Abstract
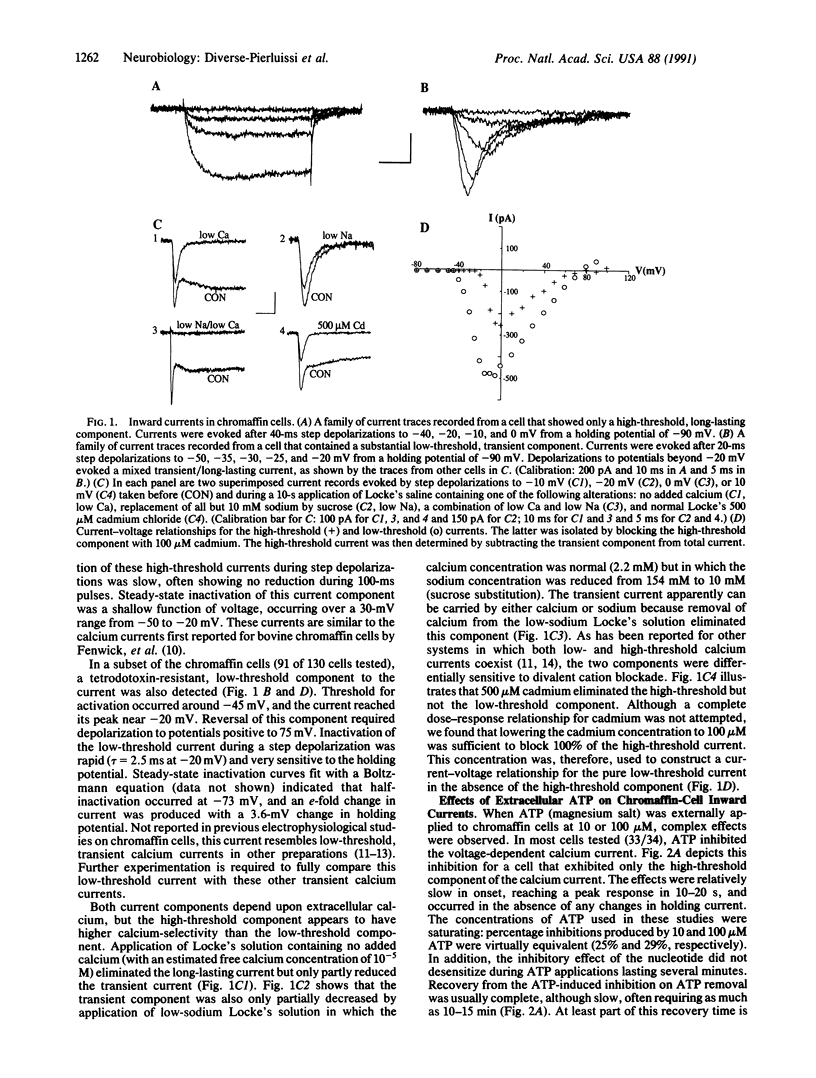
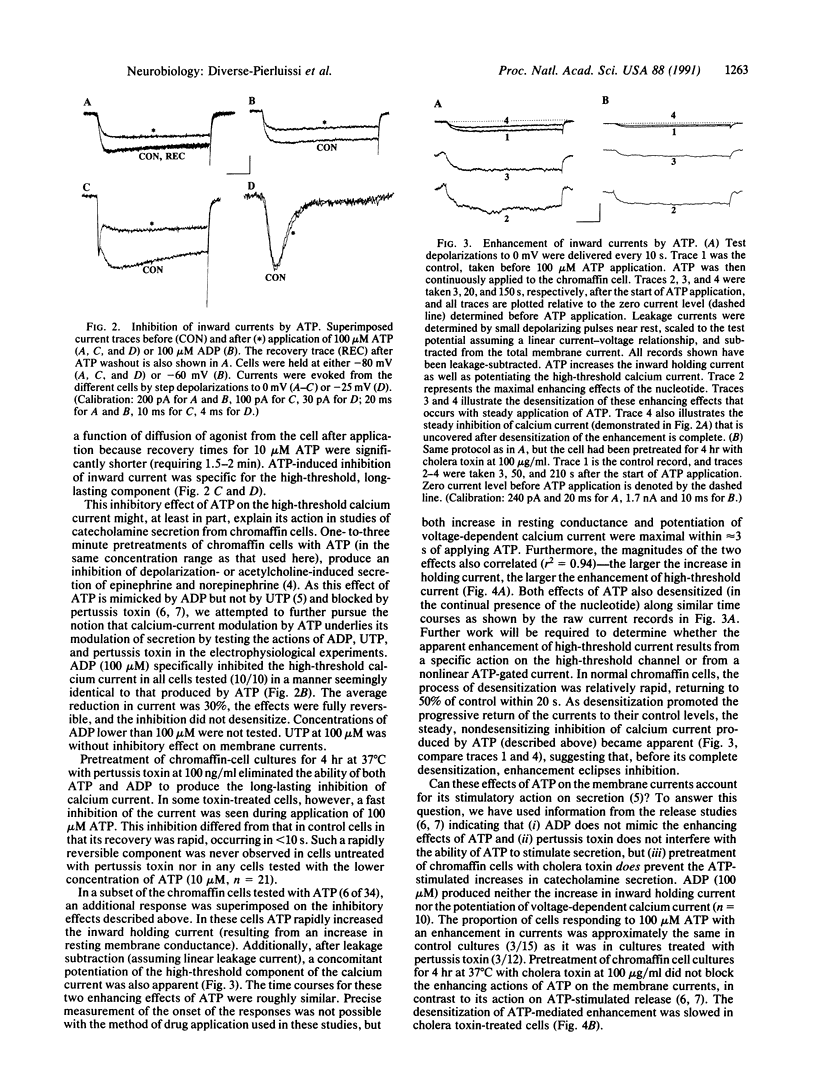
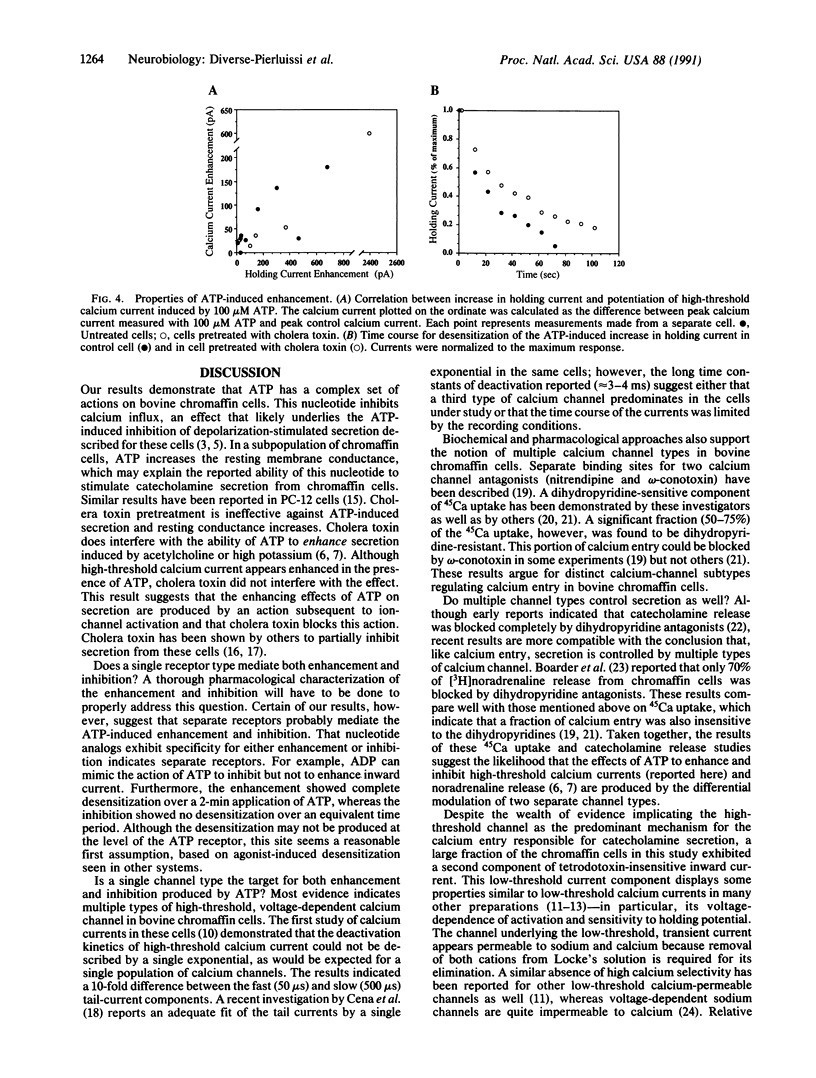
Hormone secretion from chromaffin cells is evoked by calcium influx through voltage-dependent channels in the plasma membrane. Previous studies have shown that ATP, cosecreted with catecholamines from chromaffin granules, can modulate the secretion resulting from depolarization by nicotinic agonists. The immediate effect of ATP is to enhance secretion; more prolonged exposure to the nucleotide results in inhibition. These receptor-mediated actions of ATP involve the activation of at least two separate classes of GTP-binding protein. Results from electrophysiological experiments reported here demonstrate that the modulatory actions of ATP can, in large part, be explained by the effects of the nucleotide on inward calcium current. ATP shows a rapid enhancement and a slower, persistent inhibition of the depolarization-induced inward current.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artalejo C. R., García A. G., Aunis D. Chromaffin cell calcium channel kinetics measured isotopically through fast calcium, strontium, and barium fluxes. J Biol Chem. 1987 Jan 15;262(2):915–926. [PubMed] [Google Scholar]
- Ballesta J. J., Palmero M., Hidalgo M. J., Gutierrez L. M., Reig J. A., Viniegra S., Garcia A. G. Separate binding and functional sites for omega-conotoxin and nitrendipine suggest two types of calcium channels in bovine chromaffin cells. J Neurochem. 