Summary
Visceral adipose tissue inflammation in obesity is an established risk factor for metabolic syndrome, which can include insulin resistance, type 2 diabetes, hypertension and cardiovascular diseases. With obesity and related metabolic disorders reaching epidemic proportions globally, an understanding of the mechanisms of adipose tissue inflammation is crucial. Within the immune cell cohort, dendritic cells (DC) play a key role in balancing tolerance and immunity. Despite decades of research into the characterization of DC in lymphoid and non‐lymphoid organs, their role in adipose tissue function is poorly understood. There is now an increasing interest in identification and characterization of DC in adipose tissue and understanding their function in regulating tissue metabolic homeostasis. This review provides an overview of the study of DC in adipose tissue, focusing on possible mechanisms by which DC may contribute to adipose tissue homeostasis.
Keywords: adipose tissue, dendritic cells, inflammation, metabolism, obesity
Abbreviations
- AT
adipose tissue
- ATM
adipose tissue macrophages
- BAT
brown adipose tissue
- BMDC
bone‐marrow‐derived dendritic cell
- C/EBP
CAAT/enhancer binding protein
- CD
cluster of differentiation
- cDC
conventional dendritic cell
- DC
dendritic cell
- Hif
hypoxia‐inducible factor
- IL
interleukin
- KLF4
Kruppel‐like factor 4
- pDC
plasmacytoid dendritic cell
- PPARγ
peroxisome proliferator‐activated receptor γ
- Th1
T helper type 1
- TNF‐α
tumour necrosis factor α
- WAT
white adipose tissue
Introduction
Obesity is a major health concern worldwide. The incidence of adults with a body mass index ≥ 25 kg/m2 has risen from 28·8% to 36·9% between 1980 and 2013.1 Within the UK 67% of men and 57% of women are either overweight or obese.2, 3 According to World Health Organization criteria, the increased rate is largely contributed by childhood obesity cases. Because of the increase in prevalence and established health risks, obesity has become a major health challenge.
Being overweight and obesity are major risk factors for non‐communicable diseases and predispose individuals to further risks of cardiovascular diseases, diabetes, cancer, osteoarthritis and musculoskeletal disorders. Furthermore, metabolic disorders such as non‐alcoholic fatty liver and gall stones are also suggested to be pre‐disposed due to obesity.2 An important connection between obesity and metabolic inflammation was recognized over the last two decades. Numerous studies, discussed in this review, have demonstrated that obese adipose tissue mimics an active local inflammation site. This low‐grade chronic inflammation plays a critical role in the development of obesity‐related metabolic and inflammatory disorders in individuals.4 Therefore, understanding the immune processes regulating adipose tissue homeostasis will provide a new comprehension of the pathophysiology of diabetes and related metabolic syndromes.
Adipose tissue function
Adipose tissue (AT) has long been believed to be a passive energy reservoir; however, in recent years, this notion has changed and it is now recognized as a major endocrine organ.5, 6 Broadly categorized, AT can be of two types – white adipose tissue (WAT) and brown adipose tissue (BAT). BAT obtains its name from increased vascularization and a high mitochondrial content giving it a reddish‐brown appearance. Its role is mainly implicated in maintaining and promoting thermogenesis by combusting lipids into heat. BAT is largely found in hibernating mammals, neonatal and young children and regresses with aging and obesity.7, 8 Recently it has been shown that BAT promotes clearance of triglycerides from circulation and lowers plasma glucose, reducing the risk factors for metabolic syndrome.9 On the other hand, WAT is widely distributed in the body, regulates a number of functions including hormones and nutrient metabolism. It is also the predominant form of AT that interacts with the immune cells under conditions of metabolic challenge. Therefore, this review focuses on WAT, which is hereon referred to as AT.
Adipose tissue secretes an array of bioactive mediators, collectively referred to as adipokines [e.g. adiponectin, leptin, interleukin‐6 (IL‐6), tumour necrosis factor‐α (TNF‐α)] that act both locally and systemically to control AT homeostasis and energy metabolism. The roles of these signalling molecules are critically regulated during homeostasis. AT can rapidly and efficiently respond to alterations in nutrient intake through adipocyte expansion (hypertrophy) and adipocyte differentiation (hyperplasia). This process is tightly regulated through a number of factors. By far the most studied is the regulatory process controlled by peroxisome proliferator‐activated receptor γ (PPARγ). PPARγ closely interacts with another transcription factor family – CAAT/enhancer binding protein (C/EBP) to transcribe genes during adipogenesis and adipocyte differentiation. Evidence of this is demonstrated by PPARγ and C/EBP knockout mice having reduced adipose mass, which shows the critical role of these transcription factors in AT development.10, 11, 12, 13
Adipose tissue composition in normal/lean individuals is known to account for 15–20% of the total body weight, while this increases up to 35–40% in obesity. In circumstances of over‐nutrition, adipocytes enlarge with an excess of triacylglycerol inducing hypoxia, mitochondrial dysfunction, oxidative stress and endoplasmic reticulum stress, culminating in the release of free fatty acids and adipocyte necrosis. The stressed adipocytes trigger a metabolic and immunological imbalance, with local production of IL‐6, TNF‐α and IL‐1, and recruitment of inflammatory cells.14, 15 Inflammatory responses in the early stages of obesity are first detected in the visceral AT, compared with other metabolic organs, including liver and skeletal muscle, suggesting that visceral AT chronic inflammation can either directly or indirectly influence the development of obesity‐related co‐morbidities such as insulin resistance, dyslipidaemia, hypertension, non‐alcoholic fatty liver disease and atherosclerosis.
