ABSTRACT
The adult central nervous system (CNS) was considered a comparatively static tissue with little cell turnover. It is now well established that there is more plasticity than previously thought and that astrocytes act as neural stem/precursor cells (NSPCs) in the subventricular zone (SVZ). The discovery that these NSPCs can give rise to a limited number of new neurons, reactive astrocytes and oligodendrocytes contributing to brain repair in CNS disease, has raised hopes toward harnessing these cells for therapeutic interventions. Here, we will discuss the transcriptional control of adult NSPC differentiation into astrocytes in CNS disease focusing on the helix-loop-helix transcription factor protein family. In our recent study, we reported that elevated BMP-2 levels are translated into an increase in Id3 expression in adult NSPC subpopulations after cortical injury. Id3 then heterodimerizes with the basic helix-loop-helix transcription factor E47 and releases the E47‐mediated repression of astrocyte‐specific gene expression. Consequently, adult NSPCs preferentially differentiate into astrocytes. We believe that understanding the in vivo differentiation potential and the molecular underpinnings of NSPCs in the adult mammalian brain will help us to evaluate their contributions to brain repair and may lead to new concepts in treating human CNS diseases.
KEYWORDS: astrocyte-specific genes, basic helix-loop-helix transcription factor, bone morphogenetic protein, Id protein, Notch, traumatic brain injury, vascular damage
Introduction
Interest in astrocyte function has increased dramatically in recent years. In the healthy adult central nervous system (CNS), several astrocyte functions are well accepted and include roles for astrocytes in synapse formation and function1,2 as well as contributions to blood-brain barrier (BBB) formation and integrity,3 and neurovascular coupling.4 In addition to their essential functions in the healthy CNS, astrocytes respond to CNS insults through a process called astrogliosis. Many aspects of astrocyte responses to CNS damage and disease have been summarized in excellent reviews.5-7 Astrocyte activation and scar formation was mainly associated with exerting inhibitory effects on several aspects of neuroplasticity and CNS axon regeneration.5,8 Today, it is evident that astrogliosis and scar formation also exerts numerous essential beneficial functions that improve functional outcome, including neuronal protection, BBB repair and restriction of CNS inflammation.9,10 Moreover, the dogma that the astrocyte scar inhibits CNS regeneration was recently challenged by demonstrating that scars can facilitate damaged axon regeneration.11 Nevertheless, understanding the underlying molecular mechanisms of astrogliosis and scar formation is the fundament for future therapeutic interventions to promote CNS regeneration.
Recent studies have begun to uncover a marked heterogeneity of astrocyte reactions to CNS injury, including cell morphology, regionalization and cellular function.12-17 Beside the heterogeneous response of local, individual astrocytes to brain injury, a new source of reactive astrocytes derived from a distant brain region has been identified. In CNS disease, neural stem/precursor cells (NSPCs) leave their stem cell niche in the subventricular zone (SVZ) and migrate to the damaged brain area, where they eventually differentiate into reactive astrocytes that contribute to the glial scar surrounding the injury core.18-20 However, the molecular underpinnings and functional significance of adult NSPC differentiation into newborn astrocytes in CNS disease are still poorly known.
Inhibitor of DNA binding (Id) proteins function as crucial regulators of NSPC fate determination during development.21,22 Id proteins (Id1‐4) are helix-loop-helix (HLH) proteins that act as dominant‐negative transcriptional regulators by binding to basic HLH (bHLH) transcription factors (TFs) of the E protein (class I bHLH) family (E47, E12, HEB, and E2‐2). Id proteins lack the basic DNA binding region, and thus, DNA binding of an E‐Id protein heterodimer is prevented.23 In nonlymphoid developmental systems, E proteins have the ability to bind as heterodimers with class II bHLH proteins, such as the achaete-scute family (e.g. Mash1), neurogenin family (Ngn1 and Ngn2), and NeuroD family (NeuroD1) members, which all have been described to be essential for proper differentiation and specification of NSPCs during embryonic development.24
Id protein expression is triggered rapidly by different stimuli, which include TGF‐β superfamily proteins, cytokines, and extracellular matrix proteins.25-28 Thus, Id proteins function as central hubs in translating changes of the extracellular environment, as they occur in CNS disease, into cellular responses. Yet, the implication of the HLH family in the regulation of the adult NSPC fate in CNS disease is only poorly described.
