Abstract
Aluminium salts, present in many industrial products of frequent use like antiperspirants, anti‐acid drugs, food additives and vaccines, have been incriminated in contributing to the rise in breast cancer incidence in Western societies. However, current experimental evidence supporting this hypothesis is limited. For example, no experimental evidence that aluminium promotes tumorigenesis in cultured mammary epithelial cells exists. We report here that long‐term exposure to concentrations of aluminium—in the form of aluminium chloride (AlCl3)—in the range of those measured in the human breast, transform normal murine mammary gland (NMuMG) epithelial cells in vitro as revealed by the soft agar assay. Subcutaneous injections into three different mouse strains with decreasing immunodeficiency, namely, NOD SCID gamma (NSG), NOD SCID or nude mice, revealed that untreated NMuMG cells form tumors and metastasize, to a limited extent, in the highly immunodeficient and natural killer (NK) cell deficient NSG strain, but not in the less permissive and NK cell competent NOD SCID or nude strains. In contrast, NMuMG cells transformed in vitro by AlCl3 form large tumors and metastasize in all three mouse models. These effects correlate with a mutagenic activity of AlCl3. Our findings demonstrate for the first time that concentrations of aluminium in the range of those measured in the human breast fully transform cultured mammary epithelial cells, thus enabling them to form tumors and metastasize in well‐established mouse cancer models. Our observations provide experimental evidence that aluminium salts could be environmental breast carcinogens.
Keywords: aluminium, breast cancer, antiperspirants, carcinogen, mouse models of cancer, metastasis
Short abstract
What's new?
Aluminium salts, present in many industrial products of frequent use like antiperspirants, anti‐acid drugs, food additives, and vaccines, have been incriminated in contributing to the rise in breast cancer incidence in Western societies. However, current experimental evidence supporting this hypothesis is limited. Here, the authors report that long‐term exposure to concentrations of aluminium in the range of those measured in the human breast enables normal murine mammary gland (NMuMG) epithelial cells to form tumors and metastasis in well‐established mouse cancer models. The observations indicate that aluminium salts could be environmental breast carcinogens.
Abbreviations
- HE
Hematoxylin/eosin
- NK
natural killer
- NMuMG
Normal murine mammary gland
- NSG
NOD SCID gamma
Aluminium, the most abundant metal in the Earth's crust, has no recognized role in biological systems. Due to its versatile chemistry, aluminium is included in a variety of industrial products of daily use including food additives, anti‐acid drugs, household products, vaccines and cosmetics.1
Aluminium is generally assumed to be safe, but the biological consequences of its interaction with biological systems are largely unknown. Whereas its neurotoxic potential is generally acknowledged, little data exist on the effects of aluminium on the biology of mammalian cells. Aluminium has been reported to interfere with the binding of estradiol to estrogen receptor and to enhance transcription from an estrogen‐responsive element in a reporter system.2, 3 A genotoxic effect of aluminium has been reported in several systems, including human peripheral blood lymphocytes4, 5 (references therein).
Aluminium salts, contained in antiperspirants at concentrations up to several molar units (http://www.fda.gov/RegulatoryInformation/Dockets/ucm130350.htm), have been hypothesized to reach the mammary epithelium at significant doses as a consequence of daily application to the skin of the underarm, thus resulting in a continuous exposure. Consistent with this view, aluminium is adsorbed through the human and mouse skin,6, 7, 8, 9 and significant amounts of this metal have been measured in several compartments of the human breast including breast tissue, nipple aspirate fluid, breast cysts fluids and milk.10–12
In these studies, aluminium concentrations were higher in the external part of the mammary gland in the normal breast. Also, they were higher in breast cancer cases than in controls. These observations have led to the hypothesis that aluminium from antiperspirant use might contribute to the increased incidence of breast cancer observed in the western societies in the last few decades. In addition to increased incidence, a change in the topological distribution of breast cancers has also been reported, the majority of tumours arising nowadays in the external part of the mammary gland. It has been proposed that the latter change might be due to the application of an unidentified environmental carcinogen to the underarm area, with aluminium being a potential candidate.2, 13
Due to widespread use of aluminium in antiperspirants, performing retrospective epidemiological studies on patients using versus patients non‐using aluminium salts as an antiperspirant is difficult. Therefore, the considerations on the carcinogenic potential of aluminium on the human breast have remained largely speculative in the clinics to date.
On the experimental front, existing studies have focused on the effects of aluminium on cultured mammary epithelial cells. We reported that concentrations of aluminium in the range of those measured in the human breast selectively transform MCF‐10A human mammary epithelial cells in vitro after several weeks of culture. This effect was preceded by the induction of DNA double strand breaks, whose repair is often intrinsically mutagenic, and was not reversible following aluminium withdrawal from the culture medium, thus suggesting a genetic modification of the cells. Aluminium was not detectably mutagenic in bacteria.14 In another study, aluminium increased the migratory and invasive properties of MCF‐7 or MDA‐MB‐231 human breast cancer cells in vitro.15, 16
A harmless compound is not expected to have such profound effects on the behavior of cultured cells at concentrations in the range of those measured in the organism. Experimental evidence in an animal model, however, would considerably strengthen the doubts on the safety of aluminium originating from these observations.
