Abstract
Converging evidence suggests that folate-mediated one-carbon metabolism may modulate cognitive functioning throughout the lifespan, but few studies have directly tested this hypothesis. This study examined the separate and combined effects of dietary and genetic manipulations of folate metabolism on neocortical functions in mice, modeling a common genetic variant in the MTHFD1 gene in humans. Mutant (Mthfd1gt/+) and wildtype (WT) male mice were assigned to a folate sufficient or deficient diet at weaning and continued on these diets throughout testing on a series of visual attention tasks adapted from the 5-choice serial reaction time task. WT mice on a deficient diet exhibited impulsive responding immediately following a change in task parameters that increased demands on attention and impulse control, and on trials following an error. This pattern of findings indicates a heightened affective response to stress and/or an inability to regulate negative emotions. In contrast, Mthfd1gt/+ mice (regardless of diet) exhibited attentional dysfunction and a blunted affective response to committing an error. The Mthfd1gt/+ mice also showed significantly decreased expression levels for genes encoding choline dehydrogenase and the alpha 7 nicotinic cholinergic receptor. The effects of the MTHFD1 mutation were less pronounced when combined with a deficient diet, suggesting a compensatory mechanism to the combined genetic and dietary perturbation of folate metabolism. These data demonstrate that common alterations in folate metabolism can produce functionally distinct cognitive and affective changes, and highlight the importance of considering genotype when making dietary folate recommendations.
Keywords: Folate, MTHFD1, Attention, Impulsivity, Affect
1. Introduction
Mounting evidence implicates alterations in folate-mediated one carbon metabolism in the etiology of various adult-onset disorders in addition to its well-documented role in neural tube defects. Numerous epidemiological studies have linked impairments in folate metabolism to a multitude of diseases including various cancers (Suh et al., 2001), stroke, and cardiovascular disease (MacFarlane et al., 2011), as well as neuropsychiatric (Kronenberg et al., 2009) and neurodegenerative (Van Dam and Van Gool, 2009) disorders. Most recently, research has implicated altered folate status in the etiology of age-related cognitive decline, vascular dementia, and Alzheimer's disease (Clarke et al., 2007, 1998; Kado et al., 2005; McCaddon et al., 1998; McIlroy et al., 2002; Mooijaart et al., 2005; Morris et al., 2006; Quadri et al., 2004; Ravaglia et al., 2007; Riggs et al., 1996; Seshadri, 2006, but see Ariogul et al., 2005).
There is also evidence to suggest that alterations in folate metabolism may affect cognitive and affective functioning at any age (not just during aging) because of its role in the synthesis and degradation of various neurotransmitters. Folate levels directly influence the availability of S-adenosylmethionine (SAM), the primary methyl donor for most biological methylation reactions including enzymes involved in the synthesis and degradation of the catecholamines, serotonin, and acetylcholine (Chan et al., 2008; Mischoulon and Fava, 2002). Biochemical data in animals have confirmed that a folate deficient diet for as little as five weeks after weaning can alter brain levels of SAM (Ordonez and Wurtman, 1974), dopamine (Gospe et al., 1995), norepinephrine (Kronenberg et al., 2008), acetylcholine (Chan et al., 2008), and serotonin metabolites (Gospe et al., 1995; Kronenberg et al., 2008). Based on this evidence, it follows that cognitive and affective functions modulated by these transmitter systems should be affected by altered folate metabolism. In fact, depressed patients have consistently exhibited reduced folate levels in conjunction with lower levels of SAM, serotonin, dopamine, and norepinephrine metabolites (Bottiglieri et al., 2000, 1994, 1992; Bottiglieri and Diaz-Arrastia, 2005). Nevertheless, because such studies are correlational, one cannot infer that the depression is caused by folate deficiency; experimental studies are needed that manipulate folate status and evaluate changes in cognitive and affective functioning.
Two such studies have assessed affective functioning in animal models of altered folate status. Ferguson et al. (2005) found that dietary folate deficiency during gestation in mice produced anxiety-like behaviors in the elevated plus maze when the offspring were tested as adults. Brocardo et al. (2008) found that folate supplementation of adult mice produced behavioral changes in the Porsolt swim task similar to those produced by antidepressants.
Additional studies have examined the effects of a folate deficient diet on performance in learning/memory tasks in rats. Impaired performance of the folate deficient animals has been reported for the Morris Water Maze (Kronenberg et al., 2008; Troen et al., 2008), object recognition (Li et al., 2011) and passive avoidance (Crowe and Ross, 1997) tasks, as well as active avoidance learning paradigms (Bachevalier and Botez, 1978; Bachevalier et al., 1981). However, it is unclear from these studies whether the observed effects reflect one or more aspects of cognitive functioning or instead reflect motivational, sensory, and/or affective changes.
Another limitation of these studies is that most have focused on the effects of dietary folate deficiency, notwithstanding the evidence that alterations in folate metabolism can also be caused by single nucleotide polymorphisms (SNPs) in genes involved in folate metabolism (Stover and Caudill, 2008). The study of gene–nutrient interactions is burgeoning due to the emerging understanding that the optimal level of nutrient intake may vary as a function of genotype (Carr et al., 2009; Stover and Caudill, 2008). Of the two experimental studies that have evaluated the influence of folate SNPs on cognitive functioning, both reported impaired performance of the mutant mice in tasks of learning/memory (Chan et al., 2008; Levav-Rabkin et al., 2011). The study by Chan et al. (2008) also reported that the performance impairment was more pronounced when the mutant mice were maintained on a folate deficient diet.
Thus, although several lines of evidence suggest that genetic and dietary alterations in folate metabolism influence cognitive and affective functioning throughout the lifespan, this hypothesis has yet to be fully tested, and it is still unclear which cognitive functions are impacted by these types of alterations. The present study was designed to increase our knowledge in this area by assessing the separate and combined effects of genetic and dietary alterations in folate metabolism on neocortical functions in mice, given that these functions are highly sensitive to neurotransmitter levels (Arnsten and Li, 2005; Robbins and Roberts, 2007) that are affected by folate perturbations.
This study utilized a mutant mouse that models the commonly occurring 1958G>A SNP for the methylenetetrahydrofolate dehydrogenase (MTHFD1) gene in humans, resulting in the substitution of an arginine by a glutamine at residue 653 located within the synthetase domain of the MTHFD1 enzyme (Hol et al., 1998). MTHFD1 encodes the trifunctional enzyme C1-tetrahydrofolate-synthase (C1-THF-synthase) necessary for the generation of folate-activated one-carbon units in the form of 10-formyltetrahydrofolates that are used for nucleotide synthesis, and for conversion of 10-formyltetrahydrofolate to 5,10-methylenetetrahydrofolate for use in homocysteine re-methylation, and subsequent generation of SAM in one-carbon metabolism (Tibbetts and Appling, 2010; see Supplementary Fig. A1 for the biochemical pathway). Disruption of this enzyme in mice alters the availability of these different forms of folate, leading to tissue-specific decreases in uracil content in DNA (MacFarlane et al., 2009), and alterations in hepatic SAM levels (MacFarlane et al., 2009). In humans, the MTHFD1 variant is associated with elevated serum homocysteine (Ivanov et al., 2009), and serum choline deficiency (Kohlmeier et al., 2005), both of which are thought to contribute to adverse reproductive health outcomes including congenital heart defects (Christensen et al., 2009), unexplained second trimester pregnancy loss (Parle-McDermott et al., 2006), and increased risk for neural tube defects (Brody et al., 2002; Parle-McDermott et al., 2006). No studies to date have evaluated the impact of this SNP on cognitive and affective functioning.
The goal of this study was to evaluate the impact of this genetic alteration alone and in combination with alterations in dietary folate on functions subserved by the neocortex (prefrontal, parietal and cingulate cortices): attention, inhibitory control, and the affect/cognition interface. It was hypothesized that these functions would be altered by dietary and genetic manipulations of folate metabolism because the transmitter systems known to be sensitive to altered folate availability (i.e. catecholaminergic, serotonergic and cholinergic systems) are known to modulate the functioning of this brain region (Robbins and Arnsten, 2009). To assess these functions, the mice were tested in a series of visual attention tasks adapted from the five-choice serial reaction time task (5-CSRTT), including a task that assessed affective regulation. To our knowledge, this study is the first to examine the specific dietary and genetic effects of altered folate metabolism on neocortical functions in mice.
Additionally, the present study was designed to provide information concerning the effects of the MTHFD1 mutation and dietary folate deficiency on the expression of genes involved in choline metabolism and cholinergic activity. Choline and folate are metabolically interrelated, and there is increasing evidence that choline may substitute for folate under conditions of folate deficiency (see Zeisel and da Costa, 2009 for a review). Due to the importance of cholinergic activity for various cognitive functions, it is possible that some of the cognitive effects of folate perturbations may be mediated by effects on choline metabolism and cholinergic activity. These gene expression assays may shed light on the neurochemical basis of the cognitive and/or affective changes produced by dietary folate deficiency and the MTHFD1 mutation.
2. Methods
2.1. Experimental animals and diets
All study protocols were approved by the Institutional Animal Care and Use Committee of Cornell University and conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Study animals were generated by crossing C57BI/6J female mice to 129P2/OlaHsd Mthfd1gt/+ male mice. These matings produced both heterozygous gene-trap (Mthfd1gt/+) mice and wildtype (WT) littermates. Mthfd1gt/+ have been described previously (MacFarlane et al., 2009) and the creation and genotyping of these mice is detailed in the Supplementary Methods section.
A total of 60 male littermate pairs served as subjects in the present study: 30 heterozygous gene-trap (Mthfd1gt/+) and 30 wildtype (WT) pairs, with half of each genotype maintained on a folate sufficient diet (AIN-93G with 2 mg/kg folic acid; Dyet #117814, Dyets Inc., Bethlehem, PA) and half maintained on a folate deficient diet which was the same diet but without folic acid (Dyet #117815, Dyets Inc., Bethlehem, PA). The animals were placed on these diets at weaning on postnatal day 21 and continued throughout behavioral testing.
A separate study with the same four treatment groups (WT Suf, WT Def, Mthfd1gt/+ Suf, Mthfdlgt/+ Def; n = 10 per group) was conducted to measure whole-brain gene expression levels for these animals. These animals were not behaviorally tested (see Section 2.5 below for details).
The mice were singly-housed to prevent fighting between cagemates. However, care was taken to provide enrichment in terms of daily handling and testing, as well as the use of homecage enrichment items (a gum-bone, tube, igloo, and Nestlet). The mice were housed in a room with a 12:12-h reversed light–dark cycle (lights off at 7:00 a.m.) and tested during their dark cycle. The behavioral testing room directly adjoined the housing room, preventing any light exposure during the transport of the animals between rooms.
2.2. Food restriction
The mice had ad libitum access to food until 90 days of age, at which point food intake of the behaviorally tested animals was restricted to produce body weights that were 87–93% of the animals' free-feeding weights. All mice were weighed daily. Food restriction was necessary to maintain motivation for the food reward used in the appetitively-motivated behavioral tasks. On testing days, the mice were provided liquid reinforcement during testing (liquefied AIN-76A sweetened purified chow without folic acid; “Shake and Pour”, BioServ, Frenchtown, NJ) in addition to their assigned diets after each test session. The amount fed on test days was determined by subtracting the number of calories obtained as reward in the testing chamber (Liquefied AIN-76A purified chow without folate, BioServ, Frenchtown, NJ) from the daily allowance of their respective diets.
