Abstract
The syndrome of paraganglioma (PGL), somatostatinoma (SOM), and early childhood polycythemia in patients with somatic mutations in the hypoxia-inducible factor 2 alpha (HIF2A) gene is described in only a few patients worldwide. The present study provides detailed information about the clinical aspects and course of 7 patients with this syndrome and brings these experiences into perspective with the pertinent literature. Six females and one male presented at a median age of 28 years (range 11–46). Two were found to have HIF2A somatic mosaicism. No relatives were affected. All patients were diagnosed with secondary polycythemia before age 8 and before PGL/SOM developed. PGLs were found at a median age of 17 years (range 8–38) and SOMs at 29 years (range 22–38). PGLs were multiple, recurrent, and metastatic in 100%, 100%, and 29% of all cases, and SOMs in 40%, 40%, and 60%, respectively. All PGLs were primarily norepinephrine producing. All patients had abnormal ophthalmologic findings and those with SOMs had gallbladder disease. Computed tomography (CT) and magnetic resonance imaging revealed cystic lesions at multiple sites and hemangiomas in 4 patients (57%), previously thought to be pathognomonic for von Hippel-Lindau disease. The most accurate radiopharmaceutical to detect PGL appeared to be [18F]-fluorodihydroxyphenylalanine ([18F]-FDOPA). Therefore, [18F]-FDOPA PET/CT, not [68Ga]-(DOTA)-[Tyr3]-octreotate ([68Ga]-DOTATATE) PET/CT is recommended for tumor localization and aftercare in this syndrome. The long-term prognosis of the syndrome is unknown. However, to date no deaths occurred after 6 years follow-up. Physicians should be aware of this unique syndrome and its diagnostic and therapeutic challenges.
Keywords: Pheochromocytoma, paraganglioma, somatostatinoma, polycythemia, HIF2A mutation
Introduction
Pheochromocytomas (PHEOs) and paragangliomas (PGLs) are rare catecholamine-producing neuroendocrine tumors (NETs) arising in or outside the adrenal medulla, respectively (Lenders et al. 2005). By definition, a PHEO is an intraadrenal PGL. To date, it has been recognized that up to 35%–40% of these tumors are hereditary, with about 19 causally linked mutated genes (Crona et al. 2013; Pacak and Wimalawansa 2015). Among these genes, much attention has been directed to those affecting hypoxia-signaling pathways because many of the associated tumors express a so called “pseudohypoxic signature” and most of them converge on the hypoxia-signaling pathway (Jochmanova et al. 2013).
Germline mutations in the von Hippel-Lindau (VHL) gene trigger overexpression of hypoxia inducible factor (HIF) proteins and cause VHL disease, which may predispose to various tumors such as multiple PHEOs/PGLs, hemangioblastomas of the retina and central nervous system, as well as kidney cysts, renal cell carcinoma, and polycythemia, among others (Haase 2009; Taïeb et al. 2016). Mutations in another HIF-regulating protein gene, prolyl hydroxylase domain protein 2 (PHD2) and, more recently, PHD1, which hydroxylate HIF and enable its VHL-mediated degradation, have been associated with secondary polycythemia and multiple PGLs (Yang et al. 2014). Nevertheless, the occurrence of PGL together with polycythemia is rare (Dionne et al. 2006).
Somatic mutations in HIF2A (EPAS1), affecting PHD hydroxylation and subsequent VHL degradation, were recently recognized to cause a syndrome consisting of PGL and/or somatostatinoma (SOM) associated with polycythemia in females (Pacak et al. 2013). Since then, more patients with the syndrome have been described carrying the mutations in this gene. Nevertheless, at present, the triad of PGL, SOM, and polycythemia has been exclusively found in females. Furthermore, normal tissues genomic DNA mosaicism of HIF2A mutations has recently been detected in two out of four initial patients specifically presenting with this syndrome (Yang et al. 2015).
The clinical dyad/triad of PGLs and/or SOMs associated with polycythemia, also referred to as “Pacak-Zhuang syndrome” (Toyoda et al. 2014), may be regarded as a new tumor syndrome, similar to multiple endocrine neoplasia (MEN) syndromes, VHL disease, Carney-Stratakis syndrome, Carney triad, Cowden syndrome, or the PHEO-PGL syndrome, among others (Gaal and de Krijger 2010; Ni et al. 2012). From the first studies published by our group, additional new clinical phenotypes have been identified through the follow-up of previously described and newly diagnosed patients with this syndrome.
In the present study, we provide detailed clinical information on 7 patients with the PGL, SOM, and polycythemia syndrome carrying the somatic HIF2A mutation, including a teenage boy, diagnosed and followed at the NIH for 6 years. New clinical information particularly pertains to aspects of genetics, tumor imaging, organ involvement, disease progression, and patient outcomes. We also searched the literature for studies and reports on related tumor syndromes and diseases with activating mutations of the HIF2A gene and discuss our findings in the context of this other data. As a result, we propose strategies for diagnosis and therapy of patients with PGL, SOM, and polycythemia syndrome.
Materials and Methods
Patient Evaluation
Patients were evaluated under protocol (00-CH-0093) approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. All patients provided written informed consent. We carefully reviewed all patients’ demographics, clinical manifestations, biochemical and hematological profiles, radiographic findings, and outcomes based on frequent follow-ups and very close interactions with outside physicians (Supplementary Table 1).
Laboratory Analyses
All laboratory analyses, mutation analysis, hydroxylation assays, real-time polymerase chain reaction, and chromatin immunoprecipitation were performed, as previously described (Pacak et al. 2013).
