Abstract
Context:
Individuals with chronic ankle instability (CAI) present with decreased modulation of the Hoffmann reflex (H-reflex) from a simple to a more challenging task. The neural alteration is associated with impaired postural control, but the relationship has not been investigated in individuals with CAI.
Objective:
To determine differences in H-reflex modulation and postural control between individuals with or without CAI and to identify if they are correlated in individuals with CAI.
Design:
Descriptive laboratory study.
Setting:
Laboratory.
Patients or Other Participants:
A total of 15 volunteers with CAI (9 males, 6 females; age = 22.6 ± 5.8 years, height = 174.7 ± 8.1 cm, mass = 74.9 ± 12.8 kg) and 15 healthy sex-matched volunteers serving as controls (9 males, 6 females; age = 23.8 ± 5.8 years, height = 171.9 ± 9.9 cm, mass = 68.9 ± 15.5 kg) participated.
Intervention(s):
Maximum H-reflex (Hmax) and motor wave (Mmax) from the soleus and fibularis longus were recorded while participants lay prone and then stood in unipedal stance. We assessed postural tasks of unipedal stance with participants' eyes closed for 10 seconds using a forceplate.
Main Outcome Measure(s):
We normalized Hmax to Mmax to obtain Hmax : Mmax ratios for the 2 positions. For each muscle, H-reflex modulation was quantified using the percentage change scores in Hmax : Mmax ratios calculated from prone position to unipedal stance. Center-of-pressure data were used to compute 4 time-to-boundary variables. Separate independent-samples t tests were performed to determine group differences. Pearson product moment correlation coefficients were calculated between the modulation and balance measures in the CAI group.
Results:
The CAI group presented less H-reflex modulation in the soleus (t26 = −3.77, P = .001) and fibularis longus (t25 = −2.59, P = .02). The mean of the time-to-boundary minima in the anteroposterior direction was lower in the CAI group (t28 = −2.06, P = .048). We observed a correlation (r = 0.578, P = .049) between the fibular longus modulation and mean of time-to-boundary minima in the anteroposterior direction.
Conclusions:
The strong relationship indicated that, as H-reflex amplitude in unipedal stance was less down modulated, unipedal postural control was more impaired. Given the deficits in H-reflex modulation and postural control in the CAI group, the relationship may provide insights into the neurophysiologic mechanism of postural instability.
Key Words: spinal mechanism, postural-control deficits, balance, soleus muscle, ankle muscles
Key Points
The chronic ankle instability group had decreased down modulation of the Hoffmann reflex from prone position to unipedal stance and impaired postural control in unipedal stance.
Impaired postural control was strongly correlated with altered fibularis longus modulation.
Researchers should investigate whether decreased Hoffmann-reflex modulation is present before or develops after the onset of initial ankle sprains in patients with chronic ankle instability.
Altered down modulation of the fibularis longus may be the spinal neurophysiologic mechanism responsible for postural instability associated with chronic ankle instability.
Ample evidence has shown that, after ankle sprains, a substantial proportion of patients will develop residual signs and symptoms, including subjective ankle instability, pain, swelling, muscle weakness, episodes of the ankle giving way, and recurrent injury.1−3 This condition, termed chronic ankle instability (CAI),4 has been associated with a variety of contributing factors, including postural-control deficits.5 Postural control in single-legged stance has been examined extensively in individuals with CAI over the past 5 decades because postural-control deficits derived from the initial ankle sprain are thought to play an important role in ankle instability.6,7 Despite numerous studies of postural deficits associated with CAI, the underlying mechanism that mediates the impaired postural control remains unclear.
