Abstract
Previous studies showed that the cell-surface receptor for reovirus serotype 3 (Reo3R) appears at an early stage of oligodendrocyte differentiation and that anti-Reo3R antibodies and Reo3R-binding peptides induce galactocerebroside expression by developing oligodendrocytes. In the present studies, anti-Reo3R antibodies are shown to stimulate additional features of the program of oligodendrocyte development, including the loss of the A2B5 marker and expression of myelin basic protein. In anti-Reo3R antibody-treated cultures, galactocerebroside was expressed by cells having the morphology of immature oligodendrocyte precursors. Reo3R binding did not appear directly to inhibit or stimulate proliferation of glial progenitor cells or to affect their lineage commitment. Cell-surface structures utilized as a receptor by reovirus type 3 appear to play a role in the regulation of the initiation or rate of execution of the oligodendrocyte developmental program.
Full text
PDF
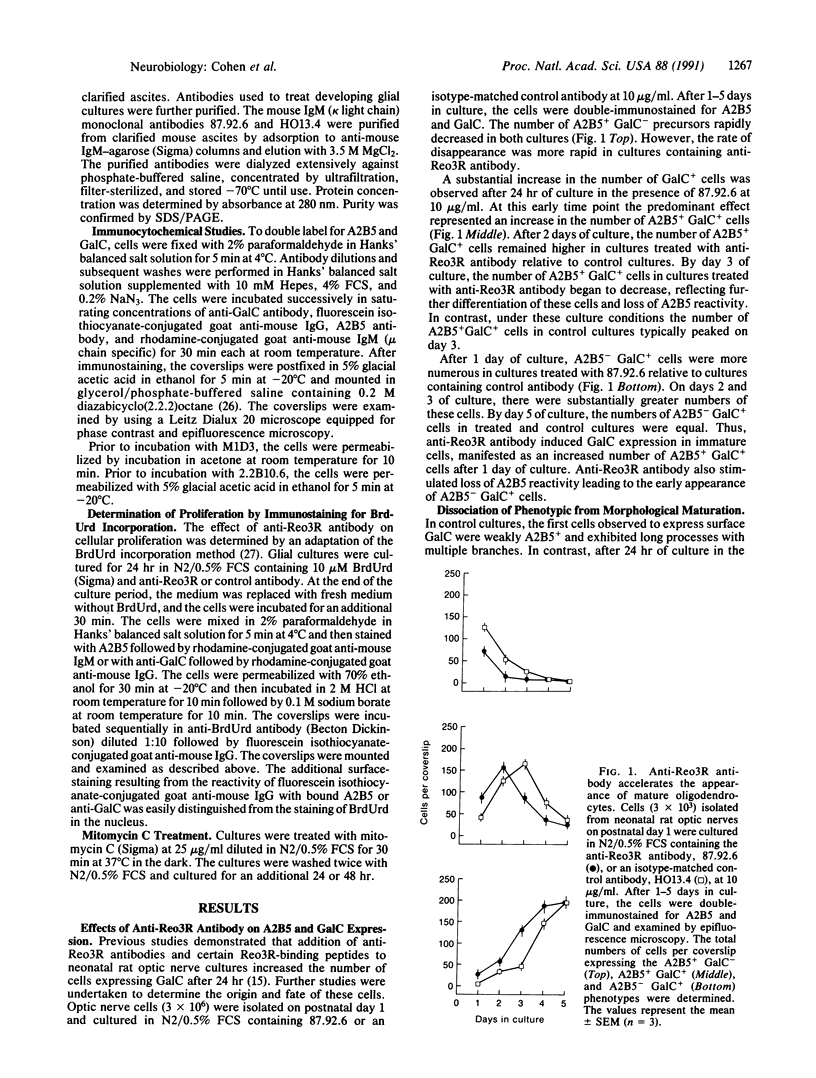
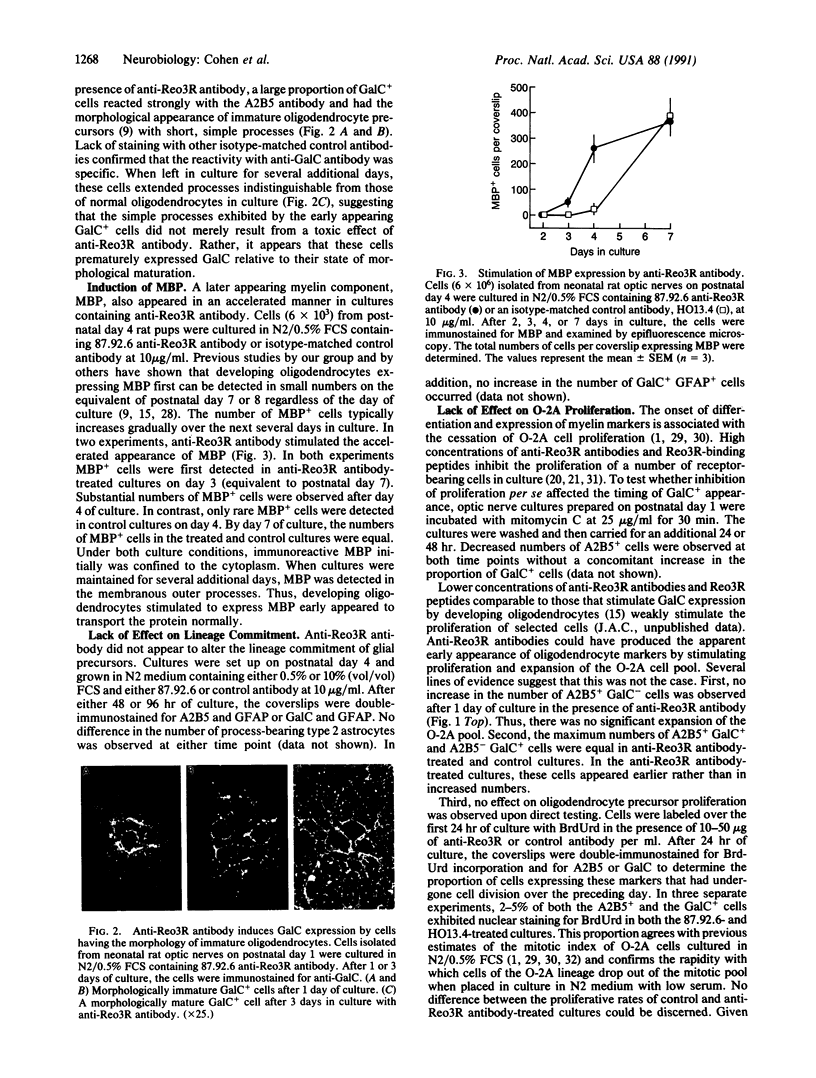


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal R., Gard A. L., Pfeiffer S. E. Stimulation of oligodendrocyte differentiation in culture by growth in the presence of a monoclonal antibody to sulfated glycolipid. J Neurosci Res. 1988 Oct-Dec;21(2-4):260–267. doi: 10.1002/jnr.490210218. [DOI] [PubMed] [Google Scholar]
- Bansal R., Pfeiffer S. E. Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6181–6185. doi: 10.1073/pnas.86.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R., Warrington A. E., Gard A. L., Ranscht B., Pfeiffer S. E. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 1989 Dec;24(4):548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck C., Co M. S., Slaoui M., Gaulton G. N., Smith T., Fields B. N., Mullins J. I., Greene M. I. Nucleic acid sequence of an internal image-bearing monoclonal anti-idiotype and its comparison to the sequence of the external antigen. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6578–6582. doi: 10.1073/pnas.83.17.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Fields B. N., Greene M. I. Isolation and biochemical characterization of the mammalian reovirus type 3 cell-surface receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1494–1498. doi: 10.1073/pnas.82.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Tominaga A., Homcy C. J., Fields B. N., Greene M. I. Structural similarities between the mammalian beta-adrenergic and reovirus type 3 receptors. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5315–5318. doi: 10.1073/pnas.82.16.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A., Sergott R. C., Geller H. M., Brown M. J., Greene M. I. Mammalian reovirus receptor expression by oligodendrocytes. Ann N Y Acad Sci. 1988;540:445–448. doi: 10.1111/j.1749-6632.1988.tb27129.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. A., Williams W. V., Weiner D. B., Geller H. M., Greene M. I. Ligand binding to the cell surface receptor for reovirus type 3 stimulates galactocerebroside expression by developing oligodendrocytes. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4922–4926. doi: 10.1073/pnas.87.13.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A., Weiner H. L. Infection of neuronal cell cultures with reovirus mimics in vitro patterns of neurotropism. Ann Neurol. 1984 Nov;16(5):603–610. doi: 10.1002/ana.410160512. [DOI] [PubMed] [Google Scholar]