Imaging Studies
Anatomical imaging using computed tomography (CT) and magnetic resonance imaging (MRI) of the neck, chest, abdomen, and pelvis were performed as previously described, along with positron emission tomography (PET)/CT studies using [18F]-fluorodeoxyglucose ([18F]-FDG), [18F]-fluorodopamine ([18F]-FDA), [18F]-fluorodihydroxyphenylalanine ([18F]-FDOPA), and [68Ga]-(DOTA)-[Tyr3]-octreotate ([68Ga]-DOTATATE) as radiopharmaceuticals (Supplementary Table 2) (Janssen et al. 2015). In addition, CT scans with negative enteric contrast were used to better detect duodenal tumors, as performed in our previous studies (Pacak et al. 2013).
Results
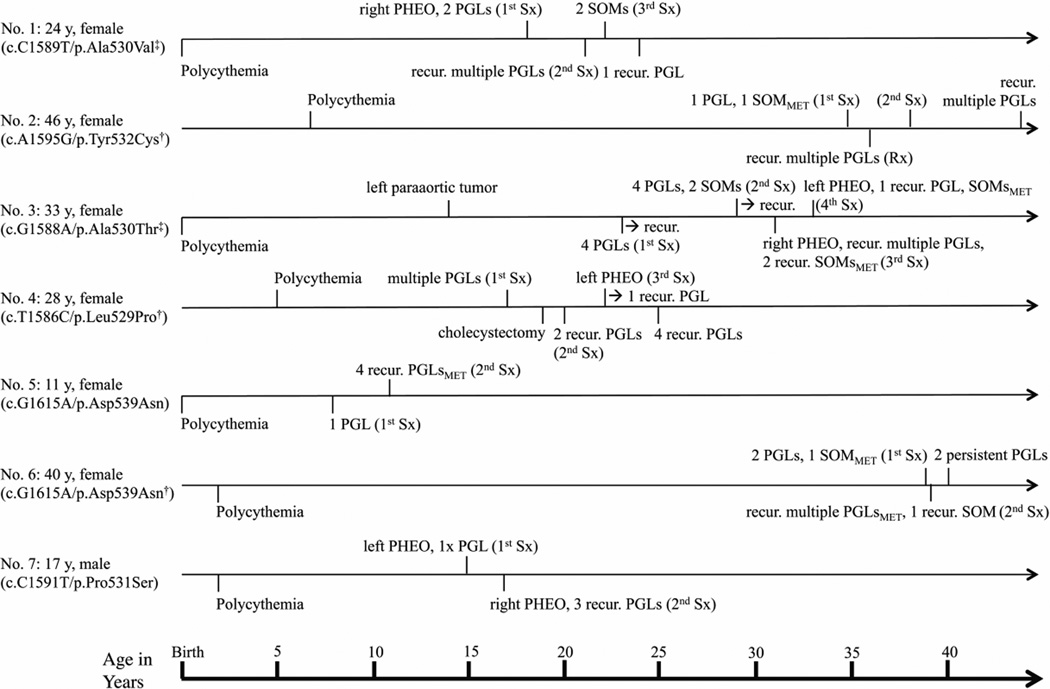
Gender, mutation status with evidence of mosaicism of HIF2A, ages at the latest outpatient visit, and ages at diagnosis of polycythemia, occurrence of PHEO/PGL, SOM and number of lesions, recurrence of lesions, as well as surgical and other treatment modalities for individual patients in chronological order are shown in Fig. 1. None of the family members had any history of polycythemia, PHEO/PGL, or SOM. Detailed clinical presentations are described in Supplementary Table 1. Patients received regular follow-up at the NIH for a median of 6 years (range 1–11) since their first tumor resection. The median age of patients at the time of their last follow-up at the NIH was 28 years (range 11–46).
Figure 1.
Ages at diagnosis of polycythemia, pheochromocytoma (PHEO)/paraganglioma (PGL) and somatostatinoma (SOM) for individual patients in chronological order. Ages at latest outpatient visit, gender, mutation status with (‡) and without (†) mosaicism of HIF2A, numbers (multiple = >3), sites (right, left) and recurrence (recur.) of lesions, presence of metastatic disease (MET), and number of surgical interventions (1st – 4th Sx, etc.) and/or radiotherapy (Rx) are indicated.
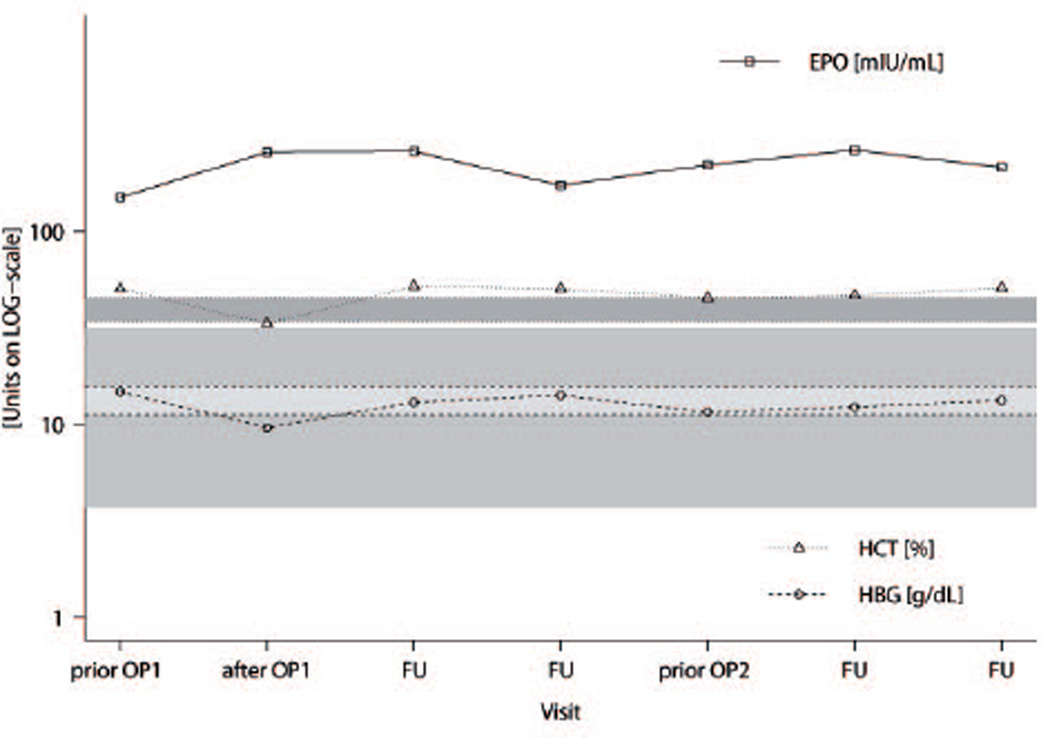
Polycythemia was detected in patients in early childhood (median 2 years, range from birth to 7 years). Erythropoietin (EPO) levels were an average of 5 times above the upper limit of normal in all patients. For patient number (No.) 3, EPO levels are plotted together with levels of hemoglobin and hematocrit over the course of the patient’s visits at the NIH (Fig. 2). There was no long-term normalization of EPO levels even after surgery in any patient.