The Hoffmann reflex (H-reflex), an electrical analogue of the monosynaptic stretch reflex, is commonly used to investigate responses of sensorimotor systems to a variety of postural tasks during changes in body orientation,8 body position,9−11 and locomotion.12,13 In healthy individuals, researchers14 have documented that H-reflex amplitudes tend to decrease as the complexity of postural tasks increases. For example, the soleus H-reflex amplitude substantially diminishes when individuals move from lying to standing position.9,11 Similar patterns of H-reflex depression have also been observed during walking compared with standing,12 with further decreases seen during running.13 These results indicate that the H-reflex amplitude needs to be down modulated during more challenging postural tasks. Down modulation has been viewed as a mechanism of motor-control shift from the spinal to the supraspinal centers, which provides finer control over more complex postural tasks.10,15
Investigators10,11,16−19 have reported that the H-reflex modulation associated with postural control, previously termed postural modulation of H-reflex, is altered in patients with postural instability. Specifically, H-reflex modulation in a more challenging postural task is altered in patients with CAI.10,18,19 Sefton et al18 reported that the soleus H-reflex modulation in bipedal and unipedal stance was altered in patients with CAI compared with healthy control participants. The patients with CAI were unable to modulate the soleus H-reflex amplitudes in unipedal stance, whereas healthy control participants modulated downward about 15%. Recently, soleus and fibularis longus H-reflex modulation associated with CAI was examined during 3 different postural transitions.10 The H-reflex modulation of both the soleus and fibularis longus in the involved limbs of the CAI group was less than that of the contralateral uninvolved limb or both limbs of the healthy control group.10 Authors10,18,19 have hypothesized that less down modulation of the H-reflex during a more challenging postural task may be hazardous due to greater reliance on spinal reflexive control during the task when finer motor control is required from the supraspinal level to sufficiently accommodate greater postural demands. From this perspective, researchers10,18,19 have speculated that the altered modulation of the H-reflex in ankle-stabilizing muscles may be linked to postural instability associated with CAI. However, this relationship has not been directly confirmed with evidence of postural-control deficits in individuals with CAI whose H-reflex modulations were diminished. Therefore, the purpose of our study was 2-fold: (1) to determine differences in H-reflex modulation during a change in body positions and postural control between individuals with or without CAI and (2) to identify if the H-reflex modulation was correlated with postural-control measures in patients with CAI. We hypothesized that patients with CAI would have less H-reflex modulation and poorer postural control and that these outcomes would be strongly correlated. Our study should provide insights into the neurophysiologic mechanism responsible for impaired postural control associated with CAI by demonstrating how much of the postural instability can be explained by the altered H-reflex modulation.
METHODS
Our study was a descriptive laboratory investigation. The first outcome variables were H-reflex modulation measures of the soleus and fibularis longus from prone position to unipedal stance. We chose this postural transition because Kim et al10 reported large effect sizes for differences in H-reflex modulation between groups with or without CAI. The second outcome variables were time-to-boundary (TTB) measures of center-of-pressure (COP) excursions recorded during unipedal stance: specifically the means and standard deviations of TTB minima in the anteroposterior (AP) and mediolateral (ML) directions.20 In addition to these primary outcome variables, the number of unsuccessful trials during a unipedal balance task was the secondary outcome variable. All tests, including prone and unipedal tests of H-reflex and unipedal balance for 10 seconds, were performed on the same day in the same order. This order of testing was standardized due to the potential effects of repetitive standing on prone maximum H-reflex (Hmax) measurements.15
Participants
A total of 15 participants with CAI (9 males, 6 females) and 15 healthy participants serving as controls (9 males, 6 females) without a history of ankle sprains were enrolled. Participant demographics are shown in Table 1. Volunteers were recruited from a university community and screened for their current ankle function and history of ankle injury. Participants reporting all of the following criteria were assigned to the CAI group: history of at least 1 lateral ankle sprain that had occurred 1 year or more before the study; repetitive episodes of the ankle giving way and feelings of ankle-joint instability as detected by 4 or more yes responses on the Modified Ankle Instability Instrument21; and self-reported symptoms of ankle disability as quantified by a score of 90% or less on the Foot and Ankle Ability Measure-Activities of Daily Living scale and a score of 80% or less on the Foot and Ankle Ability Measure–Sport, both of which have been used to detect ankle dysfunction associated with CAI.22 Volunteers denying all of the following criteria were allocated to the healthy control group: any history of ankle injury or substantial lower extremity injury or surgery and any limitation of ankle function. Individuals reporting any of the following were excluded from the study: lower extremity injury within the 6 weeks before the study and any history of lower extremity surgery, neuropathy, diabetes, balance disorder, or other conditions known to affect H-reflex and balance. If participants had bilateral CAI, we chose the ankle that they reported was more symptomatic. Participants were restricted from any intake of caffeine, alcohol, and stimulants 24 hours before the study, as these substances are known to affect H-reflex measures. The test limbs of healthy control participants were side matched to the test limbs of participants with CAI. All participants provided written informed consent, and the University of Virginia Institutional Review Board for Health Sciences Research approved the study.
Table 1. .
