Abstract
Objective
Myocardial intracellular/extracellular structure and aortic function were assessed among hypertensive left ventricular (LV) phenotypes using cardiovascular magnetic resonance (CMR).
Methods
An observational study from consecutive tertiary hypertension clinic patients referred for CMR (1.5 T) was performed. Four LV phenotypes were defined: (1) normal with normal indexed LV mass (LVM) and LVM to volume ratio (M/V), (2) concentric remodelling with normal LVM but elevated M/V, (3) concentric LV hypertrophy (LVH) with elevated LVM but normal indexed end-diastolic volume (EDV) or (4) eccentric LVH with elevated LVM and EDV. Extracellular volume fraction was measured using T1-mapping. Circumferential strain was calculated by voxel-tracking. Aortic distensibility was derived from high-resolution aortic cines and contemporaneous blood pressure measurements.
Results
88 hypertensive patients (49±14 years, 57% men, systolic blood pressure (SBP): 167±30 mm Hg, diastolic blood pressure (DBP): 96±14 mm Hg) were compared with 29 age-matched/sex-matched controls (47±14 years, 59% men, SBP: 128±12 mm Hg, DBP: 79±10 mm Hg). LVH resulted from increased myocardial cell volume (eccentric LVH: 78±19 mL/m2 vs concentric LVH: 73±15 mL/m2 vs concentric remodelling: 55±9 mL/m2, p<0.05, respectively) and interstitial fibrosis (eccentric LVH: 33±10 mL/m2 vs concentric LVH: 30±10 mL/m2 vs concentricremodelling: 19±2 mL/m2, p<0.05, respectively). LVH had worst circumferential impairment (eccentric LVH: −12.8±4.6% vs concentric LVH: −15.5±3.1% vs concentric remodelling: –17.1±3.2%, p<0.05, respectively). Concentric remodelling was associated with reduced aortic distensibility, but not with large intracellular/interstitial expansion or myocardial dysfunction versus controls.
Conclusions
Myocardial interstitial fibrosis varies across hypertensive LV phenotypes with functional consequences. Eccentric LVH has the most fibrosis and systolic impairment. Concentric remodelling is only associated with abnormal aortic function. Understanding these differences may help tailor future antihypertensive treatments.
Introduction
Hypertensive left ventricular hypertrophy (LVH) is an independent predictor of sudden cardiac death1 and heart failure.2 International hypertension guidelines3 highlight its prognostic importance. However, hypertensive left ventricular (LV) phenotypes can be further classified as: normal structure (normal LV mass (LVM) and relative wall thickness), concentric remodelling (normal LVM but elevated relative wall thickness) and LVH.4 Similar classifications can be made with cardiovascular magnetic resonance (CMR), using mass to volume ratio (M/V) in lieu of relative wall thickness.5 Hypertensive LVH and concentric remodelling are both predictors of cardiovascular morbidity and mortality.6 However, their pathophysiology is incompletely understood.
Diffuse interstitial myocardial fibrosis has been documented histologically in hypertension.7 Precontrast (native) T1-mapping is a non-invasive CMR technique that quantifies changes in myocardial intracellular and/or extracellular compartments.8 In conjunction with postcontrast T1-mapping, myocardial extracellular volume fraction (ECV) can be calculated to localise abnormality to the interstitium. T1-mapping quantification of interstitial fibrosis has been validated against histological gold standard.9
We investigated whether differences exist between hypertensive LV phenotypes in myocardial intracellular/extracellular structure and myocardial/aortic function with CMR using T1-mapping and voxel-tracking techniques. We hypothesised that the incremental adverse prognosis of LVH over the other hypertensive phenotypes would correlate with the burden of diffuse myocardial fibrosis and myocardial dysfunction.
Materials and methods
Study population
An observational study from consecutive tertiary hypertension clinic patients referred for CMR (1.5 T) between February 2012 and April 2015 was performed. The study conformed to governance arrangements for research ethics committees. Subjects provided written consent. Baseline demographic and clinical characteristics were recorded. Exclusion criteria were any concomitant myocardial pathology that may confound hypertrophy (eg, moderate–severe valvular disease, acquired/inherited cardiomyopathy) and estimated glomerular filtration rate <30 mL/min/1.73 m2. Normotensive healthy volunteers were enrolled as controls.
Average office systolic blood pressure (SBP) and diastolic blood pressure (DBP) were acquired after seated rest from both arms using standard automated sphygmomanometry. Patients were stratified by severity in accordance with European guidelines.3
CMR cine protocol and analysis
CMR was performed at 1.5 T (Avanto, Siemens, Germany). Short-axis steady-state free precession (SSFP) cines with whole LV coverage (8 mm slice thickness, no slice gap, temporal resolution 38.1 ms, echo time 1.07 ms, in-plane pixel size 1.5×0.8 mm) were used for estimating LVM and volumes, which were indexed to body surface area. A validated10 threshold-detection software (CMR42, Circle Cardiovascular Imaging, Canada) was employed to include papillary muscles/trabeculae in LVM and then include them in blood pool for volume measurements as before.11 LV dilatation and LVH were defined as indexed end-diastolic volume (EDV) and indexed LVM >95th centile of age-specific and gender-specific CMR reference ranges.11 Increased M/V was defined as >95th confidence interval (>1.16 g/mL) from previously reported data from 91 healthy volunteers.5 The mean M/V in our study, using this cut-off value was 0.89±0.13 g/mL, which is consistent with the previous reported normal values of 0.88±0.14 g/mL.5 Four hypertensive LV phenotypes were defined similar to previous echocardiographic4 and CMR studies,5 according to LVM, EDV and M/V as: normal LV (normal indexed LVM and M/V), concentric remodelling (normal indexed LVM but increased M/V), concentric LVH (elevated indexed LVM but normal EDV) and eccentric LVH (elevated indexed LVM and EDV) (table 1). CMR analysis was performed by an experienced, blinded CMR reader.
Table 1.