HLH protein family expression in adult NSPCs of the SVZ
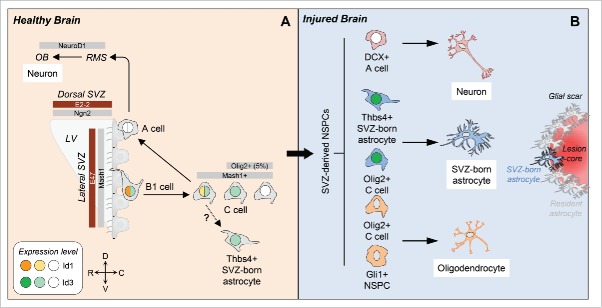
NSPCs in the SVZ constitute a heterogeneous cell population, consisting of B1 stem cells that continuously generate mobile type A neuroblasts via type C transit amplifying cells.29 High Id1 expression levels specifically define type B1 cells, the population of GFAP+ astrocytes with stem cell attributes among the SVZ astrocytes in the adult brain (Fig. 1A). Id1 expression then gradually decreases along the subventricular NSPC lineages, with no expression of Id1 in neuroblasts or neurons.30 For Id3, our results show that it is expressed at basal levels in almost all B1 and C cells and in only a small percentage of DCX+ neuroblasts in the SVZ.19 A recent study showed that the adult SVZ directly generates a small population of astrocytes, which are residing in the niche and are identified by their high expression of the glycoprotein thrombospondin 4 (Thbs4).18 In these cells, Id3 is also expressed at basal levels (Fig. 1A). Migrating DCX+ neuroblasts in the rostral migratory stream (RMS) are Id3-negative (Fig. 2), suggesting that low Id levels are mandatory for NSPC differentiation toward the neuronal lineage.
Figure 1.
The role of Id proteins in adult subventricular zone neural stem/precursor cell fate. (A) In the healthy brain, the transcriptional network is in homeostatic balance to promote neurogenesis throughout adult life. Our results show that in the adult SVZ, Id3 (light green) is expressed in almost all B1 and C cells, but only in a small percentage of type A neuroblasts (A cell, white). Id3 expression further segregates the Olig2+ C cell population into an Olig2+Id3+ and an Olig2+Id3- population. Furthermore, Id3 is expressed in almost all Thbs4+ SVZ-born astrocytes (light green). High Id1 expression levels (orange) specifically define the B1 stem cells and its expression decreases in C cells (yellow) with no expression in type A neuroblasts (white). The E proteins E47 and E2-2 (brown) and the class II bHLH transcription factors Mash1 and Ngn2 (gray) demonstrate regionally defined expression patterns in the SVZ. (B) In the injured brain, our study reveals that elevated Id3 levels (dark green) in Olig2+ C cells and Thbs4+ SVZ-born astrocytes override the transcriptional activity of the bHLH transcription factor E47 promoting NSPC differentiation into astrocytes. C, caudal; D, dorsal; LV, lateral ventricle; NSPCs, neural stem/precursor cells; OB, olfactory bulb; R, rostral; RMS, rostral migratory stream; SVZ, subventricular zone; V, ventral.
Figure 2.
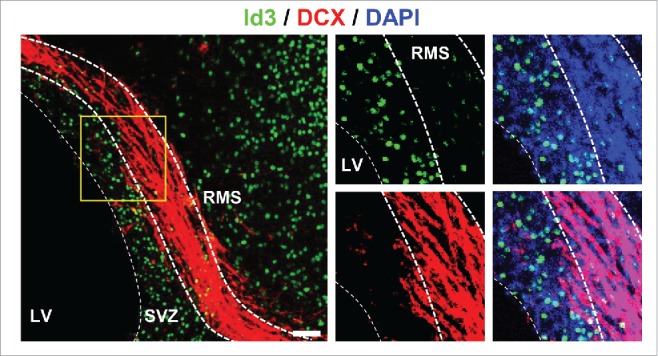
Id3 is not expressed in DCX+ type A neuroblasts in the RMS. Representative image of uninjured WT mice immunolabeled for Id3 (green) and DCX (red, marker for type A neuroblasts). Nuclei are stained with DAPI (blue). LV, lateral ventricle; RMS, rostral migratory stream; SVZ, subventricular zone. Scale bar: 50 µm.
