Abstract
The nerve growth cone is bi-directionally attracted and repelled by the same cue molecules depending on the situations, while other non-neural chemotactic cells usually show uni-directional attraction or repulsion toward their specific cue molecules. However, how the growth cone differs from other non-neural cells remains unclear. Toward this question, we developed a theory for describing chemotactic response based on a mathematical model of intracellular signaling of activator and inhibitor. Our theory was first able to clarify the conditions of attraction and repulsion, which are determined by balance between activator and inhibitor, and the conditions of uni- and bi-directional responses, which are determined by dose-response profiles of activator and inhibitor to the guidance cue. With biologically realistic sigmoidal dose-responses, our model predicted tri-phasic turning response depending on intracellular Ca2+ level, which was then experimentally confirmed by growth cone turning assays and Ca2+ imaging. Furthermore, we took a reverse-engineering analysis to identify balanced regulation between CaMKII (activator) and PP1 (inhibitor) and then the model performance was validated by reproducing turning assays with inhibitions of CaMKII and PP1. Thus, our study implies that the balance between activator and inhibitor underlies the multi-phasic bi-directional turning response of the growth cone.
During development, the connectivity of neural circuits is determined by axon guidance, a chemotactic process in which the axonal growth cone orients its migrating direction in response to extracellular guidance cues1. The motile growth cone, unlike other chemotactic cells, has the unique character of being attracted or repelled by the same guidance cue depending on its biological environment (this character denotes bi-directionality hereafter)2. These chemo-attraction and chemo-repulsion responses are dynamically regulated to achieve a mature, functional nervous system3,4. The elucidation of the molecular mechanisms by which bi-directional attractive and repulsive responses of the growth cone are regulated is key for understanding circuit formation in the developing nervous system.
Non-neural cells, such as Dictyostelium discoideum and immune cells, are persistently attracted or repelled by specific cue molecules (this character denotes uni-directionality hereafter). This uni-directional chemotaxis is correlated with the polarized accumulation of an intracellular signaling molecule, i.e., a gradient of phosphoinositol-3,4,5-trisphosphate (PIP3)5. Such polarized accumulation of PIP3 was generated by a local excitation-global inhibition (LEGI) model, in which the activator and inhibitor locally and dispersedly function, respectively6,7,8,9. It has been thought that activator and inhibitor could be phosphoinositide 3-kinase (PI3K) and the lipid phosphatase, phosphatase and tensin homolog (PTEN), each of which synthesizes PIP3 from phosphoinositol-4,5-bisphosphate (PIP2) and reciprocally metabolizes PIP3 to PIP2, respectively. Note that LEGI mechanism could also be implemented by other pathways, e.g., the small GTPase Ras8,10,11, because the cells in which all PI3Ks are knocked out still display reasonable chemotaxis with slower migration speed12. In the LEGI model, however, how a cell senses the gradient of chemorepellents has not been fully addressed.
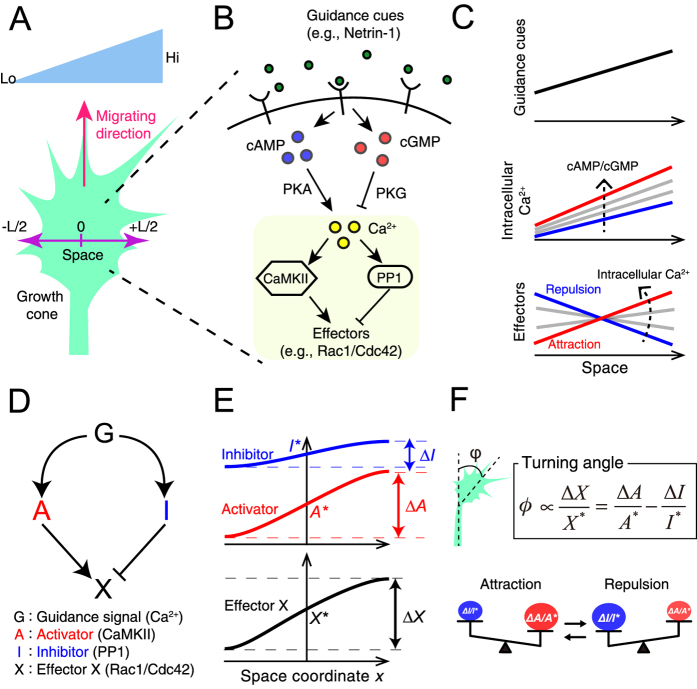
In growth cones, the intracellular levels of second messengers, cyclic nucleotides (cAMP and cGMP) and calcium ion (Ca2+), are known to regulate bi-directional turning in response to the same guidance cue. For example, Netrin-1 leads to the production of cAMP and cGMP, which activate cAMP-dependent protein kinase (PKA) and cGMP-dependent protein kinase (PKG), respectively, and, in turn, induces Ca2+ influx13 (Fig. 1A,B). Extracellular gradients of diffusible guidance signals are translated into intracellular Ca2+ gradients in the same direction as the extracellular gradients, regardless of attraction or repulsion (Fig. 1C)13,14,15,16. A gradient of Netrin-1 induces attraction by increasing both the ratio of cAMP to cGMP and the Ca2+ level in the growth cone. However, if the ratio of cAMP to cGMP or the level of Ca2+ decreases, then the same Netrin-1 gradient induces repulsion13,14. Moreover, Ca2+ imaging studies have demonstrated that high and low increases in intracellular Ca2+ gradients are generated during attraction and repulsion, respectively15. The basal (resting) level of intracellular Ca2+ has also been shown to modulate the growth cone turning direction: a high basal level of Ca2+ results in attraction, whereas a low basal level of Ca2+ results in repulsion, given the same increase in localized Ca2+ 17,18. Thus, the intracellular Ca2+ level is a key mediator that governs the bi-directional turning response of the growth cone (Fig. 1C).
Figure 1. The mathematical growth cone model encompasses an activator and inhibitor system.
(A) The schematic drawing depicting the one-dimensional model growth cone encountering an extracellular gradient of guidance cues. (B) In the growth cone, guidance cues, e.g., Netrin-1, lead to the production of cAMP and cGMP, which activate PKA and PKG, respectively, in turn inducing a Ca2+ increase. The intracellular Ca2+ up-regulates CaMKII and PP1, which function as an activator and inhibitor, respectively, of their effector to regulate growth cone motility (referred to as ‘X’ in the text and (D)), e.g., Rac1 and Cdc42. (C) Extracellular gradients of cues are transduced to intracellular gradients of Ca2+ (middle panel) in the same direction as the extracellular gradients, and the increase in the intracellular Ca2+ level that depends on the ratio of cAMP to cGMP regulates the turning direction of the growth cone, i.e., attraction or repulsion. The biased direction of the effector distribution is thought to be reversed between attraction and repulsion (lower panel). High and low levels of intracellular Ca2+ induce attraction and repulsion, respectively, as indicated by the dotted arrows. (D) An activator-inhibitor system of intracellular signaling in chemotaxis: Guidance signal (G) regulates activator (A) and inhibitor (I) in turn up- and down-regulating the effector X. G, A, I and X correspond to Ca2+, CaMKII, PP1 and Rac1/Cdc42, respectively, in the growth cone. (E) Following exposure to a gradient of the guidance cue G, the gradients of A and I are formed across the growth cone, thereby the gradient of X is also formed. A*, I* and X* represent the levels of A, I and X, respectively, at the center of the growth cone. ΔA, ΔI and ΔX represent the spatial differences of A, I and X, respectively, across the growth cone. (F) The turning angle of the growth cone is formulated as ΔX/X* based on the Weber-Fechner law. The balance between ΔA/A*and ΔI/I* governs the turning direction, which is illustrated by scales: attraction when ΔA/A*> ΔI / I* and repulsion when ΔA/A* < ΔI/I*.
Calmodulin (CaM)-dependent kinase II (CaMKII) and Protein phosphatase 1 (PP1) function in the growth cone as an activator-inhibitor system in the downstream of Ca2+, similarly as PI3K and PTEN function in non-neural cells; CaMKII and PP1 act as an activator and inhibitor, respectively, of the effectors that regulate growth cone motility, e.g., Rac1 and Cdc42 (Fig. 1B)19,20,21,22. Importantly, these downstream molecules also modulate the turning direction in response to an external cue: CaMKII triggers attraction, whereas PP1 triggers repulsion18. Thus, the downstream Ca2+ signaling pathway ultimately decodes the external signal to induce either attractive or repulsive growth cone behavior. How, then, does the activator-inhibitor system regulate the bi-directional turning behaviors of the growth cone in response to the same external cue?
Recently, two computational models have been proposed with interests in chemotactic response of growth cones. Forbes et al. proposed a model of growth cone Ca2+ signaling23, by extending the synaptic plasticity model incorporating CaMKII bi-stability24. On the other hand, Roccasalvo et al. developed a reaction-diffusion model of self-enhancement dynamics of Ca2+ in two-dimensional growth cone25. Although these models successfully reproduced bi-directional turning behaviors of growth cones, essential difference in the underlying mechanism between uni-directional chemotactic cells and bi-directional growth cones has been largely unknown.
Here, we proposed a mathematical model to generally address both uni- and bi-directional chemotactic responses, based on an activator-inhibitor system shared by many chemotactic cells. We then established a general theory that describes the mechanistic difference between non-neural chemotactic cells showing uni-directionality and growth cones showing bi-directionality. Based on the model analysis, we theoretically predicted that the turning response of the growth cone could multi-phasically change, e.g., from repulsion, attraction to repulsion, as intracellular Ca2+ increased, and experimentally validated the prediction using growth cone turning assays and Ca2+ imaging. Furthermore, we reverse engineered the model parameters to fit the growth cone turning assays, so that its predictive performance was experimentally validated by pharmacological suppression of the activator (CaMKII) or inhibitor (PP1).
Results
A mathematical model of activator-inhibitor system
We developed a mathematical model of chemotactic cells based on intracellular signaling. The model chemotactic cell migrates and encounters extracellular gradient of a guidance cue (Fig. 1A). Though the cells have three-dimensional structures in reality, we addressed an intracellular one-dimensional (1D) coordinate, which is perpendicular to the migrating direction (Fig. 1A). Note that the cells are known to persistently migrate and turn according to polarized accumulation of intracellular signals along the coordinate perpendicular to the migrating direction26,27.
