Abstract
Background
Studies have reported liver injury as a consequence of antipsychotic treatment. Very heavy alcohol drinking (ten or more drinks/day for men and eight for women) also causes liver injury. This study aims to evaluate liver injury with quetiapine extended release (XR) in very heavy drinking alcohol-dependent (AD) patients.
Methods
Two hundred and eighteen AD patients, 18–65 years of age, received 12 weeks of quetiapine XR or placebo treatment in a dose-escalated manner reaching the full dose of 400 mg/day during week 4. Blood chemistry and hematology were assessed at baseline (W0), post-titration at the end of week 3 (W4), week 8 (W8), and end of week 12 (W13). Patients were further grouped as GR.1 (no liver injury, ALT ≤40) and GR.2 (pre-existing liver injury, ALT >40) within each treatment. Drinking history, fasting blood glucose concentration (FBG), and lipid panel were used as covariates in the analyses.
Results
Liver injury and total drinks and average drinking measures from the Timeline follow-back questionnaire (TLFB) were highly associated. No significant exacerbation in liver injury was observed in patients treated with quetiapine XR in GR.2. Liver injury as determined by elevated alanine aminotransaminase (ALT) was reported in a few patients in GR.1 who received quetiapine XR; however, the occurrence was low, and the level of liver injury was not significant. FBG and lipid measures showed some elevation, but did not show any significant association with liver injury.
Conclusion
Quetiapine XR did not show any significant exacerbation of liver injury in very heavy drinking alcohol-dependent patients with pre-existing liver injury. Frequency and severity of new liver injury cases in quetiapine XR-treated patients without any pre-existing liver injury was also low. Study findings support medical management of AD patients with heavy drinking profile using quetiapine XR formulation.
1 Introduction
Efficacy of the older antipsychotic medications and their side effects have been matters of major concern [1]. Quetiapine is a dibenzothiazepine derivative acting as an antagonist of various neurotransmitter receptors in the brain [2]. It has been approved by the US Food and Drug Administration for treating bipolar disorder, schizophrenia, and depression either as monotherapy or as an add-on medication [3, 4]. Several recent studies have reported quetiapine to be a safer atypical second-generation antipsychotic drug [5, 6]. Case reports and some clinical trials have reported liver injury in patients treated with quetiapine; however, clinical studies have reported only mild liver injury to date [7–9]. Nevertheless, liver injury due to the extended-release formulation of quetiapine in a large clinical study has not been reported previously.
Alcohol drinking itself has several hazardous effects, and liver injury is one of the well-known consequences [10]. A large proportion of patients with bipolar and schizophrenia conditions report higher susceptibility to heavy alcohol drinking due to a shared genetic predisposition [11, 12]. Dual diagnosis is based on observations in a clinical population of patients suffering from both substance and mental disorders [13]. These disorders could be more persistent, severe, and treatment resistant than in patients with a single diagnosis of disorder [14, 15]. Recently, studies have reported a reduction in alcohol drinking markers in quetiapine-treated compared with placebo-treated alcoholics [16], i.e., alcohol craving and consumption and psychiatric symptoms in dually diagnosed alcoholics [17]. Thus, understanding the effects of quetiapine on the liver becomes much more important in understanding compliance with the drug that could benefit patients who drink heavily and are undergoing treatment for a mental disorder requiring quetiapine XR.
Quetiapine does not interact with the alcohol-metabolizing CYP enzyme CYP2E1, hence it does not have an additive or synergistic effect with alcohol consumption [18], although it does not necessarily mean that it might not dysregulate metabolism or would not cause injury in liver. However, drug safety recommendations for quetiapine include taking appropriate clinical assessments for liver problems before prescribing [19]. Therefore it isimportant to evaluate the incidence and level of liver injury due to the interaction of heavy alcohol drinking (in patients who are already predisposed to liver injury) and treatment with Quetiapine XR, which remains a gap both in scientific knowledge and in medical management. Antipsychotic drugs have shown altered lipid profile levels [20], with reports about causing clinically relevant elevations in the lipid panel, primarily in triglycerides [21], which could contribute to the severity of liver injury [22]. Hyperglycemia and weight gain have also been reported with second-generation antipsychotics as further adverse effects [8, 23, 24].
Furthermore, hepatic insufficiency has been shown to increase the blood concentration and half-life of quetiapine [25]; therefore, dose adjustment might become necessary in patients who show some level of hepatic impairment [26] to reduce non-compliance with treatment [27]. We postulated that this drug formulation would show better safety outcomes in patients with predisposed liver conditions. Thus, we aimed to examine the level and incidence of liver injury in two groups of heavy alcohol drinking patients who received 13 weeks of quetiapine XR treatment, one who had pre-existing liver injury, and another who had susceptibility to liver injury but did not have pre-existing liver injury. In addition, we also examined the role of BCG and lipid measures, and demographic measures in liver injury. We anticipated that the continued laboratory tests and examination during the course of the treatment would validate the changes.
2 Patients and Methods
2.1 Study Participant Population
This study is one of the investigational arms of a larger multisite protocol (ClinicalTrials.gov: NCT#00498628) that was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and was approved for publication under the NIAAA protocol CSP-1027. The Institutional Review Board of each of the sites that participated in this study approved recruitment and treatment of the patients under this multisite investigation.
