Abstract
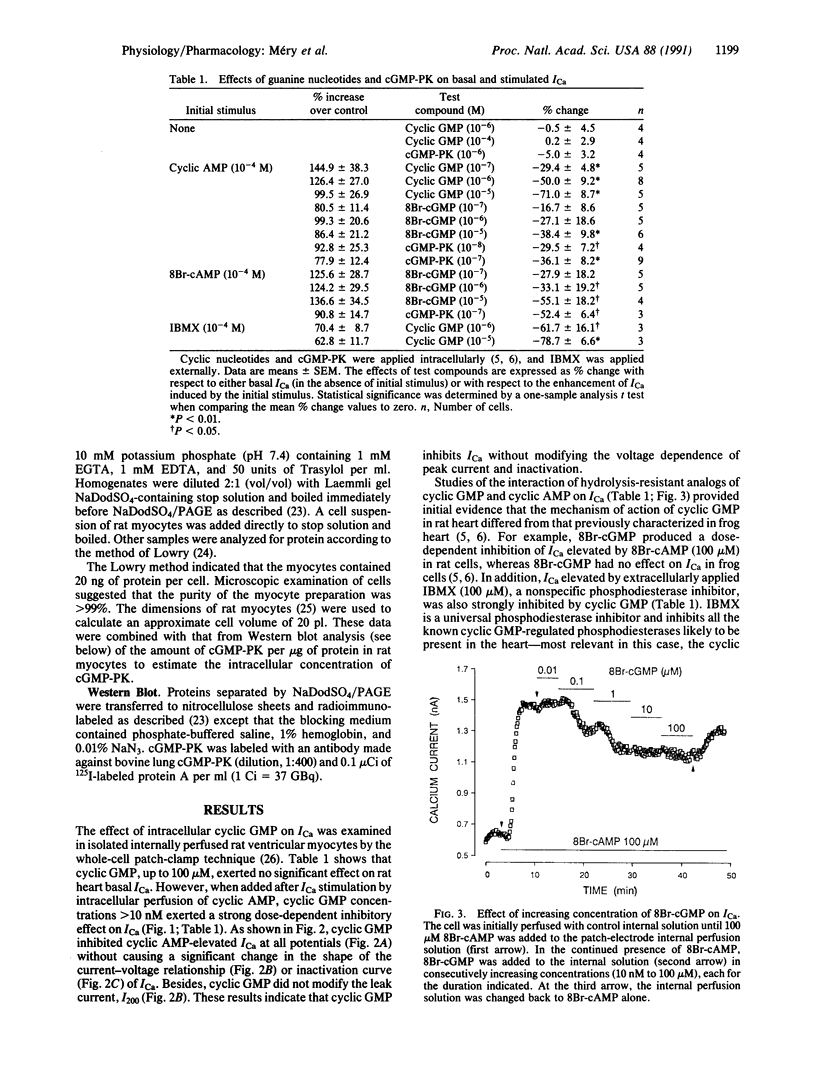
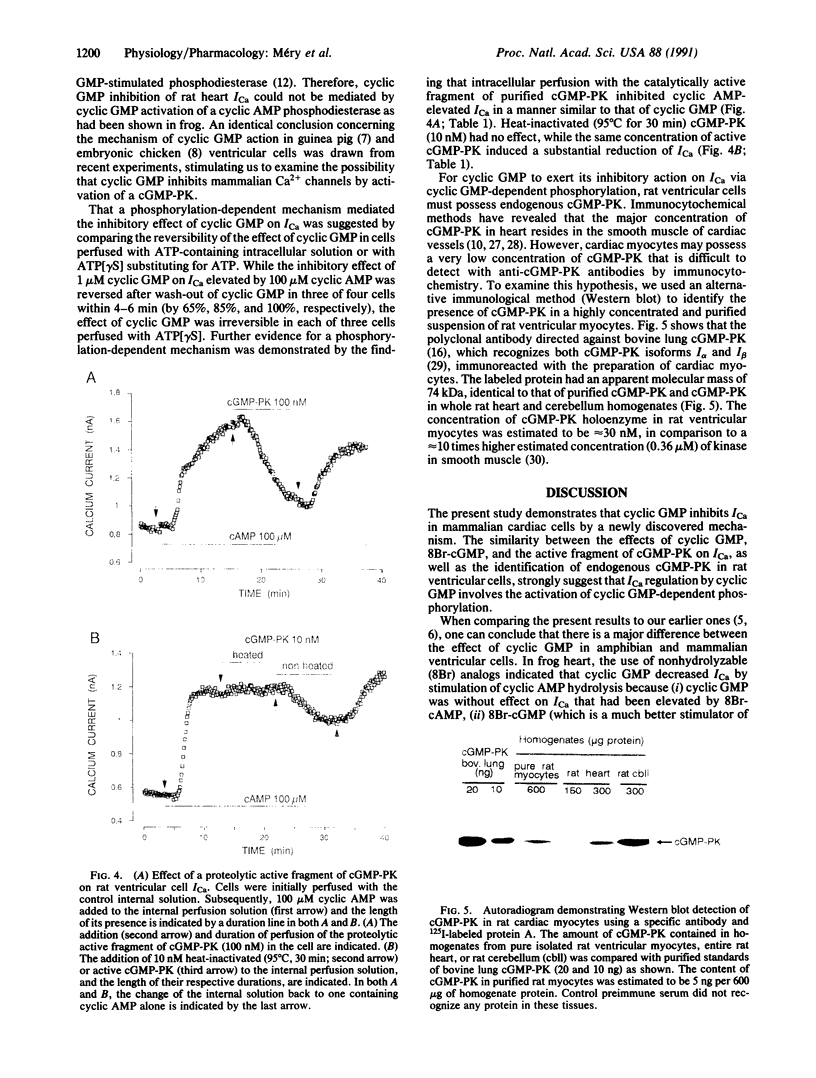
Regulation of cardiac contraction by neurotransmitters and hormones is often correlated with regulation of the L-type Ca2(+)-channel current (ICa) through the opposite actions of two second messengers, cyclic AMP and cyclic GMP. While cyclic AMP stimulation of ICa is mediated by the activation of cyclic AMP-dependent protein kinase, inhibition of ICa by cyclic GMP in frog heart is largely mediated by activation of cyclic AMP phosphodiesterase. The present patch-clamp study reveals that, in rat ventricular cells, cyclic GMP can also regulate ICa via activation of endogenous cyclic GMP-dependent protein kinase (cGMP-PK). Indeed, the effect of cyclic GMP on ICa was mimicked by intracellular perfusion with the proteolytic active fragment of purified cGMP-PK. Moreover, cGMP-PK immunoreactivity was detected in pure rat ventricular myocytes by using a specific polyclonal antibody. These results demonstrate a dual mechanism for the inhibitory action of cyclic GMP in heart, as well as a physiological role for cGMP-PK in the control of mammalian heart function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Burt J. M., Spray D. C. Inotropic agents modulate gap junctional conductance between cardiac myocytes. Am J Physiol. 1988 Jun;254(6 Pt 2):H1206–H1210. doi: 10.1152/ajpheart.1988.254.6.H1206. [DOI] [PubMed] [Google Scholar]
- Butt E., van Bemmelen M., Fischer L., Walter U., Jastorff B. Inhibition of cGMP-dependent protein kinase by (Rp)-guanosine 3',5'-monophosphorothioates. FEBS Lett. 1990 Apr 9;263(1):47–50. doi: 10.1016/0014-5793(90)80702-k. [DOI] [PubMed] [Google Scholar]
- Cramb G., Banks R., Rugg E. L., Aiton J. F. Actions of atrial natriuretic peptide (ANP) on cyclic nucleotide concentrations and phosphatidylinositol turnover in ventricular myocytes. Biochem Biophys Res Commun. 1987 Nov 13;148(3):962–970. doi: 10.1016/s0006-291x(87)80226-7. [DOI] [PubMed] [Google Scholar]