The model chemotactic cell is equipped with intracellular signaling molecules, the activator (A) and inhibitor (I), whose enzymatic activities are regulated by a guidance molecule, G (Fig. 1D). In this model, we did not specify the details of intracellular signaling, but assumed that the steady-state gradients of these intracellular signaling molecules, whose concentrations are denoted as A and I, are generated in accordance with the gradient of the extracellular guidance cue (Fig. 1E). This model is also equipped with a downstream effector X, which regulates the driving force for migration. X is directly up- and down-regulated, respectively, by the activator and inhibitor (Fig. 1D), and their regulations are assumed to be in reaction equilibrium state, in which its activity is locally determined by the ratio of A to I: X(x) = αA(x)/I(x), where X is the effector’s concentration, x denotes the one-dimensional coordinate of the model cell, and α is a positive constant. This assumption holds if the effector X is regulated by push-pull reaction (see Supplementary Information).
In the model, the migrating cell was turned based on spatial polarity of the distribution of X along the 1D coordinate, implying that X acted as a decoder that discriminated between attraction and repulsion. We here assumed that the downstream system that converts the spatial distribution of X into the growth cone turning response is endowed with adaptation property; this property was stated as the Weber-Fechner law, in which the detectable spatial polarity of X varies because of the scale of the concentration of X28. Indeed, the Weber-Fechner law has been found in several types of chemotactic cells29,30,31,32,33. We thus defined the turning angle, ω, of the growth cone as ΔX/X*, where X*denotes the effector’s level at the cellular center and ΔX is the spatial difference of X’s activity across the cell: ΔX = X(L/2)−X(−L/2), where L is the length of the cell (see Fig. 1A) and +L/2 and −L/2 represent, respectively, the coordinates at the cell’s near and far sides with respect to the gradient of G. Thus, our model shows that if ΔX > 0, the cell is attracted and migrates toward the gradient; if ΔX < 0, the cell is repelled and turns away from the gradient.
Our model addressed only 1D coordinate, although the cells usually spread their morphology on a two-dimensional (2D) culture substrate. To further check the validity of our theory in a two-dimensional space, we also developed a 2D model (see Supplementary Figure 1 and Supplementary information) and confirmed that the migrating behaviors were consistent with those by the 1D model.
Theory for chemotactic turning responses
In the presence of a shallow extracellular gradient of G, the turning angle ω is approximately derived as follows (see Methods)
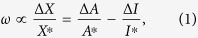 |
where A* and I* denote the levels of A and I, respectively, at the cellular center. ΔA and ΔI denote, respectively, the spatial differences of A and I across the cell: ΔA = A(L/2)−A(−L/2) and ΔI = I(L/2)−I(−L/2). Equation (1) indicates that the cellular migration direction depends on the balance between ΔA/A* and ΔI/I*, i.e., attractive (ω > 0) and repulsive (ω < 0) when ΔA/A*> ΔI/I* and ΔA/A* < ΔI/I*, respectively (Fig. 1F). This equation suggests that when the signs of ΔA and ΔI are opposite, the turning response is uni-directional (either attraction or repulsion) regardless of their magnitudes. For example, if ΔA is positive and ΔI is negative, only the attractive response occurs (ΔX > 0). This type of uni-directional chemoattractive response has commonly been observed in Dictyostelium discoideum, which is characterized by opposite intracellular gradients of PI3K and PTEN5. However, if the signs of ΔA and ΔI are the same, the migratory behaviors become bi-directional; switching occurs from attraction to repulsion and vice versa depending on the levels of A* and I*. This type of phenomenon has typically been observed in nerve growth cones13,15,18,34,35,36. We subsequently focused on the bi-directional turning responses of growth cones.
The model predicts both uni- and multi-phasic bi-directional turning responses
In the growth cone, an extracellular gradient is converted to an intracellular Ca2+ gradient, whose direction is same as the extracellular gradient, and the level of Ca2+ regulates bi-directional responses (attraction or repulsion) (Fig. 1C)15. We here specifically examined how the intracellular Ca2+ level increase affects the turning response to the extracellular gradient. Our model assumes that an intracellular gradient of Ca2+ correlates with the gradient of the guidance signal G, and that the dose-responses of both activator A and inhibitor I are monotonically up-regulated by intracellular Ca2+ level (red and blue solid lines in Fig. 2A,C,E and G) and that ΔA and ΔI are proportional, respectively, to the dose-response slopes of A and I (see Equation (7) in Methods).
Figure 2. The theoretical model is capable of producing both uni-directional and multi-phasic bi-directional turning responses.
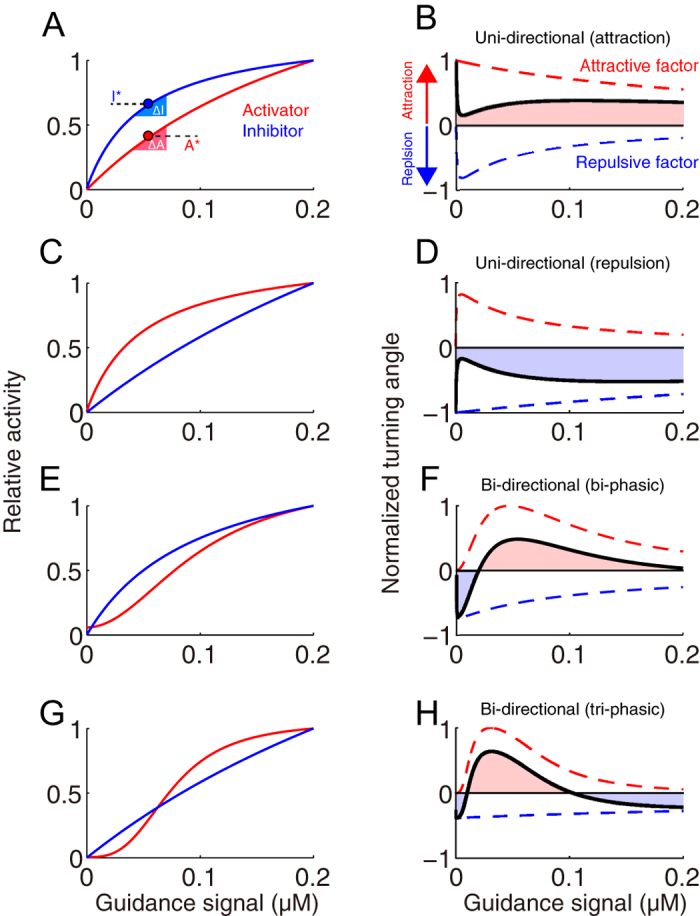
(A,C,E,G) Examples of activator and inhibitor activities in response to increasing guidance signal (intracellular Ca2+ in the case of the growth cone). Red and blue lines indicate the dose-responses of activator A and inhibitor I, respectively, to the guidance signal. The dose-response curves are given by Hill equations (Equations (8 and 9)). Parameter values are listed in Methods. (B,D,F,H) Turning responses (black lines) are calculated based on the dose-responses of activator and inhibitor, shown in (A,C,E,G) (see Methods). When the intracellular Ca2+ level is in the red and blue shaded regions, the growth cone exhibits attraction and repulsion, respectively. The turning response is given by the sum of the attractive factor (red dashed line: the first term in Equation (1)) and the repulsive factor (blue dashed line: the second term in Equation (1)). Each dashed line represents the ratio between the slope and amplitude of the dose-response curve in (B), (C), (E) or (G); e.g., the red dashed line in (B) was obtained from the slope (ΔA) and amplitude (A*) of the red solid line in (A). Thus, the theoretical model is able to produce a simple uni-directional attraction (A,B) or repulsion (C,D), bi-phasic bi-directional turning response (E,F), and tri-phasic bi-directional turning response (G,H). These complex responses are primarily due to the nonlinear responses of the activator and inhibitor to the guidance signal, typically observed in (G).
The model predicts different patterns of turning responses as the activities of A and I are progressively increased as the intracellular Ca2+ level increases: uni-directional responses, i.e., either attraction (Fig. 2B) or repulsion (Fig. 2D), and a bi-directional turning response, i.e., a change from repulsion to attraction (bi-phasic) (Fig. 2F). Interestingly, the model further predicts the existence of a tri-phasic bi-directional turning response, i.e., a change from repulsion to attraction and then back to repulsion (Fig. 2H). As assumed in Fig. 2G, CaMKII and CaN/PP1, which function as an activator and inhibitor, respectively, in the growth cone, are well known to exhibit sigmoidal dose-responses to Ca2+ 37,38. Thus, this theoretical result suggests that the intracellular Ca2+ level would be responsible for growth cone’s bi-phasic and tri-phasic turning responses.
It should be noted that these model predictions were based on the turning angle Equation (1), which was derived based on the assumption of shallow extracellular gradient in the 1D model (Fig. 1A). Here, we checked if the 1D model reproduces the 2D model (Supplementary figure 1; see Supplementary Information) at least with a 10% extracellular gradient (i.e., 10% concentration difference between the near and far sides of the growth cone), typically used in the growth cone turning assay39,40. As a result, we found that our theory based on the 1D model well characterized the 2D migration of the growth cone under a biologically realistic range of gradient like 1–10%41,42,43,44,45 (Supplementary Figures 2 and 3).
The cAMP/cGMP gradients induce tri-phasic bi-directional turning
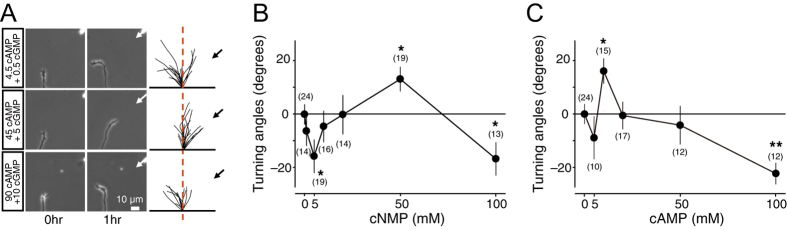
To experimentally validate our theoretical prediction of growth cone bi-directional (both bi- and tri-phasic) turning responses (Fig. 2H), we performed in vitro turning assays of Xenopus spinal neuron growth cones13,14,46 by applying a gradient of the 9:1 ratio of the membrane permeable and phosphodiesterase (PDE)-resistant cyclic nucleotide analogues, Sp-8-Br-cAMPS and 8-Br-cGMP (Fig. 3); this ratio was previously observed to induce growth cone attraction13. Note that the use of PDE-resistant cyclic nucleotides allowed us to bypass ligand-receptor interactions47 and potential interdependent signaling events between these two cyclic nucleotides, such as the effects of PDEs48.