Figure 2.
Erythropoietin (EPO), hemoglobin (HGB), and hematocrit (HCT) levels over the course of several visits for patient No. 3, starting from first presentation at NIH, prior to and after surgeries (prior OP1, after OP1, and prior OP2), and during intermediate follow-up visits (FU) before the second surgery at the NIH. Respective upper and lower reference intervals are indicated for EPO, HGB, and HCT in gray, light gray, and dark gray, respectively. EPO levels did not normalize after surgeries, strongly refuting EPO production by tumor tissue.
PGL developed later in life at a median age of 17 years (range 8–38) in all patients. PGLs were predominantly of the norepinephrine-producing biochemical phenotype. After surgery, levels of normetanephrine dropped significantly (not shown). Patients were found to have multiple (100%), recurrent (100%), and metastatic (29%) PGLs, with adrenal involvement in four cases.
Five of 7 patients had a manifestation of SOM at a median of 29 years (range 22–38). No patient was found to have SOM diagnosed before the occurrence of PGL. Patient No. 5, an 11-year-old girl, and No. 7, a 17-year-old-boy have not yet developed SOM. SOM was confirmed either histologically and/or biochemically. In the case of patient No. 4, the diagnosis of SOM was based solely on elevated levels of somatostatin along with retroperitoneal abdominal nodules on imaging studies, but without histological confirmation of the diagnosis to date since the patient has refused to have an operation. For patient No. 6, we found elevated levels of gastrin that remained high after surgery, as well as slightly elevated levels in vasoactive intestinal peptide for patient No. 3.
In four patients, we had histological proof of SOM. We found solitary (40%), multiple (40%), recurrent (40%), and metastatic SOMs in 3 cases (60%). All patients with SOM were diagnosed with gallbladder disease, with four having chronic cholecystitis and two, cholelithiasis, usually in early adulthood at a median of 29 years (range 19–39). Two patients, No. 3 and 6, were diagnosed with noninsulin-dependent diabetes at the ages of 26 and 38, respectively, which may be regarded as manifestation of PGL (Lenders et al. 2005), and which resolved in patient No. 3 after her second surgery (Supplementary Table 1).
All patients received an ophthalmology consultation because of known ocular complications occurring with this syndrome (Pacak et al. 2014). The results are summarized in Table 1. We observed optic disc fibrosis in all patients (Supplementary Figure 1). Three patients had macular changes and three peripheral retinal changes. For patient No. 5, ophthalmologic changes were noticed even before polycythemia was diagnosed and prior to the occurrence of PGL.
Table 1.
Findings on ophthalmology consultation
| Patient | Visual Acuity* |
Optic Disc Fibrosis (bilateral) |
Posterior Pole (Macular) Changes |
Peripheral Retinal Changes | Other |
|---|---|---|---|---|---|
| 1 | RE: 20/16 LE: 20/16 |
Present | Vascular tortuosity with dilated veins Few arteriolar narrowing and subtle retinal pigmentary changes |
Bilateral peripheral scattered retinal pigment epithelial (RPE) changes |
|
| 2 | RE: 20/32 LE: 20/25 |
Present | Absent | Absent | Bilateral cataract (posterior subcapsular) |
| 3◊ | RE: 20/25 LE: 20/25 |
Present | Absent | Bilateral peripheral retinal neovascularization present; LE: single hemangioblastoma-like lesion similar to VHL in the inferior temporal retina |
Myelinated nerve fiber layer in LE |
