Abstract
Purpose
The aim of this study was to compare the changes in corneal biomechanical properties following small-incision lenticule extraction (SMILE) versus Q-value–guided femtosecond laser-assisted in situ keratomileusis (Q-FS-LASIK).
Methods
In this prospective comparative study, patients with a sphere plus cylinder measurement of less than −10.00 D and cylinder measurement of less than −5.00 D were included in the study. A total of 160 patients (160 eyes) with myopia and myopic astigmatism were divided into the two groups, with 80 patients (80 eyes) allocated to SMILE and 80 patients (80 eyes) allocated to Q-FS-LASIK. Corneal hysteresis (CH) and the corneal resistance factor (CRF) were quantitatively assessed using the Ocular Response Analyzer (ORA) preoperatively and at 1 day, 2 weeks, and 1 and 3 months postoperatively.
Results
Both types of surgery were associated with statistically significant decreases in CH and the CRF at postoperative day 1 (both P < 0.01). In both groups, the decreases subsequently stabilized with no further deteriorations compared to postoperative day 1 (P > 0.05). Both groups showed similar biomechanical changes at each time point (all P > 0.05).
Conclusions
Both SMILE and Q-FS-LASIK resulted in a decrease in CH and the CRF at postoperative 1 day, with the decreases stabilizing after this point. There were no significant differences between the short term effects of SMILE and Q-FS-LASIK on corneal biomechanical properties.
Keywords: Small incision, Q-value guided, Femtosecond, Biomechanical properties
Introduction
With the rapid and extensive development of modern corneal refractive surgery, related technologies have been promoted, and new surgical procedures have been developed. Q-value–guided femtosecond laser-assisted in situ keratomileusis (Q-FS-LASIK) is a safe surgical procedure in which lower spherical aberration is introduced,1, 2, 3, 4 and has been a popular procedure for corneal refractive surgery. Small-incision lenticular extraction (SMILE) is a new kind of surgical procedure that avoids flap-related complications,5 and it is gaining more attention. Both procedures have performed well in studies in all measures of safety, efficacy, and predictability.6, 7, 8 Although some previous studies have compared corneal biomechanical properties after SMILE and LASIK, the results have been inconsistent.9, 10 The aim of this study was therefore to increase the sample number to provide a better frame of reference. In addition, and different from earlier research, we used Q-FS-LASIK, which has not previously been compared with SMILE.
In the current study, we measured corneal hysteresis (CH) and the corneal resistance factor (CRF) and evaluated the changes in corneal biomechanical properties following surgery.
Methods
The study and data accumulation were carried out with approval from HangZhou Bright Vision Hospital Independent Ethics Committee.
In this prospective, non-randomized study (patients were allocated to each surgery through their own wishes), 160 patients (160 eyes) with myopia or myopia astigmatism were treated with either SMILE or Q-FS-LASIK at HangZhou Bright Vision Hospital between January and November 2015. In patients in whom both eyes were eligible, one eye was randomly chosen for inclusion. All participants were informed about the risks and benefits of both procedures and provided written informed consent. Patient inclusion criteria included a sphere plus cylinder measurement of less than −10.00 D and a cylinder measurement of less than −5.00 D.
Preoperative assessments
Preoperative assessments included a complete medical and ophthalmologic history and a thorough ocular examination, including measurement of uncorrected visual acuity, manifest refraction, best corrected visual acuity, cycloplegic refraction, slit-lamp examination, axial length, gonioscopy, funduscopy, and intraocular pressure. In addition, corneal topography was obtained using a tomography instrument (Sirius; CSO, Florence, Italy).
Measurement of biomechanical parameters
CH and CRF were determined using the Ocular Response Analyzer (ORA, Technologies, Depew, NY, USA). The mean value of three measurements with high signal quality was used for statistical analysis. High signal quality was defined as a device waveform signal score, based on the composite index of five corneal deformation signals of more than 6.5.