Cardiovascular magnetic resonance definitions of patterns of hypertensive heart disease left ventricular (LV) phenotypes
| Indexed LVM (g/m2) | Indexed EDV (mL/m2) | M/V (g/mL) | |
|---|---|---|---|
| Normal LV | Normal | Normal | Normal |
| Concentric remodelling | Normal | ↓ | ↑ |
| Concentric LVH | ↑ | Normal | ↑ |
| Eccentric LVH | ↑ | ↑ | Normal |
EDV, end-diastolic volume; LVH, left ventricular hypertrophy; LVM, LV mass; LVM and EDV are indexed to body surface area; M/V=mass:volume ratio.
CMR late gadolinium protocol and analysis
Replacement myocardial fibrosis was assessed by late gadolinium enhancement (LGE). Inversion recovery sequences were performed 10–15 min after intravenous 0.1 mmol/kg gadobutrol (Gadovist, Bayer Pharma AG, Germany). Inversion time was optimised to achieve myocardial nulling. LGE was assessed visually by consensus between two expert CMR readers, blinded to all other data. Patients with LGE were excluded to avoid confounding effects of myocardial replacement fibrosis.
CMR T1-mapping protocol and analysis
Myocardial T1-mapping was performed using the modified look-locker inversion recovery sequence (35° flip angle, 100 ms minimum TI, 80 ms TI increment, 150 ms time delay with 5-(3)-3 heartbeat acquisition scheme).12 Using Argus software (Siemens, Germany), regions of interest were drawn within mid-septum on short-axis, motion-corrected, native T1-maps and copied onto corresponding postcontrast maps, adjusting for partial-voluming and/or artefact, as previously described.13 Analysis was performed by an experienced blinded CMR reader.
ECV was calculated using an established formula:14
where:
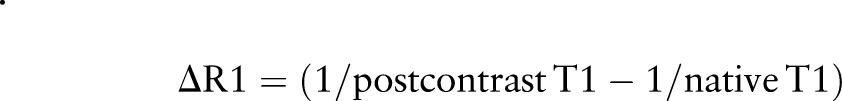 |
Indexed interstitial volume was calculated by multiplying the ECV by indexed myocardial volume (indexed LVM divided by myocardial specific gravity 1.05 g/mL). Myocardial cell volume fraction was defined, as previously,15 as (1–ECV) and multiplied by indexed LV myocardial volume to generate an estimation of indexed myocardial cell volume. Excellent reproducibility with this T1-technique has previously been demonstrated.13
CMR strain imaging
Off-line strain imaging was performed using short-axis stack SSFP cine images with Tissue Tracking Software (Circle Cardiovascular Imaging), which tracks myocardial voxels over the cardiac cycle in 2D based on a previously described algorithm.16 Strain data from age-matched and sex-matched normotensive subjects served as control data. Circumferential strain was calculated as mean values of mid-myocardial segments from the short-axis cine 2D strain model. Peak circumferential systolic and diastolic strain rates were measured. Strain analysis was performed by an experienced blinded CMR reader.
Aortic stiffness
As previously described,17 measures of ascending aortic stiffness were measured as follows:
(i) distensibility=ΔA/(Adiast×ΔP) and (ii) compliance=ΔA/ΔP
where:
ΔA(mm2) was defined Asyst–Adiast.
Asyst is the ascending aortic area, measured from cine image perpendicular to the vessel at the level of the right pulmonary artery, at end-systole.
Adiast is the ascending aortic area at end-diastole and ΔP (mm Hg) is the pulse pressure estimated from SBP–DBP at time of CMR.
Measurements were acquired by an experienced blinded CMR reader. Excellent reproducibility of these measures has previously been reported.17
Statistical analysis
Statistical analysis was performed using SPSS V.21 (Armonk, New York, USA: IBM Corp.). Categorical variables were analysed using Fisher's exact test. Data are expressed as mean±SD where appropriate. Normally distributed continuous variables were compared using one-way analysis of variance with Bonferroni correction for multiple comparisons. Continuous variables that were not normally distributed were compared by Kruskal–Wallis tests. R-values quoted are for Pearson's correlation coefficient. Post hoc multiple linear regression was used to control for covariates of age, gender, body mass index, diabetes, SBP, DBP and number of antihypertensive medications, which were significantly different between some hypertensive LV phenotypes, on the T1-mapping, myocardial strain and aortic data. Significance was defined as two-tailed p<0.05, where p values presented include Bonferroni adjustment for multiple comparisons where appropriate.
Results
Demographics
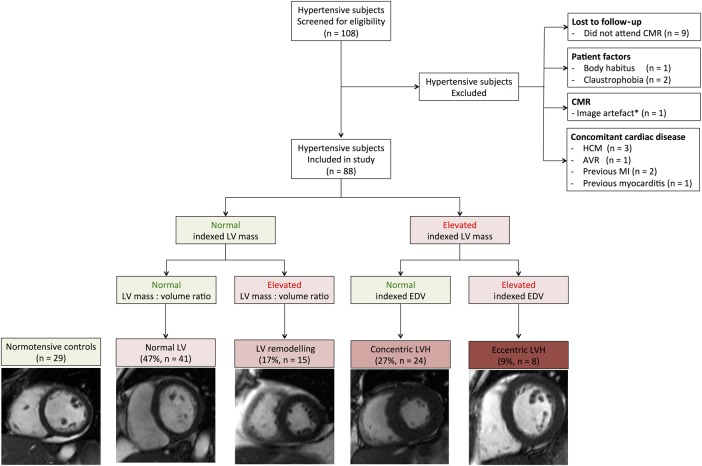
Of the 108 hypertensive subjects initially recruited who underwent CMR, 20 were excluded (figure 1), with six excluded because of LGE (three had mid-wall fibrosis and ancillary CMR features of hypertrophic cardiomyopathy, two had ischaemic LGE and one had subepicardial LGE suggesting previous myocarditis). The final hypertensive sample size was 88 (age: 49±14 years, men: 57%, office SBP: 167±30 mm Hg, office DBP: 96±14 mm Hg). Twenty-nine healthy control subjects were recruited (age: 47±14 years, men: 59%, office SBP: 128±12 mm Hg, office DBP: 79±10 mm Hg). Diabetes prevalence was similar between hypertensive subgroups (table 2).