Mash1+ C cells continuously generate mobile neuroblasts finally differentiating into interneuron populations in the olfactory bulb. However, a minor subpopulation of C cells characterized by expression of the bHLH TF Olig2 is capable to leave the stem cell niche and to differentiate into astrocytes and oligodendrocytes.31,32 We found that this subpopulation can be further segregated according to its Id3 expression into Olig2+Id3+ and Olig2+Id3- NSPCs, suggesting further heterogeneity among the C cell subpopulation (Fig. 1A). A detailed expression of Id2 and Id4 in subventricular NSPCs has not been described yet.
The expression analysis of members of the E protein family and the class II bHLH TF family in adult NSPC subpopulations of the SVZ is still incomplete. Transcriptome analyses of regionally isolated and purified SVZ-derived NSPCs revealed E protein E2-2 to be enriched in the dorsal SVZ with a suggested role in the control of NSPC proliferation and specification (Fig. 1A).33 The role of HEB in adult NSPCs is not known, but mRNA expression was detected in the dorsal SVZ.34
Our study revealed that E47 is expressed in various NSPC subpopulations at the lateral SVZ. Interestingly, B1 stem cells show the lowest percentage of E47+ cells (15%) and Thbs4+ newborn astrocytes in the SVZ show the highest percentage of E47+ cells (90%). As only a small percentage of B1 stem cells express E47, Id proteins might have different functions in these cells independent of their role as dominant‐negative transcriptional regulators of E proteins. The E protein interaction partner Mash1 is mainly expressed in mitotic C cells and is required for the generation of both neurons and oligodendrocytes,35 while expression of Ngn2 and NeuroD1 in Mash1+ precursors directs their differentiation into olfactory bulb interneurons in the healthy brain.36,37 Although we found equally distributed Id3 expression in the SVZ along the dorsoventral and anteroposterior axes in healthy animals, E proteins and pro-neurogenic bHLH TFs demonstrate overlapping, but generally discrete and transient expression patterns (Fig. 1A),33-35,38,39 suggesting spatially restricted transcriptional programs in the SVZ governing variance of NSPC progeny. Until now, Id protein expression in the human SVZ has not been examined, but their role as transcriptional regulators in cancer stem cell identity, such as in glioma stem cells, is evident.40
Recent literature examined the contribution of SVZ-derived NSPCs to tissue repair in different CNS diseases and showed that, depending on the molecular identity of the NSPCs, these SVZ-derived NSPCs can differentiate into neurons, oligodendrocytes and astrocytes (Fig. 1B).18-20,41,42 Interestingly, our data showed that upon cortical injury, Id3 was upregulated rapidly in Thbs4+ newborn astrocytes and Olig2+ C cells in the SVZ. The Id3 expression persisted in these SVZ-derived cells, which migrated toward the lesion area and differentiated into astrocytes (Fig. 1B). Gli1+ NSPCs and also Olig2+ C cells are capable to differentiate into oligodendrocytes.31,41 While we did not investigate Id3 expression in the Gli1+ cell population, we showed that the Olig2+Id3+ C cell population differentiates into astrocytes and we assume that the Olig2+Id3- cell population might be the source for oligodendrocyte differentiation (Fig. 1B). Interestingly, SVZ-derived NSPCs that differentiate into migrating DCX+ neuroblasts in the RMS do not express Id3 in the healthy brain (Fig. 2). It would be interesting to examine whether SVZ-derived NSPCs that migrate to the injured brain area and differentiate into newborn neurons are Id3-negative and if Id3 depletion is mandatory for increased neurogenesis in the injured lesion area. Overall, these data suggest that elevated levels of Id3 expression direct NSPC subpopulation toward astrocytes.
A role for E proteins and their pro-neurogenic partners of the class II bHLH TF family in adult NSPC differentiation into astrocytes after brain injury has not been characterized, yet. However, few studies provide strong evidence for an implication of bHLH TFs in the transcriptional control of SVZ NSPC fate contributing to brain repair in CNS disease. Either bHLH TF overexpression or functional abrogation suggests to be sufficient to direct cell fate. In this context, Mash1 and Olig2 are driving NSPC fate conversion toward the neuronal or oligodendrocyte lineage, respectively in the healthy and the injured brain.20,39,43,44
We identified the E protein E47 as a repressor of adult NSPC differentiation into astrocytes. We showed that E47 repressed a subset of astrocyte-specific genes by direct binding to regulatory regions of these genes in E47-overexpressing primary NSPCs and by genetic depletion of E47, which resulted in increased adult NSPC differentiation into astrocytes. Immunolabeling with an E47-detecting antibody revealed that E47 expression in NSPC subpopulations of the SVZ was not significantly altered after cortical injury compared to uninjured mice. Therefore, bHLH TFs regulate different stages of NSPC differentiation. However, their transcriptional activity can be overridden by temporally elevated Id expression in CNS disease.