Adipose tissue also hosts a proportion of immune cells that reside in the tissue contributing to organ homeostasis. In addition to the main function of energy storage, primary roles of AT are now considered to include body metabolism, coordination of immune cell functions within and outside the tissue, and regulation of glucose tolerance and insulin resistance.16, 17
Immune cells in adipose tissue
It is now clear that, beside adipocytes, AT contains a network of immune cells that work in cooperation in the maintenance of the overall metabolism and physiology of the organ. During this decade, the role of these immune cells have gained importance as they have been identified to centrally co‐ordinate immunity, metabolic pathways and tissue functioning.18 The role of different immune cells in AT was reviewed by Grant and Dixit in 2015 and is not discussed in depth here.19, 20
In normal/lean AT, macrophages comprise the majority of the immune cells contributing up to to 15% of the immune cell cohort. In addition to pathogens/toxins/debris phagocytosis and clearance, AT macrophages (ATM) fulfil essential homeostatic functions. ATM control lipid cytotoxicity by taking up triglycerides and non‐esterified fatty acids released by overstretched adipocytes and are also known to secrete high levels of IL‐10, which limits inflammatory responses and increases insulin sensitivity.21, 22 In addition to macrophages, natural killer T cells, eosinophils and regulatory T cells are also known to reside in AT, where they locally secrete IL‐4, IL‐13 and IL‐10 to maintain an anti‐inflammatory milieu under physiological conditions. This tolerogenic environment feeds back to the tissue to control glucose homeostasis and insulin sensitivity.23, 24
The first link between inflammation and obesity was published more than two decades ago by Hotamisligil and colleagues,25 showing overexpression of TNF‐α in visceral AT of obese mice. Subsequent studies demonstrated that deletion of TNF‐α could ameliorate insulin resistance. ATM were later discovered to be the prominent source of TNF‐α.25, 26 In obesity, ATM proportion increases to up to 50% of the immune cells.27, 28 This increase is accompanied by the recruitment of CD11c+ inflammatory macrophages combined with the release of pro‐inflammatory cytokines such IL‐6, IL‐12 and TNF‐α.29 Depletion of CD11c+ cells in obese mice using the conditional cell ablation system, results in reduction of AT inflammation and marked normalization of glucose and insulin tolerance.30 Albeit, most of these CD11c+ cells are F4/80+ ATM, CD11c is also a marker of dendritic cells (DC). So far, ATM have been identified by expression of F4/80 and CD11c, which are promiscuous markers. This phenotypic overlap between inflammatory ATM and DC has made it difficult to dissect their function and their contribution to AT immune responses.
Dendritic cells
Dendritic cells are professional antigen‐presenting cells. They are often referred to as messengers between adaptive and innate immune responses because they harbour the ability to either instigate or suppress immune responses depending on their maturation state. DC are present in all tissues of the body in an immature/tolerogenic state. They routinely migrate to draining lymph nodes presenting self‐antigens to lymphocytes, which mediates immunological tolerance and maintains tissue homeostasis.31 Upon environmental stimuli or ‘danger signals’, DC undergo a complex and intricate maturation process, which allows them to express surface molecules and cytokines important for T‐cell activation and the initiation of adaptive immune responses.32
Dendritic cells are broadly categorized into two types – plasmacytoid DC (pDC) and conventional DC (cDC), the latter being the focus of this review. Conventional DC can be subdivided into several subsets according to the expression of selected surface molecules, tissue localization and specialized functions. Ontogenically, there are two major cDC subsets that can be distinguished by the expression of the cell surface markers CD11b and CD8/CD103.33 Both subsets vary greatly in terms of transcriptome profiling, lineage markers and function, which depend largely on the tissue where they are localized. CD8+ and the non‐lymphoid equivalent CD103+ cDC, also termed cDC1, are transcriptionally dependent on interferon regulatory factor 8.34 The cDC1 specialize in antigen cross‐presentation and induction of T helper type 1 (Th1) responses. In contrast, CD11b+ DC, termed cDC2, are transcriptionally regulated by interferon regulatory factor 435, 36 and preferentially induce and promote Th17 and Th2 cell responses. This division of labour permits the immune system to mount distinct types of responses according to the danger/environmental signals.
Conventional DC are phenotypically distinguished by the high expression of CD11c and MHC II cell surface markers. This characterization holds true for the identification of cDC in lymphoid tissues under normal physiological conditions, e.g. spleen. It is known that cDC in steady‐state exist in an immature/semi‐mature state and that activation of cDC leads to a cascade of molecular changes that are reflected in the expression and retraction of various cell markers.37, 38 This, combined with the expression of overlapping cell surface markers such as CD11c by some macrophages and monocytes, has resulted in the lack of specific markers for the identification and characterization of cDC in vivo. This is particularly true for AT, where CD11c is highly expressed in inflammatory macrophages. Furthermore, despite some evidence of cDC presence in AT, no clear attempt has been made to determine cDC subsets and/or characterize their functions. An overview of the studies pertaining to DC in AT is discussed below.