Figure 3. The growth cone exhibits multi-phasic bi-directional turning responses when the total concentration of [cAMP + cGMP] is changed at the fixed ratio of cAMP/cGMP.
(A) Representative images of growth cones at the onset (0 hr) and after (1 hr) exposure to a gradient solution containing a mixture of the membrane permeable cyclic nucleotide analogues, [Sp-8-Br-cAMPS] and [8-Br-cGMP], at a fixed ratio of 9:1 at different concentrations (5 mM [4.5 cAMP + 0.5 cGMP]; 50 mM [45 cAMP + 5 cGMP]; and 100 mM [90 cAMP + 10 cGMP]) in the application micropipette. Superimposed traces on the right depict the total neurite trajectories examined over the 1 hr period, where the initial position of the growth cone is at the origin, and the original direction of growth is vertical. The arrows in both the representative images and trajectories mark the direction of the gradient. (B) The average growth cone turning angles in response to gradient of a mixture solution of [Sp-8-Br-cAMPS] and [8-Br-cGMP] with different concentrations (0, 1, 5, 10, 20, 50, and 100 mM) at the fixed ratio of 9:1 in the micropipette, which are indicated as “cNMP”. (C) The average growth cone turning angles in response to gradient of a solution of [Sp-8-Br-cAMPS] at different concentrations (5, 10, 20, 50, and 100 mM) in the micropipette. Positive and negative turning angles indicate attraction and repulsion, respectively. Error bars = s.e.m. (n) = number of growth cones examined. Significant differences compared to the control are indicated (*p < 0.05; **p < 0.01; Mann-Whitney U test).
The growth cones were attracted by the gradient of a 9:1 ratio of cAMP:cGMP at a total concentration of 50 mM in application micropipettes (middle panel in Fig. 3A), as demonstrated previously13. However, the growth cones were repelled by the gradient at lower and higher total concentrations (5 mM and 100 mM). Thus, a tri-phasic bi-directional turning response was observed (Fig. 3B), implying that not only the ratio of cyclic nucleotide analogues but also the magnitude of their total concentration determines the growth cone turning direction. These results also suggested that a simple increase in attractive promoting factors (e.g., cAMP) or a decrease in repulsive promoting factors (e.g., cGMP) does not always result in either exclusive attraction or repulsion, respectively. In support of this observation, an external gradient of only Sp-8-Br-cAMPS, which is an attractive promoting factor, also induced tri-phasic bi-directional turning responses (Fig. 3C).
Monotonic Ca2+ increase induces multi-phasic bi-directional turning
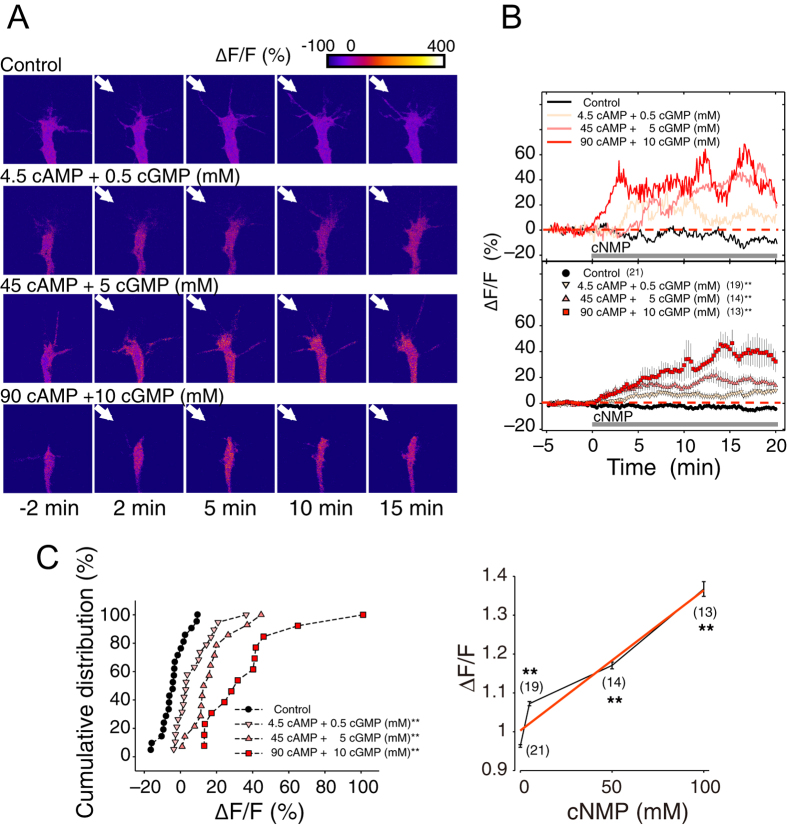
We further examined the extent to which the growth cone Ca2+ increase is associated with the multi-phasic bi-directional turning behaviors by performing growth cone Ca2+ imaging in the presence of gradients of cyclic nucleotide analogues (Fig. 4A). Within several minutes of exposure to the gradients, the Ca2+ level in the growth cones increased and remained stable throughout the imaging procedure (Fig. 4B)14. Surprisingly, the Ca2+ response was monotonically increased as the amplitude of the gradients of cyclic nucleotide analogues increased (Fig. 4C). Furthermore, this Ca2+ imaging experiment demonstrated that repulsion is not only induced in response to a low growth cone Ca2+ increase, as previously reported13,15, but also occurs at a high Ca2+ increase. Taken together, the growth cone turning assays and the Ca2+ imaging results suggested that non-linear regulation of activator and inhibitor in the downstream signaling cascade induced by Ca2+ is responsible for the multi-phasic bi-directional turning behaviors of growth cones.
Figure 4. Multi-phasic bi-directional turning depending on the concentration of cAMP/cGMP gradients is accompanied by a monotonic Ca2+ increase.
(A) Representative images of Oregon Green 488 BAPTA-1 (OGB)-dextran fluorescent growth cones obtained at various times during exposure to either culture medium (control) or gradients of different concentrations of a 9:1 ratio of cAMP/cGMP (arrows). The fluorescence intensity is expressed as pseudo-colors (top; blue = low, red = high). (B) Sample traces (top) and summary (bottom) of the percentage changes in OGB-dextran fluorescence (ΔF/F) in the growth cones exposed to culture medium (control) or to different concentration gradients of cAMP/cGMP. OGB-dextran fluorescence was normalized to the fluorescent intensity of co-injected Texas Red–dextran in the growth cones before and after exposure to the cyclic nucleotide gradients (arrows in (A)). Cyclic nucleotide (cNMP) gradients: grey horizontal bars. Error bars = s.e.m. (n) = number of growth cones examined. Significant differences compared to the control are indicated (*p < 0.05; **p < 0.01; Mann-Whitney U test). (C) Left panel: Cumulative distribution of the average ΔF/F (%) at 10–20 min after exposure to cyclic nucleotide gradients as in (B). Right panel: Monotonic increase in the averages ΔF/F at 15–20 min that resulted from growth cone exposure to cyclic nucleotide gradients as in (B). The red curve is a regression function.
System identification of CaMKII as an activator and PP1 as an inhibitor
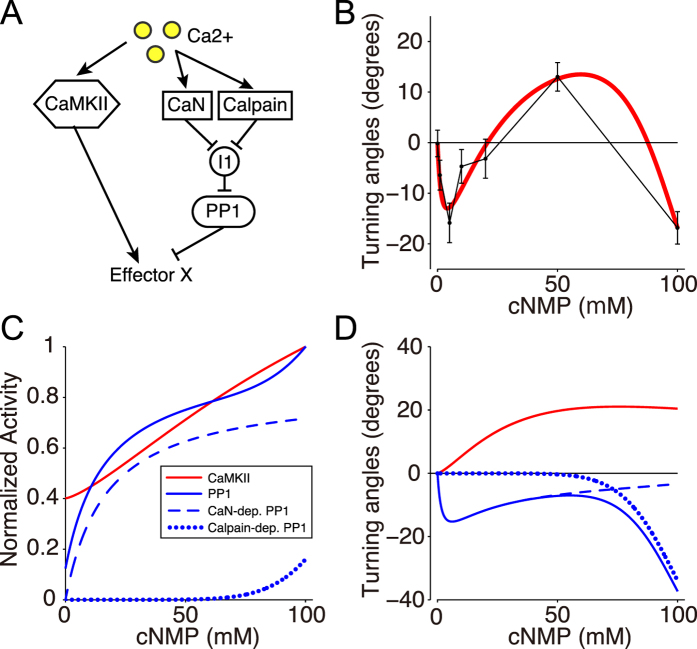
To identify how CaMKII (A; activator) and PP1 (I; inhibitor) are up-regulated in a Ca2+ -dose-dependent manner, we developed a mathematical model; the activity of CaMKII was expressed as a Hill equation of the Ca2+ concentration (Equation (10)), while the activity of PP1 was expressed as double Hill equations (Equation (11)) with lower and higher Kd values, which correspond to two distinct pathways: the CaN- and Calpain-dependent pathways, respectively (Fig. 5A), because higher level of Ca2+ is necessary for up-regulating Calpain than that for CaN49. We then took a reverse engineering approach (also called a system identification method), in which model parameters are estimated to fit the model into experimental data in general. Specifically, we optimized parameters of the Hill equations in terms of minimization of square error between experimental data of the tri-phasic turning responses (Fig. 3B) and model’s simulated turning responses as exemplified in Fig. 2, using a non-linear regression method (see Methods). The model with the estimated parameter values, called the reverse-engineered model, well reproduced the tri-phasic bi-directional turning responses of the growth cone (red line in Fig. 5B).
Figure 5. System identification of CaMKII and PP1 activities in the bi-directional turning response.
