Abstract
In this exciting era of “next-gen cytogenetics,” integrating genomic sequencing into the prenatal diagnostic setting is possible within an actionable time frame and can provide precise delineation of balanced chromosomal rearrangements at the nucleotide level. Given the increased risk of congenital abnormalities in newborns with de novo balanced chromosomal rearrangements, comprehensive interpretation of breakpoints could substantially improve prediction of phenotypic outcomes and support perinatal medical care. Herein, we present and evaluate sequencing results of balanced chromosomal rearrangements in ten prenatal subjects with respect to the location of regulatory chromatin domains (topologically associated domains [TADs]). The genomic material from all subjects was interpreted to be “normal” by microarray analyses, and their rearrangements would not have been detected by cell-free DNA (cfDNA) screening. The findings of our systematic approach correlate with phenotypes of both pregnancies with untoward outcomes (5/10) and with healthy newborns (3/10). Two pregnancies, one with a chromosomal aberration predicted to be of unknown clinical significance and another one predicted to be likely benign, were terminated prior to phenotype-genotype correlation (2/10). We demonstrate that the clinical interpretation of structural rearrangements should not be limited to interruption, deletion, or duplication of specific genes and should also incorporate regulatory domains of the human genome with critical ramifications for the control of gene expression. As detailed in this study, our molecular approach to both detecting and interpreting the breakpoints of structural rearrangements yields unparalleled information in comparison to other commonly used first-tier diagnostic methods, such as non-invasive cfDNA screening and microarray analysis, to provide improved genetic counseling for phenotypic outcome in the prenatal setting.
Introduction
Fetal material obtained through invasive methods can be assessed routinely with different techniques, including karyotyping, fluorescence in situ hybridization, and chromosomal microarray analysis (CMA).1, 2, 3 Although karyotyping remains the principal cytogenetic tool in prenatal diagnosis, CMA has the advantage of higher resolution and is the preferred method in a fetus with one or more major structural abnormalities identified by ultrasonography.1 However, unlike karyotyping, CMA cannot detect balanced chromosomal rearrangements, such as translocations, inversions, and insertions.
The risk of congenital abnormalities is two to three times higher in newborns with apparently balanced de novo chromosomal rearrangements (6.1% for translocations and 9.4% for inversions) than in a population of pregnancies tested by amniocentesis.4 The cause of the increase in abnormal phenotypes in such cases can be a submicroscopic deletion, duplication, disruption, dysregulation, or fusion of a gene(s) located at or near the breakpoints. Studies using CMA have demonstrated the presence of a cryptic imbalance in 40%–50% of subjects with an abnormal phenotype and an apparently balanced chromosomal rearrangement.5, 6, 7, 8, 9, 10, 11, 12 Massively parallel sequencing technologies can provide timely localization of chromosomal breakpoints with nucleotide-level precision in all apparently balanced rearrangements, along with information on the gain or loss of genomic material,13, 14 which could substantially improve the prediction of phenotypic outcomes and support perinatal medical care.
Outcomes of structural rearrangements changing the copy number of a gene or directly disrupting a gene can be predicted from dosage effects. However, if a balanced rearrangement occurs in a non-coding region or the regulatory effect of the rearrangement is more pertinent to an abnormal phenotype than the directly affected gene, predicting pathogenic consequences can become challenging and even erroneous when only the gene(s) with copy-number changes or disrupted gene(s) are evaluated. This is particularly important in prenatal diagnosis, because for many key developmental genes, cis-regulatory elements can extend beyond the transcription unit with an estimated median regulator-target gene distance of 120 kb,15 which can range up to 1.5 Mb.16, 17
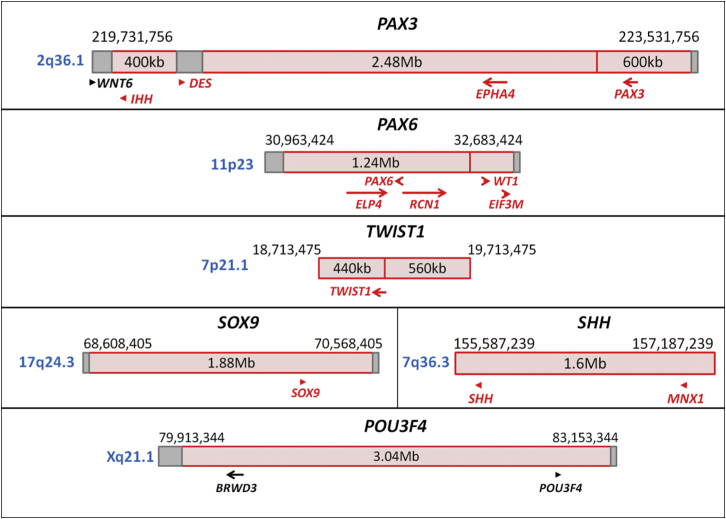
Topologically associated domains (TADs) have been elucidated as key elements of mammalian regulatory organization.18, 19 TADs are highly conserved megabase-sized genomic segments that partition the genome into large units with frequent intra-domain interactions. They are separated by topological boundary regions (TBRs), which represent “genomic insulators” by blocking the interactions between adjacent TADs. Disruption of TBRs by structural rearrangements has been demonstrated to cause rewiring of genomic regulators in the WNT6-IHH-EPHA4-PAX3 locus (MIM: 604663, 600726, 602188, and 606597) and result in human limb malformations, as described by Lupiãnez et al. (Table 1 and Figure 1).20 In this context, the developmental genes with historically well-known long-range regulation can be re-evaluated in relation to their TAD and TBR annotations (Table 2 and Figure 1). For example, disruption of PAX6 (MIM: 607108) and regulatory elements located in the same TAD as PAX6 (up to 150 kb downstream) results in isolated aniridia,21 whereas haploinsufficiency of WT1 (MIM: 194070), which is located in the TAD adjacent to PAX6, causes genitourinary anomalies without aniridia.23 Deletions of the contiguous locus containing both PAX6 and WT1, including the TBR between their two adjacent TADs, result in the autosomal-dominant WAGR syndrome (MIM: 194072) with both aniridia and genitourinary anomalies, supporting the “genomic insulator” role of TBRs. In addition, the size of an individual TAD can be relevant to the extent of long-range regulation. TWIST1 (MIM: 601622) is known to have long-range regulation up to 260 kb downstream, which is located within the same 440 kb TAD as TWIST1. Monoallelic disruption of both TWIST1 and its downstream regulatory region results in Saethre-Chotzen syndrome (MIM: 101400).24 SOX9 (MIM: 608160) is reported to have long-range regulation up to 1.5 Mb upstream, which is located within the same 1.88 Mb TAD as SOX9. Monoallelic disruption of both SOX9 and its regulatory region is associated with campomelic dysplasia (MIM: 114290) and Pierre Robin sequence (MIM: 261800).25, 28, 29 There might also be phenotype-specific regulators within the same TAD for a developmental gene depending on their distance from the gene of interest. Monoallelic disruption of regulatory elements located within the same 1.6 Mb TAD as SHH (MIM: 600725) can result in type 3 holoprosencephaly (MIM: 142945) or preaxial polydactyly (MIM: 174500), depending on the location (265 kb upstream or 1 Mb upstream of SHH, respectively).26 Lastly, in addition to the genes showing a phenotype with monoallelic disruption, regulatory regions of developmental genes located on the X chromosome or imprinted genes should also be carefully analyzed, given that disruption of a single allele through balanced rearrangements could result in an abnormal phenotype in such cases. For instance, POU3F4 (MIM: 300039) is an X-linked recessively inherited gene with long-range regulation up to 900 kb upstream27 in a 3.04 Mb TAD, and disruption of a single allele of POU3F4 or its regulatory region results in deafness in males. Overall, advances in the understanding of chromatin organization of the human genome, along with the evolving databases of phenotypes associated with structural variation, could provide a conceptual framework for the interpretation of balanced-rearrangement breakpoints and their potential cis-regulatory effects.
Table 1.
Pathological Rewiring of Genetic Regulatory Interactions
| Genomic Locus on 2q36.1 | TAD and TBR Nucleotides (hESC, GRCh37/hg19)18(Size) | Structural Rearrangement (Associated Phenotype) |
|---|---|---|
| WNT6-IHH-DES | TBR: 219,731,756–219,851,756 (120 kb), TAD: 219,851,756–220,251,756 (400 kb), TBR: 220,251,756–220,411,756 (160 kb) |
inversion or duplication altering the 160 kb TBR and bringing the centromeric portion of the EPHA4-containing TAD into proximity with WNT6 (F-syndrome [MIM: 102510]) |
| duplication or deletion altering the 160 kb TBR and bringing IHH into proximity with the centromeric portion of the EPHA4-containing TAD (polydactyly) | ||
| EPHA4 | TAD: 220,411,756–222,891,756 (2.48 Mb) | deletion involving the TBR at 222,891,756 (brachydactyly) |
| PAX3 | TAD: 222,891,756–223,491,756 (600 kb) |
This table shows the pathological rewiring of genetic regulatory interactions of enhancer EPHA4 through different structural rearrangements altering the TAD boundaries (data presented herein are modified from Lupiãnez et al.20). Abbreviations are as follows: hESC, human embryonic stem cell; TAD, topologically associated domain; and TBR, topological boundary region.
Figure 1.
Developmental Genes with Well-Known Long-Range Regulations
Schematic diagrams of representative developmental genes with well-known long-range regulations in relation to their TAD (red box) and TBR (dark-red vertical line if 0 bp or gray box if greater than 0 bp) annotations (genes in red: haploinsufficiency index < 10%).
Table 2.
TADs and TBRs of Genes with Historically Well-Known Long-Range cis-Regulation Associated with a Phenotype
| Locus (Chromosome Band) | TAD and TBR Nucleotides (hESC, GRCh37/hg19)18(Size) | Genetic Alterations | Phenotype |
|---|---|---|---|
|
PAX6-WT1 (11p23) |
TBR: 30,963,424–31,083,424 (120 kb), TAD: 31,083,424–32,323,424 (1.24 Mb), TAD: 32,323,424–32,643,424 (320 kb), TBR: 32,643,424–32,683,424 (40 kb), |
disruption of regulatory elements up to 150 kb downstream of PAX6 | aniridia21 |
| deletions involving PAX6 and WT1, which includes the TBR between the TADs of these genes | WAGR syndrome22 | ||
| haploinsufficiency of WT1 | syndromes involving genitourinary anomalies without aniridia23 | ||
|
TWIST1 (7p21.1) |
TAD: 18,713,475–19,153,475 (440 kb), TAD: 19,153,475–19,713,475 (560 kb) |
disruption of regulatory elements up to 260 kb downstream of TWIST1 | Saethre-Chotzen syndrome24 |
|
SOX9 (17q24.3) |
TAD: 68,648,405–70,528,405 (1.88 Mb) | disruption of regulatory elements up to 1.5 Mb upstream of SOX9 | Pierre Robin sequence25 |
|
SHH (7q36.3) |
TAD: 155,587,239–157,187,239 (1.6 Mb) | disruption of regulatory elements up to 265 kb upstream of SHH | HPE326 |
| disruption of regulatory elements up to 1 Mb upstream of SHH | preaxial polydactyly26 | ||
|
POU3F4 (Xq21.1) |
TAD: 80,073,344–83,113,344 (3.04 Mb) | disruption of regulatory elements up to 900 kb upstream of POU3F4 | X-linked deafness27 |
Abbreviations are as follows: hESC, human embryonic stem cell; HPE3, holoprosencephaly type 3; TAD, topologically associated domain; TBR, topological boundary region; and WAGR, Wilms tumor, aniridia, genitourinary anomalies, and mental retardation.
