Abstract
Infection is a common complication and is the second leading cause of death in hemodialysis patients. The risk of bacteremia in hemodialysis patients is 26-fold higher than in the general population, and 1/2-3/4 of the causative organisms of bacteremia in hemodialysis patients are Gram-positive bacteria. The ratio of resistant bacteria in hemodialysis patients compared to the general population is unclear. Several reports have indicated that hemodialysis patients have a higher risk of methicillin-resistant Staphylococcus aureus infection. The most common site of infection causing bacteremia is internal prostheses; the use of a hemodialysis catheter is the most important risk factor for bacteremia. Although antibiotic lock of hemodialysis catheters and topical antibiotic ointment can reduce catheter-related blood stream infection (CRBSI), their use should be limited to necessary cases because of the emergence of resistant organisms. Systemic antibiotic administration and catheter removal is recommended for treating CRBSI, although a study indicated the advantages of antibiotic lock and guidewire exchange of catheters over systemic antibiotic therapy. An infection control bundle recommended by the Center for Disease Control and Prevention succeeded in reducing bacteremia in hemodialysis patients with either a catheter or arteriovenous fistula. Appropriate infection control can reduce bacteremia in hemodialysis patients.
Keywords: Bacteremia, Hemodialysis, Blood stream infection, Epidemiology, Infection control
Core tip: Infection is common in hemodialysis patients, who are at high risk for bacteremia. The use of a hemodialysis catheter is the most important risk factor for bacteremia. Improvement of standard infection control measures, including the reduction of catheter use, appropriate catheter care, patient and staff education, and hand hygiene could reduce bacteremia in hemodialysis patients.
INTRODUCTION
Infection is the second leading cause of death in hemodialysis patients in many countries and is the leading cause of death in the first year of hemodialysis in Japan[1-3]. Furthermore, infection is a major cause of hospitalization in hemodialysis patients. In the United States, infection was observed in approximately 30% of all hospitalizations of hemodialysis patients[4,5]. These data indicate that infection is a serious threat to these patients. Hemodialysis patients have higher rates of bacteremia, whereas peritoneal dialysis patients have higher rates of peritonitis[4,6]. In a study in the United States, the rates (per 100 person-years) of specific infection-related hospitalizations of hemodialysis patients were 17.6 for septicemia, 15.3 for pulmonary infections, 3.7 for gastrointestinal infections, 12.3 for genitourinary infections, and 10.2 for soft-tissue infections[4]. In a cohort study in Denmark, the incidence of blood stream infection was 13.7 per 100 person-years in hemodialysis patients and 0.53 per 100 person-years in a population control[7]. These data indicate higher risks of bacteremia and a significant need to reduce bacteremia in hemodialysis patients. In this review, we describe the features of bacteremia, including its prevalence, microbiological features, and risk factors in hemodialysis patients. And we describe details of catheter related bacteremia, a characteristic bacteremia in hemodialysis patients. Furthermore we discuss how to reduce the risk of bacteremia in hemodialysis patients.
INCIDENCE OF BACTEREMIA IN HEMODIALYSIS PATIENTS
The incidence of bacteremia in hemodialysis patients is very high compared with its incidence in the general population. A population-based cohort study in Denmark showed that the incidence of bacteremia was 13.7 per 100 person years in hemodialysis patients, whereas that in the general population was 0.53 per 100 person years[7]. The incidence of Staphylococcus aureus (S. aureus) bacteremia in hemodialysis patients was 46.9-fold that of the general population in Denmark[8]. In studies in Canada, the relative risks of Pseudomonas aeruginosa and anaerobe infection were 123.3 and 72.7, respectively, in hemodialysis patients[9,10].
Almost all studies on bacteremia in Japan were case reports[11-13]. Sepsis was the second leading cause of death in infectious diseases in a study in Japan[14]. However, microbiological studies have not been conducted. On the other hand, the greater use of arteriovenous fistula and low catheter use are unique characteristics in Japan[15]. Eighty nine point seven percent of vascular accesses of Japanese hemodialysis patients was native arteriovenous fistula, 7.1% was arteriovenous grafts, 1.8% was superficialization of artery, and only 0.5% was long-term catheters in 2008[16]. It might be a reason why few studies have conducted on catheter related bacteremia in Japan. Further studies are required to clarify about the bacteremia in Japan.
In the Dialysis Outcomes and Practice Patterns Study (DOPPS), adjusted relative risks of mortality were 2.84 for Europe and 3.78 for the United States compared with Japan, although it was speculated because not all dialysis facilities in Japan enrolled in the study[17]. The percentage of infection in cause of death was about 18% in Japan, which is higher than North America and comparable with Europe and Australia/New Zealand in the DOPPS[18]. The other causes of death may contribute to the difference of mortality.