Potential molecular mechanisms of adult NSPC differentiation into astrocytes in CNS disease
Unlike pro-neurogenic bHLH TFs, such as Mash1, Ngn2, and NeuroD, pro-astrogenic bHLH TFs have currently not been identified. Our data suggest that in the heathy brain E47 represses a subset of astrocyte-specific genes allowing the continuous lineage progression of NSPCs into newborn neurons in the olfactory bulb. After cortical injury, elevated BMP-2 levels are translated into an increase in Id3 expression in specific NSPC subpopulations. In these cells, Id3 heterodimerizes with E47 and prevents E47-mediated repression of astrocyte-specific genes, such as the astrocyte marker GFAP and the solute carrier family member GLAST. Consequently, the NSPCs with elevated Id3 levels preferentially differentiate into astrocytes (Fig. 3A).
Figure 3.
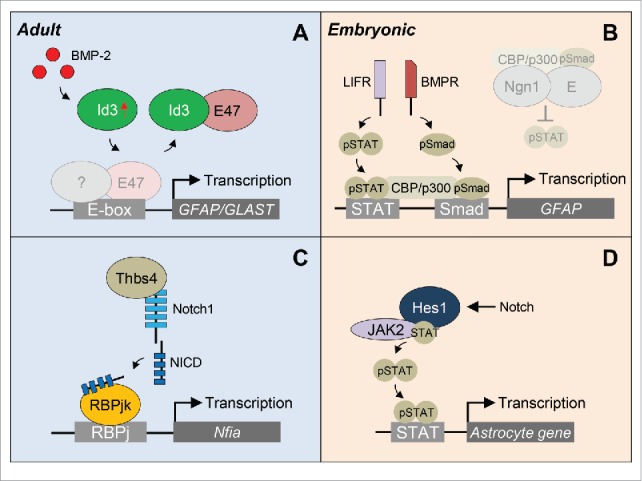
Proposed models of transcriptional regulation of NSPC differentiation into astrocytes. (A) After traumatic brain injury, elevated BMP-2 levels in the SVZ are translated into an increase in Id3 expression. Id3 then heterodimerizes with E47 and prevents the E47-mediated repression of astrocyte-specfic genes GFAP and GLAST. (B) During embryogenesis, pro-neurogenic Ngn1 inhibits astrogenesis by preventing STAT phosphorylation and occupying the CBP/p300 cofactor complex (gray shadow), the latter synergistically bridging the STAT and Smad signaling pathways. When neurogenesis is completed, Ngn1 is downregulated and in the absence of Ngn1, pSTAT recruits the CBP/p300-pSmad complex to the promoter to activate GFAP gene expression. (C) Focal stroke increases the number of Thbs4+ SVZ-born astrocytes. Thbs4 binds directly to the Notch1 receptor activating Notch signaling and expression of the Nfia transcription factor important for astrocyte differentiation. (D) During embryogenesis, Notch effector Hes1 associates with JAK2 and STAT to facilitate JAK-STAT complex formation, thus promoting STAT phosphorylation and astrocyte gene expression. BMPR, BMP receptor; LIFR, LIF receptor; NICD, Notch intracellular domain.
Transient expression of pro-neurogenic bHLH TFs, as well as the variable accessibility to astrocyte loci due to epigenetic modifications during development45,46 suggest further complexity of the molecular mechanisms governing adult NSPC fate in CNS disease. Although epigenetic mechanisms have already been described to play a critical role in stemness and lineage specification in the adult SVZ in the healthy brain, information about epigenetic modifiers of NSPC fate determination in CNS disease are still lacking.47 Investigations on embryonic NSPC differentiation into astrocytes have mainly focused on the transcriptional regulation of the GFAP promoter. Here, the timed expression of the pro-neurogenic bHLH TF Ngn1 is suggested to determine the cell fate switch between neurogenesis and astrogenesis.48 Mechanistically, Ngn1 promotes neuronal differentiation and simultaneously inhibits astrogenesis by occupying the CBP/p300 cofactor complex, which synergistically bridges the STAT and Smad signaling pathways important for inducing astrogenesis. In the presence of Ngn1, BMP stimulation is thought to promote the association of the cofactor-pSmad complex with Ngn1 to induce neurogenesis, thus converting the BMP signal from a glial-inducing cue to a neuronal-inducing cue. In the absence of Ngn1, the cofactor-pSmad complex can be recruited to the GFAP gene via pSTAT, thereby permitting gene expression and differentiation into astrocytes (Fig. 3B). As we detected binding of E47 to an E-Box in the GFAP promoter, E47 might also actively repress the expression of the GFAP gene during embryogenesis in addition to the function of the pro-neurogenic Ngn1 as a scavenger of cofactors. Furthermore, expression of Ngn1 in Mash1+ C cells in the adult SVZ might direct these cells toward the neuronal lineage beside the inhibitory astrocyte differentiation function of E47.