Identifying breakpoints of balanced chromosomal rearrangements has been the foundation of the Developmental Genome Anatomy Project (DGAP), which has sequenced more than 200 subjects. As an extension of these efforts, in this study, we sequenced ten prenatal subjects with balanced chromosomal rearrangements by using customized large-insert libraries and used publicly available databases to interpret the breakpoints on the basis of convergent genomic evidence in light of previously annotated TADs and TBRs in human embryonic stem cells.29
Material and Methods
Subjects
Ten subjects were enrolled after proper informed consent was acquired in accordance with an institutional-review-board protocol approved by Partners HealthCare System in Boston. These ten subjects represent the total of a consecutive series of DGAP prenatal referrals to date, and prior to enrollment, all had balanced chromosomal rearrangements according to karyotyping with normal CMA results. Two subjects (DGAP239 and DGAP259) have been reported in part previously.30, 31
Sequencing and Bioinformatic Analysis
Genomic DNA was extracted from amniocytes or chorionic villi with a Gentra Puregene Cell Kit (QIAGEN). Large-insert structural-variation sequencing was performed as previously described.12, 26 In brief, after the production of large-insert libraries (target size of 2–3.5 kb) and quality control, massively parallel paired-end sequencing of 25 or 50 cycles was performed with an Illumina HiSeq 2000 or 2500. Reads were processed with our customized structural-variant sequencing pipelines, which include alignment, clustering of anomalous read pairs, extensive cluster filtering, and variant screening against known structural variants.32, 33, 34, 35 Genome-wide physical coverage of inserts ranged from 35× to 68×, and DNA input ranged from 900 ng to 5 μg. For all subjects with sufficient material, DNA was amplified by PCR with primers based on sequence reads supporting the rearrangement junction for confirmation of breakpoints.
Analysis of Convergent Genomic Evidence
In addition to genes located directly at breakpoints, phenotypic associations were evaluated in relation to previously annotated TADs and TBRs in human embryonic stem cells18 for positional effects on protein-coding genes through disruption of potential regulatory elements. DECIPHER was utilized for predicting the probability of haploinsufficiency, which was determined on the basis of genes known to produce a phenotype through haploinsufficiency and genes disrupted by unambiguous loss-of-function variants in at least two apparently healthy individuals. Low haploinsufficiency indices (<10%) indicate a high predicted probability that a gene will exhibit haploinsufficiency (i.e., disruption of one allele might be pathogenic, also referred to as monoallelic).36 Within the analyzed intervals, disrupted genes, genes with a haploinsufficiency index < 10%, hemizygous or imprinted genes, and genes associated with a phenotype were evaluated in detail for each subject in relation to the disrupted TADs and TBRs. Abnormal phenotypic associations of disrupted or dysregulated regions were reviewed in the scientific literature, OMIM,37 OMIM Gene Map and Morbid Map,37 DECIPHER,38 and the Developmental Disorders Genotype-to-Phenotype (DDG2P) database.39
Expression Studies
qRT-PCR was performed with RNA extracted from cultured prenatal cells of the available subjects (amniocytes from DGAP247 and chorionic villi from DGAP248 and DGAP288) and control samples (amniocytes or chorionic villi with a normal karyotype referred for advanced maternal age) or cord blood (DGAP247 and DGAP288). qRT-PCR was performed according to standard conditions of the CFX Real-Time PCR Detection System (Bio-Rad), and transcription levels were quantified with the ΔΔCT method.30
Results
Prior to enrollment, karyotyping was performed for all pregnancies because they were considered to be high risk (e.g., advanced maternal age, abnormal first-trimester serum screening, and/or ultrasound abnormality) with normal CMA results during clinical assessment (see Supplemental Note). Among the ten subjects analyzed, four had reciprocal translocations, five had inversions, and one had a complex rearrangement according to karyotyping. Sequencing revised the initial karyotype by providing nucleotide-level resolution to the initially described chromosome bands with a size ranging from 2.8 to 53.6 Mb, encompassing 63–1,032 genes and 16–358 phenotype-associated loci for each rearrangement (Table 3 and Table S1).40 In addition to refining breakpoints, including those in a subject with a very complex karyotype (DGAP259), sequencing revealed cryptic rearrangements unapparent by karyotyping in four subjects (DGAP258, DGAP268, DGAP290, and DGAP295). All rearrangements were located within a TAD, except for one that was located in a TBR at Xq28 (DGAP285) (Figures 2, 3, and 4; Tables 4, 5, and 6; and Table S2). Five subjects had abnormal clinical outcomes, three continue to be healthy, and two were terminated prior to detection of any potential abnormal findings (Table 7).
Table 3.
Genomic Localization of the Disrupted Chromosome Bands in Comparison to Karyotypically Reported Bands
| Subject | Next-Gen Cytogenetic Nomenclature40(Short System) | G-Band | Next-Gen Band | Revised Band Range: Nucleotides (Distance) | Genesa | Phenotype-Associated Locib |
|---|---|---|---|---|---|---|
| DGAP239 | 46,XY,t(6;8)(q13;q13)dn.arr(1-22)x2,(XY)x1. seq[GRCh37/hg19] t(6;8)(q13;q12.2)dn |
6q13 | 6q13 | 6q13: 70,000,001–75,900,000 (5.9 Mb) | 63 | 16 |
| 8q13 | 8q12.2 | 8q12q21: 55,500,001–93,300,000 (37.8 Mb) | 334 | 41 | ||
| DGAP247 | 46,XY,inv(8)(q13q24.1)dn.arr(1-22)x2,(XY)x1. seq[GRCh37/hg19] inv(8)(q11.21q24.23)dn |
8q13 | 8q11.21 | 8q11q21: 45,600,001–93,300,000 (47.7 Mb) | 406 | 47 |
| 8q24.1 | 8q24.23 | 8q24: 117,700,001–146,364,022 (28.7 Mb) | 306 | 47 | ||
| DGAP248 | 46,XY,t(2;13)(p13;q14)dn.arr(1-22)x2,(XY)x1. seq[GRCh37/hg19] t(2;13)(p12;q13.2)dn |
2p13 | 2p12 | 2p14p12: 64,100,001–83,300,000 (19.2 Mb) | 225 | 32 |
| 13q14 | 13q13.2 | 13q13q21: 32,200,001–73,300,000 (41.1 Mb) | 375 | 47 | ||
| DGAP258 | 46,XY,inv(6)(p23q13)dn.arr(1-22)x2,(XY)x1. seq[GRCh37/hg19] inv(6)(p25.3q16.1)dnc |
6p23 | 6p25.3 | 6p25p22: 1–30,400,000 (30.4 Mb) | 679 | 74 |
| 6q13 | 6q16.1 | 6q11q16: 61,000,001–105,500,000 (44.5 Mb) | 293 | 44 | ||
| DGAP259 | 46,XX,t(3;18;5;7)(p25;p11.2;q13.3;q32),t(9;18)(p22;q21)dn.arr(1-22,X)x2. seq[GRCh37/hg19](3,5,7,9,18)cx,der(3)t(3;7)(p24.3;q36.3)dn,der(5)t(5;7)(q14.3;q35)t(3;7)(p24.3;q36.3) t(3;18)(p26.3;p11.31)dn,der(7)t(5;7)dn, der(9)t(9;18)(p23;q21.3)dn, der(18)t(3;18)inv(18)(p11.31q21.3)t(9;18)dn |
3p25 | 3p26.3 3p24.3 |
3p26p24:1–30,900,000 (30.9 Mb) | 277 | 49 |
| 5q13.3 | 5q14.3 | 5q12q14: 58,900,001–92,300,000 (33.4 Mb) | 323 | 358 | ||
| 7q32 | 7q35 7q36.3 |
7q31q36: 107,400,001–159,138,663 (51.8 Mb) | 693 | 80 | ||
| 9p22 | 9p23 | 9p23p21: 9,000,001–33,200,000 (24.2 Mb) | 181 | 33 | ||
| 18p11.2 | 18p11.31 | 18p11: 1–17,200,000 (17.2 Mb) | 192 | 29 | ||
| 18q21 | 18q21.3 | 18q21: 43,500,001–61,600,000 (18.1 Mb) | 172 | 33 | ||
| DGAP268 | 46,XY,inv(10)(p13q24)dn.arr(1-22)x2,(XY)x1. seq[GRCh37/hg19]inv(10)(p12.2p12.31)(p12.2q23.32)dn |
10p13 | 10p12.31 10p12.2 |
10p14p12: 6,600,001–29,600,000 (23 Mb) | 233 | 26 |
| 10q24 | 10q23.32 | 10q23q25: 82,000,001–119,100,000 (37.1 Mb) | 467 | 84 | ||
| DGAP285 | 46,Y,inv(X)(p11.2q28).arr(1-22)x2,(XY)x1. seq[GRCh37/hg19] inv(X)(p11.2q28) |
Xp11.2 | Xp11.21 | Xp11.2: 46,400,001–58,100,000 (11.7 Mb) | 274 | 65 |
| Xq28 | Xq28 | Xq28: 147,100,001–155,270,560 (8.2 Mb) | 192 | 63 | ||
| DGAP288 | 46,XX,t(6;17)(q13;q21)dn.arr(1-22,X)x2. seq[GRCh37/hg19] t(6;17)(q21;q24.3)dn |
6q13 | 6q21 | 6q11q21: 61,000,001–114,600,000 (53.6 Mb) | 404 | 57 |
| 17q21 | 17q24.3 | 17q11q24: 24,000,001–70,900,000 (46.9 Mb) | 1,032 | 138 | ||
| DGAP290 | 46,XY,t(2;7)(q33;q32)dn.arr(1-22)x2,(XY)x1. seq[GRCh37/hg19](2,7)cx, der(2)t(2;7)(q32.3;q33) inv(7)(q33q33)dn,der(7)t(2;7)dn |
2q33 | 2q32.3 | 2q32q34: 183,000,001–215,300,000 (32.3 Mb) | 313 | 51 |
| 7q32 | 7q33 | 7q31q33: 107,400,001–138,200,000 (30.8 Mb) | 291 | 49 | ||
| DGAP295 | 46,XY,t(2;11)(p13.1;p15.5)dn.arr(1-22)x2,(XY)x1. seq[GRCh37/hg19](2,11)cx,der(2)inv(11)(p15.5)inv(11)(p15.5) t(2;11)(p13.3;p15.5)dn,der(11)t(2;11)dn |
2p13.1 | 2p13.3 | 2p13: 68,600,001–75,000,000 (6.4 Mb) | 133 | 32 |
| 11p15.5 | 11p15.5 | 11p15.5: 1–2,800,000 (2.8 Mb) | 114 | 31 |
Number of genes for the presented nucleotide range (NCBI Map Viewer, annotation release 105 [GrCh37.p13]).
OMIM Phenotypic Series-specific entries for the presented nucleotide range (June 9, 2015).
Cryptic paternal inversion is not included.
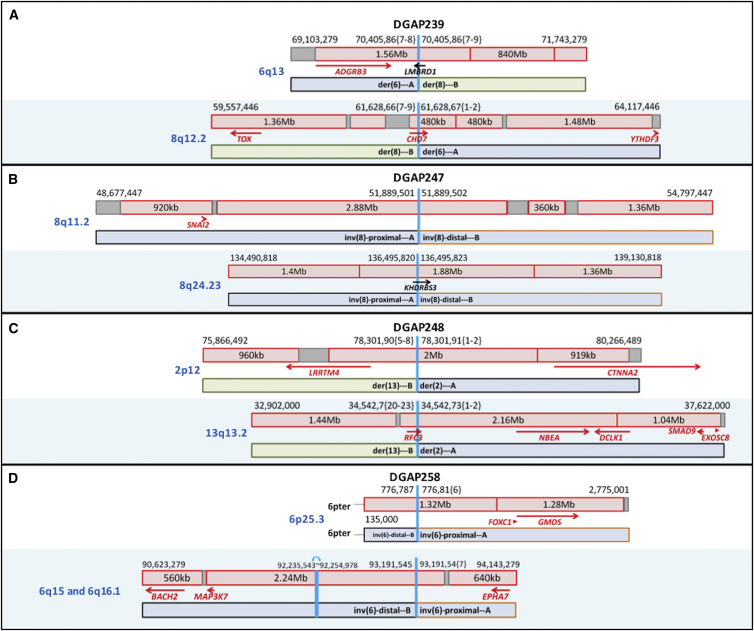
Figure 2.
