Abstract
Parabens are a class of small molecules that are regularly used as preservatives in a variety of personal care products. Several parabens, including butylparaben and benzylparaben, have been found to interfere with endocrine signaling and to stimulate adipocyte differentiation. We hypothesized these biological effects could be due to interference with the endocannabinoid system and identified fatty acid amide hydrolase (FAAH) as the direct molecular target of parabens. FAAH inhibition by parabens yields mixed-type and time-independent kinetics. Additionally, structure activity relationships indicate FAAH inhibition is selective for the paraben class of compounds and the more hydrophobic parabens have higher potency. Parabens enhanced 3T3-L1 adipocyte differentiation in a dose dependent fashion, different from two other FAAH inhibitors URB597 and PF622. Moreover, parabens, URB597 and PF622 all failed to enhance AEA-induced differentiation. Furthermore, rimonabant, a cannabinoid receptor 1 (CB1)-selective antagonist, did not attenuate paraben-induced adipocyte differentiation. Thus, adipogenesis mediated by parabens likely occurs through modulation of endocannabinoids, but cell differentiation is independent of direct activation of CB1 by endocannabinoids.
Keywords: Parabens, Benzylparaben, Fatty acid amide hydrolase, Adipocyte
1. Introduction
The biologic activity of many high production volume small molecules in consumer care products is poorly understood but is of high concern due to their daily use. Compared to environmental toxicants where individuals are unintentionally exposed to low doses of chemicals, many of these chemicals are voluntarily applied at high doses directly to the skin. Of these consumer care product chemicals, parabens are a class of small molecules commonly added to pharmaceuticals, cosmetics and food products for their antimicrobial activity (Bledzka et al., 2014). Structurally, they are esters of p-hydroxybenzoic acid and most commonly include methylparaben, propylparaben and butylparaben. They are not acutely toxic with oral LD50 values > 1 g/kg (Soni et al., 2005). Furthermore, methylparaben and propylparaben are on the Food and Drug Administration's list of chemicals “generally recognized as safe” (FDA, 1973). However, despite the low acute toxicity there has been significant concern over whether they act chronically as endocrine disrupting chemicals with studies providing evidence for (Chen et al., 2007) and against (Hoberman et al., 2008) such arguments.
In addition to their possible endocrine disrupting effects as sex hormone mimics, parabens have been reported to stimulate adipocyte differentiation (Taxvig et al., 2012; Hu et al., 2013). Adipocytes are formed early in life and the number of adipocytes does not generally increase in lean adults. However, it is possible for precursor cells to differentiate into adipocytes in adults when the demand for energy storage increases (Wang et al., 2014). Paraben-enhanced differentiation would supplement this process and increase the prevalence of obesity within exposed populations. Adipogenic effects are suggested to be mediated through the peroxisome proliferator-activated receptor gamma (PPARγ) and the glucocorticoid receptor (GR). Antagonism of either PPARγ or the GR using small molecules partially reduced the paraben-enhanced differentiation (Hu et al., 2013). Butylparaben, but not benzylparaben, activate the PPARγ receptor and all parabens tested, including butylparaben and benzylparaben, activated the GR but do not compete with dexamethasone binding to the GR (Hu et al., 2013; Pereira-Fernandes et al., 2013). Thus, while PPARγ and GR may be involved in paraben-mediated differentiation, they are not the direct targets responsible for all of these effects.
In addition to PPARγ and the GR, evidence exists to implicate the endocannabinoid (EC) system in the regulation of adipogenesis and general energy homeostasis (Silvestri and Di Marzo, 2013). Signaling through the EC system primarily occurs through two lipid mediators, arachidonoyl ethanolamide (also known as anandamide, AEA) and 2-arachidonoylglycerol (2-AG) that bind to the cannabinoid receptors (CB1 and CB2) (Pertwee, 2015). Independent of food intake, blockage of the CB1 receptor reduces lipogenesis (Vida et al., 2014). The CB1 antagonist rimonabant was used in clinical trials for the treatment of obesity, but it was pulled from the market due to serious side effects including depression (Christopoulou and Kiortsis, 2011). Pharmacologic and genetic knockout of the CB1 receptor seems to reduce or ablate glucocorticoid stimulated weight gain, indicating these effects occur downstream of the GR (Bowles et al., 2015). In addition to their action on the CB1 receptor, endocannabinoids may target additional pathways to affect metabolism. For example, AEA has been shown to bind directly to PPARγ to mediate adipocyte differentiation (Bouaboula et al., 2005; Karaliota et al., 2009).
Given the role of the EC system on adipocyte differentiation, we hypothesized previously observed effects of parabens on adipocytes may be mediated through modulation of endocannabinoid signaling. To test this hypothesis, we directly tested the ability of parabens to inhibit fatty acid amide hydrolase (FAAH), the enzyme primarily responsible for regulating concentrations of AEA. In addition, the effects of parabens, other FAAH inhibitors, and their interaction with AEA on 3T3-L1 adipocyte differentiation were explored.
2. Materials and methods
2.1. Chemicals
Methylparaben, propylparaben, butylparaben, benzylparaben and 4-hydroxybenzoic acid were all purchased from Acros Chemicals. URB597 and PF622 were purchased from Cayman Chemical and heptylparaben was purchased from Sigma-Aldrich. Other paraben-like compounds described were synthesized as described in Supplementary material. N-(6-methoxypyridin-3-yl) octanamide (OMP) and cyano(6-methoxynaphthalen-2-yl)methyl acetate (CMNA) were synthesized as previously described (Shan and Hammock, 2001; Huang et al., 2007).