Dendritic cells in adipose tissue
Considering that AT is now regarded as a metabolically active organ capable of regulating nutritional uptake, hormones and inflammatory processes, there has been an increasing interest in identification and understanding the roles of various immune cells within AT. However, given the fact that DC are closely related to other myeloid cells and are known to express overlapping cell surface markers, understanding the role of DC in AT has been challenging. Nevertheless, although inconclusive, recent studies suggest a role for DC, particularly, cDC, in the mediation of AT inflammation.39
Stefanovic‐Racic et al. demonstrated that mice fed on a high‐fat diet (HFD) have an increase in CD11c+ cells in their AT. Using multicolour flow cytometry and scanning electron microscopy they demonstrated that a proportion of these CD11c cells are DC (both cDC and pDC).40 Using gain and loss of function studies, it has also been demonstrated that the presence of cDC was essential for the recruitment of macrophages in response to metabolic challenges such as HFD.40 The authors showed that Flt3l−/− knockout mice, which lack cDC but also have reduced numbers of natural killer, regulatory T cells and B cells,41, 42 have fewer cDC in AT compared with control mice. This phenotype was rescued by administration of Flt3‐ligand, which resulted in a significant number of cDC and macrophage recruitment from a single dose. Furthermore, injection of bone‐marrow‐derived DC (BMDC) into mice also boosted immune cell recruitment to AT, thereby indicating a general role of DC in immune cell recruitment. An earlier independent study showed that Flt3−/− knockout mice have decreased numbers of cDC, pDC and CD103+ cDC in non‐lymphoid tissue,43, 44 and interestingly have tolerance against diet‐induced weight gain. This implies that cDC may play a pivotal role in regulating systemic metabolic responses and eventually contributing to AT inflammation.43, 44
In another study by Bertola et al.45 the authors identified and characterized a new subset of DC termed ‘inflammatory DC’ in both obese mice and men. These inflammatory DC were distinguished by the presence of CD11c in addition to expression of low levels of F4/80.45 However, F4/80 has been widely used as a marker for ATM, which are increased up to five times in obese mice. Hence, the identification of CD11c+ F4/80low DC as a distinct population remains arguable. Nevertheless, this study also demonstrated that these inflammatory DC could present antigen and induce a Th17 T‐cell response in vitro. Contrastingly, DC from lean/normal mice, identified as CD11c+ F4/80− cells, were shown to induce a Th1 T‐cell response, suggesting a change in DC phenotype upon HFD.45
This preferential induction of a Th17 T‐cell response by obese AT DC was later reflected in an independent study conducted by Chen et al.46 Using multicolour flow cytometry, and scanning electron microscopy it was once more confirmed that DC numbers infiltrating AT increased significantly in mice fed with HFD. In addition, it was shown that DC in adipose tissue exist in an immature/semi‐mature state expressing low levels of the maturation markers CD80 and CD86 compared with splenic cDC. Furthermore, Chen et al.46showed that AT DC from obese mice preferentially secrete higher levels of IL‐6 and IL‐23, and promote a Th17 T‐cell response compared with splenic cDC, which in contrast are known to promote Th1 T‐cell responses. It is unclear if obesity induces differentiation of immature into mature DC or is rather a recruitment of a new subset of DC, termed the ‘inflammatory DC’, into AT.
The study by Zhong et al.47 seems to suggest a mechanism by which AT DC in obesity promote tissue inflammation. The authors demonstrated that myeloid cells in AT (comprising DC and ATM) expressed dipeptidyl peptidase‐4 (or CD26). CD26 is an amino peptidase of which the major function is reducing the activity of incretin peptides, including glucagon‐like peptide‐1 and glucose‐dependent insulinotropic polypeptide, which contribute to the attenuation of insulin secretion.47, 48 Inhibitors have been shown to ameliorate type 2 diabetes and cardiovascular diseases. CD26 expression in myeloid cells was increased in the AT of obese humans and mice and its up‐regulation promoted a pro‐inflammatory environment in AT leading to insulin resistance. CD26 knockdown in both human and murine DC reversed this effect in vitro.48
Adipose tissue DC will ‘sense’ the tissue environment and travel to draining lymph nodes to meet naive T cells. For instance, the mesenteric adipose tissue is well connected to the mesenteric lymph nodes 49 (Fig. 1). Furthermore, recently it has been suggested that AT DC can uptake antigens from ‘leaky’ collective lymphatic vessels. The collective lymphatic vessels transport lymph and its content to the draining lymph nodes and do not exchange molecules and cells within the tissue. In contrast, Kuan et al. showed that collective lymphatic vessels in AT have inherent permeability and, as a consequence, soluble antigens travelling within lymph to lymph nodes can be released in AT and taken up by DC closely associated with collective lymphatic vessels.50 This implies that AT DC can also ‘sense’ the physiological status of adjacent organs.
Figure 1.
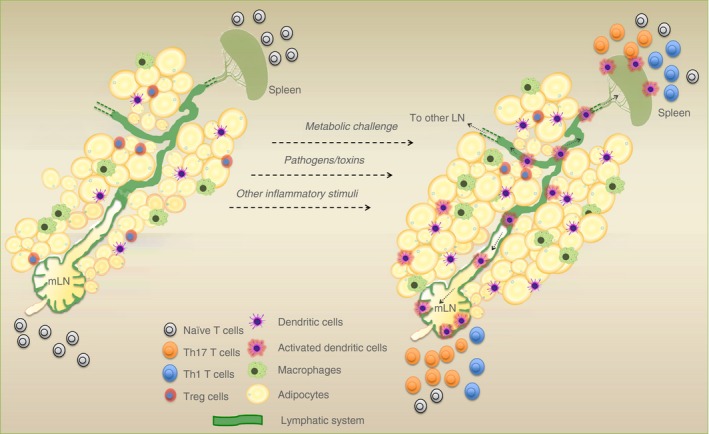
Schematic showing the potential response of stimulated adipose tissue dendritic cells (AT DC). Once activated, DC within AT may migrate along the lymphatic system (shown in green) to mesenteric lymph nodes (mLN) to activate naive T cells and bring about T‐cell responses [T helper type 17 and type 1 (Th17/Th1)].