- Ecker T., Göbel C., Hullin R., Rettig R., Seitz G., Hofmann F. Decreased cardiac concentration of cGMP kinase in hypertensive animals. An index for cardiac vascularization? Circ Res. 1989 Nov;65(5):1361–1369. doi: 10.1161/01.res.65.5.1361. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felbel J., Trockur B., Ecker T., Landgraf W., Hofmann F. Regulation of cytosolic calcium by cAMP and cGMP in freshly isolated smooth muscle cells from bovine trachea. J Biol Chem. 1988 Nov 15;263(32):16764–16771. [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic guanosine 3',5'-monophosphate regulates the calcium current in single cells from frog ventricle. J Physiol. 1987 Jun;387:453–472. doi: 10.1113/jphysiol.1987.sp016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Regulation of calcium current by low-Km cyclic AMP phosphodiesterases in cardiac cells. Mol Pharmacol. 1990 Sep;38(3):426–433. [PubMed] [Google Scholar]
- Flitney F. W., Singh J. Evidence that cyclic GMP may regulate cyclic AMP metabolism in the isolated frog ventricle. J Mol Cell Cardiol. 1981 Nov;13(11):963–979. doi: 10.1016/0022-2828(81)90472-7. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert M. P., Fischmeister R. Atrial natriuretic factor regulates the calcium current in frog isolated cardiac cells. Circ Res. 1988 Apr;62(4):660–667. doi: 10.1161/01.res.62.4.660. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Heil W. G., Landgraf W., Hofmann F. A catalytically active fragment of cGMP-dependent protein kinase. Occupation of its cGMP-binding sites does not affect its phosphotransferase activity. Eur J Biochem. 1987 Oct 1;168(1):117–121. doi: 10.1111/j.1432-1033.1987.tb13395.x. [DOI] [PubMed] [Google Scholar]
- Jahn H., Nastainczyk W., Röhrkasten A., Schneider T., Hofmann F. Site-specific phosphorylation of the purified receptor for calcium-channel blockers by cAMP- and cGMP-dependent protein kinases, protein kinase C, calmodulin-dependent protein kinase II and casein kinase II. Eur J Biochem. 1988 Dec 15;178(2):535–542. doi: 10.1111/j.1432-1033.1988.tb14480.x. [DOI] [PubMed] [Google Scholar]
- Joyce N. C., DeCamilli P., Boyles J. Pericytes, like vascular smooth muscle cells, are immunocytochemically positive for cyclic GMP-dependent protein kinase. Microvasc Res. 1984 Sep;28(2):206–219. doi: 10.1016/0026-2862(84)90018-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi R. C., Alloatti G., Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea pig ventricular myocytes. Pflugers Arch. 1989 Apr;413(6):685–687. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- Light D. B., Corbin J. D., Stanton B. A. Dual ion-channel regulation by cyclic GMP and cyclic GMP-dependent protein kinase. Nature. 1990 Mar 22;344(6264):336–339. doi: 10.1038/344336a0. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., Schwoch G., Reiser G., Port R., Walter U. Dibutyryl cAMP treatment of neuroblastoma-glioma hybrid cells results in selective increase in cAMP-receptor protein (R-I) as measured by monospecific antibodies. EMBO J. 1983;2(2):153–159. doi: 10.1002/j.1460-2075.1983.tb01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U., Miller P. E., Greengard P., De Camilli P. Immunohistochemical localization of cyclic GMP-dependent protein kinase in mammalian brain. Proc Natl Acad Sci U S A. 1981 Jan;78(1):653–657. doi: 10.1073/pnas.78.1.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall D., Fried T. A. Effect of atriopeptin II on Ca influx, contractile behavior and cyclic nucleotide content of cultured neonatal rat myocardial cells. J Mol Cell Cardiol. 1990 Feb;22(2):201–212. doi: 10.1016/0022-2828(90)91116-o. [DOI] [PubMed] [Google Scholar]
- Méry P. F., Brechler V., Pavoine C., Pecker F., Fischmeister R. Glucagon stimulates the cardiac Ca2+ current by activation of adenylyl cyclase and inhibition of phosphodiesterase. Nature. 1990 May 10;345(6271):158–161. doi: 10.1038/345158a0. [DOI] [PubMed] [Google Scholar]
- Nawrath H. Does cyclic GMP mediate the negative inotropic effect of acetylcholine in the heart? Nature. 1977 May 5;267(5606):72–74. doi: 10.1038/267072a0. [DOI] [PubMed] [Google Scholar]
- Paupardin-Tritsch D., Hammond C., Gerschenfeld H. M., Nairn A. C., Greengard P. cGMP-dependent protein kinase enhances Ca2+ current and potentiates the serotonin-induced Ca2+ current increase in snail neurones. 1986 Oct 30-Nov 5Nature. 323(6091):812–814. doi: 10.1038/323812a0. [DOI] [PubMed] [Google Scholar]
- Puceat M., Clement O., Lechene P., Pelosin J. M., Ventura-Clapier R., Vassort G. Neurohormonal control of calcium sensitivity of myofilaments in rat single heart cells. Circ Res. 1990 Aug;67(2):517–524. doi: 10.1161/01.res.67.2.517. [DOI] [PubMed] [Google Scholar]