Participant Demographics (Mean ± SD)
| Variable |
Group |
|
| Chronic Ankle Instability |
Healthy Control |
|
| Age, y | 22.6 ± 5.8 | 23.8 ± 5.8 |
| Height, cm | 174.7 ± 8.1 | 171.9 ± 9.9 |
| Mass, kg | 74.9 ± 12.8 | 68.9 ± 15.5 |
| Foot and Ankle Ability Measure–Activities of Daily Living scale, % | 82.7 ± 6.5 | 100 |
| Foot and Ankle Ability Measure–Sport scale, % | 65.0 ± 9.3 | 99.7 ± 1.3 |
| No. of previous ankle sprains | 4.9 ± 4.8 | 0 |
| Months since the latest ankle sprain | 13.5 ± 7.3 | Not applicable |
Instruments
Disposable, 10-mm pregelled Ag/AgCl surface electromyography (EMG) electrodes were used to collect H-reflex and muscle-response (motor-wave [M-wave]) measurements. The EMG signals were bandpass filtered from 10 to 500 Hz and sampled at 2000 Hz with EMG amplification at a gain of 1000. The EMG amplifier had a common mode rejection ratio of 100 dB and input impedance of 2 MΩ. Analog-to-digital signal conversion was processed using a 16-bit converter (MP150; BIOPAC Systems Inc, Goleta, CA). We used Acqknowledge software (version 3.7.3; BIOPAC Systems Inc) to capture and visualize EMG signals. A stimulator module (STIM100A; BIOPAC Systems Inc) with a 200-V–maximum stimulus isolation adaptor, a 2-mm shield disk electrode, and a 7-cm circular carbon-impregnated dispersive pad were used to elicit H-reflexes and motor responses.
An AccuSway Plus forceplate (Advanced Mechanical Technology, Inc, Watertown, MA) was used to assess postural control during unipedal stances. We used Balance Clinic Software (Advanced Mechanical Technology, Inc) to calculate COP from the 3-dimensional forces and moments at the foot–forceplate interface. The COP data were sampled at 50 Hz and filtered with a fourth-order, zero-lag low-pass filter with a cutoff frequency of 5 Hz.23,24 A custom software program processed in MATLAB (The MathWorks, Inc, Natick, MA) was used to compute TTB variables.23,24
Procedures
Hoffmann-Reflex Measurement
The H-reflexes and M-waves were collected in accordance with previously used methods for electrode placement and participant positioning.10 The skin areas for recording over the soleus and fibularis longus musculature were shaved, debrided with fine sandpaper, and cleaned with isopropyl alcohol. For the soleus, 2 recording electrodes were placed 1.75 cm apart and center to center over the midline of the muscle belly at the distal third of the lower leg. For the fibularis longus, 2 recording electrodes were positioned 2 to 3 cm distal to the fibular head. The ground electrode was placed on the ipsilateral medial malleolus. Proper electrode placement was confirmed with manual muscle testing. The stimulating electrode of a 2-mm shield disk was placed in the superior portion of the popliteal fossa to access the sciatic nerve. A 7-cm circular carbon-impregnated dispersive pad was positioned superior to the patella.
Two body positions (prone, unipedal stance) were used to obtain Hmax and maximum M-wave (Mmax) measures.10 For the prone posture, participants were positioned with the knee slightly flexed and the ankle supported on a foam roller. The head was maintained in a neutral position using a prone pillow, and the hands were placed at the sides. Participants were instructed to relax and look at a fixed object on the floor throughout the prone test. For the unipedal stance, we instructed participants to stand on the forceplate and maintain a unipedal stance on either the involved limb (CAI group) or side-matched limb (control group). The stance foot was divided equally into the AP and ML midlines of the forceplate. For consistency in multiple trials of unipedal stance, the foot position was outlined with tape.25 The unipedal H-reflex test began with participants positioned in the unipedal stance. They were instructed to close their eyes when they became stable during unipedal stance. We administered an electrical stimulus 1 to 3 seconds afterward to trigger the H-reflex. Participants were released from the unipedal stance after each stimulation. Testing the H-reflex amplitude during unipedal stance with eyes closed was important because individuals with CAI commonly demonstrate postural instability in this condition.5−7,24
After participants reached the desired testing position, we administered 1-millisecond square-wave pulses to stimulate the sciatic nerve and concurrently collected H-reflexes of both the soleus and fibularis longus. A series of stimuli were delivered in 0.2-V increments until the Hmax was identified in both muscles. At least a 12-second rest interval between stimuli was required to prevent postactivation depression.26 The stimulus intensity continued to increase until the Mmax was noted in both muscles. We recorded 5 Hmax and Mmax measurements for each of the 2 body positions.