ORA measures two applanation points during a single process. The first applanation pressure point (P1) occurs as an air puff pushes the cornea inward, while the second applanation pressure point (P2) occurs as the cornea returns from the applanated state to normal.11 CH is defined as the difference between P1 and P2. CRF is defined as a linear function of these two pressures: CRF = k1 × (P1 – 0.7 × P2) + k2, where k1 and k2 are constants.
Surgical procedures
All surgical procedures were performed by a single surgeon (L.Z.). Routine disinfection and surface anesthesia were performed before surgery.
SMILE
A total of 80 eyes underwent SMILE (VisuMax; Carl Zeiss, Oberkochen, Germany). During the procedure, a cap of 120 μm, a single side-cut incision with a circumferential length of 2.0 mm at the 120-degree position, a side-cut angle of 90°, a 3 × 3 μm point spacing of the lens surface, a 2.5 × 2.5 μm point spacing of the lens side, and a 2 × 2 μm point spacing of the side cut were created. After a femtosecond laser scan, both the front and back surfaces of the lens were separated using a micro-separator. The free lens was then removed using micro-forceps.
Q-FS-LASIK
A total of 80 eyes underwent Q-FS-LASIK with the use of the FS200 femtosecond laser and EX500 excimer laser (both Alcon, Fort Worth, Texas, USA). During flap creation, settings were adjusted to achieve a thickness of 100 μm, side-cut angle of 90°, 8 × 8 μm point spacing of the flap, and 5 × 3 μm point spacing of the side cut. After a femtosecond laser scan, the corneal stroma was ablated with a 0.2 negative adjustment of the Q value (6 mm).
Postoperative care and follow-up
After surgery, fluorometholone 0.1% and bromfenac sodium 0.1% were immediately administered topically, levofloxacin 0.3% (Cravit; Santen, Osaka, Japan) was administered topically four times a day for 1 week, and fluorometholone 0.1% was administered topically six times a day for 3 weeks, after which the frequency was steadily tapered. Patients were followed up, and the ORA measurements were repeated at 1 day, 2 weeks, and 1 and 3 months postoperatively.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, USA). Student's t test was used to compare preoperative patient demographics between the two groups. For the purpose of statistical comparisons, visual acuity measurements were converted to logarithm of the minimum angle of resolution (LogMAR) units. Repeated-measures analysis of variance was used to assess the means of CH and CRF at different examination points within and between each group. The means of CH and CRF pre- and postoperatively were confirmed to meet the homogeneity of variance, and Fisher's least significant difference (LSD) test was therefore used for multiple comparisons within each group. The means of CH and CRF pre- and postoperatively did not meet the assumption of sphericity, and multivariate statistical analysis and degree of freedom adjustment were therefore used for comparisons between each group. Pearson correlation coefficients were calculated to evaluate correlations between 3-month changes in CH and CRF and multiple variables, including spherical, cylinder, and optical zone measurements. The results are expressed as mean ± SD, and P values of <0.05 were considered statistically significant.
Results
The study enrolled 160 patients (160 eyes), with the patients divided into two equal groups. The preoperative patient demographics are summarized in Table 1. All operations were successful, and no serious complications or iatrogenic corneal ectasia were seen during the 3 months postoperatively.
Table 1.
Preoperative patient demographics.
| Parameter | SMILE | Q-FS-LASIK | t value | P value |
|---|---|---|---|---|
| Spherical (D) | −5.12 ± 1.62 | −4.87 ± 1.80 | 0.90 | 0.37 |
| Cylinder (D) | −0.77 ± 0.63 | −0.84 ± 0.76 | −0.68 | 0.50 |
| BCVA | −0.03 ± 0.06 | −0.03 ± 0.05 | 0.43 | 0.67 |
| UCVA | 1.28 ± 0.27 | 1.22 ± 0.23 | 1.54 | 0.13 |
| CCT (μm) | 550.80 ± 25.77 | 547.06 ± 29.53 | 0.85 | 0.40 |
| Optical zone (mm) | 6.43 ± 0.15 | 6.40 ± 0.17 | 0.97 | 0.33 |
Values are means ± standard deviations.
