Abstract
BACKGROUND
Germ cell depletion caused by chemical or physical toxicity, disease or genetic predisposition can occur at any age. Although semen cryopreservation is the first reflex for preserving male fertility, this cannot help out prepubertal boys. Yet, these boys do have spermatogonial stem cells (SSCs) that able to produce sperm at the start of puberty, which allows them to safeguard their fertility through testicular tissue (TT) cryopreservation. SSC transplantation (SSCT), TT grafting and recent advances in in vitro spermatogenesis have opened new possibilities to restore fertility in humans. However, these techniques are still at a research stage and their efficiency depends on the amount of SSCs available for fertility restoration. Therefore, maintaining the number of SSCs is a critical step in human fertility preservation. Standardizing a successful cryopreservation method for TT and testicular cell suspensions (TCSs) is most important before any clinical application of fertility restoration could be successful.
OBJECTIVE AND RATIONALE
This review gives an overview of existing cryopreservation protocols used in different animal models and humans. Cell recovery, cell viability, tissue integrity and functional assays are taken into account. Additionally, biosafety and current perspectives in male fertility preservation are discussed.
SEARCH METHODS
An extensive PubMED and MEDline database search was conducted. Relevant studies linked to the topic were identified by the search terms: cryopreservation, male fertility preservation, (immature)testicular tissue, testicular cell suspension, spermatogonial stem cell, gonadotoxicity, radiotherapy and chemotherapy.
OUTCOMES
The feasibility of fertility restoration techniques using frozen-thawed TT and TCS has been proven in animal models. Efficient protocols for cryopreserving human TT exist and are currently applied in the clinic. For TCSs, the highest post-thaw viability reported after vitrification is 55.6 ± 23.8%. Yet, functional proof of fertility restoration in the human is lacking. In addition, few to no data are available on the safety aspects inherent to offspring generation with gametes derived from frozen-thawed TT or TCSs. Moreover, clarification is needed on whether it is better to cryopreserve TT or TCS.
WIDER IMPLICATIONS
Fertility restoration techniques are very promising and expected to be implemented in the clinic in the near future. However, inter-center variability needs to be overcome and the gametes produced for reproduction purposes need to be subjected to safety studies. With the perspective of a future clinical application, there is a dire need to optimize and standardize cryopreservation and safety testing before using frozen-thawed TT of TCSs for fertility restoration.
Keywords: cryopreservation, testicular tissue, testicular cell suspensions, slow freezing, vitrification, fertility restoration, prepubertal boys
Introduction
Long-term preservation and storage of biological samples outside the body is achieved through cryopreservation. At low temperatures, the biological metabolism of living cells dramatically diminishes thereby stopping enzymatic and chemical reactions. Hence, long-term preservation of cells and tissues is possible. This strategy is of key importance for scientific research and many modern clinical therapies, including reproductive medicine. This emerging field encompasses cryopreservation of gametes (sperm and oocytes), embryos and reproductive cells [testicular cell suspensions (TCSs)] and tissues [ovarian tissue and testicular tissue (TT)] (Fuller and Paynter, 2004).
In clinical practice, ensuring the patient's health is the primary need. However, to face infertility in adult life undermines a patient's psychological well-being (Schover et al., 1999). Fertility preservation is of great importance to guarantee the quality of life of these patients (Oosterhuis et al., 2008). Reproductive stem cell loss begets from a variety of conditions including chemo- and radiotherapy (Thomson et al., 2002), genetic and congenital conditions such as Klinefelter’ syndrome (KS; 47,XXY) (Aksglaede et al., 2006) and cryptorchidism (Schroeder et al., 2013).
In pre- and peripubertal girls, cryopreservation of ovarian cortical tissue, followed by transplantation at adult age, has demonstrated the ability to restore fertility and to generate live births (De Vos et al., 2014; Demeestere et al., 2015).