Postural Control
Postural-control testing involved the same position of unipedal stance on the forceplate that was used for unipedal H-reflex testing, but participants performed the unipedal stance with eyes closed for 10 seconds. After 1 practice trial, a test trial was recorded if the participant maintained an initial testing position without any of the following errors: the nonstance limb touched the stance limb or forceplate or the stance limb shifted its orientation or its alignment in regard to the center of the forceplate. The number of unsuccessful trials as well as 3 successful trials were recorded.23,24
Data Processing
The average Hmax and Mmax of 5 trials were used to calculate the Hmax : Mmax ratio for the soleus and fibularis longus in each of the 2 body positions. The postural modulation of H-reflex was defined operationally as the percentage change in Hmax : Mmax ratio from prone position to unipedal stance.10 The postural modulation was calculated using the equation of Kim et al10:
The TTB analysis of COP data was performed to assess different spatiotemporal characteristics of postural control than the traditional measures.23 Researchers24,27 have reported that TTB measures are sensitive to detecting balance deficits associated with CAI. Previously described methods23,24,27 were used to compute TTB measures separately in the AP and ML directions. Boundaries of the base of support for unipedal stance were modeled as a rectangle in the same manner as previously reported,23 allowing for separation of the AP and ML components of COP. Each TTB measure was calculated using the instantaneous position and velocity of each corresponding COP point. We processed 500 COP data points (50 Hz for 10 seconds) to create a series of TTB measures in the time domain that showed a series of peaks and valleys. Each valley represented the least amount of time the COP would take to reach the boundary if it continued to move in the same direction without a change in velocity. The TTB minima, the values at each valley in the TTB data series, provide temporal margins to the boundaries of support. A smaller TTB measure indicates greater postural instability. The TTB measures serving as dependent variables included the mean and standard deviation of minima in the AP and ML directions. The mean of 3 trials was used for statistical analysis.
Statistical Analysis
Separate independent-samples t tests were performed to determine group differences (CAI, control) for measures of H-reflex modulation and postural control. We computed Cohen d effect sizes and associated 95% confidence intervals (CIs) to quantify the magnitude of the group differences. The strength of the effect size was interpreted using the guidelines of Cohen28: weak (<0.2), small (0.21–0.5), moderate (0.51–0.8), and large (>0.8). These analyses were conducted to provide evidence that the participants with CAI enrolled in the study were representative of patients with CAI described in the literature who had less modulation of H-reflex and impaired postural control. Pearson product moment correlation coefficients were calculated between H-reflex modulation and TTB measures to determine their relationships in the CAI group. A correlation coefficient (r) of 0 to 0.3 represented a weak relationship; 0.3 to 0.5, a moderate relationship; and 0.5 to 1.0, a strong relationship.28 A Mann-Whitney U test was used to determine group differences in the number of unsuccessful balance trials. The α level was set at .05. All statistical analyses were performed using SPSS software (version 19.0; IBM Corporation, Armonk, NY).
RESULTS
Descriptive data of all outcome variables are presented in Table 2. All data for H-reflex modulation in the healthy control group were included in the following results. However, 2 of the soleus data points and 3 of the fibularis longus data points in the CAI group were not included due to a lack of H-reflex responses during unipedal stance, which resulted in correlation analyses being performed with 13 data points of the soleus modulation and 12 data points of the fibularis longus modulation. In contrast, all COP measures in both groups were included in all analyses.
Table 2. .
Descriptive Summary of the Prone Position to Unipedal Hoffmann-Reflex Modulation and Center-of-Pressure Measures During Unipedal Stance
| Variable |
Group (Mean ± SD [range]) |
Effect Size (95% Confidence Interval) |
|
| Chronic Ankle Instability> |
Healthy Control |
||
| Hoffmann-reflex modulation, % | |||
| Soleusa | −5.53 ± 38.9 (−61.30–50.80) | 37.37 ± 19.50 (−6.50–64.50) | 1.43 (0.60, 2.26) |
| Fibularis longusa | 12.10 ± 27.50 (−22.20–61.40) | 33.82 ± 15.60 (12.60–55.20) | 1.00 (0.20, 1.81) |
| Center of pressure | |||
| Mean of mediolateral TTB minima, s | 2.00 ± 0.90 (0.60–3.20) | 2.05 ± 1.00 (0.80–4.70) | 0.05 (−0.66, 0.77) |
| Mean of anteroposterior TTB minima, sa | 4.75 ± 1.70 (1.60–7.30) | 5.90 ± 1.30 (2.40–7.60) | 0.76 (0.02, 1.50) |
| SD of mediolateral TTB minima, s | 1.84 ± 0.80 (0.40–3.40) | 1.91 ± 0.60 (0.60–2.80) | 0.10 (−0.62, 0.82) |
| SD of anteroposterior TTB minima, s | 3.28 ± 1.70 (1.00–6.60) | 3.68 ± 1.30 (1.20–5.90) | 0.26 (−0.45, 0.98) |
Abbreviation: TTB, time to boundary.