| 4 | RE: 20/20 LE: 20/20 |
Present | Absent | Bilateral temporal vasculature anomalies similar to familial exudative vitreoretinopathy / retinopathy of prematurity like appearance with U-turning blood vessels |
|
| 5◊ | RE: 20/25 LE: 20/32 |
Present | Bilateral macular edema** with retinal hard exudate |
Absent | Bilateral enlarged blind spot |
| 6◊ | RE: 20/20 LE: 20/63 |
Present | Absent | Absent | LE with optic nerve head drusen and amblyopia |
| 7◊ | RE: 20/250 LE: 20/20 |
Present | Macular edema*** with hard exudate RE only |
Absent |
Visual Acuity: measured on a LogMAR Visual Acuity Chart (Early Treatment Diabetic Retinopathy Study visual acuity chart) at 4 meters, best corrected
Intravitreal injection of Ranibizumab was administered
Intravitreal injection of Bevacizumab was administered
see also Pacak et al. 2014
Abbreviations: LE=left eye; RE=right eye; VHL=von Hippel-Lindau;
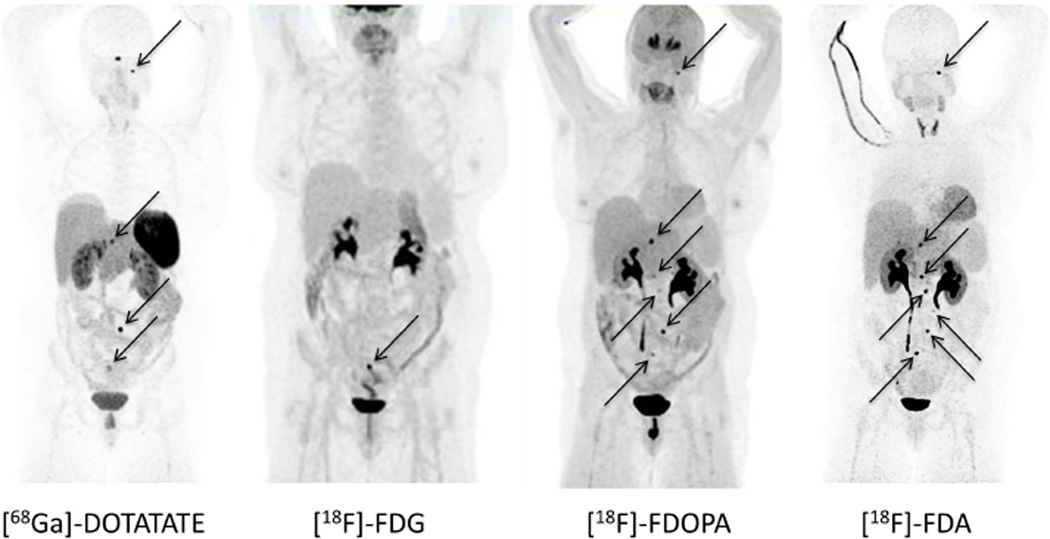
Anatomical and functional imaging characteristics are summarized in Table 2. On anatomical imaging, we found cystic lesions in 4 out of 7 patients. Cysts were localized to the kidneys, breasts, lungs, pericardium, cervix, and pancreas. In addition, patient No. 4 was found to have a liver hemangioma. Overall, the most suitable functional imaging modality that detected most of tumor lesions was [18F]-FDOPA PET/CT (according to our chosen “gold standard”, see Supplementary Table 2) closely followed by [18F]-FDA PET/CT, as exemplified by the images of patients No. 2 and 4 (Fig. 3 and Supplementary Figure 2). In contrast, overall sensitivities and positive predictive values obtained were considerably lower for [18F]-FDG PET/CT and [68Ga]-DOTATATE PET/CT, respectively.
Table 2.
Anatomical and functional imaging characteristics of patients
| Patient | Age | CT | MRI | [18F]-FDG | [18F]-FDOPA | [18F]-FDA | [68Ga]-DOTATATE* | [123I]-MIBG** |
|---|---|---|---|---|---|---|---|---|
| 1 | 18 | R adrenal, one abdominal lesion, bilateral renal cysts | + | + + + | + + + | ND | # # | |
| 22–24 | ND | pericaval focus, breast cysts | + | + + | + + | + + (+) | ND | |
| 2 | 44 – 46 | multiple retroperitoneal lesions and jugular foramen lesion | + | + + + | + + + | + + | ND | |
| lung cysts | cysts in cervix | |||||||
| 3 | 29 | multiple abdominal lesions, asc. aortic aneurysm | + + | + + + | + + + | ND | # # # | |
| 29–31 | multiple abdominal lesions, R>L adrenal lesion pericardial cyst, physiologic R pelvic cyst on MRI |
+ + | + + + | ND | ND | # # | ||
| 33 | L adrenal, 6 abdominal lesions | + + + | + + | + + | + + | ND | ||
| 4 | 25 | 2 abdominal lesions | ND | + | + + + | + + | ND | ND |
| hemangioma of the liver since age 20 | ||||||||
| 5 | 9 | mesentericadenopathy of uncertain significance | - | ND | ND | ND | ND | |
| 11 | ND | 5 abdominal lesions | + + | + + + | ND | ND | ND | |
| 6 | 39 | abdominal lesions, pancreatic cyst, liver lesion on MRI | + + | + + (+) | + + (+) | ND | ND | |
| 39–40 | ND | abdominal lesions | + (+) | + + | + + | + + | ND | |
| 7 | 17 | ND | R adrenal | ND | + + + | ND | - | ND |
Not initially performed in previous studies, [68Ga]-DOTATATE, a new functional imaging modality, was included as further means of disease localization in five out of 7 patients; [68Ga]-DOTATATE PET/CT was not performed in the teenage girl and complete series is also lacking for patient No. 4;
[123I]-MIBG scintigraphy was less accurate compared to PET/CT imaging, and was used for eligibility to MIBG treatment after approval of [68Ga]-DOTATATE for our protocol only.