CAUSATIVE ORGANISMS
Half to 3/4 of the causative organisms of bacteremia in hemodialysis patients are Gram-positive bacteria (Table 1)[19-21]. The remaining less than 1/4 are Gram-negative. Among the causative organisms, S. aureus, including methicillin-resistant S. aureus (MRSA), is the most common causative organism. Other staphylococci, including S. epidermidis and coagulase negative staphylococcus (CNS), are also common Gram-positive organisms. Escherichia coli (E. coli), Enterobacter species and Klebsiella species are the common Gram-negative organisms isolated from blood samples. The pattern of causative organisms was similar in vascular access-associated and catheter-related bacteremia[20,22]. In hemodialysis patients, rate of S. aureus was relatively high and rate of E. coli was relatively low compared with the general population[23,24]. It is unclear whether the ratio of resistant bacteria in hemodialysis patients is higher than that in the general population. In a single-center report from Brazil in 2010-2013, 38.5% of S. aureus was MRSA[25], whereas the methicillin resistance percentage was 31.0% in surveillance data from Brazil in 2005-2008[26]. However, a national surveillance report for England indicated that the relative risk of MRSA bacteremia was approximately 100-fold higher for dialysis patients than for the general population and was 8-fold higher for patients using a catheter than for those with an arteriovenous fistula[27]. In addition, another surveillance report from the United States also indicated that dialysis patients had a 100-fold higher risk of MRSA infection than the general population[28].
Table 1.
Causative organisms of bacteremia in hemodialysis patients
| Hemodialysis patients | Hemodialysis vascular access-associated | Hemodialysis catheter-related bacteremia | General population | |||||
| Ref. | Danese et al[19], 2006 | Loo et al[20], 2015 | D’Amato-Palumbo et al[21], 2013 | Aslam et al[22], 2014 | Biedenbach et al[23], 2004 | Alfandari et al[24], 2016 | ||
| Region | United States | Singapore | United States | Meta-analysis | North America | Latin America | Europe | France |
| n | 15618 | 144 | 112 | 1386 | 42857 | 11743 | 26613 | 519 |
| Gram positive | 73.6% | 73.2% | 39.7% | |||||
| Staphylococcus aureus | 38.4% | 47.2% | 50.9% | 25.9% | 26.0% | 21.6% | 19.5% | 15.4% |
| (MRSA) | 13.9% | 23.2% | 2.9% | |||||
| (MRSA/SA) | 29.4% | 45.6% | 18.8% | |||||
| Other staphylococcus | 15.4% | 20.1% | 10.7% | 23.4% | 11.5% | 13.3% | 14.6% | 8.3% |
| Streptococcus | 11.9% | 2.7% | 9.5% | 6.8% | 6.5% | 12.5% | ||
| Enterococcus | 8.9% | 10.2% | 3.3% | 7.2% | 3.5% | |||
| Gram negative | 26.4% | 23.2% | 22.0% | 55.3% | ||||
| Escherichia coli | 6.5% | 4.5% | 17.7% | 18.2% | 22.4% | 34.5% | ||
| Pseudomonas spp. | 3.6% | 9.0% | 9.8% | 4.3% | 6.5% | 6.1% | 1.5% | |
| Enterobacter spp. | 4.9% | 3.7% | 5.5% | 4.2% | 3.7% | |||
| Klebsiella spp. | 5.4% | 7.6% | 10.1% | 7.3% | 7.1% | |||
| Proteus mirabilis | 2.3% | |||||||
| Candida spp. | 1.2% | 3.6% | 6.2% | |||||
MRSA: Methicillin-resistant Staphylococcus aureus.
CAUSES OF BACTEREMIA
In seven years of hospitalization and death records of hemodialysis patients in the United States Renal Data System, the secondary diagnosis codes among all episodes of septicemia were infection/inflammation caused by internal prostheses (18%), other complications of internal prosthetic device (8%), decubitus ulcer (6%), urinary tract infections (5%), pneumonia (5%), gangrene (3%), endocarditis (2%), and cellulitis and abscess of the foot (1%). These data indicated that septicemia secondary to vascular access infection was the most common cause of septicemia[29]. In the analysis of the causes of hospitalization for infection, the leading causes of hospitalization for infection were dialysis access or central venous catheter-related infections (30%), bloodstream infections or sepsis (24%), and pulmonary infections (22%)[6]. These data also indicated that blood access infection is the most common cause of infection in hemodialysis patients.