This study was a double-blind, placebo-controlled, parallel-group design with two treatment arms: quetiapine XR as an active drug and placebo as a control. Inclusion criteria in this study included: diagnosis of alcohol dependence (using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition), age between 18 and 64 years, ten or more alcohol units/per day for men and eight or more alcohol units/per day for women (a standard drink contains 14 g of pure alcohol) for at least 40 % of the last 60 days of the 90-day drinking assessment (Time-line Follow-back; TLFB), 0.00 breath alcohol level at the time of consent, and 1.5 × 109/L or more absolute neutrophil count. Other study inclusion criteria included ALT elevation <3X the upper normal range of the ALT. Primary exclusion criteria were use of another psychoactive drug within the last year, positive urine screen for drugs, participation in another pharmacological/ behavioral study within the last 3 months, lifetime diagnosis of major depression or eating disorder, use of antidepressants (last 30 days) and antipsychotics (last 14 days) before randomization, and Clinical Institute Withdrawal Assessment of Alcohol score ≥10.
These patients were advised to not drink during the study as part of the medical management; however, the study was aimed to observe a reduction in alcohol consumption as an effect of treatment, therefore no other direct intervention was used. We further note that these patients did not show any overt clinically relevant presentation of liver injury.
2.2 Procedures and Assessments
The active drug, quetiapine XR (Seroquel XR® AstraZeneca, Wilmington, DE, USA), was provided to the participants for 3 months in 50- and 200-mg tablets with identical matching non-active pills for the placebo group titrated with a full dose of 400 mg/day [28] (in a dose-escalating manner reaching a plateau phase during week 4). Blood chemistry and clinical and subjective assessments were analyzed at baseline (0 W), end of week three (4 W), at week eight (8 W), and end of week 12 (13 W). All individuals received medical management that included assessment of medication side effects, participant education, and advice on drinking [29]. Study participants showed optimal compliance and patients who received active drug, quetiapine XR, reported overall adequate compliance as well of 96.1 % during the maintenance period. The average quetiapine dose received was 327.7 mg during the study (including the titration phase period), therefore no further validation of dose exposure was performed.
2.3 Data Collection, Statistical Paradigm, and Analysis
Individual demographics, namely age (years), sex (male or female), weight (kg), and drinking history patterns were collected at the time of baseline assessments and included in this study to estimate their role as baseline characteristics and potential factors in the quetiapine XR pharmaco-dynamics. For liver injury assessment, blood chemistry for ALT and aspartate aminotransaminase (AST) were evaluated at each time-point. Similarly, lipid panel including triglycerides and fasting blood glucose concentration (FBG) tests were also performed.
We used ALT as a reference for liver injury due to its usefulness in the evaluation of damage in hepatic tissue and management of liver injury (Medline Plus-National Institutes of Health, 2014). ALT is more specific for hepatic injury, and mildly elevated levels of ALT could be an indication of serious underlying conditions [30]. Thus, ALT was used as the primary marker for liver injury in this patient population and 40 µl was used as the upper limit of normal (no liver injury, GR.1); and >40 µl at baseline as elevated liver injury (GR.2) to study liver injury-based groups separately within each treatment arm (Fig. 1). Recent TLFB measures [31] developed from the raw data examination included total drinks in the past 90 days (TD90), number of drinking days past 90 days (NDD90), average drinks per drinking day in past 90 days (DPD90), and heavy drinking days (defined as five or more drinks per day for a man and four or more drinks per day for a woman) in the past 90 days (HDD90).
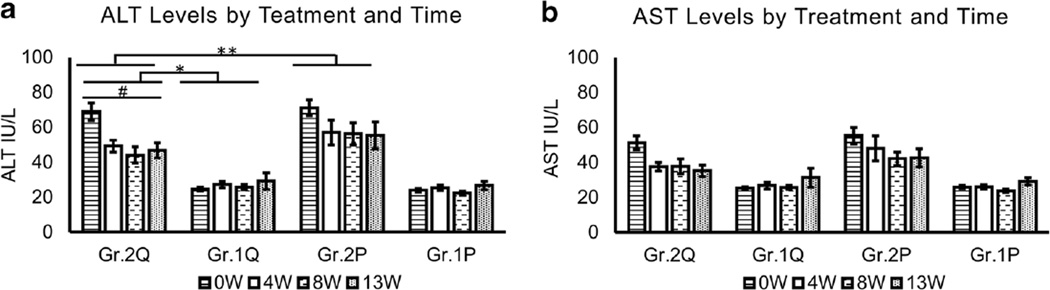
Fig. 1.
Assessment of liver injury markers during the study period. a ALT assessment. #Elevated group patients showed robust differences R2 = 0.442 in ALT levels during the medication time-course at p = 0.001. *Significant contrast in the treatment course between the baseline elevated and non-elevated groups of the quetiapine arm F(3,82) = 10.437, p < 0.01 (F statistics). **There was a significant Time × Treatment × Liver-injury group interaction. b AST assessment. There was no significant elevation in association determined between AST levels through the treatment course of the quetiapine arm. Time by Treatment repeated analysis of variance statistical test was used. Data are presented as mean ± standard error. *Statistical significance was set at p ≤ 0.05. Non-elevated group from baseline. Gr.2 elevated group from baseline. Q in Quetiapine treatment group. P in Placebo treatment group. ALT normal range 6–40 IU/L; AST normal range 10–34 IU/L
Demographic and drinking history measures were evaluated using univariate analysis. Liver enzymes were evaluated as the primary dependent measures. Association of liver injury and drinking history was performed using a linear regression model. Changes in liver injury markers were assessed using repeated measures analysis of variance (ANOVA) across the time-point measures. Age, weight, and recent and lifetime drinking history measures were included as covariates, and post-hoc analysis was performed to evaluate confounding factors on the outcomes. Lipid panel and FBG were evaluated for their association with liver injury at each time-point, and whether or not their association was contributing to liver injury in addition to drinking history. F-statistics is the test statistic for the ANOVA approach to test the significance of the model or the components in the model, which in our study was needed to estimate the association between the measures of a subdivided population.