Abbreviations: asc.=ascending; CT=computed tomography; L=left; MRI=magnetic resonance imaging; ND=Not Done; R=right; positron emission tomography (PET)/CT using: [18F]-FDG=[18F]-fluorodeoxyglucose, [18F]-FDOPA=[18F]-fluorodihydroxyphenylalanine, [18F]-FDA=[18F]-fluorodopamine, and [68Ga]-DOTATATE=[68Ga]-(DOTA)-[Tyr3]-octreotate as radiopharmaceuticals, with “-“, “+”, “+ +”, and “+ + +” signifying “no”, “weak”, “moderate”, and “good” uptake; [123I]-MIBG=[123I]-metaiodobenzylguanidinescintigraphy, with “# #” and “# # #” signifying “moderate” and “strong” uptake;
Because the pancreatic cyst of patient No. 6 developed in the interval between two surgeries and was fading to disappearance on imaging over the course of several months, there is the possibility that this cyst was iatrogenically induced by the first surgery. Patients also presented with adnexal cysts but, because of time dependent changes, these were regarded as physiological or not part of the cystic lesions related to the syndrome. For instance, patient No. 3 had been diagnosed with polycystic ovary syndrome (PCOS) in the past and we could identify a persisting pelvic cyst on MRI, patient No. 4 had an enlarged ovarian cyst (30×28 mm) at the age of 11, and patient No. 6 had been operated on for polycystic ovaries at the age of 31.
Figure 3.
Functional imaging with [68Ga]-DOTATATE, [18F]-FDG, [18F]-FDOPA, and [18F]-FDA PET/CT in patient No. 2. Radiotracer uptake is similar in [18F]-FDA PET/CT and [18F]-FDOPA, showing 5 and 6 retroperitoneal lesions, respectively. On [68Ga]-DOTATATE PET/CT only 3 retroperitoneal lesions could be identified. All of these radiopharmaceuticals showed an additional lesion at the jugular foramen (see black arrow), which was negative with [18F]-FDG. [18F]-FDG PET/CT showed only one of the retroperitoneal lesions. All retroperitoneal lesions are also indicated with black arrows.
Supplementary Table 3 provides an overview of the current literature on patients with HIF2A gain-of-function mutations and summarizes pertinent information about position and type of the mutations, presence of mosaicism, mode of inheritance, phenotypic expression, and gender distribution. Apart from the 7 patients described in the present investigation, 56 additional patients with activating HIF2A mutations were identified in prior published studies and reports between the years 2008 and 2016: one additional female patient with Pacak-Zhuang syndrome, 7 patients (3 females, 4 males) with polycythemia and PHEO/PGL but without SOM, 16 patients (12 females, 2 males) with PHEO/PGL including 2 females with gangliocytic PGL (GPGL) without polycythemia or SOM, 2 patients (1 female, 1 male) with central nervous system hemangioblastomas without polycythemia, PHEO/PGL or SOM, and 28 patients (15 females, 12 males, no record of gender in 1) with isolated sporadic or familial polycythemia plus one female offspring with inherited HIF2A mutation, but without polycythemia. One other female patient without any signs of organic disease was found to be a carrier of an activating HIF2A mutation, which she passed to her 50 year-old son who had polycythemia and PHEO/PGL. There was a remarkable partial overlap of position and type of mutations between patients with PHEO/PGL with or without polycythemia, and patients with Pacak-Zhuang syndrome. Identical mutations were also described in patients with isolated sporadic and familial polycythemia. However, there was no overlap of mutations in these latter two groups of patients compared with the former, indicating mutation specific fundamental differences in downstream signaling pathways of HIF2A leading to either isolated polycythemia or PHEO/PGL with or without polycythemia or Pacak-Zhuang syndrome.
Discussion
This report provides comprehensive new information on a 6-year follow-up of 7 patients carrying activating somatic HIF2A mutations who presented with the syndrome of PGL and/or SOM associated with polycythemia (Pacak-Zhuang syndrome), and brings these experiences into perspective with the pertinent literature. The involvement of the downstream HIF2A signaling pathway in the pathophysiology of this new syndrome is strongly supported by its association with distinct HIF2A mutations as well as by the occurrence of closely related clinical phenotypes described in some reports of patients with VHL syndrome (Karasawa et al. 2001; Haase 2009), and of polycythemia and PGL in patients with mutated PHD1 and PHD2 (Yang et al. 2014).
As a hallmark of the syndrome in all of our patients, polycythemia was diagnosed either at birth or in early childhood, and always before PHEO/PGL and/or SOM, which is typically discovered after the development of PHEO/PGL. All patients were found to have eye involvement at manifestation of the disease (Pacak et al. 2014). Furthermore, EPO levels were elevated in all patients and did not return to normal after surgical tumor removal, making paraneoplastic EPO production less likely. Mosaicism with HIF2A gain-of-function mutations in EPO-producing renal interstitial cells or hepatocytes (Haase 2013) leading to continuous stimulation of EPO synthesis may be a reasonable alternative explanation. Although, in our series, mosaicism was only found in two patients (Patients No. 1 and 3; see also Yang et al. 2015). On the other hand, there is evidence from studies in patients with familial or sporadic polycythemia harboring HIF2A gain-of-function mutations to suggest that polycythemia does not necessarily rely on increased EPO (Martini et al. 2008; Percy et al. 2012; Perrotta et al. 2013), indicating that the link between polycythemia and EPO in patients with Pacak-Zhuang syndrome may be less direct than intuitively expected. Other genetic abnormalities, including the timing of when HIF2A mutations occur, may further contribute to the full spectrum of this disease as in other hereditary syndromes.