Indicates a group difference (P ≤ .05).
The CAI group presented less H-reflex modulation from prone position to unipedal stance in both the soleus (t26 = −3.77, P = .001) and fibularis longus (t25 = −2.59, P = .02) than the healthy control group. These group differences were large, as indicated by effect-size point estimates equal to or greater than 1.00 and 95% CIs not crossing zero (Table 2). Similarly, we observed a group difference in the mean of AP TTB minima (t28 = −2.06, P = .048). Specifically, the CAI group appeared to need less time for the COP to reach the AP boundaries of the base of support than the healthy control group. The impairment was moderate, as determined by the effect size of 0.76 and 95% CI not crossing zero (Table 2). In addition, the number of unsuccessful trials during unipedal stance for 10 seconds was greater in the CAI group (median = 2, range = 0–8) than in the healthy control group (median = 0, range = 0–2; U = 55, P = .02). However, group differences were not found in other TTB measures: mean of ML TTB minima (t28 = −0.122, P = .90) and standard deviations of AP TTB (t28 = −0.723, P = .48) and ML TTB (t28 = −0.257, P = .80).
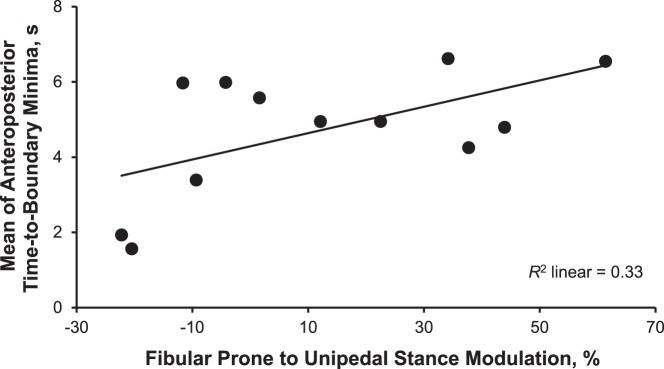
All bivariate correlations between H-reflex modulation and TTB measures in the CAI group are shown in Table 3. The strength of relationships between the soleus modulation and TTB measures ranged from r = 0.130 to r = 0.425, indicating positive and weak to moderate associations. Similar but stronger relationships were evident in the fibularis longus (r range = 0.279 to 0.578). All relationships indicated that less H-reflex modulation was associated with poorer postural control. We observed a correlation only between fibularis longus modulation and the mean of AP TTB minima (r = 0.578, P = .049). This strong relationship indicated that about 33% of the variance in the postural-control impairment was explained by the fibularis longus modulation (Figure).
Table 3. .
Pearson Product Moment Correlation Coefficients (r) Between the Hoffmann-Reflex Modulation From Prone Position to Unipedal Stance and Center-of-Pressure Measures During Unipedal Stance in Individuals With Chronic Ankle Instability
| Hoffmann-Reflex Modulation |
||||
| Soleus |
Fibularis Longus |
|||
| Center-of-Pressure Measure |
r |
P |
r |
P |
| Mean of mediolateral TTB minima, s | 0.291 | .34 | 0.473 | .12 |
| Mean of anteroposterior TTB minima, s | 0.425 | .15 | 0.578a | .049 |
| SD of mediolateral TTB minima, s | 0.130 | .67 | 0.279 | .38 |
| SD of anteroposterior TTB minima, s | 0.318 | .29 | 0.414 | .18 |
Abbreviation: TTB, time to boundary.
Indicates correlation (P ≤ .05).
Figure.
The group with chronic ankle instability showed a strong positive correlation between the fibularis longus Hoffmann-reflex modulation from prone position to unipedal stance when the mean of time-to-boundary minima were measured during unipedal stance with eyes closed for 10 seconds. The larger modulation represents better control in modulating the reflexive muscle response, and the greater time-to-boundary value indicates better postural control.