Data analysis platforms used in this study were SPSS 22.0 version (IBM, Chicago, IL, USA) and MS Office Excel 2013 (MS Corporation, Redmond, WA, USA). Statistical significance was set at p ≤ 0.05. Specific significances or trend levels are mentioned individually wherever needed.
3 Results
3.1 Patient Characterization and Drinking History
One hundred and seventy-nine men and 45 women were randomized in this study and 218 started the treatment (Table 1). There were no significant differences in the demographic measures between the treatment arms and subgroups (Table 1). Frequency of heavy drinking days (HDD90) showed a significant (p = 0.029) treatment by sex interaction, with lower values observed in the placebo male group compared to the males of the active drug group. In the placebo group, none of the female participants exhibited liver injury at the baseline.
Table 1.
Patient demographics and timeline for last 90 days for drinking history collected at baseline by treatment arm and sex
| Measures | Quetiapine XR |
Placebo |
||
|---|---|---|---|---|
| Males (n = 78) | Females (n = 29) | Males (n = 101) | Females (n = 16) | |
| Age (years) | 45.6 ± 10.5 | 47.0 ± 7.6 | 44.5 ± 9.1 | 47.6 ± 10.0 |
| Height (cm) | 170.87 ± 29.2 | 168.57 ± 33.1 | 168.91 ± 36.3 | 175.59 ± 8.6 |
| Weight (kg) | 85.63 ± 17.6 | 85.78 ± 20.0 | 87.02 ± 21.9 | 80.20 ± 24.3 |
| Recent drinking history (TLFB90) | ||||
| Total drinks (TD90)* | 1296.7 ± 499.4 | 1023.3 ± 367.0 | 1227.9 ± 497.1 | 919.0 ± 395.9 |
| Number of drinking days (NDD90)* | 81.0 ± 14.3 | 83.4 ± 9.4 | 78.9 ± 14.3 | 80.7 ± 15.0 |
| Average drinks/day (AvgDD90)*) | 16.3 ± 6.0 | 12.4 ± 4.9 | 15.8 ± 6.5 | 11.5 ± 4.6 |
| Number of heavy drinking days (NDD90)* | 71.0 ± 19.2 | 74.4 ± 17.2 | 61.7 ± 23.2 | 80.7 ± 11.2 |
Data presented as mean ± standard deviation. Statistical significance was set at p ≤ 0.05
There was a significant treatment × sex interaction in TD90, AvgDPD90 and HDD90 drinking measures. Statistical significance at p ≤ 0.05
3.2 Association of Liver injury with Drinking History
TD90 and AvgDPD90 showed a robust association with baseline liver enzymes (ALT and AST) in the quetiapine-treated patients (p < 0.01), suggesting liver injury likely due to the drinking profile at baseline. Other drinking measures did not show any association with liver injury in the quetiapine-treated patients.
3.3 Evaluation of Liver injury by Treatment and Time
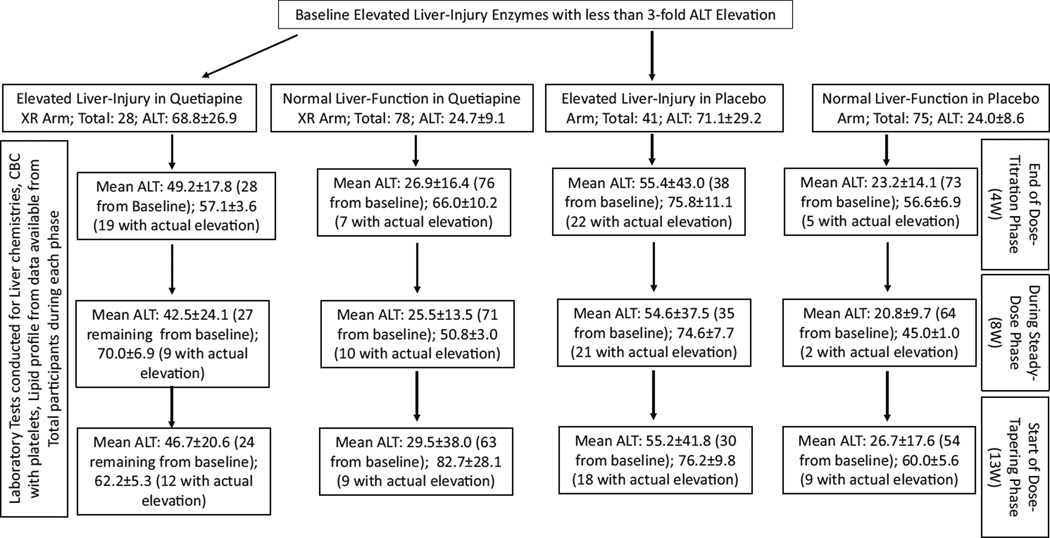
Baseline ALT was 2.5-fold higher in the GR.2 (baseline elevated-ALT group) than the patients with ALT in the normal range (GR.1) in both the treatment arms. Based on the study design, we observed expected significant main effect of ALT in the liver injury group (Gr.2) between each treatment arm (Fig. 1a). Only 67 % of the participants who had baseline elevated ALT levels in the quetiapine arm continued to show elevated ALT by the end of titration (4 W). This decrease was at 33 % during steady-state in the same group (8 W); however, there was a slight increase up to 50 % at the end of steady phase (13 W). Importantly, there was a significant decrease in ALT levels in quetiapine-treated patients in GR.2.