Figure 1.
Study size and exclusion criteria. AVR, aortic valve replacement; CMR, cardiovascular magnetic resonance; EDV, end-diastolic volume; HCM,hypertrophic cardiomyopathy; LV, left ventricular; LVH, left ventricular hypertrophy; MI, myocardial infarction. *Image artefact from implantable loop recorder device precluding volumetric analysis.
Table 2.
Demographic data for hypertensive subjects and normotensive controls
| Hypertensive subjects (n=88) | |||||
|---|---|---|---|---|---|
| Normal indexed LVM (n=56) | Elevated indexed LVM (n=32) | ||||
| Controls (n=29) | Normal LV (n=41) | Concentric remodelling (n=15) | Concentric LVH (n=24) | Eccentric LVH (n=8) | |
| Age (years) | 47±13 | 45±16 | 56±12*1 | 48±12 | 56±11*2 |
| Gender (% male) | 59 | 41*3 | 60 | 71 | 88 |
| Ethnicity | |||||
| Caucasian | 93*4 | 73 | 93 | 83 | 87 |
| Black African | 3 | 0 | 0 | 9 | 13 |
| Black Caribbean | 0 | 10 | 0 | 4 | 0 |
| Oriental | 0 | 2 | 0 | 0 | 0 |
| South East Asian | 3 | 12 | 0 | 0 | 0 |
| Mixed | 0 | 2 | 7 | 4 | 0 |
| BMI (kg/m2) | 26±5*5 | 30±6 | 33±5 | 31±6 | 32±7 |
| Diabetes (%) | 0*6 | 5 | 13 | 17 | 20 |
| Heart rate (bpm) | 67±12 | 72±12 | 76±16*7 | 68±9 | 65±13 |
| Office SBP (mm Hg) | 128±12*8 | 161±26 | 175±33 | 170±30 | 172±37 |
| Office DBP (mm Hg) | 79±10*8 | 94±11 | 94±15 | 99±14 | 102±21 |
| ESH/ESC office BP grade | |||||
| Controlled (%) | … | 5 | 7 | 13 | 10 |
| High normal (%) | … | 7 | 7 | 0 | 0 |
| Grade 1 (%) | … | 39 | 13 | 25 | 10 |
| Grade 2 (%) | … | 22 | 27 | 29 | 30 |
| Grade 3 (%) | … | 22 | 47 | 33 | 50 |
| Isolated systolic HTN (%) | … | 5 | 7 | 0 | 0 |
| No. antihypertensive medications | 0*8 | 2±1 | 2±2 | 3±2*9 | 4±3*10 |
| ACEi/ARB (%) | 0*8 | 68 | 80 | 83 | 100*11 |
*1Concentric remodelling versus Normal LV: p=0.007 and Concentric remodelling versus Controls: p=0.037.
*2Eccentric LVH versus Normal LV: p=0.035.
*3Normal LV versus Concentric LVH: p=0.020 and Normal LV versus Eccentric LVH: p=0.016.
*4Controls versus Normal LV: p=0.027.
*5Controls versus Normal LV: p=0.017, Controls versus Concentric remodelling: p<0.0001, Controls versus Concentric LVH: p=0.001 and Controls versus Eccentric LVH: p=0.009.
*6Controls versus Concentric LVH: p=0.030 and Controls versus Eccentric LVH: p=0.025.
*7Concentric remodelling versus Controls: p=0.032, Concentric remodelling versus Concentric LVH: p=0.043 and Concentric remodelling versus Eccentric LVH: p=0.041.
*8Controls versus Normal LV: p<0.0001, Controls versus Concentric remodelling: p<0.0001, Controls versus Concentric LVH: p<0.0001 and Controls versus Eccentric LVH: p<0.0001.
*9Concentric LVH versus Normal LV: p=0.011.
*10Eccentric LVH versus Concentric remodelling p=0.016 and Eccentric LVH versus Normal LV: p=0.001.
*11Eccentric LVH versus Normal LV: p=0.035.
European Society of Hypertension/European Society of Cardiology (ESH/ESC) Office BP grade—Controlled SBP: 120–129 and/or DBP 80–84, High normal SBP: 130–139 mm Hg and/or DBP 85–89 mm Hg, Grade 1 SBP 140–159 and/or DBP 90–99, Grade 2 SBP 160–179 and/or DBP 100–109, Grade 3 SBP ≥180 and/or DBP ≥110, Isolated systolic hypertension SBP ≥140 mm Hg and DBP <90 mm Hg.
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BP, blood pressure; BMI, body mass index; DBP, diastolic blood pressure; HTN, hypertension; LV, left ventricular; LVH, left ventricular hypertrophy; SBP, systolic blood pressure.
Findings in eccentric LVH
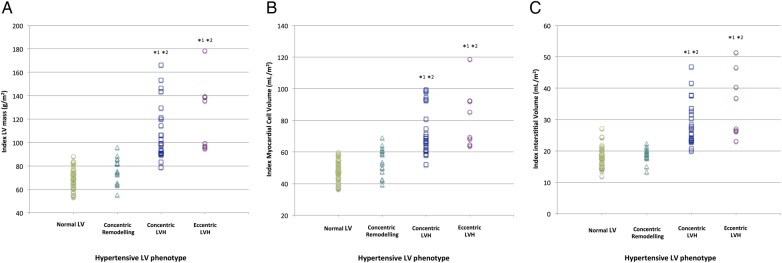
Eccentric LVH was present in 9% (n=8). Subjects with eccentric LVH had the most advanced hypertrophy, with indexed LVM (122±30 g/m2) higher than those classified as concentric LVH (108±24 g/m2), concentric remodelling (75±10 g/m2) and normal LV (70±9 g/m2) (table 3). Indexed LVM correlated positively, albeit weakly, with native T1 (R=0.352, p=0.001) and ECV (R=0.318, p=0.003) (see online supplementary figure S1A, B). The elevated native T1 and ECV values for eccentric LVH compared with the other subgroups persisted after correction for covariates (table 4). The elevated indexed LVM in eccentric LVH was a result of increases in both intracellular and interstitial myocardial components (figure 2).