A recent study provides novel evidence of regulatory mechanisms that influence differentiation and development of adipocytes by DC.51 Pamir et al. report that granulocyte–macrophage colony‐stimulating factor is essential for adipocyte development and this was demonstrated using granulocyte–macrophage colony‐stimulating factor deficient mice (Csf2−/− mice). Furthermore, in vitro adipocyte differentiation was observed to be inhibited when cells were pre‐treated with DC‐conditioned medium, in contrast to macrophage‐conditioned medium.51 Csf2 is notably a critical cytokine for the generation of monocyte‐derived DC in vitro. Csf2 has also been shown to be necessary for cDC survival in non‐lymphoid tissues, where it is highly expressed. However, this regulation is seldom observed in cDC within lymphoid tissues.52 Given the non‐lymphoid nature of AT, the possibility that cDC can control adipocyte expansion and differentiation when necessary, is interesting. Moreover, the up‐regulation of MHC II (another classical DC marker) has been reported to occur in AT before tissue inflammation and deposition of fat. Mice lacking MHC II were shown to be marginally resistant to obesity when fed an HFD.53 Conversely, adipocyte stem cells have also been showed to control AT DC function. Work by Peng et al.54 demonstrated that adipocyte stem cells could inhibit DC maturation and restrict T‐cell responses in vitro, suggesting a tolerogenic effect of adipocyte stem cells. Overall, these studies demonstrate a role of CD11c+ MHC II+ cells (potentially cDC) in AT biology (Fig. 2).
Figure 2.
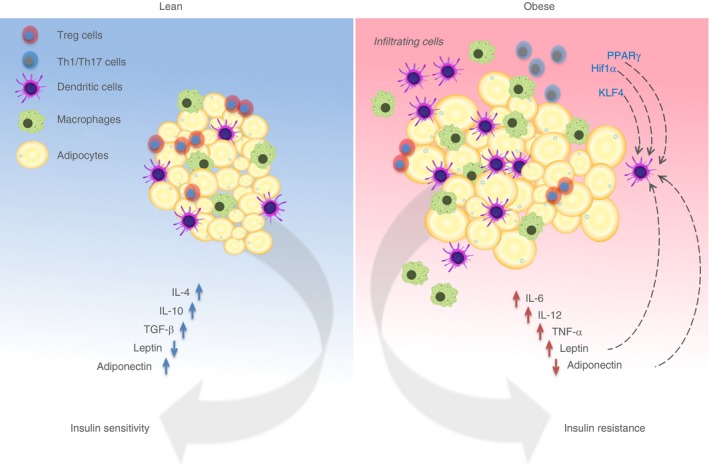
Schematic depicting adipose tissue homeostasis and its response in obesity. Under normal/lean conditions, immune cells and adipocytes work in cooperation to develop a tolerogenic milieu to maintain tissue homeostasis. In response to obesity‐induced chronic inflammation, immune cells including dendritic cells (DC) and macrophages are recruited to adipose tissue, promoting a pro‐inflammatory response. Dotted black arrows indicate possible transcriptional and systemic regulation of DC, which may result in anti‐ or pro‐inflammatory responses. HIF‐1α, hypoxia‐inducible factor‐1α; KLF4, Kruppel‐like factor 4; IL‐4, interleukin‐4; PPARγ, peroxisome proliferator‐activated receptor γ; TGF‐β, transforming growth factor‐β; TNF‐α, tumour necrosis factor‐α.
Table 1 summarizes some of the key findings on identification and role of DC within AT.
Table 1.
Summary of key findings on adipose tissue dendritic cells
| Key finding | DC population investigated | Model | Reference |
|---|---|---|---|
| Depletion of CD11c+ cell rescues glucose and insulin sensitivity | CD11c cells | Mouse | 30 |
| HFD promotes increase in cDC numbers in AT. Depletion of cDC in visceral AT protects from HFD‐induced inflammation | cDC in visceral AT | Mouse | 40 |
| ‘Inflammatory DC’ are identified in obese AT. These DC are capable of inducing Th17 response in vitro | Inflammatory DC (CD11chi, F4/80low) | Mouse and human | 45 |
| HFD increases AT DC recruitment. DC from AT preferentially promote Th17 response | MHCIIhi, CD11chi cells | Mouse | 46 |
| DC can regulate differentiation of adipocytes via GM‐CSF signalling | MHCIIhi, CD11chi cells | Mouse | 51 |
| AT DC express CD26 which is required for enhanced T‐cell proliferation. Expression of CD26 is elevated in obese AT | MHCIIhi, CD11chi cells | Mouse and human | 48 |
| Activation of PPARγ inhibits maturation of DC | Bone‐marrow‐derived DC | Mouse | 55, 56 |
Abbreviations: AT, adipose tissue; cDC, conventional dendritic cells; DC, dendritic cells; GM‐CSF, granulocyte–macrophage colony‐stimulating factor; HFD, high‐fat diet; PPARγ, peroxisome proliferator‐activated receptor γ; Th17, T helper type 17.
