Summary
Autoimmune uveitis is an intraocular inflammatory disorder in developed countries. Understanding the mechanisms underlying the development and modulation of immune reaction in uveitic eyes is critical for designing therapeutic interventions. Here we investigated the role of Notch signalling in regulatory T‐cell (Treg cell) function during experimental autoimmune uveitis (EAU). Using the Foxp3‐GFP reporter mouse strain, the significance of Notch signalling for the function of infiltrating Treg cells was characterized in an EAU model. We found that infiltrating Treg cells substantially expressed Notch‐1, Notch‐2, JAG1 and DLL1 in uveitic eyes. Activation of Notch signalling, represented by expression of HES1 and HES5, was enhanced in infiltrating Treg cells. Treatment with JAG1 and DLL1 down‐regulated Foxp3 expression and immunosuppressive activity of isolated infiltrating Treg cells in vitro, whereas neutralizing antibodies against JAG1 and DLL1 diminished Notch ligand‐mediated negative effects on Treg cells. To investigate the significance of Notch signalling for Treg cell function in vivo, lentivirus‐derived Notch short hairpin RNAs were transduced into in vitro expanded Treg cells before adoptive transfer of Treg cells into EAU mice. Transfer of Notch‐1‐deficient Treg cells remarkably reduced pro‐inflammatory cytokine production and inflammatory cell infiltration in uveitic eyes. Taken together, Notch signalling negatively modulates the immunosuppressive function of infiltrating Treg cells in mouse EAU.
Keywords: Delta‐like ligand 1, experimental autoimmune uveitis, Jagged 1, Notch, regulatory T cells
Abbreviations
- APC
allophycocyanin
- CFSE
carboxyfluorescein succinimidyl ester
- DLL1
Delta‐like ligand 1
- EAU
experimental autoimmune uveitis
- Foxp3
forkhead box p3
- GFP
green fluorescence protein
- IFN‐γ
interferon γ
- IL
interleukin
- JAG
Jagged 1
- PD‐1
programmed cell death protein 1
- PE
phycoerythrin
- shRNA
short hairpin RNA
- Teff cells
effector T cells
- TGF‐β
transforming growth factor β
- Th1
T helper type 1
- Treg cells
regulatory T cells
Introduction
Clinically, uveitis describes a group of intraocular inflammatory disorders which are classified anatomically as anterior, intermediate, posterior or panuveitis, depending on which anatomical structures of the eye are involved.1 All of these forms are characterized by an inflammatory cellular infiltrate. Uveitis may affect the iris, ciliary body, choroid, retina, retinal blood vessels, the vitreous and the optic nerve,2 and may be caused by infections and/or autoimmunity. Uveitis related to autoimmune disease is more common in developed countries, whereas overt infectious disease causes are more frequent in the developing world.3 Approximately 70–90% of sight‐threatening uveitis in developed countries is reported to be non‐infectious.4, 5
To recreate uveitis in animals, the most commonly used model is experimental autoimmune uveoretintis (EAU).6 In this model, autoreactive T cells activated in peripheral lymphoid organs cross the blood–retina barrier, and immune responses associated with intraocular inflammation and damage occur. Among these autoreactive T cells, CD4+ T cells exhibiting a T helper type 1 (Th1) phenotype are thought to play a critical role in the pathogenesis of EAU.7, 8 Another CD4+ effector lineage, Th17, characterized as secreting interleukin‐17 (IL‐17), has been described as contributing to the pathogenesis of uveitis.9 Importantly, CD4+ regulatory T cells, which are critical for immune homeostasis and inhibition of autoimmune diseases, also play a remarkable role in inhibiting EAU. Previous studies indicated that progressive increase of Foxp3+ regulatory T (Treg) cell to T effector (Teff) cell ratio in uveitic eyes correlated with resolution of EAU.10 Systemic depletion of Treg cells at peak disease delayed resolution of EAU, and their depletion after resolution triggered a relapse.10 Retinal antigen‐specific regulatory T cells protect against spontaneous and autoimmunity‐mediated uveitis induced by interphotoreceptor retinoid‐binding protein.11 Hence, increasing Treg cell number, as well as promoting immunosuppressive and anti‐inflammatory activity of Treg cells, might be beneficial for patients with uveitis.
Notch signalling is an evolutionarily conserved cell‐to‐cell communication cascade mediated by Notch ligand–receptor interactions between neighbouring cells.12 Mammals possess four receptors (Notch‐1 to Notch‐4) that are bound by five ligands of the Jagged family and Delta‐like family. Notch was initially shown to be a key determinant of cell‐lineage commitment in developing lymphocytes. Moreover, the roles of Notch in adaptive immunity have expanded to include the regulation of T‐cell differentiation and function.13 With regard to the role of Notch signalling in Treg cells, contradictory results are present in previous studies, including either positive or negative effects on Treg cell homeostasis and function.14, 15, 16 However, the exact role of Notch signalling in the modulation of uveitic Treg cells, especially infiltrating Treg cells in uveitic eyes, has not been specified.
In this study, we investigated the effect of Notch signalling on the immunosuppressive activity of infiltrating Treg cells in a murine experimental uveitis model. Using genetically engineered mouse strains, we found that infiltrating Treg cells expressed both Notch ligands and Notch receptors. The expression of Notch ligands, Jagged 1 (JAG1) and Delta‐like ligand 1 (DLL1), was stable on infiltrating Treg cells, whereas Notch‐1 and Notch‐2 were up‐regulated on infiltrating Treg cells. Notch ligands down‐regulated expression of forkhead box p3 (Foxp3), programmed cell death protein 1 (PD‐1) and immunosuppressive cytokines in Treg cells in vitro, so inhibiting the immunosuppressive activity of Treg cells. Furthermore, Notch‐1 was important for Treg cells to suppress EAU, as knockdown of Notch‐1 by lentiviral short hairpin (sh)RNA transduction increased Foxp3 and PD‐1 expression in infiltrating Treg cells. Notch‐1‐deficient Treg cells showed a higher potency of alleviating EAU severity. Our study revealed the significant role of Notch signalling in the modulation of uveitic Treg cells. This discovery might provide a clue for the development of new therapeutic strategies for treating autoimmune uveitis.
Materials and methods
Mice and EAU model
All animal experiments were conducted in compliance with institutional guidelines and Tongji University Guidelines for the Use of Animals. All animal procedures were approved by the Tongji University School of Medicine Animal Care and Use Committee. Foxp3‐green fluorescence protein (GFP) reporter mice (C57BL/6J background), C57BL/6J and C57BL/6.SJL mice were purchased from The Jackson Laboratory (Sacramento, CA, USA). Mice were raised under pathogen‐free conditions and were used at 6–8 weeks of age. EAU induction was conducted following the established method.10, 17, 18 Briefly, mice were immunized with an emulsion of complete Freund's adjuvant with 2 mg/ml desiccated Mycobacterium tuberculosis (Sigma‐Aldrich, St Louis, MO, USA) and 300 μg human interphotoreceptor retinoid‐binding protein (1–20). The mice received a subcutaneous injection of the emulsion (200 μl) into three sites on the lower back, followed by an intraperitoneal injection of 0·3 μg pertussis toxin. To check the inflammatory response, eyes were enucleated from mice, fixed in 10% buffered formalin, dehydrated through graded alcohols, embedded in paraffin, and serially transverse‐sectioned through the pupillary optic nerve plane. Tissue sections (5 μm) were deparaffinized in xylene, rehydrated through a graded alcohol series, and stained with haematoxylin & eosin.
Isolation of T cells from uveitic eyes
Isolation of T cells from uveitic eyes was performed according to a previous protocol with some modifications.19 In brief, the tissue around the eyeball was removed, and the eyeball was dissected to remove the cornea and lens. The remaining portion of the eye (including the iris, ciliary body, retina, choroids) was minced with scissors and shaken in medium supplemented with 0·5 mg/ml of collagenase type D (Roche R&D Center, Shanghai, China) at 37°C for 40 min. As a basic medium, we used RPMI‐1640 (Life Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (Life Technologies, Carlsbad, CA, USA), 100 U/ml penicillin, 100 μg/ml streptomycin, 5 × 10−5 m 2‐mercaptoethenol and 5 mg/ml HEPES buffer. The cell dispersion was passed through metal meshes (70‐μm window) and washed three times before sorting with flow cytometry.