Diagrams of DGAP239, DGAP247, DGAP248, and DGAP258 Rearrangements
Schematic diagrams of the breakpoints of DGAP239 (A), DGAP247 (B), DGAP248 (C), and DGAP258 (D) in relation to their TAD (red box) and TBR (dark-red vertical line if 0 bp or gray box if greater than 0 bp) annotations (genes in red: haploinsufficiency index < 10%).
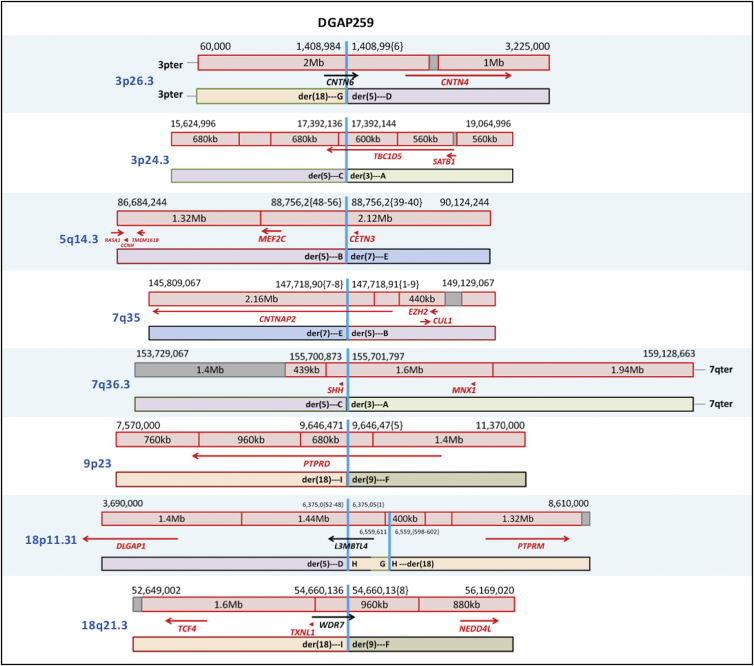
Figure 3.
Diagrams of DGAP259 Rearrangements
Schematic diagrams of the breakpoints of DGAP259 in relation to their TAD (red box) and TBR (dark-red vertical line if 0 bp or gray box if greater than 0 bp) annotations (genes in red: haploinsufficiency index < 10%).
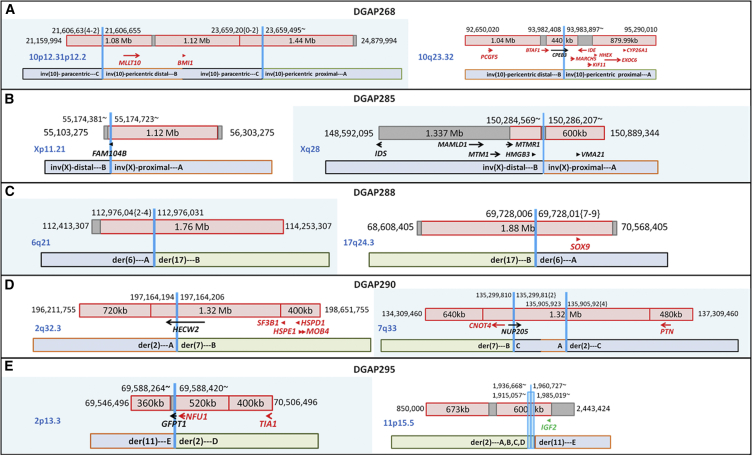
Figure 4.
Diagrams of DGAP268, DGAP285, DGAP288, DGAP290, and DGAP290 Rearrangements
Schematic diagrams of the breakpoints of DGAP268 (A), DGAP285 (B), DGAP288 (C), DGAP290 (D), and DGAP290 (E) in relation to their TAD (red box) and TBR (dark-red vertical line if 0 bp or gray box if greater than 0 bp) annotations (genes in red: haploinsufficiency index < 10%; green: imprinted).
Table 4.
DGAP239, DGAP247, DGAP248 and DGAP258: Significant Protein-Coding Genes Surrounding the Breakpoints according to TADs and Convergent Genomic Evidence
| Gene | Nucleotides (GRCh37/hg19) | Description | OMIM37 | OMIM Morbid37 | DDG2P39 | HI (%)36 | Notes |
|---|---|---|---|---|---|---|---|
| DGAP239: 6q13 Breakpoints on Rearrangement_A (70,405,86{7-8}) and Rearrangement_B (70,405,86{7-9}) | |||||||
| ADGRB3 | 69,345,259–70,099,403 | adhesion G protein-coupled receptor B3 | 602684 | − | − | 3.02 | no reported phenotype association; homologous to ADGRB1, an angiogenesis inhibitor that is a candidate for involvement in development of glioblastoma41 |
|
LMBRD1 (disrupted) |
70,385,694–70,507,003 | LMBR1 domain containing 1 | 612625 | + | + | 12.92 | biallelic loss of function (autosomal recessive) associated with methylmalonic aciduria and homocystinuria, cblF type42 (no phenotypic overlap with DGAP239) |
| DGAP239: 8q12.2 Breakpoints on Rearrangement_A (61,628,67{1-2}) and Rearrangement_B (61,628,66{7-9}) | |||||||
|
CHD7 (disrupted) |
61,591,337–61,779,465 | chromodomain helicase DNA binding protein 7 | 608892 | + | + | 2.4 | haploinsufficiency (autosomal dominant, monoallelic) reported to be associated with CHARGE syndrome, such that mutations in >90% of subjects meet diagnostic criteria of CHARGE syndrome43 (consistent with the clinical diagnosis of CHARGE syndrome during the postnatal period of DGAP239) |
| DGAP247: 8q11.2 Breakpoints on Rearrangement_A (51,889,501) and Rearrangement_B (51,889,502) | |||||||
| No significant gene within the same TAD as the breakpoints | |||||||
| DGAP247: 8q24.23 Breakpoints on Rearrangement_A (136,495,820) and Rearrangement_B (136,495,823) | |||||||
| KHDRBS3 | 136,469,700–136,668,965 | KH domain containing, RNA binding, signal transduction associated 3 | 610421 | − | − | 10.52 | no reported phenotype association |
| DGAP248: 2p12 Breakpoints on Rearrangement_A (78,301,91{1-2}) and Rearrangement_B (78,301,90{8-5}) | |||||||
| LRRTM4 | 76,974,845–77,820,445 | leucine rich repeat transmembrane neuronal 4 | 610870 | − | − | 7.26 | no reported phenotype association; structure and expression profile of LRRTM mRNAs in mice suggest role in development and maintenance of the vertebrate nervous system44 |
| DGAP248: 13q13.2 breakpoints on Rearrangement_A (34,542,73{2-1}) and Rearrangement_B (34,542,7{20-23}) | |||||||
|
RFC3 (disrupted) |
34,392,186–34,540,695 | replication factor C subunit 3 | 600405 | − | − | 4.93 | no reported phenotype association |
| NBEA | 35,516,424–36,247,159 | neurobeachin | 6084889 | − | − | 6.83 | disrupted in a subject with a de novo translocation and idiopathic autism,45 and haploinsufficiency causes autism-like behaviors in animal models46, 47 |
| DGAP258: 6p25.3 Breakpoints on Rearrangement_A (776,81{6}) and Rearrangement_B (776,787) | |||||||
| No significant gene within the same TAD as the breakpoints | |||||||
| DGAP258: 6q16.1 Breakpoints on Rearrangement_A (93,191,54{7}) and Rearrangement_B (93,191,545) | |||||||
| MAP3K7 | 91,223,292–91,296,764 | mitogen-activated protein kinase kinase kinase 7 | 602614 | − | − | 2.75 | no reported phenotype association |
Abbreviations are as follows: DDG2P, Developmental Disorders Genotype-to-Phenotype database; and HI, haploinsufficiency index.
Table 5.
