermatitis with focal sarcoidal features associated with recombinant interferon beta‐1b injections. J. Am. Acad. Dermatol. 39:1024–1028. [DOI] [PubMed] [Google Scholar]
- 18. O'Reilly, S. , White A., and Florian A.. 2005. Mediastinal sarcoidosis in a patient receiving interferon‐beta for multiple sclerosis. Chest 128:450S–a451S. [Google Scholar]
- 19. Petousi, N. , and Thomas E.. 2012. Interferon‐ß‐induced pulmonary sarcoidosis in a 30‐year‐old woman treated for multiple sclerosis. J. Med. Case Rep. 6:344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdi, E. , Nguyen G., and Ludwig R.. 1987. Pulmonary sarcoidosis following interferon therapy for advanced renal cell carcinoma. Cancer 59:896–900. [DOI] [PubMed] [Google Scholar]