For adult men and boys with ongoing spermatogenesis, sperm banking must always be offered as a first line treatment (Palermo et al., 1992). In men, this is a validated and non-invasive procedure to preserve fertility (Tournaye et al., 2014). Assisted reproduction techniques with semen cryopreserved before the onset of gonadotoxic treatment has shown good fertility outcome (Agarwal et al., 2004; Anderson et al., 2015). However, for prepubertal boys not producing sperm, this option does not exist. Nonetheless, these boys do have spermatogonial stem cells (SSCs) able to produce sperm from puberty onwards, giving them the alternative to cryopreserve TT or TCSs to safeguard their chances of having offspring (Goossens et al., 2013).
Cryopreservation of a testicular biopsy provides several options after thawing: (i) autologous SSC transplantation (SSCT); (ii) grafting TT to the testis or to a heterotopic area; or (iii) in vitro spermatogenesis (IVS) (Sato et al., 2011; Zhou et al., 2016). Alternatively, TCSs can be cryopreserved, although this excludes the possibility of a graft. To keep all options available, TT sampling has been recommended. These fertility restoration techniques have been successfully applied in several animal models (Dobrinski et al., 1999a; Shinohara et al., 2002; Hermann et al., 2012; Sato et al., 2013) and are considered to be very promising for future clinical applications (Fig. 1).
Figure 1.
Clinical set-up for spermatogonial stem cell (SSC) preservation in prepubertal, adolescent and adult patients at high risk of infertility. Before the start of the gonadotoxic treatment or expected SSC loss, according to the age and pubertal stage, tissue or semen samples are retrieved. SSCs are cryopreserved during the time of therapy and recovery. Different cryopreservation techniques (e.g. controlled and uncontrolled freezing and vitrification) are being optimized to effectively preserve these samples. Later when the patient is in full remission and fertility restoration techniques are available, tissue pieces or cell suspensions can be thawed for autologous transplantation or in vitro spermatogenesis. In the best-case scenario, SSCs could (recolonize the seminiferous tubules and) reinitiate spermatogenesis, leading to mature spermatozoa.
Who should be offered SSC preservation?
Infertility can have a dramatic psychosocial impact during adulthood. For a large group of male patients without the alternative of sperm cryopreservation, SSC banking represents an option to prevent this distress. Several groups of patients might benefit from SSC banking.
Patients facing cancer treatment
Of children diagnosed with cancer, 80% are expected to survive their disease (Hudson, 2010). Since 30% of male childhood cancer survivors are azoospermic at adult age (Thomson et al., 2002), TT banking is recommended.
A variety of cancer conditions indicate for TT banking. Children and adolescents with testicular cancer, leukemia or Ewing sarcoma are at the highest risk of developing permanent sterility after cancer treatment (Tournaye et al., 2014).
The effect of chemotherapy on spermatogenesis varies substantially depending on the combination of drugs used and potential negative effects on future fertility should be followed up continuously during adolescence. Nitrogen mustard derivatives, alkylating drugs and cisplatin seem to have the most detrimental effect on germ cell proliferation (Loren et al., 2013; Tournaye et al., 2014).
Irradiation injures the germ-cell pool in a dose-dependent manner (Rowley et al., 1974; Wallace et al., 2005). Doses as low as 0.1 Gy to more than 4 Gy can result in oligozoospermia or even complete sterility (Howell and Shalet, 2005; Wyns et al., 2010). Testicular irradiation with doses over 20 Gy might cause Leydig cell dysfunction in prepubertal boys (Shalet et al., 1989). Moreover, dose fractionation and most importantly the cumulated dose are major risk factors for permanent sterility (Ash, 1980; Tournaye et al., 2014).
Although spermatogenesis is not yet active in prepubertal boys, the testicular environment is not quiescent. There is a constant turnover of germ cells and SSCs continuously divide to populate the growing seminiferous tubules. Since chemotherapy kills dividing cells, the SSCs of children are also at risk (Chemes, 2001; Jahnukainen et al., 2011; Goossens et al., 2013).
