Abstract
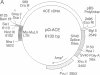
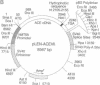
The testis isozyme of angiotensin-converting enzyme (ACE; EC 3.4.15.1) is a membrane-bound protein that, apart from the first 35 N-terminal residues, is identical to the C-terminal half of somatic ACE and contains the same putative C-terminal membrane anchor. Stable transfection of Chinese hamster ovary (CHO) cells with an expression vector containing the full-length human testis ACE cDNA results in the expression of two forms of recombinant human testis ACE (hTACE): membrane-bound ACE and, surprisingly, large quantities (up to 3 mg/liter) of soluble hTACE in the conditioned medium. Both forms are fully active and are physicochemically similar. However, by phase separation in Triton X-114, the soluble enzyme is hydrophilic, as is an anchor-minus mutant hTACE recovered from the medium of CHO cells transfected with a vector that contains a 3'-truncated testis ACE cDNA lacking the sequence encoding the membrane anchor. In contrast, the membrane-bound hTACE is amphipathic but is converted to a hydrophilic form on treatment with trypsin. The data establish that in ACE the hydrophobic sequence near the C terminus is necessary for membrane anchoring. Moreover, in CHO cells, membrane-bound hTACE is apparently solubilized by proteolytic cleavage of this anchor. A similar mechanism may account for the release of endothelial ACE in vivo to generate serum ACE and more generally for the constitutive processing and solubilization of analogously anchored proteins such as the amyloid precursor protein, among others. The release of membrane-bound ACE in CHO cells may, therefore, provide a useful system for the study of membrane-protein-solubilizing proteases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein K. E., Martin B. M., Edwards A. S., Bernstein E. A. Mouse angiotensin-converting enzyme is a protein composed of two homologous domains. J Biol Chem. 1989 Jul 15;264(20):11945–11951. [PubMed] [Google Scholar]
- Bicknell R., Holmquist B., Lee F. S., Martin M. T., Riordan J. F. Electronic spectroscopy of cobalt angiotensin converting enzyme and its inhibitor complexes. Biochemistry. 1987 Nov 17;26(23):7291–7297. doi: 10.1021/bi00397a014. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Ehlers M. R., Fox E. A., Strydom D. J., Riordan J. F. Molecular cloning of human testicular angiotensin-converting enzyme: the testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7741–7745. doi: 10.1073/pnas.86.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M. R., Maeder D. L., Kirsch R. E. Rapid affinity chromatographic purification of human lung and kidney angiotensin-converting enzyme with the novel N-carboxyalkyl dipeptide inhibitor N-[1(S)-carboxy-5-aminopentyl]glycylglycine. Biochim Biophys Acta. 1986 Sep 4;883(2):361–372. doi: 10.1016/0304-4165(86)90329-6. [DOI] [PubMed] [Google Scholar]
- Ehlers M. R., Riordan J. F. Angiotensin-converting enzyme: new concepts concerning its biological role. Biochemistry. 1989 Jun 27;28(13):5311–5318. doi: 10.1021/bi00439a001. [DOI] [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. The angiotensin I-converting enzyme. Lab Invest. 1987 Apr;56(4):345–348. [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Friedland J., Silverstein E. A sensitive fluorimetric assay for serum angiotensin-converting enzyme. Am J Clin Pathol. 1976 Aug;66(2):416–424. doi: 10.1093/ajcp/66.2.416. [DOI] [PubMed] [Google Scholar]
- Gliniak B. C., Kabat D. Leukemogenic membrane glycoprotein encoded by Friend spleen focus-forming virus: transport to cell surfaces and shedding are controlled by disulfide-bonded dimerization and by cleavage of a hydrophobic membrane anchor. J Virol. 1989 Sep;63(9):3561–3568. doi: 10.1128/jvi.63.9.3561-3568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Holmquist B., Bünning P., Riordan J. F. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal Biochem. 1979 Jun;95(2):540–548. doi: 10.1016/0003-2697(79)90769-3. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Keen J., Pappin D. J., Turner A. J. Pig kidney angiotensin converting enzyme. Purification and characterization of amphipathic and hydrophilic forms of the enzyme establishes C-terminal anchorage to the plasma membrane. Biochem J. 1987 Oct 1;247(1):85–93. doi: 10.1042/bj2470085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R. S., Kusari J., Roy S. N., Soffer R. L., Sen G. C. Structure of testicular angiotensin-converting enzyme. A segmental mosaic isozyme. J Biol Chem. 1989 Oct 5;264(28):16754–16758. [PubMed] [Google Scholar]
- Lanzillo J. J., Stevens J., Dasarathy Y., Yotsumoto H., Fanburg B. L. Angiotensin-converting enzyme from human tissues. Physicochemical, catalytic, and immunological properties. J Biol Chem. 1985 Dec 5;260(28):14938–14944. [PubMed] [Google Scholar]
- Lattion A. L., Soubrier F., Allegrini J., Hubert C., Corvol P., Alhenc-Gelas F. The testicular transcript of the angiotensin I-converting enzyme encodes for the ancestral, non-duplicated form of the enzyme. FEBS Lett. 1989 Jul 31;252(1-2):99–104. doi: 10.1016/0014-5793(89)80897-x. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Schofield P. R., Kuang W. J., Seeburg P. H., Mason A. J., Henzel W. J. Molecular cloning and amino acid sequence of rat enkephalinase. Biochem Biophys Res Commun. 1987 Apr 14;144(1):59–66. doi: 10.1016/s0006-291x(87)80475-8. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Kutny R. M. Structure-function relationships for the IL 2-receptor system. IV. Analysis of the sequence and ligand-binding properties of soluble Tac protein. J Immunol. 1987 Aug 1;139(3):855–862. [PubMed] [Google Scholar]
- Soubrier F., Alhenc-Gelas F., Hubert C., Allegrini J., John M., Tregear G., Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Taljanidisz J., Stewart L., Smith A. J., Klinman J. P. Structure of bovine adrenal dopamine beta-monooxygenase, as deduced from cDNA and protein sequencing: evidence that the membrane-bound form of the enzyme is anchored by an uncleaved signal peptide. Biochemistry. 1989 Dec 26;28(26):10054–10061. doi: 10.1021/bi00452a026. [DOI] [PubMed] [Google Scholar]
- Turner A. J., Hooper N. M. Phosphatidylinositol-glycan-tailed membrane proteins: the biochemistry of glycolipid anchors. Biochem Soc Trans. 1989 Oct;17(5):864–866. doi: 10.1042/bst0170864. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]