Due to the known difficulty of growing human cells in mouse models, we investigated the capacity of normal murine mammary gland (NMuMG) epithelial cells cultured in the presence of concentrations of aluminium in the range of those measured in the human breast to form tumors in well‐established mouse models of cancer. Like MCF‐10A cells, NMuMG cells are spontaneously immortalized and do not form malignant lesions in nude mice or in the mouse strain they were originally isolated from (NAMRU).17,18
Material and Methods
Cell culture
NMuMG cells17, 18 were kindly provided by Prof. R. Montesano (University of Geneva) or purchased from LGC Standards/ATCC (Molsheim Cedex, France) (cat. no. CRL‐1636) and used immediately in the experiments reported in this manuscript. The cells were cultured in Dulbecco's modified Eagle's medium (Sigma‐Aldrich, Buchs, Switzerland) (cat. no. D5796) supplemented with 10% heat‐inactivated fetal calf serum (Amimed/Bioconcept, Allschwil, Switzerland; cat. no. 2‐01F10‐I), 100 units/ml penicillin and 0.1 mg/ml streptomycin (Sigma‐Aldrich, cat. no. P0781). Aluminium chloride hexahydrate (Sigma‐Aldrich, cat. no. 06232) was dissolved in Milli‐Q H2O at the concentration of 1M and immediately diluted at the intermediate concentrations of 100 mM, 30 mM or 10 mM in Milli‐Q H2O. These stocks were stored at 4°C until used. The stocks, or Milli‐Q H2O as a control, were diluted 1:1,000 in fresh culture medium twice a week, at the time the medium was added to the cells.
Under standard culture conditions, NMuMG cells retained the rounded cobblestone‐like growth pattern typical of the original cell line throughout the time period required to perform the experiments reported in this study. NMuMG cells transformed by AlCl3 were the only transformed mouse cell line present in our laboratory.
Western blotting
Approximately 80% confluent (E‐cadherin) or 50% confluent (N‐cadherin) NMuMG cells cultured for 6 months in the presence of AlCl3 100 μM, or of the same dilution—1/1,000—of solvent (H2O) alone as a control, were lysed in RIPA buffer (Sigma‐Aldrich, cat. no. R0278) supplemented with proteinase inhibitors (Sigma‐Aldrich, cat. no. P8340) and analysed by Western Blotting, using standard procedures. Antibodies (E‐cadherin: cat. no. ab76055; N‐cadherin: cat. no. ab76011; β‐actin: cat. no. ab6276; all from Abcam, Cambridge, UK) were used according to manufacturer's instructions. First antibody/horseradish peroxidase‐conjugate second antibody complexes were revealed with SuperSignal™ West Pico Chemiluminescent Substrate (Thermofisher cat. no. 34080) in a myECL imager (Thermofisher). Bands were quantified using myImageAnalysis version 2.01 (Thermofisher) and normalized with respect to β‐actin levels.
Soft agar assay
Cells were resuspended in agarose gels at the density of 2 × 104 cells/ml and grown for 14 days in the presence of complete culture medium as described.19 AlCl3 was not added to the soft agar assays.
Xenografts
In all the xenograft experiments described, 5 × 106 PBS‐washed cells were resuspended in 200 μl of growth factor reduced Matrigel (BD Biosciences, Allschwil, Switzerland; cat. no. 354230) at the final concentration of 6.5–7.0 mg/ml (depending on the specific lot used) and injected subcutaneously into one flank of 6‐week‐old female mice of the indicated strain. Except where indicated, five mice/condition were used. Mice were purchased from Charles River Laboratories (Saint‐Germain‐sur‐l'Arbresle, France) and maintained in a specific pathogen free area. Experiments were performed according to Institutional ethics guidelines. For the NOD SCID gamma (NSG) experiment, the indicated organs were embedded in paraffin using standard procedures. Two hundreds 5 μm‐thick consecutive sections were cut. Of these, one in every ten was stained with Hematoxylin‐eosin (HE). For the Swiss nude experiment, the procedure was the same, except that 100 5 μm‐thick consecutive sections were cut, and one every ten was stained with HE. HE stained sections were screened for metastasis with the help of an expert pathologist. Immunohistochemistry for cytokeratin 7 or 19 used antibodies ab181598 or ab133496, respectively (both from Abcam, Cambridge, UK), and was performed according to manufacturer's instructions.
Whole‐exome sequencing of NMuMG cells
Whole‐exome sequencing was conducted on NMuMG cells cultured for 7 months in the presence of AlCl3 100 μM or the same dilution of solvent (H2O) alone as a control. Libraries were prepared with the Agilent SureSelect Target Enrichment technology. Next generation sequencing was performed on an Illumina Hiseq 2500 using paired‐end 100 bp run. The variants identified were filtered through the “spin‐off” tool specific for somatic cancer mutations, MuTect (https://www.broadinstitute.org/cancer/cga/mutect). The indicated mutations were verified by PCR followed by Sanger sequencing, using standard procedures.
Results
NMuMG cells were routinely cultured in the presence of AlCl3 100 μM, or of the same dilution—1/1,000—of solvent (H2O) alone as a control. AlCl3 had no immediate effects on the morphology of NMuMG cells. After 14–16 weeks of continuous exposure to AlCl3—corresponding approximately to 42 population doublings—however, NMuMG cells acquired a fibroblastoid morphology and a scattered growth pattern reminiscent of epithelial‐mesenchymal transition (EMT), a hallmark of malignant transformation20 (Fig. 1 a, bottom). Control NMuMG cells, in contrast, exhibited the rounded cobblestone‐like growth pattern typical of these cells (Fig. 1 a, top). Decrease of E‐cadherin and increase of N‐cadherin expression are two well‐established markers of EMT.20 Western Blotting revealed, on average, a 63% decrease in E‐cadherin and a 3.7‐fold increase in N‐cadherin expression in NMuMG cells incubated in the presence of AlCl3, compared to controls (Figs. 1 b and 1 c). In the soft agar assay, a well‐characterized method to assess cellular transformation in vitro, AlCl3‐treated NMuMG cells formed larger colonies compared to controls (Figs. 2 a and 2 b). NMuMG cells from another source (ATCC) gave similar results in the soft agar assay (Fig. 2 c). These findings confirm and extend the results we obtained with MCF‐10A human mammary epithelial cells.14
Figure 1.