BCVA = best corrected visual acuity, CCT = central corneal thickness, UCVA = uncorrected visual acuity.
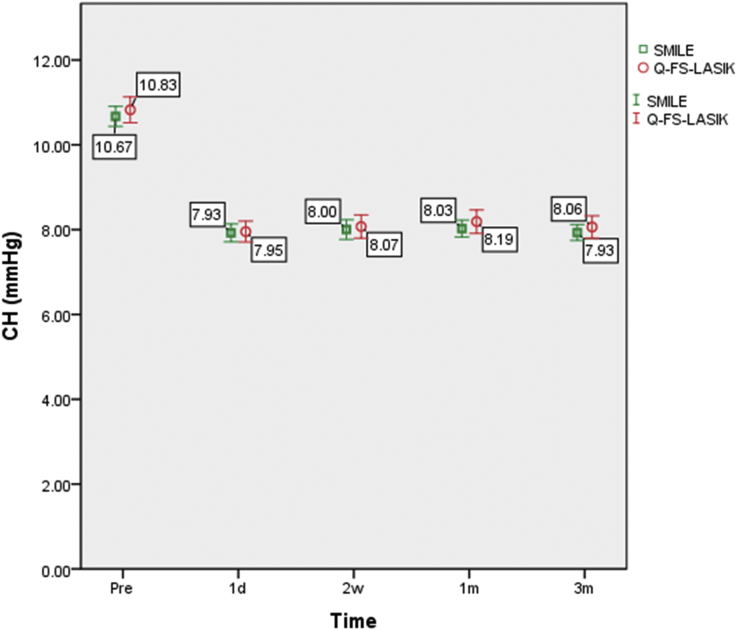
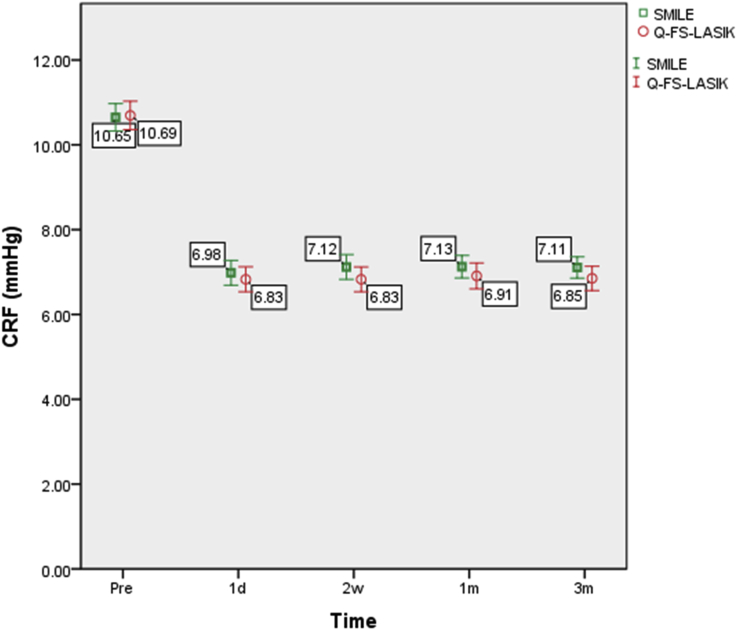
The corneal biomechanical parameters are summarized in Table 2. The changes in CH and CRF following SMILE and Q-FS-LASIK are shown in Fig. 1, Fig. 2, respectively.
Table 2.