2.3. Testing apparatus
Testing was conducted in six automated Plexiglas chambers, with chamber assignment constant for each animal throughout the study. Each chamber was controlled by a computer and situated in an insulated, sound-attenuating enclosure. The testing chambers were custom-designed but adapted from the nine-hole operant chambers developed to assess attention in mice (Humby et al., 1999). Within the chamber, a slightly curved rear wall contained five circular response ports, 1 cm in diameter, located 2 cm above the floor, and 5 mm apart. Embedded on the back surface of each port was a green 4 mA light-emitting diode (LED), which served as the visual discrimination cue. Responses to the ports were detected by infrared photodiodes, positioned inside each port, 0.5 cm from the opening. On the chamber wall opposite the response ports was an alcove (15 mm wide, 2 cm above the floor) containing a dipper (ENV0302M, MED Associates, Inc., St. Albans, VT), which dispensed .01 ml of the liquid food reward. Access to the dipper alcove was controlled by a thin metal door, activated by a motor on the outside of the testing chamber. As with the ports, nose pokes into the alcove were monitored by infrared photodiodes. Each chamber was fitted with an exhaust system that transported the air from each chamber directly to the room exhaust ventilator system at a rate of four complete air changes per minute.
2.4. Behavioral testing
Beginning on PND 120, each mouse was administered one daily test session, six days per week, always at the same time each day and with the same experimenter. Treatment groups were counterbalanced for time of day. The individuals conducting the behavioral testing were blind to the treatment groups of the mice.
2.4.1. Shaping
The mice initially completed a four-stage training procedure designed to shape the general response sequence required for completion of each trial in the visual attention tasks. Briefly, during these four stages the mice learned that the door to the dipper alcove would be raised at the start of each trial, and a nose poke into the dipper port would initiate the start of the each trial; they then learned that following trial initiation, a nose poke into one of the five response ports would produce the delivery of 0.01 ml of the liquid diet in the dipper alcove. During the final training stage, each mouse was required to respond a fixed number of trials in each of the five response ports, to eliminate preferences or aversions to any of the ports. These training stages required a total of 10 to 15 sessions on average. For a more detailed description of these stages, see Driscoll et al. (2004).
2.4.2. Attention tasks
Following training, the mice were administered a series of visual attention tasks, described below in the order in which they were administered, and summarized in Table 1. In the visual discrimination task, they learned that a nose poke into an illuminated port (a correct response) would be rewarded with access to sweetened liquid food in the dipper alcove. Subsequent tasks placed greater demands on attentional capabilities and impulse control, as outlined below. All testing sessions were terminated after 30 min or 70 response trials, whichever came first.
Table 1.
Task parameters and sequence of tasks.
| Task | Pre-cue Delay | Cue Duration | Number of sessions |
|---|---|---|---|
| Visual discrimination task | 0 sa | 32 sa | Criterionc |
| Attention Task 1 | 0 sa | 2 sa | 8 |
| Attention Task 2 | 0 sa | 1 sa | 15 |
| Attention Task 3 | 0, 2, 4 sb | 1 sa | 20 |
| Attention Task 4 | 0, 2, 4 sb | 0.8, 1.0, 1.4 sb | 20 |
| Attention Task 5 | 0, 4, 8 sb | 0.4, 0.8, 1.0 sb | 6 |
Constant across trials.
Variable across trials.
80% correct for two out of three consecutive sessions with at least 50 response trials in each testing session.
2.4.3. Five-choice visual discrimination task
On each trial, one of the five port LEDs was illuminated and the mouse was rewarded for making a nose-poke in the illuminated port. The location of the visual cue was pseudorandomized across trials, such that the number of cue presentations in each port was equal across each daily test session. A 2 s delay separated trial initiation and cue onset; this delay (termed the “turn-around time”) allowed time for the mouse to turn around and orient toward the ports before cue illumination. The LED remained illuminated until the mouse made a response or until 32 s elapsed, whichever came first.
Each mouse was tested on this task until the learning criterion was reached, defined as 80% correct responses for two out of three consecutive sessions, containing a minimum of 50 trials per session. The dependent measures in this task were the number of trials and number of errors to reach criterion. Once an individual mouse reached criterion it was moved on to the subsequent task.
2.4.4. Task characteristics and performance measures
The subsequent attention tasks were identical in concept and basic procedures to the visual discrimination task but introduced two novel task characteristics: (1) an increasing and variable delay prior to cue onset (pre-cue delay), and (2) a decreasing and variable cue duration, which together have been shown to increase demands on impulse control (Blondeau and Dellu-Hagedorn, 2007; Puumala and Sirvio, 1998) and selective attention (Blondeau and Dellu-Hagedorn, 2007) in this task. All combinations of cue location, cue duration, and pre-cue delay were balanced across the 70 trials in each session.
Several error types were analyzed to gain insight into the basis of group differences. A premature response was scored when an animal made a nose-poke in one of the response ports prior to cue onset, indicative of impaired impulse control (Dalley et al., 2008). A nose-poke into a non-illuminated port was scored as an inaccurate response. An omission error was tallied if a mouse initiated a trial but did not make a nose poke in any of the five response ports within 5 s after cue presentation. Both inaccurate responses and omission errors are indicative of attentional dysfunction, after the basic task contingencies have been mastered (Blondeau and Dellu-Hagedorn, 2007; Puumala and Sirvio, 1998). Each of these three error types was followed by a five second time-out period, signaled by the illumination of a three-watt house light located on the ceiling of the chamber. Finally, if an animal failed to initiate a trial within 60 s of the dipper alcove door being raised at trial onset, a nontrial was tallied. Nontrials, which were rare, were not counted as an error nor a correct response, and were deleted from analyses.
2.4.5. Attention Tasks 1 and 2
In Attention Task 1, the duration of cue illumination was reduced to 2 s; this task was administered for 8 sessions. Cue duration was further reduced to 1 s in Attention Task 2, which was administered for 15 sessions. The purpose of these tasks was to train the mice for subsequent tasks, described below, which placed greater demands on attentional resources and inhibitory control.
2.4.6. Attention Task 3
In this task, a delay of varying duration (0, 2, or 4 s) was imposed between trial initiation and cue onset; cue duration was constant at 1 s. This task was administered for 20 sessions. This task assessed several functions: (1) the ability of the mice to learn that the cue would be presented after a delay on some trials, (2) their ability to wait for the cue (inhibitory control), as well as (3) their ability to maintain attention while waiting for the cue.
2.4.7. Attention Task 4
In Attention Task 4, both cue duration and pre-cue delay varied randomly across trials. The pre-cue delays were the same as in the previous task (0, 2, or 4 s), but now cue duration also varied across trials between 0.8, 1.0, and 1.4 s. The mice were tested on this task for 20 sessions. Varying both cue duration and pre-cue delay across trials was designed to increase demands on attention and inhibitory control.
2.4.8. Attention Task 5
In Attention Task 5, both cue duration and pre-cue delay again varied randomly across trials, but entailed shorter durations and longer pre-cue delays than the prior task. Pre-cue delay varied between 0, 4, and 8 s and cue duration varied between 0.4, 0.8, and 1.2 s. This task, administered for eight sessions, was designed to further increase demands on attention and inhibitory control.
2.5. Red blood cell folate concentrations and cholinergic gene expression
The same four treatment groups were included in this separate study (n = 10/group) of whole brain expression for genes involved in cholinergic activity and metabolism including: choline dehydrogenase (CHDH), high affinity choline transporter (CHT1); nicotinic acetylcholine receptor alpha 7 (nAChR7), choline acetyltransferase (ChAT), acetylcholinesterase (AChE), and phospholipase D2 (PLD2). Animals were provided their respective diets at three weeks of age and maintained on these diets for a total of five weeks, a period of time sufficient for the folate deficient diet to produce physiological signs of folate deficiency (MacFarlane et al., 2009). At eight weeks of age, mice were sacrificed by cervical dislocation after 12 h of food deprivation. Blood was collected via cardiac puncture for four animals per group to assess red blood cell folate levels and fresh whole brain was collected from all animals for gene expression analyses. The procedures for these assays are delineated below. The gene expression assays were performed on whole brain homogenates because whole brain was needed for other outcome measures of interest, [e.g., proteins, S-adenosylmethionine (SAM), and S-adenosyl-homocysteine (SAH)], processed for other studies.
2.5.1. Red blood cell (RBC) folate concentrations
RBC folate concentrations were quantified using the Lactobacillus casei assay, which has been previously described (Suh et al., 2000). Folate concentrations were normalized to total protein, which was determined using the Lowry assay (Herbig et al., 2002).
2.5.2. Quantitative real-time PCR (qPCR)
Expression levels of the genes were assessed in brain tissue using quantitative real-time PCR. Total RNA was extracted from frozen tissue by RNeasy® Mini kit (Qiagen) and reverse transcription was performed by the ImProm-II Reverse Transcription System™ (Promega) according to the manufacturer's instructions. Quantitative PCR was conducted with the HotStart-IT SYBR Green system (Affymetrix). The reaction conditions were as follows: 95 °C for 5 min, followed by 40 cycles with 15 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C. All primers were designed by GeneRunner software (www.generunner.net; see Supplementary Table A1). Expression levels were normalized by the delta Ct method (Livak and Schmittgen, 2001) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the housekeeping gene.
2.6. Statistical analyses
Statistical analyses were conducted using the Statistical Analysis System (Version 9.1; SAS Institute, Cary, NC).
2.6.1. Behavioral measures
The initial visual discrimination task was analyzed using a oneway analysis of variance with group as the predictor variable. Analyses were conducted for total number of errors to criterion and total trials to criterion, both measures of learning rate. For errors to criterion, premature responses, inaccurate responses, and omission errors were all counted as errors. However, because the cue light was illuminated immediately at trial onset and stayed on for 32 s, the animals rarely committed premature responses (only if they responded during the “turn-around” time), and rarely if ever committed omission errors due to the long illumination duration. Thus, in this task, the errors were almost exclusively “inaccurate responses” (responses to a port that was not illuminated).
The subsequent attention tasks were analyzed using PROC GLIMMIX, a generalized linear mixed models procedure for conducting repeated measures analyses for various probability distributions including normal data (Wolfinger and O'Connell, 1993). The statistical models included Genotype (WT or Mthfd1gt/+), Diet [folate deficient (Def) or folate sufficient (Suf)], Pre-cue Delay, Cue Duration, and Session-block, as appropriate for each task (see Table 1). The number of sessions included in each session-block varied by task, depending on the shape of the learning curves for that task. The analysis of Attention Task 4 also included a variable denoting the outcome of the previous trial (correct or error) to permit an assessment of group differences in the degree of disruption produced by committing an error (discussed in Strupp and Beaudin, 2006). It was deemed optimal to assess this aspect of performance in this task because the basic rules had been mastered by the time Task 4 was presented and performance was relatively constant across sessions, facilitating the interpretation of performance as a function of the outcome of the prior trial (correct or incorrect).
The following dependent measures were analyzed: percentage of correct responses, percentage of inaccurate responses, percentage of premature responses, and percentage of omission errors. Each dependent variable was calculated as a percentage of the total number of responses of each type (correct, inaccurate, premature, and omission) for each session-block, pre-cue delay and/or cue duration condition divided by the total number of response trials within that condition. Nontrials were not considered to be “response trials” and were not included in the calculation of these dependent measures. Analyses were conducted using the mean percentage of each response type for each animal.
In general, contrasts to compare specific groups were conducted only if the F test for the omnibus main effect of Genotype or Diet or higher order interactions involving these variables was statistically significant at the 0.05 level. However, because this study was viewed as hypothesis-generating (rather than confirmatory), some effects indicated by marginally significant omnibus tests are reported.