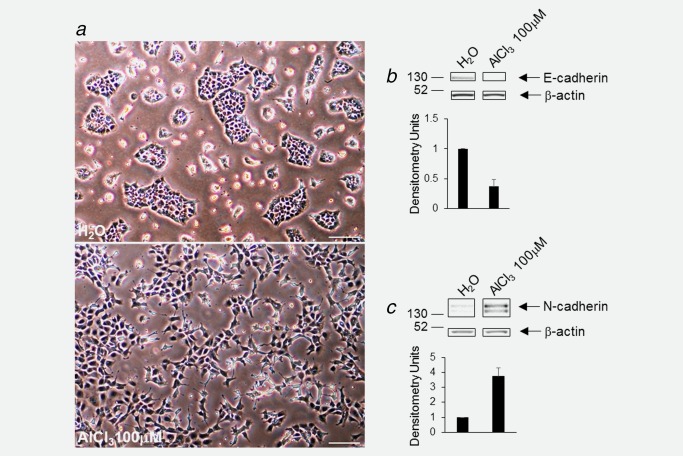
(a) Phase contrast view of NMuMG cells incubated for 4 months in the presence of AlCl3 100 μM (bottom) or the same volume (1/1,000) of solvent (H2O) alone as a control (top). AlCl3 or H2O were renewed twice a week with fresh culture medium. Bar = 100 μm. (b) Approximately 80% confluent NMuMG cells cultured for 6 months in the presence of AlCl3 100 μM, or of the same dilution—1/1,000—of solvent (H2O) alone as a control were analysed for the expression of E‐cadherin, or β‐actin as a loading control, by Western Blotting. One representative gel (out of 6) is shown. The bands presented were from the same gel. The graph shows the mean values for E‐cadherin expression, obtained in densitometric analysis, ± SEM, from six experiments, normalized with respect to β‐actin levels. Control (H2O) was set to 1. p H2O versus AlCl3 100 μM = 0.00038 (two‐tailed t‐test). (c) Same experiment as in (b), except that (i) cells were approximately 50% confluent; (ii) analysis was for N‐cadherin; (iii) the number of experiments was 4; (iv) p H2O versus AlCl3 100 μM = 0.0023 (two‐tailed t‐test). [Color figure can be viewed at wileyonlinelibrary.com.]
Figure 2.
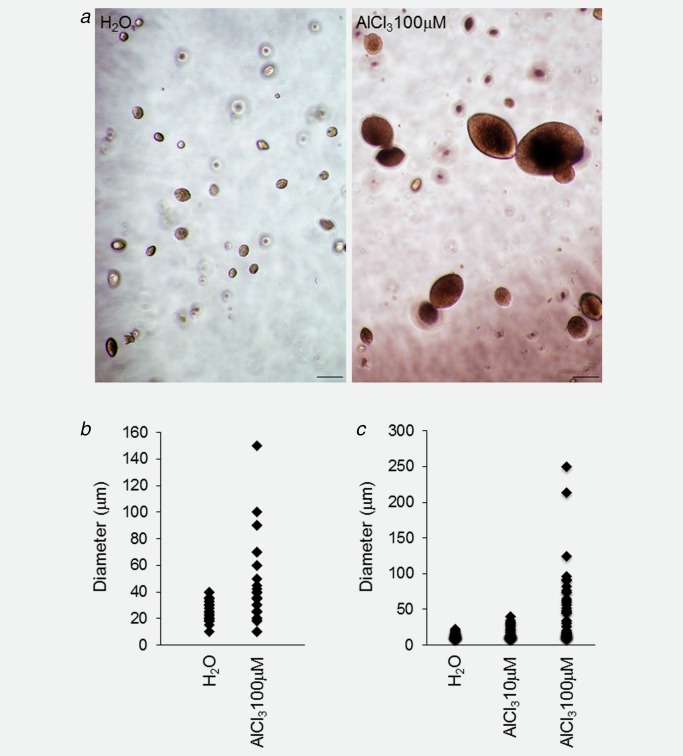
AlCl3 transforms NMuMG cells in vitro. (a) NMuMG cells cultured for 4 months in the presence of AlCl3 100 μM or the equivalent volume of H2O as control were resuspended in agarose gels at the density of 2 × 104 cells/ml and grown for 14 days in the presence of complete culture medium. AlCl3 was not added to the soft agar assay. Bar = 100 μm. (b) The growth in agarose gels was quantified by measuring the diameter of the structures (single cells or multicellular colonies) formed after 14 days. At least 50 randomly selected structures (single cells or multicellular colonies) from two independent experiments/condition were measured. p AlCl3 versus H2O = 6.71E‐04 (two‐tailed t‐test). (c) The graph shows the growth in agarose of NMuMG cells from a different source (ATCC), cultured for 7 months in the presence of AlCl3 10 μM, AlCl3 100 μM or the equivalent volume of H2O as control, as indicated. The soft agar assay was as described in a, b. p AlCl3 10 μM versus H2O = 0.02; p AlCl3 100 μM versus H2O = 2.79E‐05 (two‐tailed t‐test). [Color figure can be viewed at wileyonlinelibrary.com.]