2.2. Preparation of enzyme extracts
The transgenic production of the FAAH enzyme and other esterases in baculovirus are previously described (Nishi et al., 2006; Huang et al., 2007). A crude preparation of enzyme was prepared by centrifuging cells at 1000 rpm, 15 min, 4 °C and re-suspending the pellet in 50 mM tris buffer (pH = 8.0) with 1 mM benzamidine and 1 mM EDTA. The solution was homogenized (3 × 15 s) and centrifuged (9000g, 20 min). The resulting pellet was re-suspended in 50 mM tris/HCl buffer (pH = 8.0) with 1 mM CHAPS and 10% glycerol and kept frozen at 80 °C until use. The amount of FAAH in the crude extract was estimated to be 5% by SDS-PAGE.
For measuring FAAH inhibition, rat and mouse microsomes were prepared from frozen brain tissue. Tissue was homogenized (3 × 15 s) in 20 mM phosphate buffer (pH = 7.4) with 5 mM EDTA and centrifuged (9,000g, 20 min). The soluble fraction (S9) was collected and centrifuged again (100,000g, 1 h). The pellet containing microsomes was resuspended in 10 mM phosphate buffer with 2.5 mM EDTA and 20% glycerol and kept frozen at 80 °C until use. Treatment with 50 nM of the potent FAAH inhibitor URB597 reduced OMP hydrolysis to less than 20%, indicating most if not all of the activity measured is from FAAH.
2.3. Fluorimetric enzyme assays
FAAH activity was measured in 0.1 M sodium phosphate buffer at pH = 8 with 0.1-0.2 mg/mL of bovine serum albumin (BSA) using OMP as the substrate (Huang et al., 2007). Formation of the fluorescent methoxypyridine product was measured kinetically at λexcitation = 303 nm and λemission = 394 nm while reaction solutions were kept at 37 °C. To determine IC50 values, final reaction solutions contained approximately 0.9 μg of crude FAAH extract, [S]final = 50 μM and inhibitor in no more than 2% DMSO solution. Esterase activity (hCE1, hCE2 and AADAC) was measured under the same conditions using CMNA as the substrate and measuring the liberation of 6-methoxynaphthaldehyde at λexcitation = 330 nm and λemission = 465 nm (Morisseau et al., 2009). All experiments were run in either duplicate or triplicate and values reported as average ± SD represent at least 3 independent experiments.
2.4. Cell culture, induction of adipocyte differentiation and chemical treatments
Murine 3T3-L1 fibroblasts (ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% calf serum (Hyclone) in a 5% CO2, 37 °C environment until they reach confluence. To study the potentiating effects of parabens and other chemicals on adipocyte differentiation, the standard differentiation protocol was modified using a weaker GR agonist cortisone as previously described (Hu et al., 2013). Briefly, on the day of reaching confluence (designated as day 0), cells were treated with DMEM containing 10% fetal bovine serum (FBS, Atlas Biologicals), 0.5 mM methylisobutylxanthine (MIX), 170 nM insulin and 5 μM cortisone for 3 days. The cells were then grown in maintenance DMEM containing 10% FBS and 170 nM insulin for additional two days until day 5 followed by in DMEM containing 10% FBS until day 7.
Parabens and other chemicals were added in the differentiation media of 3T3-L1. Parabens and other chemicals were applied at each change of the media at day 0, day 3, day 5 during the 7-day process.
2.5. Oil O red staining and quantification
To quantify lipid accumulation, differentiated cells were fixed with 4% paraformaldehyde overnight, then rinsed with deionized water and stained with Oil Red O solution (60% Oil Red O in isopropanol) for 10 min. After staining, the plates were rinsed with deionized water. The images of the stained cell morphology were taken by an integrated digital camera linked to a Micro-master inverted digital microscope (Thermo Fisher Scientific). To quantify the staining, the Oil Red O was eluted with 100% isopropanol for 10 min and OD absorbance at 500 nm was measured with GloMax-Multi Detection System (Promega, Madison, WI).
2.6. RT-PCR
Total RNA was isolated using TRI reagent (Molecular Research Center Inc., Cincinnati, OH) according to the manufacturer's instructions. Total RNA abundance was quantified using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Reverse transcription was carried out using High Capacity cDNA Reverse Transcription kits (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's instructions. mRNA expression of adipocyte marker genes and loading control 36B4 were measured quantitatively using Absolute Blue QPCR SYBR Green ROX mix (Thermo Fisher Scientific, Waltham, MA). Primer Sequences are available upon request. PCR were run in a 96-well format using an ABI 7300HT instrument (Thermo Fisher Scientific, Waltham, MA). Cycle conditions were 50 °C 2 min, 95 °C 15 min, then 40 cycles of 95 °C for 15 s/60 °C for 1 min. Relative gene expression was calculated using the 2(−ΔΔCt) method, which normalizes against 36B4.