Patients with life-threatening non-malignant diseases
Gonadotoxic treatments are also used as a conditioning treatment and/or to cure a variety of life-threatening non-malignant conditions (Slavin et al., 2002; Passweg and Rabusin, 2008; Lukusa et al., 2009). Common treatments for drepanocytosis, thalassemia, idiopathic medulla aplasia and granulomatous disease are full body radiotherapy to deplete the blood stem cell line before hematopoietic stem cell transplantation and hydroxyurea regimens (Bernaudin et al., 2007; Picton et al., 2015). Any condition requiring bone marrow transplantation involves a high infertility risk (>80%) for prepubertal boys (Sadri-Ardekani and Atala, 2014a).
Additionally, patients dealing with severe autoimmune diseases (e.g. juvenile systemic lupus and systemic sclerosis) (Anserini et al., 2002; Oktay and Oktem, 2009; Sadri-Ardekani and Atala, 2014a) that necessitate administration of high dose chemotherapy may opt for TT cryopreservation.
Patients enduring genetic and congenital conditions
Of men experiencing azoospermia, 15% suffer from KS (Van Assche et al., 1996; Foresta et al., 1999). KS is the most common sex chromosome abnormality in humans (1/600 live births). Yet, only 10% of KS patients are diagnosed before puberty (Bojesen et al., 2003). In a retrospective study for TT banking, analysis of seven non-mosaic 47,XXY adolescents, aged between 13 and 16 years, demonstrated massive fibrosis and hyalinization of the testis. Spermatogonia were detected in five out of seven patients. Only in the youngest patient, the spermatogonia were located in non-degenerating seminiferous tubules (Van Saen et al., 2012a). Indeed, early development of the testis appears to be normal in boys with KS, yet, SSC depletion occurs in mid-puberty, leading to infertility (Gies et al., 2012a). In adult KS patients, spermatogenic foci may remain, although spermatozoa can be retrieved by TESE in less than 50% (Tournaye et al., 1996; Sciurano et al., 2009). This implies that freezing of semen samples or TT sampling should be offered to boys with KS preferably before puberty, at the onset of puberty, or as soon as it is diagnosed (Wikström et al., 2007; Gies et al., 2012a,b; Van Saen et al., 2012a,b). However, to this date, no clinical parameters are available to efficiently diagnose and detect patients who might benefit from these techniques (Gies et al., 2012b). Retrieval of SSCs in prepubertal boys with KS should therefore still be viewed as experimental and patients and their parents must be counseled accordingly (Van Saen et al., 2012a,b).
Cryobiology and cryopreservation-induced cell death
SSC cryopreservation is a cost-effective and efficient method to preserve genetic material for decades (Lee et al., 2014a). Success in tissue and/or cell cryopreservation is built upon the understanding of biophysical fundamentals underlying any cryobiological protocol (Fuller and Paynter, 2004).
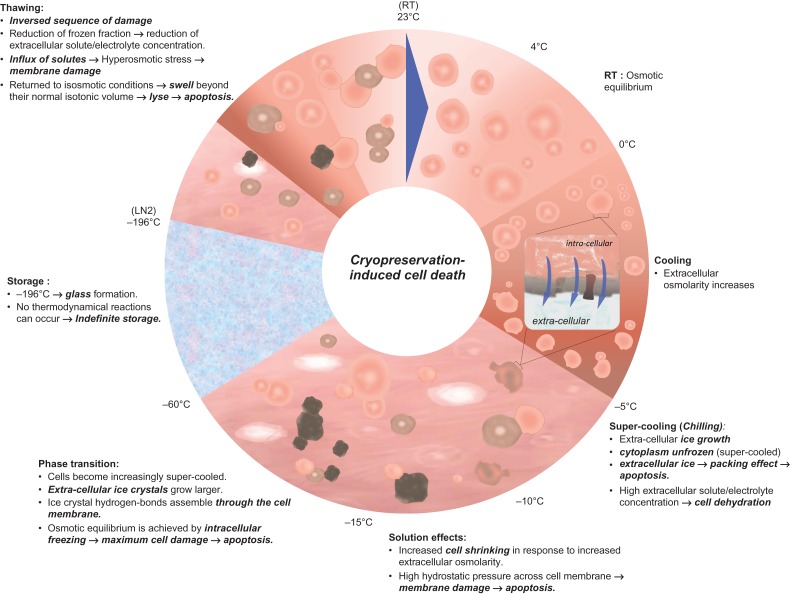
As summarized in Fig. 2, along cooling, cells and tissues lose osmotic equilibrium within their medium. Extracellular medium starts freezing with temperatures around −5°C, yet, the cytoplasm remains unfrozen. Between −5 and −10°C, cells supercool and the growth of extracellular ice leads to an increase of solute (electrolyte) concentration in the extracellular medium. The cells equilibrate with the medium by losing water causing severe cell dehydration and shrinkage. Between −10 and −15°C, the extracellular ice expands, increasing cell-ice and cell–cell contacts. These