DISCUSSION
Not only did we find decreased down modulation of the H-reflex from prone position to unipedal stance but also deficits in postural control during unipedal stance in the CAI group. The impaired postural control was identified by less time needed for the COP to reach the boundaries of the base of support in the AP directions, and it was confirmed with greater numbers of unsuccessful balance trials. These group differences appeared to be clear and meaningful because of the moderate to large effect sizes with their corresponding CIs not crossing zero. Our results were consistent with previous findings,10,18,19,24,27 thus ensuring that our sample of participants with CAI was representative of patients with CAI described in the literature. More importantly, we observed a strong, positive correlation of the fibularis longus H-reflex modulation with unipedal balance in the CAI group. Specifically, less down regulation of the fibularis longus H-reflex amplitudes in unipedal stance from prone position was largely associated with less time needed for the COP to reach the AP boundaries of the base of support. These findings are unique because postural-control deficits can be partially explained (33%) by the alterations in spinal-level motor control (Figure), which provide greater insights into a neurophysiologic mechanism of impaired postural control associated with CAI and may help us to develop a specific intervention directly addressing the neural alterations for better postural-control outcomes.
The relationships of fibularis longus H-reflex modulation from prone position to unipedal stance with postural control during unipedal stance were moderate to strong, indicating that a range of 8% to 33% of the variance in TTB measures can be explained by the variance in the H-reflex modulation. These results provide insights into the neurophysiologic mechanism of postural-control deficits associated with CAI. Convincing evidence indicates that postural-control deficits during unipedal stance are present in patients with CAI,6,7 and the impairments have been demonstrated consistently using TTB measures that appear to be more sensitive than traditional COP measures.24,27 The underlying neurophysiologic mechanism of postural-control deficits in patients with CAI, however, has been unclear despite substantial research efforts over the past several decades. Loss of proprioception due to mechanoreceptors that are disrupted after initial ankle sprains has long been proposed as a primary source of postural instability in patients with CAI,4,5 but evidence is emerging for deficits in control of postural tasks that could not be explained solely by the proprioceptive deficits, such as bilateral postural instability in individuals with unilateral CAI,24 decreased α motor-neuron activation,25 and altered lower extremity activity before initial contact during gait.29 These findings suggest central alterations in the neuromuscular system.5,24,25,29 The H-reflex, as a means of assessing α motor-neuron excitability, may provide insights into the neurophysiologic mechanism, as it has commonly been linked to postural instability in the literature.11,15−17,30 Specifically, many researchers10,11,16−19 have demonstrated a loss of or decrease in down modulation of the H-reflex during changes in body positions in patients with postural instability, including those with CAI. Elderly people11 and patients with neurologic diseases16,17 have shown the decreased down modulation of H-reflex that was correlated with control of standing posture. These results suggest that postural instability in these populations may be largely explained by the alteration in H-reflex modulation.11,16,17 This may be true in patients with CAI, as we found decreased down modulation of the H-reflex and poor balance performance during unipedal stance in the CAI group (Table 2). In addition, the altered H-reflex modulation of the fibularis longus was strongly correlated with postural instability. Our results suggested that the decreased down modulation of the fibularis longus plays an important role in postural-control impairment associated with CAI, as 33% of the variance in the impaired postural control was explained solely by the variance in H-reflex modulation (Figure).
We observed that soleus H-reflex modulation had weak to moderate relationships with TTB measures, accounting for 2% to 19% of the variance. In particular, the soleus modulation explained close to 20% of the variance in the mean of the AP TTB minima measure that discriminated between groups with or without CAI. Whereas 80% of the variance in the AP TTB minima may be explained by other factors, the soleus H-reflex modulation remains important in regulating the spinal-level motor control that is critical to postural tasks.11,16,17 Lack of differences in the soleus may be due to the higher variability found in the H-reflex modulation measure of the soleus than in the fibularis longus, which was correlated with the AP TTB minima (Table 2). A subsequent study with a larger sample size may be warranted to determine the latent relationship of the soleus H-reflex modulation with postural-control measures.
Our result of the strong relationship of the H-reflex modulation of the fibularis longus with postural control during unipedal stance may be clinically important for providing a better understanding of postural instability associated with CAI and allowing for the development of more effective approaches to treating postural instability. We observed that participants with CAI who down modulated the H-reflex amplitude of the fibularis longus less in unipedal stance had more difficulty maintaining postural stability during the stance. The decreased down modulation may reflect the decreased ability of the sensorimotor system to shift motor control of a more challenging postural task from the spinal and supraspinal systems; researchers10,15 believe that motor-control centers at the supraspinal level provide finer adjustments to sufficiently accommodate greater postural demands in a more challenging postural task, such as unipedal stance. Inadequate suppression of the reflexive muscle responses may result in greater reflexive control at the spinal level, which may not be ideal for maintaining postural stability during unipedal stance, as the reflexive responses may act as destabilizing oscillations and disturb postural stability.10,15 From this standpoint, the decreased down modulation of the H-reflex may be the spinal neurophysiologic mechanism responsible for postural instability associated with CAI.