Future scope
Transcriptional regulation of DC within AT
Adipose tissue is an active endocrine organ playing a role in metabolic regulation. Interestingly, cDC activation and function can also be controlled metabolically. PPARγ is the master regulator of AT differentiation and homeostasis. It plays a central role in regulating lipid and glucose metabolism. PPARγ is a member of the nuclear hormone receptor superfamily and functions heterodimerically with the retinoic X receptor.57 In addition to its metabolic role, PPARγ is known to mediate and promote anti‐inflammatory responses in various immune cell populations. Indeed, PPARγ deficiency in macrophages results in increased obesity‐induced AT inflammation and insulin resistance.58 Function of DC is also suggested to be heavily regulated by PPARγ. Various studies have demonstrated the effects of PPARγ‐dependent regulation of DC main functions including antigen uptake, maturation and migration.59 In cDC and BMDC, it has been reported to suppress the release of pro‐inflammatory IL‐12.55 In addition, PPARγ agonists were shown to suppress maturation of BMDC by negatively regulating the nuclear factor‐κB/mitogen‐activated protein kinase pathways.56, 60 Concurrently, PPARγ‐deficient BMDC showed significant increased expression of co‐stimulatory molecules and superior antigen‐presenting capacity compared with control cells.55 Given the tolerogenic state of cDC in lean AT, investigating the role of PPARγ activation and its potential effects on AT cDC function is critical. However, there are currently no studies that shed light on the role of PPARγ in AT cDC.
In addition to PPARγ, another nuclear receptor, Kruppel‐like factor 4 (KLF4) is known to control adipogenesis through its effects on another intermediate transcription factor – C/EBPβ. KLF4 is an early transcription factor that is essential for the development of adipocytes. Knockdown of KLF4 has been shown to stall adipogenesis, which results in down‐regulation of C/EBPβ and therefore that of PPARγ.61 KLF4 has also been implicated in the control of ATM phenotype. KLF4 expression is down‐regulated upon lipopolysaccharide stimulation and KLF4‐specific deletion switches macrophages from an anti‐inflammatory state to a pro‐inflammatory one. Recently it has been demonstrated that KLF4 mediates Th2 priming capabilities of the CD11b+ cDC subset. It is suggested that KLF4 regulates the expression of interferon regulatory factor 4 transcription factor, which is critical in lineage‐specific and function‐specific development of this cDC subset.62
Several studies demonstrate the interplay between hypoxia and obese AT.63, 64 In obesity, hypoxia is suggested to result from a number of factors such as reduced blood supply, nutrient deprivation and endoplasmic reticulum stress.65, 66 It is has been reviewed that exposure of adipocytes to hypoxia can result in changes of over 1000 genes affecting metabolic cycles, cytokine production and cellular signalling.63 Hypoxic conditions are determined by the ready induction of hypoxia‐inducible factor 1‐α (Hif‐1α). Hif‐1α is a transcription factor that regulates genes to stabilise and restore normal oxygen levels (normoxia) within the tissue. In BMDC, Hif‐1α up‐regulation results in increased production of stimulatory molecules, followed by released of cytokines as cDC mature.67, 68 It has been suggested that under certain maturation stimuli, maturation of cDC is contributed to by Hif‐1α transcription, which is in turn induces a metabolic switch to fulfil energy demands during activation.69, 70 Given that normal cDC function can be regulated by Hif‐1α,71, 72, 73 and that hypoxic conditions are present in obesity, understanding the interplay between Hif‐1α and cDC in the biology of obese AT is essential. In this regard, the development of hypoxia within AT may potentially instigate AT cDC to induce a Th17 response. The possible interplay between the transcriptional regulation of adipocyte differentiation/expansion and DC function remains unexplored.
Systemic regulation of DC in AT
Two of the most important secreted cytokines from adipose tissue are adiponectin and leptin. Adiponectin is a hormone highly secreted by white adipocytes. It is known to enhance free fatty acid oxidation and insulin sensitivity in liver and muscle. The effects of adiponectin are diverse, which includes insulin sensitization,74 prevention of atherosclerotic plaques and regulation of metabolism. In obesity and in obesity‐related disease models, levels of adiponectin have been demonstrated to decrease rapidly compared with controls.75 Yamauchi et al.76 demonstrated that insulin‐resistant mice show significant improvement towards insulin sensitivity upon adiponectin administration. Apart from its metabolic functions, adiponectin has also been shown to regulate immune cell functions.77, 78 It has been suggested that adiponectin plays a role in the inhibition of TNF‐α activity79 promoting and maintaining tissue homeostasis. Tsang et al. showed that adiponectin could alter BMDC phenotype and cytokine production.80 It was reported that BMDC pre‐treated with adiponectin preferentially induced T‐cell regulatory responses.80 This is suggestive of a tolerogenic function of DC in response to an adipokine that is abundantly produced under normal conditions.80 In sharp contrast, Jung et al.81 demonstrated that DC pre‐treated with adiponectin can induce both Th1 and Th17 cells and that pre‐treatment of DC leads to maturation and secretion of pro‐inflammatory cytokines such as IL‐12. With contradictory evidence, the effect of adiponectin in cDC and AT cDC function, remains to be elucidated.
Leptin is another adipokine with a primary role in energy metabolism. Secretion of leptin is dependent on food intake,82 which then negatively regulates feeding to prevent excessive energy balance. The role of leptin in adipocyte function is evident from the knockout studies where mice lacking leptin expression gain weight rapidly and become diabetic. This effect is reversed by regular administration of leptin.83, 84 This leptin‐mediated reduction of insulin resistance, diabetes and hyperglycaemia was also observed in murine lipodystrophy models. Administration of recombinant leptin to lipodystrophic mice resulted in reduced weight gain and improved insulin sensitivity, indicating that this adipose‐tissue hormone is capable of regulating diverse metabolic functions and is not restricted to adipogenesis.85 There is some evidence demonstrating a link between leptin and DC function. Mattioli et al.86 show that leptin treatment improves monocyte‐derived DC maturation and boosts their T‐cell priming capabilities in vitro. This was corroborated in another two studies where BMDC lacking leptin had reduced antigen presentation capabilities and increased regulatory T cell induction.87, 88 It is clear that both leptin and adiponectin play a pivotal role affecting DC phenotype and function. Hence understanding their effect in visceral AT DC function and their control of AT homeostasis will be beneficial.