Compared to baseline, fewer participants showed elevated ALT levels at the subsequent time-points in the quetiapine arm who had baseline liver injury (Fig. 1a and Suppl. Fig 1). Even the sub-group who exhibited baseline elevated liver injury (GR.2) and received placebo, showed a lower frequency of liver injury (Suppl. Fig. 1). Repeated ANOVA analysis of the quetiapine-administered elevated liver injury group patients showed robust significant differences, adjusted R2 = 0.442 (p = 0.001), in ALT levels during the medication time-course (Fig. 1a). Furthermore, there was a significant ALT difference between the Gr. 1 and Gr. 2 quetiapine-treated patients across all the time-points, F(3, 82) = 10.437, p ≤ 0.01 (Fig. 1a), between GR.1 and GR.2. In post-hoc analysis, this significance was observed in GR.1, in which there was an increase in the number of participants with elevated ALT at later time points, although overall, the mean ALT values were not clinically relevant. Body mass index (BMI) did not show any augmentation of results. Thus, quetiapine XR showed an effect by elevating liver injury markers over the time course, but still within the normal range, and clinically these elevations were not important.
Placebo group participants showed a similar drop both in ALT levels and in number of participants exhibiting ALT elevation (Suppl. Fig. 1). During the titration phase, minor elevation of ALT was observed in patients with no pre-existing liver injury in the placebo arm, although not clinically significant. This elevation was comparable to the level of liver injury in the same cohort in the quetiapine arm (Fig. 1a). No specific finding was observed in liver injury by time in the placebo group. No significant quetiapine XR versus placebo treatment differences in AST levels were observed. In the quetiapine group, AST levels were lowered through each phase and did not go above the upper limit of the normal range in GR.1 patients (Fig. 1b).
We assessed the distribution of patients on quetiapine who showed ALT elevation at each time-point during the course of treatment using a Chi-squared test (Table 2). There was a moderate increase in probability (albeit only showing a trend level of significance)/likelihood of liver injury at 8 W in GR.2 patients (with baseline liver injury) treated with quetiapine when compared with the same cohort of the placebo arm (Table 2). We conducted the same assessment on GR.1 patients without pre-existing liver injury and a moderate increase in the likelihood of liver injury was observed at 8 W only in quetiapine-treated GR.1 patients (Table 2) compared to the same cohort of the placebo arm. At week 4 (4 W) or week 13 (13 W) we did not find any such changes in the distribution in both the analyses. Furthermore, these elevations were not clinically significant in that they required no medical management. Further, we also reviewed the role of lipid markers on blood glucose concentration. There was no statistical significance determined using weight as a covariate in the analyses.
Table 2.
Assessment of elevation of ALT by treatment during the study course at four (4 W), eight (8 W) and 13 weeks (13 W) in patients
| Timeline | Pearson’s Chi-square | Likelihood ratio |
|---|---|---|
| (A) GR.2 Patients with elevated ALT at baseline | ||
| 4-week | 0.680 (p = 0.410) | 0.685 (p = 0.408) |
| 8-week | 5.279 (p = 0.071) | 5.351 (p = 0.069) |
| 13-week | 1.778 (p = 0.411) | 1.826 (p = 0.401) |
| (B) GR.1 Patients with normal ALT at baseline | ||
| 4-week | 0.142 (p = 0.707) | 0.142 (p = 0.706) |
| 8-week | 4.8 (p = 0.029) | 5.221 (p = 0.022) |
| 13-week | 0.190 (p = 0.663) | 0.189 (p = 0.664) |
p-value that does not support relevance of the elevation is depicted in bold
A: Analysis was conducted in patients with baseline elevated ALT receiving different study treatment (active drug vs. placebo). Chi-square test was conducted between treatment and frequency of all patients with elevated or non-elevated ALT at each time-point
B: Analysis was conducted in patients with baseline normal ALT receiving a different study treatment (active drug vs. placebo). Chi-square test was conducted between treatment and frequency of all patients with elevated or non-elevated ALT at each time-point. Statistical significance was set at p ≤ 0.05
At 4-week and 13-week, likelihood of occurrence of ALT elevation did not reach any significant probability between the quetiapine and placebo sub-arms in either group (A [GR.2] vs. B [GR.1]). At 8-week, there was moderate probability of ALT elevation between the quetiapine and placebo sub-arms that was significant, however it was similar in both the groups (A [GR.2] vs. B [GR.1])
3.4 Role of Lipid Profile
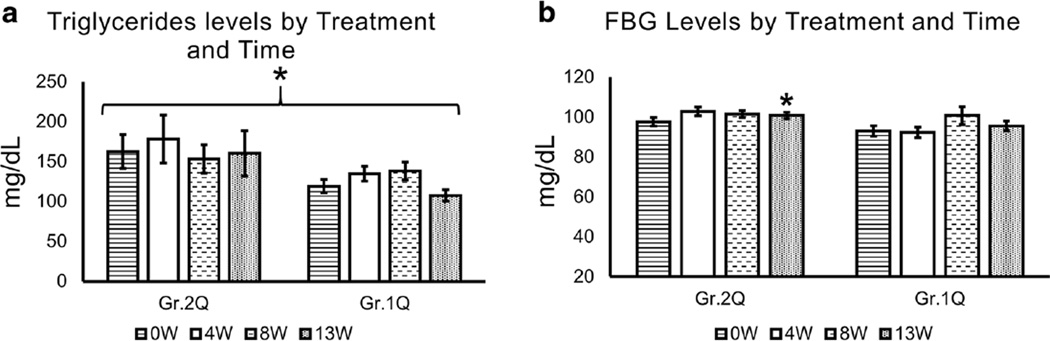
Among the GR.2 patients (those with baseline high ALTs), triglycerides were approximately 1.5-fold higher at baseline in each treatment arm (Fig. 2a). In all quetiapine-treated patients, there was a significant elevation in triglycerides during the study period, with mild elevation at 4 W (titration end) and 13 W (end of medication at steady dose) in both groups of the quetiapine arm. However, when we conducted a linear regression analysis to estimate the association between triglycerides and liver injury markers, ALT and AST independently, we did not find any significant correlations at any given timeline with quetiapine treatment. Other lipid markers were not clinically significant.