(A) The schematic drawing depicting the regulation of CaMKII and PP1, which up- and down-regulate the effector X, respectively, in the growth cone model. (B) Simulation of the multi-phasic bi-directional turning response by a mathematical model where the parameters were identified by the reverse engineering approach (solid red line). Points and error bars represent the experimental results (the same as in Fig. 3B). Goodness of fitting was evaluated by the Pearson’s chi-square test (p = 0.78), indicating no significant difference. (C) Reverse-engineered dose-responses of CaMKII (Red line) and PP1 (blue line) to the total cAMP:cGMP concentration (cNMP). The dose-responses were normalized. The blue line is the sum of CaN- and Calpain-dependent PP1 (blue dashed and dotted lines). The estimated parameter values are listed in Methods. (D) Contributions of the activator (A; CaMKII) and the inhibitor (I; PP1) to growth cone turning (black line in (B)) depending on the cNMP level. Attractive and repulsive influences of the activator and inhibitor are represented by red and blue solid lines, respectively, which correspond to the first and second terms in Equation (1). The PP1-induced repulsion (blue solid line) is given by the sum of the CaN- and Calpain- dependent factors (dashed and dotted blue lines, respectively).
Bi-directionality depends on the balance between CaMKII and PP1
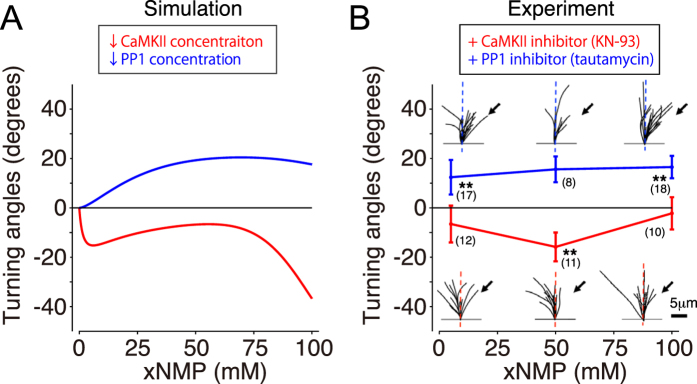
This reverse-engineered model prediction was further validated by the growth cone turning assays with the suppression of either CaMKII or PP1. First, we simulated the reverse-engineered model in which total CaMKII activity was reduced while other parameters were held constant, leading the prediction that the growth cones exclusively show repulsion with CaMKII suppression (red line in Fig. 6A). Next, we confirmed by growth cone turning assays with bath application of KN-93 (CaMKII inhibitor) that the tri-phasic bi-directional response observed in Fig. 3B became a repulsive uni-directional response (red line in Fig. 6B). Note that the reverse-engineered model was independent of data obtained from down-regulation experiments (Fig. 6B).
Figure 6. Bi-directional turning depends on the molecular switching between CaMKII and PP1.
(A) Model simulation of growth cone turning responses during the inhibition of either the activator, CaMKII (red line) or the inhibitor, PP1 (blue line). Inhibition of CaMKII or PP1 activity was simulated by a 90% reduction of the estimated parameter for each concentration. (B) Growth cone turning responses were experimentally observed in response to a gradient solution that contained [Sp-8-Br-cAMPS] and [8-Br-cGMP] at a fixed ratio (9:1) at different concentrations in the presence of KN-93 (0.5 μM; CaMKII inhibitor, red line) or tautomycin (4 nM; PP1 inhibitor, blue line) in the assay medium. Significant differences compared to the control are indicated (*p < 0.05; **p < 0.01; Mann-Whitney U test). Goodness of fitting of model simulation to experiment was evaluated by p-value of the Pearson’s chi-square test (p < 0.01 with KN-93; p = 0.38 with tautomycin).
Alternatively, the simulated turning with the suppression of PP1 activity in the model resulted in the disappearance of the tri-phasic bi-directional turning response so that the model predicted only uni-directional attraction (blue line in Fig. 6A). Congruently, the growth cone turning assays with bath application of tautomycin (PP1 inhibitor) resulted exclusively in attraction (blue line in Fig. 6B). Therefore, the model predictions, together with the experimental results, supported the idea that the balance between the activator, CaMKII, and the inhibitor, PP1, determines whether the growth cone turning response is either uni-directional or bi-directional, and, furthermore, bi-phasic or tri-phasic bi-directional.
Discussion
Intracellular Ca2+ signaling regulates growth cone bi-directional turning responses to many diffusible guidance cues. However, its molecular signaling mechanism is not well understood. In this study, we presented a mathematical model that identifies the growth cone chemotactic attraction and repulsion governed by the balance between the activator and inhibitor, as expressed by Equation (1) (Fig. 1F). We used both theoretical and experimental approaches to demonstrate how CaMKII and PP1, respectively, as activator and inhibitor, are non-linearly regulated depending on the intracellular Ca2+ and are responsible for multi-phasic growth cone turning in response to extracellular cues.
Our theoretical model was developed based on the intracellular signaling pathways shared by chemotactic cells. This model has the following characteristics: First, it assumes only “activator” and “inhibitor” as the intracellular signaling molecules that are commonly present in all chemotactic cells (Fig. 1D)50. Therefore, the theory is applicable not only to the growth cones, but also to the other chemotactic cells. Second, the theory is based on arbitrary gradients of the activator and inhibitor. Thus, it allows the detailed biochemical processes in intracellular signaling to be bypassed. Third, because this model only considers a few parameters, the reverse engineering approach was feasible (Fig. 5). These characteristics provide great advantage for identifying how the activator (e.g., CaMKII) and inhibitor (e.g., PP1) are regulated by an extracellular signal (Fig. 5) and the potential for experimental validation of the model (Fig. 6).
Multi-phasic turning response
Our mathematical model generated various patterns of turning responses (uni- or bi-directional and bi- or tri-phasic) depending on the dose-response patterns of the activator and inhibitor (Fig. 2 and Supplementary figure 2). Such counterintuitive turning responses can be understood by our theory based on Equation (1); the turning response was determined by inequality between attractive factor (ΔA/A*) and repulsive factor (ΔI/I*) (see ΔA, ΔI, A* and I* in Fig. 2A). In Fig. 2E, for example, the attractive factor increased and then decreased when changing the guidance signal (red dashed lines), because its dose-response (A*) is a sigmoidally saturating function of the guidance signal, accompanied by transient changes of its slope (ΔA) as shallow, steep and then shallow. On the other hand, the repulsive factor monotonically decreased (blue dashed lines), because the dose-response (I*) and its slope (ΔI) are monotonically increasing and decreasing functions, respectively. Then, tug-of-war between these attractive and repulsive factors generated the bi-phasic bi-directional turning response (black line). In this way, our theory based on Equation (1) described the mechanism of turning responses, which was dependent on the dose-responses of A and I.
Uni- and bi-directional gradient sensing
What is the critical difference between the uni-directional and bi-directional turning responses? Subsequently, we discuss their prerequisites.
Uni-directional gradient sensing: Our model can be expressed as a linear model equipped with an activator and inhibitor that exhibit linear dose-response patterns to a guidance signal G: A = dAG and I = dIG, where dA and dI denote positive sensitivity constants. The LEGI model exemplifies this type of linear model6. This linear model manifests a phenomenon called adaptation, in which the effector exhibits a constant steady-state response regardless of the magnitude of guidance gradients. This phenomenon occurs because of the constant nature of A*/I*. ΔA and ΔI are also constants that are determined by the diffusion coefficients of A and I, which are independent of the level of the guidance signal G* (see Equation (S19) in Supplementary Information). In this linear model, the turning response ω in Equation (1) becomes ω = cA/dAG*−cI/dIG*, where cA and cI are positive constants defined in Methods. This equation states that the direction (sign) of the turning response is independent of the concentration of the guidance signal G. Thus, a chemotactic system with linear dose-response patterns would always exhibit uni-directional responses (either attraction or repulsion) in an adaptive manner. Then, what determines whether the uni-directional turning response is attractive or repulsive? Intuitively, when the inhibitor rapidly diffuses out, as assumed in the LEGI model, the inhibitor will be distributed almost uniformly over the growth cone with a small ΔI, which results in an attractive response (ΔA/A* > ΔI/I*), and vice versa. A typical example of a chemotactic cell is Dictyostelium discoideum, which responds uni-directionally to a chemoattractant gradient at a wide range of concentrations (e.g., 5 pM-5 μM of extracellular cAMP)51.
Bi-directional gradient sensing: Our model typically expresses non-linear, sigmoidal-like responses of the activator and inhibitor as demonstrated in Figs 2 and 5. Because the dose-response of the effector X depends non-linearly on the concentration of guidance signal G, it no longer exhibits adaptation. The balance between ΔA/A* and ΔI/I* in Equation (1) thus can be switched from positive to negative and vice versa, resulting in bi-directional gradient sensing.
Therefore, our mathematical model can characterize the two basic and distinctive forms of chemotaxis: (1) a linear system with adaptation exhibits only uni-directional chemotaxis and (2) a non-linear system with non-adaptive character is required for exhibiting a bi-directional turning response, as observed in growth cones. Accordingly, there should be a trade-off between adaptation and bi-directionality.
The reverse-engineered model
Our reverse-engineered model both qualitatively and quantitatively captured the tri-phasic bi-directional turning responses of the growth cone (no significant difference, p = 0.78, according to the Pearson’s chi-square test) (Fig. 5B). In the condition with pharmacological inhibition of CaMKII or PP1, the model’s predictive performance was also quantitatively evaluated, indicating that there was no significant difference with inhibition of PP1, but there was of CaMKII (p < 0.01 for CaMKII inhibition; p = 0.38 for PP1 inhibition according to the Pearson’s chi-square test). However, the model still qualitatively predicted actual growth cone turning even in these conditions; the model actually reproduced uni-directional repulsion and attraction with inhibition of CaMKII and PP1, respectively (Fig. 6).
In the reverse-engineered model, PP1 was up-regulated by two distinct CaN- and Calpain-dependent pathways. In the CaN-dependent pathway, Ca2+ -bound Calmodulin (CaM) up-regulates CaN, which subsequently up-regulates PP1 by the suppression of Inhibitor 1 (I1)52. In the Calpain-dependent pathway, higher level of Ca2+ than that required for CaN activation up-regulates Calpain49, which converts p35 to p25 and causes prolonged activation of cyclin dependent kinase 5 (Cdk5), which then inhibits I1 and subsequently up-regulates PP153,54. In addition, CaMKII and PP1 were independently addressed in the model though PP1 was known to inhibit CaMKII55. On the other hand, Ca2+ -dependent dose-response of CaMKII was a monotonically increasing function even with PP1, while PP1 affected its Kd value56. Thus, CaMKII dose-response to Ca2+ in the model included the effect of PP1.