RISK FACTORS
The most important risk factor for bacteremia in hemodialysis patients is the use of central venous catheters. Hemodialysis catheter uses were at higher risk of bacteremia compared with arteriovenous fistula or graft uses. A single center study in the United States indicated that the rate of positive blood cultures in patients with central venous catheters was 1.86/1000 d and 0.08/1000 d in patients with an arteriovenous fistula and 0.31/1000 d in patients with an arteriovenous graft[30]. Rate of infection related hospitalization was higher in the patients with catheters or arteriovenous grafts compared with arteriovenous fistula, the rate ratios were 1.59 and 1.37, respectively[5]. Analysis of the United States Renal Data System showed that hemodialysis patients with a temporary catheter had a 50% higher risk of septicemia than patients with a native fistula. Patients with a GOR-TEX or bovine graft had a 33% higher risk of septicemia than patients with a native fistula during throughout seven years of follow-up[29]. In a retrospective study of a hospital in Brazil, a multiple regression analysis showed that the use of a central venous catheter was associated with an 11.2-fold increased risk of bloodstream infections compared with arteriovenous fistula for vascular access[25]. In addition, a second leading risk factor was previous hospitalizations, which had an odds ratio of 6.63 in a multiple logistic regression analysis[25]. In patient characteristics, the adjusted risk ratios of age > 65 years, diabetes mellitus, and serum albumin < 3.5 were 1.61, 1.26 and 1.66, respectively[29]. The patients who reused dialyzers had a 28% higher risk of septicemia than patients who did not reuse membranes[29].
CATHETER-RELATED BLOOD STREAM INFECTIONS
Long-term catheters are essential for hemodialysis patients whose blood access site is limited. Catheters are a major risk factor for bacteremia as described above, and they cannot be easily changed. Therefore, especially careful handling is needed to prevent catheter-related blood stream infections (CRBSI).
Diagnosis of CRBSI
In the guidelines for the diagnosis and management of intravascular catheter-related infection by the Infectious Diseases Society of America, a definitive diagnosis of CRBSI requires: (1) a set of peripheral blood cultures; (2) blood cultures from a peripheral vein and from a culture of the catheter tip; or (3) cultures from an arterial and venous catheter hub that meet differential time-to-positivity criteria[31]. A report from Canada indicated that blood cultures drawn from a hemodialysis circuit were the most sensitive, specific, and accurate for diagnosing CRBSIs when all culture data and clinical information were factored into the assessment[32]. For the hemodialysis circuit, the values for sensitivity, specificity, and accuracy were 93.5%, 100% and 95%, respectively, whereas peripheral veins had a sensitivity of 93.9%, a specificity of 92.5%, and an accuracy of 93%.
Antibiotic lock for hemodialysis catheter
There are many reports and a meta-analysis of antibiotic lock for hemodialysis catheters[33-39]. The antibiotics include gentamycin, minocycline, cefotaxime, cefazolin, and vancomycin (Table 2). Moreover, antiseptics including taurolidine and trisodium citrate have also been tested. Antibiotic lock therapy significantly reduced CRBSI in all studies. In a subgroup analysis of each antibiotic, the reductions in bacteremia rates remained significant for locks containing gentamicin, minocycline, cefotaxime, and vancomycin and gentamicin, but not for those containing taurolidine, or cefazolin and gentamicin, or citrate[33,39]. Although they are associated with a significant reduction of CRBSI, the guidelines of the Centers for Disease Control and Prevention (CDC) do not recommend the routine use of antibiotic lock, and limit this treatment to patients with long-term catheters who have a history of multiple CRBSIs despite optimal maximal adherence to aseptic techniques because of the potential for side effects, toxicity, allergic reactions, or the emergence of resistance to the antimicrobial agent[40].
Table 2.
Antibiotic concentrations applied in locks[31]
| Dosage (mg/mL) | Heparin or saline, IU/mL | |
| Vancomycin | 2.5 | 2500 or 5000 |
| Vancomycin | 2.0 | 10 |
| Vancomycin | 5.0 | 0 or 5000 |
| Ceftazidime | 0.5 | 100 |
| Cefazolin | 5.0 | 2500 or 5000 |
| Ciprofloxacin | 0.2 | 5000 |
| Gentamicin | 1.0 | 2500 |
| Ampicillin | 10.0 | 10 or 5000 |
| 70% ethanol | 0 |
Topical antibiotics
The prophylactic effects of the application of topical antibiotics, including mupirocin and polysporin triple antibiotic ointments, to the exit sites of the catheter were also investigated. These topical antibiotics significantly reduced CRBSI in hemodialysis patients. A randomized trial indicated that the topical use of polysporin triple antibiotic ointment to catheter exit sites reduced the relative risk of bacteremia by 60%, as well as the relative risk of mortality by 78%[41]. Mupirocin ointment is also effective to reduce CRBSI. In a randomized controlled trial, application of mupirocin ointment to the catheter exit sites reduced CRBSI by 85%[42]. However, the rapid emergence of resistant S. aureus and CNS has been reported[43-45]. Based on this evidence, the CDC guidelines recommend the antibiotic ointment for only hemodialysis patients.