Corneal biomechanical parameters.
| Parameter | Preoperative | Postoperative |
F value | P value | |||
|---|---|---|---|---|---|---|---|
| 1 day | 2 weeks | 1 month | 3 months | ||||
| Corneal hysteresis (mm Hg) | |||||||
| SMILE | 10.64 ± 1.09 | 7.91 ± 1.06 | 7.94 ± 1.08 | 8.00 ± 0.99 | 7.91 ± 0.92 | 109.84 | 0.001 |
| Q-FS-LASIK | 10.83 ± 1.60 | 7.98 ± 1.17 | 8.07 ± 1.37 | 8.17 ± 1.31 | 8.00 ± 1.32 | 66.38 | 0.001 |
| Corneal resistance factor (mm Hg) | |||||||
| SMILE | 10.54 ± 1.53 | 6.88 ± 1.47 | 7.01 ± 1.38 | 7.08 ± 1.34 | 7.07 ± 1.27 | 102.30 | 0.001 |
| Q-FS-LASIK | 10.71 ± 1.74 | 6.85 ± 1.42 | 6.87 ± 1.45 | 6.88 ± 1.46 | 6.82 ± 1.40 | 105.83 | 0.001 |
Values are means ± standard deviations.
Fig. 1.
CH changes following SMILE and Q-FS-LASIK. Changes in corneal hysteresis (CH) over time. Bars represent standard deviations. The differences in CH between the SMILE and Q-FS-LASIK groups were not statistically significant.
Fig. 2.
CRF changes following SMILE and Q-FS-LASIK. Changes in the corneal resistance factor (CRF) over time. Bars represent standard deviations. The differences in CRF between the SMILE and Q-FS-LASIK groups were not statistically significant.
In the SMILE group, the variation in CH was statistically significant (P < 0.001). Fisher's LSD test showed that CH at each postoperative time point was statistically significantly lower than the preoperative value (P < 0.001). However, the difference between each postoperative time point was not significant (Table 3).
Table 3.
Multiple comparisons within SMILE group.
| Parameter | P value | |
|---|---|---|
| Corneal hysteresis | ||
| Preoperative | 1 day postoperative | 0.001 |
| 2 weeks postoperative | 0.001 | |
| 1 month postoperative | 0.001 | |
| 3 months postoperative | 0.001 | |
| 1 day postoperative | 2 weeks postoperative | 0.84 |
| 1 month postoperative | 0.60 | |
| 3 months postoperative | 0.99 | |
| 2 weeks postoperative | 1 month postoperative | 0.74 |
| 3 months postoperative | 0.86 | |
| 1 month postoperative | 3 months postoperative | 0.61 |
| Corneal resistance factor | ||
| Preoperative | 1 day postoperative | 0.001 |
| 2 weeks postoperative | 0.001 | |
| 1 month postoperative | 0.001 | |
| 3 months postoperative | 0.001 | |
| 1 day postoperative | 2 weeks postoperative | 0.55 |
| 1 month postoperative | 0.37 | |
| 3 months postoperative | 0.40 | |
| 2 weeks postoperative | 1 month postoperative | 0.77 |
| 3 months postoperative | 0.81 | |
| 1 month postoperative | 3 months postoperative | 0.96 |
The variation in CRF in the SMILE group was also statistically significant (P < 0.001). Fisher's LSD test showed that the CRF at each postoperative time point was statistically significantly lower than the preoperative value (P < 0.001). However, the difference between each postoperative time point was not significant (Table 3).
In Q-FS-LASIK group, the variation in CH was again statistically significant (P < 0.001). Fisher's LSD test showed that CH at each postoperative time point was statistically significantly lower than the preoperative value (P < 0.001). However, the difference between each postoperative time point was not significant (Table 4).
Table 4.
Multiple comparisons within Q-FS-LASIK group.