lead to a packing effect and may result in cell damage. The major hurdle for cells to surpass is the water to ice phase transition. Indeed, between −15 and −60°C, cells become increasingly supercooled. Extracellular ice crystals grow larger, and exceptionally, ice crystal hydrogen-bonds assemble through the cell membrane, leading to osmotic equilibrium via intracellular freezing. Intracellular ice freezing is considered the major degree of cryopreservation-induced cell damage. Hence, the ability of cells and tissues to endure the lethality of this intermediate zone (between −15 and −60°C), that they must traverse twice during cooling and warming, is crucial for their survival (Mazur, 1970, 1977).
Figure 2.
Schematic of physical events underlying the freezing, storing and thawing.
There is evidence that the intrinsic response of cells to cryopreservation is different depending upon whether the cells are part of a tissue or whether they are isolated in a cell suspension. Indeed, scaling up of cryopreservation from a microscopic cellular level to a macroscopic tissue level will introduce heat and mass transfer phenomena (Karlsson and Toner, 1996). Heat transfer limitations relate to thermal conductivity of a tissue sample. Generally it is more difficult to achieve rapid cooling and warming rates in tissues compared with cell suspensions, implying non-uniform rates of cooling throughout a tissue sample. Hence, the temperature will change more slowly in the interior of the sample compared with the surface. Mass transfer and the subsequent redistribution of water are determined by membrane-limited water transport in the case of individual cells, whereas cell–cell interactions and the diffusive processes must be taken into consideration for multicellular tissues (Levin et al., 1977; Karlsson and Toner, 1996).
To improve cell survival during freezing and thawing, cryoinjury caused by intracellular ice crystal formation has to be avoided. Addition of cryoprotective agents (CPAs) and controlling freezing and thawing rates are intended to diminish the width of the possible damage window during cryopreservation by lowering ice formation temperatures to −40°C. Two classes of CPAs exist, permeating CPAs (e.g. dimethyl sulfoxide [DMSO], glycerol, formamide and propanediol) and non-permeating CPAs including sugars (e.g. sucrose, trehalose, dextran, lactose and d-mannitol) and high molecular weight compounds (e.g. polyethylene glycol and hydroxyethyl starch). Their cryoprotective action consists of decreasing the concentration of solutes and increasing membrane stability during the dehydration and rehydration phases (Lee et al., 2014b).
Vitrification may be a promising alternative to freezing. It leads to utrarapid liquid solidification and thus, avoids ice formation. In this technique, very high concentrations of CPAs are combined with ultra-rapid cooling rates (>106 °C/min) with the intention of preventing crystal formation in the solution and allowing the cells to shrink slowly enough to avoid membrane damage. This is followed by direct plunging of the samples into liquid nitrogen to avoid phase transition damage (Yavin and Arav, 2007).
The major steps in a cryopreservation process can be summarized as follows: (i) addition of CPAs to cells/tissues before cooling; (ii) cooling of the cells/tissues toward a low temperature (e.g. −196°C, the liquid nitrogen temperature) at which the cells/tissues are stored; (iii) warming the cells/tissues; and (iv) removal of the CPAs from the cells/tissues after thawing.