Antibiotics/antiseptics coated catheters
Antibiotics or antiseptics impregnated or coated catheters can reduce the catheter related bacteremia[46,47]. The duration of catheter use in these studies were within a month, and the efficacy in long term use of these catheter has not been established[48].
Treatment of catheter related bacteremia
Generally, the treatment of bacteremia is the administration of systemic antibiotics and the care of the infection site; for CRBSI, this necessitates catheter removal. Initial treatment is empiric systemic antibiotics and antibiotic lock (Figure 1, Tables 2 and 3)[31]. Recommended empirical antibiotics are vancomycin plus empirical gram-negative rod coverage based on local antibiogram data. Catheter removal is required in cases that bacteremia or clinical symptom persists, or in cases that causative organisms could colonize in the surface of catheters (Figure 1). For hemodialysis patients, catheters sometimes cannot be removed due to limited blood access sites. Antibiotic lock therapy and guidewire exchange of the catheter are attempted in those cases. A meta-analysis compared systemic antibiotics, antibiotic lock therapy and guidewire exchange of the catheter to treat patients with tunneled hemodialysis catheter-related bacteremia[22]. The cure proportions were significantly higher with antibiotic lock therapy than with systemic antibiotics (OR = 2.08; 95%CI: 1.25-3.45; P < 0.01) and were higher with guidewire exchange than with systemic antibiotics (OR = 2.88; 95%CI: 1.82-4.55; P < 0.001). In particular, for those with S. aureus infections, guidewire exchange achieved a significantly higher cure proportion than both systemic antibiotics and antibiotic lock therapy (OR = 3.33, 95%CI: 1.17-9.46, P = 0.02; OR = 4.72, 95%CI: 1.79-12.46, P = 0.002). However, guidelines recommend systemic antibiotics and catheter removal, especially for infection with S. aureus, P. aeruginosa, or Candida species[31,48]. Guidewire exchange is considered to be an alternative only if another insertion site is not available. This study described above was a meta-analysis of observational studies. Randomized controlled studies are required to obtain further insight.
Figure 1.
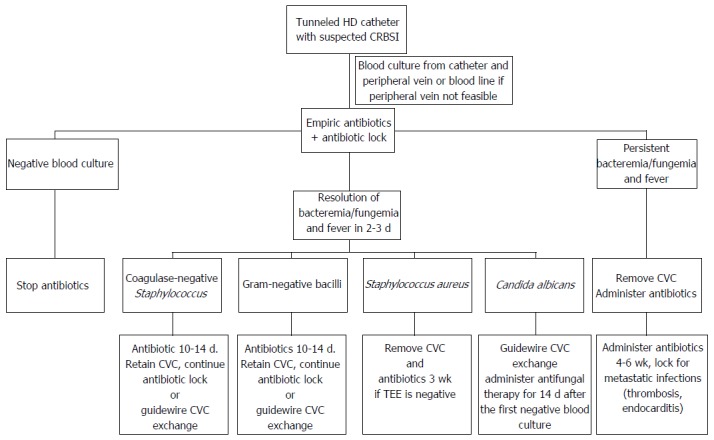
Catheter-related blood stream infection among patients who are undergoing hemodialysis with tunneled catheters[31]. CVC: Central venous catheter; TEE: Transesophageal echocardiograph; HD: Hemodialysis; CRBSI: Catheter-related blood stream infection.
Table 3.
Antibiotic dosing for patients who are undergoing hemodialysis[31]
| Empirical dosing pending culture results |
| Vancomycin plus empirical gram-negative rod coverage based on local antibiogram data |
| Or |
| Vancomycin plus gentamicin (Cefazolin may be used in place of vancomycin in units with a low prevalence of methicillin-resistant staphylococci) |
| Vancomycin: 20 mg/kg loading dose infused during the last hour of the dialysis session, and then 500 mg during the last 30 min of each subsequent dialysis session |
| Gentamicin (or tobramycin): 1 mg/kg, not to exceed 100 mg after each dialysis session |
| Ceftazidime: 1 g iv after each dialysis session |
| Cefazolin: 20 mg/kg iv after each dialysis session |
| For Candida infection |
| An echinocandin (caspofungin 70 mg iv loading dose followed by 50 mg iv daily; intravenous micafungin 100 mg iv daily; or anidulafungin 200 mg iv loading dose, followed by 100 mg iv daily); fluconazole (200 mg orally daily); or amphotericin-B |
iv: Intravenous.