1989 Oct;53(4):1050–1056. doi: 10.1111/j.1471-4159.1989.tb07394.x. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Boarder M. R., Marriott D., Adams M. Stimulus secretion coupling in cultured chromaffin cells. Dependency on external sodium and on dihydropyridine-sensitive calcium channels. Biochem Pharmacol. 1987 Jan 1;36(1):163–167. doi: 10.1016/0006-2952(87)90394-7. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceña V., Nicolas G. P., Sanchez-Garcia P., Kirpekar S. M., Garcia A. G. Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience. 1983 Dec;10(4):1455–1462. doi: 10.1016/0306-4522(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Ceña V., Stutzin A., Rojas E. Effects of calcium and Bay K-8644 on calcium currents in adrenal medullary chromaffin cells. J Membr Biol. 1989 Dec;112(3):255–265. doi: 10.1007/BF01870956. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Cyclic AMP inhibits both nicotine-induced actin disassembly and catecholamine secretion from bovine adrenal chromaffin cells. J Biol Chem. 1987 Aug 25;262(24):11663–11666. [PubMed] [Google Scholar]
- Chern Y. J., Kim K. T., Slakey L. L., Westhead E. W. Adenosine receptors activate adenylate cyclase and enhance secretion from bovine adrenal chromaffin cells in the presence of forskolin. J Neurochem. 1988 May;50(5):1484–1493. doi: 10.1111/j.1471-4159.1988.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to metal cations in myelinated nerve. J Gen Physiol. 