Table 3.
Cardiovascular magnetic resonance volumetric, T1-mapping and myocardial strain data for hypertensive subjects and normotensive controls
| Hypertensive subjects (n=88) | |||||
|---|---|---|---|---|---|
| Normal indexed LVM (n=56) | Elevated indexed LVM (n=32) | ||||
| Controls (n=29) | Normal LV (n=41) | Concentric remodelling (n=15) | Concentric LVH (n=24) | Eccentric LVH (n=8) | |
| LV volumetrics | |||||
| Ejection fraction (%) | 66±7 | 67±7 | 73±7*1 | 67±7 | 54±15*2 |
| Indexed EDV (mL/m2) | 77±18 | 76±12 | 55±8*3 | 81±11 | 109±14*2 |
| Indexed ESV (mL/m2) | 27±9 | 25±7 | 15±5*3 | 29±10 | 51±21*2 |
| Indexed SV (mL/m2) | 50±11 | 51±10 | 40±7*4 | 54±9 | 58±13 |
| Indexed LV mass (g/m2) | 61±11*5 | 70±9 | 75±10 | 108±24*6 | 122±30*7 |
| Mass:volume ratio (g/mL) | 0.80±0.12*8 | 0.92±0.10*9 | 1.38±0.22*10 | 1.39±0.38*11 | 1.08±0.20 |
| T1-mapping | |||||
| Native T1 (ms) | 1024±41 | 1031±35 | 1029±45 | 1054±41*12 | 1062±41*13 |
| Extracelluar volume fraction (%) | … | 27±3 | 26±3 | 29±4*14 | 30±3*15 |
| Myocardial cell volume fraction (%) | … | 73±3 | 74±3 | 71±4*14 | 70±3*15 |
| Circumferential myocardial function | |||||
| Peak strain (%) | −17.4±2.6 | −17.6±3.0 | −17.1±3.2 | −15.5±3.1*16 | −12.8±4.6*17 |
| Peak systolic strain rate (%/s) | −101±13 | −107±28 | −115±38 | −98±20*18 | −70±20*19 |
| Peak diastolic strain rate (%/s) | 101±26 | 102±26 | 90±24 | 82±23*20 | 65±21*21 |
| Aortic function | |||||
| Compliance (mm2/mm Hg) | 2.27±1.13*22 | 1.62±1.21 | 0.99±0.70 | 1.60±1.09 | 1.27±0.72 |
| Distensibility (mm2/mm Hg ×103) | 3.48±2.14*23 | 2.26±1.71 | 1.22±0.82*24 | 1.84±1.46 | 1.28±0.72 |
*1Concentric remodelling versus Controls: p=0.002, Concentric remodelling versus Normal LV: p=0.006, Concentric remodelling versus Concentric LVH: p=0.009 and Concentric remodelling versus Eccentric LVH: p<0.0001.
*2Eccentric LVH versus Controls: p<0.0001, Eccentric LVH versus Normal LV: p<0.0001 and Eccentric LVH versus Concentric LVH: p<0.0001.
*3Concentric remodelling versus Controls: p<0.0001, Concentric remodelling versus Normal LV: p<0.0001, Concentric remodelling versus Concentric LVH: p<0.0001 and LV remodelling versus Eccentric LVH: p<0.0001.
*4Concentric remodelling versus Controls: p=0.001, Concentric remodelling versus Normal LV: p<0.0001, Concentric remodelling versus Concentric LVH: p<0.0001 and Concentric remodelling versus Eccentric LVH: p<0.0001.
*5Controls versus Normal LV: p=0.022, Controls versus Concentric remodelling: p=0.002, Controls versus Concentric LVH: p<0.0001 and Controls versus Eccentric LVH: p<0.0001.
*6Concentric LVH versus Normal LV: p<0.0001, Concentric LVH versus Concentric remodelling: p<0.0001 and Concentric LVH versus Eccentric LVH: p=0.030.
*7Eccentric LVH versus Normal LV: p<0.0001 and Eccentric LVH versus Concentric remodelling: p<0.0001.
*8Controls versus Normal LV: p=0.019, Controls versus Concentric remodelling: p<0.0001, Controls versus Concentric LVH: P<0.0001 and Controls versus Eccentric LVH: p<0.0001.
*9Normal LV versus Concentric remodelling: p<0.0001, Normal LV versus Concentric LVH: p<0.0001 and Normal LV versus Eccentric LVH: p=0.018.
*10Concentric remodelling versus Eccentric LVH: p=0.002.
*11Concentric LVH versus Eccentric LVH: p=0.002.
*12Concentric LVH versus Controls: p=0.007 and Concentric LVH versus Normal LV: p=0.023.
*13Concentric LVH versus Controls: p=0.017 and Concentric LVH versus Normal LV: p=0.042.
*14Concentric LVH versus Concentric remodelling: p=0.012.
*15Eccentric LVH versus Concentric remodelling: p=0.021.
*16Concentric LVH versus Controls: p=0.028, Concentric LVH versus Normal LV: p=0.010 and Concentric LVH versus Eccentric LVH: p=0.030.
*17Eccentric LVH versus Controls: p<0.0001, Eccentric LVH versus Normal LV: p<0.0001 and Eccentric LVH versus Concentric remodelling: p=0.002.
*18Concentric versus Concentric remodelling: p=0.037 and Concentric LVH versus Eccentric LVH: p=0.006.
*19Eccentric LVH versus Controls: p=0.002, Eccentric LVH versus Normal LV: p<0.0001 and Eccentric LVH versus Concentric remodelling: p<0.0001.
*20Concentric LVH versus Controls: p=0.007 and Concentric LVH versus Normal LV: p=0.002.
*21Eccentric LVH versus Controls: p=0.001, Eccentric LVH versus Normal LV: p<0.0001 and Eccentric LVH versus LV remodelling: p=0.024.