Improvement of infection control measures
In 2009, the CDC established a collaborative project to prevent bloodstream infection in hemodialysis patients[49]. Core interventions included surveillance and feedback using the National Healthcare Safety Network, audits of hand hygiene, observation of vascular access care, and other infection control measures (Table 4). In the analysis of 17 outpatient hemodialysis facilities that participated in the project, in the pre-intervention period, the pooled mean blood stream infections (BSI) and access-related BSI rates were 1.09 and 0.73 events per 100 patient-months, respectively. After the intervention, these rates decreased to 0.89 and 0.42 events per 100 patient-months, respectively[49]. Furthermore, in a report using positive deviance to improve the infection control measures in addition to the collaborative interventions of the CDC, the incidence of all access-related BSIs reduced from 2.04 per 100 patient-months pre-intervention to 0.75 after employing the collaborative interventions and to 0.24 after augmenting the collaborative interventions with positive deviance[50]. Positive deviance is a behavioral change process based on the observation of those with uncommon, beneficial practices who consequently experience better outcomes than their neighbors who share similar risks[51]. These data indicated that the improvement of infection control practices could reduce bacteremia in hemodialysis patients.
Table 4.
Core interventions of dialysis blood stream infections prevention in collaboration with the Centers for Disease Control and Prevention
| Surveillance and feedback using NHSN |
| Conduct monthly surveillance for BSIs and other dialysis events using NHSN-Dialysis Surveillance. Calculate facility rates and compare to rates in other NHSN facilities. Actively share results with front-line clinical staff. See Data Reports on this website (available from: URL: http://www.cdc.gov/dialysis/reports-news/data-reports.html) |
| Hand hygiene observations |
| Perform observations of hand hygiene opportunities monthly and share results with clinical staff. See observation protocols for hand hygiene and glove use on this website (available from: URL: http://www.cdc.gov/dialysis/PDFs/collaborative/Hemodialysis-Hand-Hygiene-Observations.pdf) |
| Catheter/vascular access care observations |
| Perform observations of vascular access care and catheter accessing quarterly. Assess staff adherence to aseptic technique when connecting and disconnecting catheters and during dressing changes. Share results with clinical staff |
| Staff education and competency |
| Train staff on infection control topics, including access care and aseptic technique. Perform competency evaluation for skills such as catheter care and accessing every 6-12 mo and upon hire. See staff education on this website (available from: URL: http://www.cdc.gov/dialysis/clinician/index.html) |
| Patient education/engagement |
| Provide standardized education to all patients on infection prevention topics including vascular access care, hand hygiene, risks related to catheter use, recognizing signs of infection, and instructions for access management when away from the dialysis unit. See patient education on this website (available from: URL: http://www.cdc.gov/dialysis/clinician/index.html) |
| Catheter reduction |
| Incorporate efforts (e.g., through patient education, vascular access coordinator) to reduce catheters by identifying and addressing barriers to permanent vascular access placement and catheter removal |
| Chlorhexidine for skin antisepsis |
| Use an alcohol-based chlorhexidine (> 0.5%) solution as the first line skin antiseptic agent for central line insertion and during dressing changes. Povidone-iodine (preferably with alcohol) or 70% alcohol are alternatives for patients with chlorhexidine intolerance |
| Catheter hub disinfection |
| Scrub catheter hubs with an appropriate antiseptic after cap is removed and before accessing. Perform every time catheter is accessed or disconnected. If closed needleless connector device is used, disinfect connector device per manufacturer’s instructions |
| Antimicrobial ointment |
| Apply antibiotic ointment or povidone-iodine ointment to catheter exit sites during dressing change. See information on selecting an antimicrobial ointment for hemodialysis catheter exit sites (selecting an antimicrobial ointment). Use of chlorhexidine-impregnated sponge dressing might be an alternative |
NHSN: National Healthcare Safety Network; BSIs: Blood stream infections.
CONCLUSION
The prevalence of blood stream infection in hemodialysis patients is much higher than in the general population. Furthermore, bacteremia is sometimes life-threatening. Improvement of basic infection control measures, including appropriate hand hygiene, catheter care, and education for medical staff and patients, could reduce the occurrence of bacteremia, although this is difficult because the blood streams of these patients are frequently exposed to extracorporeal devices.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: No conflicts of interest are associated with this article.