| Parameter | P value | |
|---|---|---|
| Corneal hysteresis | ||
| Preoperative | 1 day postoperative | 0.001 |
| 2 weeks postoperative | 0.001 | |
| 1 month postoperative | 0.001 | |
| 3 months postoperative | 0.001 | |
| 1 day postoperative | 2 weeks postoperative | 0.66 |
| 1 month postoperative | 0.37 | |
| 3 months postoperative | 0.92 | |
| 2 weeks postoperative | 1 month postoperative | 0.65 |
| 3 months postoperative | 0.74 | |
| 1 month postoperative | 3 months postoperative | 0.43 |
| Corneal resistance factor | ||
| Preoperative | 1 day postoperative | 0.001 |
| 2 weeks postoperative | 0.001 | |
| 1 month postoperative | 0.001 | |
| 3 months postoperative | 0.001 | |
| 1 day postoperative | 2 weeks postoperative | 0.95 |
| 1 month postoperative | 0.90 | |
| 3 months postoperative | 0.90 | |
| 2 weeks postoperative | 1 month postoperative | 0.94 |
| 3 months postoperative | 0.85 | |
| 1 month postoperative | 3 months postoperative | 0.80 |
The variation in the CRF in the Q-FS-LASIK group was also statistically significant (P < 0.001). Fisher's LSD test showed that the CRF at each postoperative time point was statistically significantly lower than the preoperative value (P < 0.001). However, the difference between each postoperative time point was not significant (Table 4).
The differences in CH and CRF between the SMILE and Q-FS-LASIK groups were not statistically significant (P = 0.044 and P = 0.64, respectively).
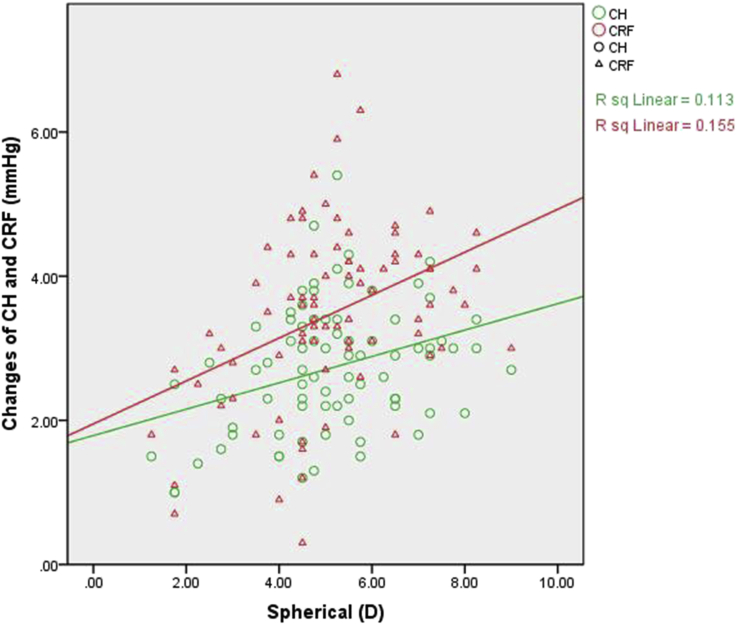
In the SMILE group, the 3-month changes in CH and CRF were correlated with spherical measurements only (P = 0.002 and P < 0.001, respectively) (Table 5, Fig. 3).
Table 5.
Correlation analysis.
| R value | P value | ||
|---|---|---|---|
| SMILE | |||
| Change of Corneal hysteresis | Spherical | 0.34 | 0.002 |
| Cylinder | −0.16 | 0.17 | |
| Optical zone | −0.03 | 0.76 | |
| Change of Corneal resistance factor | Spherical | 0.39 | 0.001 |
| Cylinder | 0.05 | 0.67 | |
| Optical zone | −0.07 | 0.54 | |
| Q-FS-LASIK | |||
| Change of Corneal hysteresis | Spherical | 0.54 | 0.001 |
| Cylinder | 0.03 | 0.82 | |
| Optical zone | −0.09 | 0.43 | |
| Change of Corneal resistance factor | Spherical | 0.49 | 0.001 |
| Cylinder | 0.17 | 0.13 | |
| Optical zone | −0.13 | 0.27 | |
Fig. 3.
Correlation analysis within the SMILE group. Scatterplot showing the correlation of spherical measurements with the difference between pre-operation and 3-month CH and CRF following SMILE. The changes in CH and CRF were both positively correlated with spherical measurements.