Fig. 2.
Serum triglyceride and fasting blood glucose evaluation of study participants by Time and Treatment. Within the quetiapine arm, triglyceride levels were higher in patients with baseline liver injury, and showed significant between-subjects effects at each time-point, p = 0.041, when TD90 was co-varied. *Significant time × treatment effect in observed in glucose level in quetiapine arm, p = 0.054, post hoc test showed an effect at 13 W. Time by Treatment repeated analysis of variance statistical test was used. Data are presented as mean ± standard error. *Statistical significance was set at p ≤ 0.05. Gr.1 Non-elevated group from baseline. Gr.2 Elevated group from baseline. Q in Quetiapine treatment group. P in Placebo treatment group. Triglycerides: normal <150, borderline-high range 150–199, high triglycerides range 200–499, and very high triglycerides ≥500. Normal fasting blood glucose (FBG) range was 60–115 mg/dl
3.5 Role of Glucose
There was a significant difference between the Gr.2 and Gr.1 treatment effect on FBG levels, p = 0.037 (Fig. 2b). However, we did not find any significant association between the FBG level and liver injury (as assessed by ALT) at any time points. We also tested BCG as a covariate in the elevated ALT group (GR.2) using repeated ANOVA by treatment and time to evaluate augmentation of results; however, FBG did not show any augmentation of the results.
4 Discussion
Study results supported our primary aim that a quetiapine XR formulation did not exacerbate liver injury in patients with pre-existing liver injury. Notably, there was a decrease in ALT levels from baseline in the elevated ALT group (Gr.2) of the active drug (quetiapine XR) arm (Fig. 1). This gave support for the fact that the drug was well tolerated in these patients. We also found liver marker ALT elevated above normal levels in a few patients who otherwise had normal liver enzymes at the pre-treatment stage (GR.1). However, this increase was observed only in a small fraction of patients (Table 2) and this increase was mild (Suppl. Fig. 1). In our study, quetiapine XR could be compared with a placebo-level incidence of hepatotoxicity with an indistinguishable effect from placebo in ALT levels at the corresponding course of treatment. Drug-induced liver injury (DILI) is described as liver test abnormalities due to the pharmacological action of the drugs administered, and it accounts for 7–15 % of the cases of acute liver failure in the USA and Europe alone, leading to withdrawal of approved drugs [32, 33]. DILI is a common consequence of medications [34], and we noticed a mild form of it in our study too.
One study in patients with major depression did not report any liver injury in any of the quetiapine XR-treated patients [21]; however, none of the patients in that study were alcohol dependent, drinking heavily, or had any clinical determination of pre-existing liver injury. In our study, the significance of the incidence in liver injury as new cases was observed only at the end of titration (Table 2); however, this was moderate and limited to new liver injury cases. Among the patients with pre-existing liver injury, the significance of incidence was moderate; however, it was not significantly different to the placebo arm. Therefore, our results show the likeliness of such an observation (in liver injury) is most probable after titration or when the dose level is ascending (Fig. 3). Oxcarbazepine [35] and baclofen [36] have also shown a low hepatic impact; it is to be noted that the patients were detoxified prior to treatment in the oxcarbazepine study, and patients in the baclofen study drank relatively less at baseline assessment compared to our study cohort. Gabapentin and pregabalin treatment for neuropathic pain has been reported in a few cases to cause liver injury [37, 38]; however, one review concludes it to be safe in mild alcoholic liver disease [39]. In this context quetiapine XR provides therapy in an alcohol-drinking population where hepatic injury is likely, and interaction of the treatment could potentially result in liver injury or exacerbation of ongoing liver injury.
Fig. 3.
Evaluation of liver injury by incidence and level during the study period. “Total” shows records included in the study from the baseline number of participants at each study phase. Data presented as Mean ± SE
Heavy drinking is a well accepted cause of liver injury. Appropriate assessment of drinking history as a potential risk factor should be accounted for in estimating the risk of exacerbation in pre-existing liver-injury during treatment with quetiapine XR. Assessment of the drinking profile followed up with routine blood chemistry in order to identify liver injury in patients who report heavy drinking during the intake may be useful to develop a treatment plan to estimate the potential interaction of drugs in such patients.
Investigations into the second generation antipsychotics have shown nonalcoholic steatosis of the liver in animal models [40]. An elevated lipid panel has been reported with quetiapine XR treatment [41], which was observed in our study as well (primarily in triglycerides); however, we did not find any significant correlation with liver injury during the treatment course. We also found a similar elevation in FBG [42]; however, this was also not associated with the liver injury. Such laboratory findings on lipid panel and blood glucose levels could be valuable in making decisions for prescribing quetiapine XR.