3. Results
3.1. Parabens inhibit FAAH
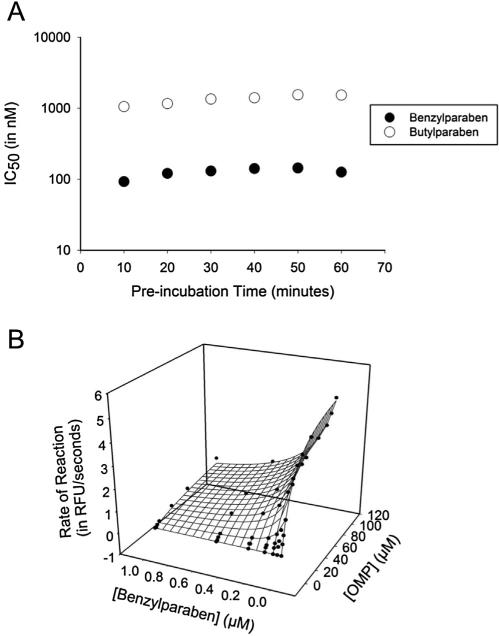
The ability of several relevant parabens to inhibit FAAH was tested on a recombinant preparation of the enzyme and compared against the well-characterized FAAH inhibitor URB597. Inhibition potencies (IC50) are reported in Table 1. Consistent with previous findings, URB597 is highly potent, with a low nanomolar IC50, and its potency increases significantly over time (an order of magnitude better between 5 and 60 min pre-incubation), as it forms a covalent complex with the enzyme (Kathuria et al., 2003). Benzylparaben is one and two orders of magnitude more potent than butyl- and propylparaben, respectively, while methylparaben is completely inactive. Additionally, the hydrolysis product, 4-hydroxybenzoic acid, is also completely inactive. After a 5 min incubation, benzylparaben is only 3-fold less potent than URB597. Compared to the time-dependent inhibition by URB597 that occurs by a covalent intermediate, 1 h pre-incubation did not decrease the IC50 of parabens, indicating inhibition does not occur through a covalent intermediate. A more extensive analysis of time-dependence demonstrates potency does not appreciably change over an hour-long period (Fig. 1A). To explore its mechanism of inhibition, kinetic constants for benzylparaben were measured (Fig. 1B). Both the apparent Vmax and the apparent Km changes as inhibitor concentration increases and therefore the relationship between enzyme, substrate and inhibitor was analyzed as a linear, mixed-type model (Segel, 1993). This model derived a Ki value of 52 ± 14 nM with an α value of 9.7 ± 6.6, indicating at saturating inhibitor concentrations, the relative in vivo Km of AEA will change by up to one order of magnitude.
Table 1.
Inhibition of fatty acid amide hydrolase (FAAH) by parabens and 4-hydroxybenzoate.
| pre-incubation (min) | 5 | 60 |
|---|---|---|
| Compound | IC50 (μM) | |
| URB597 | 0.040 ± 0.011 | 0.0030 ± 0.0005 |
| 4-HO-benzoate | >100 | >100 |
| Methylparaben | >100 | >100 |
| Propylparaben | 9.4 ± 1.0 | 17.1 ± 1.8 |
| Butylparaben | 1.1 ± 0.2 | 2.4 ± 0.5 |
| Benzylparaben | 0.14 ± 0.03 | 0.28 ± 0.03 |
Fig. 1.
Enzyme kinetics associated with FAAH inhibition by butylparaben and benzylparaben. (A) Inhibition of FAAH is independent on incubation time of enzyme with either butylparaben or benzylparaben. (B) Benzylparaben inhibits FAAH through a mixed type mechanism as evidenced by a change in Vmaxapp (5.5 RFU/s to 2.0 RFU/s) and KMapp (18 μM to 98 μM). The calculated Ki and α assuming a linear mixed-type model of inhibition is 52 ± 14 nM and 9.7 ± 6.6, respectively.
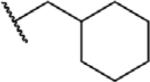
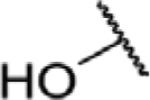
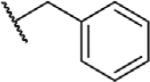
To determine which structural features confer potency, several analogs of benzylparaben were synthesized and tested (Table 2). Modification of the phenol to the methylaminophenyl (1) decreases potency 100-fold, while modification to the anisole (2), phenyl (3) or nitrophenyl (4) completely removed potency towards FAAH. This general trend suggests the p-hydroxyl is an essential component for activity against FAAH. Replacement of the benzyl alcohol with cyclohexyl-methanol (5) decreased potency 2-fold while replacement to the heptylparaben (6) had no change in potency, indicating the potency is primarily determined by the hydrophobicity of the alcohol portion of the ester. Finally, modification of the ester to an amide (7) reduced potency 100-fold, indicating the ester is an essential component for potency.
Table 2.
Structure activity relationships of paraben-liked molecules on fatty acid amide hydrolase (FAAH) inhibition.
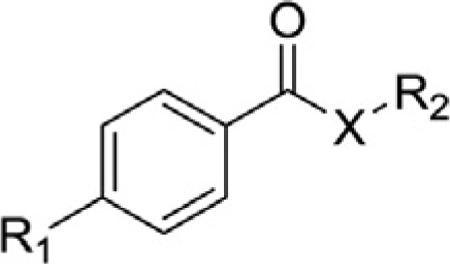
| ||||
|---|---|---|---|---|
| Compound | R1 | R2 | X | IC50 (μM) (5 min pre-incubation) |
| Benzylparaben |
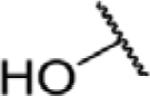
|
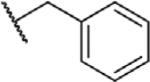
|
O | 0.14 ± 0.03 |
| 1 |
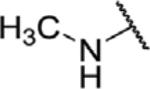
|
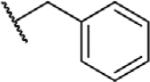
|
O | 18.2 ± 4.9 |
| 2 |
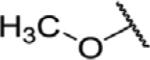
|
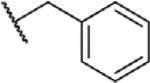
|
O | >100 |
| 3 |
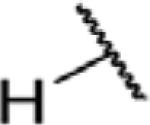
|
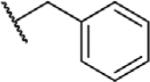
|
O | >100 |
| 4 |
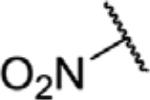
|
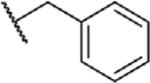
|
O | >100 |
| 5 |
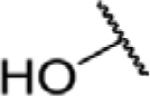
|
|
O | 0.36 ± 0.03 |
| 6 |
|
|
O | 0.15 ± 0.04 |
| 7 |
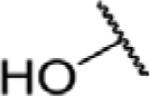
|
|
N | 8.6 ± 1.3 |
3.2. Inhibition by parabens is selective for FAAH and is not species-dependent
To test the possibility that parabens inhibit other serine hydrolases, the potencies of butylparaben and benzylparaben were compared with recombinant preparations of human carboxylesterase 1 and 2 (hCE1 and hCE2) and arylacetamide deacetylase (AADAC) using the general esterase substrate CMNA (Shan and Hammock, 2001) (Table 3). For all enzymes, the IC50 of both parabens are above 10 μM (the highest concentration tested), suggesting that it is unlikely that parabens inhibit serine hydrolases in general.