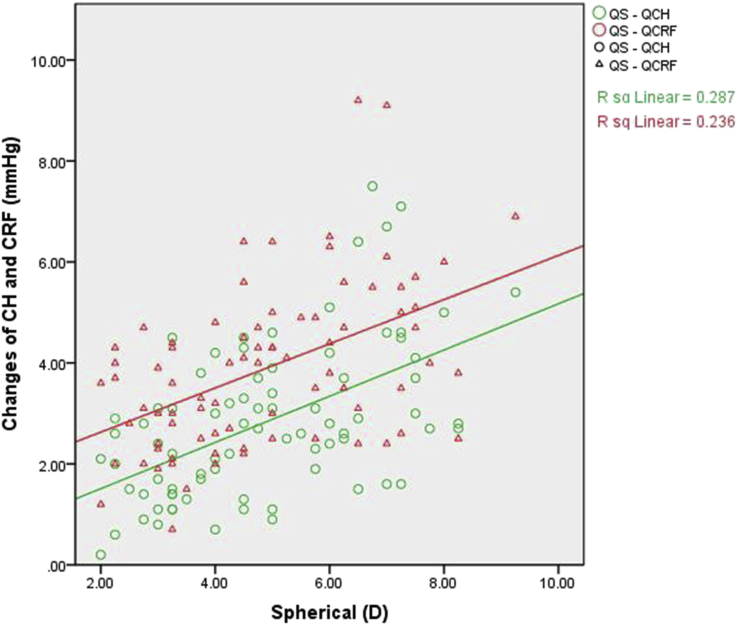
In the Q-FS-LASIK group, the 3-month changes in CH and CRF were also correlated with spherical measurements only (both P < 0.001) (Table 5, Fig. 4).
Fig. 4.
Correlation analysis within the Q-FS-LASIK group. Scatterplot showing the correlation of spherical measurements with the difference between pre-operation and 3-month CH and CRF following Q-FS-LASIK. The changes in CH and CRF were both positively correlated with spherical measurements.
Discussion
Q-FS-LASIK, which is safe and associated with low spherical aberrations, has been a popular procedure for corneal refractive surgery. SMILE is a new kind of surgical procedure that avoids flap-related complications and is gaining more attention. Since the first case of corneal ectasia following LASIK, published by Seiler et al,12 the biomechanical properties of the cornea have become an important indicator of safety13, 14, 15 and are attracting more consideration. The ORA, a dynamic bidirectional applanation device, has been used in corneal refractive surgery to calculate the values of CH and the CRF, allowing a quantitative description of the cornea's biomechanical properties16 by measuring, recording, and analyzing changes in corneal shape. CH represents the cornea's absorption ability against external energy, while the CRF is an indicator of the total reaction of the cornea, incorporating corneal elastic resistance. Both CH and the CRF, as inherent attributes of the cornea, can be used in the diagnosis of keratoconus.17
The results of this study suggest that the cornea's absorption ability against external energy and corneal elastic resistance decreased by day 1 postoperatively following both SMILE and Q-FS-LASIK. This shows that both procedures affect the cornea's biomechanical properties, especially in highly myopic patients in whom greater amounts of corneal tissue are removed. This also indirectly shows that CH and the CRF are inherent attributes of the cornea, and that postoperative healing and mild morphologic changes have little effect on them. Consistent with other studies, highly myopic patients have larger risk of corneal ectasia following LASIK.18, 19
Corneal biomechanical properties are also affected by other surgical parameters, in addition to the amount of corneal stroma removed. Kirwan and O'Keefe reported that the decrease in CH was not statistically significantly different after LASIK or laser-assisted subepithelial keratectomy.20 Wu et al reported that LASIK was associated with greater changes in corneal biomechanical properties than SMILE.10 Furthermore, Medeiros et al reported, using a porcine model, that CH and the CRF did not change significantly after the creation of a 100 mm thin flap, but significantly decreased after the creation of a 300 mm thick flap.21
In the current study, we also found that CH and the CRF were very stable at 1 day, 2 weeks, and 1 and 3 months postoperatively following both SMILE and Q-FS-LASIK. Furthermore, there were no significant differences in CH and the CRF between the two groups at any postoperative time point.