If a significant interaction including both Genotype and Diet was found, four post-hoc comparisons of interest were examined: (1) WT Suf (the control group) vs. WT Def, indicating the effect of diet within WT mice; (2) WT Suf vs. Mthfd1gt/+ Suf, indicating the effect of the mutation for mice consuming normal levels of dietary folate; (3) WT Suf vs. Mthfd1gt/+ Def, indicating the combined effect of the mutation and the folate deficient diet relative to the WT Suf group; and (4) Mthfd1gt/+ Suf vs. Mthfd1gt/+ Def, indicating the effect of diet within the mutant groups of mice. A Bonferroni correction was applied for these four comparisons, yielding an alpha level of 0.05/4 or 0.0125, for these contrasts.
2.6.2. RBC folate concentrations and gene expression assays
Because of the small sample sizes for the assessments of RBC folate and gene expression, a nonparametric Wilcoxon rank-sum test was used to assess group differences for these endpoints.
2.6.3. Body weight analysis
A one-way ANOVA was used to assess group differences in body weights for behaviorally-tested animals.
3. Results
3.1. Sample size
The final sample sizes for the various tasks ranged from 9 to 13 per group depending on the task. In some instances, the sample size for a given group varied across tasks due to the exclusion of animals for reasons that would affect only one task (e.g., apparatus malfunction, experimenter error, animal illness).
3.2. Body weights
Body weights of all behaviorally-tested mice were monitored daily throughout testing. There were no significant differences in body weight for any of the treatment groups across any of the behavioral tasks (p's ≥ 0.503).
3.3. Nontrial analysis
There were no significant differences in the percentage of nontrials (trials on which the mouse did not initiate a trial by making a nose poke into the dipper alcove at trial onset) for any of the treatment groups across any of the behavioral tasks (p's ≥ 0.254), indicating that appetitive motivation was comparable for all groups.
3.4. Five-choice visual discrimination task (no pre-cue delay, cue duration: 32 s)
The four groups did not differ significantly in the number of errors to criterion [F(3, 46) = 0.52, p = 0.671], nor trials to criterion [F(3, 46) = 0.52, p = 0.673, see Table 2]. All mice met the learning criterion.
Table 2.
Learning rate measures for the visual discrimination task.
| Group | Errors to criterion, M ± SEM | Trials to criterion, M ± SEM |
|---|---|---|
| WT Suf | 175.92 ± 17.21 | 430.15 ± 29.91 |
| WT Def | 168.62 ± 19.09 | 401.54 ± 31.98 |
| gt/+ Suf | 196.36 ± 19.01 | 447.36 ± 42.41 |
| gt/+ Def | 167.54 ± 16.20 | 397.54 ± 25.46 |
Data presented as means ± SEM. There were no significant group differences for either measure of learning rate.
Abbreviations: WT Suf: wildtype mice fed a folate sufficient diet; WT Def: wildtype mice fed a folate deficient diet; gt/+ Suf: Mthfd1gt/+ mice fed a folate sufficient diet; gt/+ Def: Mthfd1gt/+ mice fed a folate deficient diet.
3.5. Attention Tasks 1 and 2
Due to a number of instances of apparatus malfunction during Attention Tasks 1 and 2, several days of data could not be analyzed and therefore statistical analyses were limited to the final three sessions of Attention Task 2, a point at which these problems had been resolved. There were no significant treatment group differences in percentage of correct responses during these sessions [F(3, 137) = 0.30, p = 0.821]; all groups attained approximately the same high level of performance: (WT Suf: 86.8%; WT Def: 87.1%; Mthfd1gt/+ Suf: 86.4%; Mthfd1gt/+ Def: 87.6%).
3.6. Performance on Attention Tasks 3–5
In order to streamline the presentation of results, the overall main effects of the task variables (e.g., pre-cue delay, cue duration) are presented first, followed by the presentation of Genotype and/or Diet combinations for each task. For each task, the analysis of the percentage of correct responses is reported first, followed by a description of the specific error types that characterized group differences. For clarity, only those error types that best capture the phenotypes of the Genotype, Diet, or their combination are discussed below.
3.7. Effects of task variables
In Attention Tasks 3–5, the main effect of pre-cue delay was significant (p's < 0.001), indicating that as the pre-cue delay increased, the percentage of correct responses declined; this pattern reflects the intensifying demands on inhibitory control and focused attention with increasing pre-cue delay. Similarly, in those tasks in which cue duration varied across trials (Attention Tasks 4 and 5), the main effect of cue duration was significant (p's < 0.0001), revealing that with decreasing cue duration, the overall performance declined, reflecting the increased demand on focused attention with briefer cues. Finally, there was a significant main effect of session-block in Tasks 3 and 5 (p's < 0.001), showing increasing proficiency in these tasks with extended training.
3.8. Attention Task 3 (pre-cue delay: 0, 2, 4 s; cue duration: 1 s)
3.8.1. Correct responses
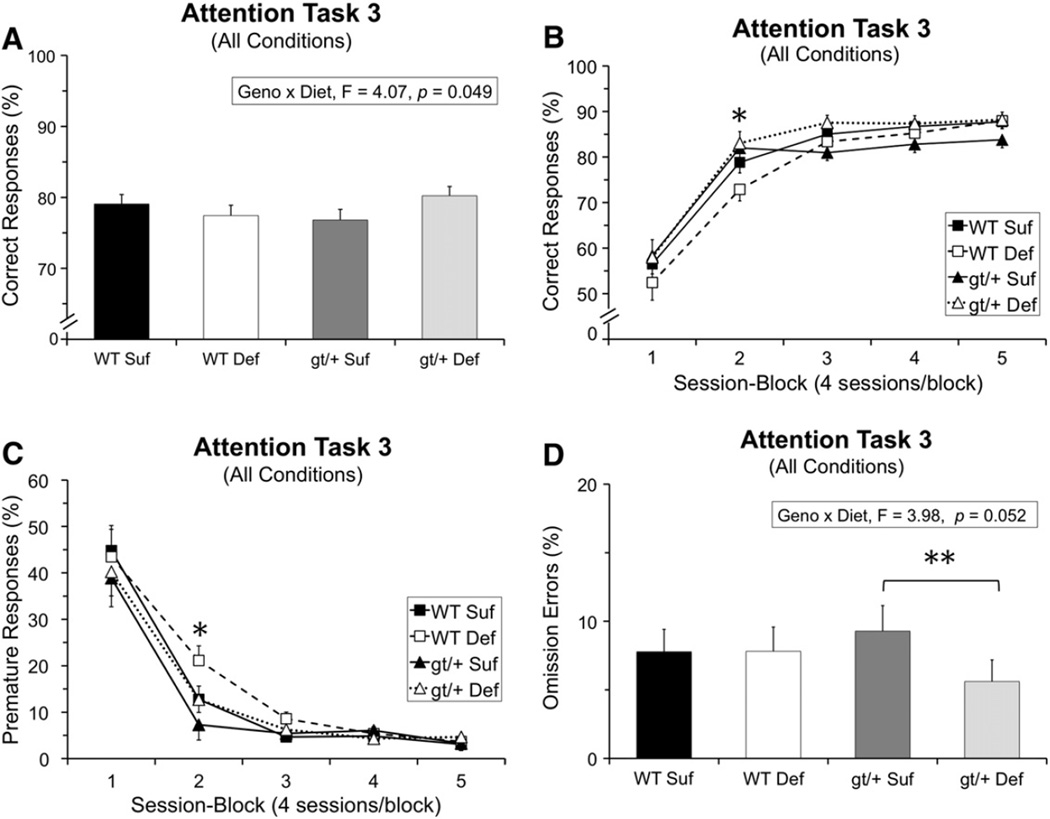
Analysis of the percentage of correct responses revealed several significant interactions involving Genotype and/or Diet. First, a significant interaction of Genotype and Diet was found [F(1, 43.37) = 4.07, p = 0.049], reflecting opposite effects of the Def diet (relative to the Suf diet) for the two genotypes. As depicted in Fig. 1A, the Def diet tended to impair performance of the WT mice, but improve the performance of the Mthfd1gt/+ mice.
Fig. 1.
Task 3 (pre-cue delay: 0, 2, 4 s; cue duration: 1 s) performance: (A) Percentage of correct responses averaged across all conditions; the significant Genotype × Diet interaction indicates that the Def diet tended to impair performance of the WT mice but improve performance of the gt/+ mice; (B) percentage of correct responses as a function of session-block; the gt/+ mice performed significantly better than the WT mice early in testing (block 2: gt/+ > WT, p = 0.013); (C) percentage of premature responses as a function of session-block, revealing effects of both Diet and Genotype during session-block 2: (1) a higher percentage of premature responses for the mice maintained on the Def diet (relative to those on the Suf diet) for trials with either a 2 s (p = 0.014) or 4 s delay (p = 0.017), and (2) a higher percentage of premature responses for the WT mice than the gt/+ mice (p = 0.025); and (D) percentage of omission errors averaged across all conditions, showing that the gt/+ Suf mice make a significantly higher percentage of omission errors than gt/+ Def mice (p = 0.008). Abbreviations: WT Suf: wildtype mice fed a folate sufficient diet; WT Def: wildtype mice fed a folate deficient diet; gt/+ Suf: Mthfd1gt/+ mice fed a folate sufficient diet; gt/+ Def: Mthfd1gt/+ mice fed a folate deficient diet; gt/+: Mthfd1gt/+ mice, WT: wildtype mice; *indicates significance ≤ 0.05. Data points are means ± SEM.
Second, Genotype and Diet each exerted effects on performance that varied across the 20 testing sessions (5 session-blocks). A significant interaction between Genotype and Session-block was found [F(4, 481.1) = 5.04, p = 0.0005], reflecting superior performance of the Mthfd1gt/+ mice early in testing (session-block 2, p = 0.013), with the two genotypes not differing (on average) during the later testing sessions (Fig. 1B). Additionally, a significant interaction between Diet and Session-block was also found [F(4, 481.1) = 2.60, p = 0.035; see Fig. 1B], due to the tendency of the Def diet to impair performance (relative to the Suf diet) during the early test sessions (primarily for the WT mice) but improve it during the later session-blocks (for the Mthfd1gt/+ mice). Contrasts revealed only trends, however (Def vs. Suf: session-block 3, p = 0.068; session-block 5, p = 0.070). As discussed below, the effects of the mutation and diet on performance reflected different types of errors, and hence different types of influences on brain function.
3.8.2. Premature responses
The analysis of percent premature responses revealed a significant interaction of Diet and Session-block [F(4, 136.9) = 4.46, p = 0.002], and Diet, Session-block, and Pre-cue Delay [F(4, 232.7) = 3.43, p = 0.001]. Contrasts revealed that during session-block 2, at the 2 and 4 s pre-cue delays, the Def diet increased premature responding, relative to the Suf diet (2 s: p = 0.014; 4 s: p = 0.017), indicative of impulsive responding (Fig. 1C). Although this pattern was seen in both genotypes the effect appeared to be somewhat more pronounced in the WT mice.
The analysis of premature responses also revealed a significant interaction between Genotype and Session-block [F(4, 136.9) = 3.06, p = 0.019; Fig. 1C]. Contrasts revealed that the Mthfd1gt/+ mice committed a lower percentage of premature responses than WT mice early in testing (significant only in session-block 2; p = 0.025), with the groups converging in later test sessions.