Peer-review started: July 1, 2016
First decision: August 5, 2016
Article in press: September 22, 2016
P- Reviewer: Bernieh B, Burdette SD, Watanabe T S- Editor: Ji FF L- Editor: A E- Editor: Li D
References
- 1.Masakane I, Nakai S, Ogata S, Kimata N, Hanafusa N, Hamano T, Wakai K, Wada A, Nitta K. An Overview of Regular Dialysis Treatment in Japan (As of 31 December 2013) Ther Apher Dial. 2015;19:540–574. doi: 10.1111/1744-9987.12378. [DOI] [PubMed] [Google Scholar]
- 2.2015 USRDS Annual Data Report. Volume 2 - ESRD in the United States Chapter 6: Mortality. Am J Kidney Dis. 2016;67:S219–S226. [Google Scholar]
- 3.Pruthi R, Steenkamp R, Feest T. UK Renal Registry 16th annual report: chapter 8 survival and cause of death of UK adult patients on renal replacement therapy in 2012: national and centre-specific analyses. Nephron Clin Pract. 2013;125:139–169. doi: 10.1159/000360027. [DOI] [PubMed] [Google Scholar]
- 4.Dalrymple LS, Johansen KL, Chertow GM, Cheng SC, Grimes B, Gold EB, Kaysen GA. Infection-related hospitalizations in older patients with ESRD. Am J Kidney Dis. 2010;56:522–530. doi: 10.1053/j.ajkd.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalrymple LS, Mu Y, Nguyen DV, Romano PS, Chertow GM, Grimes B, Kaysen GA, Johansen KL. Risk Factors for Infection-Related Hospitalization in In-Center Hemodialysis. Clin J Am Soc Nephrol. 2015;10:2170–2180. doi: 10.2215/CJN.03050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalrymple LS, Mu Y, Romano PS, Nguyen DV, Chertow GM, Delgado C, Grimes B, Kaysen GA, Johansen KL. Outcomes of infection-related hospitalization in Medicare beneficiaries receiving in-center hemodialysis. Am J Kidney Dis. 2015;65:754–762. doi: 10.1053/j.ajkd.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skov Dalgaard L, Nørgaard M, Jespersen B, Jensen-Fangel S, Østergaard LJ, Schønheyder HC, Søgaard OS. Risk and Prognosis of Bloodstream Infections among Patients on Chronic Hemodialysis: A Population-Based Cohort Study. PLoS One. 2015;10:e0124547. doi: 10.1371/journal.pone.0124547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen LH, Jensen-Fangel S, Benfield T, Skov R, Jespersen B, Larsen AR, Østergaard L, Støvring H, Schønheyder HC, Søgaard OS. Risk and prognosis of Staphylococcus aureus bacteremia among individuals with and without end-stage renal disease: a Danish, population-based cohort study. BMC Infect Dis. 2015;15:6. doi: 10.1186/s12879-014-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ngo JT, Parkins MD, Gregson DB, Pitout JD, Ross T, Church DL, Laupland KB. Population-based assessment of the incidence, risk factors, and outcomes of anaerobic bloodstream infections. Infection. 2013;41:41–48. doi: 10.1007/s15010-012-0389-4. [DOI] [PubMed] [Google Scholar]
- 10.Parkins MD, Gregson DB, Pitout JD, Ross T, Laupland KB. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection. 2010;38:25–32. doi: 10.1007/s15010-009-9145-9. [DOI] [PubMed] [Google Scholar]
- 11.Okada A, Hangai M, Oda T. Bacteremia with an iliopsoas abscess and osteomyelitis of the femoral head caused by Enterococcus avium in a patient with end-stage kidney disease. Intern Med. 2015;54:669–674. doi: 10.2169/internalmedicine.54.3576. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama A, Yamanaka K, Hayashi H, Ohkusu K. Moraxella lacunata infection associated with septicemia, endocarditis, and bilateral septic arthritis in a patient undergoing hemodialysis: a case report and review of the literature. J Infect Chemother. 2014;20:61–64. doi: 10.1016/j.jiac.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Kenzaka T, Takamura N, Kumabe A, Takeda K. A case of subacute infective endocarditis and blood access infection caused by Enterococcus durans. BMC Infect Dis. 2013;13:594. doi: 10.1186/1471-2334-13-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakasugi M, Kawamura K, Yamamoto S, Kazama JJ, Narita I. High mortality rate of infectious diseases in dialysis patients: a comparison with the general population in Japan. Ther Apher Dial. 2012;16:226–231. doi: 10.1111/j.1744-9987.2012.01062.x. [DOI] [PubMed] [Google Scholar]
- 15.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis. 2009;53:475–491. doi: 10.1053/j.ajkd.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 16.Kukita K, Ohira S, Amano I, Naito H, Azuma N, Ikeda K, Kanno Y, Satou T, Sakai S, Sugimoto T, et al. 2011 update Japanese Society for Dialysis Therapy Guidelines of Vascular Access Construction and Repair for Chronic Hemodialysis. Ther Apher Dial. 2015;19 Suppl 1:1–39. doi: 10.1111/1744-9987.12296. [DOI] [PubMed] [Google Scholar]