- Dorfman S. H., Fry J. M., Silberberg D. H. Antiserum induced myelination inhibition in vitro without complement. Brain Res. 1979 Nov 9;177(1):105–114. doi: 10.1016/0006-8993(79)90921-1. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M. Characterization of a slowly proliferative cell along the oligodendrocyte differentiation pathway. EMBO J. 1987 Sep;6(9):2587–2595. doi: 10.1002/j.1460-2075.1987.tb02549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulton G. N., Greene M. I. Inhibition of cellular DNA synthesis by reovirus occurs through a receptor-linked signaling pathway that is mimicked by antiidiotypic, antireceptor antibody. J Exp Med. 1989 Jan 1;169(1):197–211. doi: 10.1084/jem.169.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. E., Geier S. S., Hirano M. Differentiation of astrocytes and oligodendrocytes from germinal matrix cells in primary culture. J Neurosci. 1986 Jan;6(1):52–60. doi: 10.1523/JNEUROSCI.06-01-00052.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Page C. D., Wu H. L., Schlaepfer W. W. Monoclonal antibodies to gel-excised glial filament protein and their reactivities with other intermediate filament proteins. J Neurochem. 1984 Jan;42(1):25–32. doi: 10.1111/j.1471-4159.1984.tb09692.x. [DOI] [PubMed] [Google Scholar]
- Liu J., Co M. S., Greene M. I. Reovirus type 3 and [125I]-iodocyanopindolol bind to distinct domains on the beta-adrenergic like receptor. Immunol Res. 1988;7(3):232–238. doi: 10.1007/BF02918138. [DOI] [PubMed] [Google Scholar]
- Maly P., Lüthi C. Purification of the type I insulin-like growth factor receptor from human placenta. Biochem Biophys Res Commun. 1986 Jun 13;137(2):695–701. doi: 10.1016/0006-291x(86)91134-4. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- McMorris F. A., Dubois-Dalcq M. Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J Neurosci Res. 1988 Oct-Dec;21(2-4):199–209. doi: 10.1002/jnr.490210212. [DOI] [PubMed] [Google Scholar]
- McMorris F. A., Smith T. M., DeSalvo S., Furlanetto R. W. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci U S A. 1986 Feb;83(3):822–826. doi: 10.1073/pnas.83.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Jarnagin K., Roth R. A. Purification and characterization of the receptor for insulin-like growth factor I. Biochemistry. 1986 Sep 23;25(19):5560–5564. doi: 10.1021/bi00367a032. [DOI] [PubMed] [Google Scholar]