In our patients PHEOs/PGLs were detected at a median age of 17 years, some 15 years after the diagnosis of polycythemia, and similar time intervals have been reported in other studies on patients with Pacak-Zhuang syndrome with or without established SOMs (Taïeb et al. 2013; Comino-Mendez et al. 2013; Buffet et al. 2014; Toyoda et al. 2014), as well as in one patient with familial polycythemia and PGL (Lorenzo et al. 2013). Together, these observations are in support of the concept that activating HIF2A mutations predispose to, but may not be sufficient for, the development of PHEOs/PGLs (Lorenzo et al. 2013). Of note, sporadic PHEOs/PGLs and other solid tumors with activating somatic HIF2A mutations occurring in the adult age do not appear to be preceded or accompanied by polycythemia, despite largely overlapping genetic changes compared to patients with Pacak-Zhuang syndrome or familial polycythemia with PHEO/PGL (Favier et al. 2012; Comino-Mendez et al. 2013; Toledo et al. 2013; Welander et al. 2014; Taïeb et al. 2016). It is therefore conceivable, that patients with Pacak-Zhuang syndrome and familial polycythemia with PHEO/PGL may harbor additional, yet unidentified tumor promoting aberrations, which are not present in sporadic PHEOs/PGLs later in life, the most obvious phenotypic reflections of which are polycythemia and increased EPO. Another explanation may be due to the precise timing of when an activating HIF2A mutation occurs during embryogenesis. It is anticipated that deep sequencing may allow a more definitive answer.
Considerations such as the above may also explain the distinct clinical features of SOMs in the patients described in the present study and by others (Buffet et al. 2014), which resemble those seen in conjunction with hereditary tumor syndromes, specifically neurofibromatosis type 1 (von Recklinghausen disease-NF1), MEN type 1, and VHL disease. Similarities include the nearly two decades earlier age of onset, lower rate of malignancy (despite early metastases in regional lymph nodes), and the preferential duodenal localization compared to sporadic SOMs (Klöppel et al. 2004; Marini et al. 2009), with the notable exception of VHL disease, where SOMs are predominantly found in the pancreas (Hammel et al. 2000). However, sporadic SOMs and SOMs associated with NF1, MEN1 or VHL gene mutations are clearly distinguished from SOMs in patients with Pacak-Zhuang syndrome by their substantially lower incidence and equal gender distribution. Therefore, even assuming the presence of an acquired genetic predisposition, the observation of a highly efficient and exclusive expression of SOMs in women remains a puzzling feature of Pacak-Zhuang syndrome.
Tumor location and biochemical phenotype of our patients fit well into the entity of “pseudohypoxic” cluster 1 tumors, as is the case for VHL, PHD, and for patients harboring succinate dehydrogenase (SDHx) mutations (Eisenhofer et al. 2011; Jochmanova et al. 2013; Richter et al. 2013). As in these patients (Neumann et al. 2002), HIF2A gain-of-function mutations may manifest with head and neck PGL. This was the case in patient No. 2 with an undefined skull-based lesion (Fig. 3).
Patients with SDHB mutations are especially prone to malignant disease (Gimenez-Roqueplo et al. 2003). In contrast, expression of tumor promoting genes seems to be less pronounced in patients with activating HIF2A mutations (Favier et al. 2012), resulting in a less aggressive PHEO/PGL phenotype. Indeed, none of our patients with metastatic PGLs thus far required chemotherapy or repeated radiotherapy, and none have died even 11 years after the first surgery. Therefore, these patients can be reassured that their overall outlook is favorable, although recurrent disease at multiple sites is highly probable and will require repeated surgeries, beginning at a young age.
The best functional imaging tracer for localization of tumors within Pacak-Zhuang syndrome in our series was [18F]-FDOPA, and in line with recent recommendations for localization of NETs with a low Ki-67 Index (van Essen et al. 2014). [18F]-FDG appeared to perform best in patients with mainly metastatic NET (Supplementary Table 2). In contrast, accuracy of [68Ga]-DOTATATE was low. This finding points to substantial differences in tumor biology between patients with HIF2A and SDHB mutations, where [18F]-FDG PET/CT and now [68Ga]-DOTATATE PET/CT provide superior results (Timmers et al. 2007; Timmers et al. 2009; Janssen et al. 2015). The demonstration of cystic and hemangiomatous lesions in all of our patients but the two teenage subjects suggests shared phenotypic features in patients harboring mutated VHL (Haase 2009; Taïeb et al. 2016).
Although limited by a small number of patients in this case series, our findings call for several recommendations (Neumann and Eng 2009): a) consider genetic testing for HIF2A mutations in congenital polycythemia; b) screen for PHEO/PGL starting from the age of about eight; c) perform yearly measurement of plasma or urine metanephrines; d) perform regular (every 1–2 years) whole body or at least abdominal imaging, MRI in children; e) consider a surgical approach since no specific therapies are currently known; f) PGLs are predominantly norepinephrine-producing and, therefore, patients should preferably be on an α-adrenoceptor blockade; g) screen for SOM starting from about the age of 20; h) measure somatostatin levels in all patients with HIF2A mutations; i) in all SOMs larger than 1 cm metastatic potential is high and therefore, attempt early endoscopic or surgical removal; j) use a negative enteric contrast CT scan or endoscopic examination for SOMs; k) base follow-up for SOMs on the measurement of plasma somatostatin levels and imaging; l) search for other, especially gastrin- but also other neurohormone-secreting NETs.
In summary, we present the most updated follow-up of 7 patients with HIF2A mutations and Pacak-Zhuang syndrome consisting of PHEO/PGL and/or SOM associated with polycythemia, and discuss these experiences in the context of the pertinent literature. We conclude that due to its multi-facetted manifestations an interdisciplinary approach to the patient with this syndrome is imperative in order to allow for the best possible outcome. Current treatment options are exclusively surgical.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health; the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Institute of Neurological Disorders and Stroke; and the German Research Foundation (DFG) (grant number DA 1630/1-1 to R.D.).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author contributions
RD, JN, and KP designed the study with JDR, IJ, MM, IJ, KTA, AL, CCC, EC, CY, EK, ZZ. KP, MT, BB, JTP, RML, AST, VP, DM, FRP, MRG, MF, NN, DT, CAS, TF were involved in data collection, analysis, interpretation, and review, in the preparation of the manuscript, as well as subsequent revisions.