DGAP259: Significant Protein-Coding Genes Surrounding the Breakpoints according to TADs and Convergent Genomic Evidence
| Gene | Nucleotides (GRCh37/hg19) | Description | OMIM37 | OMIM Morbid37 | DDG2P39 | HI (%)36 | Notes |
|---|---|---|---|---|---|---|---|
| 3p26.3 Breakpoints on Rearrangement_D (1,408,99{6}) and Rearrangement_G (1,408,984) | |||||||
|
CNTN6 (disrupted) |
1,134,260–1,445,901 | contactin 6 | 607220 | − | − | 39.69 | no reported phenotype association; neural adhesion molecule48 |
| CNTN4 | 2,140,497–3,099,645 | contactin 4 | 607280 | − | − | 6.9 | disrupted in a subject with a 3p deletion syndrome (autosomal-dominant) phenotype49 (cerebral and renal malformation phenotype of DGAP259) |
| 3p26.3 Breakpoints on Rearrangement_D (1,408,99{6}) and Rearrangement_G (1,408,984) | |||||||
|
TBC1D5 (disrupted) |
17,198,654–18,486,309 | TBC1 domain family member 5 | 615740 | − | − | 5.84 | no reported phenotype association |
| SATB1a | 18,386,879–18,487,080 | SATB homeobox 1 | 602075 | − | − | 2.15 | global genome organizer50, 51 (complex chromosomal rearrangement of DGAP259); role in neuronal plasticity of cortical neurons and regulation of key neuronal genes52, 53 (cerebral malformation phenotype of DGAP259) |
| 5q14.3 Breakpoints on Rearrangement_B (88,756,2{48-56}) and Rearrangement_E (88,756,2{39-40}) | |||||||
| MEF2C | 88,013,975–88,199,922 | myocyte enhancer factor 2C | 600662 | + | + | 0.26 | haploinsufficiency (autosomal dominant, monoallelic) associated with mental retardation, stereotypic movements, epilepsy, and cerebral malformations (MIM: 613443)47, 54 (cerebral malformation and hypoplastic corpus callosum phenotype of DGAP259); role in synaptic plasticity and hippocampal-dependent learning and memory55 (9p23 breakpoints of DGAP259 disrupt PTPRD1 with similar role) |
| CETN3 | 89,688,078–89,705,603 | centrin 3 | 602907 | − | − | 5.94 | present in centrosomes and important role in early cleavage of frog embryos56 (complex chromosomal rearrangement of DGAP259) |
| 7q35 Breakpoints on Rearrangement_B (147,718,91{1-9}) and Rearrangement_E (147,718,90{7-8}) | |||||||
|
CNTNAP2 (disrupted) |
145,813,453–148,118,090 | contactin associated protein-like 2 | 604569 | + | + | 4.94 | susceptibility to autism type 15;57 homozygous or compound-heterozygous mutations cause Pitt-Hopkins-like syndrome 1 (MIM: 610042)58 (cerebral malformation phenotype of DGAP259; 18q21 breakpoints are one TAD downstream of TCF4, associated with Pitt-Hopkins syndrome) |
| CUL1a | 148,395,006–148,498,128 | cullin 1 | 603134 | − | − | 4.3 | regulates the mammalian G1/S transition59 |
| EZH2a | 148,504,475–148,581,413 | enhancer of zeste 2 polycomb repressive complex 2 subunit | 601573 | + | + | 3.07 | has a critical role during normal and perturbed development of the hematopoietic and central nervous systems,60 maintains homeotic gene repression, and is thought to control gene expression by regulating chromatin61 (cerebral malformation and complex chromosomal rearrangement of DGAP259) |
| 7q36.3 Breakpoints on Rearrangement_A (155,701,797) and Rearrangement_C (155,700,873) | |||||||
| SHH | 155,592,680–155,604,967 | sonic hedgehog | 600725 | + | + | 0.66 | haploinsufficiency (autosomal dominant, monoallelic) associated with HPE3,62 which has a long-range regulation-associated phenotype63 (cerebral malformation phenotype of DGAP259) |
| 9p23 Breakpoints on Rearrangement_F (9,646,47{5}) and Rearrangement_I (9,646,471) | |||||||
|
PTPRD (disrupted) |
8,314,246–10,612,723 | protein tyrosine phosphatase, receptor type D | 601598 | − | − | 0.14 | homozygous microdeletion causes trigonocephaly, hearing loss, and intellectual disability, which overlap the autosomal-dominant 9p deletion syndrome64 (cerebral malformation phenotype of DGAP259); role in synaptic plasticity and hippocampal-dependent learning and memory65 (5q14.3 breakpoints are within the same TAD as MEF2C with similar role) |
| 18p11.31 Breakpoints on Rearrangement_D (6,375,05{1}), Rearrangement_G (6,559,611), and Rearrangement_H (6,375,0{52-48} and 6,559,{598-602}) | |||||||
|
L3MBTL4 (disrupted) |
5,954,705–6,415,236 | L(3)Mbt-like 4 (Drosophila) | − | − | − | 59.07 | no reported phenotype association |
| 18q21.3 Breakpoints on Rearrangement_F (54,660,13{8}) and Rearrangement_I (54,660,136) | |||||||
| TCF4a | 52,889,562–53,332,018 | transcription factor 4 | 602272 | + | + | 0.38 | haploinsufficiency (autosomal dominant, monoallelic) is associated with Pitt-Hopkins syndrome66 (cerebral malformation phenotype of DGAP259, 7q35 breakpoints disrupt CNTNAP2, related to Pitt-Hopkins-like syndrome58) |
|
WDR7 (disrupted) |
54,318,574–54,698,828 | WD repeat domain 7 | 613473 | − | − | 14.85 | no reported phenotype association; localized to synaptic vesicles in rat and mouse brain67 |
| NEDD4La | 55,711,599–56,068,772 | neural precursor cell expressed, developmentally down-regulated 4-like, E3 ubiquitin protein ligase | 606384 | − | − | 8.66 | regulator of renal sodium channels; involved in induction of mesoendodermal fates in mouse embryonic stem cells68 (renal malformation phenotype of DGAP259) |
Abbreviations are as follows: DDG2P, Developmental Disorders Genotype-to-Phenotype database; HI, haploinsufficiency index; and HPD3, holoprosencephaly type 3.
Although not located within the same hESC TAD18 as the breakpoint, these genes might be relevant to the phenotype of DGAP259 given the complexity of the rearrangement.
Table 6.
DGAP268, DGAP285, DGAP288, DGAP290, and DGAP295: Significant Protein-Coding Genes Surrounding the Breakpoints according to TADs and Convergent Genomic Evidence
| Gene | Nucleotides (GRCh37/hg19) | Description | OMIM37 | OMIM Morbid37 | DDG2P39 | HI (%)36 | Notes |
|---|---|---|---|---|---|---|---|
| DGAP268: 10p12.31 Breakpoints on Rearrangement_B (21,606,655) and Rearrangement_C (21,606,63{4-2}) | |||||||
| No significant gene within the same TAD as the breakpoints | |||||||
| DGAP268: 10p12.2 Breakpoints on Rearrangement_A (23,659,495∼) and Rearrangement_C (23,659,20{0-2}) | |||||||
| No significant gene within the same TAD as the breakpoints | |||||||
| DGAP268: 10q23.32 Breakpoints on Rearrangement_A (93,983,897∼) and Rearrangement_B (93,982,408) | |||||||
|
CPEB3 (disrupted) |
93,806,449–94,050,844 | cytoplasmic polyadenylation element binding protein 3 | 610606 | − | − | 12.96 | no reported phenotype association |
| DGAP285: Xp11.21 Breakpoints on Rearrangement_A (55,174,723∼) and Rearrangement_B (55,174,381∼) | |||||||
|
FAM104B (disrupted) |
55,169,535–55,187,743 | family with sequence similarity 104 member B | − | − | − | 93.08 | no reported phenotype association |
| DGAP285: Xq28 Breakpoints on Rearrangement_A (150,286,207∼) and Rearrangement_B (150,284,569∼) | |||||||
| MTM1 | 149,737,069–149,841,795 | myotubularin 1 | 300415 | + | + | 12.54 | hemizygous loss of function (X-linked recessive) associated with X-linked myotubular myopathy69 (overlapping the phenotype of DGAP285) |
| DGAP288: 6q21 Breakpoints on Rearrangement_A (112,976,04{2-4}) and Rearrangement_B (112,976,031) | |||||||
| No significant gene within the same TAD as the breakpoints | |||||||
| DGAP288: 17q24.3 Breakpoints on Rearrangement_A (69,728,01{7-9}) and Rearrangement_B (69,728,006) | |||||||
| SOX9 | 70,117,161–70,122,561 | SRY-box 9 | 608160 | + | + | 0.56 | haploinsufficient (autosomal dominant, monoallelic) long-range cis-regulation associated with Pierre Robin sequence28 (overlapping the phenotype of DGAP288) |
| DGAP290: 2q32.3 Breakpoints on Rearrangement_A (197,164,194) and Rearrangement_B (197,164,206) | |||||||
|
HECW2 (disrupted) |
197,059,094–197,458,416 | HECT, C2, and WW domain containing E3 ubiquitin protein ligase 2 | − | − | − | 18.5 | no reported phenotype association |
| DGAP290: 7q33 Breakpoints on Rearrangement_A (135,905,923), Rearrangement_B (135,299,810), and Rearrangement_C (135,299,81{2} and 135,905,92{4}) | |||||||
|
NUP205 (disrupted) |
135,242,667–135,333,505 | nucleoporin 205 | 614352 | − | − | 11.41 | no reported phenotype association |
| DGAP295: 2p13.3 Breakpoints on Rearrangement_D (69,588,420∼) and Rearrangement_E (69,588,264∼) | |||||||
|
GFPT1 (disrupted) |
69,546,905–69,614,382 | glutamine-fructose-6-phosphate transaminase 1 | 138292 | + | − | 22.36 | biallelic loss of function (autosomal recessive) associated with congenital myasthenia type 1270 (no overlap with the phenotype of DGAP295) |
| DGAP295: 11p15.5 Breakpoints on Rearrangement_A (1,915,057∼ and 1,936,993∼), Rearrangement_B (1,960,727∼ and 1,936,668∼), Rearrangement_C (1,915,843∼ and 1,961,361∼), Rearrangement_D (1,984,895∼), and Rearrangement_E (1,985,019∼) | |||||||
| IGF2 | 2,150,342–2,170,833 | insulin-like growth factor 2 | 147470 | + | + | 79.01 | imprinted loss of function (epimutation) associated with GRDF71 and Silver-Russell syndrome72 (overlapping the phenotype of DGAP295) |
Abbreviations are as follows: DDG2P, Developmental Disorders Genotype-to-Phenotype database; GRDF, growth restriction with distinctive facies; and HI, haploinsufficiency index.
Table 7.
Lessons Learned: Next-Generation Sequencing of Ten Prenatal Subjects
| Subject | Gene(s) of Interest according to Sequencing Results | Interpretation of the Sequencing Results | Clinical Significance | Clinical Outcome |
|---|---|---|---|---|
| DGAP239 |
CHD7 (disrupted), LMBRD1 (disrupted) |
disruption of an autosomal-dominant gene with a low haploinsufficiency index and associated with CHARGE syndrome (pathogenic) and an autosomal-recessive gene (non-contributory) | pathogenic | CHARGE syndrome |
| DGAP247 | KHDRBS3 (disrupted) | disruption of a single gene without pathogenicity | unknown, likely to be benign | healthy newborn |
| DGAP248 | LRRTM4, RFC3 (disrupted), NBEA | disruption of a gene with a low haploinsufficiency index but no reported pathogenicity; potential dysregulation of an additional gene with a low haploinsufficiency index and reported to be associated with autism-like behaviors in animal models and disrupted in a subject with idiopathic autism45, 46 | unknown | termination prior to communication of sequencing results |
| DGAP258 | – | non-genic breakpoints with cryptic paternal inversion not at the karyotypically detected breakpoint | unknown, likely to be benign | healthy newborns |
| DGAP259 | CNTN6 (disrupted), CNTN4, TBC1D5 (disrupted), SATB1, MEF2C, CETN3, CNTNAP2 (disrupted), CUL1, EZH2, SHH, PTPRD (disrupted), L3MBTL4 (disrupted), TCF4, WDR7 (disrupted), NEDD4L | complex rearrangement with potential dysregulation of genes with a low haploinsufficiency index and associated with malformation in the CNS and chromatin organization | pathogenic | termination due to multiple abnormal prenatal findings (bilateral ventriculomegaly and colpocephaly with partial agenesis of the corpus callosum) |
| DGAP268 |
CPEB3 (disrupted) |
disruption of a single gene without known pathogenicity and a cryptic inversion at non-genic breakpoints | unknown, likely to be benign | healthy newborn |
| DGAP285 |
FAM104B (disrupted), MTM1 |
disruption of a single gene without known pathogenicity; disruption of a TBR with potential dysregulation of a gene associated with X-linked myotubular myopathy, a prenatal-onset fatal disease | unknown, likely to be pathogenic | intrauterine fetal demise (overlapping findings with X-linked myotubular myopathy include decreased fetal movements, hydrocephalus, and stillbirth) |
| DGAP288 | SOX9 | non-genic breakpoints with dysregulation of a gene with a low haploinsufficiency index and known to be associated with Pierre Robin sequence | pathogenic | Pierre Robin sequence |
| DGAP290 |
HECW2 (disrupted), NUP205 (disrupted) |
disruption of two genes without known pathogenicity; non-genic cryptic inversion in one of the breakpoints | unknown, likely to be benign | termination after communication of sequencing results |
| DGAP295 | GFPT1 (disrupted), IGF2 | complex rearrangement with potential dysregulation of an imprinted gene associated with Silver-Russell syndrome (pathogenic) and a recessively inherited syndromic gene (noncontributory) | pathogenic | small birth weight and failure to thrive (findings consistent with Silver-Russell syndrome) |
DGAP239
DGAP23930 (46,XY,t(6;8)(q13;q13)dn.arr(1-22)x2,(XY)x1.seq[GRCh37/hg19] t(6;8)(q13;q12.2)dn) had multisystemic abnormalities detected by imaging studies starting in the second trimester and was diagnosed clinically with CHARGE syndrome (MIM: 214800) only after birth. Sequencing the prenatal DNA sample identified translocation breakpoints (designated as t(6;8)(q13;q13) by karyotyping) disrupting CHD7 (MIM: 608892) at 8q12.2 and LMBRD1 (MIM: 612625) at 6q13 (Figure 2A and Table 4). Whereas biallelic losses of LMBRD1 are associated with methylmalonic aciduria and homocystinuria, cblF type (MIM: 277380) (no phenotypic overlap with DGAP239),42 monoallelic loss of CHD7 is well known to be associated with CHARGE syndrome (it is mutated in more than 90% of subjects), correlating with the low haploinsufficiency index of CHD7 and the clinical outcome of DGAP239 (see Supplemental Note and Tables S3 and S4).43
DGAP247
DGAP247 (46,XY,inv(8)(q13q24.1)dn.arr(1-22)x2,(XY)x1.seq[GRCh37/hg19] inv(8)(q11.21q24.23)dn) had normal prenatal findings without complications during the perinatal period. At 31 months of age, he continues to be healthy. Sequencing of the prenatal DNA sample identified inversion breakpoints (designated as inv(8)(q13q24.1) by karyotyping) within a non-genic region at 8q11.2 and disruption of KHDRBS3 (MIM: 610421) at 8q24.23 (Figure 2B and Table 4). Although KHDRBS3 has a borderline haploinsufficiency index and showed decreased RNA expression in the prenatal sample (see Supplemental Note, Figures S1 and S2, and Tables S5 and S6), it is not reported to be associated with a developmental role and/or abnormal phenotype, and no additional genes located in the rearranged TADs have been implicated in a phenotype or developmental role, correlating with the normal clinical phenotype.