*22Controls versus Normal LV: p=0.019, Controls versus Concentric remodelling: p<0.0001, Controls versus Concentric LVH: p=0.033 and Controls versus Eccentric LVH: p=0.025.
*23Controls versus Normal LV: p=0.004, Controls versus Concentric remodelling: p<0.0001, Controls versus Concentric LVH: p=0.001 and Controls versus Eccentric LVH: p=0.001.
*24Concentric remodelling versus Normal LV: p=0.037.
EDV, end-diastolic volume; LV, left ventricular; ESV, end-systolic volume; LVH, left ventricular hypertrophy; LVM, left ventricular mass; SV, stroke volume.
Table 4.
T1-mapping, myocardial strain and aortic function data corrected for covariates* for hypertensive subjects
| Hypertensive subjects (n=88) | ||||
|---|---|---|---|---|
| Normal indexed LVM (n=56) | Elevated indexed LVM (n=32) | |||
| Normal LV (n=41) | Concentric remodelling (n=15) | Concentric LVH (n=24) | Eccentric LVH (n=8) | |
| T1-mapping | ||||
| Native T1 (ms) | 1031±6 | 1025±10 | 1054±8*1 | 1067±15*2 |
| Extracellular volume fraction (%) | 27±1 | 26±1 | 29±1*3 | 30±1*4 |
| Circumferential myocardial function | ||||
| Peak strain (%) | −16.9±0.5 | −17.4±0.8 | −16.1±0.6 | −14.2±1.1*5 |
| Peak systolic strain rate (%/s) | −104±4 | −120±7 | −99±5*6 | −76±10*7 |
| Peak diastolic strain rate (%/s) | 95±4 | 97±6 | 85±5 | 80±8 |
| Aortic function | ||||
| Compliance (mm2/mm Hg) | 1.61±0.19 | 0.93±0.28*8 | 1.73±0.23 | 1.47±0.40 |
| Distensibility (mm2/mm Hg ×103) | 2.27±0.26 | 1.05±0.39*9 | 2.04±0.30 | 1.57±0.55 |
*Multiple linear regression accounting for the covariates of age, gender, body mass index, diabetes, office systolic blood pressure and diastolic blood pressure and number of antihypertensive medications. Data are presented as mean±SE.
*1Concentric LVH versus normal LV: p=0.033 and concentric LVH versus concentric remodelling: p=0.028.
*2Eccentric LVH versus normal LV: p=0.031 and eccentric LVH versus concentric remodelling: p=0.018.
*3Concentric LVH versus normal LV: p=0.013 and concentric LVH versus concentric remodelling: p=0.001.
*4Eccentric LVH versus normal LV: p=0.022 and eccentric LVH versus concentric remodelling: p=0.001.
*5Eccentric LVH versus normal LV: p=0.047 and eccentric LVH versus concentric remodelling: p=0.024.
*6Concentric LVH versus concentric remodelling: p=0.025 and concentric LVH versus eccentric LVH: p=0.038.
*7Eccentric LVH versus normal LV: p=0.016 and eccentric LVH versus concentric remodelling: p=0.002.
*8Concentric remodelling versus concentric LVH: p=0.028.
*9Concentric remodelling versus normal LV: p=0.020 and concentric remodelling versus concentric LVH: p=0.048.
LV, left ventricular; LVH, left ventricular hypertrophy; LVM, left ventricular mass.
Figure 2.
Dotplots showing differences in (A) indexed LV mass, (B) indexed myocardial cell volume and (C) indexed interstitial volume between hypertensive LV phenotypes. *1Versus Normal LV: p<0.0001, *2Versus Concentric remodelling: p<0.0001. LV, left ventricular; LVH, left ventricular hypertrophy.
heartjnl-2016-309576supp_figures.pdf (1.3MB, pdf)
There was a weak positive correlation between SBP and native T1 that was statistically significant (R=0.267, p=0.012). There were no significant correlations between SBP and ECV (R=0.143, p=0.185), DBP and native T1 (R=0.089, p=0.411) or DBP and ECV (R=−0.112, p=0.300) (see online supplementary figure S2A–D).
Eccentric LVH had the lowest peak circumferential strain values (−12.8±4.6%) compared with concentric LVH (−15.5±3.1%), concentric remodelling (−17.1±3.2%), normal LV (−17.6±3.0%) and controls (−17.4±2.6%), with evidence of both systolic and diastolic strain impairment (table 3). These associations persisted after correction for covariates (table 4).
Findings in concentric LVH
Concentric LVH was present in 27% (n=24) and associated with elevated indexed LVM (108±24 g/m2) compared with concentric remodelling (75±10 g/m2), normal LV (70±9 g/m2) and controls (61±11 g/m2). Concentric LVH subjects had elevated native T1 values (1054±41 ms) compared with those with LV remodelling (1029±45 ms), normal LV (1031±35 ms) and controls (1024±41 ms) (table 3). These findings for native T1 and ECV persisted after correction for covariates (table 4). The increased indexed LVM in concentric LVH compared with subjects with concentric remodelling and normal LV was due to expansion of both the myocardial interstitium and the myocardial cell volume (figure 2).
Myocardial functional changes accompanied the changes in myocardial structure in concentric LVH, with lower peak circumferential strain values (−15.5±3.1%) compared with concentric remodelling (−17.1±3.2%), normal LV (−17.6±3.0%) and controls (−17.4±2.6%) despite no statistically significant differences in LV ejection fraction (table 3).
Findings in concentric remodelling
Subjects with concentric remodelling had no differences in SBP, DBP or prevalence of European Society of Hypertension (ESH)/European Society of Cardiology (ESC) grade 3 blood pressure (BP) compared with other hypertensive subgroups (table 2). Concentric remodelling was not associated with large intracellular/extracellular myocardial changes compared with controls (native T1 1031±35 vs 1024±41 ms, p=0.465). There were no large differences in myocardial strain in concentric remodelling compared with controls (table 3). Aortic compliance and distensibility were reduced in all hypertensive phenotypes, including normal LV, compared with controls. However, concentric remodelling was associated with the lowest aortic distensibility and compliance (tables 3 and 4).