As a next step, we wished to determine if NMuMG cells cultured in the presence of AlCl3 form tumors in mice. The NAMRU mouse strain being no longer available, we used three mouse models having different degrees of immunodeficiency. For these experiments, we selected NMuMG cells grown in the presence of 100 μM AlCl3, as they exhibit the most marked signs of cellular transformation in vitro (Figs. 1 a and 2). NMuMG cells grown in parallel in the presence of the same dilution of H2O were used as controls.
For the first experiment, we used the NOD.Cg‐PrkdcscidIl2rgtm1Wjl/SzJ strain, commonly referred to as NOD SCID gamma (NSG). NSG mice lack mature T cells, B cells, and functional natural killer (NK) cells, and are deficient in cytokine signaling, leading to successful engraftment of normal and transformed cell types. In the NSG strain, both NMuMG cells cultured in the presence of AlCl3 100 μM and their corresponding controls formed palpable tumors at the site of injection. However, tumors formed by AlCl3‐treated cells were significantly larger (Fig. 3 a).
Figure 3.
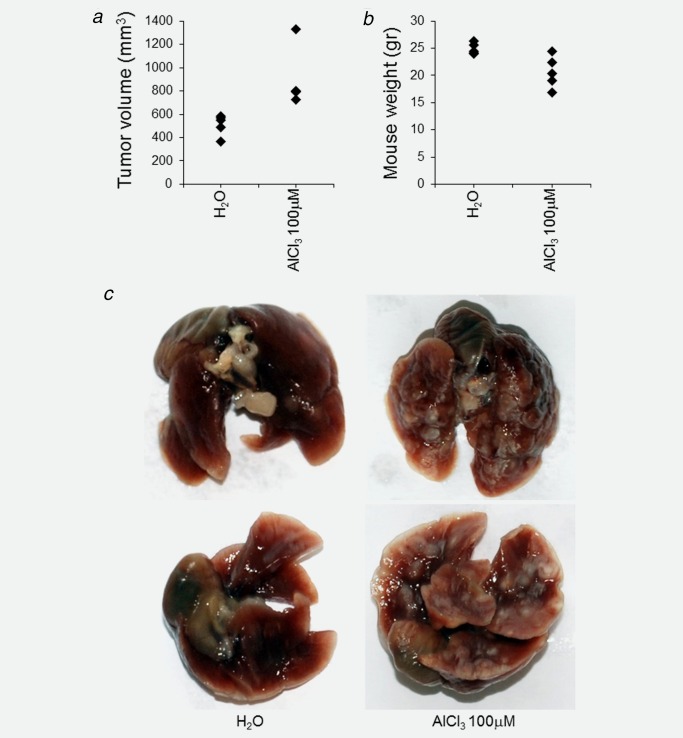
AlCl3 promotes tumorigenesis and lung metastasis in NMuMG cells injected subcutaneously into NSG mice. Five million NMuMG cells cultured for 6 months in the presence of AlCl3 100 μM or the equivalent volume of H2O as control were resuspended in 200 μl of Matrigel and injected subcutaneously into the flank of 6‐week‐old NSG female mice. Five mice/condition were used. The mice were sacrificed 6 weeks after injection. The graphs show (a) the volume of each tumor (calculated according to the formula 4/3π × a × b × c, with a, b, c corresponding each to ½ of the three axis of the tumor, approximated to an ellipsoid, respectively) or (b) the weight of each mouse at the time of sacrifice. Tumor incidence was 100% (all the mice formed tumors). Statistics were as follows. For tumor volume, H2O versus AlCl3 100 μM: p = 0.01, two‐tailed t‐test. For mouse weight, H2O versus AlCl3 100 μM: p = 0.01, two‐tailed t‐test. (c) Macroscopic apical view (upper panel) or ventral view (lower panel) of the lungs of a NSG female mouse injected with AlCl3‐treated NMuMG cells or H2O‐treated NMuMG cells, as indicated, at the time of sacrifice.
One week before sacrifice—that is, 5 weeks after injection—mice injected with AlCl3‐treated cells had lost weight and looked sick. They became progressively unable to move around and to eat. In contrast, mice injected with control cells retained normal weight and exhibited the usual behavior of healthy animals. At the time of sacrifice, mice injected with control cells weighted 24.9 ± 0.4 (mean ± SEM) g, whereas mice injected with AlCl3‐treated cells weighted 20.6 ± 1.3 (mean ± SEM) g (Fig. 3 b).
At the time of dissection, the lightest mouse injected with AlCl3‐treated cells had massive, macroscopically visible metastasis in the lungs (Fig. 3 c, right; Fig. 4 b). This mouse also had liver and brain metastasis (Supporting Information Fig. S1 and data not shown). Of the four remaining mice injected with AlCl3 –treated NMuMG cells, all had massive metastasis in the lungs. These frequently presented with a central area of necrosis (Fig. 4 b). One of the remaining four mice injected with AlCl3—treated NMuMG cells also had metastasis in the cerebellum (Fig. 5). Like the tumors grown at the site of injection, metastases were labeled by the epithelial markers cytokeratin 7 and 19 (Supporting Information Fig. S1, 5 and data not shown). In mice injected with control cells, only lung metastases were observed (Fig. 4 a). These were smaller and less numerous than in mice injected with AlCl3‐treated NMuMG cells. By counting metastases in the lungs of either groups on randomly selected HE stained sections, we found 2.4 ± 0.9 (mean ± SEM) metastasis/45 mm2 in the group injected with control NMuMG cells, and 11.8 ± 2.0 (mean ± SEM) metastasis/45 mm2 in the group injected with AlCl3‐treated NMuMG cells (p = 0.003, two‐tailed t test; n = 5 mice/group). HE staining found no metastases in the heart, spleen or kidneys of the two experimental groups.