1972 Jun;59(6):637–658. doi: 10.1085/jgp.59.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Nakazawa K., Fujimori K., Takanaka A. Extracellular adenosine 5'-triphosphate-evoked norepinephrine secretion not relating to voltage-gated Ca channels in pheochromocytoma PC12 cells. Neurosci Lett. 1989 Dec 4;106(3):294–299. doi: 10.1016/0304-3940(89)90179-1. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Ledbetter F. H., Carson K. A., Kirshner A. G., Slepetis R., Kirshner N. Stability of bovine adrenal medulla cells in culture. J Neurochem. 1980 Sep;35(3):679–692. doi: 10.1111/j.1471-4159.1980.tb03707.x. [DOI] [PubMed] [Google Scholar]
- Kim K. T., Westhead E. W. Cellular responses to Ca2+ from extracellular and intracellular sources are different as shown by simultaneous measurements of cytosolic Ca2+ and secretion from bovine chromaffin cells. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9881–9885. doi: 10.1073/pnas.86.24.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott D., Adams M., Boarder M. R. Effect of forskolin and prostaglandin E1 on stimulus secretion coupling in cultured bovine adrenal chromaffin cells. J Neurochem. 1988 Feb;50(2):616–623. doi: 10.1111/j.1471-4159.1988.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. An ATP-activated conductance in pheochromocytoma cells and its suppression by extracellular calcium. J Physiol. 1990 Sep;428:257–272. doi: 10.1113/jphysiol.1990.sp018211. [DOI] [PMC free article] [PubMed] [Google Scholar]