Determinants of myocardial systolic strain
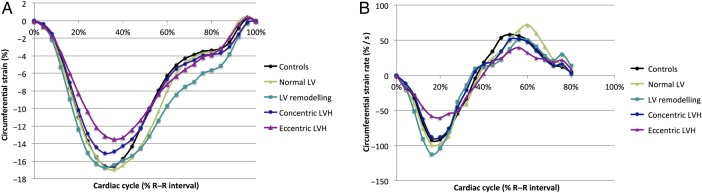
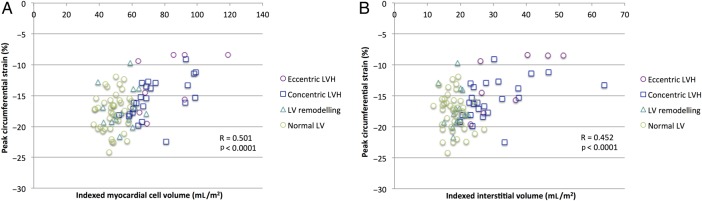
Mean circumferential strain data are demonstrated in figure 3. Increasing indexed myocardial cell volume (R=0.507, p<0.0001) and increasing indexed interstitial volume (R=0.452, p<0.0001) both correlated with worsening peak circumferential strain (figure 4). As there was significant difference in circumferential strain between concentric and eccentric LVH, post hoc analysis with a one-way analysis of covariance (ANCOVA) was conducted to determine whether the concomitant differences in indexed LVM and/or indexed EDV were responsible. The predicted main effect of indexed LVM was significant, F(2,29)=12.3, p=0.002, but that of indexed EDV was not significant, F(2,29)=0.25, p=0.621. Increased LVM is the likely reason for impaired circumferential strain in eccentric LVH compared with concentric LVH and this is consistent with our other results demonstrating significant positive correlations of both indexed myocardial cell volume and interstitial volume with peak circumferential strain.
Figure 3.
(A) Mean circumferential strain of the mid-myocardium over the cardiac cycle (B) Mean circumferential strain rate of the mid-myocardium over the cardiac cycle. LV, left ventricular; LVH, left ventricular hypertrophy.
Figure 4.
Peak circumferential strain versus (A) indexed myocardial cell volume (R=0.501, p<0.0001) and versus (B) indexed interstitial volume (R=0.452, p<0.0001). LV, left ventricular; LVH, left ventricular hypertrophy.
Discussion
This study investigates changes at the intracellular/extracellular myocardial structural level between the different hypertensive heart disease phenotypes and investigates whether such changes are associated with myocardial and aortic functional consequences. We show that: (i) hypertensive LVH is associated with elevated indexed LVM due to significant expansion of the interstitium as well as the myocardial cell component. It is associated with significant systolic and diastolic circumferential strain impairment. These significant findings occur both in eccentric LVH and concentric LVH but are most advanced in the former. (ii) In hypertensive concentric remodelling, there was no large association with increased myocardial interstitial fibrosis or myocardial systolic strain impairment relative to controls, but these subjects demonstrated the most aortic stiffness.
Left ventricular hypertrophy
Hypertensive LVH was associated with significantly elevated native T1 compared with normotensive controls and significantly elevated native T1 and ECV compared with hypertensive subjects without LVH, which is consistent with work by Kuruvilla et al18 and Treibel et al19 in their studies of 43 and 40 hypertensive subjects, respectively. Our larger sample size of 88 hypertensive subjects may provide further insights into the pathophysiology of the spectrum of hypertensive heart disease. Concentric and eccentric hypertensive LVH are associated with adverse cardiovascular prognosis.6 We show that the eccentric form of LVH is associated with most advanced intracellular and interstitial myocardial expansion.
We also explored the functional implications of hypertensive LVH with CMR myocardial strain analysis. Eccentric and concentric LVH subgroups exhibited significant circumferential strain impairment compared with other hypertensive phenotypes. Our findings are consistent with previous echocardiographic studies.20 However, we additionally demonstrate that both increasing indexed myocardial cell and interstitial volume correlate significantly with worsening circumferential strain values. A putative mechanism to explain the relationship between strain and interstitial expansion is that LV stiffness increases with increased interstitial fibrosis, culminating in reduced end-diastolic muscle fibre length and, in turn, reduced myocardial contraction and LV systolic strain.21 The relationship between myocardial cell volume with strain may be explained by the fact that there is no significant change in external LV diameter during systole22 and conservation of myocardial volume, with only negligible capillary bed compression, over the cardiac cycle. Consequently, in a hypertrophied LV with elevated end-diastolic wall thickness, less endocardial displacement (the output from myocardial strain) may be required to achieve the same stroke volume.23
Concentric remodelling and LVH: a spectrum or distinct entities?
Hypertensive patients with normal indexed LVM may have normal LV structure (with normal M/V) or concentric remodelling (with elevated M/V). The latter is associated with adverse prognosis.6 Interestingly, in our study, hypertensive subjects with concentric remodelling had no intracellular or extracellular myocardial expansion compared with normotensives and no evidence of significant myocardial systolic strain dysfunction. The lack of strong or significant positive correlation between SBP or DBP and native T1 or ECV suggests the development of myocardial fibrosis in hypertensive heart disease in our cohort is not simply linearly related to arterial pressure. Excess sympathetic activity may drive myocardial changes independently of BP.24 Myocardial mechanical stress in response to pressure overload can upregulate pro-fibrotic and pro-hypertrophic genetic pathways25 and may also be implicated. It is possible the reduction in EDV is a compensatory mechanism to ‘unload’ the pressure-loaded LV.4
Concentric remodelling subjects had increased aortic stiffness. Age and female gender are associated with increased aortic stiffness.26 Although our concentric remodelling cohort were significantly older than controls and hypertensive subjects with normal LV structure, there were no significant differences in age or gender between concentric remodelling and concentric LVH or eccentric LVH. Furthermore, differences in aortic distensibility persisted after correcting for the covariates of age and gender. Severity of elevated BP (office BP and prevalence of ESH/ESC grade 3 hypertension) was not different between concentric remodelling and LVH, but we were not able to investigate the impact of time spent at elevated BPs in the current study, which may be implicated. The interaction with the autonomic nervous system may, again, be important. Sympathetic neural mechanisms may have an arterial stiffening effect. Carotid–femoral pulse wave velocity, a marker of aortic stiffness, has been demonstrated to be linked to muscle sympathetic nerve activity (MSNA) in human subjects.27 The degree of MSNA can vary among hypertensive subjects. A putative explanation is that subjects with high MSNA have resultant increased aortic stiffness, and then the left ventricle remodels in response. Further longitudinal cohort studies are required to determine whether LV remodelling occurs in response to hypertension or whether it predates and is implicated in the aetiology of the hypertension.