This mechanism may be more evident in previous findings30−32 that a therapeutic intervention aimed at addressing the decreased H-reflex modulation led to substantial improvements in postural control. For example, Mynark and Koceja30 developed a 2-day balance-training protocol using an electrical perturbation with soleus H-reflex responses aimed at restoring the altered soleus H-reflex modulation seen in elderly individuals. The decreased H-reflex modulation was alleviated after training in which the participants were instructed to maintain their bipedal stance on a platform that moved in the AP direction after each of multiple perturbations. The training also improved postural control, as indicated by a 10% decrease in postural sway.30 Similar training was effective for spastic patients.32 This type of intervention involving balance training and an electrical or mechanical perturbation has been termed perturbation-based balance training, and evidence that this new type of balance training is more effective than the traditional type involving only voluntary exercises is convincing.31 Perturbation-based balance training aimed at restoring the decreased down-modulation of the H-reflex may improve postural control in patients with CAI.
The H-reflex measures allow for assessment of spinal-level control of an upright posture.14 Given that postural control during standing is influenced by both spinal and supraspinal motor-control mechanisms,15 inclusion of supraspinal measures, such as motor-evoked potentials and electroencephalogram in addition to the H-reflex, would be beneficial to providing a more complete picture of the neurophysiologic mechanism of postural instability associated with CAI. In addition, examining a change in the relationship between H-reflex modulation and postural control after different therapeutic intervention regimens would provide greater insights into the neurophysiologic mechanism.
CONCLUSIONS
The CAI group presented decreased down modulation of the H-reflex in the ankle muscles from prone position to unipedal stance and impaired postural control during unipedal stance. More importantly, the impaired postural control was strongly correlated with the altered fibularis longus modulation; as down modulation of the H-reflex amplitude in unipedal stance decreased, postural control was more impaired. Further studies are warranted to determine a causal link between H-reflex modulation and postural control because the interval validity of our study was limited due to its retrospective design. Therefore, researchers need to investigate if decreased H-reflex modulation is present before an initial ankle sprain or if the altered modulation tends to develop after the initial injury in individuals who develop CAI. In addition, blinding examiners would provide unbiased results. Our results suggested that the altered down modulation of the fibularis longus H-reflex may be the spinal neurophysiologic mechanism responsible for postural instability associated with CAI, which provides insights into developing a more effective approach (eg, perturbation-based balance training) to treating the postural-control deficits that are common after joint injury.
ACKNOWLEDGMENTS
This study was funded by a doctoral dissertation grant from the National Athletic Trainers' Association Research & Education Foundation (K.-M.K.).
REFERENCES
- 1. Anandacoomarasamy A, Barnsley L. Long term outcomes of inversion ankle injuries. Br J Sports Med. 2005; 39 3: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braun BL. Effects of ankle sprain in a general clinic population 6 to 18 months after medical evaluation. Arch Fam Med. 1999; 8 2: 143– 148. [DOI] [PubMed] [Google Scholar]
- 3. Konradsen L, Bech L, Ehrenbjerg M, Nickelsen T. Seven years follow-up after ankle inversion trauma. Scand J Med Sci Sports. 2002; 12 3: 129– 135. [DOI] [PubMed] [Google Scholar]
- 4. Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002; 37 4: 364– 375. [PMC free article] [PubMed] [Google Scholar]
- 5. Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med. 2008; 27 3: 353– 370, vii. [DOI] [PubMed] [Google Scholar]
- 6. Munn J, Sullivan SJ, Schneiders AG. Evidence of sensorimotor deficits in functional ankle instability: a systematic review with meta-analysis. J Sci Med Sport. 2010; 13 1: 2– 12. [DOI] [PubMed] [Google Scholar]
- 7. Wikstrom EA, Naik S, Lodha N, Cauraugh JH. Bilateral balance impairments after lateral ankle trauma: a systematic review and meta-analysis. Gait Posture. 2010; 31 4: 407– 414. [DOI] [PubMed] [Google Scholar]