Our study had some limitations. One limitation was that we did not include continued alcohol drinking as a covariate in the analyses for treatment and liver injury; however, the injury was not significantly different from that of the placebo group. Our study objective was to monitor the overall level of injury from the treatment along with drinking. Moreover, drinking assessment during the treatment did not change much [28]. Furthermore, our study was not intended to investigate liver injury of individual effects of drinking as a factor, which would not have improved the results. There were threefold more men in this study than women, though since this occurred in all the groups evenly, this did not affect the analysis; however, conducting a sex-difference analysis was not optimal. There was a limited enrolment of females that restricted analysis as to potential sex differences in our study. A few patients did not continue with the study (less than 5 %). Moreover, tests for some patients were unavailable in this investigation, and we could not include these patients in the analysis.
Results from our investigation provided valuable information on the safety of a susceptible liver condition with quetiapine XR treatment that could be compared with reports on liver safety assessment with other antipsychotics in previously published studies (Table 3) either with quetiapine or other second-generation drugs. This qualitative analysis supports the use of quetiapine XR in patients who are vulnerable to liver injury due to heavy drinking (Table 3).
Table 3.
Hepatotoxicity report from published articles of second-generation antipsychotic drugs in human subjects. Our study was conducted in patients who were actively drinking and had a heavy drinking profile
| Study | Drug and dose | Treatment population (baseline liver status) |
New hepatotoxicity incidence % |
Level of Liver injury reported (ALT IU/l) |
Patients actively drinking (yes/ no) |
|---|---|---|---|---|---|
| Kurz et al. [45] | Clozapine (193.7 mg/d) | 167 Patient (normal liver tests) |
W1–6: (18/59) 30.5%; W13–18: (7/59) 11.9%; |
W1–6: 69.5 ± 28.1 W13–18: 62.35 ± 20.6 (only with liver injury) |
No |
| Atasoy et al. [43] | Olanzapine (14.8 mg/d) | 33 Patients (normal liver tests) |
4 W: (4/33) 12.1% | 4 W: 27.8 ± 14.4 24 W: 24.5 ± 14.3 (adjusted overall mean) |
No |
| Atasoy et al. [43] | Risperidone (2.9 mg/d) | 29 Patients (normal liver tests) |
4 W: (3/29) 10.3% | 4 W: 20.6 ± 13.9 24 W: 26.7 ± 14.0 (adjusted overall mean) |
No |
| Atasoy et al. [43] | Quetiapine (244 mg/d) | 48 Patients (normal liver tests) |
4 W: (4/48) 8.3% | 4 W: 23.2 ± 14.0 24 W: 26.1 ± 13.9 (adjusted overall mean) |
No |
| Wang et al. [21] | Quetiapine XR (50–300 mg/d titrated) |
157 Patients (normal liver tests) |
None reported | None reported | No |
| Present Study (CSP-1027) |
Quetiapine XR (50–400 mg/d titrated) |
78 Patients (normal liver tests) |
4 W: 7/ 76 (9%); 8 W: 10/ 71 (14%); 13 W: 9/ 63 (14%). |
4 W: 66.0 ± 10.2; 8 W: 50.8 ± 3.0; 13 W: 82.7 ± 28.1 (Only with liver injury) |
Yes |
| Present Study (CSP-1027) |
Quetiapine XR (50–400 mg/d titrated) |
29 Patients (confirmed liver injury) |
4 W: 19/ 28 (68%); 8 W: 9/ 27 (33%); 13 W: 12/ 24 (50%). |
4 W: 57.1 ± 3.6; 8 W: 70.0 ± 6.9; 13 W: 62.2 ± 5.3 (Only with liver injury) |
Yes |
mg milligram, d day, W weeks, CSP Clinical Study Protocol
To monitor changes in patients with a susceptible liver state, time-course laboratory analyses are needed before and during treatment. One larger clinical study supported obtaining baseline liver injury before using antipsychotic therapy [43]; however, their patients did not have any susceptibility to liver injury. Our study corroborates the significance of evaluating patients by identifying predisposition in liver condition by screening them before treatment. Therefore pre-treatment screening could help patients who have underlying liver disease [44] or who exhibit vulnerability in liver condition in obtaining appropriate antipsychotic treatment.
5 Conclusions
In this study, alcohol-dependent patients susceptible to liver injury were treated with quetiapine XR and investigated for medication safety. Exacerbation of liver injury in quetiapine XR-treated alcohol-dependent patients (with pre-existing liver injury) was not significant; also new cases of liver injury in patients treated with quetiapine XR, who did not have pre-existing liver injury, were not higher in incidence or severity as reported from other studies. Quetiapine XR causes elevation in lipid panel and blood glucose concentrations; however, this was not clinically significant and not associated with liver injury. Quetiapine XR could prove to be a safer medication for patients with predisposed liver injury due to heavy alcohol drinking. Routine laboratory assessment for liver, lipid panel, and blood glucose could enhance the safety of treatment with quetiapine XR.
Supplementary Material
Key Points.
Exacerbation of liver injury in quetiapine XR-treated alcohol-dependent patients (with pre-existing liver injury) was not significant; furthermore, new cases of liver injury in patients treated with quetiapine XR who did not have pre-existing liver injury were not higher in incidence or severity as reported in other studies.
Quetiapine XR causes elevation in lipid panel and blood glucose concentration, however, this was not clinically significant and not associated with liver injury.
Quetiapine XR could be a safer medication for patients with predisposed liver injury due to heavy alcohol drinking.
Routine laboratory assessment for liver, lipid panel, and blood glucose could enhance the safety of treatment with quetiapine XR.
Acknowledgments
Study evaluation was conducted at UofL Alcohol Research Center. We thank Ms. Marion McClain for editing the manuscript.
Compliance with Ethical Standards
Funding This study was supported by the NIH - National Institute of Alcohol Abuse and Alcoholism: CSP-1027 (VV) and Z99-AA999999 (VV); P50-AA024337-01, U01-AA021901 and R37-AA010762 (CJM); and ZIA AA000466 (VAR).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s40261-016-0439-2) contains supplementary material, which is available to authorized users.