Beside the transcriptional control of bHLH TFs, Notch signaling has important implications in NSPC fate determination. After photothrombotic cortical injury, the number of newborn astrocytes in the SVZ increases. These cells are characterized by their high expression levels of Thbs4, which is an adhesive glycoprotein that mediates cell-to-cell and cell-to-matrix interactions.18 These SVZ-newborn Thbs4+ astrocytes respond to injury and are capable to migrate long distances to the lesion area. Here, Thbs4 has been shown to bind to the Notch1 receptor and subsequent pathway activation leads to increased expression of the transcription factor Nfia important for further astrocyte differentiation (Fig. 3C). Interestingly, we also found elevated Id3 levels in these Thbs4+ astrocytes after cortical injury, which might probably further sustain astrocyte identity and function. However, although Notch activity and the Id3-E47 axis share the regulation of astrocyte genes, they modulate different target genes, such as the solute carrier family and Nfia. In addition, our microarray gene expression analyses revealed that Cadherin 20, Mmp14, EGFR and FGFR3 were down-regulated in BMP-treated Id3−/− NSPCs,19 all genes potentially involved in migration. Therefore, variable combination of active pathways regulating astrocyte differentiation could ultimately specify distinct astrocyte subtypes with different functions for brain repair.
During embryonic development, the Notch effector Hes1 has been demonstrated to facilitate STAT activation by binding to STAT and JAK2, ultimately promoting astrocyte differentiation (Fig. 3D).49 In the adult SVZ, Hes1 and Hes5 expression is limited to the B1 cells.50,51 Furthermore, Notch signaling is thought to specifically promote the proliferation and maintenance of the activated B1 cell pool.52 However, it would be intriguing to know, whether Notch signaling is extended to other SVZ NSPC subpopulations under pathologic conditions, such as the Olig2+ C cells, and whether Hes and Id proteins exert synergistic or different functionality in NSPC differentiation into astrocytes in CNS disease.
Conclusion
Lineage plasticity and heterogeneity of adult NSPCs of the SVZ in CNS disease has important implications for the development of cell replacement strategies. However, we still do not know much about the extracellular cues, the fine-tuned transcriptional network, and the regional molecular identity of NSPC subpopulations in the SVZ stem cell niche that determine SVZ-derived newborn astrocyte morphology and function in CNS disease.
Our findings revealed that the bHLH TF E47 acts as a repressor of NSPC differentiation into astrocytes and that under pathological conditions, BMP-2-induced high Id3 expression in a subset of NSPCs captures E47 releasing its repression of astrocyte-specific gene transcription and directing the SVZ-derived NSPCs toward the lesion area and astrocyte differentiation. We propose that the altered NSPC environment upon cortical injury is a key event in directing NSPC differentiation53 and that rapid and robust upregulation of Id3 acts as the central player for overriding NSPC intrinsic programming resulting in NSPC differentiation into astrocytes. Cytokines of the TGF-β superfamily, which regulate the Id abundance, have been described to be elevated in lesion areas of mouse models for CNS disease and in human CNS pathologies.54-57 Future studies will show whether human CNS diseases alter the SVZ stem cell environment and regulate Id3 levels to direct NSPC differentiation into astrocytes.
An emerging concept is to control NSPC fate and functions tailored to the different pathological conditions at the site of tissue damage for CNS repair.58 Distinct transcriptional programs of NSPCs within regionally separated SVZ microdomains offer a high variability of NSPC responses in a disease-specific context, which can be further modulated by rapid changes of transcriptional regulators, such as by the Id protein family. Thus, a targeted therapy against specific Id proteins to modulate adult NSPC identity could be a beneficial therapeutic intervention for brain repair in the future.