References
- Alaikov T, Ivanova M, Shivarov V. EPAS1 p.M535T mutation in a Bulgarian family with congenital erythrocytosis. Hematology. 2016:1–4. doi: 10.1080/10245332.2016.1192394. [DOI] [PubMed] [Google Scholar]
- Buffet A, Smati S, Mansuy L, Menara M, Lebras M, Heymann MF, Simian C, Favier J, Murat A, Cariou B, et al. Mosaicism in HIF2A-related polycythemia-paraganglioma syndrome. J Clin Endocrinol Metab. 2014;99:E369–E373. doi: 10.1210/jc.2013-2600. [DOI] [PubMed] [Google Scholar]
- Comino-Mendez I, de Cubas AA, Bernal C, Alvarez-Escola C, Sanchez-Malo C, Ramirez-Tortosa CL, Pedrinaci S, Rapizzi E, Ercolino T, Bernini G, et al. Tumoral EPAS1 (HIF2A) mutations explain sporadic pheochromocytoma and paraganglioma in the absence of erythrocytosis. Hum Mol Genet. 2013;22:2169–2176. doi: 10.1093/hmg/ddt069. [DOI] [PubMed] [Google Scholar]
- Crona J, Verdugo AD, Granberg D, Welin S, Stålberg P, Hellman P, Björklund P. Next-generation sequencing in the clinical genetic screening of patients with pheochromocytoma and paraganglioma. Endocr Connect. 2013;2:104–111. doi: 10.1530/EC-13-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne JM, Wu JK, Heran M, Murphy JJ, Jevon G, White CT. Malignant hypertension, polycythemia, and paragangliomas. J Pediatr. 2006;148:540–545. doi: 10.1016/j.jpeds.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, Mannelli M, Friberg P, Grebe SK, Timmers HJ, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18:97–111. doi: 10.1677/ERC-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier J, Buffet A, Gimenez-Roqueplo AP. HIF2A mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367:2161. doi: 10.1056/NEJMc1211953. author reply 2161–2162. [DOI] [PubMed] [Google Scholar]
- Furlow PW, Percy MJ, Sutherland S, Bierl C, McMullin MF, Master SR, Lappin TR, Lee FS. Erythrocytosis-associated HIF-2alpha mutations demonstrate a critical role for residues C-terminal to the hydroxylacceptor proline. J Biol Chem. 2009;284:9050–9058. doi: 10.1074/jbc.M808737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal J, de Krijger RR. Neuroendocrine tumors and tumor syndromes in childhood. Pediatr Dev Pathol. 2010;13:427–441. doi: 10.2350/09-04-0635-CP.1. [DOI] [PubMed] [Google Scholar]
- Gale DP, Harten SK, Reid CD, Tuddenham EG, Maxwell PH. Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2 alpha mutation. Blood. 2008;112:919–921. doi: 10.1182/blood-2008-04-153718. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre X, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615–5621. [PubMed] [Google Scholar]
- Haase VH. The VHL tumor suppressor: master regulator of HIF. Curr Pharm Des. 2009;15:3895–3903. doi: 10.2174/138161209789649394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27:41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel PR, Vilgrain V, Terris B, Penfornis A, Sauvanet A, Correas JM, Chauveau D, Balian A, Beigelman C, O'Toole D, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087–1095. doi: 10.1053/gast.2000.18143. [DOI] [PubMed] [Google Scholar]
- Janssen I, Blanchet EM, Adams K, Chen CC, Millo C, Herscovitch P, Taïeb D, Kebebew E, Lehnert H, Fojo AT, et al. Superiority of [68Ga]-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2015;21:3888–3895. doi: 10.1158/1078-0432.CCR-14-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochmanova I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105:1270–1283. doi: 10.1093/jnci/djt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa Y, Sakaguchi M, Minami S, Kitano K, Kawa S, Aoki Y, Itoh N, Sakurai A, Miyazaki M, Watanabe T, et al. Duodenal somatostatinoma and erythrocytosis in a patient with von Hippel-Lindau disease type 2A. Intern Med. 2001;40:38–43. doi: 10.2169/internalmedicine.40.38. [DOI] [PubMed] [Google Scholar]
- Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- Lorenzo FR, Yang C, Ng Tang Fui M, Vankayalapati H, Zhuang Z, Huynh T, Grossmann M, Pacak K, Prchal JT. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med (Berl) 2013;91:507–512. doi: 10.1007/s00109-012-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini F, Falchetti A, Luzi E, Tonelli F, Maria Luisa B. Multiple Endocrine Neoplasia Type 1 (MEN1) Syndrome. In: Riegert-Johnson DL, Boardman LA, Hefferon T, Roberts M, editors. Cancer Syndromes. Bethesda (MD): National Center for Biotechnology Information (US); 2009. [PubMed] [Google Scholar]
- Martini M, Teofili L, Cenci T, Giona F, Torti L, Rea M, Foa R, Leone G, Larocca LM. A novel heterozygous HIF2AM535I mutation reinforces the role of oxygen sensing pathway disturbances in the pathogenesis of familial erythrocytosis. Haematologica. 2008;93:1068–1071. doi: 10.3324/haematol.13210. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–1466. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Eng C. The approach to the patient with paraganglioma. J Clin Endocrinol Metab. 2009;94:2677–2683. doi: 10.1210/jc.2009-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, He X, Chen J, Moline J, Mester J, Orloff MS, Ringel MD, Eng C. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependant destabilization of p53. Hum Mol Genet. 2012;21:300–310. doi: 10.1093/hmg/ddr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Chew EY, Pappo AS, Yang C, Lorenzo FR, Wilson MW, Aronow MB, Young JA, Popovic V, Zhuang Z. Ocular manifestations of hypoxia-inducible factor-2a paraganglioma-somatostatinoma-polycythemia syndrome. Ophthalmology. 2014;121:2291–2293. doi: 10.1016/j.ophtha.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Jochmanova I, Prodanov T, Yang C, Merino MJ, Fojo T, Prchal JT, Tischler AS, Lechan RM, Zhuang Z. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol. 2013;31:1690–1698. doi: 10.1200/JCO.2012.47.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Wimalawansa SJ. Pheochromocytoma and paraganglioma. Endocr Pract. 2015;21:406–412. doi: 10.4158/EP14481.RA. [DOI] [PubMed] [Google Scholar]