DGAP248
DGAP248 (46,XY,t(2;13)(p13;q14)dn.arr(1-22)x2,(XY)x1.seq[GRCh37/hg19] t(2;13)(p12;q13.2)dn) had normal first-trimester screening. At 19.4 weeks, the pregnancy was terminated before the sequencing results were available. Sequencing of the prenatal DNA sample identified translocation breakpoints (designated as t(2;13)(p13;q14) by karyotyping) within a non-genic region at 2p12 and disrupting RFC3 (MIM: 600405) at 13q13.2 (Figure 1C). The 2p12 breakpoint is located within a TAD that includes LRRTM4 (MIM: 610870), a gene with a low haploinsufficiency index and no reported abnormal phenotypic association. However, structure and expression profiles of LRRTM mRNAs in mice suggest a role in development and maintenance of the vertebrate nervous system.44 RFC3 has a low haploinsufficiency index and showed decreased RNA expression in the prenatal sample (Figure S3).36 In addition, NBEA (MIM: 6084889), a candidate autism gene with a low haploinsufficiency score,45, 46 is located within the same 2.16 Mb TAD and 973 kb downstream of the breakpoints (Figure 2C and Table 4). Given the presence of two genes with low haploinsufficiency indices—one associated with a phenotype and located within the 13q13.2 rearrangement TAD (NBEA) and the other implicated in nervous system development and located within the 2p12 rearrangement TAD (LRRTM4)—but the lack of strong evidence for a phenotypic correlation, these results are interpreted as “unknown clinical significance. Clinical follow-up was not possible because the pregnancy was terminated (see Supplemental Note and Tables S7 and S8). Of note, the pregnancy was terminated prior to communication of the sequencing results on the basis of an informed decision after karyotyping, CMA, and genetic counseling.
DGAP258
DGAP258 (46,XY,inv(6)(p23q13)dn.arr(1-22)x2,(XY)x1.seq[GRCh37/hg19] inv(6)(p25.3q16.1)dn(q15q15)pat or 46,XY,inv(6)(p23q13)dn.arr(1-22)x2,(XY)x1.seq[GRCh37/hg19] inv(6)(p25.3q16.1)dn,inv(6)(q15q15)pat) was a monozygotic twin pregnancy, and amniocentesis was performed as a result of abnormal first-trimester serum screening. Other than minor complications due to a twin pregnancy, there were no abnormal clinical findings during the perinatal period. At 2.5 years of age, the twins continue to be healthy. Sequencing of the prenatal DNA sample identified inversion breakpoints (designated as inv(6)(p23q13) by karyotyping) within non-genic regions at both 6p25.3 and 6q16.1. In addition, a paternally inherited cryptic non-genic rearrangement at 6q15 was detected (Figure 2D and Table 4). Because of the length of the sequencing reads, it was not possible to determine whether both of the breakpoints on 6q reside in the same paternally inherited chromosome; however, given their relative proximity and localization within the same 2.21 Mb TAD, this is a likely possibility. Analysis of protein-coding genes localized in the same TAD as the breakpoints did not reveal any additional genes associated with an abnormal phenotype or a developmental role, correlating with the normal clinical phenotype of DGAP258 (see Supplemental Note and Tables S9 and S10).
DGAP259
DGAP25931 (46,XX,t(3;18;5;7)(p25;p11.2;q13.3;q32),t(9;18)(p22;q21)dn.arr(1-22,X)x2.seq[GRCh37/hg19](3,5,7,9,18)cx,der(3)t(3;7)(p24.3;q36.3)dn,der(5)t(5;7)(q14.3;q35)t(3;7)(p24.3;q36.3)t(3;18)(p26.3;p11.31)dn,der(7)t(5;7)dn,der(9)t(9;18)(p23;q21.3)dn,der(18)t(3;18)inv(18)(p11.31q21.3)t(9;18)dn) had abnormal prenatal findings of bilateral ventriculomegaly and colpocephaly with partial agenesis of the corpus callosum and a complex amniotic fluid karyotype designated as 46,XX,t(3;18;5;7)(p25;p11.2;q13.3;q32),t(9;18)(p22;q21)dn. The pregnancy was terminated at 22 weeks as a result of the abnormal findings. Sequencing of the prenatal DNA sample identified nine rearrangement sequences located at 3p26.3, 3p24.3, 5q14.3, 7q35, 7q36.3, 9p23, 18p11.31, and 18q21.3 with small deletions and duplications less than 1 kb (Figure 3 and Table 5). Among six disrupted protein-coding genes, TBC1D5 (MIM: 615740) and CNTNAP2 (MIM: 604569) reside in the vicinity of well-known genome-organizer- and chromatin-regulator-encoding regions—SATB1 (MIM: 602075)50 and EZH2 (MIM: 601573)61 at 3p24.3 and 7q35, respectively—which might be relevant to the complex chromosomal aberration of DGAP259 (all four of these genes are predicted to have low haploinsufficiency indices). Breakpoints at 7q36.3 disrupt the regulatory region of SHH, which has a low haploinsufficiency index. Monoallelic disruption of this SHH regulatory region is associated with holoprosencephaly,26 which is consistent with the cerebral malformation phenotype of DGAP259. Breakpoints at 5q14.3 are located within the same TAD as MEF2C (MIM: 600662), another gene that has a low haploinsufficiency index and is associated with cerebral malformation and hypoplastic corpus callosum,47, 54 as observed in DGAP259 (see Supplemental Note and Tables S11–S18).
DGAP268
DGAP268 (46,XY,inv(10)(p13q24)dn.arr(1-22)x2,(XY)x1.seq[GRCh37/hg19] inv(10)(p12.2p12.31)(p12.2q23.32)dn) had abnormal nuchal translucency detected in the first trimester, and there were no complications during the perinatal period. At 1 year of age, he continues to be healthy. Sequencing of the prenatal DNA sample identified a complex inversion with breakpoints (designated as inv(10)(p13q24) by karyotyping) within non-genic regions at 10p12.31 and 10p12.2 and disruption CPEB3 (MIM: 610606) at 10q23.32 (Figure 4A and Table 6). CPEB3 does not have a low haploinsufficiency index and does not have any abnormal phenotypic association. Analysis of protein-coding genes localized in the same TAD as the breakpoints also did not reveal any genes associated with an abnormal phenotype, correlating with the normal clinical phenotype of DGAP268 (see Supplemental Note and Tables S19–S21).
DGAP285
DGAP285 (46,Y,inv(X)(p11.2q28).arr(1-22)x2,(XY)x1.seq[GRCh37/hg19] inv(X)(p11.21q28)) showed abnormal prenatal imaging findings, including hydrocephalus, starting at 22.5 weeks and fetal demise at 31.4 weeks after decreased fetal movements. Sequencing of the prenatal DNA sample identified inversion breakpoints (designated as inv(X)(p11.2q28) by karyotyping) disrupting FAM104B at Xp11.21 and within a non-genic region at Xq28 (Figure 4B and Table 6). Breakpoints at Xq28 disrupt a TBR, which could result in genomic rewiring of the surrounding TADs and TBRs. MTM1 (MIM: 300415) is an X-linked recessively inherited gene associated with centronuclear myopathy (MIM: 310400), a prenatal-onset fatal disease with clinical findings including decreased fetal movements, hydrocephalus, and stillbirth.73, 74, 75 MTM1 is located in a TBR upstream of the TBR at the Xq28 rearrangement, and therefore dysregulation of MTM1 might contribute to the phenotype of DGAP285 (see Supplemental Note and Tables S22 and S23).
DGAP288
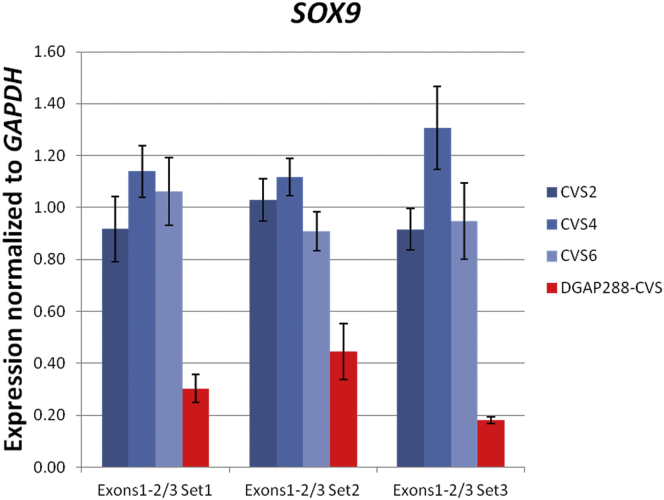
DGAP288 (46,XX,t(6;17)(q13;q21)dn.arr(1-22,X)x2.seq[GRCh37/hg19] t(6;17)(q21;q24.3)dn) had cystic hygroma at 11.1 weeks, followed by prenatal imaging findings consistent with Pierre Robin sequence, which were confirmed during the postnatal period. Sequencing of the prenatal DNA sample identified translocation breakpoints (designated as t(6;17)(q13;q21) by karyotyping) within non-genic regions at 6q21 and 17q24.3 (Figure 4C and Table 6). Breakpoints at 17q24.3 were in a 1.88 Mb TAD corresponding to an upstream cis-regulatory region of SOX9 (MIM: 608160), a region known to be associated with Pierre Robin sequence as a result of dysregulation of SOX9, an autosomal-dominantly inherited gene with a low haploinsufficiency index.25, 28, 29 The prenatal sample showed decreased RNA expression of SOX9 (Figure 5), correlating with the clinical outcome of DGAP288 (see Supplemental Note and Tables S24 and S25).
Figure 5.
SOX9 Expression of DGAP288
Decreased expression of SOX9 in the chorionic villus sample (CVS) of DGAP288 in comparison to three CVS controls (three different primer sets were used for the expression assessment of exons 1 and 2 out of 3, normalized to GAPDH). Error bars represent the SE of the normalized ratios.