In summary, understanding the in vivo differentiation potential and the molecular underpinnings of neural and glial precursor cells in the embryonic and adult mammalian brain will help us to evaluate their contributions to neural repair and may lead to new concepts in treating human CNS diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the International Graduate Academy fellowship to C.B., and the Fritz Thyssen Stiftung, and the German Research Foundation Grants SCHA 1442/3-2, and SCHA 1442/5-1 to C.S.
References
- [1].Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci 2013; 14:311-21; PMID:23595014; http://dx.doi.org/ 10.1038/nrn3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Allen NJ. Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol 2014; 30:439-63; PMID:25288116; http://dx.doi.org/ 10.1146/annurev-cellbio-100913-013053 [DOI] [PubMed] [Google Scholar]
- [3].Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7:41-53; PMID:16371949; http://dx.doi.org/ 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- [4].Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron 2011; 71:782-97; PMID:21903073; http://dx.doi.org/ 10.1016/j.neuron.2011.08.009 [DOI] [PubMed] [Google Scholar]
- [5].Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 2010; 119:7-35; PMID:20012068; http://dx.doi.org/ 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol 2014; 253:197-207; PMID:24424280; http://dx.doi.org/ 10.1016/j.expneurol.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 2015; 16:249-63; PMID:25891508; http://dx.doi.org/ 10.1038/nrn3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci 2004; 5:146-56; PMID:14735117; http://dx.doi.org/ 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- [9].Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. Astrocytes: a central element in neurological diseases. Acta Neuropathol 2016; 131:323-45; PMID:26671410; http://dx.doi.org/ 10.1007/s00401-015-1513-1 [DOI] [PubMed] [Google Scholar]
- [10].Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist 2005; 11:400-7; PMID:16151042; http://dx.doi.org/ 10.1177/1073858405278321 [DOI] [PubMed] [Google Scholar]
- [11].Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016; 532:195-200; PMID:27027288; http://dx.doi.org/ 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, et al.. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 2013; 16:580-6; PMID:23542688; http://dx.doi.org/ 10.1038/nn.3371 [DOI] [PubMed] [Google Scholar]
- [13].Molofsky AV, Deneen B. Astrocyte development: A Guide for the Perplexed. Glia 2015; 63:1320-9; PMID:25963996; http://dx.doi.org/ 10.1002/glia.22836 [DOI] [PubMed] [Google Scholar]
- [14].Molofsky AV, Kelley KW, Tsai HH, Redmond SA, Chang SM, Madireddy L, Chan JR, Baranzini SE, Ullian EM, Rowitch DH. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature 2014; 509:189-94; PMID:24776795; http://dx.doi.org/ 10.1038/nature13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sabelstrom H, Stenudd M, Reu P, Dias DO, Elfineh M, Zdunek S, Damberg P, Goritz C, Frisen J. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science 2013; 342:637-40; PMID:24179227; http://dx.doi.org/ 10.1126/science.1242576 [DOI] [PubMed] [Google Scholar]
- [16].Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, et al.. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 2012; 337:358-62; PMID:22745251; http://dx.doi.org/ 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 2013; 33:12870-86; PMID:23904622; http://dx.doi.org/ 10.1523/JNEUROSCI.2121-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature 2013; 497:369-73; PMID:23615612; http://dx.doi.org/ 10.1038/nature12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bohrer C, Pfurr S, Mammadzada K, Schildge S, Plappert L, Hils M, Pous L, Rauch KS, Dumit VI, Pfeifer D, et al.. The balance of Id3 and E47 determines neural stem/precursor cell differentiation into astrocytes. EMBO J 2015; 34:2804-19; PMID:26438726; http://dx.doi.org/ 10.15252/embj.201591118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Faiz M, Sachewsky N, Gascon S, Bang KW, Morshead CM, Nagy A. Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke. Cell Stem Cell 2015; 17:624-34; PMID:26456685; http://dx.doi.org/ 10.1016/j.stem.2015.08.002 [DOI] [PubMed] [Google Scholar]