Author Contribution VV is the project PI, and designed this study. VV and MS were responsible for data management. VV conducted the data analysis. VV interpreted the data analysis. VV wrote this manuscript. MS, SSB, AP, MCC, VAR, and CJM provided significant scientific input into this project. All authors have approved the final submission version of this manuscript.
Vijay A Ramchandani and Craig J. McClain are senior authors.
Declaration/Conflict of interest Vatsalya Vatsalya, Akash Pandey, Melanie L. Schwandt, Shirish S. Barve, Matthew C. Cave, Vijay A. Ramchandani, and Craig J. McClain declare that they have no conflicts of interest.
Ethical Approval All procedures in this study were in accordance with the 1964 Helsinki Declaration (and its amendments).
Ethics Committee This study was approved by the IRB of specific sites that recruited patients.
Informed Consent All study patients enrolled in this study consented to participate in this treatment study.
NIH and Public Access Policy This article is a US Government-sponsored work and is in the public domain in the USA.
References
- 1.Goldstein JM. Quetiapine fumarate (Seroquel): a new atypical antipsychotic. Drugs Today (Barcelona, Spain: 1998) 1999;35(3):193–210. doi: 10.1358/dot.1999.35.3.533849. [DOI] [PubMed] [Google Scholar]
- 2.Balestrieri M, Vampini C, Bellantuono C. Efficacy and safety of novel antipsychotics: a critical review. Human Psychopharmacol Clin Experim. 2000;15(7):499–512. doi: 10.1002/1099-1077(200010)15:7<499::AID-HUP194>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Kane JM. Pharmacologic treatment of schizophrenia. Biol Psychiatry. 1999;46(10):1396–1408. doi: 10.1016/s0006-3223(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 4.Thase ME, Macfadden W, Weisler RH, Chang W, Paulsson B, Khan A, Calabrese JR. Bolder II. Study Group. Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study) J Clin Psychopharmacol. 2006;26(6):600–609. doi: 10.1097/01.jcp.0000248603.76231.b7. [DOI] [PubMed] [Google Scholar]
- 5.Findling RL, Pathak S, Earley WR, Liu S, DelBello MP. Efficacy and safety of extended-release quetiapine fumarate in youth with bipolar depression: an 8 week, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2014;24(6):325–335. doi: 10.1089/cap.2013.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao K, Wu R, Kemp DE, Chen J, Karberg E, Conroy C, Chan P, Ren M, Serrano MB, Ganocy SJ. Efficacy and safety of quetiapine-XR as monotherapy or adjunctive therapy to a mood stabilizer in acute bipolar depression with generalized anxiety disorder and other comorbidities: a randomized, placebo-controlled trial. J Clin Psychiatry. 2014;75(10):1–478. doi: 10.4088/JCP.13m08847. [DOI] [PubMed] [Google Scholar]
- 7.Naharci MI, Karadurmus N, Demir O, Bozoglu E, Ak M, Doruk H. Fatal hepatotoxicity in an elderly patient receiving low-dose quetiapine. Am J Psychiatry. 2011;168(2):212. doi: 10.1176/appi.ajp.2010.10091292. [DOI] [PubMed] [Google Scholar]
- 8.Rettenbacher MA, Baumgartner S, Eder-Ischia U, Edlinger M, Graziadei I, Hofer A, Huber R, Hummer M, Kemmler G, Weiss E, Fleischhacker WW. Association between antipsychotic-in-duced elevation of liver enzymes and weight gain: a prospective study. J Clin Psychopharmacol. 2006;26(5):500–503. doi: 10.1097/01.jcp.0000236654.85791.ae. [DOI] [PubMed] [Google Scholar]
- 9.El Hajj I, Sharara AI, Rockey DC. Subfulminant liver failure associated with quetiapine. Eur J Gastroenterol Hepatol. 2004;16(12):1415–1418. doi: 10.1097/00042737-200412000-00029. [DOI] [PubMed] [Google Scholar]
- 10.Reid MC, Fiellin DA, O’Connor PG. Hazardous and harmful alcohol consumption in primary care. Arch Intern Med. 1999;159(15):1681–1689. doi: 10.1001/archinte.159.15.1681. [DOI] [PubMed] [Google Scholar]
- 11.Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry. 2002;59(12):1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- 12.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- 13.Kessler RC, Merikangas KR. The National Comorbidity Survey Replication (NCS-R): background and aims. Int J Methods Psychiatr Res. 2004;13(2):60–68. doi: 10.1002/mpr.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolese HC, Malchy L, Negrete JC, Tempier R, Gill K. Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr Res. 2004;67(2):157–166. doi: 10.1016/S0920-9964(02)00523-6. [DOI] [PubMed] [Google Scholar]
- 15.Brady KT, Back SE, Coffey SF. Substance abuse and posttraumatic stress disorder. Curr Dir Psychol Sci. 2004;13(5):206–209. [Google Scholar]
- 16.Kampman KM, Pettinati HM, Lynch KG, Whittingham T, Macfadden W, Dackis C, Tirado C, Oslin DW, Sparkman T, O’Brien CP. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007;27(4):344. doi: 10.1097/JCP.0b013e3180ca86e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinotti GI, Andreoli S, Di Nicola MA, Di Giannantonio M, Sarchiapone M, Janiri L. Quetiapine decreases alcohol consumption, craving, and psychiatric symptoms in dually diagnosed alcoholics. Human Psychopharmacol Clin Experim. 2008;23(5):417–424. doi: 10.1002/hup.944. [DOI] [PubMed] [Google Scholar]
- 18.DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine. Clin Pharmacokinet. 2001;40(7):509–522. doi: 10.2165/00003088-200140070-00003. [DOI] [PubMed] [Google Scholar]