- 8. Trimble MH. Postural modulation of the segmental reflex: effect of body tilt and postural sway. Int J Neurosci. 1998; 95 1−2: 85– 100. [DOI] [PubMed] [Google Scholar]
- 9. Katz R, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain. 1988; 111 pt 2: 417– 437. [DOI] [PubMed] [Google Scholar]
- 10. Kim KM, Ingersoll CD, Hertel J. Altered postural modulation of Hoffmann reflex in the soleus and fibularis longus associated with chronic ankle instability. J Electromyogr Kinesiol. 2012; 22 6: 997– 1002. [DOI] [PubMed] [Google Scholar]
- 11. Koceja DM, Markus CA, Trimble MH. Postural modulation of the soleus H reflex in young and old subjects. Electroencephalogr Clin Neurophysiol. 1995; 97 6: 387– 393. [DOI] [PubMed] [Google Scholar]
- 12. Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986; 6 5: 1308– 1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol. 1987; 392: 513– 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zehr PE. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002; 86 6: 455– 468. [DOI] [PubMed] [Google Scholar]
- 15. Taube W, Gruber M, Gollhofer A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol (Oxf). 2008; 193 2: 101– 116. [DOI] [PubMed] [Google Scholar]
- 16. Hayashi R, Tokuda T, Tako K, Yanagisawa N. Impaired modulation of tonic muscle activities and H-reflexes in the soleus muscle during standing in patients with Parkinson's disease. J Neurol Sci. 1997; 153 1: 61– 67. [DOI] [PubMed] [Google Scholar]
- 17. Tokuda T, Tako K, Hayashi R, Yanagisawa N. Disturbed modulation of the stretch reflex gain during standing in cerebellar ataxia. Electroencephalogr Clin Neurophysiol. 1991; 81 6: 421– 426. [DOI] [PubMed] [Google Scholar]
- 18. Sefton JM, Hicks-Little CA, Hubbard TJ, et al. Segmental spinal reflex adaptations associated with chronic ankle instability. Arch Phys Med Rehabil. 2008; 89 10: 1991– 1995. [DOI] [PubMed] [Google Scholar]
- 19. Sefton JM, Hicks-Little CA, Hubbard TJ, et al. Sensorimotor function as a predictor of chronic ankle instability. Clin Biomech (Bristol, Avon). 2009; 24 5: 451– 458. [DOI] [PubMed] [Google Scholar]
- 20. Krause BA, Hopkins JT, Ingersoll CD, Cordova ML, Edward JE, Merrick MA. The effects of ankle and axillary cooling on the human soleus Hoffmann reflex [abstract]. J Athl Train. 2000; 35 suppl 2: S58. [Google Scholar]
- 21. Docherty CL, Gansneder BM, Arnold BL, Hurwitz SR. Development and reliability of the ankle instability instrument. J Athl Train. 2006; 41 2: 154– 158. [PMC free article] [PubMed] [Google Scholar]
- 22. Carcia CR, Martin RL, Drouin JM. Validity of the Foot and Ankle Ability Measure in athletes with chronic ankle instability. J Athl Train. 2008; 43 2: 179– 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hertel J, Olmsted-Kramer LC, Challis JH. Time-to-boundary measures of postural control during single leg quiet standing. J Appl Biomech. 2006; 22 1: 67– 73. [DOI] [PubMed] [Google Scholar]
- 24. Hertel J, Olmsted-Kramer LC. Deficits in time-to-boundary measures of postural control with chronic ankle instability. Gait Posture. 2007; 25 1: 33– 39. [DOI] [PubMed] [Google Scholar]
- 25. McVey ED, Palmieri RM, Docherty CL, Zinder SM, Ingersoll CD. Arthrogenic muscle inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int. 2005; 26 12: 1055– 1061. [DOI] [PubMed] [Google Scholar]
- 26. Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004; 39 3: 268– 277. [PMC free article] [PubMed] [Google Scholar]
- 27. McKeon PO, Hertel J. Spatiotemporal postural control deficits are present in those with chronic ankle instability. BMC Musculoskelet Disord. 2008; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Association; 1988. [Google Scholar]
- 29. Delahunt E, Monaghan K, Caulfield B. Altered neuromuscular control and ankle joint kinematics during walking in subjects with functional instability of the ankle joint. Am J Sports Med. 2006; 34 12: 1970– 1976. [DOI] [PubMed] [Google Scholar]
- 30. Mynark RG, Koceja DM. Down training of the elderly soleus H reflex with the use of a spinally induced balance perturbation. J Appl Physiol (1985). 2002; 93 1: 127– 133. [DOI] [PubMed] [Google Scholar]
- 31. Granacher U, Muehlbauer T, Zahner L, Gollhofer A, Kressig RW. Comparison of traditional and recent approaches in the promotion of balance and strength in older adults. Sports Med. 2011; 41 5: 377– 400. [DOI] [PubMed] [Google Scholar]
- 32. Hoseini N, Koceja DM, Riley ZA. The effect of operant-conditioning balance training on the down-regulation of spinal H-reflexes in a spastic patient. Neurosci Lett. 2011; 504 2: 112– 114. [DOI] [PubMed] [Google Scholar]