- Ruth P., Röhrkasten A., Biel M., Bosse E., Regulla S., Meyer H. E., Flockerzi V., Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989 Sep 8;245(4922):1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Sandberg M., Natarajan V., Ronander I., Kalderon D., Walter U., Lohmann S. M., Jahnsen T. Molecular cloning and predicted full-length amino acid sequence of the type I beta isozyme of cGMP-dependent protein kinase from human placenta. Tissue distribution and developmental changes in rat. FEBS Lett. 1989 Sep 25;255(2):321–329. doi: 10.1016/0014-5793(89)81114-7. [DOI] [PubMed] [Google Scholar]
- Scamps F., Mayoux E., Charlemagne D., Vassort G. Calcium current in single cells isolated from normal and hypertrophied rat heart. Effects of beta-adrenergic stimulation. Circ Res. 1990 Jul;67(1):199–208. doi: 10.1161/01.res.67.1.199. [DOI] [PubMed] [Google Scholar]
- Sorbera L. A., Morad M. Atrionatriuretic peptide transforms cardiac sodium channels into calcium-conducting channels. Science. 1990 Feb 23;247(4945):969–973. doi: 10.1126/science.2154853. [DOI] [PubMed] [Google Scholar]
- Wahler G. M., Rusch N. J., Sperelakis N. 8-Bromo-cyclic GMP inhibits the calcium channel current in embryonic chick ventricular myocytes. Can J Physiol Pharmacol. 1990 Apr;68(4):531–534. doi: 10.1139/y90-076. [DOI] [PubMed] [Google Scholar]
- Walter U. Cyclic-GMP-regulated enzymes and their possible physiological functions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:249–258. [PubMed] [Google Scholar]
- Walter U., Miller P., Wilson F., Menkes D., Greengard P. Immunological distinction between guanosine 3':5'-monophosphate-dependent and adenosine 3':5'-monophosphate-dependent protein kinases. J Biol Chem. 1980 Apr 25;255(8):3757–3762. [PubMed] [Google Scholar]
- Walter U. Physiological role of cGMP and cGMP-dependent protein kinase in the cardiovascular system. Rev Physiol Biochem Pharmacol. 1989;113:41–88. doi: 10.1007/BFb0032675. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., White R. L., Ginzberg R. D., Spray D. C. Effect of calcium on the dissociation of the mature rat heart into individual and paired myocytes: electrical properties of cell pairs. Circ Res. 1986 Aug;59(2):143–150. doi: 10.1161/01.res.59.2.143. [DOI] [PubMed] [Google Scholar]