Table 3.
Relative selectivity of butylparaben and benzylparaben on FAAH in rodent species and on other recombinant enzymes.
| Enzyme Source | IC50 (μM) (5 min pre-incubation) |
||
|---|---|---|---|
| URB597 | Butylparaben | Benzylparaben | |
| Human Recombinant hCE1b | 2.2 | >10 | >10 |
| Human Recombinant hCE2b | 0.73 | >10 | >10 |
| Human Recombinant AADACb | >10 | >10 | >10 |
| Human Recombinant FAAHa | 0.040 ± 0.011 | 1.1 ± 0.2 | 0.14 ± 0.03 |
| Mouse Brain Microsomesa | <0.001 | 1.6 | 0.21 |
| Rat Brain Microsomesa | 0.005 | 1.1 | 0.23 |
Measured by hydrolysis of OMP, [S] = 50 μM.
Measured by hydrolysis of CMNA, [S] = 50 μM.
In addition to selectivity against other enzymes, potency of parabens was compared between human and rodent FAAH. For rodent data, brain microsome preparations were used instead of recombinant enzyme preparations. URB597 was used as a control to confirm the same fluorescence-based substrate could be used with brain microsome preparations. URB597 inhibits over 80% of the activity at 50 nM in both rat and mouse microsomes, demonstrating FAAH accounts for most of N-(6-methoxypyridin-3-yl) octanamide (OMP) hydrolysis and the IC50 from these preparations represents approximate values of parabens on mouse and rat FAAH. The values for both benzylparaben and butylparaben had a less than 2-fold difference between human and both rodent species (Table 3).
3.3. The effects of FAAH inhibition by parabens and other compounds, and interaction with AEA on 3T3-L1 adipocyte differentiation
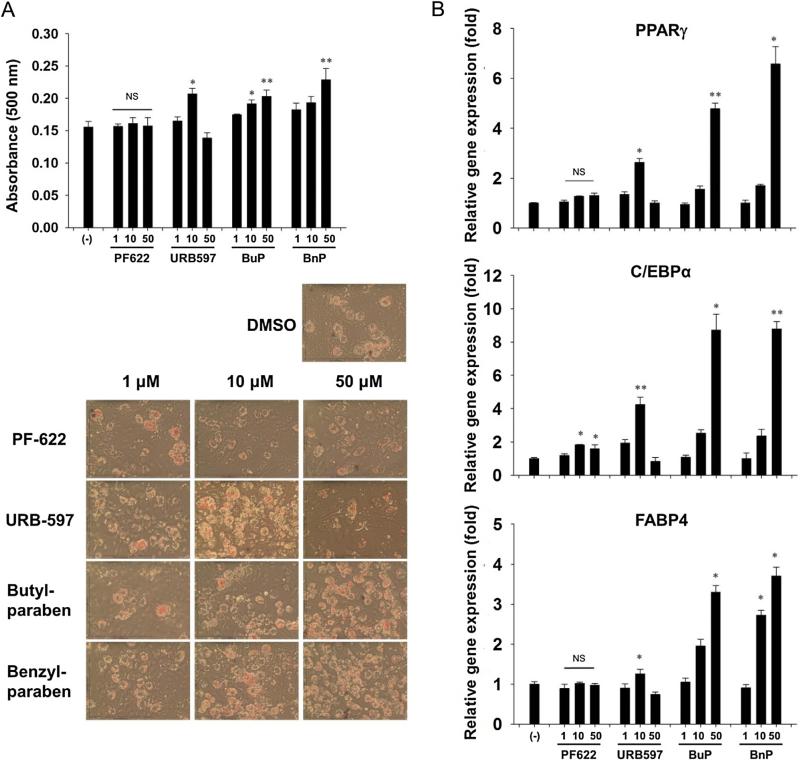
To determine whether previously observed actions of parabens on pre-adipocytes are mediated by FAAH inhibition (Hu et al., 2013), we first tested whether FAAH inhibition could plausibly induce differentiation. Although two inhibitors, URB597 and PF622, were tested on pre-adipocytes, only exposure to 10 μM of URB597 resulted in increased lipid accumulation as shown by Oil Red O staining (Fig. 2A). This stimulation was supported by increased PPARγ, C/EBPα and FABP4 mRNA expression (Fig. 2B), which are common biomarkers of adipocyte differentiation. As previously observed, butylparaben and benzylparaben dose-dependently enhanced 3T3-L1 adipocyte differentiation, as shown by increased Oil Red O staining and enhanced PPARγ, C/EBPα and FABP4 mRNA expression.
Fig. 2.
3T3-L1 cells were induced to differentiate in the presence of PF622, URB597, butylparaben (BuP), or benzylparaben (BnP) (1, 10, 50 μM) for 7 days. (A) Parabens dose dependently increase adipocyte differentiation as measured by oil red O while URB597 increases differentiation at 10 μM. (B) Parabens and URB597 increase mRNA expression of the adipocyte markers PPARγ, C/EBPα and FABP4 (n = 3).