- Nepom J. T., Weiner H. L., Dichter M. A., Tardieu M., Spriggs D. R., Gramm C. F., Powers M. L., Fields B. N., Greene M. I. Identification of a hemagglutinin-specific idiotype associated with reovirus recognition shared by lymphoid and neural cells. J Exp Med. 1982 Jan 1;155(1):155–167. doi: 10.1084/jem.155.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M., Murray K. Purified astrocytes promote the in vitro division of a bipotential glial progenitor cell. EMBO J. 1984 Oct;3(10):2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M., Murray K., Stroobant P., Waterfield M. D., Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988 Jun 9;333(6173):560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Noseworthy J. H., Fields B. N., Dichter M. A., Sobotka C., Pizer E., Perry L. L., Nepom J. T., Greene M. I. Cell receptors for the mammalian reovirus. I. Syngeneic monoclonal anti-idiotypic antibody identifies a cell surface receptor for reovirus. J Immunol. 1983 Nov;131(5):2533–2538. [PubMed] [Google Scholar]
- Pringle N., Collarini E. J., Mosley M. J., Heldin C. H., Westermark B., Richardson W. D. PDGF A chain homodimers drive proliferation of bipotential (O-2A) glial progenitor cells in the developing rat optic nerve. EMBO J. 1989 Apr;8(4):1049–1056. doi: 10.1002/j.1460-2075.1989.tb03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Fok-Seang J. Reconstitution of a developmental clock in vitro: a critical role for astrocytes in the timing of oligodendrocyte differentiation. Cell. 1985 Aug;42(1):61–69. doi: 10.1016/s0092-8674(85)80101-x. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Lillien L. E., Richardson W. D., Burne J. F., Noble M. D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988 Jun 9;333(6173):562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raible D. W., McMorris F. A. Cyclic AMP regulates the rate of differentiation of oligodendrocytes without changing the lineage commitment of their progenitors. Dev Biol. 1989 Jun;133(2):437–446. doi: 10.1016/0012-1606(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Ranscht B., Clapshaw P. A., Price J., Noble M., Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R., Wilkin G. P. Development of macroglial cells in rat cerebellum. II. An in situ immunohistochemical study of oligodendroglial lineage from precursor to mature myelinating cell. Development. 1988 Feb;102(2):409–425. doi: 10.1242/dev.102.2.409. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Pringle N., Mosley M. J., Westermark B., Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988 Apr 22;53(2):309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978 Apr 25;253(8):2769–2776. [PubMed] [Google Scholar]
- Saneto R. P., Low K. G., Melner M. H., de Vellis J. Insulin/insulin-like growth factor I and other epigenetic modulators of myelin basic protein expression in isolated oligodendrocyte progenitor cells. J Neurosci Res. 1988 Oct-Dec;21(2-4):210–219. doi: 10.1002/jnr.490210213. [DOI] [PubMed] [Google Scholar]
- Sawutz D. G., Bassel-Duby R., Homcy C. J. High affinity binding of reovirus type 3 to cells that lack beta adrenergic receptor activity. Life Sci. 1987 Jan 26;40(4):399–406. doi: 10.1016/0024-3205(87)90142-1. [DOI] [PubMed] [Google Scholar]