3.8.3. Omission errors
The analysis of percent omission errors revealed a main effect of Diet [F(1, 43.8) = 3.87, p = 0.05], as well as a significant interaction between Genotype and Diet [F(1, 43.8) = 3.98, p = 0.05]. Contrasts revealed that the Def diet decreased omission errors for the Mthfd1gt/+ mice (relative to the Suf diet; p = 0.008), but had no effect on omission errors for the WT mice the Def mice (Fig. 1D).
3.8.4. Inaccurate responses
Percentage of inaccurate responses did not reveal significant effects of Genotype, Diet, nor their interaction.
3.9. Attention Task 4 (pre-cue delay: 0, 2, 4 s; cue duration: 0.8, 1.0, 1.4 s)
The analyses of the dependent measures for Task 4 contained a variable denoting the outcome of the previous trial (correct vs. error) to assess group differences in reactivity to committing an error on the previous trial (Prev), an index of emotional reactivity (see the Introduction section). For all dependent measures (correct responses, premature responses, inaccurate responses, omission errors), there was a significant main effect of Prev (all p's < 0.0001), reflecting that performance was significantly better on trials following a correct response than on trials following an error, signifying the disruptive effect of committing an error on the previous trial.
3.9.1. Correct responses
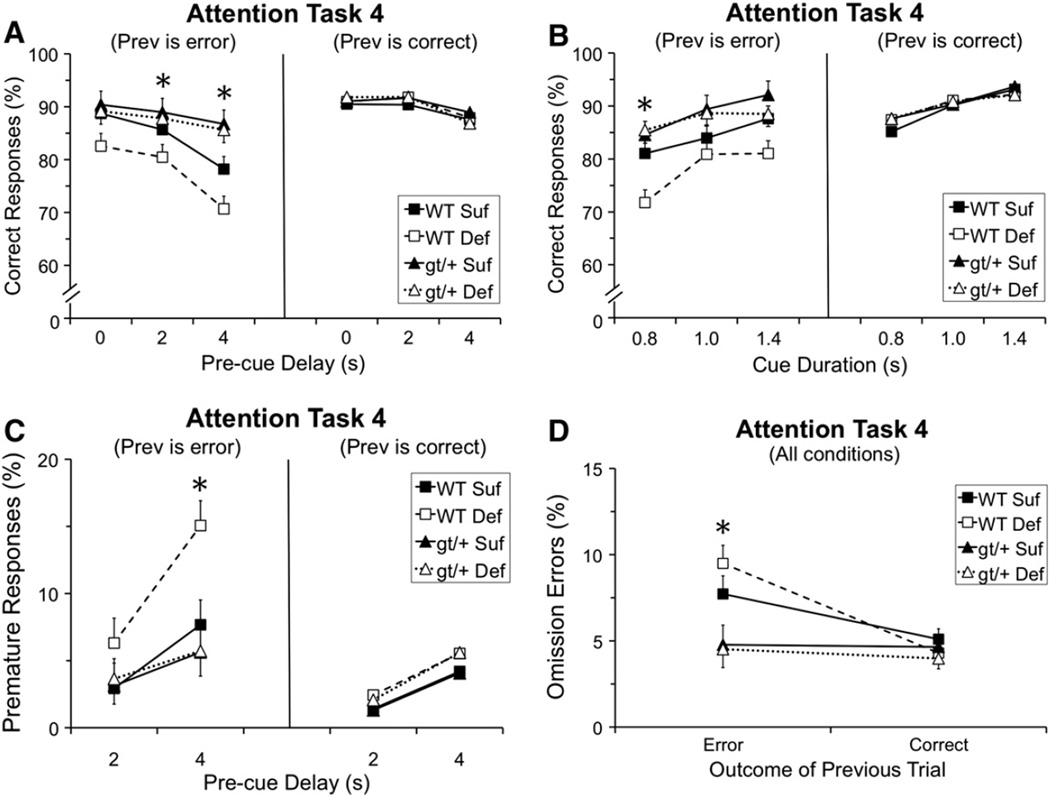
Analysis of the percentage of correct responses revealed a significant main effect of Genotype [F(1, 38.9) = 10.58, p = 0.002], as well as several interactions including: Genotype and Pre-cue Delay [F(2, 627.5) = 4.82, p = 0.008], Genotype and Prev [F(1, 42.2) = 8.98, p = 0.005], and Genotype, Pre-cue Delay, and Prev [F(2, 627.5) = 6.40, p = 0.002]. As seen in Fig. 2A (right panel), there were no group differences in performance following a correct response. In contrast, as seen in Fig. 2A (left panel), on trials that followed an error and included a pre-cue delay (2 s or 4 s), the Mthfd1gt/+ mice performed better than the WT mice (2 s delay, p = 0.030; 4 s delay, p = 0.0001). Note that the superiority of the Mthfd1gt/+ mice under these conditions reflects the fact that these mice did not exhibit any disruption of performance following an error, contrary to the WT mice.
Fig. 2.
Task 4 (pre-cue delay: 0, 2, 4 s; cue duration: 0.8, 1.0, 1.4 s) performance as a function of the outcome of the prior trial (Prev) i.e., correct or incorrect: (A) Percentage of correct responses as a function of pre-cue delay and Prev; no group differences were seen for trials following a correct response whereas for trials following an error and including a pre-cue delay, the gt/+ mice performed significantly better than the WT mice (2 s delay: WT < gt/+, p = 0.030; 4 s delay: WT < gt/+, p = 0001); (B) percentage of correct responses as a function of cue duration and Prev, showing that the WT Def mice performed more poorly than WT Suf specifically on trials that both followed an error and included the briefest cue (p = 0.0079); (C) percentage of premature responses as a function of pre-cue delay and Prev; the WT mice made a significantly higher percentage of premature responses than the gt/+ mice on trials that both followed an error and included the longest delay (p = 0.004), although this effect appears driven by the WT Def mice; and (D) percentage of omission errors as a function of Prev (averaged across all other conditions), indicating that the percentage of omission errors was significantly higher for the WT mice than the gt/+ mice specifically on trials that followed an error (p = 0.0005). Abbreviations: WT Suf: wildtype mice fed a folate sufficient diet; WT Def: wildtype mice fed a folate deficient diet; gt/+Suf: Mthfd1gt/+ mice fed a folate sufficient diet; gt/+ Def: Mthfd1gt/+ mice fed a folate deficient diet; gt/+: Mthfd1gt/+ mice, WT: wildtype mice; *indicates significance ≤ 0.05. Data points are means ± SEM.
In addition, a trend was seen for an interaction involving Genotype, Diet, Cue Duration and Prev [F(2, 627.2) = 2.29, p = 0.10]. Group differences were not seen for trials following a correct response (Fig. 2B, right panel), whereas group differences were seen on trials that both followed an error and included a cue of 0.8 s duration (Fig. 2B, left panel). Both groups of WT mice exhibited a drop in performance for trials that followed an error (relative to after a correct response), contrary to the Mthfd1gt/+ mice. This error- induced disruption in performance was more pronounced for the WT Def mice than for the WT Suf mice for trials with the briefest cue (p = 0.0079). The pattern of findings indicates that for the WT mice, committing an error on the prior trial transiently disrupted attention, an effect that was exacerbated by dietary folate deficiency.
3.9.2. Premature responses
The analysis of percent premature responses revealed a significant interaction of Genotype and Pre-cue Delay [F(1, 427.4) = 7.79, p = 0.006], Genotype and Prev [F(1, 42.21) = 4.86, p = 0.033], and Genotype, Pre-cue Delay, and Prev [F(1, 427.2) = 9.36, p = 0.002]. As depicted in Fig. 2C, the Mthfd1gt/+ mice made a significantly lower percentage of premature responses than the WT mice on trials that both followed an error and included a 4 s pre-cue delay (p = 0.004). However, as shown in this figure, the increase in premature responding for the WT mice was primarily driven by the animals on the Def diet. In fact, it is notable that for both percentage correct and percent premature responses, the pattern of effects (Fig. 2, panels A–C) suggests that the disruption produced by committing an error on the prior trial was most pronounced for the WT mice maintained on the Def diet.
3.9.3. Omission errors
In the analysis of percentage of omission errors, a significant main effect of Genotype [F(1, 47.97) = 8.42, p = 0.006] was seen, as well as a significant interaction between Genotype and Prev [F(1, 119.1) = 15.35, p = 0.0001]. As seen in Fig. 2D, the two genotypes did not differ in omission errors for trials that followed a correct response, whereas for trials following an error, the Mthfd1gt/+ mice committed a significantly lower percentage of omission errors (p = 0.0005), again indicating that these Mthfd1gt/+ mice did not exhibit the normal error-induced disruption seen in the WT mice.
3.9.4. Inaccurate responses
The analysis of percent inaccurate responses revealed a significant interaction of Diet and Prev [F(1, 53.57) = 4.15, p = 0.046; Fig. A2]. For trials that followed a correct response, no group differences were seen, whereas for trials that followed an error, the animals on a Def diet committed a significantly higher percentage of inaccurate responses than the animals on a Suf diet (p = 0.0001). The average difference between groups was, however, very small (1.05 percentage points), which corresponds to a 28% increase in this type of error.
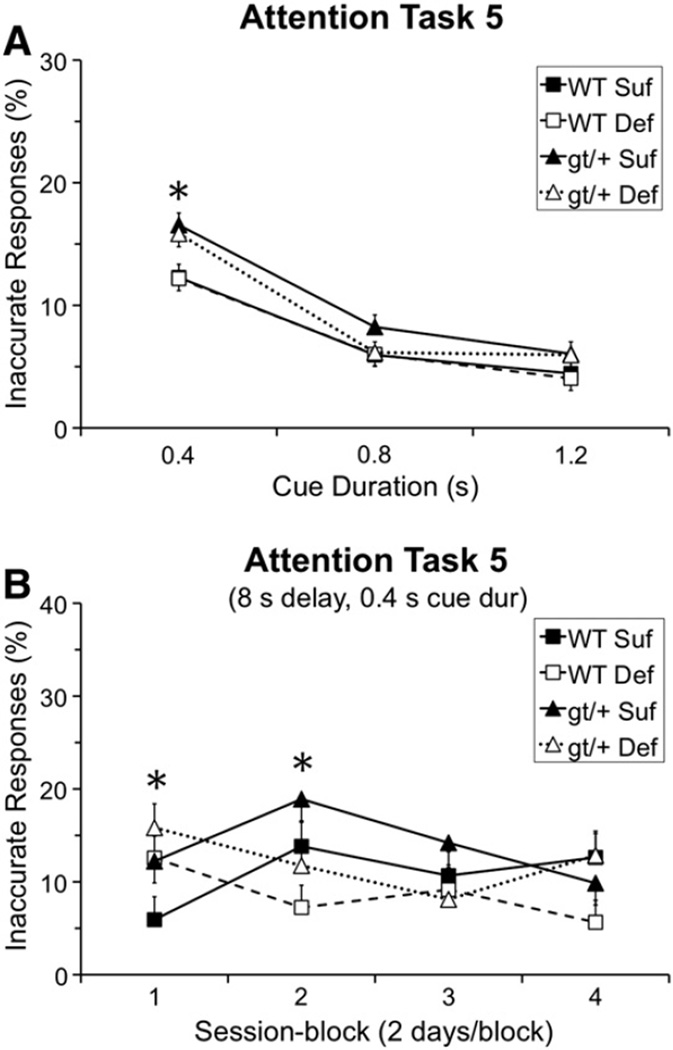
3.10. Attention Task 5 (pre-cue delay: 0, 4, 8 s; cue duration: 0.4, 0.8, 1.2 s)
3.10.1. Correct responses
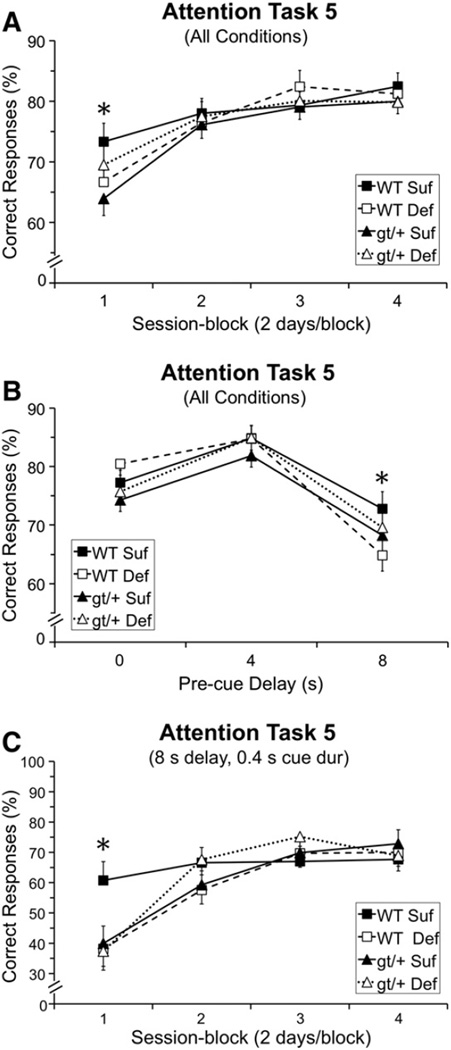
Analysis of the percentage of correct responses revealed a significant interaction involving Genotype, Diet, and Session-block [F(3, 391.5) = 2.94, p = 0.033]. As seen in Fig. 3A, during session-block 1 (1st two testing sessions), all three experimental groups performed more poorly than the WT Suf group. Moreover, the pattern of effects indicated that reducing the folate content of the diet exerted qualitatively different effects for the two genotypes: for the WT mice, the Def diet impaired performance (relative to the Suf diet), whereas for Mthfd1gt/+ mice, the mice maintained on the Def diet performed better than those on the Suf diet. Contrasts did not demonstrate any significant group differences following the Bonferroni correction, although the comparison between the Mthfd1gt/+ Suf and WT Suf mice was suggestive (p = 0.032).