Flow cytometry and cell sorting
The following anti‐mouse antibodies were used for detection and sorting of Treg cells: allophycocyanin (APC) anti‐CD3 (17A2), APC‐Cy7 anti‐CD4 (GK1.5), Peridinin chlorophyll protein anti‐CD8a (53‐6.7), phycoerythrin (PE) anti‐Notch‐2 (16F11), PE anti‐DLL1 (30B11.1), PE‐Cy5 anti‐CD45 (30‐F11), Alexa Fluor® 647 anti‐Foxp3 (R16‐715), PE anti‐CD45.1 (A20), PE‐Cy7 anti‐CD45.2 (104) and PE Annexin V were purchased from BD Pharmingen (San Diego, CA, USA). APC anti‐T‐cell receptor‐β (H57‐597), PE‐Cy7 anti‐CD154 (MR1), APC anti‐Helios (22F6), PE‐Cy5 anti‐CD25 (FC), PE anti‐Notch‐1 (HMN1‐12), PE anti‐Notch‐3 (HMN3‐133), and PE anti‐JAG1 (HMJ1‐29) were purchased from Biolegend (San Diego, CA, USA). eFluor450 anti‐CD137 (17B5) and eFluor450 anti‐PD‐1 (J43) were purchased from eBioscience (San Diego, CA, USA).
For staining, cells were incubated with the above antibodies (5 μg/ml each) in PBS for 30 min at 4°. Dead cells were excluded by staining with propidium iodide (5 μg/ml). For apoptosis assay, cells were stained with PE Annexin V following the manufacturer's instructions. For Foxp3 or Helios staining, a Foxp3 fix/perm buffer (Biolegend) set was used according to the manufacturer's instructions. Dead cells were excluded with the LIVE/DEAD fixable blue stain kit (Thermo Fisher Scientific, Waltham, MA, USA). Cells were analysed on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA, USA). Cell sorting was performed on a BD FACSAria™ III cell sorter (BD Biosciences).
RNA isolation, reverse transcription and real‐time PCR
Total RNA was extracted from cells or tissues using the RNeasy Mini Kit (Qiagen, Hilden, North Rhine‐Westphalia, Germany). Synthesis of cDNA was performed using SuperScript® III First‐Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Real‐time PCR was performed using SYBR® Green (Bio‐Rad, Hercules, CA, USA) on a QuantStudio 3 Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). Primer sequences for each gene are shown in the Supplementary material (Table S1). PCR conditions used for all primer sets were as follows: 95° hot start for 10 min, followed by 40 amplification cycles of 95° for 15 seconds, 60° for 1 min. Relative abundance of RNA was analysed using 2−ΔΔCt method.
Immunoblot assay
The following antibodies were used in Immunoblot: anti‐β‐actin, anti‐HES1, anti‐Notch‐1 and anti‐Notch‐2 were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti‐HES5 antibody was purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
In vitro treatment with Notch ligands
Immobilization of Notch ligands was conducted based on a previous study.20 Briefly, 96‐well culture plates (Corning Inc, Corning, NY, USA) were pre‐coated with 25 μg/ml human IgG antibody (Abcam, Cambridge, United Kingdom) and subsequently treated with blocking buffer (PBS plus BSA 1%) for 1 hr at room temperature. After removal of excess blocking buffer, each well was coated with 5μg/ml recombinant mouse JAG1‐Fc chimera protein (MyBioSource, San Diego, CA, USA) and 5 μg/ml recombinant mouse DLL1‐Fc chimera protein (R&D Systems, Minneapolis, MN, USA) for 2 hr at room temperature. CD4+ Foxp3(GFP)+ Treg cells were sorted and pooled from uveitic eyes, and suspended at 5 × 105 cells/ml in complete RPMI medium (RPMI‐1640 medium containing 10% fetal bovine serum, 100 IU/ml penicillin and 100 μg/ml streptomycin) with 50 U recombinant mouse IL‐2, and then were seeded in 96‐well plates pre‐coated with JAG1 and DLL1. 24 or 48 hr later, Treg cells underwent flow cytometry analysis or real‐time PCR. In some wells, before Treg cells were added, pre‐coated wells were pre‐treated with a mixture of 20 μg/ml neutralizing antibody against JAG1 (HMJ1‐29, Biolegend) and 20 μg/ml neutralizing antibody against DLL1 (HMD1‐5, eBioscience) for 1 hr at 37°.
Suppression assay
To detect the immunosuppressive effect of Treg cells on Teff cells, a 96‐well round‐bottom plate (Corning) was coated with 5 μg/ml anti‐CD3 antibody (17A2, eBioscience). CD4+ CD25− Teff cells were sorted from C57BL/6SJL mouse spleens. They were labelled with 5 μm CFSE (Molecular Probes, Eugene, OR, USA) following the manufacturer's manual; 1 × 105 CFSE‐labelled Teff cells were seeded into each well in the presence of 2 μg/ml anti‐CD28 antibody (37.51, eBioscience). One day later, GFP+ infiltrating Treg cells, which were pre‐treated with Notch ligands as described above, were added into each well and co‐cultured with Teff cells. The ratio between Treg and Teff was 1 : 2. Two days after addition of Treg cells, Teff cells were stained with APC‐Cy7 anti‐CD45.1 antibody (A20, Biolegend). CFSE dilution in Teff cells was analysed by flow cytometry.
In vitro Treg cell expansion
Six‐well plates were coated with 5 μg/ml anti‐CD3 antibody (17A2, eBioscience). 1·5 × 106 splenic CD4+ CD25hi Treg‐enriched cells (from C57BL/6SJL mice) or CD4+ GFP+ Treg cells (from Foxp3‐GFP reporter mice) were added into each well with 2 μg/ml anti‐CD28 antibody (37.51, eBioscience) and 100 U recombinant mouse IL‐2 (R&D Systems). Treg cells were re‐seeded into new pre‐coated cells every 5 days and incubated for a total of 10 days.
Lentivirus preparation and transduction
Notch‐1 siRNA/shRNA/RNAi Lentivector (designated L‐sh‐N‐1), Notch‐2 siRNA/shRNA/RNAi Lentivector (designated L‐sh‐N‐2), and Scrambled siRNA GFP Lentivector (designated L‐SC) were purchased from Applied Biological Materials Inc. (Richmond, BC, Canada). These lentivectors are based on the same plasmid backbone (piLenti‐siRNA‐GFP). L‐SC was modified by restriction endonuclease digestion with XmaI and KpnI to remove the GFP sequence. Then the vector was treated with Klenow Fragment to form blunt ends, followed by ligation with T4 DNA Ligase. Therefore, L‐SC vector lost GFP sequence whereas other sequences were not influenced. Restriction endonucleases, Klenow Fragment and T4 DNA Ligase were purchased from New England Biolabs (Ipswich, MA, USA) (NEB). All molecular cloning procedures were conducted following the NEB protocols.
For lentivirus packaging, 1·2 × 107 293T cells were cultured in a 10‐cm dish overnight until cells were 70% confluent. Lentivectors were mixed with 3rd Generation Packaging Mix (Applied Biological Materials Inc.) and transfection was performed according to the manufacturer's manual. The lentivirus‐containing supernatants were harvested 48 and 72 hr after transfection. The harvest was filtered through 0·45‐μm sterile filters (Millipore, Billerica, MA, USA). Lentivirus were purified with Lenti‐X Maxi Purification Kit (Takara Biomedical Technology, Beijing, China) following the manufacturer's manual. Viral titres were determined by Lenti‐X qRT‐PCR Titration Kit (Clontech) following the manufacturer's manual.