Dendritic cells in human AT
Studies to date have been focused on determining DC populations within murine AT. This, however, has not yet been extensively translated to human AT. In humans, DC have been characterized into three groups – CD1c+, CD141+ and CD303+.89 Using mesenchymal stem cells, numerous studies have demonstrated the effects of adipocyte and adipocyte‐derived factors on myeloid cells, including monocyte‐derived DC. Results from these studies imply a role of these cells in maintaining a tolerogenic phenotype.27, 78, 90 More recently, Bertola et al.45 conclusively demonstrated that identification and phenotyping of different subsets of human DC populations was possible within human subcutaneous AT. Despite encouraging evidence from subcutaneous AT, this was not translated to human visceral fat.
Conclusion
Adipose tissue has gained increasing attention and understanding in recent years particularly in the field of immuno‐metabolism. Despite compelling evidence, the identification of cDC and their role in AT maintenance has remained unclear and has not been investigated in depth. With emerging cDC‐specific models at hand, there appears to be an opportunity to address these issues and to understand how cDC can influence AT homeostasis and their response to metabolic challenge.
Disclosures
The authors declare that they have no competing interests.
Acknowledgement
SSR drafted the manuscript and prepared the figures and table and MPL provided critical review. SSR is funded by BHF grant FS/13/49/30421 and MPL is funded by the BHF intermediate fellowship FS/13/49/30421 and the Marie Curie Cascade Fellowship CF‐2013‐11‐003‐LONGHI.
References
- 1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:766–81. Epub 2014/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bluher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab 2013; 27:163–77. Epub 2013/06/05. [DOI] [PubMed] [Google Scholar]
- 3. Rennie KL, Jebb SA. Prevalence of obesity in Great Britain. Obes Rev 2005; 6:11–2. Epub 2005/01/19. [DOI] [PubMed] [Google Scholar]
- 4. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444:860–7. Epub 2006/12/15. [DOI] [PubMed] [Google Scholar]
- 5. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010; 316:129–39. Epub 2009/09/03. [DOI] [PubMed] [Google Scholar]
- 6. Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol 2012; 9:689–702. Epub 2012/11/15. [DOI] [PubMed] [Google Scholar]
- 7. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277–359. Epub 2004/01/13. [DOI] [PubMed] [Google Scholar]
- 8. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013; 9:191–200. Epub 2013/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al Brown adipose tissue activity controls triglyceride clearance. Nat Med 2011; 17:200–5. Epub 2011/01/25. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPδ gene. EMBO J 1997; 16:7432–43. Epub 1998/02/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al PPAR γ is required for the differentiation of adipose tissue in vivo and in vitro . Mol Cell 1999; 4:611–7. Epub 1999/11/05. [DOI] [PubMed] [Google Scholar]
- 12. Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz‐Lozano P, Chien KR, et al PPAR γ is required for placental, cardiac, and adipose tissue development. Mol Cell 1999; 4:585–95. Epub 1999/11/05. [DOI] [PubMed] [Google Scholar]
- 13. Linhart HG, Ishimura‐Oka K, DeMayo F, Kibe T, Repka D, Poindexter B, et al C/EBPα is required for differentiation of white, but not brown, adipose tissue. Proc Natl Acad Sci USA 2001; 98:12532–7. Epub 2001/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol 2011; 57:2461–73. Epub 2011/06/18. [DOI] [PubMed] [Google Scholar]
- 15. Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells 2014; 37:365–71. Epub 2014/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gavrilova O, Marcus‐Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, et al Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 2000; 105:271–8. Epub 2000/02/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 2011; 121:2094–101. Epub 2011/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lolmede K, Duffaut C, Zakaroff‐Girard A, Bouloumie A. Immune cells in adipose tissue: key players in metabolic disorders. Diabetes Metab 2011; 37:283–90. Epub 2011/04/22. [DOI] [PubMed] [Google Scholar]
- 19. Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015; 23:512–8. Epub 2015/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015; 161:146–60. Epub 2015/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796–808. Epub 2003/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al MCP‐1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116:1494–505. Epub 2006/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest 2013; 123:939–44. Epub 2013/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low‐grade inflammation. J Endocrinol 2014; 222:R113–27. Epub 2014/07/10. [DOI] [PubMed] [Google Scholar]
- 25. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor‐α: direct role in obesity‐linked insulin resistance. Science 1993; 259:87–91. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 26. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity‐induced insulin resistance in mice lacking TNF‐α function. Nature 1997; 389:610–4. [DOI] [PubMed] [Google Scholar]
- 27. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117:175–84. Epub 2007/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet‐induced obesity. Diabetes 2007; 56:16–23. Epub 2006/12/29. [DOI] [PubMed] [Google Scholar]
- 29. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Invest 2003; 112:1821–30. Epub 2003/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c‐positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 2008; 8:301–9. Epub 2008/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013; 31:563–604. Epub 2013/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joffre OP, Segura E, Savina A, Amigorena S. Cross‐presentation by dendritic cells. Nat Rev Immunol 2012; 12:557–69. Epub 2012/07/14. [DOI] [PubMed] [Google Scholar]