- 17.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 18.Hecking M, Bieber BA, Ethier J, Kautzky-Willer A, Sunder-Plassmann G, Säemann MD, Ramirez SP, Gillespie BW, Pisoni RL, Robinson BM, et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS) PLoS Med. 2014;11:e1001750. doi: 10.1371/journal.pmed.1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danese MD, Griffiths RI, Dylan M, Yu HT, Dubois R, Nissenson AR. Mortality differences among organisms causing septicemia in hemodialysis patients. Hemodial Int. 2006;10:56–62. doi: 10.1111/j.1542-4758.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- 20.Loo LW, Liew YX, Choong HL, Tan AL, Chlebicki P. Microbiology and audit of vascular access-associated bloodstream infections in multi-ethnic Asian hemodialysis patients in a tertiary hospital. Infect Dis (Lond) 2015;47:225–230. doi: 10.3109/00365548.2014.986193. [DOI] [PubMed] [Google Scholar]
- 21.D’Amato-Palumbo S, Kaplan AA, Feinn RS, Lalla RV. Retrospective study of microorganisms associated with vascular access infections in hemodialysis patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:56–61. doi: 10.1016/j.oooo.2012.08.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aslam S, Vaida F, Ritter M, Mehta RL. Systematic review and meta-analysis on management of hemodialysis catheter-related bacteremia. J Am Soc Nephrol. 2014;25:2927–2941. doi: 10.1681/ASN.2013091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997-2002) Diagn Microbiol Infect Dis. 2004;50:59–69. doi: 10.1016/j.diagmicrobio.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Alfandari S, Cabaret P, Nguyen S, Descamps D, Vachée A, Cattoen C, Van Grunderbeeck N. Evaluating the management of 493 patients presenting with bacteremia in 23 northern French hospitals. Med Mal Infect. 2016;46:194–199. doi: 10.1016/j.medmal.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Fram D, Okuno MF, Taminato M, Ponzio V, Manfredi SR, Grothe C, Belasco A, Sesso R, Barbosa D. Risk factors for bloodstream infection in patients at a Brazilian hemodialysis center: a case-control study. BMC Infect Dis. 2015;15:158. doi: 10.1186/s12879-015-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gales AC, Sader HS, Ribeiro J, Zoccoli C, Barth A, Pignatari AC. Antimicrobial susceptibility of gram-positive bacteria isolated in Brazilian hospitals participating in the SENTRY Program (2005-2008) Braz J Infect Dis. 2009;13:90–98. doi: 10.1590/s1413-86702009000200004. [DOI] [PubMed] [Google Scholar]
- 27.Fluck R, Wilson J, Davies J, Blackburn R, O’Donoghue D, Tomson CR. UK Renal Registry 11th Annual Report (December 2008): Chapter 12 Epidemiology of Methicillin Resistant Staphylococcus aureus bacteraemia amongst patients receiving Renal Replacement Therapy in England in 2007. Nephron Clin Pract. 2009;111 Suppl 1:c247–c256. doi: 10.1159/000210001. [DOI] [PubMed] [Google Scholar]
- 28.Invasive methicillin-resistant Staphylococcus aureus infections among dialysis patients--United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:197–199. [PubMed] [Google Scholar]
- 29.Powe NR, Jaar B, Furth SL, Hermann J, Briggs W. Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int. 1999;55:1081–1090. doi: 10.1046/j.1523-1755.1999.0550031081.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Burr RA, Sheth HS, Piraino B. Organism-specific bacteremia by hemodialysis access. Clin Nephrol. 2016;86:141–146. doi: 10.5414/CN108633. [DOI] [PubMed] [Google Scholar]
- 31.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quittnat Pelletier F, Joarder M, Poutanen SM, Lok CE. Evaluating Approaches for the Diagnosis of Hemodialysis Catheter-Related Bloodstream Infections. Clin J Am Soc Nephrol. 2016;11:847–854. doi: 10.2215/CJN.09110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Li Z, Zhang L, Yang J, Yang Y, Tang Y, Fu P. Citrate versus heparin lock for hemodialysis catheters: a systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis. 2014;63:479–490. doi: 10.1053/j.ajkd.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Yahav D, Rozen-Zvi B, Gafter-Gvili A, Leibovici L, Gafter U, Paul M. Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta-analysis of randomized, controlled trials. Clin Infect Dis. 2008;47:83–93. doi: 10.1086/588667. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Wang C, Zhao H, Zhang J, Ma J, Hou Y, Zou H. RETRACTED ARTICLE: Anticoagulant agents for the prevention of hemodialysis catheter-related complications: systematic review and meta-analysis of prospective randomized controlled trials. Int Urol Nephrol. 2016;48:1111. doi: 10.1007/s11255-015-1143-5. [DOI] [PubMed] [Google Scholar]