Animal studies have underlined the pivotal role of viable and functional SSCs on the efficiency of any of the prospective fertility restoration procedures (TTG, SSCT and IVS). Comparisons of functional assays using fresh to cryopreserved samples indicate loss of SSCs during the cryopreservation procedure. Thus, the effectiveness of the cryopreservation procedures is critical and should be improved (Izadyar et al., 2002a,b; Shinohara et al., 2002; Frederickx et al., 2004; Baert et al., 2012; Hermann et al., 2012; Jahnukainen et al., 2012; Sá et al., 2012; Pacchiarotti et al., 2013; Sato et al., 2013).
This review describes the basics of male reproductive tissue and cell cryopreservation. It gives an overview of existing cryopreservation protocols used in different animal models. Important parameters such as cell recovery, cell viability, tissue integrity and functional assays are taken into account. Additionally, biosafety and current perspectives in male fertility preservation are discussed. As an increasing number of centers start to cryopreserve TT samples, the paramount goal of this work is to inform the reader about the need to develop and standardize a proper procedure to cryopreserve TT and TCSs in order to optimize the success rates of future clinical procedures (Ruutiainen et al., 2013; Picton et al., 2015).
Methods
A broad PubMED and MEDline database search was conducted. Search terms were: cryopreservation, male fertility preservation, (immature)testicular tissue, testicular cell suspension, spermatogonial stem cell, gonadotoxicity, radiotherapy and chemotherapy. An initial search query lead to a total of 4154 papers associated to the topic. Selection criteria based on relevance to the topic, outcome of interest (i.e. percentage of viable cells after testicular cell suspension cryopreservation, tissue morphology, and functional test by SSCT) and potential clinical application, resulted in a total of 352 scientific and review articles. Finally, 54 studies linked to the main subject of interest, published in English or French between 1996 and 2015, were referenced in this review (Fig. 3).
Figure 3.
Analysis diagram for inclusion and exclusion criteria of studies in this review.
Results
Testicular tissue cryopreservation
In the 1990s, cryopreservation of TT emerged as a safe and effective means to facilitate the treatment of couples with azoospermia. From one procedure, it enables the storage of enough spermatozoa for multiple In-vitro fertilization (IVF)/Intra-cytoplasmic sperm injection (ICSI) cycles while reducing patient costs and risks of repeated surgeries (Hovatta et al., 1996; Trombetta et al., 2000; Garg et al., 2008). The main purpose of early TT cryopreservation protocols was thus to preserve the most mature stages of spermiogenesis. The freezing media contained glycerol as this is used worldwide as the preservative of choice for spermatozoa, although it is not optimal for TT (Crabbe et al., 1999). However, the pioneering work of Brinster and colleagues brought TT cryopreservation in a new light. By elegantly showing that spermatogenesis could be restored by transplanting cryopreserved SSCs in the seminiferous tubules of sterilized mice (Avarbock et al., 1996), it was quickly recognized that TT cryopreservation could also be helpful in preserving the fertility of prepubertal boys who are undergoing gonadotoxic chemotherapy or radiotherapy (Nugent et al., 1997). Therefore, adapted TT cryopreservation protocols were needed with a focus on preserving SSCs and their supportive cells rather than mature germ cells. To find such, the rationale was to strive for minimal cryoinjury and maximal cell recovery. This posed a challenge, owing to the various cell types in TT, each differing in dimension, complexity, water permeability, and hence requiring different optimal cryopreservation protocols. The major achievements made during the last two decades regarding tissue preparation, cooling, cryoprotection, warming after storage and storage vessels to cryopreserve TT are reviewed here.