Fig. 3.
Task 5 (pre-cue delay: 0, 4, 8 s; cue duration: 0.4, 0.8, 1.2 s) percentage of correct responses: (A) Percentage of correct responses as a function of session block; the performance of the gt/+ Suf mice was significantly lower than that of the WT Suf mice in session-block 1 (p = 0.032); (B) percentage of correct responses as a function of pre-cue delay; the WT Def mice performed significantly more poorly than WT Suf mice for trials with the longest pre-delay (8 s delay: WT Def < WT Suf, p = 0.038); and (C) percentage correct for the most demanding condition (trials with an 8 s pre-cue delay and 0.4 s cue duration), as a function of session-block; all groups performed significantly more poorly than the WT Suf group in the first block of testing (WT Suf > WT Def, p = 0.011; WT Suf > gt/+ Def, p = 0.015; WT Suf > gt/+ Suf, p = 0.018). Abbreviations: WT Suf: wildtype mice fed a folate sufficient diet; WT Def: wildtype mice fed a folate deficient diet; gt/+ Suf: Mthfd1gt/+ mice fed a folate sufficient diet; gt/+ Def: Mthfd1gt/+ mice fed a folate deficient diet; gt/+: Mthfd1gt/+ mice, WT: wildtype mice; *indicates significance ≤ 0.05. Data points are means ± SEM.
The analysis of the percentage of correct responses also revealed significant interactions between Diet and Pre-cue delay [F(2, 328.6) = 4.15, p = 0.017], as well Genotype, Diet, and Pre-cue Delay [F(3, 328.6) = 4.03, p = 0.019]. As seen in Fig. 3B, no group differences were seen at the two shorter pre-cue delays (0 and 4 s), whereas at the longest delay (8 s), all three experimental groups performed less well than the WT Suf group, with the contrast between the WT Suf and WT Def groups just falling short of significance following the Bonferroni correction (p = 0.038).
An analysis including only the most-demanding condition of this task (trials with an 8 s pre-cue delay and 0.4 s cue duration) for percentage of correct responses revealed a marginally significant interaction including Genotype, Diet, and Session-Block [F(3, 92.45) = 2.54, p = 0.061]. As seen in Fig. 3C, in session-block 1, each of the three experimental groups performed significantly more poorly than the WT Suf group (WT Suf vs. WT Def: p = 0.011; WT Suf vs. Mthfd1gt/+ Suf: p = 0.018; WT Suf vs. Mthfd1gt/+ Def: p = 0.015).
3.10.2. Premature responses
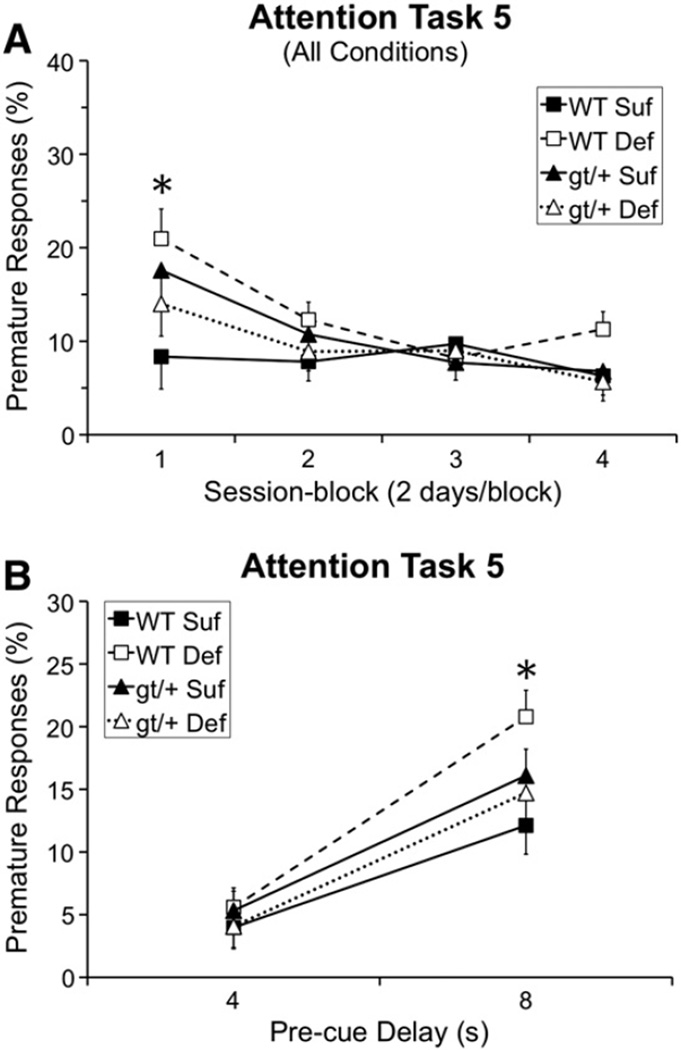
The analysis of percentage premature responses revealed a significant interaction of Genotype and Diet [F(1, 44.1) = 3.76, p = 0.059], Genotype, Diet, and Session-block [F(3, 196.3) = 3.39, p = 0.019], and Genotype, Diet, and Pre-cue delay [F(1, 176.8) = 3.34, p = 0.069]. As shown in Fig. 4A, in session-block 1, the WT Def mice committed a significantly higher percentage of premature responses than WT Suf mice (WT Suf vs. WT Def: p = 0.001), with a similar trend seen in the Mthfd1gt/+ Suf mice (p = 0.050). Furthermore, as shown in Fig. 4B, at the longest delay (8 s), WT Def mice committed a significantly higher percentage of premature responses than WT Suf mice (p = 0.007).
Fig. 4.
Task 5 (pre-cue delay: 0, 4, 8 s; cue duration: 0.4, 0.8, 1.2 s) percentage of premature responses: (A) Percentage of premature responses as a function of session-block, revealing that the WT Def (p = 0.001) and gt/+ Suf (p = 0.050) groups committed a significantly higher percentage of premature responses than the WT Suf group in session-block 1; and (B) percent premature responses as a function of pre-cue delay; for trials with an 8 s pre-cue delay, the WT Def mice committed a significantly higher percentage of premature responses than WT Suf mice (p = 0.007). Abbreviations: WT Suf: wildtype mice fed a folate sufficient diet; WT Def: wildtype mice fed a folate deficient diet; gt/+ Suf: Mthfd1gt/+ mice fed a folate sufficient diet; gt/+ Def: Mthfd1gt/+ mice fed a folate deficient diet; *indicates significance ≤ 0.05. Data points are means ± SEM.
3.10.3. Omission errors
Analysis of omission errors revealed a significant Genotype by Session-block interaction [F(3, 1497) = 2.75, p = 0.042], although the two genotypes did not differ significantly during any session-block (Supplementary Fig. A3).
3.10.4. Inaccurate responses
The analysis of percentage inaccurate responses revealed a significant interaction of Genotype and Cue-duration [F(2, 1453) = 4.85, p = 0.008]. As seen in Fig. 5A, the Mthfd1gt/+ mice committed a significantly higher percentage of inaccurate responses than the WT mice for trials with the briefest cue (0.4 s: p = 0.0002), but did not differ for trials with more prolonged cues, indicative of attentional dysfunction.
Fig. 5.
Task 5 (pre-cue delay: 0, 4, 8 s; cue duration: 0.4, 0.8, 1.2 s) percentage of inaccurate responses: (A) Percentage of inaccurate responses as a function of cue duration; the gt/+ mice made a significantly higher percentage of inaccurate responses than the WT mice specifically for trials with the briefest cue (p = 0.0002); and (B) percentage of inaccurate responses for the most demanding trials (8 s delay, 0.4 s cue duration), as a function of session-block, revealing a significantly higher percentage of inaccurate responses for mice on the Def diet relative to the Suf diet in session-block 1 (p = 0.041), whereas this pattern was reversed in session-block 2 (p = 0.006), before the groups converged in session-blocks 3 and 4. Abbreviations: WT Suf: wildtype mice fed a folate sufficient diet; WT Def: wildtype mice fed a folate deficient diet; gt/+ Suf: Mthfd1gt/+ mice fed a folate sufficient diet; gt/+ Def: Mthfd1gt/+ mice fed a folate deficient diet; gt/+: Mthfd1gt/+ mice, WT: wildtype mice; Def: folate deficient diet, Suf: folate sufficient diet; *indicates significance ≤ 0.05. Data points are means ± SEM.
Additionally, a significant interaction of Diet, Session-block, Pre-cue Delay, and Cue Duration [F(12, 1453) = 1.74, p = 0.05] was found, reflecting group differences specifically for the most demanding trials; i.e., those with an 8 s pre-cue delay and 0.4 cue duration. In session-block 1, for these demanding trials, the mice maintained on the Def diet made a significantly higher percentage of inaccurate errors than those on the Suf diet (p = 0.041; Fig. 5B). This pattern was reversed during session-block 2, with the mice on the Suf diet exhibiting a higher percentage of inaccurate errors than the Def mice (p = 0.006). Group differences were not seen for session-blocks 3 and 4. This pattern reflects improvement of the Def mice from session-blocks 1 to block 2, but an unexpected increase in this error-type for the Suf mice across these session-blocks. This latter pattern may reflect the concomitant decrease in premature responses across this time.