Figure 4.
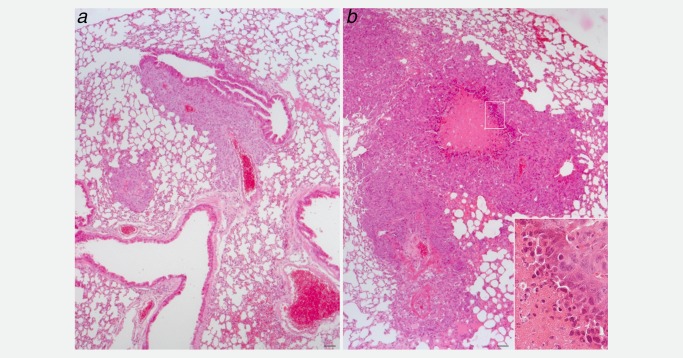
HE stained sections of the lungs of a NSG female mouse injected with (b) NMuMG cells cultured in the presence of AlCl3 100 μM for 6 months or (a) NMuMG cells incubated for 6 months in the presence of a 1/1,000 dilution of H2O. The mice were from the same experiment described in Figure 3. In (b), the inset shows necrotic cells. Bar in a and b = 50 μm.
Figure 5.
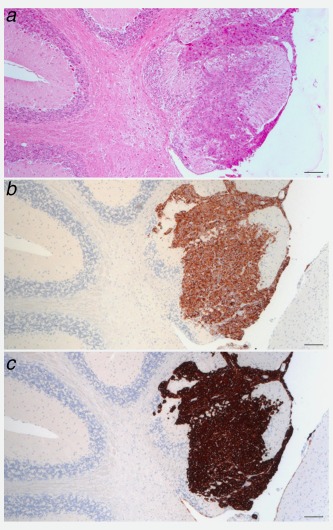
AlCl3 promotes tumorigenesis and brain metastasis in NMuMG cells injected subcutaneously into NSG mice. (a) HE stained sections of the cerebellum of a NSG female mouse injected with NMuMG cells cultured in the presence of AlCl3 100 μM for 6 months. The mouse was from the same experiment described in Figure 3. (b) Cytokeratin 7 or (c) cytokeratin 19 immunohistochemistry on adjacent sections. Bar in a, b, c = 50 μm.
As a next step, we injected AlCl3‐treated NMuMG cells, or their untreated counterparts, in the NOD.CB17‐Prkdcscid/J mouse strain (commonly referred to as NOD SCID). This strain is characterized by an absence of functional T cells and B cells, lymphopenia, hypogammaglobulinemia and a normal hematopoietic microenvironment. In contrast to NSG mice, NOD SCID mice have NK cells.
In the NOD SCID strain, AlCl3‐treated NMuMG cells formed palpable tumors with a kinetics similar to that observed in the NSG strain. In contrast to NSG mice, however, control NMuMG cells did not grow beyond the volume of injection during the same period of time (Fig. 6 a). At the time of sacrifice, the general health status of the animals was similar in the two experimental groups. Also, the weight of the mice was not significantly different between the two groups (mice injected with AlCl3‐treated NMuMG cells weighted 23.4 ± 1.0 (mean ± SEM) g, whereas mice injected with H2O‐treated NMuMG cells weighted 22.7 ± 0.5 (mean ± SEM) g, p = 0.56, two‐tailed t test, n = 5 mice/group). At dissection, metastases were macroscopically visible in the lungs of mice injected with AlCl3‐treated cells, but not in the lungs of mice injected with control NMuMG cells (data not shown).
Figure 6.
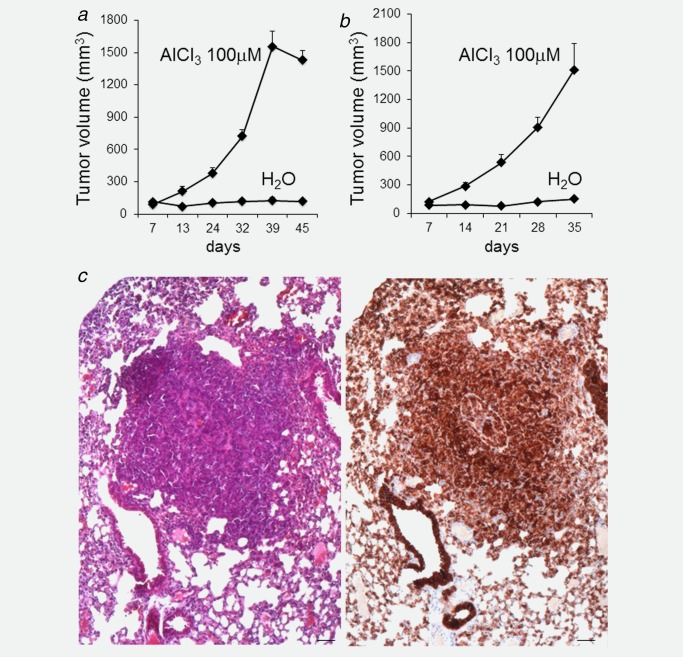
AlCl3 promotes tumorigenesis and metastasis in NMuMG cells injected subcutaneously into NOD SCID and nude mice. (a) Five million NMuMG cells cultured for 8 months in the presence of AlCl3 100 μM or the equivalent volume of H2O as control were resuspended in 200 μl of Matrigel and injected subcutaneously into the flank of 6‐week‐old NOD SCID female mice. Five mice/condition were used. Cell growth at the site of injection was measured with a caliper. The graph shows the volume ± SEM of such growth (calculated according to the formula V (mm3) = d 2 (mm2) × D (mm)/2, where d and D are the smallest and largest tumor diameters, respectively) at the indicated number of days after injection. Tumor incidence was 100% (all the mice injected with AlCl3‐treated cells formed tumors). p values (two‐tailed t‐test) comparing the two experimental groups were as follows: 7 days: p = 0.37; 13 days: p = 0.02; 24 days: p = 0.001; 32, 39 and 45 days: p < 0.001. (b) Five million NMuMG cells cultured for 8 months in the presence of AlCl3 100 μM or the equivalent volume of H2O as control were resuspended in 200 μl of Matrigel and injected subcutaneously into the flank of 6‐week‐old Swiss nude female mice. Five mice were used for AlCl3‐treated cells, and four mice for control cells (the fifth mouse planned to be injected with control cells died during anesthesia at the time of injection). Cell growth at the site of injection was measured with a caliper. The graph shows the volume ± SEM of such growth (calculated according to the formula V (mm3) = d 2 (mm2) × D (mm)/2, where d and D are the smallest and largest tumor diameters, respectively) at the indicated number of days after injection. Tumor incidence was 100% (all the mice injected with AlCl3‐treated cells formed tumors). p values comparing the two experimental groups were as follows: 7 days: p = 0.11; 14 days: p = 0.0049; 21 days: p = 0.0017; 28 and 35 days: p < 0.003 (two‐tailed t‐test). (c) HE stained section (left) or cytokeratin 19 immunohistochemistry on an adjacent section (right) showing a lung metastasis in a Swiss nude mouse injected with AlCl3‐treated NMuMG cells. Bar = 50 μm.