We followed patients for 3 months, because it has been previously reported that there are no significant changes in biomechanical properties between 3 and 6 months.22 However, it is possible that further biomechanical changes might occur over time, and long-term follow-up studies are therefore required.
The observations from our study have several limitations. First, the sample size of our study population was relatively modest. Second, other surgical procedures were not considered as an independent factor or control group. Third, the non-randomized method of treatment allocation resulted is a small imbalance in some baseline patient characteristics between both groups. Selected bias was at least partially offset by having each patient select surgical procedure, however it could not be completely ruled out. Fourth, the study follow-up was limited to 3 months, which does not rule out the possibility of subsequent regression. Further studies are needed to elucidate long-term biomechanical changes.
In summary, both SMILE and Q-FS-LASIK were associated with decreases in corneal biomechanics. These changes were seen on postoperative day 1 and were approximately stable thereafter. In addition, there were no significant differences between SMILE and Q-FS-LASIK in their effects on the biomechanical properties of the cornea.
Acknowledgments
The authors received financial support for this article from the Hangzhou Science and Technology Development Committee, Hangzhou, China.
Footnotes
The authors have no financial or proprietary interest in any products, methods, or materials described herein.
This article has previously been presented at the 12th National Symposium on Refractive Surgery (CSORS), Wu Xi, JiangSu, China 2015-11-26.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Somani S., Tuan K.A., Chernyak D. Corneal asphericity and retinal image quality: a case study and simulations. J Refract Surg. 2004;20(5):S581–S585. doi: 10.3928/1081-597X-20040901-32. [DOI] [PubMed] [Google Scholar]
- 2.Amigó A., Bonaque-González S., Guerras-Valera E. Control of induced spherical aberration in moderate hyperopic LASIK by customizing corneal asphericity. J Refract Surg. 2015;31(12):802–806. doi: 10.3928/1081597X-20151111-03. [DOI] [PubMed] [Google Scholar]
- 3.Goyal J.L., Garg A., Arora R., Jain P., Goel Y. Comparative evaluation of higher-order aberrations and corneal asphericity between wavefront-guided and aspheric LASIK for myopia. J Refract Surg. 2014;30(11):777–784. doi: 10.3928/1081597X-20141021-10. [DOI] [PubMed] [Google Scholar]
- 4.Pajic B., Vastardis I., Pajic-Eggspuehler B., Gatzioufas Z., Hafezi F. Femtosecond laser versus mechanical microkeratome-assisted flap creation for LASIK: a prospective, randomized, paired-eye study. Clin Ophthalmol. 2014;8(9):1883–1889. doi: 10.2147/OPTH.S68124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chansue E., Tanehsakdi M., Swasdibutra S., McAlinden C. Safety and efficacy of VisuMax® circle patterns for flap creation and enhancement following small incision lenticule extraction. Eye Vis (Lond) 2015;2(12):21. doi: 10.1186/s40662-015-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koller T., Iseli H.P., Hafezi F. Q factor customized ablation profile for the correction of myopic astigmation. J Cataract Refract Surg. 2006;32(4):584–589. doi: 10.1016/j.jcrs.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya K., Shimizu K., Igarashi A., Kobashi H. Visual and refractive outcomes of femtosecond lenticule extraction and small incision lenticule extraction for myopia. Am J Ophthalmol. 2014;157(1):128–134. doi: 10.1016/j.ajo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Liu M., Chen Y., Wang D. Clinical outcomes after SMILE and femtosecond laser-assisted LASIK for myopia and myopic astigmatism: a prospective randomized comparative study. Cornea. 2016;35(2):210–216. doi: 10.1097/ICO.