- Schachner M., Kim S. K., Zehnle R. Developmental expression in central and peripheral nervous system of oligodendrocyte cell surface antigens (O antigens) recognized by monoclonal antibodies. Dev Biol. 1981 Apr 30;83(2):328–338. doi: 10.1016/0012-1606(81)90478-4. [DOI] [PubMed] [Google Scholar]
- Skoff R. P., Price D. L., Stocks A. Electron microscopic autoradiographic studies of gliogenesis in rat optic nerve. I. Cell proliferation. J Comp Neurol. 1976 Oct 1;169(3):291–312. doi: 10.1002/cne.901690303. [DOI] [PubMed] [Google Scholar]
- Skoff R. P., Price D. L., Stocks A. Electron microscopic autoradiographic studies of gliogenesis in rat optic nerve. II. Time of origin. J Comp Neurol. 1976 Oct 1;169(3):313–334. doi: 10.1002/cne.901690304. [DOI] [PubMed] [Google Scholar]
- Small R. K., Riddle P., Noble M. Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987 Jul 9;328(6126):155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981 Apr 30;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian T., Sprinkle T. J., Dawson G., Szuchet S. Oligodendrocyte substratum adhesion modulates expression of adenylate cyclase-linked receptors. Proc Natl Acad Sci U S A. 1988 Feb;85(3):939–943. doi: 10.1073/pnas.85.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventimiglia R., Greene M. I., Geller H. M. Localization of beta-adrenergic receptors on differentiated cells of the central nervous system in culture. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5073–5077. doi: 10.1073/pnas.84.14.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E. M., Maratos-Flier E., Carpentier J. L., Kahn C. R. Persistent infection with a nontransforming RNA virus leads to impaired growth factor receptors and response. J Cell Physiol. 1986 Sep;128(3):457–465. doi: 10.1002/jcp.1041280315. [DOI] [PubMed] [Google Scholar]
- Williams W. V., Guy H. R., Rubin D. H., Robey F., Myers J. N., Kieber-Emmons T., Weiner D. B., Greene M. I. Sequences of the cell-attachment sites of reovirus type 3 and its anti-idiotypic/antireceptor antibody: modeling of their three-dimensional structures. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6488–6492. doi: 10.1073/pnas.85.17.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. V., Moss D. A., Kieber-Emmons T., Cohen J. A., Myers J. N., Weiner D. B., Greene M. I. Development of biologically active peptides based on antibody structure. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5537–5541. doi: 10.1073/pnas.86.14.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yong V. W., Kim S. U. A new double labelling immunofluorescence technique for the determination of proliferation of human astrocytes in culture. J Neurosci Methods. 1987 Sep;21(1):9–16. doi: 10.1016/0165-0270(87)90098-7. [DOI] [PubMed] [Google Scholar]
- Zeller N. K., Behar T. N., Dubois-Dalcq M. E., Lazzarini R. A. The timely expression of myelin basic protein gene in cultured rat brain oligodendrocytes is independent of continuous neuronal influences. J Neurosci. 1985 Nov;5(11):2955–2962. doi: 10.1523/JNEUROSCI.05-11-02955.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