- 33. Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity 2014; 40:642–56. Epub 2014/05/20. [DOI] [PubMed] [Google Scholar]
- 34. Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non‐lymphoid tissue dendritic cells in mice. Immunol Rev 2010; 234:55–75. Epub 2010/03/03. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, et al Critical roles of interferon regulatory factor 4 in CD11bhighCD8α – dendritic cell development. Proc Natl Acad Sci USA 2004; 101:8981–6. Epub 2004/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O'Shea JJ, et al IFN regulatory factor‐4 and ‐8 govern dendritic cell subset development and their functional diversity. J Immunol 2005; 174:2573–81. Epub 2005/02/25. [DOI] [PubMed] [Google Scholar]
- 37. Schraml BU, Reis e Sousa C. Defining dendritic cells. Curr Opin Immunol 2015; 32:13–20. Epub 2015/01/02. [DOI] [PubMed] [Google Scholar]
- 38. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18:767–811. Epub 2000/06/03. [DOI] [PubMed] [Google Scholar]
- 39. Majdoubi A, Kishta OA, Thibodeau J. Role of antigen presentation in the production of pro‐inflammatory cytokines in obese adipose tissue. Cytokine 2016; 82:112–21. Epub 2016/02/09. [DOI] [PubMed] [Google Scholar]
- 40. Stefanovic‐Racic M, Yang X, Turner MS, Mantell BS, Stolz DB, Sumpter TL, et al Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity‐associated increases in CD11c+ cells in adipose tissue and liver. Diabetes 2012; 61:2330–9. Epub 2012/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Darrasse‐Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, et al Feedback control of regulatory T cell homeostasis by dendritic cells in vivo . J Exp Med 2009; 206:1853–62. Epub 2009/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, et al Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000; 95:3489–97. Epub 2000/05/29. [PubMed] [Google Scholar]
- 43. Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, et al Murine epidermal Langerhans cells and langerin‐expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci USA 2009; 106:3312–7. Epub 2009/02/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waskow C, Liu K, Darrasse‐Jeze G, Guermonprez P, Ginhoux F, Merad M, et al The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol 2008; 9:676–83. Epub 2008/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, et al Identification of adipose tissue dendritic cells correlated with obesity‐associated insulin‐resistance and inducing Th17 responses in mice and patients. Diabetes 2012; 61:2238–47. Epub 2012/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Y, Tian J, Tian X, Tang X, Rui K, Tong J, et al Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One 2014; 9:e92450. Epub 2014/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fan H, Meng W, Kilian C, Grams S, Reutter W. Domain‐specific N‐glycosylation of the membrane glycoprotein dipeptidylpeptidase IV (CD26) influences its subcellular trafficking, biological stability, enzyme activity and protein folding. Eur J Biochem 1997; 246:243–51. Epub 1997/05/15. [DOI] [PubMed] [Google Scholar]
- 48. Zhong J, Rao X, Deiuliis J, Braunstein Z, Narula V, Hazey J, et al A potential role for dendritic cell/macrophage‐expressing DPP4 in obesity‐induced visceral inflammation. Diabetes 2013; 62:149–57. Epub 2012/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. von der Weid PY, Rainey KJ. Review article: lymphatic system and associated adipose tissue in the development of inflammatory bowel disease. Aliment Pharmacol Ther 2010; 32:697–711. Epub 2010/07/20. [DOI] [PubMed] [Google Scholar]
- 50. Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, et al Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node‐homing adipose tissue dendritic cells. J Immunol 2015; 194:5200–10. Epub 2015/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pamir N, Liu NC, Irwin A, Becker L, Peng Y, Ronsein GE, et al Granulocyte/macrophage colony‐stimulating factor‐dependent dendritic cells restrain lean adipose tissue expansion. J Biol Chem 2015; 290:14656–67. Epub 2015/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo‐Cantero J, et al GM‐CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity 2012; 36:1031–46. Epub 2012/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, et al Class II major histocompatibility complex plays an essential role in obesity‐induced adipose inflammation. Cell Metab 2013; 17:411–22. Epub 2013/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peng W, Gao T, Yang ZL, Zhang SC, Ren ML, Wang ZG, et al Adipose‐derived stem cells induced dendritic cells undergo tolerance and inhibit Th1 polarization. Cell Immunol 2012; 278:152–7. Epub 2012/09/18. [DOI] [PubMed] [Google Scholar]
- 55. Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, Paul B, et al Peroxisome proliferator‐activated receptor γ control of dendritic cell function contributes to development of CD4+ T cell anergy. J Immunol 2007; 178:2122–31. Epub 2007/02/06. [DOI] [PubMed] [Google Scholar]
- 56. Zand H, Rahimipour A, Salimi S, Shafiee SM. Docosahexaenoic acid sensitizes Ramos cells to γ‐irradiation‐induced apoptosis through involvement of PPAR‐γ activation and NF‐κB suppression. Mol Cell Biochem 2008; 317:113–20. Epub 2008/06/21. [DOI] [PubMed] [Google Scholar]
- 57. Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab 2014; 25:293–302. Epub 2014/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR‐γ dependent and independent effects on macrophage‐gene expression in lipid metabolism and inflammation. Nat Med 2001; 7:48–52. Epub 2001/01/03. [DOI] [PubMed] [Google Scholar]