DGAP290
DGAP290 (46,XY,t(2;7)(q33;q32)dn.arr(1-22)x2,(XY)x1.seq[GRCh37/hg19](2,7)cx,der(2)t(2;7)(q32.3;q33)inv(7)(q33q33)dn,der(7)t(2;7)dn) was a high-risk pregnancy according to first-trimester screening, which showed normal imaging up to 18 weeks. The parents decided to terminate the pregnancy at 23 weeks because of uncertainty of the clinical significance of the balanced rearrangement. Sequencing of the prenatal DNA sample identified translocation breakpoints (designated as t(2;7)(q33;q32) by karyotyping) disrupting HECW2 at 2q32.3 and NUP205 (MIM: 614352) at 7q33 and an additional non-genic disruption at 7q33 (Figure 4D and Table 6). Neither disrupted gene had a low haploinsufficiency index, and analysis of protein-coding genes in the same TAD as the breakpoints did not reveal any genes associated with an abnormal phenotype. These results are interpreted as “unknown clinical significance, likely to be benign”; however, clinical correlation was not possible because the pregnancy was terminated (see Supplemental Note and Tables S26 and S27).
DGAP295
DGAP295 (46,XY,t(2;11)(p13.1;p15.5)dn.arr(1-22)x2,(XY)x1.seq[GRCh37/hg19](2,11)cx,der(2)inv(11)(p15.5)inv(11)(p15.5)t(2;11)(p13.3;p15.5)dn,der(11)t(2;11)dn) had abnormal first-trimester screening, which showed an abnormal prenatal imaging finding of growth restriction starting from 19 weeks, and weighed 450 g upon delivery at 31 weeks. Sequencing of the prenatal DNA sample identified translocation breakpoints (designated as t(2;11)(p13.1;p15.5) by karyotyping) disrupting GFPT1 (MIM: 138292) at 2p13.3 and multiple non-genic regions at 11p15.5 within a 70 kb distribution (Figure 4E and Table 6). The complex breakpoints at 11p15.5 are within the same 600 kb TAD as IGF2 (MIM: 147470), an imprinted region known to be associated with growth restriction with distinctive facies (GRDF [MIM: 616489])71 and Silver-Russell syndrome (MIM: 180860),72 consistent with the growth restricted phenotype of DGAP295 (see Supplemental Note and Tables S28 and S29).
Discussion
We report whole-genome sequencing of ten prenatal subjects with balanced chromosomal rearrangements with “normal” CMA results and their phenotypic interpretation through publicly available resources. Each subject has contributed uniquely to our experience in the evolution of this approach to a new standard of care in prenatal diagnosis by providing further insight into prognosis through incorporation of an understanding of the regulatory genome (Table 7).
In the evaluation of the pathogenic outcomes of balanced rearrangements, disruption or dysregulation of a single allele is of particular significance when it involves a region known to be hemizygous for X-linked traits, haploinsufficient (autosomal dominant), or imprinted and associated with an abnormal phenotype. Next-generation sequencing can identify the disrupted regions at the nucleotide level; however, predicting the dysregulation of the genes in the vicinity of the breakpoints is more challenging. Advances in the understanding of large-scale regulatory chromatin domains (TADs) contribute to overcoming this obstacle. A recent study analyzing the WNT6-IHH-EPHA4-PAX3 locus and three related congenital genetic disorders has provided multiple layers of evidence for the significance of these megabase-sized regulatory domains and their contribution to abnormal phenotypes through genomic rewiring of the regulatory boundaries resulting from structural rearrangements.20 It is well established that the cis-regulatory elements for many key developmental genes can extend beyond the transcription unit in the range of 120 kb to 1.5 Mb,15, 16, 17, 76, 77 which could be explained by these regulatory associations. Therefore, we analyzed the aforementioned characteristics (hemizygosity, haploinsufficiency, and imprinting) of the disrupted genes at the breakpoints, as well as the protein-coding genes located in the regulatory domains and boundaries (TADs and TBRs, respectively) associated with the breakpoints to identify the dysregulated regions. Then, we evaluated the phenotypic and developmental significance of these genes of interest. None of the three subjects with normal outcomes (DGAP247, DGAP258, and DGAP268) had disrupted genes or were predicted to have dysregulated genes involved with an abnormal phenotype. Among five subjects with abnormal outcomes, one (DGAP239) had a disrupted syndromic gene with a low haploinsufficiency index, one (DGAP285) had a disrupted TBR and was predicted to have a dysregulated X-linked recessively inherited syndromic gene, one (DGAP288) had a dysregulated gene involved with an abnormal phenotype, one (DGAP295) was predicted to have a dysregulated imprinted gene involved with a syndrome, and lastly, in one chromothripsis-affected subject (DGAP259), multiple genes associated with CNS malformations and genomic organization were disrupted and predicted to be dysregulated. All showed abnormal phenotypes overlapping the predicted outcomes of the sequencing results. Of note, two of the five subjects with abnormal phenotypes (DGAP239 and DGAP295) had additional disrupted genes involved in autosomal-recessive syndromes and did not show any clinical features associated with these syndromes. However, in such cases, a potential “carrier” status for the relevant syndromes might be considered in future genetic counseling of the newborn if the outcome is otherwise normal. Among the two terminated pregnancies without any abnormal phenotypes prior to termination, one subject (DGAP248) is interpreted as having a rearrangement predicted to be of unknown clinical significance, and the other (DGAP290) is interpreted has having a rearrangement predicted to be likely benign.
Although karyotyping remains the standard of care for prenatal diagnosis, advances in genomic technologies are rapidly transitioning into clinical practice. Non-invasive cfDNA screening and CMA in invasive testing are increasingly popular methods in the field of prenatal genetics.78, 79, 80 Non-invasive prenatal testing of cfDNA offers tremendous potential as a screening tool, particularly for fetal aneuploidies. Although this next-generation-sequencing-based approach has been shown to reliably demonstrate copy-number variations greater than 5 Mb,81 it currently remains a screening method.2, 3 Current guidelines recommend offering CMA to any woman choosing to undergo prenatal invasive diagnostic testing and recommend CMA as the primary test (replacing conventional karyotype) if the prenatal diagnostic test is performed for an indication of a structural abnormality detected by prenatal imaging studies.33 Nonetheless, CMA cannot assess balanced rearrangements and, if performed alone in the present study, would have “missed” all five prenatal subjects with abnormal outcomes (each of whom had abnormal prenatal imaging findings), including a subject with complex chromothripsis (DGAP259).
Karyotyping remains superior to CMA for the detection of balanced rearrangements, despite its megabase-sized resolution. Next-generation sequencing using large-insert libraries provides precise delineation of the breakpoints of structural rearrangements while detecting additional high-resolution cryptic rearrangements, as well as copy-number alterations that could potentially be detected by CMA and not karyotyping. Although cfDNA screening is also a sequence-based approach, given the fragmented nature of cell-free DNA, it would be cumbersome to analyze truly balanced rearrangements with the current cfDNA technology. Another sequence-based approach in the field of prenatal genetics is whole-exome sequencing.82, 83 Although this method provides higher nucleotide-level coverage and therefore can more reliably detect nucleotide-level mutations in the exome than our large-insert library method, given the presence of non-genic breakpoints in structural rearrangements, a whole-genome paired-end sequencing approach using large-insert libraries, as presented herein, would be most useful in detecting structural rearrangements. Currently, we would recommend using this method in subjects with a normal CMA and a karyotype with a balanced rearrangement (the order of CMA and karyotyping depends on the clinical scenario). In subjects with an abnormal CMA and/or a karyotype without a balanced rearrangement that fails to explain an abnormal phenotype, our method could still be valuable for identifying cryptic rearrangements in the appropriate clinical setting. We believe next-generation sequencing technologies will eventually be proposed as a first-line diagnostic method because they can provide details on structural rearrangements that cannot be detected by either karyotyping or CMA.
As with other genomic testing methods, whole-genome sequencing also raises the issue of variants of unknown clinical significance. The topic of “unknown clinical significance” is not a new problem for the field of prenatal diagnosis, whether it be a subtle imaging finding such as mildly enlarged ventricles or the detection of a balanced chromosomal rearrangement by karyotyping. Sequencing provides additional understanding of the breakpoints involved in a balanced chromosomal rearrangement. Although this information could fundamentally influence genetic counseling, clinical management, and decision making, it could also bring additional pressure to managing unknown findings on the basis of current genomic evidence. Eventually, evolving annotation of the human genome—including the discovery of disease-associated genes or other predictors of regulatory effect, such as pathogenic increases in gene expression—along with guidelines from expert committees, could close these gaps of interpretation, as has been the case with improved clinical reporting of CMA results over the past decade.84
In conclusion, detecting balanced chromosomal rearrangements with whole-genome sequencing provides nucleotide-level precision incomparable to currently employed prenatal genetic-testing methods, thus enabling the regulatory genome to be evaluated in such a way that could prove invaluable in clinical interpretation.
Acknowledgments
We are grateful to the families of the subjects for their participation. We thank Christina Jabbour, Amelia Lindgren, Samantha Schilit, and Drs. Ozden Altiok Clark and Frederick Bieber for their contributions. We dedicate this article in loving memory of Dorothy Warburton, a co-author on this study and the author of an article also published in The Journal,4 which has served as a foundation for the Developmental Genome Anatomy Project. This study was funded by the NIH (GM061354 to C.C.M., M.E.T., and J.F.G.; HD081256 and March of Dimes 6-FY15-255 to M.E.T.).
Published: October 13, 2016
Footnotes
Supplemental Data include a Supplemental Note, 3 figures, and 29 tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.08.022.