The reverse-engineered model elucidated the following underlying mechanisms responsible for inducing multi-phasic bi-directional turning responses (Fig. 5C–D): (1) A low level of Ca2+ increase favorably triggers the CaN-dependent activation of PP1 over either the Calpain-dependent activation of PP1 or CaMKII, which then induces repulsion. (2) A moderate Ca2+ increase favorably triggers CaMKII over CaN- and Calpain-dependent activation of PPI, which then induces attraction. Importantly, we argue that the Ca2+ increase associated with growth cone attraction is considerably lower (i.e., 100 to 200 nM)17,57,58 than the extent to which Ca2+ recruits the autophosphorylation of CaMKII24,59,60, suggesting that CaMKII autophosphorylation is not a critical requirement for growth cone bi-directional turning behaviors. (3) A high Ca2+ increase strongly induces the Calpain-dependent activation of PP1, while sustaining the activation of both CaMKII- and CaN-dependent PP1. Consistently, a previous study indicated that Calpain activation requires a high level of Ca2+ (>10 μM)49, although this high level of Ca2+ increase was not normally induced by guidance cues14,15,57,58,61. Increasing evidence has indicated that Calpain activation can occur at physiological Ca2+ concentrations62,63,64. Thus, the modulation of growth cone repulsion by the Calpain-PP1 pathway may occur under some physiological conditions.
Comparison with previous computation models
There have been many studies of computational models for chemotaxis. Most of them were interested in gradient sensing mechanisms with an emphasis on non-neural cells, e.g., Dictyostelium discoideum6,7,8,9,65,66,67,68,69,70. These models intended primarily to describe uni-directional attractive gradient sensing by means of global inhibition, e.g., a rapidly diffusing inhibitor. For example, the LEGI model6,7,9,70 elucidates the mechanism of “adaptation”, in which chemotactic cells exhibit greater sensitivity to the gradient of a chemoattractant while exhibiting insensitivity to the magnitude of the chemoattractant gradient. On the other hand, our model showed that non-adaptive character is required for realizing bi-directional turning responses.
The growth cone has also been computational modeled by several aspects71. Motivations to model the growth cone cover formation of the extracellular gradient that the growth cone senses42,72,73, axonal pathfinding by the gradient cone74,75,76,77,78,79,80, the growth cone movement in three-dimensional space25,81,82,83, axonal specification during neuronal polarization84,85,86,87,88 and the gradient sensing based on intracellular reaction23,25,74,89 or on Bayesian information approach90,91.
Related to our current study, Forbes et al.23 and Roccasalvo et al.25 addressed bi-directional turning responses of the growth cone based on models of intracellular Ca2+ signaling. While these models were specialized in the growth cone, we also intended to establish the general theory that describes the mechanistic difference between uni-directional chemotaxis of non-neural cells and bi-directional chemotaxis of growth cones. In Roccasalvo et al., intracellular Ca2+ dynamics was described as a 2D reaction-diffusion system which was based on the Gierer-Meinhartd model74,89 with Turing instability92. The growth cone was turned according to spatial polarity of Ca2+ in their model. The turning response was, on the other hand, determined by spatial polarity of Ca2+ downstream effector, e.g., Rac1 and Cdc42, in our 1D model, in which the spatial polarity was represented by sign and magnitude of ΔX; the latter is natural, because the study of growth cone turning assay using Ca2+ uncaging showed that attraction and repulsion were both induced by intracellular Ca2+ elevation with the same polarity, but attraction or repulsion was determined by the elevation level18. In addition, other Ca2+ imaging studies clarified that intracellular Ca2+ constitutes the gradient of the same direction as the extracellular gradient during both attraction and repulsion15. Moreover, Rac1 and Cdc42 participate in membrane protrusion of the growth cone93,94 via regulating actin dynamics of filopodia and lamellipodia95,96, regulated by Ca2+-dependent CaMKII and PP122,97,98. Thus, as addressed in our model, Rac1 and Cdc42 in the Ca2+ downstream should be important determinants for the growth cone turning. In comparison to the existing 2D model, our 1D model is advantageous, because it has the ability to explain the basic mechanism in the molecular level that allows the growth cone to exhibit bi-directional and even multi-phasic turning behaviors with the help of simple linear approximation. We also confirmed simple extension of our theory to 2D, i.e., a 2D model, well showed turning behaviors of the growth cone (Supplementary Figure 2).
Forbes et al. simulated Ca2+ signaling pathway including CaMKII, CaN, and PP1 in the growth cone and described the tri-phasic bi-directional turning responses; their model was an extension of existing model for signaling pathway in synaptic plasticity24, reflecting the fact that CaMKII, CaN, and PP1 are also equipped in a dendritic spine99,100. In contrast to our model with PP1 as an inhibitor, Forbes et al.’s model considered CaN as an inhibitor and PP1 as an inhibitory modulator of CaMKII. Their model predicted that activity changes of PKA, which negatively regulates PP1, induce shift of the turning response depending on Ca2+, still maintaining the bi-directionality. On the other hand, our growth cone turning assays with suppression of PP1 showed that the bi-directionality disappeared and only attraction was induced (Fig. 6B). This experimental result has clearly shown that the molecular tug-of-war between PP1 and CaMKII is crucial for tri-phasic bi-directional turning. In addition, CaMKII possesses bi-stability in their model, in which the model’s intracellular signaling was adopted from a previous model with CaMKII bi-stability24. It should be noted here that CaMKII has sometimes been hypothesized to function as a bi-stable memory element at postsynaptic spines during the induction of long-term potentiation24,59,60,101, while such bi-stable character has not been observed by an in vivo imaging of CaMKII activity in dendritic spines102. Related to this controversy, our reverse-engineered model suggested monotonical dose-response of CaMKII, instead of more complicated one like with hysteresis; the former never produces bi-stability.
Methods
Theoretical methods
Chemotactic turning response
The chemotactic cells have been known to detect shallow extracellular gradients (e.g., few percent difference of concentrations in Dictyostelium discoideum51,103 and the growth cone41,42,43,44,45). Then, the chemotactic turning response is modeled under a shallow extracellular gradient. In response to the extracellular gradient, intracellular gradients of A and I, A(x) and I(x), are supposed to be produced in a shallow manner across the growth cone (Fig. 1E). In this situation, it can be considered that at a specific position x′ on the cellular coordinate, the activities of A and I, A(x = x′) and I(x = x′), are slightly perturbed from their activities at the cellular center x = 0, i.e., A(x = 0) and I(x = 0), respectively. Due to the assumption of shallowness, the activity of X at the position x′, X′, can be approximately linearized as
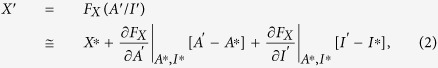 |
where FX(A/I) represents the activity of X given a ratio of A to I; A′, I′ and X′ indicate A(x = x′), I(x = x′), X(x = x′), respectively; A*, I* and X* indicate A(x = 0), I(x = 0) and X(x = 0), respectively. Here, the first-order Taylor expansion with respect to A′ and I′ around A* and I* was used, where A′−A* and I′−I* represent small perturbations. Because the position x′ can be arbitrarily selected, this equation can be generalized for any position x as
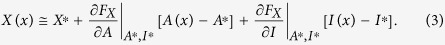 |
The spatial difference of X across the cell was simply calculated by ΔX = X(L/2)−X(−L/2), where L indicates the length of the cell; L/2 and −L/2 indicate the spatial coordinates at two ends facing higher and lower extracellular guidance signal, respectively (Fig. 1A); X(L/2) and X(−L/2) were obtained by substituting L/2 and −L/2 for x in Equation (3), respectively. Then, ΔX becomes
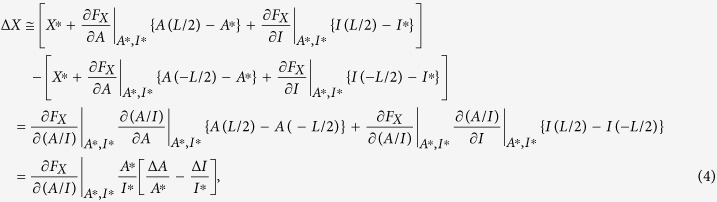 |
where ΔA and ΔI indicate the spatial differences of the activator and inhibitor, respectively, across the growth cone coordinate (Fig. 1E). We also derived Equation (4) in different way (see Supporting Information).
In the model, the turning angle ω of the chemotactic cell was proportionally determined by ΔX/X* according to the Weber-Fechner law, which states that alterations of a detectable gradient are based on the scale of the concentration X*:
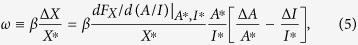 |
where β denotes a positive constant (the coefficient of Equation (1)). Note that the sign of the turning angle ω (attraction or repulsion) is not affected even if X non-linearly depends on A/I, though the amplitude is changed due to the non-linearity. If X is proportional to A/I (FX(A/I) = αA/I),
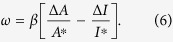 |
Note that in Equation (6), α was cancelled out.
The turning response to the extracellular gradient
Based on Equation (6), we evaluated the turning angle in response to an extracellular gradient G(x). Suppose that the activities of A and I are regulated by the guidance signal G in a dose-dependent manner as A = FA(G) and I = FI(G), respectively (Fig. 2A,C,E,G). A* and I* in Equation (6) can be replaced by FA(G*) and FI(G*). ΔA and ΔI in Equation (6) were simply set to be proportional to the derivatives of FA(G) and FI(G) with respective to G, i.e., dFA/dGG* and dFI/dGG*, respectively. Then, Equation (6) was rewritten by
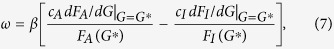 |
where cA and cI are positive constants which describe the sensitivity of A and I to the extracellular gradient, depending on their diffusion constants. Note that the assumption used here (ΔA = cAdFA/dGG* and ΔI = cIdFI/dGG*) can result from intracellular reaction-diffusion dynamics of A and I (see Supporting Information).