- 59. Szatmari I, Rajnavolgyi E, Nagy L. PPARγ, a lipid‐activated transcription factor as a regulator of dendritic cell function. Ann N Y Acad Sci 2006; 1088:207–18. Epub 2006/12/29. [DOI] [PubMed] [Google Scholar]
- 60. Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, et al Cytokines suppress adipogenesis and PPAR‐γ function through the TAK1/TAB 1/NIK cascade. Nat Cell Biol 2003; 5:224–30. Epub 2003/02/25. [DOI] [PubMed] [Google Scholar]
- 61. Birsoy K, Chen Z, Friedman J. Transcriptional regulation of adipogenesis by KLF4. Cell Metab 2008; 7:339–47. Epub 2008/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tussiwand R, Everts B, Grajales‐Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, et al Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 2015; 42:916–28. Epub 2015/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 2013; 93:1–21. Epub 2013/01/11. [DOI] [PubMed] [Google Scholar]
- 64. Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, et al Disruption of hypoxia‐inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high‐fat diet‐fed mice. Diabetes 2011; 60:2484–95. Epub 2011/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56:901–11. Epub 2007/03/31. [DOI] [PubMed] [Google Scholar]
- 66. Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, et al Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 2005; 280:847–51. Epub 2004/10/29. [DOI] [PubMed] [Google Scholar]
- 67. Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, et al Hypoxia and hypoxia‐inducible factor‐1α modulate lipopolysaccharide‐induced dendritic cell activation and function. J Immunol 2008; 180:4697–705. Epub 2008/03/21. [DOI] [PubMed] [Google Scholar]
- 68. Wobben R, Husecken Y, Lodewick C, Gibbert K, Fandrey J, Winning S. Role of hypoxia inducible factor‐1α for interferon synthesis in mouse dendritic cells. Biol Chem 2013; 394:495–505. Epub 2013/01/31. [DOI] [PubMed] [Google Scholar]
- 69. Everts B, Pearce EJ. Metabolic control of dendritic cell activation and function: recent advances and clinical implications. Front Immunol 2014; 5:203. Epub 2014/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 2015; 25:771–84. Epub 2015/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Winning S, Fandrey J. Dendritic cells under hypoxia: how oxygen shortage affects the linkage between innate and adaptive immunity. J Immunol Res 2016; 2016:5134329. Epub 2016/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pantel A, Teixeira A, Haddad E, Wood EG, Steinman RM, Longhi MP. Direct type I IFN but not MDA5/TLR3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly IC stimulation. PLoS Biol 2014; 12:e1001759. Epub 2014/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kohler T, Reizis B, Johnson RS, Weighardt H, Forster I. Influence of hypoxia‐inducible factor 1α on dendritic cell differentiation and migration. Eur J Immunol 2012; 42:1226–36. Epub 2012/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev 2005; 6:13–21. Epub 2005/01/19. [DOI] [PubMed] [Google Scholar]
- 75. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005; 26:439–51. Epub 2005/05/18. [DOI] [PubMed] [Google Scholar]
- 76. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al The fat‐derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7:941–6. Epub 2001/08/02. [DOI] [PubMed] [Google Scholar]
- 77. Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 2000; 96:1723–32. Epub 2000/08/29. [PubMed] [Google Scholar]
- 78. Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti‐inflammatory cytokines IL‐10 and IL‐1RA in human leukocytes. Biochem Biophys Res Commun 2004; 323:630–5. Epub 2004/09/17. [DOI] [PubMed] [Google Scholar]
- 79. Zhang H, Park Y, Zhang C. Coronary and aortic endothelial function affected by feedback between adiponectin and tumor necrosis factor α in type 2 diabetic mice. Arterioscler Thromb Vasc Biol 2010; 30:2156–63. Epub 2010/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tsang JY, Li D, Ho D, Peng J, Xu A, Lamb J, et al Novel immunomodulatory effects of adiponectin on dendritic cell functions. Int Immunopharmacol 2011; 11:604–9. Epub 2010/11/26. [DOI] [PubMed] [Google Scholar]
- 81. Jung MY, Kim HS, Hong HJ, Youn BS, Kim TS. Adiponectin induces dendritic cell activation via PLCγ/JNK/NF‐κB pathways, leading to Th1 and Th17 polarization. J Immunol 2012; 188:2592–601. Epub 2012/02/22. [DOI] [PubMed] [Google Scholar]
- 82. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998; 395:763–70. Epub 1998/10/31. [DOI] [PubMed] [Google Scholar]
- 83. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al Weight‐reducing effects of the plasma protein encoded by the obese gene. Science 1995; 269:543–6. Epub 1995/07/28. [DOI] [PubMed] [Google Scholar]
- 84. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996; 379:632–5. Epub 1996/02/15. [DOI] [PubMed] [Google Scholar]
- 85. Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 1999; 401:73–6. Epub 1999/09/15. [DOI] [PubMed] [Google Scholar]
- 86. Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol 2005; 174:6820–8. Epub 2005/05/21. [DOI] [PubMed] [Google Scholar]
- 87. Lam QL, Liu S, Cao X, Lu L. Involvement of leptin signaling in the survival and maturation of bone marrow‐derived dendritic cells. Eur J Immunol 2006; 36:3118–30. Epub 2006/11/28. [DOI] [PubMed] [Google Scholar]
- 88. Moraes‐Vieira PM, Larocca RA, Bassi EJ, Peron JP, Andrade‐Oliveira V, Wasinski F, et al Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur J Immunol 2014; 44:794–806. Epub 2013/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology 2013; 140:22–30. Epub 2013/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gonzalez‐Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009; 58:929–39. Epub 2009/01/13. [DOI] [PubMed] [Google Scholar]