Web Resources
DECIPHER, https://decipher.sanger.ac.uk/
OMIM, http://www.omim.org
Supplemental Data
References
- 1.American College of Obstetricians and Gynecologists Committee on Genetics Committee Opinion No. 581: the use of chromosomal microarray analysis in prenatal diagnosis. Obstet. Gynecol. 2013;122:1374–1377. doi: 10.1097/01.AOG.0000438962.16108.d1. [DOI] [PubMed] [Google Scholar]
- 2.(2016). Practice Bulletin No. 162 Summary: Prenatal Diagnostic Testing for Genetic Disorders. Obstet. Gynecol. 127, 976–978. [DOI] [PubMed]
- 3.(2016). Practice Bulletin No. 163: Screening for Fetal Aneuploidy. Obstet. Gynecol. 127, e123–e137. [DOI] [PubMed]
- 4.Warburton D. De novo balanced chromosome rearrangements and extra marker chromosomes identified at prenatal diagnosis: clinical significance and distribution of breakpoints. Am. J. Hum. Genet. 1991;49:995–1013. [PMC free article] [PubMed] [Google Scholar]
- 5.Gribble S.M., Prigmore E., Burford D.C., Porter K.M., Ng B.L., Douglas E.J., Fiegler H., Carr P., Kalaitzopoulos D., Clegg S. The complex nature of constitutional de novo apparently balanced translocations in patients presenting with abnormal phenotypes. J. Med. Genet. 2005;42:8–16. doi: 10.1136/jmg.2004.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Gregori M., Ciccone R., Magini P., Pramparo T., Gimelli S., Messa J., Novara F., Vetro A., Rossi E., Maraschio P. Cryptic deletions are a common finding in “balanced” reciprocal and complex chromosome rearrangements: a study of 59 patients. J. Med. Genet. 2007;44:750–762. doi: 10.1136/jmg.2007.052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sismani C., Kitsiou-Tzeli S., Ioannides M., Christodoulou C., Anastasiadou V., Stylianidou G., Papadopoulou E., Kanavakis E., Kosmaidou-Aravidou Z., Patsalis P.C. Cryptic genomic imbalances in patients with de novo or familial apparently balanced translocations and abnormal phenotype. Mol. Cytogenet. 2008;1:15. doi: 10.1186/1755-8166-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baptista J., Mercer C., Prigmore E., Gribble S.M., Carter N.P., Maloney V., Thomas N.S., Jacobs P.A., Crolla J.A. Breakpoint mapping and array CGH in translocations: comparison of a phenotypically normal and an abnormal cohort. Am. J. Hum. Genet. 2008;82:927–936. doi: 10.1016/j.ajhg.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins A.W., Alkuraya F.S., Bosco A.F., Brown K.K., Bruns G.A., Donovan D.J., Eisenman R., Fan Y., Farra C.G., Ferguson H.L. Characterization of apparently balanced chromosomal rearrangements from the developmental genome anatomy project. Am. J. Hum. Genet. 2008;82:712–722. doi: 10.1016/j.ajhg.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schluth-Bolard C., Delobel B., Sanlaville D., Boute O., Cuisset J.M., Sukno S., Labalme A., Duban-Bedu B., Plessis G., Jaillard S. Cryptic genomic imbalances in de novo and inherited apparently balanced chromosomal rearrangements: array CGH study of 47 unrelated cases. Eur. J. Med. Genet. 2009;52:291–296. doi: 10.1016/j.ejmg.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Gijsbers A.C., Bosch C.A., Dauwerse J.G., Giromus O., Hansson K., Hilhorst-Hofstee Y., Kriek M., van Haeringen A., Bijlsma E.K., Bakker E. Additional cryptic CNVs in mentally retarded patients with apparently balanced karyotypes. Eur. J. Med. Genet. 2010;53:227–233. doi: 10.1016/j.ejmg.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Feenstra I., Hanemaaijer N., Sikkema-Raddatz B., Yntema H., Dijkhuizen T., Lugtenberg D., Verheij J., Green A., Hordijk R., Reardon W. Balanced into array: genome-wide array analysis in 54 patients with an apparently balanced de novo chromosome rearrangement and a meta-analysis. Eur. J. Hum. Genet. 2011;19:1152–1160. doi: 10.1038/ejhg.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korbel J.O., Urban A.E., Affourtit J.P., Godwin B., Grubert F., Simons J.F., Kim P.M., Palejev D., Carriero N.J., Du L. Paired-end mapping reveals extensive structural variation in the human genome. Science. 2007;318:420–426. doi: 10.1126/science.1149504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talkowski M.E., Ernst C., Heilbut A., Chiang C., Hanscom C., Lindgren A., Kirby A., Liu S., Muddukrishna B., Ohsumi T.K. Next-generation sequencing strategies enable routine detection of balanced chromosome rearrangements for clinical diagnostics and genetic research. Am. J. Hum. Genet. 2011;88:469–481. doi: 10.1016/j.ajhg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanyal A., Lajoie B.R., Jain G., Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–113. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatia S., Kleinjan D.A. Disruption of long-range gene regulation in human genetic disease: a kaleidoscope of general principles, diverse mechanisms and unique phenotypic consequences. Hum. Genet. 2014;133:815–845. doi: 10.1007/s00439-014-1424-6. [DOI] [PubMed] [Google Scholar]
- 17.Kleinjan D.A., van Heyningen V. Long-range control of gene expression: emerging mechanisms and disruption in disease. Am. J. Hum. Genet. 2005;76:8–32. doi: 10.1086/426833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon J.R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., Hu M., Liu J.S., Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sexton T., Cavalli G. The role of chromosome domains in shaping the functional genome. Cell. 2015;160:1049–1059. doi: 10.1016/j.cell.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 20.Lupiáñez D.G., Kraft K., Heinrich V., Krawitz P., Brancati F., Klopocki E., Horn D., Kayserili H., Opitz J.M., Laxova R. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161:1012–1025. doi: 10.1016/j.cell.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatia S., Bengani H., Fish M., Brown A., Divizia M.T., de Marco R., Damante G., Grainger R., van Heyningen V., Kleinjan D.A. Disruption of autoregulatory feedback by a mutation in a remote, ultraconserved PAX6 enhancer causes aniridia. Am. J. Hum. Genet. 2013;93:1126–1134. doi: 10.1016/j.ajhg.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto T., Togawa M., Shimada S., Sangu N., Shimojima K., Okamoto N. Narrowing of the responsible region for severe developmental delay and autistic behaviors in WAGR syndrome down to 1.6cMb including PAX6, WT1, and PRRG4. Am. J. Med. Genet. A. 2014;164A:634–638. doi: 10.1002/ajmg.a.36325. [DOI] [PubMed] [Google Scholar]
- 23.Lehnhardt A., Karnatz C., Ahlenstiel-Grunow T., Benz K., Benz M.R., Budde K., Büscher A.K., Fehr T., Feldkötter M., Graf N. Clinical and molecular characterization of patients with heterozygous mutations in wilms tumor suppressor gene 1. Clin. J. Am. Soc. Nephrol. 2015;10:825–831. doi: 10.2215/CJN.10141014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai J., Goodman B.K., Patel A.S., Mulliken J.B., Van Maldergem L., Hoganson G.E., Paznekas W.A., Ben-Neriah Z., Sheffer R., Cunningham M.L. Increased risk for developmental delay in Saethre-Chotzen syndrome is associated with TWIST deletions: an improved strategy for TWIST mutation screening. Hum. Genet. 2003;114:68–76. doi: 10.1007/s00439-003-1012-7. [DOI] [PubMed] [Google Scholar]
- 25.Gordon C.T., Tan T.Y., Benko S., Fitzpatrick D., Lyonnet S., Farlie P.G. Long-range regulation at the SOX9 locus in development and disease. J. Med. Genet. 2009;46:649–656. doi: 10.1136/jmg.2009.068361. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez B.A., Siegel-Bartelt J., Herbrick J.A., Teshima I., Scherer S.W. Holoprosencephaly and cleidocranial dysplasia in a patient due to two position-effect mutations: case report and review of the literature. Clin. Genet. 2005;68:349–359. doi: 10.1111/j.1399-0004.2005.00498.x. [DOI] [PubMed] [Google Scholar]
- 27.de Kok Y.J., Vossenaar E.R., Cremers C.W., Dahl N., Laporte J., Hu L.J., Lacombe D., Fischel-Ghodsian N., Friedman R.A., Parnes L.S. Identification of a hot spot for microdeletions in patients with X-linked deafness type 3 (DFN3) 900 kb proximal to the DFN3 gene POU3F4. Hum. Mol. Genet. 1996;5:1229–1235. doi: 10.1093/hmg/5.9.1229. [DOI] [PubMed] [Google Scholar]
- 28.Benko S., Fantes J.A., Amiel J., Kleinjan D.J., Thomas S., Ramsay J., Jamshidi N., Essafi A., Heaney S., Gordon C.T. Highly conserved non-coding elements on either side of SOX9 associated with Pierre Robin sequence. Nat. Genet. 2009;41:359–364. doi: 10.1038/ng.329. [DOI] [PubMed] [Google Scholar]
- 29.Amarillo I.E., Dipple K.M., Quintero-Rivera F. Familial microdeletion of 17q24.3 upstream of SOX9 is associated with isolated Pierre Robin sequence due to position effect. Am. J. Med. Genet. A. 2013;161A:1167–1172. doi: 10.1002/ajmg.a.35847. [DOI] [PubMed] [Google Scholar]
- 30.Talkowski M.E., Ordulu Z., Pillalamarri V., Benson C.B., Blumenthal I., Connolly S., Hanscom C., Hussain N., Pereira S., Picker J. Clinical diagnosis by whole-genome sequencing of a prenatal sample. N. Engl. J. Med. 2012;367:2226–2232. doi: 10.1056/NEJMoa1208594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macera M.J., Sobrino A., Levy B., Jobanputra V., Aggarwal V., Mills A., Esteves C., Hanscom C., Pereira S., Pillalamarri V. Prenatal diagnosis of chromothripsis, with nine breaks characterized by karyotyping, FISH, microarray and whole-genome sequencing. Prenat. Diagn. 2015;35:299–301. doi: 10.1002/pd.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills R.E., Walter K., Stewart C., Handsaker R.E., Chen K., Alkan C., Abyzov A., Yoon S.C., Ye K., Cheetham R.K., 1000 Genomes Project Mapping copy number variation by population-scale genome sequencing. Nature. 2011;470:59–65. doi: 10.1038/nature09708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudmant P.H., Rausch T., Gardner E.J., Handsaker R.E., Abyzov A., Huddleston J., Zhang Y., Ye K., Jun G., Hsi-Yang Fritz M., 1000 Genomes Project Consortium An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. doi: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brand H., Collins R.L., Hanscom C., Rosenfeld J.A., Pillalamarri V., Stone M.R., Kelley F., Mason T., Margolin L., Eggert S. Paired-Duplication Signatures Mark Cryptic Inversions and Other Complex Structural Variation. Am. J. Hum. Genet. 2015;97:170–176. doi: 10.1016/j.ajhg.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brand H., Pillalamarri V., Collins R.L., Eggert S., O’Dushlaine C., Braaten E.B., Stone M.R., Chambert K., Doty N.D., Hanscom C. Cryptic and complex chromosomal aberrations in early-onset neuropsychiatric disorders. Am. J. Hum. Genet. 2014;95:454–461. doi: 10.1016/j.ajhg.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang N., Lee I., Marcotte E.M., Hurles M.E. Characterising and predicting haploinsufficiency in the human genome. PLoS Genet. 2010;6:e1001154. doi: 10.1371/journal.pgen.1001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amberger J.S., Bocchini C.A., Schiettecatte F., Scott A.F., Hamosh A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43:D789–D798. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Firth H.V., Richards S.M., Bevan A.P., Clayton S., Corpas M., Rajan D., Van Vooren S., Moreau Y., Pettett R.M., Carter N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009;84:524–533. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bragin E., Chatzimichali E.A., Wright C.F., Hurles M.E., Firth H.V., Bevan A.P., Swaminathan G.J. DECIPHER: database for the interpretation of phenotype-linked plausibly pathogenic sequence and copy-number variation. Nucleic Acids Res. 2014;42:D993–D1000. doi: 10.1093/nar/gkt937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ordulu Z., Wong K.E., Currall B.B., Ivanov A.R., Pereira S., Althari S., Gusella J.F., Talkowski M.E., Morton C.C. Describing sequencing results of structural chromosome rearrangements with a suggested next-generation cytogenetic nomenclature. Am. J. Hum. Genet. 2014;94:695–709. doi: 10.1016/j.ajhg.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiratsuchi T., Nishimori H., Ichise H., Nakamura Y., Tokino T. Cloning and characterization of BAI2 and BAI3, novel genes homologous to brain-specific angiogenesis inhibitor 1 (BAI1) Cytogenet. Cell Genet. 1997;79:103–108. doi: 10.1159/000134693. [DOI] [PubMed] [Google Scholar]