As a next step, AlCl3‐treated NMuMG cells, or their corresponding controls, were injected subcutaneously into Swiss nude mice (Crl:NU‐Foxn1nu strain). Of the mouse strains used in our experiments, this is the less immunodeficient one. Swiss nude mice are athymic and hairless as a result of the recessive nu mutation. T cell precursors exist but development is blocked in the absence of a thymus. In contrast, they have normal B cells and normal numbers and functions of macrophages, NK cells and antigen presenting cells.
In Swiss nude mice, AlCl3‐treated NMuMG cells formed palpable tumors with a kinetics similar to that observed in NOD SCID mice (Figs. 6a, 6b). In contrast, control NMuMG cells did not grow beyond the volume of injection during the same period of time (Fig. 6 b). At the time of sacrifice, mouse weight was similar in the two groups (mice injected with control cells weighted 24.17 ± 1.28 (mean ± SEM) g whereas the mice injected with AlCl3‐treated cells weighted 26.34 ± 0.79 (mean ± SEM) g (p = 0.20, two‐tailed t test; n = 4 mice for control NMuMG cells; n = 5 mice for AlCl3‐treated NMuMG cells)).
The lungs of 3/5 Swiss nude mice injected with AlCl3‐treated NMuMG cells had metastases as assessed by HE staining and cytokeratin 19 staining (Fig. 6 c). In contrast, 4/4 mice injected with control NMuMG cells did not have lung metastasis. No metastases were observed in HE stained sections of the brain, kidneys, spleen, heart or liver of either groups.
After the initial culture in the presence of AlCl3, AlCl3 was not added to the soft agar or tumor cell xenograft assays, where NMuMG cells exhibit a proliferative advantage. Similarly, growth in soft agar of MCF‐10A cells cultured in the presence of AlCl3 was not reduced after AlCl3 withdrawal for 5 weeks.14 This suggests that the growth advantage observed in mammary epithelial cells cultured in the presence of AlCl3 could be due to mutations induced by AlCl3. We previously reported that AlCl3 induces DNA double strand breaks, a common source of mutation, in cultured mammary epithelial cells.14 To explore the possibility that AlCl3 had induced mutations in NMuMG cells, whole‐exome sequencing was conducted on AlCl3‐treated NMuMG cells or NMuMG cells grown in parallel in the presence of the same dilution of solvent (H2O) alone as a control.
We found 48 mutations affecting 43 genes in AlCl3‐treated versus control NMuMG cells (Supporting Information Table S1). Direct sequencing on 18 such mutations was successful in 14 cases and confirmed the mutation in 13 cases (92.8%; Supporting Information Table S1) consistent with the very low false positive rate of MuTect (https://www.broadinstitute.org/cancer/cga/mutect). The mutations affect genes regulating cellular proliferation, migration, metastasis and apoptosis, including Max‐binding protein Mnt 21, 22 and T‐lymphoma invasion and metastasis‐inducing protein 2 (Tiam2)23 (Supporting Information Table S1). Although AlCl3‐treated NMuMG cells clearly exhibit a proliferative advantage in the soft agar and xenograft assays, AlCl3 does not increase proliferation in the conventional, two‐dimensional culture system used to produce these cells (Supporting Information Fig. S2), thus ruling out the possibility that the mutations found in AlCl3‐treated NMuMG cells are simply a consequence of increased proliferation.
Discussion
Although it is generally acknowledged that aluminium is neurotoxic, its widespread distribution in industrial products of daily use seems to reflect a general belief that, at least when used at certain concentrations and in certain formulations, aluminium is innocuous to human health. By several variations of the Ames test, one of the most common biological assays used in the industry to assess the mutagenic potential of chemical compounds, aluminium is not detectably mutagenic in bacteria.14 Whereas this result might seem reassuring, accumulating experimental evidence on the effects of aluminium in mammalian systems is opening an unsuspected scenario.