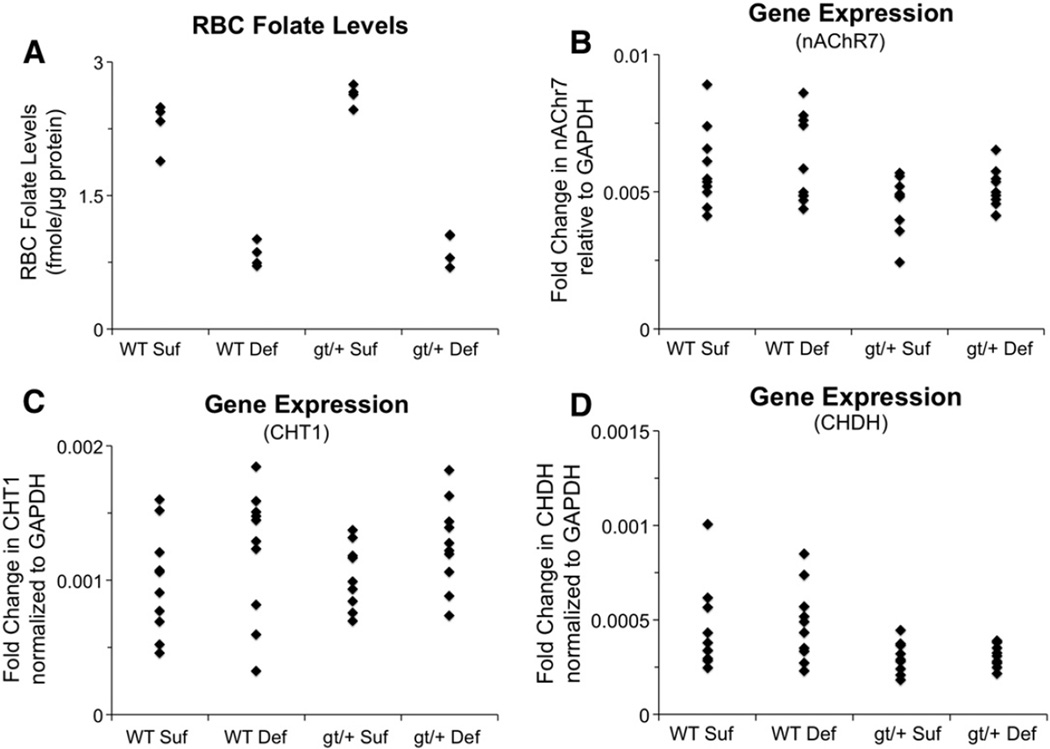
3.11. RBC folate concentrations
The mice maintained on the folate deficient diet had significantly lower RBC folate concentrations than the mice on the sufficient diet, (Wilcoxon rank-sum test, p = 0.0009), as shown in Fig. 6A. There was no effect of Genotype. These findings are consistent with the patterns seen in a previous study examining Mthfd1gt/+ mice on a similar folate deficient diet (MacFarlane et al., 2009).
Fig. 6.
Dot plots for red blood cell (RBC) folate levels and cholinergic gene expression data: (A) RBC folate levels normalized to total protein levels, indicating that mice on the Def diet had significantly lower RBC levels than those on the Suf diet (p = 0.0009); (B) fold change in nAChR7 expression levels relative to GAPDH expression, showing significantly reduced nAChR7 expression for the gt/+ mice relative to the WT mice (p = 0.027), although this effect was less pronounced for the gt/+ Def mice; (C) fold change in CHT1 expression levels relative to GAPDH expression, indicating significantly higher expression for mice maintained on the Def diet (Def > Suf, p = 0.040); and (D) fold change in CHDH expression levels relative to GAPDH expression, revealing significantly reduced expression for the gt/+ mice (gt/+ < WT, p = 0.011). Abbreviations: WT Suf: wildtype mice fed a folate sufficient diet; WT Def: wildtype mice fed a folate deficient diet; gt/+ Suf: Mthfd1gt/+ mice fed a folate sufficient diet; gt/+ Def: Mthfd1gt/+ mice fed a folate deficient diet; gt/+: Mthfd1gt/+ mice; WT: wildtype mice; CHDH: choline dehydrogenase, nAChR7: nicotinic acetylcholine receptor alpha 7; CHT1: high affinity choline transporter; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
3.12. Gene expression analyses
The analysis of nAChR7 expression revealed an effect of Genotype (p = 0.027). As shown in Fig. 6B, the Mthfd1gt/+ mice exhibited reduced expression of this gene relative to the WT mice. There was no significant effect of Diet, although inspection of the data (Fig. 6B) suggests that the Def diet tended to “normalize” nAChR7 expression in the Mthfd1gt/+ mice, relative to the Suf diet, similar to the behavioral data.
The analysis of CHT1 expression revealed an effect of Diet (p = 0.040). As shown in Fig. 6C, the mice maintained on the Def diet had higher levels of CHT1 expression than those on the Suf diet. There was no significant effect of Genotype.
The analysis of CHDH expression revealed an effect of Genotype, (p = 0.011), as shown in Fig. 5C. CHDH expression was lower in Mthfd1gt/+ mice than WT mice. There was no effect of Diet.
4. Discussion
4.1. Impulsivity caused by dietary folate deficiency
The constellation of effects seen in the WT mice maintained on the folate deficient diet was qualitatively different than that exhibited by the Mthfd1gt/+ mice. The WT Def mice did not differ from the WT Suf group in their rate of learning the initial visual discrimination task nor in the percentages of omission errors or inaccurate responses in the attention tasks, indicative of normal attentional function. These findings also rule out sensory or motivational deficits as potential causes of the instances of impaired performance that were observed. Specifically, the WT Def mice exhibited impulsive responding (relative to the WT Suf mice) under two conditions: (1) during the initial sessions of new tasks which placed greater demands on impulse control (i.e., longer pre-cue delays), and initially produced a substantial drop in performance (i.e., likely causing stress and frustration) [Task 3 (Fig. 1C), and Task 5 (Figs. 3 & 4)], and (2) on trials that immediately followed an error (Task 4: Fig. 2C).
In Task 3, the first task with relatively long pre-cue delays on some trials, all groups initially showed a substantial drop in performance, due primarily to a high percentage of premature responses as they learned to wait for the cue. This high rate of premature responses declined more slowly for the mice maintained on the folate deficient diet, with the result that in session-block 2, their percentage of premature responses was significantly higher than that of the mice on the folate sufficient diet, an effect that appeared to be most pronounced in the WT mice (Fig. 1C). Similarly, in Task 5, in which demands on both impulse control and attention were substantially increased and performance dropped, the WT Def mice again exhibited a pronounced but transient increase in premature responding (Figs. 3 & 4).
One explanation for this pattern of findings is that these particular testing conditions – a novel and demanding task coupled with a pronounced drop in reinforcement – produced stress and frustration. Dietary folate deficiency appeared to increase the reactivity of the mice to this type of stressor, manifesting as impulsive responding. This interpretation gains support from the second circumstance in which the WT Def mice exhibited impulsive responding: on trials immediately following an error. In Task 4, performance was evaluated not only as a function of pre-cue delay and cue duration but also as a function of the outcome of the previous trial (correct or incorrect). Importantly, the groups did not differ on trials following a correct response, regardless of the pre-cue delay. Rather, WT Def mice exhibited an increase in premature responding (relative to WT Suf mice) on trials that both followed an error and included a 4 s pre-cue delay (Fig. 2C). WT Suf mice performed significantly worse on trials following an error than on trials following a correct response, indicative of error-induced disruption in performance, consistent with many prior studies involving rats (e.g. Beaudin et al., 2007; Gendle et al., 2003; Morgan et al., 2002; Stangle et al., 2007), mice (e.g. Moon et al., 2010, 2008), and humans (see Segalowitz and Dywan, 2009 for a review). This error-induced disruption in performance was exacerbated by dietary folate deficiency, suggesting an affective response that is either heightened or less well regulated.
Although the neural basis of the putative affective dysfunction for WT Def mice is unknown, one plausible underlying mechanism is altered 5-HT activity, based on the evidence that (1) dietary folate deficiency for as little as three months can reduce 5-HT levels and turnover in specific areas of the brain including the hippocampus, amygdala, striatum (Kronenberg et al., 2008) and hypothalamus (Gospe et al., 1995), and (2) impulsive responding has been reported in rats following manipulations that alter 5-HT activity (Dalley et al., 2008; Passetti et al., 2002; Puumala and Sirvio, 1998).
4.2. Dietary deficiency alters CHT1 gene expression
This study also found that dietary folate deficiency increased expression for CHT1, the gene encoding the choline transporter, a rate-limiting step in synthesis of acetylcholine (ACh). Choline and folate are metabolically interrelated and there is growing evidence from both animal (Chew et al., 2011; Craciunescu et al., 2010; Crivello et al., 2010; Field et al., 2013; Kim et al., 1994; Schwahn et al., 2003; Troen et al., 2008; Varela-Moreiras et al., 1995; Zeisel, 2008) and human (Ivanov et al., 2009; Jacob et al., 1999) studies that altering metabolism of one of these nutrients can result in compensatory changes in the other. The present finding that dietary folate deficiency increases expression of CHT1 is consistent with the prior report that maintaining rats on a folate deficient diet for ten weeks increases ACh levels in the frontal cortex. Future research is needed to further elucidate the mechanism(s) underlying the effects of dietary folate deficiency on choline and cholinergic activity, and how this may, in turn, affect cognitive and affective functioning.
4.3. The Mthfd1 mutation produces abnormal error-reactivity in mice
One striking behavioral phenotype of the Mthfd1gt/+ mice, was that unlike the WT mice, their performance was unaffected by committing an error on the prior trial. On post-error trials, WT Suf and WT Def mice committed a higher percentage of omission errors than on trials following a correct response (Fig. 2), indicating that committing an error disrupted attention on the following trial (as reported previously for mice, rats, and humans). Interestingly, for the Mthfd1gt/+ mice, performance on post-error trials was as proficient as on trials following a correct response (Fig. 2), indicating that they either were unaware that they committed an error on the previous trial or that the affective response to the error was diminished or absent. This blunted response to stress or negative life events not only explains the superior performance of the Mthfd1gt/+ mice on trials following an error but also is the likely explanation for the superior performance of these mice during the early sessions of Task 3, a period of testing which was likely to be both frustrating and stressful, due to the novel imposition of long delays and the concomitant dramatic increase in errors for all groups.
4.4. The MTHFD1 mutation produces attentional dysfunction in mice
Both groups of Mthfd1gt/+ mice also exhibited attentional dysfunction. During the first session-block of Task 5, under the most challenging task conditions (8 s pre-cue delay, 0.4 s cue duration), both Mthfd1gt/+ groups performed significantly worse than the WT Suf group (Fig. 3C), due to a higher percentage of inaccurate responses (both groups; Fig. 5A) and premature responses (Fig. 4A), although in this latter case only the Mthfd1gt/+ Suf group differed from WT Suf group; the Mthfd1gt/+ Def group did not. As suggested by this last finding, the impairment of the Mthfd1gt/+ mice was, in many instances, more pronounced when they were maintained on a folate sufficient diet; i.e., the folate deficient diet was, interestingly, somewhat “therapeutic” for these mice. This was also seen in Attention Task 3; here the Mthfd1gt/+ Suf mice exhibited a significantly higher percentage of omission errors than the Mthfd1gt/+ Def mice (Fig. 1D).