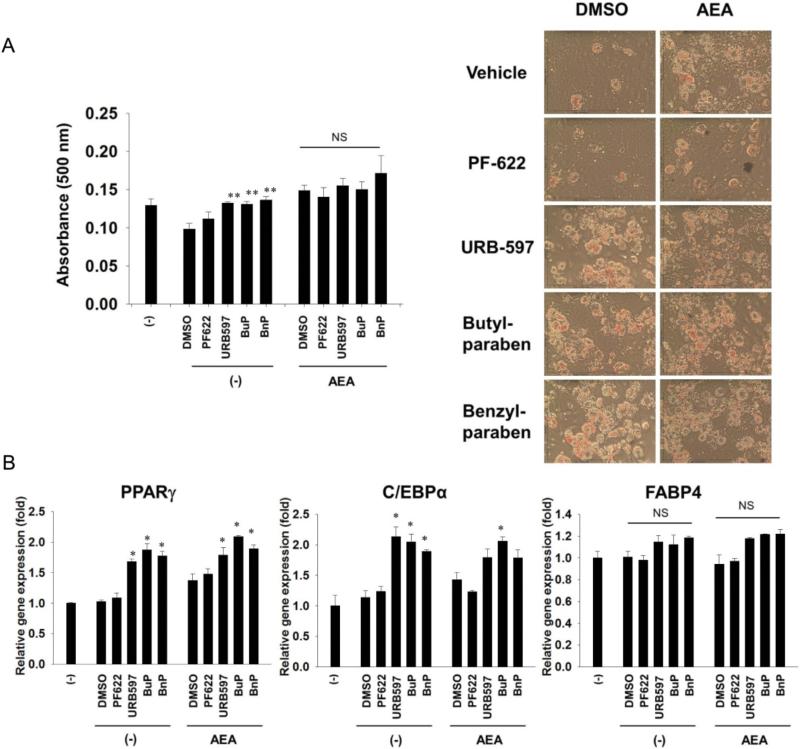
If these effects were mediated through FAAH inhibition, we speculated the biologically active substrates of FAAH would enhance paraben- or other FAAH inhibitor-induced differentiation. Paraben, PF622, or URB597 did not further increase AEA-mediated Oil Red O staining (Fig. 3A). Neither co-treatment of AEA with paraben, PF622, or URB597 further enhanced adipocyte marker gene expression (Fig. 3B). This could indicate that a FAAH substrate different from AEA might account for the effects of the FAAH inhibitors or the lack of additive effect could be due to saturation of AEA in the cell system.
Fig. 3.
3T3-L1 cells were pretreated with 10 μM of PF622, URB597, or parabens for two hours. The cells were then induced to differentiate in the presence of arachidonoylethanolamide (AEA) (10 μM) or vehicle (absolute alcohol, EtOH) while maintained in 10 μM of PF622, URB597, butylparaben (BuP), benzylparaben (BnP) or vehicle (DMSO) for 7 days. (A) AEA and parabens, PF622 or URB597 do not synergize to increase adipocyte differentiation as measured by oil red O. (B) AEA does not affect the regulation of mRNA expression of the adipocyte markers PPARγ, C/EBPα and FABP4 in the presence or absence of parabens or URB597 (n = 3). *p < 0.05 compared to the respective control within the treatment.
3.4. 3T3-L1 adipocyte differentiation by FAAH inhibitors is CB1R-independent
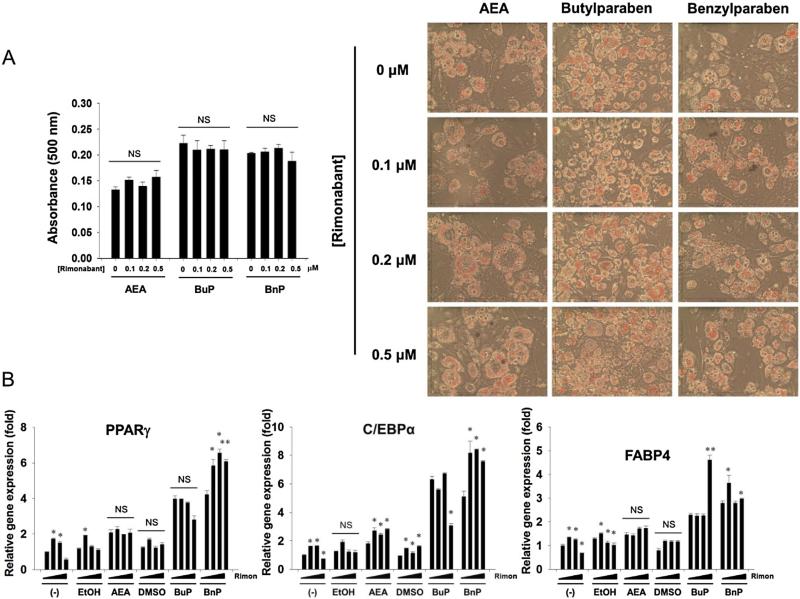
Most of the physiologic effects of FAAH inhibitors are believed to be mediated through activation of one or both of the cannabinoid receptors (CB1 and CB2). To test whether the effects of parabens on adipocyte differentiation were mediated in total or in part by the CB1 receptor, we performed a co-treatment of butylparaben, benzylparabens and AEA with the potent CB1 antagonist rimonabant (Rinald-Carmona et al., 1994) (Fig. 4). None of the tested concentrations of rimonabant reduced Oil Red O staining in either the AEA or paraben groups, although highest dose of rimonabant reduced C/EBPα expression in the butylparaben group. Interestingly, rimonabant treatment seemed to actually result in a robust increase in several markers, including C/EBPα expression in the AEA group, PPARγ and C/EBPα expression in the benzylparaben group and FABP4 in the butylparaben group (Fig. 4B).
Fig. 4.