- Percy MJ, Beer PA, Campbell G, Dekker AW, Green AR, Oscier D, Rainey MG, van Wijk R, Wood M, Lappin TR, et al. Novel exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood. 2008a;111:5400–5402. doi: 10.1182/blood-2008-02-137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy MJ, Chung YJ, Harrison C, Mercieca J, Hoffbrand AV, Dinardo CL, Santos PC, Fonseca GH, Gualandro SF, Pereira AC, et al. Two new mutations in the HIF2A gene associated with erythrocytosis. Am J Hematol. 2012;87:439–442. doi: 10.1002/ajh.23123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, Lee FS. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008b;358:162–168. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta S, Stiehl DP, Punzo F, Scianguetta S, Borriello A, Bencivenga D, Casale M, Nobili B, Fasoli S, Balduzzi A, et al. Congenital erythrocytosis associated with gain-of-function HIF2A gene mutations and erythropoietin levels in the normal range. Haematologica. 2013;98:1624–1632. doi: 10.3324/haematol.2013.088369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Qin N, Pacak K, Eisenhofer G. Role of hypoxia and HIF2a in development of the sympathoadrenal cell lineage and chromaffin cell tumors with distinct catecholamine phenotypic features. Adv Pharmacol. 2013;68:285–317. doi: 10.1016/B978-0-12-411512-5.00014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taïeb D, Barlier A, Yang C, Pertuit M, Tchoghandjian A, Rochette C, Zattara-Canoni H, Figarella-Branger D, Zhuang Z, Pacak K, et al. Somatic gain-of-function HIF2A mutations in sporadic central nervous system hemangioblastomas. J Neurooncol. 2016;126:473–481. doi: 10.1007/s11060-015-1983-y. [DOI] [PubMed] [Google Scholar]
- Taïeb D, Yang C, Delenne B, Zhuang Z, Barlier A, Sebag F, Pacak K. First report of bilateral pheochromocytoma in the clinical spectrum of HIF2A-related polycythemia-paraganglioma syndrome. J Clin Endocrinol Metab. 2013;98:E908–E913. doi: 10.1210/jc.2013-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- Toledo RA, Qin Y, Srikantan S, Morales NP, Li Q, Deng Y, Kim SW, Pereira MA, Toledo SP, Su X, et al. In vivo and in vitro oncogenic effects of HIF2A mutations in pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2013;20:349–359. doi: 10.1530/ERC-13-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Hirayama J, Sugimoto Y, Uchida K, Ohishi K, Hirayama M, Komada Y. Polycythemia and Paraganglioma With a Novel Somatic HIF2A Mutation in a Male. Pediatrics. 2014;133:e1787–e1791. doi: 10.1542/peds.2013-2419. [DOI] [PubMed] [Google Scholar]
- van Essen M, Sundin A, Krenning EP, Kwekkeboom DJ. Neuroendocrine tumours: the role of imaging for diagnosis and therapy. Nat Rev Endocrinol. 2014;10:102–114. doi: 10.1038/nrendo.2013.246. [DOI] [PubMed] [Google Scholar]
- van Wijk R, Sutherland S, Van Wesel AC, Huizinga EG, Percy MJ, Bierings M, Lee FS. Erythrocytosis associated with a novel missense mutation in the HIF2A gene. Haematologica. 2010;95:829–832. doi: 10.3324/haematol.2009.017582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welander J, Andreasson A, Brauckhoff M, Backdahl M, Larsson C, Gimm O, Soderkvist P. Frequent EPAS1/HIF2alpha exons 9 and 12 mutations in non-familial pheochromocytoma. Endocr Relat Cancer. 2014;21:495–504. doi: 10.1530/ERC-13-0384. [DOI] [PubMed] [Google Scholar]
- Yang C, Hong CS, Prchal JT, Balint MT, Pacak K, Zhuang Z. Somatic mosaicism of EPAS1 mutations in the syndrome of paraganglioma and somatostatinoma associated with polycythemia. Human Genome Variation. 2015;2:15053. doi: 10.1038/hgv.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Sun MG, Matro J, Huynh TT, Rahimpour S, Prchal JT, Lechan R, Lonser R, Pacak K, Zhuang Z. Novel HIF2A mutations disrupt oxygen sensing, leading to polycythemia, paragangliomas, and somatostatinomas. Blood. 2013;121:2563–2566. doi: 10.1182/blood-2012-10-460972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhuang Z, Fliedner SM, Shankavaram U, Sun MG, Bullova P, Zhu R, Elkahloun AG, Kourlas PJ, Merino M, et al. Germ-line PHD1 and PHD2 mutations detected in patients with pheochromocytoma/paraganglioma-polycythemia. J Mol Med (Berl) 2014;93:93–104. doi: 10.1007/s00109-014-1205-7. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367:922–930. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Yang C, Ryska A, Ji Y, Hou Y, Graybill SD, Bullova P, Lubensky IA, Klöppel G, Pacak K. HIF2A gain-of-function mutations detected in duodenal gangliocytic paraganglioma. Endocr Relat Cancer. 2016;23:L13–L16. doi: 10.1530/ERC-16-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.