- [21].Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, et al.. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 1999; 401:670-7; PMID:10537105; http://dx.doi.org/ 10.1038/44334 [DOI] [PubMed] [Google Scholar]
- [22].Niola F, Zhao X, Singh D, Castano A, Sullivan R, Lauria M, Nam HS, Zhuang Y, Benezra R, Di Bernardo D, et al.. Id proteins synchronize stemness and anchorage to the niche of neural stem cells. Nat Cell Biol 2012; 14:477-87; PMID:22522171; http://dx.doi.org/ 10.1038/ncb2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 1990; 61:49-59; PMID:2156629; http://dx.doi.org/ 10.1016/0092-8674(90)90214-Y [DOI] [PubMed] [Google Scholar]
- [24].Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci 2002; 3:517-30; PMID:12094208; http://dx.doi.org/ 10.1038/nrn874 [DOI] [PubMed] [Google Scholar]
- [25].Benezra R. Role of Id proteins in embryonic and tumor angiogenesis. Trends Cardiovasc Med 2001; 11:237-41; PMID:11673054; http://dx.doi.org/ 10.1016/S1050-1738(01)00117-7 [DOI] [PubMed] [Google Scholar]
- [26].Chen X, Zankl A, Niroomand F, Liu Z, Katus HA, Jahn L, Tiefenbacher C. Upregulation of ID protein by growth and differentiation factor 5 (GDF5) through a smad-dependent and MAPK-independent pathway in HUVSMC. J Mol Cell Cardiol 2006; 41:26-33; PMID:16716349; http://dx.doi.org/ 10.1016/j.yjmcc.2006.03.421 [DOI] [PubMed] [Google Scholar]
- [27].Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 1999; 274:19838-45; PMID:10391928; http://dx.doi.org/ 10.1074/jbc.274.28.19838 [DOI] [PubMed] [Google Scholar]
- [28].Kang Y, Chen CR, Massague J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell 2003; 11:915-26; PMID:12718878; http://dx.doi.org/ 10.1016/S1097-2765(03)00109-6 [DOI] [PubMed] [Google Scholar]
- [29].Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97:703-16; PMID:10380923; http://dx.doi.org/ 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- [30].Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell 2009; 5:515-26; PMID:19896442; http://dx.doi.org/ 10.1016/j.stem.2009.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 2006; 26:7907-18; PMID:16870736; http://dx.doi.org/ 10.1523/JNEUROSCI.1299-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marshall CA, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci 2005; 25:7289-98; PMID:16093378; http://dx.doi.org/ 10.1523/JNEUROSCI.1924-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Azim K, Hurtado-Chong A, Fischer B, Kumar N, Zweifel S, Taylor V, Raineteau O. Transcriptional Hallmarks of Heterogeneous Neural Stem Cell Niches of the Subventricular Zone. Stem Cells 2015; 33:2232-42; PMID:25827345; http://dx.doi.org/ 10.1002/stem.2017 [DOI] [PubMed] [Google Scholar]
- [34].Fischer B, Azim K, Hurtado-Chong A, Ramelli S, Fernandez M, Raineteau O. E-proteins orchestrate the progression of neural stem cell differentiation in the postnatal forebrain. Neural Dev 2014; 9:23; PMID:25352248; http://dx.doi.org/ 10.1186/1749-8104-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J 2004; 23:4495-505; PMID:15496983; http://dx.doi.org/ 10.1038/sj.emboj.7600447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Boutin C, Hardt O, de Chevigny A, Core N, Goebbels S, Seidenfaden R, Bosio A, Cremer H. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc Natl Acad Sci U S A 2010; 107:1201-6; PMID:20080708; http://dx.doi.org/ 10.1073/pnas.0909015107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci 2009; 12:1090-2; PMID:19701197; http://dx.doi.org/ 10.1038/nn.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Azim K, Fiorelli R, Zweifel S, Hurtado-Chong A, Yoshikawa K, Slomianka L, Raineteau O. Three-dimensional examination of the adult mouse subventricular zone reveals lineage-specific microdomains. PLoS One 2012; 7:e49087; PMID:23166605; http://dx.doi.org/ 10.1371/journal.pone.0049087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Roybon L, Deierborg T, Brundin P, Li JY. Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci 2009; 29:232-43; PMID:19200230; http://dx.doi.org/ 10.1111/j.1460-9568.2008.06595.x [DOI] [PubMed] [Google Scholar]
- [40].Lasorella A, Benezra R, Iavarone A. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer 2014; 14:77-91; PMID:24442143; http://dx.doi.org/ 10.1038/nrc3638 [DOI] [PubMed] [Google Scholar]