Treatment implications
Hypertension is the strongest modifiable risk factor for cardiac morbidity and mortality.1 LVH can regress with appropriate antihypertensive treatment. However, a risk of heart failure persists after LVH regression,28 suggesting that current treatment strategies fail to tackle adverse myocardial changes beyond LVM. In a mouse model of hypertensive LVH, antihypertensive treatment caused interstitial fibrosis and cardiomyocyte hypertrophy regression.29 However, there has been failure to translate significant benefits of antifibrotic type therapies into patients with heart failure with preserved ejection fraction, who are often elderly and have long-standing hypertension. Large-scale human studies will be required to assess whether regression in myocyte mass and/or interstitial fibrosis occur with targeted antihypertensive agents and to categorically determine their relative importance with regard to: (i) regain of regional myocardial functional and (ii) improvement in overall cardiovascular prognosis. Such studies would help clarify whether myocardial interstitial fibrosis in hypertensive heart disease represents a viable therapeutic target. Understanding why altered aortic function occurs more in certain hypertensive phenotypes may have important treatment implications too; for example, antihypertensive agents with vasodilatory properties may be less effective in subjects with concentric remodelling and the stiffest aortas.
Limitations
Contrast medium was not administered to our normotensive control cohort. Consequently, there is no corresponding ECV data. However, the lack of significant difference between native T1 values between controls and hypertensive subjects with normal LV suggests the ECV is normal in this hypertensive subgroup, which essentially acts as hypertensive controls.
Our sample size of 88 hypertensive subjects is modest but represents the largest study of T1-mapping in hypertensive subjects until now. Nevertheless, we were unable to determine the impact of hypertension duration, or antihypertensive treatment strategies, on the variables investigated.
We do not have prognostic data in our cohort due to the short follow-up time and overall low event rate but prognostic data related to LV geometry are well established from large prospective, population-based studies.6
The voxel-tracking software used to generate strain values makes assumptions regarding conservation of myocardial mass over the cardiac cycle to derive strain values. Contemporaneous echocardiographic data were not available in all subjects, but have previously been investigated.19
Conclusion
In hypertensive heart disease, structural differences exist at the intracellular/extracellular myocardial level across hypertensive phenotypes and are associated with functional consequences. Concentric and eccentric LVH are associated with significant intracellular and interstitial expansion with significant systolic and diastolic strain impairment. Concentric remodelling is associated with normal intracellular/extracellular myocardial structure and function but increased aortic stiffness. Our results may help explain why LVH, in particular eccentric LVH, has poor cardiovascular prognosis. Native T1 and myocardial ECV may become novel imaging biomarkers to characterise hypertensive heart disease and eventually help guide and monitoring treatment response with antifibrotic agents in certain hypertensive individuals.
Key messages.
What is already known on this subject?
Hypertensive heart disease has a spectrum of left ventricular (LV) phenotypes that are associated with varying cardiovascular prognosis but the underlying pathophysiological mechanisms leading to these differences are incompletely understood.
What might this study add?
This study demonstrates differences in diffuse myocardial interstitial fibrosis, myocardial circumferential strain and aortic distensibility and compliance across the various hypertensive heart disease LV phenotypes, using multiparametric cardiovascular magnetic resonance (CMR). Our findings may help explain, at least in part, the differing cardiovascular prognosis observed in the different LV phenotypes.
How might this impact on clinical practice?
The non-invasive detection of subclinical abnormalities with CMR, such as the burden of diffuse myocardial interstitial and abnormal myocardial strain and aortic distensibility, may represent novel biomarkers in arterial hypertension to guide targeted pharmacological interventions in future.
Acknowledgments
This work was supported by the Bristol NIHR Cardiovascular Biomedical Research Unit at the Bristol Heart Institute. The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), National Institute for Health Research or Department of Health. We thank Christopher Lawton, Superintendent Radiographer, and the Bristol Heart Institute CMR radiographers for their expertise in performing the CMRs. JCLR: Clinical Society of Bath Postgraduate Research Bursary 2014 and Royal College of Radiologists Kodak Research Scholarship 2014. ECH and JFRP are funded by the British Heart Foundation.
Footnotes
Twitter: Follow Jonathan Rodrigues at @JCLRodrigues
Contributors: JCLR conceived and designed the study, acquired data, analysed data, drafted the manuscript and revised it critically for important intellectual content. AMA, AGD, GS, SL, CG, LEKR and AEB acquired data and revised the work for important intellectual content. ECH, MCKH, AKN and JFRP contributed to the interpretation of data and revised the work for important intellectual content. NEM and CB-D contributed to the design of the work, analysed data and revised the work for critically important intellectual content. All authors gave final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CB-D is the overall guarantor for the work.
Funding: Bristol NIHR Cardiovascular Biomedical Research Unit in The Bristol Heart Institute, University of Bristol and University Hospitals Bristol NHS Foundation Trust.
Competing interests: CB-D is a consultant for Circle Cardiovascular Imaging.
Patient consent: Obtained.