Activator-inhibitor dose-response model
In Fig. 2, the dose-response curves of the activator (A) and inhibitor (I) were given by Hill equations:
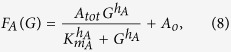 |
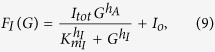 |
where Atot and Itot indicate maximum activities of A and I, respectively; Ao and Io indicate basal activities of A and I, respectively; ha and hI are Hill coefficients of the dose-responses of A and I, respectively, which regulate the non-linearity of sigmoidal curves; KmA and KmI indicate Ca2+ concentrations required for half activation of A and I, respectively. Parameter values used in the Hill equations above, to draw the Fig. 2’s panels, are listed below:
Figure 2A: Atot = 1, hA = 1, KmA = 0.25, Ao = 0, Itot = 1, hI = 1, KmI = 0.05, Io = 0.01.
Figure 2C: Atot = 1, hA = 1, KmA = 0.05, Ao = 0.01, Itot = 1, hI = 1, KmI = 0.5, Io = 0.
Figure 2E: Atot = 1, hA = 2, KmA = 0.1, Ao = 0.05, Itot = 1, hI = 1, KmI = 0.1, Io = 0.001.
Figure 2G: Atot = 1, hA = 3, KmA = 0.075, Ao = 0.1, Itot = 1, hI = 1, KmI = 0.5, Io = 0.01.
In Fig. 5A, the activities of CaMKII (A) and PP1 (I) are expressed by dose-response functions of the Ca2+ concentration C:
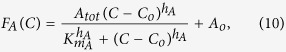 |
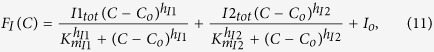 |
where Atot, I1tot and I2tot indicate maximum activities of CaMKII, CaN-dependent PP1 and Calpain-dependent PP1, respectively; Ao and Io indicate basal activities of CaMKII and PP1, respectively; ha, hI1 and hI2 are Hill coefficients of the dose-responses of CaMKII, CaN-dependent PP1 and Calpain-dependent PP1, respectively; KmA, KmI1 and KmI2 indicate Ca2+ concentrations required for half activation of CaMKII, CaN-dependent PP1 and Calpain-dependent PP1, respectively. The dose-response of CaMKII is expressed as a Hill equation with the Ca2+-independent basal activity Ao, whereas that of PP1 consists of double Hill equations with the basal activity Io. The first and second right-hand-side terms of Equation (11) correspond to activations by CaN- and Calpain-dependent PP1 pathways, respectively. Co is the basal Ca2+ concentration.
The Ca2+ concentration C is also a function of the total concentration of the cyclic nucleotide analogue, N, which is represented as C(N). In this model, C(N) was obtained by a fitting function based on the Ca2+ imaging data as 0.0036110N + 1.00308 (right panel in Fig. 4C). The turning angle ω was calculated as described above with the following minor modification. ΔA and ΔI in Equation (6) were assumed to be proportional, respectively, to both the derivatives of A and I with respect to N, and to the total concentration of cyclic nucleotide analogue N, as ΔA = cAN(dFA/dC)(dC/dN) and ΔI = cIN(dFI/dC)(dC/dN). Because the slope of the extracellular gradient of cyclic nucleotide analogue in the experimental paradigm should depend proportionally on its applied concentration and this extracellular gradient is translated into the intracellular Ca2+ gradient, ΔA and ΔI should be proportional to the total concentration of the cyclic nucleotide analogue.
Parameters of Equations (7, 10 and 11) were estimated by a reverse-engineered approach and used for depicting Fig. 5. Those values were:
γ = 42.20511, cA = 1, cI = 0.76617, Atot = 1, hA = 1.35144, KmA = 0.48443, Ao = 0.26922, I1tot = 1, hI1 = 1.00000, KmI1 = 0.061014, I2tot = 1.40063, hI2 = 7.59427, KmI2 = 0.46046, Io = 0.14789, Co = 1.00308
Estimation of parameters
The parameters, KmA, hA, Ao, KmI1, hI1, I2tot, KmI2, hI2, Io in Equations (10 and 11) and γ, cI in Equation (7), which are denoted in total as Θ, were estimated by a non-linear regression to minimize the quadratic error function as follows:
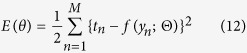 |
where f(yn; Θ) is a function that analytically describes the turning angle of the growth cone based on Equation (7), yn and tn denote the input variable (total concentration of Sp-8-Br-cAMPS and 8-Br-cGMP) and the target variable (turning angle of the growth cone), respectively, of the nth data sample obtained by the turning assays, and M indicates the total number of data samples. Note that Atot, I1tot and cA were not optimized and set to 1 and Co was set to basal value of ΔF/F (right panel in Fig. 4C). We used a simplex Nelder–Mead algorithm to minimize the error function (12). Although this optimization had an issue of local minima because of its non-linearity regarding the parameters to be estimated, we repeated its solution many times by changing its initial condition and then selected the best fit.
Experimental methods
Neuronal cultures
Cultures of Xenopus spinal neurons were prepared from neural tubes of stage 22 embryos and were used for growth cone turning assays and Ca2+ imaging at 14–18 h after incubation at 23–25 °C as previously described13,14,46. The culture medium consisted of 49% (v/v) LB medium (Gibco), 1% (v/v) FBS (HyClone) and 50% (v/v) Ringer’s solution (in mM: 115 NaCl, 2 CaCl2, 2.5 KCl and 10 HEPES (pH 7.4)). All experiments were in accordance with protocols approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine.
Chemical gradients and pharmacological usage
The membrane-permeable analogues of cNMP, CaMKII inhibitor (KN-93) and PP1 inhibitor (tautomycin) were purchased from Calbiochem. The microscopic gradients of the membrane-permeable analogues of the cNMP solutions were generated through a micropipette with a tip opening of 1 μm by applying repetitive pressure ejection, as described previously14. Pharmacological inhibitors (KN-93: 0.5 μM; tautomycin: 4 nM) were applied in the culture medium (4 ml total volume) at least 30 min before the start of each experiment and were present throughout the experiments.
Growth cone turning assay
The growth cone turning assay was accomplished as described previously13,14,46. Briefly, a micropipette tip that contained the chemical solutions was placed 100 μm away from the palm of the growth cone at an angle of 45° with respect to the initial direction of neurite extension (indicated by the last 10-μm segment of the neurite). Images of both the initial and final growth cones were recorded using a charge-coupled device (CCD) camera (Hitachi KP-M2U) attached to a phase contrast microscope (Olympus CKX-41). Growth cones with a net extension >10 μm over the 1-h period were analyzed using NIH ImageJ software. The final turning angle (expressed in degrees), which represents the angle between the initial direction of the neurite extension and a straight line connecting the positions of the growth cone at the onset and the end of the 1-h exposure to the cNMP solutions, was measured.
Calcium imaging
Calcium imaging of the growth cones was performed as described previously14. The soma of isolated Xenopus spinal neurons were microinjected with 200 μM Oregon Green 488 BAPTA-1 and Texas Red conjugated to 10-kDa dextran (Molecular Probes, Inc.) at least 3 hours before the imaging, using an Eppendorf pressure injection system (Transjector 5246)15,16. Calcium imaging was performed using a Yokogawa confocal system (CSU-22, Perkin Elmer) equipped with an Ar/Kr gas laser. Excitation at 488 nm and 568 nm was controlled by an acousto-optical tunable filter (AOTF), and Oregon green BAPTA and Texas red fluorescence emission signals were collected, respectively, at 520–540 nm and 614–642 nm by an EM-CCD camera (Hamamatsu) through a 100× objective (UPlanSApo, N.A. 1.4, Olympus). Fluorescence images were collected sequentially in pairs every 5 s and analyzed using UltraView (Perkin Elmer) and ImageJ software. The mean fluorescence intensity at a growth cone was measured over an area that covered the entire growth cone. Oregon Green fluorescence was normalized to Texas Red fluorescence to control for experimental fluctuations, e.g., growth cone volumes or focal plane changes. The fluorescence ratio at each sampling time was normalized to the average fluorescence ratio measured during the initial 5-min baseline period.
Additional Information
How to cite this article: Naoki, H. et al. Multi-phasic bi-directional chemotactic responses of the growth cone. Sci. Rep. 6, 36256; doi: 10.1038/srep36256 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by the Collaborative Research in Computational Neuroscience (K.H., M.M. and S.I.) from National Science Foundation and National Institutes of Health, USA, as well as the Platform Project for Supporting in Drug Discovery and Life Science Research (Platform for Dynamic Approaches to Living System) (H.N. and S.I.), the Program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (S.I.), both from Japan Agency for Medical Research and Development, and CREST (S.I.) from Japan Science and Technology Agency. We thank Drs W. Jelinek, H. Urakubo and K. Nakae for their valuable comments.
Footnotes
Author Contributions H.N., Y.I. and S.I. developed the theoretical model. M.N., K.H., H.N. and S.I. conceived the experimental project. H.N. performed the computational analysis. M.N., K.H. and K.T. performed the experiments. H.N., M.N., S.I. and K.H. wrote the manuscript.