For transduction of Treg cells with lentivirus, Treg cells were expanded in vitro as described above. At day 8 after expansion, polybrene was added into the medium to 5 μg/ml. Then cells were transduced with lentivirus at a multiplicity of infection of 10 overnight. Cells were washed and expanded for a further 2 days. GFP expression and Notch expression were evaluated by flow cytometry and Immunoblot, respectively.
Adoptive transfer
EAU was induced in Fxop3‐GFP donor mice. Splenic CD4+ GFP+ Treg cells were sorted by flow cytometry at day 14 of EAU, expanded in vitro and transduced with L‐SC, L‐sh‐N1 or L‐sh‐N‐2, respectively, as described above. Then 2 × 106 Treg cells (in 200 μl PBS) were injected into the tail vein of C57BL/6J recipient mice in whom EAU had been induced 7 days earlier. Cytokines in uveitic eyes and histological changes were evaluated at 1 week after adoptive transfer.
Statistics
Most data were presented as mean ± SEM and analysed by statistical software (prism 6.0, graphpad software; La Jolla, CA, USA). Student's t‐test or one‐way analysis of variance were used for comparison of mean between the groups. P values < 0·05 were considered significant.
Results
The presence of infiltrating Treg cells in uveitic eyes
To characterize the infiltrating Treg cells, EAU was induced in Foxp3‐GFP reporter mice. Single‐cell suspensions were prepared from pooled eyes and were analysed by flow cytometry. As shown in Fig. 1(a), very few Treg cells were found in normal eyes (day 0), and the percentage of Foxp3+ Treg cells in infiltrating CD4+ T cells increased from day 14 to day 28 after immunization, in association with increased proportions of the infiltrating CD3+ T cells. The ratio of CD4+ versus CD8+ T cells was not significantly altered. Consistently, the absolute number of infiltrating Treg cells was also increased during the progression of EAU (Fig. 1b). As shown in Fig. 1(c–f), infiltrating Treg cells substantially expressed immunoregulatory cytokines such as IL‐10, transforming growth factor β (TGF‐β) and IL‐35 (IL‐12a with IL‐27b), suggesting that they were functionally immunosuppressive. However, expression of these cytokines progressively decreased over time, probably due to resolution of inflammation or immune reaction. Notably, expression of Foxp3 was also moderately reduced at day 21 and day 28 after EAU (Fig. 1g).
Figure 1.
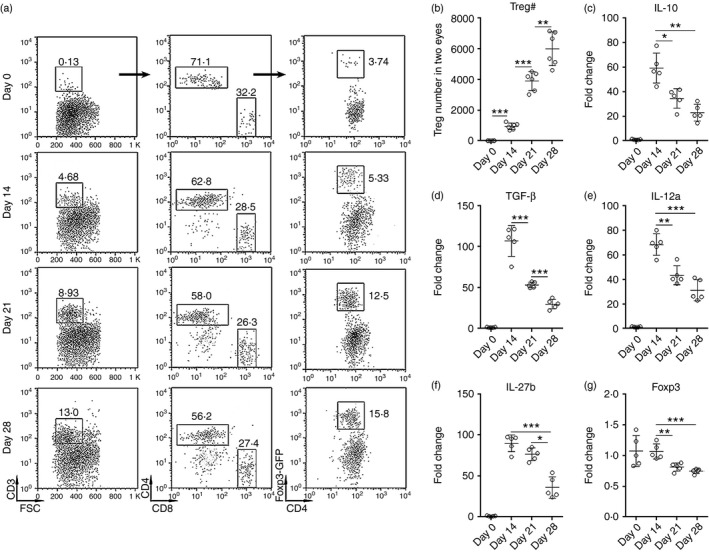
The presence of regulatory T (Treg) cells in uveitic eyes. (a) Representative dot plots of Treg cells in isolated eye cells. CD3+ T cells were gated in the whole isolated single cells from eyes of Foxp3‐GFP mice at day 0, day 14, day 21 and day 28 after immunization with human interphotoreceptor retinoid‐binding protein (IRBP). Then CD4+ and CD8+ subpopulations were gated in CD3+ T cells. GFP + Treg cells were further gated in CD4+ T cells. Numbers in the plots are percentages of gated populations in their mother populations. (b) Infiltrating Treg cell numbers in two eyes at different time‐points of experimental autoimmune uveitis (EAU). (c–g) mRNA levels of indicated cytokines and Foxp3 in Treg cells sorted from eyes. *P < 0·05; **P < 0·01; ***P < 0·001. n = 5 or n = 6 per group. Each circle represents a mouse.
Infiltrating Treg cells express both Notch receptors and ligands
To determine the expression of Notch receptors, infiltrating Treg cells were stained with antibodies against Notch‐1 to Notch‐3, respectively. Notch expression at day 0 was not shown because the Treg number was too low at this time‐point. As shown in Fig. 2, Notch‐1 was expressed on Treg cells at day 14 and its expression was even higher at days 21 and 28. Notch‐2 was also expressed on Treg cells, but its expression was only moderately elevated at day 28. Notch‐3 staining was quite low and unchanged on Treg cells at different time‐points. We also found that infiltrating Treg cells expressed Notch ligands – JAG1 and DLL1 in uveitic eyes, and their expression levels were stable during the whole EAU process (Fig. 3). Therefore, infiltrating Treg cells constitutively express JAG1 and DLL1.
Figure 2.
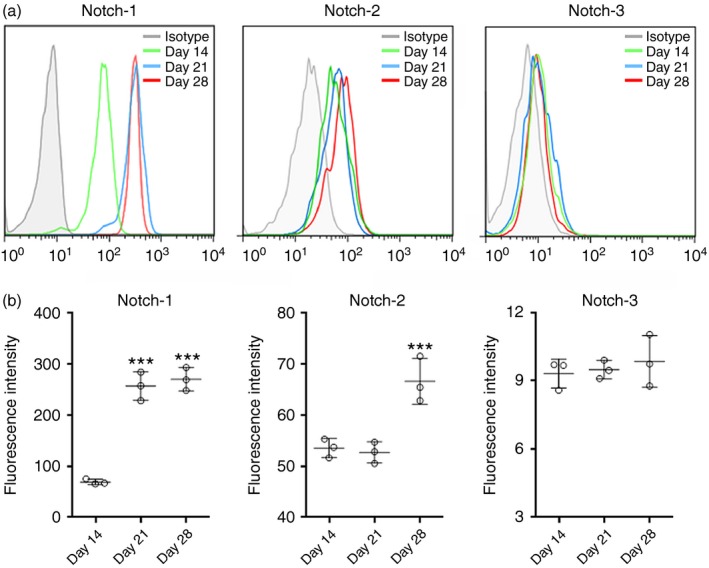
Expression of Notch receptors on infiltrating regulatory T (Treg) cells in experimental autoimmune uveitis (EAU). (a) Representative histograms of Notch‐1, Notch‐2 and Notch‐3 on GFP + Treg cells in uveitic eyes. (b) Statistical analysis for the fluorescent intensity of Notch‐1, Notch‐2 and Notch‐3 on GFP + Treg cells in uveitic eyes. Isotype, isotype antibody control. ***P < 0·001. n = 3 per group. Each circle represents a mouse.
Figure 3.