- [41].Samanta J, Grund EM, Silva HM, Lafaille JJ, Fishell G, Salzer JL. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature 2015; 526:448-52; PMID:26416758; http://dx.doi.org/ 10.1038/nature14957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002; 8:963-70; PMID:12161747; http://dx.doi.org/ 10.1038/nm747 [DOI] [PubMed] [Google Scholar]
- [43].Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci 2005; 8:865-72; PMID:15951811; http://dx.doi.org/ 10.1038/nn1479 [DOI] [PubMed] [Google Scholar]
- [44].Nakatani H, Martin E, Hassani H, Clavairoly A, Maire CL, Viadieu A, Kerninon C, Delmasure A, Frah M, Weber M, et al.. Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J Neurosci 2013; 33:9752-68; PMID:23739972; http://dx.doi.org/ 10.1523/JNEUROSCI.0805-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Freeman MR. Specification and morphogenesis of astrocytes. Science 2010; 330:774-8; PMID:21051628; http://dx.doi.org/ 10.1126/science.1190928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sloan SA, Barres BA. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol 2014; 27:75-81; PMID:24694749; http://dx.doi.org/ 10.1016/j.conb.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lim DA, Alvarez-Buylla A. Adult neural stem cells stake their ground. Trends Neurosci 2014; 37:563-71; PMID:25223700; http://dx.doi.org/ 10.1016/j.tins.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, Greenberg ME. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 2001; 104:365-76; PMID:11239394; http://dx.doi.org/ 10.1016/S0092-8674(01)00224-0 [DOI] [PubMed] [Google Scholar]
- [49].Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat Cell Biol 2004; 6:547-54; PMID:15156153; http://dx.doi.org/ 10.1038/ncb1138 [DOI] [PubMed] [Google Scholar]
- [50].Giachino C, Basak O, Lugert S, Knuckles P, Obernier K, Fiorelli R, Frank S, Raineteau O, Alvarez-Buylla A, Taylor V. Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells 2014; 32:70-84; PMID:23964022; http://dx.doi.org/ 10.1002/stem.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 2010; 467:323-7; PMID:20844536; http://dx.doi.org/ 10.1038/nature09347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Basak O, Giachino C, Fiorini E, Macdonald HR, Taylor V. Neurogenic subventricular zone stem/progenitor cells are Notch1-dependent in their active but not quiescent state. J Neurosci 2012; 32:5654-66; PMID:22514327; http://dx.doi.org/ 10.1523/JNEUROSCI.0455-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schildge S, Bohrer C, Pfurr S, Mammadzada K, Schachtrup K, Schachtrup C. Instructions from the vascular system - directing neural stem cell fate in health and disease. Curr Med Chem 2014; 21:2190-207; PMID:24372220; http://dx.doi.org/ 10.2174/0929867321666131227162215 [DOI] [PubMed] [Google Scholar]
- [54].Ara J, See J, Mamontov P, Hahn A, Bannerman P, Pleasure D, Grinspan JB. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res 2008; 86:125-35; PMID:17722066; http://dx.doi.org/ 10.1002/jnr.21462 [DOI] [PubMed] [Google Scholar]
- [55].Crews L, Adame A, Patrick C, Delaney A, Pham E, Rockenstein E, Hansen L, Masliah E. Increased BMP6 levels in the brains of Alzheimer disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J Neurosci 2010; 30:12252-62; PMID:20844121; http://dx.doi.org/ 10.1523/JNEUROSCI.1305-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Deininger M, Meyermann R, Schluesener H. Detection of two transforming growth factor-β-related morphogens, bone morphogenetic proteins-4 and -5, in RNA of multiple sclerosis and Creutzfeldt-Jakob disease lesions. Acta Neuropathol 1995; 90:76-9; PMID:7572083; http://dx.doi.org/ 10.1007/BF00294462 [DOI] [PubMed] [Google Scholar]
- [57].Fuller ML, DeChant AK, Rothstein B, Caprariello A, Wang R, Hall AK, Miller RH. Bone morphogenetic proteins promote gliosis in demyelinating spinal cord lesions. Ann Neurol 2007; 62:288-300; PMID:17696121; http://dx.doi.org/ 10.1002/ana.21179 [DOI] [PubMed] [Google Scholar]
- [58].Martino G, Pluchino S, Bonfanti L, Schwartz M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev 2011; 91:1281-304; PMID:22013212; http://dx.doi.org/ 10.1152/physrev.00032.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]