Although the attentional impairment of the Mthfd1gt/+ mice was transient in Task 5, it is likely that this testing situation underestimates the severity and persistence of the attentional dysfunction produced by this MTHFD1 mutation (or human SNP). These tasks assessed the ability to attend to a single stimulus in relatively barren testing conditions. Each task was administered for many sessions, allowing the characteristics of the task to become rote and predictable, with only pre-cue delay and cue duration varying. These characteristics contrast with everyday life where one is constantly bombarded with new, complex stimuli engaging all sensory modalities. Thus, it is likely that the dysfunction of these mice would be more pronounced and persistent if assessed in a more complex environment, in which contingencies and cues frequently changed (i.e., conditions that more closely approximate the complexity of the real world).
4.5. Possible causes of the attentional dysfunction of the Mthfd1gt/+ mice
It is possible that altered cholinergic activity may contribute to the attentional dysfunction of the Mthfd1gt/+ mice. Although only the Mthfd1gt/+ Def displayed a reduction in RBC folate levels (Fig. 5A), both Mthfd1gt/+ groups exhibited reduced expression of the gene encoding the nicotinic acetylcholine receptor, subunit α7 (nAChR7; Fig. 5B), relative to WT mice. As this receptor subunit has been implicated in modulating attentional performance in normal rats (Day et al., 2007; Hahn et al., 2003; Hoyle et al., 2006; Lambe et al., 2005; Young et al., 2004), it is possible that a deficiency in the expression of this gene may contribute to the attentional dysfunction exhibited by these mice.
The finding that the Mthfd1gt/+ Def mice were less impaired than their counter-parts on a sufficient diet suggests that the combination of the MTHFD1 mutation and the deficient diet may have triggered a compensatory response which lessened the impact of the mutation on attentional functioning. One possible mechanism pertains to SAM. A prior study demonstrated that maintaining Mthfd1gt/+ mice on a diet deficient in both folate and choline lessened the effect of the mutation on SAM levels relative to Mthfd1gt/+ mice maintained on a diet with sufficient levels of these nutrients (MacFarlane et al., 2009, 2011). Similar compensatory effects have been reported for mice with mutations in genes that alter other aspects of folate metabolism (Elmore et al., 2007; Matthews and Elmore, 2007). SAM plays an important role as a methyl donor, thus affecting DNA methylation (Ulrey et al., 2005) as well as the metabolism of the catecholamines, serotonin, and acetylcholine (Alpert et al., 2002; Chan et al., 2008; Zhu, 2002), all transmitter systems that project to and modulate neocortical functions (Robbins and Arnsten, 2009). Thus, although still a working hypothesis, maintenance on a deficient diet may reduce the effect of the Mthfd1 mutation on SAM levels, which in turn could produce less disruption of neurotransmitter levels and activity, thereby preserving neocortical functions.
4.6. Mthfd1gt/+ mice exhibited reduced expression of the gene encoding choline dehydrogenase (CHDH)
The MTHFD1 mutation not only reduced expression of nAChR7 in brain tissue, but also produced a significant decrease in the expression of the gene encoding CHDH (Fig. 6D). CHDH is involved in the irreversible oxidation of choline to form betaine (Zeisel, 2006). Although not highly expressed in the brain (Johnson et al., 2010), reduced expression of CHDH may represent a mechanism to redirect choline availability from betaine production to other pathways, such as acetylcholine synthesis. Consistent with this hypothesis, a recent study found that plasma homocysteine levels were higher in Mthfd1gt/+ mice (Field et al., 2013), a pattern which would be predicted following suppression of CHDH and reduction in betaine, an important methyl donor normally used to convert homocysteine to methionine. This suggests collectively that individuals with MTHFD1 SNPs may require increased choline intake, consistent with other studies (Field et al., 2013; Ivanov et al., 2009; Kohlmeier et al., 2005).
5. Summary and conclusions
The present findings suggest that alterations in folate metabolism produced by dietary folate deficiency and/or a common SNP affecting a gene in the folate metabolic pathway can impact attention and affect regulation. Moreover, the results demonstrate qualitatively different effects depending on the type of alteration, likely due to different neurobiological mechanisms. The WT mice maintained on a folate deficient diet exhibited impulsive responding under two conditions: (1) immediately following a change in task parameters that both increased demands on impulse control and attention, and which substantially increased the error rate, and (2) on trials following an error. This pattern of findings indicates a heighted affective response to stress or negative life events, or an inability to regulate these negative emotions. In contrast, the Mthfd1 mutation produced a blunted affective response to committing an error as well as attentional dysfunction. This latter effect was, interestingly, less pronounced when dietary folate levels were very low; i.e., for these Mthfd1gt/+ mice, the Def diet was somewhat protective, suggesting a compensatory mechanism in the face of the combined genetic and dietary perturbation of folate metabolism. The Mthfd1gt/+ mice also showed significantly decreased expression levels for genes encoding CHDH or nAChR7, both of which are related to attentional functioning. Dietary folate deficiency also increased expression of the gene encoding the high affinity choline transporter. Due to the exploratory nature of this study, definitive conclusions must await replication of the findings. These types of gene–diet interaction studies are crucial for informing nutrition recommendations for individuals based on their specific genotype in order to achieve optimal cognitive functioning.
Supplementary Material
Acknowledgments
We would like to thank Dr. Martha Field and Cheryll A. Perry for their roles in conducting biochemical and genotyping analyses for the mice used in this study; Dr. Field also provided valuable comments regarding the interpretation of the results. We would also like to thank Elaina Chen for her help with mouse colony maintenance and supervising the behavioral testing. Finally, we are indebted to the many Cornell undergraduates who participated in the behavioral testing of the mice.
Role of the funding source
This study was supported by the USDA Cooperative State Research, Education and Extension Service (CSREES), Special Research Grant No. 00444528 contracted to Dr. Marie Caudill and the Public Health Service Grant (PHS) HD059120 contracted to Dr. Patrick Stover. These sources had no involvement in study design, collection, analysis or interpretation of data, in writing of the report, or in the decision to submit the article for publication.
Footnotes
Conflict of interest statement
There are no conflicts of interest to report.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ntt.2013.05.002.
References
- Alpert JE, Mischoulon D, Rubenstein GE, Bottonari K, Nierenberg AA, Fava M. Folinic acid (leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002;14:33–38. doi: 10.1023/a:1015271927517. [DOI] [PubMed] [Google Scholar]
- Ariogul S, Cankurtaran M, Dagli N, Khalil M, Yavuz B. Vitamin B12, folate, homocysteine and dementia: are they really related? Arch Gerontol Geriatr. 2005;40:139–146. doi: 10.1016/j.archger.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Botez MI. Avoidance behavior in folate-deficient rats. Tohoku J Exp Med. 1978;126:111–116. doi: 10.1620/tjem.126.111. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Joyal C, Botez MI. Blood thiamine and blood folate levels. A comparative study in control, alcoholic and folate-deficient subjects. Int J Vitam Nutr Res. 1981;51:205–210. [PubMed] [Google Scholar]
- Beaudin SA, Stangle DE, Smith DR, Levitsky DA, Strupp BJ. Succimer chelation normalizes reactivity to reward omission and errors in lead-exposed rats. Neurotoxicol Teratol. 2007;29:188–202. doi: 10.1016/j.ntt.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Blondeau C, Dellu-Hagedorn F. Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry. 2007;61:1340–1350. doi: 10.1016/j.biopsych.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Bottiglieri T, Diaz-Arrastia R. Hyperhomocysteinemia and cognitive function: more than just a causal link? Am J Clin Nutr. 2005;82:493–494. doi: 10.1093/ajcn.82.3.493. [DOI] [PubMed] [Google Scholar]
- Bottiglieri T, Hyland K, Laundy M, Godfrey P, Carney MW, Toone BK, et al. Folate deficiency, biopterin and monoamine metabolism in depression. Psychol Med. 1992;22:871–876. doi: 10.1017/s0033291700038447. [DOI] [PubMed] [Google Scholar]
- Bottiglieri T, Hyland K, Reynolds EH. The clinical potential of ademetionine (S-adenosylmethionine) in neurological disorders. Drugs. 1994;48:137–152. doi: 10.2165/00003495-199448020-00002. [DOI] [PubMed] [Google Scholar]
- Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69:228–232. doi: 10.1136/jnnp.69.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocardo PS, Budni J, Kaster MP, Santos AR, Rodrigues AL. Folic acid administration produces an antidepressant-like effect in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology. 2008;54:464–473. doi: 10.1016/j.neuropharm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Brody LC, Conley M, Cox C, Kirke PN, McKeever MP, Mills JL, et al. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. Am J Hum Genet. 2002;71:1207–1215. doi: 10.1086/344213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DF, Whiteley G, Alfirevic A, Pirmohamed M. Investigation of inter-individual variability of the one-carbon folate pathway: a bioinformatic and genetic review. Pharmacogenomics J. 2009;9:291–305. doi: 10.1038/tpj.2009.29. [DOI] [PubMed] [Google Scholar]
- Chan A, Tchantchou F, Graves V, Rozen R, Shea TB. Dietary and genetic compromise in fo-late availability reduces acetylcholine, cognitive performance and increases aggression: critical role of S-adenosyl methionine. J Nutr Health Aging. 2008;12:252–261. doi: 10.1007/BF02982630. [DOI] [PubMed] [Google Scholar]
- Chew TW, Jiang X, Yan J, Wang W, Lusa AL, Carrier BJ, et al. Folate intake, MTHFR genotype, and sex modulate choline metabolism in mice. J Nutr. 2011;141:1475–1481. doi: 10.3945/jn.111.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KE, Rohlicek CV, Andelfinger GU, Michaud J, Bigras JL, Richter A, et al. The MTHFD1 p.Arg653Gln variant alters enzyme function and increases risk for congenital heart defects. Hum Mutat. 2009;30:212–220. doi: 10.1002/humu.20830. [DOI] [PubMed] [Google Scholar]
- Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, Scott J, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr. 2007;86:1384–1391. doi: 10.1093/ajcn/86.5.1384. [DOI] [PubMed] [Google Scholar]
- Craciunescu CN, Johnson AR, Zeisel SH. Dietary choline reverses some, but not all, effects of folate deficiency on neurogenesis and apoptosis in fetal mouse brain. J Nutr. 2010;140:1162–1166. doi: 10.3945/jn.110.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivello NA, Blusztajn JK, Joseph JA, Shukitt-Hale B, Smith DE. Short-term nutritional folate deficiency in rats has a greater effect on choline and acetylcholine metabolism in the peripheral nervous system tan in the brain, and this effect escalates with age. Nutr Res. 2010;30:722–730. doi: 10.1016/j.nutres.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe SF, Ross CK. Effect of folate deficiency and folate and B12 excess on memory functioning in young chicks. Pharmacol Biochem Behav. 1997;56:189–197. doi: 10.1016/s0091-3057(96)00175-x. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Day M, Pan JB, Buckley MJ, Cronin E, Hollingsworth PR, Hirst WD, et al. Differential effects of ciproxifan and nicotine on impulsivity and attention measures in the 5-choice serial reaction time test. Biochem Pharmacol. 2007;73:1123–1134. doi: 10.1016/j.bcp.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Driscoll LL, Carroll JC, Moon J, Crnic LS, Levitsky DA, Strupp BJ. Impaired sustained attention and error-induced stereotypy in the aged Ts65Dn mouse: a mouse model of Down syndrome and Alzheimer's disease. Behav Neurosci. 2004;118:1196–1205. doi: 10.1037/0735-7044.118.6.1196. [DOI] [PubMed] [Google Scholar]