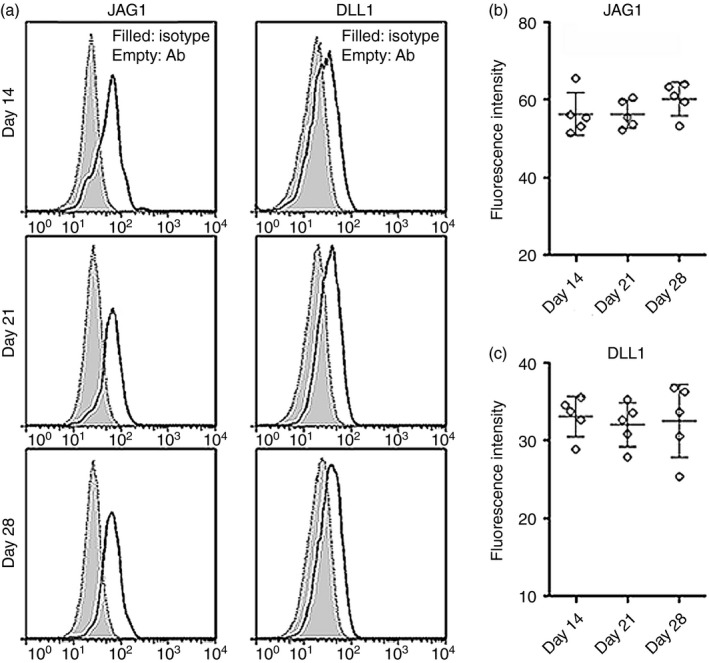
Expression of Jagged 1 (JAG1) and Delta‐like ligand 1 (DLL1) on infiltrating regulatory T (Treg) cells in experimental autoimmune uveitis (EAU). (a) Representative histograms of JAG1 and DLL1 on GFP + Treg cells in uveitic eyes. (b, c) Statistical analysis for the fluorescent intensity of JAG1 and DLL1 on GFP + Treg cells. Isotype, isotype antibody control. Ab, antibody against JAG1 or DLL1. n = 5 per group. Each circle represents a mouse.
Notch signalling down‐regulates Foxp3 expression in Treg cells in vitro
The expression of Notch receptors on infiltrating Treg cells suggests the possible role of Notch signalling in the modulation of Treg cell function in uveitic eyes. To test activation of Notch signalling, we first determined the expression of Notch signalling target genes in infiltrating Treg cells. HES1 and HES5 levels were significantly up‐regulated in infiltrating Treg cells at day 21 and day 28, in comparison with day 14 (Fig. 4a), suggesting that the Notch signal pathway was activated in infiltrating Treg cells. To further analyse the Notch signalling profile in antigen‐reactive Treg cells and naive Treg cells in uveitic eyes, we stained infiltrating Treg cells with antibodies against CD137, CD154 and Helios, according to a previous study distinguishing Treg subpopulations with these markers.21 As shown in Fig. 4(b,c), total infiltrating Treg cells were divided into three subpopulations: CD137+ CD154−, CD137+ CD154+ and CD137− CD154− cells. Helios expression profiles suggested that CD137+ CD154− and CD137− CD154− cells were natural Treg (nTreg) cells, whereas CD137+ CD154+ were predominantly induced Treg (iTreg) cells. As CD137 expression defines antigen‐reactive Treg cells,21 we considered that CD137+ CD154− cells were antigen‐reactive nTreg cells, CD137+ CD154+ cells were antigen‐reactive iTreg cells, and CD137− CD154− cells were naive nTreg cells. HES1 expression was comparable in CD137+ CD154− and CD137− CD154− cells, but was lower in CD137+ CD154+ cells (Fig. 4d). Therefore, in our settings, Notch signalling was equally activated in infiltrating nTreg cells regardless of antigen specificity. Furthermore, Notch signalling was weaker in iTreg cells than in nTreg cells. The differential Notch signalling prompted us to assess the expression of Notch receptors on these Treg subpopulations. As shown in Fig. 4(e), Notch‐1 expression was comparable on CD137+ CD154− and CD137− CD154− nTreg cells, whereas CD137+ CD154+ iTreg cells expressed lower Notch‐1. The similar expression pattern was also observed with regard to Notch‐2. To identify the effect of Notch signalling on infiltrating Treg cells, we treated Treg cells with Notch ligands in the presence or absence of neutralizing antibodies against Notch ligands. Notch ligands up‐regulated HES1 and HES5 levels, whereas the neutralizing antibodies diminished the up‐regulation of HES1 and HES5 (Fig. 4f). Moreover, Foxp3 expression was remarkably decreased in Treg cells treated with Notch ligands, in comparison with Treg cells cultured alone, and the neutralizing antibodies effectively sustained Foxp3 level (Fig. 4g,h). Therefore, Notch signalling negatively regulates Foxp3 expression. The role of Notch signalling in modulating Treg cell apoptosis was also examined. We found that Notch ligands increased Treg cell apoptosis, and the neutralizing antibodies counteracted the pro‐apoptotic effect of Notch ligands (Fig. 4i).
Figure 4.
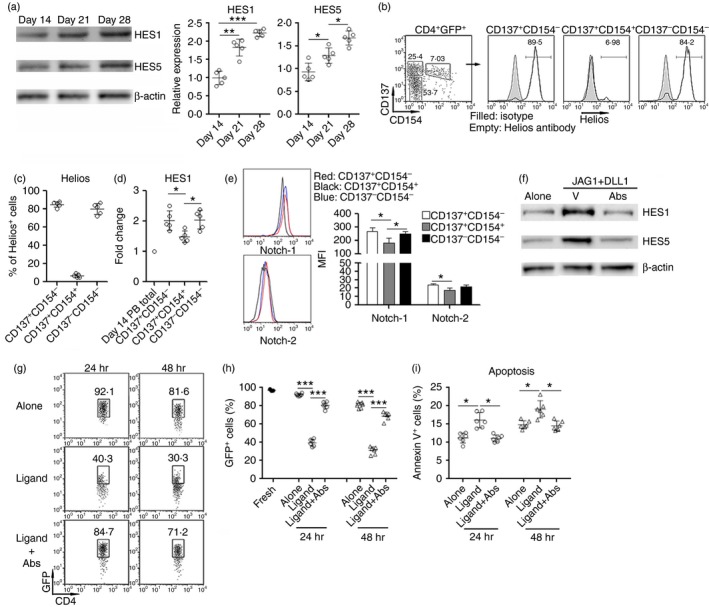
Notch signalling down‐regulates Foxp3 in infiltrating regulatory T (Treg) cells. (a) HES1 and HES5 expression in infiltrating Treg cells. Left panel: representative Immunoblot image. Right panel: statistical analysis for the expression of HES1 and HES5. (b, c) Staining of CD137, CD154 and Helios in infiltrating Treg cells. CD4+ GFP + Treg cells were sorted from uveitic eyes 21 days after experimental autoimmune uveitis (EAU) induction. Then expression of CD137, CD154 and Helios were evaluated in Treg cells. Representative dot plot and histograms are presented in (b), and percentages of Helios+ cells in Treg subpopulations are shown in (c). Numbers in (b) are percentages of gated populations. (d) Expression of HES1 in Treg subpopulations was quantified by real‐time PCR. Note that peripheral blood total Treg cells at day 14 were used as control. Day 14 PB total, peripheral blood total Treg cells at day 14. (e) Expression of Notch‐1 and Notch‐2 on Treg subpopulations. Left panel: representative histograms. Right panel: statistics for mean fluorescence intensity of Notch‐1 and Notch‐2. n = 3 per group. (f) The blocking effect of neutralizing antibodies against JAG1 and DLL1 on Notch signalling in Treg cells. Splenic Treg cells were cultured in a microplate pre‐coated with JAG1 and DLL1 in the presence or absence of mixed neutralizing antibodies against JAG1 and DLL1 for 12 hr. HES1 and HES5 were detected by Immunoblot. This is a representative image of two independent experiments. V, vehicle. Abs, mixed neutralizing antibodies. (g, h) Foxp3 expression in infiltrating Treg cells after in vitro culture for 24 and 48 hr. (g) Representative dot plots. The number in each plot is the percentage of GFP + cells. (h) Statistical analysis for the proportion of GFP + cells. (i) Apoptosis of Treg cells after culture. Fresh, freshly sorted Treg cells. Alone, Treg cells alone. Ligand, Treg cells cultured with immobilized JAG1 and DLL1. Ligand+Abs, Treg cells cultured with immobilized JAG1 and DLL1 in the presence of neutralizing antibodies against JAG1 and DLL1. *P < 0·05; **P < 0·01; ***P < 0·001. Each circle represents a mouse.