- 19.Food and Drug Administration [Internet] Silver Spring (MD: Medication Guide Seroquel; [[cited 2013, October]]. [Google Scholar]
- 20.Melkersson KI, Hulting AL, Brismar KE. Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychoses. J Clin Psychiatry. 2000;61(10):742–749. doi: 10.4088/jcp.v61n1006. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, McIntyre A, Earley WR, Raines SR, Eriksson H. A randomized, double-blind study of the efficacy and tolerability of extended-release quetiapine fumarate (quetiapine XR) monotherapy in patients with major depressive disorder. Neuropsychiatr Dis Treat. 2014;10:201. doi: 10.2147/NDT.S50248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 23.Fertig MK, Brooks VG, English CW. Hyperglycemia associated with olanzapine. J Clin Psychiatry. 1998;59(12):1–478. doi: 10.4088/jcp.v59n1208c. [DOI] [PubMed] [Google Scholar]
- 24.Suppes T, Vieta E, Liu S, Brecher M, Paulsson B. Trial 127 Investigators. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127) Am J Psychiatry. 2009;166(4):476–488. doi: 10.1176/appi.ajp.2008.08020189. [DOI] [PubMed] [Google Scholar]
- 25.DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine. Clin Pharmacokinet. 2001;40(7):509–522. doi: 10.2165/00003088-200140070-00003. [DOI] [PubMed] [Google Scholar]
- 26.AstraZeneca [Internet] [[cited 2013, October]];Wilmington (DE). Prescribing information for SEROQUEL XR including Boxed Warnings. 2013 [Google Scholar]
- 27.Masand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289–2304. doi: 10.1016/s0149-2918(03)80220-5. [DOI] [PubMed] [Google Scholar]
- 28.Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, Murtaugh C, Ciraulo D, Green AI, Johnson B, Pettinati H. A, Double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406–416. doi: 10.1111/j.1530-0277.2011.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56(10):785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Phys. 2005;71(6):1105. [PubMed] [Google Scholar]
- 31.Sobell LC, Sobell MB, Connors GJ, Agrawal S. Assessing drinking outcomes in alcohol treatment efficacy studies: selecting a yardstick of success. Alcohol Clin Exp Res. 2003;27(10):1661–1666. doi: 10.1097/01.ALC.0000091227.26627.75. [DOI] [PubMed] [Google Scholar]
- 32.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 33.Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, Lenoir C, Lemoine A, Hillon P. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36(2):451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 34.Kaplowitz N. Drug-induced liver disorders. Drug Saf. 2001;24(7):483–490. doi: 10.2165/00002018-200124070-00001. [DOI] [PubMed] [Google Scholar]
- 35.Martinotti G, Di Nicola M, Romanelli R, Andreoli S, Pozzi G, Moroni N, Janiri L. High and low dosage oxcarbazepine versus naltrexone for the prevention of relapse in alcohol-dependent patients. Human Psychopharmacol Clin Experim. 2007;22(3):149–156. doi: 10.1002/hup.833. [DOI] [PubMed] [Google Scholar]
- 36.Addolorato G, Leggio L, Ferrulli A, Cardone S, Bedogni G, Caputo F, Gasbarrini G, Landolfi R. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312–317. doi: 10.1093/alcalc/agr017. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y, Yamazaki Y, Hashizume H, Kobayashi T, Ohyama T, Horiguchi N, Sato K, Kakizaki S, Kusano M, Yamada M. Endoscopic treatment for esophageal varices complicated by Isaacs’ syndrome involving difficulty with conventional sedation. Clin J Gastroenterol. 2016;9(1):27–31. doi: 10.1007/s12328-016-0626-y. [DOI] [PubMed] [Google Scholar]
- 38.Sendra JM, Junyent TT, Pellicer MJ. Pregabalin-induced hepatotoxicity. Ann Pharmacother. 2011;45(6):e32. doi: 10.1345/aph.1Q032. [DOI] [PubMed] [Google Scholar]
- 39.Addolorato G, Mirijello A, Barrio P, Gual A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt RH, Jokinen JD, Massey VL, Falkner KC, Shi X, Yin X, Zhang X, Beier JI, Arteel GE. Olanzapine activates hepatic mammalian target of rapamycin: new mechanistic insight into metabolic dysregulation with atypical antipsychotic drugs. J Pharmacol Exp Ther. 2013;347(1):126–135. doi: 10.1124/jpet.113.207621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaren KD, Marangell LB. Special considerations in the treatment of patients with bipolar disorder and medical co-morbidities. Ann Gen Psychiatry. 2004;3(1):7. doi: 10.1186/1475-2832-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirshing DA, Boyd JA, Meng LR, Ballon JS, Wirshing WC. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry. 2002;63(10):1–478. doi: 10.4088/jcp.v63n1002. [DOI] [PubMed] [Google Scholar]
- 43.Atasoy N, Erdogan A, Yalug I, Ozturk U, Konuk N, Atik L, Ustundag Y. A review of liver function tests during treatment with atypical antipsychotic drugs: a chart review study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1255–1260. doi: 10.1016/j.pnpbp.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 45.Kurz M, Hummer M, Oberbauer H, Fleischhacker WW. Extrapyramidal side effects of clozapine and haloperidol. Psychopharmacology. 1995;118(1):52–56. doi: 10.1007/BF02245249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.