Tissue preparation
The macroscopic physical dimension of the tissue is a major point to be defined in a cryopreservation protocol. It is key to achieving equal distribution with rapid in and out diffusion of CPAs and uniform rates of temperature change to limit cryoinjury (Karlsson and Toner, 1996). This is especially true for vitrification, as the sample size is a critical variable in the probability of successful solidification of the aqueous milieu of the cells/tissue into a non-crystalline glassy phase. It is also important in the prevention of devitrification which occurs during warming and is characterized by the formation of ice crystals (Yavin and Arav, 2007). Yet, few data exist on the effect of sample size on TT cryopreservation. Nevertheless, some important observations have been made. For instance, it seemed that results of the studies differed depending on the donor species. It was shown that whole testes with pricked tunica albuginea were better preserved than testes with intact tunica, testes without tunica and testis halves, after rapid freezing of immature mouse TT (Gouk et al., 2011). In agreement, another rodent study proved that the original tissue size can influence the outcome of TT cryopreservation as immature rat tissue showed fewer morphological alterations when 7.5 mg fragments were slow frozen compared to 15 mg fragments, albeit using a similar cryopreservation protocol (Travers et al., 2011). Nevertheless, the cell viability of immature porcine testicular tissues undergoing the same cryopreservation treatment was not affected by the original size of the tissue fragment (5, 15, 20 or 30 mg) (Abrishami et al., 2010). Also, for the cryopreservation of tissue from prepubertal boys, varying fragments sizes (2–9 mm³) were successfully frozen (Keros et al., 2007; Wyns et al., 2007, 2008; Curaba et al., 2011a; Poels et al., 2013, 2014; Picton et al., 2015). As evidenced by studies using whole ovaries, it would be interesting to see if whole human testes can be cryopreserved (Pfeifer et al., 2014).
Cooling, cryoprotection and warming after storage
The two most commonly used cryopreservation procedures are slow (controlled or uncontrolled) freezing and vitrification. Rapid freezing has been reported as well, although without great success (Gouk et al., 2011). Slow freezing and vitrification differ mainly in concentrations of CPAs and cooling rates used. Slow freezing with controlled-freezing rates allows modifying the soaking temperature to master cell dehydration before reaching temperatures at which intracellular ice nucleation occurs, thereby minimizing the probability of harmful intracellular ice crystal formation. A recent study found that soaking at −9°C better preserved the ability of frozen-thawed mouse SSCs to generate haploid germ cells after in vitro maturation than −7°C and −8°C (Arkoun et al., 2015). Initiating ice-nucleation manually after soaking is often performed during controlled slow freezing as an additional protective step, although this may not be necessary and may even be harmful (Milazzo et al., 2008; Baert et al., 2013). Further down the line, slow freezing with either controlled or uncontrolled cooling rates takes advantage of the regulatory properties of extracellular ice formation to gradually dehydrate cells when the temperature slowly decreases. This further reduces the risk of intracellular ice. In addition to aspects related to the freezing rate, slow freezing avoids cytotoxicity because this procedure generally requires low CPA concentrations. Freezing solutions yielding good results in animal and human models contain penetrating CPAs like DMSO (0.7–3 M), ethylene glycol (EG, 1.5 M) or glycerol (1 M) with or without the addition of sucrose (0.05–0.1 M) or serum (5–80%) (Table I). As discussed above, the formation of extracellular ice, may not pose a problem for cryopreservation of cell suspensions, but is likely to be the main problem in tissues. Therefore, during vitrification, ice crystal formation is bypassed by using ultrafast cooling rates and higher concentrations of CPAs, e.g. 6.2 M EG combined with 24 M polyvinylpyrrolidone and 0.3 M trehalose. Often a combination of multiple CPAs is used to minimize their individual cytotoxicity (2.1–2.6 M DMSO besides 2.7–2.8 M EG with or without 0.5 M sucrose and 20–25% serum) (Table I). This should, in theory, avoid injuries associated with mechanical disfiguration. Incomplete vitrification, though, it may induce the damaging mechanism associated with fast cooling rates. Because cells are only partially dehydrated at fast cooling rates, cell injury is attributed to intracellular ice formation or membrane rupture due to strong osmotic fluxes (Mazur, 1970; Muldrew and McGann, 1994).
Table I.