The results obtained in cultured mammary epithelial cells are perhaps the most intriguing ones. In the large majority of commercial antiperspirants, aluminium is present as AlCl3 or, more frequently, as aluminium chlorohydrate (Al2Cl(OH)5) at concentrations up to several molar units (see Introduction). In aqueous solutions at pH 7.0, both salts yield aluminium hydroxide and they are absorbed through the human and mouse skin.6–9 It is, therefore, conceivable that the daily application of antiperspirants to the skin of the underarm represents a major source of exposure of the human mammary epithelium to aluminium. Although aluminium concentrations in the body are generally low, levels measured in the breast area are significantly higher. More specifically, Al concentrations were in the range of 150–520 μg/l (mean 268.4 ± 28.1 μg/l), corresponding to 5.6–19.3 μM, in nipple aspirate fluids11; in the range of 80–330 μg/l (median 150 μg/l) corresponding to 3.0–12.2 μM, in type I breast cyst fluids10; or in the range of 4–437 nmol/g dry weight, corresponding to 0.8–87 nmol/g wet weight in human breast tissue.12 Assuming that 1 g of tissue has a volume of 1 ml, then overall concentrations of aluminium in the human breast are in the range of 0.8–87 μM. It appears relevant that the reported transforming and invasion promoting effects of aluminium on cultured mammary epithelial cells14–16 (this study) occur at aluminium concentrations within o very close to the range of those measured in the human breast.
Like MCF‐10A cells,14 NMuMG cells cultured in the presence of AlCl3 10–100 μM undergo cellular transformation in vitro, as assessed by the soft agar assay. Regarding the xenografts experiments, although in the NSG model both AlCl3‐treated and control NMuMG cells form tumors and metastasize, tumors formed by AlCl3‐treated NMuMG cells were larger than those formed by control cells. Metastases to the lungs were fivefold more numerous in mice injected with AlCl3 treated cells, compared to the lungs of mice injected with control cells. In addition, in NSG mice, AlCl3‐treated cells metastasized to the brain and the liver, whereas control cells did not. Therefore, in the three mouse models used, including nude mice, the most used mouse strain in experimental oncology and drug testing, AlCl3‐treated NMuMG cells are markedly more aggressive than their controls. In addition, our results demonstrate that continuous exposure of mammary epithelial cells to aluminium enables them to evade the immunological barrier represented by NK cells and the other immune tumor suppressive cells present in NOD SCID or nude mice, a key step in malignant tumor progression. These effects are likely to be explained at least in part by the mutagenic activity of AlCl3, because selection for mutations that provide a proliferative advantage is a well‐recognized mechanism of malignancy. EMT induced in NMuMG cells by AlCl3 is also likely to contribute to their invasive phenotype.
Aluminium is not the only metal with carcinogenic properties. Known examples include certain forms of arsenic, beryllium, chromium, nickel and cadmium. For some of these metals—in particular arsenic and hexavalent chromium—the carcinogenic effect seems to rely on their mutagenic potential, although other mechanisms cannot be excluded.24 In the case of aluminium, its transforming effect is preceded by the dose‐dependent appearance of DNA double strand breaks at the same concentrations that transform cells on long‐term culture.14 As the repair of DNA double strand breaks is error‐prone, and the transformed phenotype of MCF‐10A cells cultured in the presence of AlCl3 is not reverted by AlCl3 withdrawal,14 it appears conceivable that the transforming effect of aluminium relies at least in part on a mutagenic effect. Consistent with this interpretation, by full exome sequencing we found several mutations in AlCl3‐treated NMuMG cells compared to controls. These mutations occurred in genes regulating cellular proliferation, migration, metastasis and apoptosis, Max‐binding protein Mnt 21, 22 and T‐lymphoma invasion and metastasis‐inducing protein 2 (Tiam2)23 being the most interesting mutated cancer genes in the list presenting with a high PolyPhen‐2 score (Supporting Information Table S1). The actual contribution of these mutations to the transforming effect of aluminium, if any, remains to be demonstrated.
Conclusive evidence on the carcinogenic potential of aluminium requires epidemiological studies in humans and in vivo experiments were aluminium is directly applied to the skin of mice. In addition, it would be interesting to isolate and grow primary cultures of mouse mammary epithelial cells in the presence of AlCl3, prior to tumorigenicity assays in syngenic mice. Whereas these evidences are awaited, the in vivo observations reported in this study consolidate our previous in vitro findings14 and provide additional experimental evidence that aluminium salts could be environmental breast carcinogens.
Supporting information
Supporting Information Figure 1.
Supporting Information Table 1.
Acknowledgements
We thank Dr. J.‐C. Tille for expert advice in histopathology; C. Barraclough, N. Civic and M. Docquier of the iGE3 Genomics Platform of the University of Geneva for the whole‐exome sequencing; Nathalie Lin‐Marq for immunohistochemical analysis; D. Marino and L. Lesne for technical advice; Mr. F. del Coso for support; Prof. D. Belin for critically reading the manuscript and for support. Author Contributions: SJM planned the experiments, analyzed the data, supervised the work and wrote the manuscript. MT planned and performed the experiments, and analyzed the data. PF performed the experiments and analyzed the data. APS planned the experiments, supervised the work and wrote the manuscript.
Conflict of Interest: The authors declare no conflict of interest in relation to the work presented in this manuscript.