- 42.Rutsch F., Gailus S., Miousse I.R., Suormala T., Sagné C., Toliat M.R., Nürnberg G., Wittkampf T., Buers I., Sharifi A. Identification of a putative lysosomal cobalamin exporter altered in the cblF defect of vitamin B12 metabolism. Nat. Genet. 2009;41:234–239. doi: 10.1038/ng.294. [DOI] [PubMed] [Google Scholar]
- 43.Janssen N., Bergman J.E., Swertz M.A., Tranebjaerg L., Lodahl M., Schoots J., Hofstra R.M., van Ravenswaaij-Arts C.M., Hoefsloot L.H. Mutation update on the CHD7 gene involved in CHARGE syndrome. Hum. Mutat. 2012;33:1149–1160. doi: 10.1002/humu.22086. [DOI] [PubMed] [Google Scholar]
- 44.Laurén J., Airaksinen M.S., Saarma M., Timmusk T. A novel gene family encoding leucine-rich repeat transmembrane proteins differentially expressed in the nervous system. Genomics. 2003;81:411–421. doi: 10.1016/s0888-7543(03)00030-2. [DOI] [PubMed] [Google Scholar]
- 45.Castermans D., Wilquet V., Parthoens E., Huysmans C., Steyaert J., Swinnen L., Fryns J.P., Van de Ven W., Devriendt K. The neurobeachin gene is disrupted by a translocation in a patient with idiopathic autism. J. Med. Genet. 2003;40:352–356. doi: 10.1136/jmg.40.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wise A., Tenezaca L., Fernandez R.W., Schatoff E., Flores J., Ueda A., Zhong X., Wu C.F., Simon A.F., Venkatesh T. Drosophila mutants of the autism candidate gene neurobeachin (rugose) exhibit neuro-developmental disorders, aberrant synaptic properties, altered locomotion, and impaired adult social behavior and activity patterns. J. Neurogenet. 2015;29:135–143. doi: 10.3109/01677063.2015.1064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nuytens K., Gantois I., Stijnen P., Iscru E., Laeremans A., Serneels L., Van Eylen L., Liebhaber S.A., Devriendt K., Balschun D. Haploinsufficiency of the autism candidate gene Neurobeachin induces autism-like behaviors and affects cellular and molecular processes of synaptic plasticity in mice. Neurobiol. Dis. 2013;51:144–151. doi: 10.1016/j.nbd.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Lee S., Takeda Y., Kawano H., Hosoya H., Nomoto M., Fujimoto D., Takahashi N., Watanabe K. Expression and regulation of a gene encoding neural recognition molecule NB-3 of the contactin/F3 subgroup in mouse brain. Gene. 2000;245:253–266. doi: 10.1016/s0378-1119(00)00031-7. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez T., Morgan T., Davis N., Klin A., Morris A., Farhi A., Lifton R.P., State M.W. Disruption of contactin 4 (CNTN4) results in developmental delay and other features of 3p deletion syndrome. Am. J. Hum. Genet. 2004;74:1286–1293. doi: 10.1086/421474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohwi-Shigematsu T., Kohwi Y., Takahashi K., Richards H.W., Ayers S.D., Han H.J., Cai S. SATB1-mediated functional packaging of chromatin into loops. Methods. 2012;58:243–254. doi: 10.1016/j.ymeth.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvarez J.D., Yasui D.H., Niida H., Joh T., Loh D.Y., Kohwi-Shigematsu T. The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev. 2000;14:521–535. [PMC free article] [PubMed] [Google Scholar]
- 52.Balamotis M.A., Tamberg N., Woo Y.J., Li J., Davy B., Kohwi-Shigematsu T., Kohwi Y. Satb1 ablation alters temporal expression of immediate early genes and reduces dendritic spine density during postnatal brain development. Mol. Cell. Biol. 2012;32:333–347. doi: 10.1128/MCB.05917-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Close J., Xu H., De Marco García N., Batista-Brito R., Rossignol E., Rudy B., Fishell G. Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J. Neurosci. 2012;32:17690–17705. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Meur N., Holder-Espinasse M., Jaillard S., Goldenberg A., Joriot S., Amati-Bonneau P., Guichet A., Barth M., Charollais A., Journel H. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J. Med. Genet. 2010;47:22–29. doi: 10.1136/jmg.2009.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbosa A.C., Kim M.S., Ertunc M., Adachi M., Nelson E.D., McAnally J., Richardson J.A., Kavalali E.T., Monteggia L.M., Bassel-Duby R., Olson E.N. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc. Natl. Acad. Sci. USA. 2008;105:9391–9396. doi: 10.1073/pnas.0802679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Middendorp S., Paoletti A., Schiebel E., Bornens M. Identification of a new mammalian centrin gene, more closely related to Saccharomyces cerevisiae CDC31 gene. Proc. Natl. Acad. Sci. USA. 1997;94:9141–9146. doi: 10.1073/pnas.94.17.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arking D.E., Cutler D.J., Brune C.W., Teslovich T.M., West K., Ikeda M., Rea A., Guy M., Lin S., Cook E.H., Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am. J. Hum. Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zweier C., de Jong E.K., Zweier M., Orrico A., Ousager L.B., Collins A.L., Bijlsma E.K., Oortveld M.A., Ekici A.B., Reis A. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am. J. Hum. Genet. 2009;85:655–666. doi: 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu Z.K., Gervais J.L., Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc. Natl. Acad. Sci. USA. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Meglio T., Kratochwil C.F., Vilain N., Loche A., Vitobello A., Yonehara K., Hrycaj S.M., Roska B., Peters A.H., Eichmann A. Ezh2 orchestrates topographic migration and connectivity of mouse precerebellar neurons. Science. 2013;339:204–207. doi: 10.1126/science.1229326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen H., Rossier C., Antonarakis S.E. Cloning of a human homolog of the Drosophila enhancer of zeste gene (EZH2) that maps to chromosome 21q22.2. Genomics. 1996;38:30–37. doi: 10.1006/geno.1996.0588. [DOI] [PubMed] [Google Scholar]
- 62.Belloni E., Muenke M., Roessler E., Traverso G., Siegel-Bartelt J., Frumkin A., Mitchell H.F., Donis-Keller H., Helms C., Hing A.V. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat. Genet. 1996;14:353–356. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- 63.Anderson E., Devenney P.S., Hill R.E., Lettice L.A. Mapping the Shh long-range regulatory domain. Development. 2014;141:3934–3943. doi: 10.1242/dev.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choucair N., Mignon-Ravix C., Cacciagli P., Abou Ghoch J., Fawaz A., Mégarbané A., Villard L., Chouery E. Evidence that homozygous PTPRD gene microdeletion causes trigonocephaly, hearing loss, and intellectual disability. Mol. Cytogenet. 2015;8:39. doi: 10.1186/s13039-015-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uetani N., Kato K., Ogura H., Mizuno K., Kawano K., Mikoshiba K., Yakura H., Asano M., Iwakura Y. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 2000;19:2775–2785. doi: 10.1093/emboj/19.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zweier C., Peippo M.M., Hoyer J., Sousa S., Bottani A., Clayton-Smith J., Reardon W., Saraiva J., Cabral A., Gohring I. Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins syndrome) Am. J. Hum. Genet. 2007;80:994–1001. doi: 10.1086/515583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawabe H., Sakisaka T., Yasumi M., Shingai T., Izumi G., Nagano F., Deguchi-Tawarada M., Takeuchi M., Nakanishi H., Takai Y. A novel rabconnectin-3-binding protein that directly binds a GDP/GTP exchange protein for Rab3A small G protein implicated in Ca(2+)-dependent exocytosis of neurotransmitter. Genes Cells. 2003;8:537–546. doi: 10.1046/j.1365-2443.2003.00655.x. [DOI] [PubMed] [Google Scholar]
- 68.Gao S., Alarcón C., Sapkota G., Rahman S., Chen P.Y., Goerner N., Macias M.J., Erdjument-Bromage H., Tempst P., Massagué J. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol. Cell. 2009;36:457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buj-Bello A., Biancalana V., Moutou C., Laporte J., Mandel J.L. Identification of novel mutations in the MTM1 gene causing severe and mild forms of X-linked myotubular myopathy. Hum. Mutat. 1999;14:320–325. doi: 10.1002/(SICI)1098-1004(199910)14:4<320::AID-HUMU7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 70.Senderek J., Müller J.S., Dusl M., Strom T.M., Guergueltcheva V., Diepolder I., Laval S.H., Maxwell S., Cossins J., Krause S. Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am. J. Hum. Genet. 2011;88:162–172. doi: 10.1016/j.ajhg.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Begemann M., Zirn B., Santen G., Wirthgen E., Soellner L., Büttel H.M., Schweizer R., van Workum W., Binder G., Eggermann T. Paternally Inherited IGF2 Mutation and Growth Restriction. N. Engl. J. Med. 2015;373:349–356. doi: 10.1056/NEJMoa1415227. [DOI] [PubMed] [Google Scholar]
- 72.Gicquel C., Rossignol S., Cabrol S., Houang M., Steunou V., Barbu V., Danton F., Thibaud N., Le Merrer M., Burglen L. Epimutation of the telomeric imprinting center region on chromosome 11p15 in Silver-Russell syndrome. Nat. Genet. 2005;37:1003–1007. doi: 10.1038/ng1629. [DOI] [PubMed] [Google Scholar]
- 73.Jungbluth H., Wallgren-Pettersson C., Laporte J. Centronuclear (myotubular) myopathy. Orphanet J. Rare Dis. 2008;3:26. doi: 10.1186/1750-1172-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joseph M., Pai G.S., Holden K.R., Herman G. X-linked myotubular myopathy: clinical observations in ten additional cases. Am. J. Med. Genet. 1995;59:168–173. doi: 10.1002/ajmg.1320590211. [DOI] [PubMed] [Google Scholar]
- 75.Starr J., Lamont M., Iselius L., Harvey J., Heckmatt J. A linkage study of a large pedigree with X linked centronuclear myopathy. J. Med. Genet. 1990;27:281–283. doi: 10.1136/jmg.27.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeong Y., El-Jaick K., Roessler E., Muenke M., Epstein D.J. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development. 2006;133:761–772. doi: 10.1242/dev.02239. [DOI] [PubMed] [Google Scholar]
- 77.Gordon C.T., Attanasio C., Bhatia S., Benko S., Ansari M., Tan T.Y., Munnich A., Pennacchio L.A., Abadie V., Temple I.K. Identification of novel craniofacial regulatory domains located far upstream of SOX9 and disrupted in Pierre Robin sequence. Hum. Mutat. 2014;35:1011–1020. doi: 10.1002/humu.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bianchi D.W., Parker R.L., Wentworth J., Madankumar R., Saffer C., Das A.F., Craig J.A., Chudova D.I., Devers P.L., Jones K.W., CARE Study Group DNA sequencing versus standard prenatal aneuploidy screening. N. Engl. J. Med. 2014;370:799–808. doi: 10.1056/NEJMoa1311037. [DOI] [PubMed] [Google Scholar]
- 79.Norton M.E., Jacobsson B., Swamy G.K., Laurent L.C., Ranzini A.C., Brar H., Tomlinson M.W., Pereira L., Spitz J.L., Hollemon D. Cell-free DNA analysis for noninvasive examination of trisomy. N. Engl. J. Med. 2015;372:1589–1597. doi: 10.1056/NEJMoa1407349. [DOI] [PubMed] [Google Scholar]
- 80.Wapner R.J., Martin C.L., Levy B., Ballif B.C., Eng C.M., Zachary J.M., Savage M., Platt L.D., Saltzman D., Grobman W.A. Chromosomal microarray versus karyotyping for prenatal diagnosis. N. Engl. J. Med. 2012;367:2175–2184. doi: 10.1056/NEJMoa1203382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arbabi A., Rampášek L., Brudno M. Cell-free DNA fragment-size distribution analysis for non-invasive prenatal CNV prediction. Bioinformatics. 2016;32:1662–1669. doi: 10.1093/bioinformatics/btw178. [DOI] [PubMed] [Google Scholar]
- 82.Drury S., Williams H., Trump N., Boustred C., Lench N., Scott R.H., Chitty L.S., GOSGene Exome sequencing for prenatal diagnosis of fetuses with sonographic abnormalities. Prenat. Diagn. 2015;35:1010–1017. doi: 10.1002/pd.4675. [DOI] [PubMed] [Google Scholar]
- 83.Pangalos C., Hagnefelt B., Lilakos K., Konialis C. First applications of a targeted exome sequencing approach in fetuses with ultrasound abnormalities reveals an important fraction of cases with associated gene defects. PeerJ. 2016;4:e1955. doi: 10.7717/peerj.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kearney H.M., Thorland E.C., Brown K.K., Quintero-Rivera F., South S.T., Working Group of the American College of Medical Genetics Laboratory Quality Assurance Committee American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet. Med. 2011;13:680–685. doi: 10.1097/GIM.0b013e3182217a3a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.