Notch signalling inhibits immunosuppressive function of infiltrating Treg cells
To examine the impact of Notch signalling on Treg cell function, expression of immunosuppressive molecules in Notch ligand‐treated Treg cells was detected by real‐time PCR. As shown in Fig. 5(a–e), mRNA levels of TGF‐β, IL‐12a, IL‐27b and PD‐1 were markedly lower after treatment with Notch ligands, whereas the neutralizing antibodies restored expression of these molecules. Notably, PD‐1 has been recently shown to be critical for the immunoregulatory activity of Treg cells in EAU.17 Interestingly, Notch ligands induced higher interferon‐γ (IFN‐γ) expression in Treg cells, suggesting that Notch signalling might influence the plasticity of Treg cells (Fig. 5f). Expression of IL‐4 and IL‐17a was not altered by Notch ligands (Fig. 5g,h). In the Teff cell proliferation assay, compared with untreated Treg cells, Notch ligand‐treated Treg cells were less potent to inhibit Teff cell proliferation (Fig. 6a,b). Moreover, Notch ligand‐treated Treg cells were less competent to suppress IFN‐γ and tumour necrosis factor‐α production in Teff cells (Fig. 6c,d). Taken together, our data suggest that Notch signalling inhibits Treg cell function.
Figure 5.
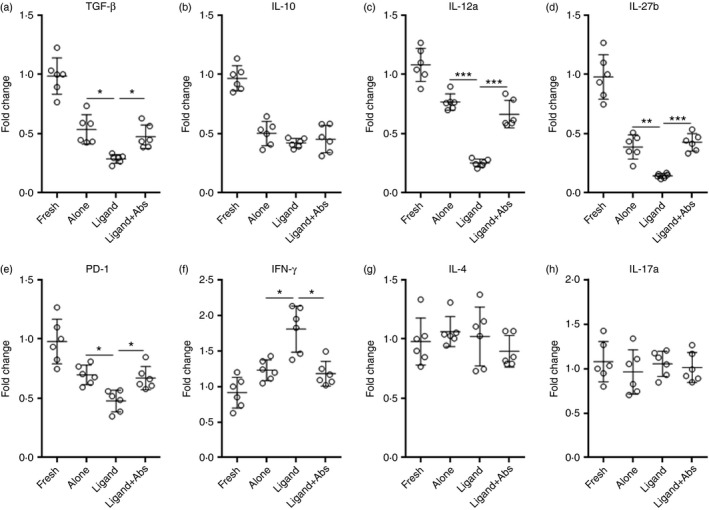
Notch signalling inhibits expression of immunoregulatory molecules in infiltrating regulatory T (Treg) cells. Infiltrating Treg cells were cultured for 24 hr the same way as described in Fig. 4(c). (a–e) Expression of indicated cytokines and programmed cell death protein 1 (PD‐1) in infiltrating Treg cells after culture was determined by real‐time PCR. (f–h) Expression of interferon‐γ (IFN‐γ), interleukin‐4 (IL‐4) and IL‐17a in cultured Treg cells. Fresh, freshly sorted Treg cells. Alone, Treg cells alone. Ligand, Treg cells cultured with immobilized Jagged‐1 (JAG1) and Delta‐like ligand 1 (DLL1). Ligand+Abs, Treg cells cultured with immobilized JAG1 and DLL1 in the presence of neutralizing antibodies against JAG1 and DLL1. *P < 0·05; **P < 0·01; ***P < 0·001.
Figure 6.
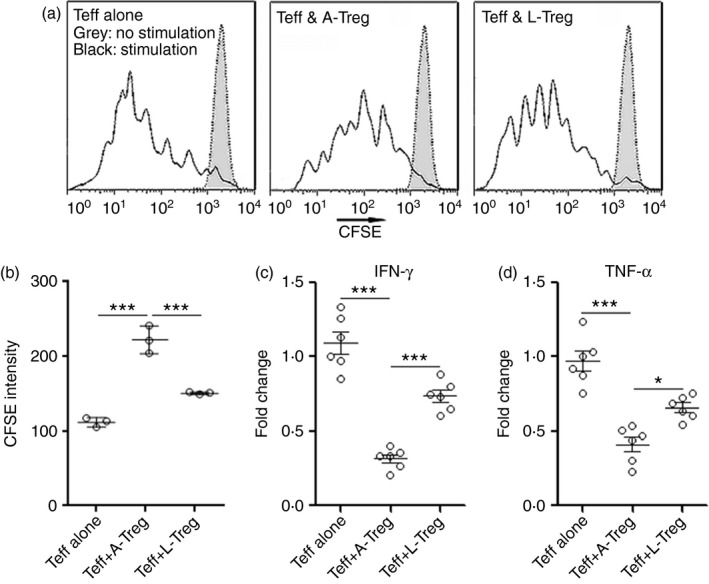
Notch signalling inhibits regulatory T (Treg) cell‐mediated suppression of effector T (Teff) cells. (a, b) Teff cell proliferation assay. Teff cells (CD4+ CD25−) were sorted from C57BL/6.SJL mouse spleens. They were labelled with CFSE before stimulation with agonistic antibodies. One day later, in vitro cultured infiltrating Treg cells were added into Teff cell culture and incubated for a further 2 days before CFSE dilution was evaluated in CD45.1+ Teff cells. Representative CFSE histograms were shown in (a). Statistical analysis for CFSE intensity was shown in (b). (c, d) Expression of cytotoxic mediators in Teff cells was determined by real‐time PCR. A‐Treg, Treg cells previously cultured alone. L‐Treg, Treg cells previously pre‐treated with Jagged 1 (JAG1) and Delta‐like ligand 1 (DLL1). *P < 0·05; ***P < 0·001. n = 3 or n = 6 per group. Each circle represents a mouse.
Notch‐1 is crucial for the inhibition of Treg cell function in EAU
To determine which Notch receptor is critical for modulating Treg cells in EAU, we isolated splenic CD4+ CD25hi T cells from C57BL/6SJL mice at day 14 after EAU induction. These Treg cells were expanded in vitro for 10 days with agonistic CD3 and CD28 antibodies and IL‐2. Two days before the end of expansion, these cells were transduced with lentivirus expressing shRNAs for Notch‐1 or Notch‐2, respectively. Lentiviral transduction notably down‐regulated Notch‐1 and Notch‐2 expression (Fig. 7a), but did not remarkably influence expression of PD‐1 or Foxp3 (see Supplementary material, Figs S1 and S2). It is possible that both CD3 and IL‐2 signalling compensated for the reduced Notch signalling and maintained Foxp3 and PD‐1 expression in vitro. Treg cells transduced with L‐sh‐N‐1 (GFP+) were mixed with Treg cells transduced with L‐SC (GFP−) at 1 : 1. Similarly, Treg cells transduced with L‐sh‐N‐2 (GFP+) were mixed with Treg cells transduced with L‐SC (GFP−) at 1 : 1. Then mixed Treg cells were adoptively transferred into recipient C57BL/6J mice in whom EAU was induced 7 days ago (Fig. 7b). One week after adoptive transfer, donor‐derived infiltrating CD45.1+ cells were detected in the cells isolated from uveitic eyes of the recipient mice, and the proportions of GFP+ and GFP− cells in infiltrating CD45.1+ cells were calculated. We found that the average proportion of either GFP+ or GFP− cells was almost 50% (Fig. 7c and see Supplementary material, Fig. S3), suggesting that the deficiency of Notch‐1 or Notch‐2 did not impair Treg cell transmigration into uveitic eyes. Further investigation of expression of Foxp3 and PD‐1 revealed that Notch‐1‐deficient Treg cells expressed higher Foxp3 and PD‐1, in comparison with L‐SC‐transduced Treg cells, whereas Notch‐2‐deficient Treg cells expressed similar Foxp3 and PD‐1 levels to L‐SC‐transduced Treg cells (Fig. 7d). Moreover, Notch‐1‐deficient Treg cells expressed higher TGF‐β, IL‐12a and IL‐27b than L‐SC‐transduced Treg cells, whereas Notch‐2‐deficient Treg cells expressed comparable levels of these molecules to L‐SC‐transduced Treg cells (Fig. 7e). Notch‐1 deficiency also inhibited IFN‐γ expression in infiltrating Treg cells. Therefore, Notch‐1 is more important for Treg cell function in EAU.