3T3-L1 cells were pretreated with Rimonabant (0.1, 0.2, or 0.5 μM) or vehicle (DMSO) for two hours. Then, the cells were induced to differentiate in the presence of AEA (10 μM), butylparaben (50 μM) or benzylparaben (50 μM), while maintained in rimonabant for 7 days. (A) Increasing concentrations of rimonabant had no effect on paraben-enhanced adipocyte differentiation as measured by oil red O. (B) Effects of rimonabant on parabens-mediated upregulation of adipocyte marker PPARγ, C/EBPα, and FABP4. The dark triangles indicate the increasing doses of rimonabant shown in (A). *p < 0.05 compared with the control (0 μM of rimonabant) within each treatment group. NS, not significant.
4. Discussion
In this study, we identified benzylparaben as a mixed-type, time-independent inhibitor of fatty acid amide hydrolase. Other parabens, including butylparaben, also inhibit FAAH, although their potency decreases with decreasing chain length. We calculated the Ki of benzylparaben towards FAAH to be 52 ± 14 nM and the α value, which indicates the maximum fold-difference possible to the Km or Ki, was 9.7 ± 6.6. Since substrates for FAAH, such as AEA, are produced on demand in response to stimuli (Piomelli and Sasso, 2014), this means at a basal state the relative binding affinity of benzylparabens for FAAH will be relatively high but as substrates are produced, the relative affinity will decrease. However, since the interaction is mixed-type instead of competitive, the relative Ki will never be higher than 1 μM. Isoflavones, including Biochanin A, have been reported to inhibit FAAH in a similar mixed-type fashion (Thors et al., 2010). Several of these isoflavones have similar structural features including a para phenol to the carbonyl group. Additionally, both parabens and biochanin A have potencies that are comparable between human and rodent species. Given these similarities, it is possible parabens and biochanin A may interact with a well-conserved binding site on FAAH close to but distinct from the active site that could be used in future endeavors for designing novel inhibitors.
Since parabens were previously reported to enhance adipocyte differentiation with a comparable structure-activity relationship (Hu et al., 2013), we hypothesized that differentiation could be mediated by FAAH inhibition. Here, we tested adipogenic effects of two FAAH inhibitors, PF622 and URB597 alongside with parabens at dose range of 1–50 μM. We found only URB597 increased differentiation and only at a concentration of 10 μM, not at 50 μM, and a weaker FAAH inhibitor PF622 had no effects at any of the doses tested. Both of these are in contrast to the dose-dependent adipogenic effects of parabens. The endogenous FAAH substrate arachidonoyl ethanolamide (AEA) was reported to stimulate adipocyte differentiation of 3T3-L1 cells (Bouaboula et al., 2005) and rat primary preadipocytes (Karaliota et al., 2009). In rat preadipocytes, adipogenic effects of AEA were inhibited by FAAH inhibitor URB597 (3 μM) and the COX-2 inhibitor indomethacin, suggesting that adipogenic effects of AEA might be due to the AEA metabolites derived from both FAAH and COX-2 (Karaliota et al., 2009). Here, we found AEA increased expression of several markers of differentiation (PPARγ and C/EBPα) but its effects were not significantly changed with FAAH inhibition by URB597, butylparaben or benzylparaben in 3T3-L1 cells. Although no significant changes were observed from FAAH inhibition when URB597 or paraben were added to AEA-treated cells, this may be due to saturation of AEA in the cell system. On the other hand, AEA-induced 3T3-L1 adipocyte differentiation was reported to be dependent on direct binding and activation of PPARγ (Bouaboula et al., 2005). Therefore, it remains to be determined whether AEA promotes adipocyte differentiation via its metabolites from FAAH (or COX-2) or itself as a PPARγ agonist. To test further whether differentiation could be due to CB1R activation, we treated 3T3-L1 cells with rimonabant, a CB1R antagonist, and found both AEA and parabens’ adipogenic effects are independent of CB1R activation. Activation of the CB1 receptor stimulates adipogenesis (Bellocchio et al., 2008) while CB2 activation attenuates adipogenesis (Verty et al., 2015; Rossi et al., 2016); thus, the CB2 receptor is unlikely to be responsible for the paraben-enhanced adipogenesis. However, the fact that CB2 receptor was not excluded as a potential target is a limitation of our study and this hypothesis should be examined in future studies. Taken together, our results suggest that adipogenic effects of FAAH inhibition by parabens or other inhibitors in 3T3-L1 cells may be due to accumulation of AEA, leading to more PPARγ activation.
Although studies have implicated parabens in endocrine disruption (Chen et al., 2007), their use in cosmetics has been considered safe by the United States (U.S. FDA, 2007). This is due, in part, to their low metabolic stability and fast excretion with 81–85% excreted in the urine after the first 24 h and over half of that excreted as p-hydroxyhippuric acid, the primary metabolite (Moos et al., 2015). Despite the rapid metabolism, the high prevalence of these products may result in regular daily exposure, as evidenced by a high incidence of detection in urine (Ye et al., 2006; Tefre de Renzy-Martin et al., 2014). In a survey of personal care products, methylparaben is used at the highest concentrations, while propyl- and butylparaben are regularly used but at lower concentrations and benzylparaben is rarely used (Guo and Kannan, 2013). This general trend approximately matches the relative concentrations of individual parabens in the urine (Ye et al., 2006; Tefre de Renzy-Martin et al., 2014). Urinary concentrations of butylparaben reach 0.1–0.5 μM (Calafat et al., 2010; Tefre de Renzy-Martin et al., 2014), which is in the range relevant to FAAH inhibition but not adipocyte differentiation. While benzylparaben is more biologically active on FAAH, butylparaben is more relevant from the perspective of human exposure.