References
- Dickson B. J. Molecular mechanisms of axon guidance. Science 298, 1959–1964, doi: 10.1126/science.1072165 (2002). [DOI] [PubMed] [Google Scholar]
- Hopker V. H., Shewan D., Tessier-Lavigne M., Poo M. & Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature 401, 69–73, doi: 10.1038/43441 (1999). [DOI] [PubMed] [Google Scholar]
- Kennedy T. E., Wang H., Marshall W. & Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J. Neurosci. 26, 8866–8874, doi: 10.1523/JNEUROSCI.5191-05.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamarino S. A. & Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell 81, 621–629 (1995). [DOI] [PubMed] [Google Scholar]
- Janetopoulos C., Ma L., Devreotes P. N. & Iglesias P. A. Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proceedings of the National Academy of Sciences of the United States of America 101, 8951–8956, doi: 10.1073/pnas.0402152101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko A. & Iglesias P. A. Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophysical journal 82, 50–63, doi: 10.1016/S0006-3495(02)75373-3 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Huang C. H., Iglesias P. A. & Devreotes P. N. Cells navigate with a local-excitation, global-inhibition-biased excitable network. Proc Natl Acad Sci USA 107, 17079–17086, doi: 10.1073/pnas.1011271107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A., Ishihara S., Imoto D. & Sawai S. Rectified directional sensing in long-range cell migration. Nat Commun 5, 5367, doi: 10.1038/ncomms6367 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoge M. et al. Cellular memory in eukaryotic chemotaxis. Proc Natl Acad Sci USA 111, 14448–14453, doi: 10.1073/pnas.1412197111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A. T., Chun C., Takeda K. & Firtel R. A. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol 167, 505–518, doi: 10.1083/jcb.200406177 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortholt A., Keizer-Gunnink I., Kataria R. & Van Haastert P. J. Ras activation and symmetry breaking during Dictyostelium chemotaxis. J Cell Sci 126, 4502–4513, doi: 10.1242/jcs.132340 (2013). [DOI] [PubMed] [Google Scholar]
- Hoeller O. & Kay R. R. Chemotaxis in the absence of PIP3 gradients. Curr Biol 17, 813–817, doi: 10.1016/j.cub.2007.04.004 (2007). [DOI] [PubMed] [Google Scholar]
- Nishiyama M. et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 423, 990–995, doi: 10.1038/nature01751 (2003). [DOI] [PubMed] [Google Scholar]
- Hong K., Nishiyama M., Henley J., Tessier-Lavigne M. & Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403, 93–98, doi: 10.1038/47507 (2000). [DOI] [PubMed] [Google Scholar]
- Nishiyama M., von Schimmelmann M. J., Togashi K., Findley W. M. & Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nature neuroscience 11, 762–771, doi: 10.1038/nn.2130 (2008). [DOI] [PubMed] [Google Scholar]
- Togashi K. et al. Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron 58, 694–707, doi: 10.1016/j.neuron.2008.03.017 (2008). [DOI] [PubMed] [Google Scholar]
- Zheng J. Q. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature 403, 89–93, doi: 10.1038/47501 (2000). [DOI] [PubMed] [Google Scholar]
- Wen Z., Guirland C., Ming G. L. & Zheng J. Q. A CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron 43, 835–846, doi: 10.1016/j.neuron.2004.08.037 (2004). [DOI] [PubMed] [Google Scholar]
- Gomez T. M. & Zheng J. Q. The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Neurosci. 7, 115–125, doi: 10.1038/nrn1844 (2006). [DOI] [PubMed] [Google Scholar]
- Walter J., Allsopp T. E. & Bonhoeffer F. A common denominator of growth cone guidance and collapse? Trends Neurosci 13, 447–452 (1990). [DOI] [PubMed] [Google Scholar]
- Mortimer D., Fothergill T., Pujic Z., Richards L. J. & Goodhill G. J. Growth cone chemotaxis. Trends Neurosci 31, 90–98, doi: 10.1016/j.tins.2007.11.008 (2008). [DOI] [PubMed] [Google Scholar]
- Gomez T. M. & Zheng J. Q. The molecular basis for calcium-dependent axon pathfinding. Nature reviews. Neuroscience 7, 115–125, doi: 10.1038/nrn1844 (2006). [DOI] [PubMed] [Google Scholar]
- Forbes E. M., Thompson A. W., Yuan J. & Goodhill G. J. Calcium and cAMP levels interact to determine attraction versus repulsion in axon guidance. Neuron 74, 490–503, doi: 10.1016/j.neuron.2012.02.035 (2012). [DOI] [PubMed] [Google Scholar]
- Graupner M. & Brunel N. STDP in a bistable synapse model based on CaMKII and associated signaling pathways. PLoS computational biology 3, e221, doi: 10.1371/journal.pcbi.0030221 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccasalvo I. M., Micera S. & Sergi P. N. A hybrid computational model to predict chemotactic guidance of growth cones. Sci Rep 5, 11340, doi: 10.1038/srep11340 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T. et al. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nature neuroscience 10, 58–66, doi: 10.1038/nn1814 (2007). [DOI] [PubMed] [Google Scholar]
- Tojima T., Itofusa R. & Kamiguchi H. Asymmetric clathrin-mediated endocytosis drives repulsive growth cone guidance. Neuron 66, 370–377, doi: 10.1016/j.neuron.2010.04.007 (2010). [DOI] [PubMed] [Google Scholar]
- Keller E. F. & Segel L. A. Traveling bands of chemotactic bacteria: a theoretical analysis. Journal of theoretical biology 30, 235–248 (1971). [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J. Sensory adaptation of Dictyostelium discoideum cells to chemotactic signals. J Cell Biol 96, 1559–1565 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Mayo A. & Alon U. Logarithmic and power law input-output relations in sensory systems with fold-change detection. PLoS Comput Biol 10, e1003781, doi: 10.1371/journal.pcbi.1003781 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Wissler J. H., Hess M. W. & Cottier H. Distinct chemokinetic and chemotactic responses in neutrophil granulocytes. Eur J Immunol 8, 1–7, doi: 10.1002/eji.1830080102 (1978). [DOI] [PubMed] [Google Scholar]
- Levit M. N. & Stock J. B. Receptor methylation controls the magnitude of stimulus-response coupling in bacterial chemotaxis. J Biol Chem 277, 36760–36765, doi: 10.1074/jbc.M204325200 (2002). [DOI] [PubMed] [Google Scholar]
- Lazova M. D., Ahmed T., Bellomo D., Stocker R. & Shimizu T. S. Response rescaling in bacterial chemotaxis. Proc Natl Acad Sci USA 108, 13870–13875, doi: 10.1073/pnas.1108608108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H. Conversion of Neuronal Growth Cone Responses from Repulsion to Attraction by Cyclic Nucleotides. Science 281, 1515–1518, doi: 10.1126/science.281.5382.1515 (1998). [DOI] [PubMed] [Google Scholar]
- Ming G. L. et al. cAMP-dependent growth cone guidance by netrin-1. Neuron 19, 1225–1235 (1997). [DOI] [PubMed] [Google Scholar]
- Song H. J., Ming G. L. & Poo M. M. cAMP-induced switching in turning direction of nerve growth cones. Nature 388, 275–279, doi: 10.1038/40864 (1997). [DOI] [PubMed] [Google Scholar]
- De Koninck P. Sensitivity of CaM Kinase II to the Frequency of Ca2+ Oscillations. Science 279, 227–230, doi: 10.1126/science.279.5348.227 (1998). [DOI] [PubMed] [Google Scholar]
- Stemmer P. M. & Klee C. B. Dual calcium ion regulation of calcineurin by calmodulin and calcineurin B. Biochemistry 33, 6859–6866 (1994). [DOI] [PubMed] [Google Scholar]
- Lohof A. M., Quillan M., Dan Y. & Poo M. M. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci 12, 1253–1261 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. Q., Felder M., Connor J. A. & Poo M. M. Turning of nerve growth cones induced by neurotransmitters. Nature 368, 140–144, doi: 10.1038/368140a0 (1994). [DOI] [PubMed] [Google Scholar]
- Bagnard D., Thomasset N., Lohrum M., Puschel A. W. & Bolz J. Spatial distributions of guidance molecules regulate chemorepulsion and chemoattraction of growth cones. J Neurosci 20, 1030–1035 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhill G. J. & Baier H. Axon guidance: stretching gradients to the limit. Neural Comput 10, 521–527 (1998). [DOI] [PubMed] [Google Scholar]
- Xu J., Rosoff W. J., Urbach J. S. & Goodhill G. J. Adaptation is not required to explain the long-term response of axons to molecular gradients. Development 132, 4545–4552, doi: 10.1242/dev.02029 (2005). [DOI] [PubMed] [Google Scholar]
- Baier H. & Bonhoeffer F. Axon guidance by gradients of a target-derived component. Science 255, 472–475 (1992). [DOI] [PubMed] [Google Scholar]
- Mortimer D. et al. Axon guidance by growth-rate modulation. Proc Natl Acad Sci USA 107, 5202–5207, doi: 10.1073/pnas.0909254107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K. et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97, 927–941 (1999). [DOI] [PubMed] [Google Scholar]
- Piper M., Salih S., Weinl C., Holt C. E. & Harris W. A. Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nature neuroscience 8, 179–186, doi: 10.1038/nn1380 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly M. et al. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science 327, 547–552, doi: 10.1126/science.1179735 (2010). [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S. M., Yuan Y., Cooray P., Salem H. H. & Jackson S. P. Calpain cleavage of focal adhesion proteins regulates the cytoskeletal attachment of integrin alphaIIbbeta3 (platelet glycoprotein IIb/IIIa) and the cellular retraction of fibrin clots. J Biol Chem 272, 1694–1702 (1997). [DOI] [PubMed] [Google Scholar]
- Iglesias P. A. & Devreotes P. N. Navigating through models of chemotaxis. Current opinion in cell biology 20, 35–40, doi: 10.1016/j.ceb.2007.11.011 (2008). [DOI] [PubMed] [Google Scholar]
- Fisher P. R., Merkl R. & Gerisch G. Quantitative analysis of cell motility and chemotaxis in Dictyostelium discoideum by using an image processing system and a novel chemotaxis chamber providing stationary chemical gradients. The Journal of cell biology 108, 973–984 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z. & Storm D. R. The role of calmodulin as a signal integrator for synaptic plasticity. Nature reviews. Neuroscience 6, 267–276, doi: 10.1038/nrn1647 (2005). [DOI] [PubMed] [Google Scholar]
- Kerokoski P., Suuronen T., Salminen A., Soininen H. & Pirttila T. Influence of phosphorylation of p35, an activator of cyclin-dependent kinase 5 (cdk5), on the proteolysis of p35. Brain research. Molecular brain research 106, 50–56 (2002). [DOI] [PubMed] [Google Scholar]