Figure 7.
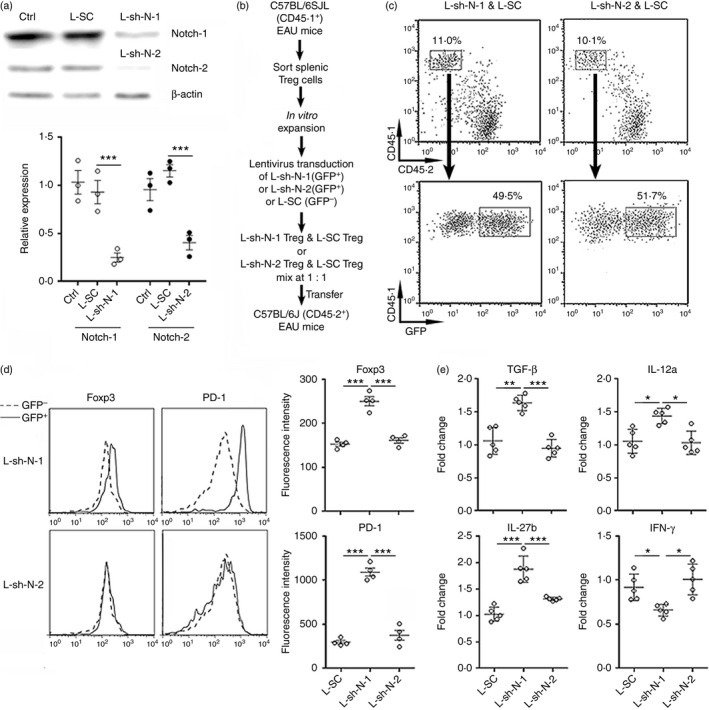
Notch‐1 is crucial for regulatory T (Treg) cell function in experimental autoimmune uveitis (EAU). (a) Knockdown of Notch‐1 or Notch‐2 in Treg cells. Upper panel: representative Immunoblot image. Lower panel: statistical analysis for Notch‐1 and Notch‐2. (b) Experimental design for (c–e). (c) Representative dot plots of donor‐derived Treg cells in uveitic eyes. (d) Expression of Foxp3 and programmed cell death protein 1 (PD‐1) in donor‐derived Treg cells in uveitic eyes. Left panel: representative histograms. Right panel: statistical analysis. (e) mRNA levels of transforming growth factor‐β (TGF‐β), interleukin‐12a (IL‐12a), IL‐27b and interferon‐γ (IFN‐γ) in donor‐derived Treg cells in uveitic eyes. L‐SC, Treg cells transduced with L‐SC. L‐sh‐N‐1, Treg cells transduced with L‐sh‐N‐1. L‐sh‐N‐2, Treg cells transduced with L‐sh‐N‐2. *P < 0·05; **P < 0·01; ***P < 0·001. n = 3 or n = 5 per group. Each circle represents a mouse.
Notch‐1 deficiency in Treg cells contributes to suppression of EAU
To investigate the impact of Treg‐cell‐specific Notch‐1 deficiency on EAU, splenic Treg cells were sorted from Foxp3‐GFP donor mice at day 14 after EAU induction. These Treg cells were in vitro expanded and transduced with L‐SC or L‐sh‐N‐1 as described in the Materials and methods. Lentivirus‐transduced Treg cells were adoptively transferred into C57BL/6J mice at day 7 of EAU. The expression of pro‐inflammatory cytokines in uveitic eyes such as tumour necrosis factor‐α, IFN‐γ, IL‐17a and IL‐2, which reflects the extent of immune reaction, was determined 7 days after transfer. As shown in Fig. 8(a–d), Transfer of L‐SC‐transduced Treg cells reduced all tested cytokine levels in comparison with mice receiving vehicle only, suggesting that normal Treg cells inhibited immune reaction in uveitic eyes. Transfer of Notch‐1‐deficient Treg cells more robustly inhibited production of these cytokines, suggesting that Notch‐1 weakened Treg cell‐mediated immunosuppression in EAU. Haematoxylin & eosin staining revealed remarkable inflammatory cell infiltration in the vitreous, photoreceptor cell damage and retinal folds in uveitic eyes (Fig. 8e). Adoptive transfer of L‐SC‐transduced Treg cells alleviated uveitis, demonstrated by modest tissue damage and less inflammatory infiltrate. Moreover, transfer of Notch‐1‐deficient Treg cells further diminished tissue damage and infiltrating inflammatory cells (Fig. 8e), suggesting that lack of Notch‐1 promoted Treg cell‐mediated suppression of EAU. Hence, inhibition of Notch‐1 is important for Treg‐cell‐mediated suppression of EAU.
Figure 8.
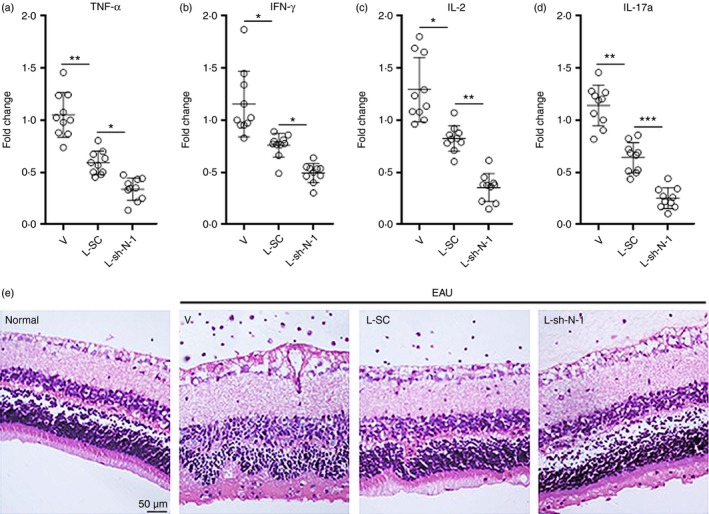
Notch‐1‐deficiency in regulatory T (Treg) cells contributes to suppression of experimental autoimmune uveitis (EAU). (a–d) Expression of immune reactive cytokines in eyes. Splenic Treg cells were sorted from Foxp3‐GFP donor mice at day 14 after EAU induction. These Treg cells were expanded and transduced with L‐SC or L‐sh‐N‐1 as described above. Then they were transferred into C57BL/6J recipient mice at day 7 of EAU. After 1 week, indicated cytokines in the whole eye were detected by real‐time PCR. *P < 0·05; **P < 0·01; ***P < 0·001. n = 8 or n = 10 per group. Each circle represents a mouse. (e) Haematoxylin & eosin staining at day 7 after adoptive transfer (Day 14 after EAU induction). Normal, normal eyes. V, mice receiving PBS. L‐SC, mice receiving L‐SC‐transduced Treg cells. L‐sh‐N‐1, mice receiving L‐sh‐N‐1‐transduced Treg cells.
Discussion
Regulatory T cells comprise a subset of CD4+ lymphocytes that suppresses activation, proliferation and effector responses of both innate and adaptive immune cells.22 They suppress exuberant immune system activation, promote immunological tolerance, and restrain excessive inflammatory response. The role of Treg cells in uveitis, especially in EAU, has been revealed by recent studies. In human uveitis patients, a decrease in Treg cells is associated with active uveitis, but the percentage of Treg cells increases with clinical remission.23 In EAU, progressive increase of Foxp3+ Treg to Teff cell ratio in uveitic eyes correlated with resolution of disease. Importantly, systemic depletion of Treg cells at peak disease delayed resolution of EAU, and their depletion after resolution triggered a relapse.10 One study indicated that in post‐EAU spleen, inducible Treg cells are generated and suppress EAU when transferred to recipient mice.17 Therefore, modulation of Treg cell amount and function might be a promising approach for treating uveitis. In our study, we confirmed the accumulation of Treg cells in uveitic eyes, which is consistent with a recent report.10 These infiltrating Treg cells expressed immunosuppressive cytokines such as IL‐10, TGF‐β and IL‐35.