It is difficult to determine, based on human exposure, whether the potencies of these parabens toward FAAH and adipocyte differentiation are substantial enough to cause major physiologic changes. Although the potent FAAH inhibitor PF-04457845 was well tolerated in the clinic with no major adverse side effects (Huggins et al., 2012), the death of a volunteer in a Phase I trial of the FAAH inhibitor BIA 10-2474 has increased safety concerns related to FAAH inhibition (von Schaper, 2016). While the potency of parabens is relatively weak compared to drugs optimized to target FAAH, the efficacy of FAAH inhibition may be enhanced by other factors. Recent reports have demonstrated FAAH inhibition synergizes with other targets including soluble epoxide hydrolase (sEH) (Sasso et al., 2015) and cyclooxygenase (COX) (Naidu et al., 2009). While these studies investigate synergy in the context of nociception, both sEH and COX are generally relevant to adipo-genesis and the regulation of inflammation (Karaliota et al., 2009; De Taeye et al., 2010). Triclocarban, a common antibacterial found in consumer care products, similarly inhibits sEH (Schebb et al., 2011). Thus, while exposure to either triclocarban or parabens alone may not be substantial enough to have a major impact on human health, combined exposure to triclocarban and either butyl- or benzylparaben could have significant biological effects. Similarly, cyclooxygenase inhibitors, including aspirin, remain highly used pharmaceuticals whose potency may be enhanced due to synergism with parabens. Further studies will be necessary to validate whether synergism occurs from exposure to these commonly used consumer and pharmaceutical products.
5. Conclusion
In conclusion, parabens, preservatives found in personal care products, inhibit the endocannabinoid enzyme FAAH. Based on the structure activity relationship, activity requires a para-hydroxy benzoic ester and potency increases as hydrophobicity increases. While FAAH inhibition may account for paraben-enhanced adipocyte differentiation, the adipogenic activity is independent of the CB1 receptor. Further experiments are needed to determine whether FAAH inhibitor activities are required for parabens’ adipogenic effects. Moreover, further experiments are needed to determine whether doses relevant to human exposure can elicit physiologic effects in vivo.
Supplementary Material
HIGHLIGHTS.
Parabens inhibit the endocannabinoid enzyme fatty acid amide hydrolase (FAAH).
Paraben inhibition has time-independent, mixed-type kinetics.
Benzylparaben, the most potent paraben, inhibits FAAH with sub-micromolar potency.
Endocannabinoids may mediate paraben-enhanced adipogenesis but not through CB1 activation.
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences grant R01 ES002710 and the National Institute of Environmental Health Sciences Superfund Research Program Grant P42 ES004699. SDK was supported by a NIGMS-funded Pharmacology Training Program (T32GM099608).
Abbreviations
- AADAC
arylacetamide deacetylase
- AEA
arachidonoyl ethanolamide
- BnP
benzylparaben
- BuP
butylparaben
- CB1R
cannabinoid receptor 1
- CB2R
cannabinoid receptor 2
- CMNA
cyano(6-methoxynaphthalen-2-yl)methyl acetate
- EC
endocannabinoid
- FAAH
fatty acid amide hydrolase
- GR
glucocorticoid receptor
- hCE1
carboxylesterase 1
- hCE2
carboxylesterase 2
- OMP
N-(6-methoxypyridin-3-yl) octanamide
- PF-622
N-phenyl-4-(quinolin-2-ylmethyl)piperazine-1-carboxamide
- PPARγ
peroxisome proliferator-activated receptor γ
- URB597
3′-carbamoyl-[1,1′-biphenyl]-3-yl cyclohexylcarbamate
Footnotes
SDK and BDH are authors on a patent disclosure filed through the University of California, Davis for fatty acid amide hydrolase inhibitors that are unrelated to the work described here.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxlet.2016.09.011.
References
- Bellocchio L, Cervino C, Vicennati V, Pasquali R, Pagotto U. Cannabinoid type 1 receptor: another arrow in the adipocytes’ bow. J. Neuroendocrinol. 2008;20(Suppl. 1):130–138. doi: 10.1111/j.1365-2826.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- Bledzka D, Gromadzinska J, Wasowicz W. Parabens. From environmental studies to human health. Environ. Int. 2014;67:27–42. doi: 10.1016/j.envint.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur. J. Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Bowles NP, Karatsoreos IN, Li X, Vemuri VK, Wood JA, Li Z, Tamashiro KL, Schwartz GJ, Makriyannis AM, Kunos G, Hillard CJ, McEwen BS, Hill MN. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc. Natl. Acad. Sci. U. S. A. 2015;112:285–290. doi: 10.1073/pnas.1421420112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ. Health Perspect. 2010;118:679–685. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ahn KC, Gee NA, Gee SJ, Hammock BD, Lasley BL. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol. Appl. Pharmacol. 2007;221:278–284. doi: 10.1016/j.taap.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulou FD, Kiortsis DN. An overview of the metabolic effects of rimonabant in randomized controlled trials: potential for other cannabinoid 1 receptor blockers in obesity. J. Clin. Pharm. Ther. 2011;36:10–18. doi: 10.1111/j.1365-2710.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, Yang J, Murphy SB, Friedman DB, Hammock BB, Vaughan DE. Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity (Silver, Spring) 2010;18:489–498. doi: 10.1038/oby.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Methyl paraben and propyl paraben. Affirmation of GRAS status of direct human food ingredients. Fed. Regist. 1973;38:20048–20050. [Google Scholar]
- Guo Y, Kannan K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Technol. 2013;47:14442–14449. doi: 10.1021/es4042034. [DOI] [PubMed] [Google Scholar]