- Nguyen C. et al. Regulation of protein phosphatase inhibitor-1 by cyclin-dependent kinase 5. J Biol Chem 282, 16511–16520, doi: 10.1074/jbc.M701046200 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J., Schulman H. & Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature reviews. Neuroscience 3, 175–190, doi: 10.1038/nrn753 (2002). [DOI] [PubMed] [Google Scholar]
- Bradshaw J. M., Kubota Y., Meyer T. & Schulman H. An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling. Proc Natl Acad Sci USA 100, 10512–10517, doi: 10.1073/pnas.1932759100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley J. R., Huang K. H., Wang D. & Poo M. M. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron 44, 909–916, doi: 10.1016/j.neuron.2004.11.030 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan C. B., Xu H. T., Jin M., Yuan X. B. & Poo M. M. Long-range Ca2+ signaling from growth cone to soma mediates reversal of neuronal migration induced by slit-2. Cell 129, 385–395, doi: 10.1016/j.cell.2007.01.051 (2007). [DOI] [PubMed] [Google Scholar]
- Zhabotinsky A. M. Bistability in the Ca(2+)/calmodulin-dependent protein kinase-phosphatase system. Biophysical journal 79, 2211–2221, doi: 10.1016/S0006-3495(00)76469-1 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H. & Ichikawa K. Switching characteristics of a model for biochemical-reaction networks describing autophosphorylation versus dephosphorylation of Ca2+/calmodulin-dependent protein kinase II. Biological cybernetics 82, 35–47 (2000). [DOI] [PubMed] [Google Scholar]
- Ming G. L. et al. Adaptation in the chemotactic guidance of nerve growth cones. Nature 417, 411–418, doi: 10.1038/nature745 (2002). [DOI] [PubMed] [Google Scholar]
- Kaczmarek J. S., Riccio A. & Clapham D. E. Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse. Proceedings of the National Academy of Sciences of the United States of America 109, 7888–7892, doi: 10.1073/pnas.1205869109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A. et al. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Molecular and cellular biology 24, 2499–2512 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y. et al. Intracellular calcium level required for calpain activation in a single myocardial cell. Journal of molecular and cellular cardiology 33, 1133–1142, doi: 10.1006/jmcc.2001.1373 (2001). [DOI] [PubMed] [Google Scholar]
- Naoki H., Sakumura Y. & Ishii S. Stochastic control of spontaneous signal generation for gradient sensing in chemotaxis. Journal of theoretical biology 255, 259–266, doi: 10.1016/j.jtbi.2008.08.012 (2008). [DOI] [PubMed] [Google Scholar]
- Narang A. Spontaneous polarization in eukaryotic gradient sensing: a mathematical model based on mutual inhibition of frontness and backness pathways. Journal of theoretical biology 240, 538–553, doi: 10.1016/j.jtbi.2005.10.022 (2006). [DOI] [PubMed] [Google Scholar]
- Skupsky R., Losert W. & Nossal R. J. Distinguishing modes of eukaryotic gradient sensing. Biophysical journal 89, 2806–2823, doi: 10.1529/biophysj.105.061564 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma M. & Van Haastert P. J. A diffusion-translocation model for gradient sensing by chemotactic cells. Biophysical journal 81, 1314–1323, doi: 10.1016/S0006-3495(01)75788-8 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. K. & Narang A. A mechanistic model for eukaryotic gradient sensing: spontaneous and induced phosphoinositide polarization. Journal of theoretical biology 231, 49–67, doi: 10.1016/j.jtbi.2004.05.024 (2004). [DOI] [PubMed] [Google Scholar]
- Ma L., Janetopoulos C., Yang L., Devreotes P. N. & Iglesias P. A. Two complementary, local excitation, global inhibition mechanisms acting in parallel can explain the chemoattractant-induced regulation of PI(3,4,5)P3 response in dictyostelium cells. Biophysical journal 87, 3764–3774, doi: 10.1529/biophysj.104.045484 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A. Using theoretical models to analyse neural development. Nat Rev Neurosci 12, 311–326, doi: 10.1038/nrn3031 (2011). [DOI] [PubMed] [Google Scholar]
- Goodhill G. J. Diffusion in axon guidance. Eur J Neurosci 9, 1414–1421 (1997). [DOI] [PubMed] [Google Scholar]
- Goodhill G. J. Mathematical guidance for axons. Trends Neurosci 21, 226–231 (1998). [DOI] [PubMed] [Google Scholar]
- Bauer R., Zubler F., Hauri A., Muir D. R. & Douglas R. J. Developmental origin of patchy axonal connectivity in the neocortex: a computational model. Cereb Cortex 24, 487–500, doi: 10.1093/cercor/bhs327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisyuk R., Cooke T. & Roberts A. Stochasticity and functionality of neural systems: mathematical modelling of axon growth in the spinal cord of tadpole. Biosystems 93, 101–114, doi: 10.1016/j.biosystems.2008.03.012 (2008). [DOI] [PubMed] [Google Scholar]
- Zubler F. & Douglas R. A framework for modeling the growth and development of neurons and networks. Front Comput Neurosci 3, 25, doi: 10.3389/neuro.10.025.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krottje J. K. & van Ooyen A. A mathematical framework for modeling axon guidance. Bull Math Biol 69, 3–31, doi: 10.1007/s11538-006-9142-4 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel H. G. & van Ooyen A. Models of axon guidance and bundling during development. Proc Biol Sci 266, 2231–2238, doi: 10.1098/rspb.1999.0913 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisyuk R., Al Azad A. K., Conte D., Roberts A. & Soffe S. R. A developmental approach to predicting neuronal connectivity from small biological datasets: a gradient-based neuron growth model. PLoS One 9, e89461, doi: 10.1371/journal.pone.0089461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev R. & Ben-Jacob E. Generic modeling of chemotactic based self-wiring of neural networks. Neural Netw 13, 185–199 (2000). [DOI] [PubMed] [Google Scholar]
- Sergi P. N., Morana Roccasalvo I., Tonazzini I., Cecchini M. & Micera S. Cell guidance on nanogratings: a computational model of the interplay between PC12 growth cones and nanostructures. PLoS One 8, e70304, doi: 10.1371/journal.pone.0070304 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua J. S. et al. Extending neurites sense the depth of the underlying topography during neuronal differentiation and contact guidance. Biomaterials 35, 7750–7761, doi: 10.1016/j.biomaterials.2014.06.008 (2014). [DOI] [PubMed] [Google Scholar]
- Sergi P. N., Marino A. & Ciofani G. Deterministic control of mean alignment and elongation of neuron-like cells by grating geometry: a computational approach. Integr Biol (Camb) 7, 1242–1252, doi: 10.1039/c5ib00045a (2015). [DOI] [PubMed] [Google Scholar]
- Tomba C., Braini C., Wu B., Gov N. S. & Villard C. Tuning the adhesive geometry of neurons: length and polarity control. Soft Matter 10, 2381–2387, doi: 10.1039/c3sm52342j (2014). [DOI] [PubMed] [Google Scholar]
- Naoki H., Nakamuta S., Kaibuchi K. & Ishii S. Flexible search for single-axon morphology during neuronal spontaneous polarization. PLoS One 6, e19034, doi: 10.1371/journal.pone.0019034 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivaz M., Bandara S., Inoue T. & Meyer T. Robust neuronal symmetry breaking by Ras-triggered local positive feedback. Curr Biol 18, 44–50, doi: 10.1016/j.cub.2007.11.051 (2008). [DOI] [PubMed] [Google Scholar]
- Samuels D. C., Hentschel H. G. & Fine A. The origin of neuronal polarization: a model of axon formation. Philos Trans R Soc Lond B Biol Sci 351, 1147–1156, doi: 10.1098/rstb.1996.0099 (1996). [DOI] [PubMed] [Google Scholar]
- Toriyama M., Sakumura Y., Shimada T., Ishii S. & Inagaki N. A diffusion-based neurite length-sensing mechanism involved in neuronal symmetry breaking. Mol Syst Biol 6, 394, doi: 10.1038/msb.2010.51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. Journal of cell science 112 (Pt 17), 2867–2874 (1999). [DOI] [PubMed] [Google Scholar]
- Mortimer D. et al. Bayesian model predicts the response of axons to molecular gradients. Proc Natl Acad Sci USA 106, 10296–10301, doi: 10.1073/pnas.0900715106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer D., Dayan P., Burrage K. & Goodhill G. J. Bayes-optimal chemotaxis. Neural Comput 23, 336–373, doi: 10.1162/NECO_a_00075 (2011). [DOI] [PubMed] [Google Scholar]
- Turing A. M. The chemical basis of morphogenesis. 1953. Bull Math Biol 52, 153–197, discussion 119–152 (1990). [DOI] [PubMed] [Google Scholar]
- Dickson B. J. Rho GTPases in growth cone guidance. Curr Opin Neurobiol 11, 103–110 (2001). [DOI] [PubMed] [Google Scholar]
- Gallo G. & Letourneau P. C. Regulation of growth cone actin filaments by guidance cues. J Neurobiol 58, 92–102, doi: 10.1002/neu.10282 (2004). [DOI] [PubMed] [Google Scholar]
- Yamao M. et al. Distinct predictive performance of Rac1 and Cdc42 in cell migration. Sci Rep 5, 17527, doi: 10.1038/srep17527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S., Naoki H. & Ishii S. A multiphysical model of cell migration integrating reaction-diffusion, membrane and cytoskeleton. Neural Netw 24, 979–989, doi: 10.1016/j.neunet.2011.06.009 (2011). [DOI] [PubMed] [Google Scholar]
- Fleming I. N., Elliott C. M., Buchanan F. G., Downes C. P. & Exton J. H. Ca2+/calmodulin-dependent protein kinase II regulates Tiam1 by reversible protein phosphorylation. J Biol Chem 274, 12753–12758 (1999). [DOI] [PubMed] [Google Scholar]
- Murakoshi H., Wang H. & Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472, 100–104, doi: 10.1038/nature09823 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakubo H., Honda M., Froemke R. C. & Kuroda S. Requirement of an allosteric kinetics of NMDA receptors for spike timing-dependent plasticity. J Neurosci 28, 3310–3323, doi: 10.1523/JNEUROSCI.0303-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoki H., Sakumura Y. & Ishii S. Local signaling with molecular diffusion as a decoder of Ca2+ signals in synaptic plasticity. Mol Syst Biol 1, 2005 0027, doi: 10.1038/msb4100035 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakubo H., Sato M., Ishii S. & Kuroda S. In vitro reconstitution of a CaMKII memory switch by an NMDA receptor-derived peptide. Biophys J 106, 1414–1420, doi: 10.1016/j.bpj.2014.01.026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Escobedo-Lozoya Y., Szatmari E. M. & Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458, 299–304, doi: 10.1038/nature07842 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L. et al. Dictyostelium discoideum chemotaxis: threshold for directed motion. Eur J Cell Biol 85, 981–989, doi: 10.1016/j.ejcb.2006.01.012 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.