- 36.Zacharioudakis IM, Zervou FN, Arvanitis M, Ziakas PD, Mermel LA, Mylonakis E. Antimicrobial lock solutions as a method to prevent central line-associated bloodstream infections: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:1741–1749. doi: 10.1093/cid/ciu671. [DOI] [PubMed] [Google Scholar]
- 37.Labriola L, Crott R, Jadoul M. Preventing haemodialysis catheter-related bacteraemia with an antimicrobial lock solution: a meta-analysis of prospective randomized trials. Nephrol Dial Transplant. 2008;23:1666–1672. doi: 10.1093/ndt/gfm847. [DOI] [PubMed] [Google Scholar]
- 38.Jaffer Y, Selby NM, Taal MW, Fluck RJ, McIntyre CW. A meta-analysis of hemodialysis catheter locking solutions in the prevention of catheter-related infection. Am J Kidney Dis. 2008;51:233–241. doi: 10.1053/j.ajkd.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 39.James MT, Conley J, Tonelli M, Manns BJ, MacRae J, Hemmelgarn BR. Meta-analysis: antibiotics for prophylaxis against hemodialysis catheter-related infections. Ann Intern Med. 2008;148:596–605. doi: 10.7326/0003-4819-148-8-200804150-00004. [DOI] [PubMed] [Google Scholar]
- 40.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lok CE, Stanley KE, Hux JE, Richardson R, Tobe SW, Conly J. Hemodialysis infection prevention with polysporin ointment. J Am Soc Nephrol. 2003;14:169–179. doi: 10.1097/01.asn.0000038688.76195.a4. [DOI] [PubMed] [Google Scholar]
- 42.Johnson DW, MacGinley R, Kay TD, Hawley CM, Campbell SB, Isbel NM, Hollett P. A randomized controlled trial of topical exit site mupirocin application in patients with tunnelled, cuffed haemodialysis catheters. Nephrol Dial Transplant. 2002;17:1802–1807. doi: 10.1093/ndt/17.10.1802. [DOI] [PubMed] [Google Scholar]
- 43.Zakrzewska-Bode A, Muytjens HL, Liem KD, Hoogkamp-Korstanje JA. Mupirocin resistance in coagulase-negative staphylococci, after topical prophylaxis for the reduction of colonization of central venous catheters. J Hosp Infect. 1995;31:189–193. doi: 10.1016/0195-6701(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 44.Netto dos Santos KR, de Souza Fonseca L, Gontijo Filho PP. Emergence of high-level mupirocin resistance in methicillin-resistant Staphylococcus aureus isolated from Brazilian university hospitals. Infect Control Hosp Epidemiol. 1996;17:813–816. [PubMed] [Google Scholar]
- 45.Miller MA, Dascal A, Portnoy J, Mendelson J. Development of mupirocin resistance among methicillin-resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infect Control Hosp Epidemiol. 1996;17:811–813. doi: 10.1086/647242. [DOI] [PubMed] [Google Scholar]
- 46.Raad I, Darouiche R, Dupuis J, Abi-Said D, Gabrielli A, Hachem R, Wall M, Harris R, Jones J, Buzaid A, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med. 1997;127:267–274. doi: 10.7326/0003-4819-127-4-199708150-00002. [DOI] [PubMed] [Google Scholar]
- 47.Chatzinikolaou I, Finkel K, Hanna H, Boktour M, Foringer J, Ho T, Raad I. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am J Med. 2003;115:352–357. doi: 10.1016/s0002-9343(03)00367-x. [DOI] [PubMed] [Google Scholar]
- 48.Vanholder R, Canaud B, Fluck R, Jadoul M, Labriola L, Marti-Monros A, Tordoir J, Van Biesen W. Diagnosis, prevention and treatment of haemodialysis catheter-related bloodstream infections (CRBSI): a position statement of European Renal Best Practice (ERBP) NDT Plus. 2010;3:234–246. doi: 10.1093/ndtplus/sfq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel PR, Yi SH, Booth S, Bren V, Downham G, Hess S, Kelley K, Lincoln M, Morrissette K, Lindberg C, et al. Bloodstream infection rates in outpatient hemodialysis facilities participating in a collaborative prevention effort: a quality improvement report. Am J Kidney Dis. 2013;62:322–330. doi: 10.1053/j.ajkd.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Lindberg C, Downham G, Buscell P, Jones E, Peterson P, Krebs V. Embracing collaboration: a novel strategy for reducing bloodstream infections in outpatient hemodialysis centers. Am J Infect Control. 2013;41:513–519. doi: 10.1016/j.ajic.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 51.Marsh DR, Schroeder DG, Dearden KA, Sternin J, Sternin M. The power of positive deviance. BMJ. 2004;329:1177–1179. doi: 10.1136/bmj.329.7475.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]