- Hoberman AM, Schreur DK, Leazer T, Daston GP, Carthew P, Re T, Loretz L, Mann P. Lack of effect of butylparaben and methylparaben on the reproductive system in male rats. Birth Defects Res B Dev. Reprod. Toxicol. 2008;83:123–133. doi: 10.1002/bdrb.20153. [DOI] [PubMed] [Google Scholar]
- Hu P, Chen X, Whitener RJ, Boder ET, Jones JO, Porollo A, Chen J, Zhao L. Effects of parabens on adipocyte differentiation. Toxicol. Sci. 2013;131:56–70. doi: 10.1093/toxsci/kfs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Nishi K, Tsai HJ, Hammock BD. Development of highly sensitive fluorescent assays for fatty acid amide hydrolase. Anal. Biochem. 2007;363:12–21. doi: 10.1016/j.ab.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–1846. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Karaliota S, Siafaka-Kapadai A, Gontinou C, Psarra K, Mavri-Vavayanni M. Anandamide increases the differentiation of rat adipocytes and causes PPARgamma and CB1 receptor upregulation. Obesity (Silver Spring) 2009;17:1830–1838. doi: 10.1038/oby.2009.177. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Moos RK, Angerer J, Dierkes G, Bruning T, Koch HM. Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage. Arch. Toxicol. 2015 doi: 10.1007/s00204-015-1636-0. doi: http://dx.doi.org/10.1007/s00204-015-1636-0. [DOI] [PubMed]
- Morisseau C, Merzlikin O, Lin A, He G, Feng W, Padilla I, Denison MS, Pessah IN, Hammock BD. Toxicology in the fast lane: application of high-throughput bioassays to detect modulation of key enzymes and receptors. Environ. Health Perspect. 2009;117:1867–1872. doi: 10.1289/ehp.0900834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J. Pharmacol. Exp. Ther. 2009;329:48–56. doi: 10.1124/jpet.108.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K, Huang H, Kamita SG, Kim IH, Morisseau C, Hammock BD. Characterization of pyrethroid hydrolysis by the human liver carboxylesterases hCE-1 and hCE-2. Arch. Biochem. Biophys. 2006;445:115–123. doi: 10.1016/j.abb.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Fernandes A, Demaegdt H, Vandermeiren K, Hectors TL, Jorens PG, Blust R, Vanparys C. Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS One. 2013;8:e77481. doi: 10.1371/journal.pone.0077481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Endocannabinoids and their pharmacological actions. Handb. Exp. Pharmacol. 2015;231:1–37. doi: 10.1007/978-3-319-20825-1_1. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci. 2014;17:164–174. doi: 10.1038/nn.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinald-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Fur GL. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rossi F, Bellini G, Luongo L, Manzo I, Tolone S, Tortora C, Bernardo ME, Grandone A, Conforti A, Docimo L, Nobili B, Perrone L, Locatelli F, Maione S, Del Giudice EM. Cannabinoid receptor 2 as anti-obesity target: inflammation, fat storage and browning modulation. J. Clin. Endocrinol. Metab. 2016:jc20154381. doi: 10.1210/jc.2015-4381. [DOI] [PubMed] [Google Scholar]
- Sasso O, Wagner K, Morisseau C, Inceoglu B, Hammock BD, Piomelli D. Peripheral FAAH and soluble epoxide hydrolase inhibitors are synergistically antinociceptive. Pharmacol. Res. 2015;97:7–15. doi: 10.1016/j.phrs.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebb NH, Inceoglu B, Ahn KC, Morisseau C, Gee SJ, Hammock BD. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ. Sci. Technol. 2011;45:3109–3115. doi: 10.1021/es103650m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. Wiley; New York: 1993. [Google Scholar]
- Shan G, Hammock BD. Development of sensitive esterase assays based on alpha-cyano-containing esters. Anal. Biochem. 2001;299:54–62. doi: 10.1006/abio.2001.5388. [DOI] [PubMed] [Google Scholar]
- Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of phydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005;43:985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, Boergesen M, Mandrup S, Vinggaard AM. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Mol. Cell. Endocrinol. 2012;361:106–115. doi: 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Tefre de Renzy-Martin K, Frederiksen H, Christensen JS, Boye Kyhl H, Andersson AM, Husby S, Barington T, Main KM, Jensen TK. Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction. 2014;147:443–453. doi: 10.1530/REP-13-0461. [DOI] [PubMed] [Google Scholar]
- Thors L, Burston JJ, Alter BJ, McKinney MK, Cravatt BF, Ross RA, Pertwee RG, Gereau RW, Wiley JL, Fowler CJ. Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase. Br. J. Pharmacol. 2010;160:549–560. doi: 10.1111/j.1476-5381.2010.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verty AN, Stefanidis A, McAinch AJ, Hryciw DH, Oldfield B. Anti-obesity effect of the CB2 receptor agonist JWH-015 in diet-induced obese mice. PLoS One. 2015;10:e0140592. doi: 10.1371/journal.pone.0140592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida M, Rivera P, Gavito AL, Suarez J, Pavon FJ, Arrabal S, Romero-Cuevas M, Bautista D, Martinez A, de Fonseca FR, Serrano A, Baixeras E. CB1 blockade potentiates down-regulation of lipogenic gene expression in perirenal adipose tissue in high carbohydrate diet-induced obesity. PLoS One. 2014;9:e90016. doi: 10.1371/journal.pone.0090016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Scherer PE, Gupta RK. Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. J. Lipid Res. 2014;55:605–624. doi: 10.1194/jlr.R046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. Parabens as urinary biomarkers of exposure in humans. Environ. Health Perspect. 2006;114:1843–1846. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schaper E. Bial incident raises FAAH suspicions. Nat. Biotechnol. 2016;34:223. doi: 10.1038/nbt0316-223a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.