- Elmore CL, Wu X, Leclerc D, et al. Metabolic derangement of methionine and folate metabolism in mice deficient in methionine synthase reductase. Mol Genet Metab. 2007;91:85–97. doi: 10.1016/j.ymgme.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA, Berry KJ, Hansen DK, Wall KS, White G, Antony AC. Behavioral effects of prenatal folate deficiency in mice. Birth Defects Res A Clin Mol Teratol. 2005;73:249–252. doi: 10.1002/bdra.20111. [DOI] [PubMed] [Google Scholar]
- Field MS, Kelsey SS, Abarinov EV, Malysheva OV, Allen RH, Stabler SP, et al. Reduced MTHFD1 activity in male mice perturbs folate and choline dependent one-carbon metabolism as well as transsulfuration. J Nutr. 2013;143:41–45. doi: 10.3945/jn.112.169821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Impaired sustained attention and altered reactivity to errors in an animal model of prenatal cocaine exposure. Brain Res Dev Brain Res. 2003;147:85–96. doi: 10.1016/j.devbrainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gospe sM, Jr, Gietzen DW, Summers PJ, Lunetta JM, Miller JW, Selhub J, et al. Behavioral and neurochemical changes in folate-deficient mice. Physiol Behav. 1995;58:935–941. doi: 10.1016/0031-9384(95)00156-d. [DOI] [PubMed] [Google Scholar]
- Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44:1054–1067. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Herbig K, Chiang EP, Lee LR, Hills J, Shane B, Stover PJ. Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosynthesis. J Biol Chem. 2002;277:38381–38389. doi: 10.1074/jbc.M205000200. [DOI] [PubMed] [Google Scholar]
- Hol FA, van der Put NM, Geurds MP, Heil SG, Trijbels FJ, Hamel BC, et al. Molecular genetic analysis of the gene encoding the trifunctional enzyme MTHFD (methylenetetrahydrofolate-dehydrogenase, methenyltetrahydrofolate-cyclohydrolase, formyltetrahydrofolate synthetase) in patients with neural tube defects. Clin Genet. 1998;53:119–125. doi: 10.1111/j.1399-0004.1998.tb02658.x. [DOI] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharma-cology (Berl) 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Nash-Barboza S, Hinkis S, Caudill MA. Genetic variants in phosphatidyleth-anolamine N-methyltransferase and methylenetetrahydrofolate dehydrogenase influence biomarkers of choline metabolism when folate intake is restricted. J Am Diet Assoc. 2009;109:313–318. doi: 10.1016/j.jada.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr. 1999;129:712–717. doi: 10.1093/jn/129.3.712. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Craciunescu CN, Guo Z, Teng YW, Thresher RJ, Blusztajn JK, et al. Deletion of murine choline dehydrogenase results in diminished sperm motility. FASEB J. 2010;24:2752–2761. doi: 10.1096/fj.09-153718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado DM, Karlamangla AS, Huang MH, Troen A, Rowe JW, Selhub J, et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur studies of successful aging. Am J Med. 2005;118:161–167. doi: 10.1016/j.amjmed.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Kim YI, Miller JW, da Costa KA, et al. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J Nutr. 1994;124:2197–2203. doi: 10.1093/jn/124.11.2197. [DOI] [PubMed] [Google Scholar]
- Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci U S A. 2005;102:16025–16030. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Harms C, Sobol RW, Cardozo-Pelaez F, Linhart H, Winter B, et al. Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase. J Neurosci. 2008;28:7219–7230. doi: 10.1523/JNEUROSCI.0940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Colla M, Endres M. Folic acid, neurodegenerative and neuropsychiatric disease. Curr Mol Med. 2009;9:315–323. doi: 10.2174/156652409787847146. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J Neurosci. 2005;25:5225–5229. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levav-Rabkin T, Blumkin E, Galron D, Golan HM. Sex-dependent behavioral effects of Mthfr deficiency and neonatal GABA potentiation in mice. Behav Brain Res. 2011;216:505–513. doi: 10.1016/j.bbr.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Li JT, Su YA, Guo CM, Feng Y, Yang Y, Huang RH, et al. Persisting cognitive deficits induced by low-dose, subchronic treatment with MK-801 in adolescent rats. Eur J Pharmacol. 2011;652:65–72. doi: 10.1016/j.ejphar.2010.10.074. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- MacFarlane AJ, Perry CA, Girnary HH, Gao D, Allen RH, Stabler SP, et al. Mthfd1 is an essential gene in mice and alters biomarkers of impaired one-carbon metabolism. J Biol Chem. 2009;284:1533–1539. doi: 10.1074/jbc.M808281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane AJ, Perry CA, McEntee MF, Lin DM, Stover PJ. Mthfd1 is a modifier of chemically-induced Intestinal carcinogenesis. Carcinogenesis. 2011;32:427–433. doi: 10.1093/carcin/bgq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RG, Elmore CL. Defects in homocysteine metabolism: diversity among hyperhomocyst(e)inemias. Clin Chem Lab Med. 2007;45:1700–1703. doi: 10.1515/CCLM.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13:235–239. doi: 10.1002/(sici)1099-1166(199804)13:4<235::aid-gps761>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke. 2002;33:2351–2356. doi: 10.1161/01.str.0000032550.90046.38. [DOI] [PubMed] [Google Scholar]
- Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76:1158S–1161S. doi: 10.1093/ajcn/76/5.1158S. [DOI] [PubMed] [Google Scholar]
- Mooijaart SP, Gussekloo J, Frölich M, Jolles J, Stott DJ, Westendorp RG, et al. Homocys-teine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: the Leiden 85-Plus study. Am J Clin Nutr. 2005;82:866–871. doi: 10.1093/ajcn/82.4.866. [DOI] [PubMed] [Google Scholar]
- Moon J, Ota KT, Driscoll LL, Levitsky DA, Strupp BJ. A mouse model of fragile X syndrome exhibits heightened arousal and/or emotion following errors or reversal of contingencies. Dev Psychobiol. 2008;50:473–485. doi: 10.1002/dev.20308. [DOI] [PubMed] [Google Scholar]
- Moon J, Chen M, Gandhy SU, Strawderman M, Levitsky DA, Maclean KN, et al. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav Neurosci. 2010;124:346–361. doi: 10.1037/a0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RE, Garavan HP, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ. Enduring effects of prenatal cocaine exposure on attention and reaction to errors. Behav Neurosci. 2002;116:624–633. doi: 10.1037//0735-7044.116.4.624. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Schneider JA, Tangney CC, Bienias JL, Aggarwal NT. Dietary folate and vitamins B-12 and B-6 not associated with incident Alzheimer's disease. J Alzheimers Dis. 2006;9:435–443. doi: 10.3233/jad-2006-9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez LA, Wurtman RJ. Folic acid deficiency and methyl group metabolism in rat brain: effects of L-dopa. Arch Biochem Biophys. 1974;160:372–376. doi: 10.1016/0003-9861(74)90410-x. [DOI] [PubMed] [Google Scholar]
- Parle-McDermott A, Kirke PN, Mills JL, Molloy AM, Cox C, O'Leary VB, et al. Confirmation of the R653Q polymorphism of the trifunctional C1-synthase enzyme as a maternal risk for neural tube defects in the Irish population. Eur J Hum Genet. 2006;14:768–772. doi: 10.1038/sj.ejhg.5201603. [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Puumala T, Sirvio J. Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience. 1998;83:489–499. doi: 10.1016/s0306-4522(97)00392-8. [DOI] [PubMed] [Google Scholar]
- Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, et al. Homocyste-ine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr. 2004;80:114–122. doi: 10.1093/ajcn/80.1.114. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Montesi F, Rietti E, Pisacane N, et al. Risk factors for dementia: data from the Conselice study of brain aging. Arch Gerontol Geriatr. 2007;44(Suppl. 1):311–320. doi: 10.1016/j.archger.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Riggs KM, Spiro A, III, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. AmJ Clin Nutr. 1996;63:306–314. doi: 10.1093/ajcn/63.3.306. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17(Suppl. 1):151–160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- Schwahn BC, Chen Z, Laryea MD, Wendel U, Lussier-Cacan S, Genest J, Jr, et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003;17:512–514. doi: 10.1096/fj.02-0456fje. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Dywan J. Individual differences and developmental change in the ERN response: implications for models of ACC function. Psychol Res. 2009;73:857–870. doi: 10.1007/s00426-008-0193-z. [DOI] [PubMed] [Google Scholar]
- Seshadri S. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer's disease? J Alzheimers Dis. 2006;9:393–398. doi: 10.3233/jad-2006-9404. [DOI] [PubMed] [Google Scholar]
- Stangle DE, Smith DR, Beaudin SA, Strawderman MS, Levitsky DA, Strupp BJ. Succimer chelation improves learning, attention, and arousal regulation in lead-exposed rats but produces lasting cognitive impairment in the absence of lead exposure. Environ Health Perspect. 2007;115:201–209. doi: 10.1289/ehp.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover PJ, Caudill MA. Genetic and epigenetic contributions to human nutrition and health: managing genome-diet interactions. J Am Diet Assoc. 2008;108:1480–1487. doi: 10.1016/j.jada.2008.06.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupp BJ, Beaudin S. Assessing the neurobehavioral effects of early toxicant exposure: a perspective from animal research. In: Bellinger D, editor. Human developmental neurotoxicology. New York: Taylor & Francis Group; 2006. pp. 415–445. [Google Scholar]
- Suh JR, Oppenheim EW, Girgis S, Stover PJ. Purification and properties of a folate-catabolizing enzyme. J Biol Chem. 2000;275:35646–35655. doi: 10.1074/jbc.M005864200. [DOI] [PubMed] [Google Scholar]
- Suh JR, Herbig AK, Stover PJ. New perspectives on folate catabolism. Annu Rev Nutr. 2001;21:255–282. doi: 10.1146/annurev.nutr.21.1.255. [DOI] [PubMed] [Google Scholar]
- Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- Troen AM, Chao WH, Crivello NA, D'Anci KE, Shukitt-Hale B, Smith DE, et al. Cognitive impairment in folate-deficient rats corresponds to depleted brain phosphatidylcholine and is prevented by dietary methionine without lowering plasma homocysteine. J Nutr. 2008;138:2502–2509. doi: 10.3945/jn.108.093641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrey CL, Liu L, Andrews LG, Tollefsbol TO. The impact of metabolism on DNA methylation. Hum Mol Genet. 2005;14(Spec No 1):R139–R147. doi: 10.1093/hmg/ddi100. [DOI] [PubMed] [Google Scholar]
- Van Dam F, Van Gool WA. Hyperhomocysteinemia and Alzheimer's disease: a systematic review. Arch Gerontol Geriatr. 2009;48:425–430. doi: 10.1016/j.archger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Varela-Moreiras G, Ragel C, Perez de Miguelsanz J. Choline deficiency and methotrexate treatment induces marked but reversible changes in hepatic folate concentrations, serum homocysteine and DNA methylation rates in rats. J Am Coll Nutr. 1995;14:480–485. doi: 10.1080/07315724.1995.10718539. [DOI] [PubMed] [Google Scholar]
- Wolfinger R, O' Connell M. Generalized linear mixed models — a pseudo-likelihood approach. J Stat Comput Simul. 1993;48:233–243. [Google Scholar]
- Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, et al. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Genetic polymorphisms in methyl-group metabolism and epigenetics: lessons from humans and mouse models. Brain Res. 2008;1237:5–11. doi: 10.1016/j.brainres.2008.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu BT. Catechol-O-methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab. 2002;3:321–349. doi: 10.2174/1389200023337586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.