Ethics approval: NHS research ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Haider AW, Larson MG, Benjamin EJ, et al. . Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998;32:1454–9. 10.1016/S0735-1097(98)00407-0 [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Castelli WP, McNamara PM, et al. . Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med 1972;287:781–7. 10.1056/NEJM197210192871601 [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, Fagard R, Narkiewicz K, et al. . 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–219. 10.1093/eurheartj/eht151 [DOI] [PubMed] [Google Scholar]
- 4.Ganau A, Devereux RB, Roman MJ, et al. . Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 1992;19:1550–8. 10.1016/0735-1097(92)90617-V [DOI] [PubMed] [Google Scholar]
- 5.Dweck MR, Joshi S, Murigu T, et al. . Left ventricular remodeling and hypertrophy in patients with aortic stenosis: insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:50 10.1186/1532-429X-14-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol 1995;25:879–84. 10.1016/0735-1097(94)00473-4 [DOI] [PubMed] [Google Scholar]
- 7.Querejeta R, Varo N, López B, et al. . Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation 2000;101:1729–35. 10.1161/01.CIR.101.14.1729 [DOI] [PubMed] [Google Scholar]
- 8.Flett AS, Hayward MP, Ashworth MT, et al. . Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 2010;122:138–44. 10.1161/CIRCULATIONAHA.109.930636 [DOI] [PubMed] [Google Scholar]
- 9.Miller CA, Naish JH, Bishop P, et al. . Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging 2013;6:373–83. 10.1161/CIRCIMAGING.112.000192 [DOI] [PubMed] [Google Scholar]
- 10.Childs H, Ma L, Ma M, et al. . Comparison of long and short axis quantification of left ventricular volume parameters by cardiovascular magnetic resonance, with ex-vivo validation. J Cardiovasc Magn Reson 2011;13:40 10.1186/1532-429X-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maceira AM, Prasad SK, Khan M, et al. . Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:417–26. 10.1080/10976640600572889 [DOI] [PubMed] [Google Scholar]
- 12.Messroghli DR, Greiser A, Fröhlich M, et al. . Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 2007;26:1081–6. 10.1002/jmri.21119 [DOI] [PubMed] [Google Scholar]
- 13.Pica S, Sado DM, Maestrini V, et al. . Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2014;16:99 10.1186/s12968-014-0099-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah AS, Chin CW, Vassiliou V, et al. . Left ventricular hypertrophy with strain and aortic stenosis. Circulation 2014;130:1607–16. 10.1161/CIRCULATIONAHA.114.011085 [DOI] [PubMed] [Google Scholar]
- 15.Flett AS, Sado DM, Quarta G, et al. . Diffuse myocardial fibrosis in severe aortic stenosis: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging 2012;13:819–26. 10.1093/ehjci/jes102 [DOI] [PubMed] [Google Scholar]
- 16.Bistoquet A, Oshinski J, Skrinjar O. Myocardial deformation recovery from cine MRI using a nearly incompressible biventricular model. Med Image Anal 2008;12:69–85. 10.1016/j.media.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 17.Groenink M, de Roos A, Mulder BJ, et al. . Biophysical properties of the normal-sized aorta in patients with Marfan syndrome: evaluation with MR flow mapping. Radiology 2001;219:535–40. 10.1148/radiology.219.2.r01ma01535 [DOI] [PubMed] [Google Scholar]
- 18.Kuruvilla S, Janardhanan R, Antkowiak P, et al. . Increased extracellular volume and altered mechanics are associated with LVH in hypertensive heart disease, not hypertension alone. JACC Cardiovasc Imaging 2015;8:172–80. 10.1016/j.jcmg.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treibel TA, Zemrak F, Sado DM, et al. . Extracellular volume quantification in isolated hypertension—changes at the detectable limits? J Cardiovasc Magn Reson 2015;17:74 10.1186/s12968-015-0176-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuguchi Y, Oishi Y, Miyoshi H, et al. . Concentric left ventricular hypertrophy brings deterioration of systolic longitudinal, circumferential, and radial myocardial deformation in hypertensive patients with preserved left ventricular pump function. J Cardiol 2010;55:23–33. 10.1016/j.jjcc.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 21.McLenachan JM, Dargie HJ. Ventricular arrhythmias in hypertensive left ventricular hypertrophy. Relationship to coronary artery disease, left ventricular dysfunction, and myocardial fibrosis. Am J Hypertens 1990;3:735–40. [DOI] [PubMed] [Google Scholar]
- 22.Emilsson K, Brudin L, Wandt B. The mode of left ventricular pumping: is there an outer contour change in addition to the atrioventricular plane displacement? Clin Physiol 2001;21:437–46. 10.1046/j.1365-2281.2001.00343.x [DOI] [PubMed] [Google Scholar]
- 23.MacIver DH, Adeniran I, Zhang H. Left ventricular ejection fraction is determined by both global myocardial strain and wall thickness. IJC Hear Vasc 2015;7: 113–18. 10.1016/j.ijcha.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seravalle G, Lonati L, Buzzi S, et al. . Sympathetic nerve traffic and baroreflex function in optimal, normal, and high-normal blood pressure states. J Hypertens 2015;33:1411–17. 10.1097/HJH.0000000000000567 [DOI] [PubMed] [Google Scholar]
- 25.Adams JW, Sakata Y, Davis MG, et al. . Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA 1998;95:10140–5. 10.1073/pnas.95.17.10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nethononda RM, Lewandowski AJ, Stewart R, et al. . Gender specific patterns of age-related decline in aortic stiffness: a cardiovascular magnetic resonance study including normal ranges. J Cardiovasc Magn Reson 2015;17:20 10.1186/s12968-015-0126-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swierblewska E, Hering D, Kara T, et al. . An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens 2010;28:979–84. 10.1097/HJH.0b013e328336ed9a [DOI] [PubMed] [Google Scholar]
- 28.Devereux RB, Wachtell K, Gerdts E, et al. . Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004;292:2350–6. 10.1001/jama.292.19.2350 [DOI] [PubMed] [Google Scholar]
- 29.Coelho-Filho OR, Shah RV, Neilan TG, et al. . Cardiac magnetic resonance assessment of interstitial myocardial fibrosis and cardiomyocyte hypertrophy in hypertensive mice treated with spironolactone. J Am Heart Assoc 2014;3:e000790–e000790. 10.1161/JAHA.114.000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2016-309576supp_figures.pdf (1.3MB, pdf)