Stefano J. Mandriota's and André‐Pascal Sappino's current address is: Laboratoire de cancérogenèse environnementale, Fondation des Grangettes, 110/c Route de Chêne, 1224 Chêne‐Bougeries, Switzerland
André‐Pascal Sappino's current address in the clinic: Clinique des Grangettes, 7 chemin des Grangettes, 1224 Chêne‐Bougeries, Switzerland
References
- 1. Exley C. Human exposure to aluminium. Environ Sci Process Impacts 2013; 15:1807–16. [DOI] [PubMed] [Google Scholar]
- 2. Darbre PD. Aluminium, antiperspirants and breast cancer. J Inorg Biochem 2005; 99:1912–9. [DOI] [PubMed] [Google Scholar]
- 3. Darbre PD. Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol 2006; 26:191–7. [DOI] [PubMed] [Google Scholar]
- 4. Banasik A, Lankoff A, Piskulak A, et al. Aluminum‐induced micronuclei and apoptosis in human peripheral‐blood lymphocytes treated during different phases of the cell cycle. Environ Toxicol 2005; 20:402–6. [DOI] [PubMed] [Google Scholar]
- 5. Lankoff A, Banasik A, Duma A, et al. A comet assay study reveals that aluminium induces DNA damage and inhibits the repair of radiation‐induced lesions in human peripheral blood lymphocytes. Toxicol Lett 2006; 161:27–36. [DOI] [PubMed] [Google Scholar]
- 6. Flarend R, Bin T, Elmore D, et al. A preliminary study of the dermal absorption of aluminium from antiperspirants using aluminium‐26. Food Chem Toxicol 2001; 39:163–8. [DOI] [PubMed] [Google Scholar]
- 7. Guillard O, Fauconneau B, Olichon D, et al. Hyperaluminemia in a woman using an aluminum‐containing antiperspirant for 4 years. Am J Med 2004; 117:956–9. [DOI] [PubMed] [Google Scholar]
- 8. Anane R, Bonini M, Grafeille JM, et al. Bioaccumulation of water soluble aluminium chloride in the hippocampus after transdermal uptake in mice. Arch Toxicol 1995; 69:568–71. [DOI] [PubMed] [Google Scholar]
- 9. Anane R, Bonini M, Creppy EE. Transplacental passage of aluminum from pregnant mice to fetus organs after maternal transcutaneous exposure. Hum Exp Toxicol 1997; 16:501–4. [DOI] [PubMed] [Google Scholar]
- 10. Mannello F, Tonti GA, Darbre PD. Concentration of aluminium in breast cyst fluids collected from women affected by gross cystic breast disease. J Appl Toxicol 2009; 29:1–6. [DOI] [PubMed] [Google Scholar]
- 11. Mannello F, Tonti GA, Medda V, et al. Analysis of aluminium content and iron homeostasis in nipple aspirate fluids from healthy women and breast cancer‐affected patients. J Appl Toxicol 2011; 31:262–9. [DOI] [PubMed] [Google Scholar]
- 12. Exley C, Charles LM, Barr L, et al. Aluminium in human breast tissue. J Inorg Biochem 2007; 101:1344–6. [DOI] [PubMed] [Google Scholar]
- 13. Darbre PD. Underarm cosmetics and breast cancer. J Appl Toxicol 2003; 23:89–95. [DOI] [PubMed] [Google Scholar]
- 14. Sappino AP, Buser R, Lesne L, et al. Aluminium chloride promotes anchorage‐independent growth in human mammary epithelial cells. J Appl Toxicol 2012; 32:233–43. [DOI] [PubMed] [Google Scholar]
- 15. Darbre PD, Bakir A, Iskakova E. Effect of aluminium on migratory and invasive properties of MCF‐7 human breast cancer cells in culture. J Inorg Biochem 2013; 128:245–9. [DOI] [PubMed] [Google Scholar]
- 16. Bakir A, Darbre PD. Effect of aluminium on migration of oestrogen unresponsive MDA‐MB‐231 human breast cancer cells in culture. J Inorg Biochem 2015; 152:180–5. [DOI] [PubMed] [Google Scholar]
- 17. Owens RB, Smith HS, Hackett AJ. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst 1974; 53:261–9. [DOI] [PubMed] [Google Scholar]
- 18. Hynes NE, Jaggi R, Kozma SC, et al. New acceptor cell for transfected genomic DNA: oncogene transfer into a mouse mammary epithelial cell line. Mol Cell Biol 1985; 5:268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mandriota SJ, Buser R, Lesne L, et al. Ataxia telangiectasia mutated (ATM) inhibition transforms human mammary gland epithelial cells. J Biol Chem 2010; 285:13092–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT‐inducing transcription factors. Nat Cell Biol 2014; 16:488–94. [DOI] [PubMed] [Google Scholar]
- 21. Link JM, Hurlin PJ. The activities of MYC, MNT and the MAX‐interactome in lymphocyte proliferation and oncogenesis. Biochim Biophys Acta 2015; 1849:554–62. [DOI] [PubMed] [Google Scholar]
- 22. Toyo‐oka K, Bowen TJ, Hirotsune S, et al. Mnt‐deficient mammary glands exhibit impaired involution and tumors with characteristics of myc overexpression. Cancer Res 2006; 66:5565–73. [DOI] [PubMed] [Google Scholar]
- 23. Chen JS, Su IJ, Leu YW, et al. Expression of T‐cell lymphoma invasion and metastasis 2 (TIAM2) promotes proliferation and invasion of liver cancer. Int J Cancer 2012; 130:1302–13. [DOI] [PubMed] [Google Scholar]
- 24. Léonard A, Bernard A. Biomonitoring exposure to metal compounds with carcinogenic properties. Environ Health Perspect 1993; 101: 127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.
Supporting Information Table 1.