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 9.Agca A., Ozgurhan E.B., Demirok A. Comparison of corneal hysteresis and corneal resistance factor after small incision lenticule extraction and femtosecond laser-assisted LASIK: a prospective fellow eye study. Cont Lens Anterior Eye. 2014;37(2):77–80. doi: 10.1016/j.clae.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Wu D., Wang Y., Zhang L., Wei S., Tang X. Corneal biomechanical effects: small-incision lenticule extraction versus femtosecond laser-assisted laser in situ keratomileusis. J Cataract Refract Surg. 2014;40(6):954–962. doi: 10.1016/j.jcrs.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Shin J., Lee J.W., Kim E.A., Caprioli J. The effect of corneal biomechanical properties on rebound tonometer in patients with normal-tension glaucoma. Am J Ophthalmol. 2015;159(1):144–154. doi: 10.1016/j.ajo.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Seiler T., Koufala K., Richter G. Iatrogenic keratectasia after laser in situ keratomileusis. J Refract Surg. 1998;14(3):312–317. doi: 10.3928/1081-597X-19980501-15. [DOI] [PubMed] [Google Scholar]
- 13.Ambrósio R., Jr., Dawson D.G., Salomao M., Guerra F.P., Caiado A.L., Belin M.W. Corneal ectasia after LASIK despite low preoperative risk: tomographic and biomechanical findings in the unoperated, stable, fellow eye. J Refract Surg. 2010;26(11):906–911. doi: 10.3928/1081597X-20100428-02. [DOI] [PubMed] [Google Scholar]
- 14.Qazi M.A., Sanderson J.P., Mahmoud A.M., Yoon E.Y., Roberts C.J., Pepose J.S. Postoperative changes in intraocular pressure and corneal biomechanical metrics; laser in situ keratomileusis versus laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2009;35(10):1774–1788. doi: 10.1016/j.jcrs.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 15.Kamiya K., Shimizu K., Ohmoto F. Comparison of the changes in corneal biomechanical properties after photorefractive keratectomy and laser in situ keratomileusis. Cornea. 2009;28(7):765–769. doi: 10.1097/ICO.0b013e3181967082. [DOI] [PubMed] [Google Scholar]
- 16.Shin J., Kim T.W., Park S.J., Yoon M., Lee J.W. Changes in biomechanical properties of the cornea and intraocular pressure after myopic laser in situ keratomileusis using a femtosecond laser for flap creation determined using ocular response analyzer and Goldmann applanation tonometry. J Glaucoma. 2015;24(3):195–201. doi: 10.1097/IJG.0b013e31829da1ec. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadpour M., Etesami I., Yavari Z., Naderan M., Abdollahinia F., Jabbarvand M. Ocular response analyzer parameters in healthy, keratoconus suspect and manifest keratoconus eyes. Oman J Ophthalmol. 2015;8(2):102–106. doi: 10.4103/0974-620X.159255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao S.N., Epstein R.J. Early onset ectasia following laser in situ keratomileusus: case report and literature review. J Refract Surg. 2002;18(2):177–184. doi: 10.3928/1081-597X-20020301-13. [DOI] [PubMed] [Google Scholar]
- 19.Rad A.S., Jabbarvand M., Saifi N. Progressive keratectasia after laser in situ keratomileusis. J Refract Surg. 2004;20(5):S718–S722. doi: 10.3928/1081-597X-20040903-18. [DOI] [PubMed] [Google Scholar]
- 20.Kirwan C., O'Keefe M. Corneal hysteresis using the Reichert ocular response analyser: findings pre- and post-LASIK and LASEK. Acta Ophthalmol. 2008;86(2):215–218. doi: 10.1111/j.1600-0420.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros F.W., Sinha-Roy A., Alves M.R., Dupps W.J., Jr. Biomechanical corneal changes induced by different flap thickness created by femtosecond laser. Clinics (Sao Paulo) 2011;66(6):1067–1071. doi: 10.1590/S1807-59322011000600025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamiya K., Shimizu K., Ohmoto F. Time course of corneal biomechanical parameters after laser in situ keratomileusis. Ophthalmic Res. 2009;42(3):167–171. doi: 10.1159/000230670. [DOI] [PubMed] [Google Scholar]