In the past decades, the role of Notch signalling in modulating Treg cell function has been explored. The Notch pathway is a highly conserved cell‐signalling system that regulates multiple aspects of cell biology.12 Notch‐1 signalling cooperates with TGF‐β signalling to regulate Foxp3 expression and Treg cell maintenance both in vitro and in vivo.14 A former study showed blockade of Notch‐1 signalling with an anti‐JAG1 or a blocking anti‐Notch‐1 antibody inhibits Treg suppressor function in vitro.24 However, a recent study specifically interrupting Notch signalling in Treg cells revealed that Notch signalling is a negative regulator for Treg cell polarization and function.16 Lineage‐specific deletion of components of the Notch pathway enhanced Treg cell‐mediated suppression of Th1 cell responses and protected against their Th1 skewing and apoptosis. In contrast, expression in Treg cells of a gain‐of‐function transgene encoding the Notch‐1 intracellular domain resulted in lymphoproliferation, exacerbated Th1 responses and autoimmunity.16 This study further found that cell‐intrinsic canonical Notch signalling impaired Treg cell fitness and promoted the acquisition by Treg cells of a Th1 cell‐like phenotype, whereas non‐canonical Notch signalling dependent on the adaptor Rictor activated the kinase AKT–transcription factor Foxo1 axis and impaired the epigenetic stability of Foxp3.16 However, other independent research concluded that inactivation of Notch signalling has no significant impact on Treg cells and Foxp3 mRNA expression in patients with immune thrombocytopenia.15 Hence, it is likely that the role of Notch signalling in Treg cells depends on distinct experimental systems and diseases. In our study, we found that infiltrating Treg cells expressed Notch‐1 and Notch‐2 in uveitic eyes, whereas Notch‐3 expression was relatively low. Interestingly, Notch‐1 expression on Treg cells was up‐regulated at day 21 and day 28. Considering that EAU inflammation in C57BL/6J mice generally resolves at the third week after induction, it is possible that up‐regulation of Notch‐1 reflects down‐regulation of Treg cell activation in a relatively homeostatic environment. Furthermore, it has been shown that several cytokines increase expression of Notch‐1 and Notch‐2 through nuclear factor‐κB and mitogen‐activated protein kinase pathways in some cell types such as nucleus pulposus cells and macrophages.25, 26 Hence, it is also possible that Notch‐1 expression was enhanced by inflammatory cytokines in uveitic eyes. It would be necessary to test whether inflammatory cytokines up‐regulate Notch‐1 expression in future research.
We observed relatively stable expression of Notch ligands JAG1 and DLL1 on infiltrating Treg cells, posing the possibility that adjacent Treg cells might modulate one another through engagement of Notch ligands and receptors. Our finding is consistent with a former study showing distribution of Notch receptors and ligands on peripheral Treg cells.27 In addition, there might be other sources of Notch ligands in eyes. For example, Notch ligands are expressed in retina, optic vesicle, ciliary body and lens, with patterns that changed over time.28 In the developing retina, DLL1 is expressed primarily in progenitor cells, DLL4 is expressed in newly differentiated neurons.29 However, which cell type expresses Notch ligands in mature eyes remains elusive. Expression of Notch ligands such as DLL1, DLL4 and JAG have been reported in mature Müller glia.30 Retinal astrocytes could be another source of Notch ligands. Previous studies indicated expression of JAG and DLL on reactive brain astrocytes, and astrocytes can induce Notch signalling by themselves or with other cell types such as neural stem cells.31, 32, 33, 34 Moreover, a recent study demonstrated that optic nerve astrocytes express JAG1 with no significant expression of DLL.35 Since retinal astrocytes are immigrants from the optic nerve,36 it is possible that they also express Notch ligands. Our future study will check the types and expression levels of Notch ligands on distinctive eye cells. It would also be valuable to determine whether inflammatory insults change the expression pattern of both Notch receptors and ligands in eyes.
Notch signalling is important for Treg cell development and function. Here we identified the negative role of Notch signalling in Foxp3 expression in infiltrating Treg cells. Furthermore, activation of Notch signalling is associated with lower expression of immunosuppressive cytokines and PD‐1 in Treg cells, as well as weaker immunosuppressive activity of Treg cells on Teff cells. However, the molecular mechanisms underlying the effect of Notch signalling is still unclear. In future it will be helpful to determine if the same mechanisms stated in the previous research16 are also responsible for the alterations observed in our model. Notch signalling might independently or cooperatively alter Treg cell function, depending on whether other cell signal pathways interact with Notch pathways. It is also likely that engagement of different Notch ligands with receptors exert differential effects. Additionally, our ongoing study is testing the effect of Notch signalling on other uveitic immune cells including T‐cell subsets, because the both Notch ligands and receptors have been reported in distinctive immune cell populations such as dendritic cells, Teff cells and macrophages.37, 38, 39 It is possible that Notch signalling favours the differentiation of Th1 and Th17 cells through inhibition of Foxp3 expression in infiltrating Teff cells in the acute phase of uveitis.
Furthermore, we used lentivirus‐induced gene silencing to demonstrate the critical role of Notch‐1 signalling for Treg cell activity to inhibit EAU. Infiltrating Notch‐1‐deficient Treg cells showed higher expression of Foxp3, PD‐1 and immunosuppressive cytokines, which was consistent with our in vitro results. PD‐1 has been proved to be necessary for the suppressive activity of inducible Treg cells in EAU.17 Interestingly, Notch‐2 knockdown had very limited influence on Treg cell function. This might be explained by relatively low expression of Notch‐2 on infiltrating Treg cells, or the compensation for Notch‐2 signalling by Notch‐1. In general, our data indicated that Notch‐1 was more important than Notch‐2 in inhibiting infiltrating Treg cell function. It will be interesting to test whether constitutive Notch‐1 activation in genetically modified Treg cells would profoundly promote EAU.
Author contribution
Hua Rong and Hongjie Shen carried out most experiments and analysed data. Yueli Xu conducted lentivirus preparation and transduction. Hai Yang designed the study and prepared the manuscript.
Disclosures
The authors have no financial or commercial conflicts of interest.
Supporting information
Figure S1. Sorting of CD4+ CD25hi regulatory T (Treg) cell‐enriched cells from spleen. The purity of Treg cells in sorted cells was determined by Foxp3 staining. CD4+ CD25− T cells did not express Foxp3.
Figure S2. (a) GFP expression in regulatory T (Treg) cells after lentiviral transduction with L‐SC, L‐sh‐N1 and L‐sh‐N‐2. (b) Expression of PD‐1 and Foxp3 in Treg cells after lentiviral transduction with L‐SC, L‐sh‐N1 and L‐sh‐N‐2.
Figure S3. Statistics of the proportions of transferred regulatory T (Treg) cells in uveitic eyes.
Table S1. Quantitative RT‐PCR primer sequences.
Acknowledgements
This study was supported by a grant from the Science and Technology Projects of Pudong New Area of Shanghai Health Bureau (PW2013B‐3).
References
- 1. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 2005; 140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guly CM, Forrester JV. Investigation and management of uveitis. BMJ 2010; 341:c4976. [DOI] [PubMed] [Google Scholar]
- 3. Lee RW, Nicholson LB, Sen HN, Chan CC, Wei L, Nussenblatt RB et al Autoimmune and autoinflammatory mechanisms in uveitis. Semin Immunopathol 2014; 36:581–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grange LK, Kouchouk A, Dalal MD, Vitale S, Nussenblatt RB, Chan CC et al Neoplastic masquerade syndromes in patients with uveitis. Am J Ophthalmol 2014; 157:526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, Papo T et al Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore) 2001; 80:263–70. [DOI] [PubMed] [Google Scholar]
- 6. Forrester JV, Klaska IP, Yu T, Kuffova L. Uveitis in mouse and man. Int Rev Immunol 2013; 32:76–96. [DOI] [PubMed] [Google Scholar]
- 7. Atalla L, Linker‐Israeli M, Steinman L, Rao NA. Inhibition of autoimmune uveitis by anti‐CD4 antibody. Invest Ophthalmol Vis Sci 1990; 31:1264–70. [PubMed] [Google Scholar]
- 8. Xu H, Rizzo LV, Silver PB, Caspi RR. Uveitogenicity is associated with a Th1‐like lymphokine profile: cytokine‐dependent modulation of early and committed effector T cells in experimental autoimmune uveitis. Cell Immunol 1997; 178:69–78. [DOI] [PubMed] [Google Scholar]
- 9. Amadi‐Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB et al TH17 cells contribute to uveitis and scleritis and are expanded by IL‐2 and inhibited by IL‐27/STAT1. Nat Med 2007; 13:711–8. [DOI] [PubMed] [Google Scholar]
- 10. Silver PB, Horai R, Chen J, Jittayasothorn Y, Chan CC, Villasmil R et al Retina‐specific T regulatory cells bring about resolution and maintain remission of autoimmune uveitis. J Immunol 2015; 194:3011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McPherson SW, Heuss ND, Pierson MJ, Gregerson DS. Retinal antigen‐specific regulatory T cells protect against spontaneous and induced autoimmunity and require local dendritic cells. J Neuroinflammation 2014; 11:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guruharsha KG, Kankel MW, Artavanis‐Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet 2012; 13:654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Radtke F, MacDonald HR, Tacchini‐Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol 2013; 13:427–37. [DOI] [PubMed] [Google Scholar]
- 14. Samon JB, Champhekar A, Minter LM, Telfer JC, Miele L, Fauq A et al Notch1 and TGFβ1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood 2008; 112:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu S, Liu C, Li L, Tian T, Wang M, Hu Y et al Inactivation of Notch signaling reverses the Th17/Treg imbalance in cells from patients with immune thrombocytopenia. Lab Invest 2015; 95:157–67. [DOI] [PubMed] [Google Scholar]
- 16. Charbonnier LM, Wang S, Georgiev P, Sefik E, Chatila TA. Control of peripheral tolerance by regulatory T cell‐intrinsic Notch signaling. Nat Immunol 2015; 16:1162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee DJ, Taylor AW. Recovery from experimental autoimmune uveitis promotes induction of antiuveitic inducible Tregs. J Leukoc Biol 2015; 97:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang L, Bell BA, Yu M, Chan CC, Peachey NS, Fung J et al Complement anaphylatoxin receptors C3aR and C5aR are required in the pathogenesis of experimental autoimmune uveitis. J Leukoc Biol 2016; 99:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sonoda KH, Sasa Y, Qiao H, Tsutsumi C, Hisatomi T, Komiyama S et al Immunoregulatory role of ocular macrophages: the macrophages produce RANTES to suppress experimental autoimmune uveitis. J Immunol 2003; 171:2652–9. [DOI] [PubMed] [Google Scholar]
- 20. Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F et al Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci USA 2008; 105:15529–34. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Schoenbrunn A, Frentsch M, Kohler S, Keye J, Dooms H, Moewes B et al A converse 4‐1BB and CD40 ligand expression pattern delineates activated regulatory T cells (Treg) and conventional T cells enabling direct isolation of alloantigen‐reactive natural Foxp3+ Treg. J Immunol 2012; 189:5985–94. [DOI] [PubMed] [Google Scholar]
- 22. Singer BD, King LS, D'Alessio FR. Regulatory T cells as immunotherapy. Front Immunol 2014; 5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruggieri S, Frassanito MA, Dammacco R, Guerriero S. Treg lymphocytes in autoimmune uveitis. Ocul Immunol Inflamm 2012; 20:255–61. [DOI] [PubMed] [Google Scholar]
- 24. Asano N, Watanabe T, Kitani A, Fuss IJ, Strober W. Notch1 signaling and regulatory T cell function. J Immunol 2008; 180:2796–804. [DOI] [PubMed] [Google Scholar]
- 25. Wang H, Tian Y, Wang J, Phillips KL, Binch AL, Dunn S et al Inflammatory cytokines induce NOTCH signaling in nucleus pulposus cells: implications in intervertebral disc degeneration. J Biol Chem 2013; 288:16761–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monsalve E, Perez MA, Rubio A, Ruiz‐Hidalgo MJ, Baladron V, Garcia‐Ramirez JJ et al Notch‐1 up‐regulation and signaling following macrophage activation modulates gene expression patterns known to affect antigen‐presenting capacity and cytotoxic activity. J Immunol 2006; 176:5362–73. [DOI] [PubMed] [Google Scholar]
- 27. Ou‐Yang HF, Zhang HW, Wu CG, Zhang P, Zhang J, Li JC et al Notch signaling regulates the FOXP3 promoter through RBP‐J‐ and Hes1‐dependent mechanisms. Mol Cell Biochem 2009; 320:109–14. [DOI] [PubMed] [Google Scholar]
- 28. Bao ZZ, Cepko CL. The expression and function of Notch pathway genes in the developing rat eye. J Neurosci 1997; 17:1425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nelson BR, Reh TA. Relationship between Delta‐like and proneural bHLH genes during chick retinal development. Dev Dyn 2008; 237:1565–80. [DOI] [PubMed] [Google Scholar]
- 30. Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Muller glia. J Neurosci 2010; 30:3101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilhelmsson U, Faiz M, de Pablo Y, Sjoqvist M, Andersson D, Widestrand A et al Astrocytes negatively regulate neurogenesis through the Jagged1‐mediated Notch pathway. Stem Cells 2012; 30:2320–9. [DOI] [PubMed] [Google Scholar]
- 32. Lebkuechner I, Wilhelmsson U, Mollerstrom E, Pekna M, Pekny M. Heterogeneity of Notch signaling in astrocytes and the effects of GFAP and vimentin deficiency. J Neurochem 2015; 135:234–48. [DOI] [PubMed] [Google Scholar]
- 33. Nardai S, Dobolyi A, Pal G, Skopal J, Pinter N, Lakatos K et al Selegiline promotes NOTCH‐JAGGED signaling in astrocytes of the peri‐infarct region and improves the functional integrity of the neurovascular unit in a rat model of focal ischemia. Restor Neurol Neurosci 2015; 33:1–14. [DOI] [PubMed] [Google Scholar]
- 34. Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C et al Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 1998; 21:63–75. [DOI] [PubMed] [Google Scholar]
- 35. Valapala M, Hose S, Gongora C, Dong L, Wawrousek EF, Samuel Zigler J Jr et al Impaired endolysosomal function disrupts Notch signalling in optic nerve astrocytes. Nat Commun 2013; 4:1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature 1988; 332:834–7. [DOI] [PubMed] [Google Scholar]
- 37. Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY et al Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 2010; 70:4840–9. [DOI] [PubMed] [Google Scholar]
- 38. Yuan JS, Kousis PC, Suliman S, Visan I, Guidos CJ. Functions of notch signaling in the immune system: consensus and controversies. Annu Rev Immunol 2010; 28:343–65. [DOI] [PubMed] [Google Scholar]
- 39. Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity 2010; 32:14–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sorting of CD4+ CD25hi regulatory T (Treg) cell‐enriched cells from spleen. The purity of Treg cells in sorted cells was determined by Foxp3 staining. CD4+ CD25− T cells did not express Foxp3.
Figure S2. (a) GFP expression in regulatory T (Treg) cells after lentiviral transduction with L‐SC, L‐sh‐N1 and L‐sh‐N‐2. (b) Expression of PD‐1 and Foxp3 in Treg cells after lentiviral transduction with L‐SC, L‐sh‐N1 and L‐sh‐N‐2.
Figure S3. Statistics of the proportions of transferred regulatory T (Treg) cells in uveitic eyes.
Table S1. Quantitative RT‐PCR primer sequences.