Overview of experimental cryopreservation protocols for mammalian testicular tissue.
| Protocol | Species | (Im)mature | Cryoprotectant | Freezing rate | Main outcome | Reference |
|---|---|---|---|---|---|---|
| Controlled | Mouse | Mature | 1.5 M DMSO + 0.1 M sucorse + 1% HSA | Start: 0°C, −2°C/min to −7°C, seeding, −0.3°C/min to −40°C, −10°C/min to −140°C, LN2 | Spz in isografts | Schlatt et al. (2002) |
| Immature | 1.5 M DMSO + 0.1 M sucorse + 1% HSA | Start: 0°C, −2°C/min to −7°C, seeding, −0.3°C/min to −40°C, −10°C/min to −140°C, LN2 | Spz in isografts | Schlatt et al. (2002) | ||
| DMSO + FCS (concentrations not mentioned) | Start: 4°C, −2°C/min to −7°C, hold 10 min, −0.3°C/min to −30°C, LN2 | Offspring using spz from allografts | Shinohara et al. (2002) | |||
| 1.5 M DMSO + 0.05 M sucrose + 10% FCS | Start: 5°C, −2°C/min to −9°C, hold 7 min, −0.3°C/min to −40°C, 25°C/min to −150°C, LN2 | Maintenance of tissue integrity and activity in organotypic tissue culture, spz in allografts | Milazzo et al. (2008, 2010), Travers et al. (2011) | |||
| 0.7 M DMSO + 5% HSA | Start: 4°C, −1°C/min to −0°C, hold 5 min, −0.5°C/min to −8°C, seeding + hold 10 min, −0.5°C/min to −40°C, hold 10 min, −7°C/min to −70°C, LN2 | Spz in allografts | Yildiz et al. (2013) | |||
| Rat | Mature | 1 M DMSO + 20% FBS | Start: RT, −2°C/min to −9°C, hold 5 min + seeding, −0.3°C/min to −40°C, 10°C/min to −140°C, LN2 | Preservation of testicular cells | Unni et al. (2012) | |
| Immature | 1.5 M DMSO + 0.05 M sucrose + 10% FCS | Start: 5°C, −2°C/min to −9°C, hold 7 min, −0.3°C/min to −40°C, 25°C/min to −150°C, LN2 | Maintenance of tissue integrity and activity in organotypic tissue culture | Travers et al. (2011) | ||
| 1 M DMSO + 20% FBS | Start: RT, −2°C/min to −9°C, hold 5 min + seeding, −0.3°C/min to −40°C, 10°C/min to −140°C, LN2 | Preservation of testicular cells | Unni et al. (2012) | |||
| Porcine | Immature | 1 M glycerol + 5% FBS | Start: 4°C, −1°C/min to −0°C, hold 5 min, −0.5°C/min to −8°C, hold 15 min + seeding, −0.5°C/min to −40°C, hold 10 min, −7°C/min to −80°C, LN2 | Spz in xenografts | Abrishami et al. (2010) | |
| Human | Mature | 0.7 M DMSO + 5% patient serum | Start: 4°C, −1°C/min to −0°C, hold 5 min, −0.5°C/min to −8°C, hold 15 min + seeding, −0.5°C/min to −40°C, hold 10 min, −7°C/min to −80°C, LN2 | Maintenance of tissue integrity and activity in organotypic tissue culture | Keros et al. (2005) | |
| 0.5 M EG + 20% FBS | Start: RT, −2°C/min to −9°C, hold 5 min + seeding, −0.3°C/min to −40°C, 10°C/min to −140°C, LN2 | Preservation of testicular cells | Unni et al. (2012) | |||
| 1.4 M DMSO + 10% HSA + 1% Dextran | Start: 5°C, −1°C/min to −80°C,−50°C/min to −120°C, LN2 | Preservation of testicular cells | Pacchiarotti et al. (2013) | |||
| Immature | 1.5 M EG + 0.1 M surcorse + 10% HSA | Start: 1°C, −2°C/min to −9°C, hold 5 min + seeding, −0.3°C/min to −40°C, −10°C/min to −140°C, LN2 | Maintenance of tissue integrity and activity in organotypic tissue culture | Kvist et al. (2006) | ||
| 0.7 M DMSO + 5% HSA | Start: 4°C, −1°C/min to −0°C, hold 5 min, −0.5°C/min to −8°C, seeding + hold 10 min, −0.5°C/min to −40°C, hold 10 min, −7°C/min to −70°C, LN2 | Maintenance of tissue integrity and activity in organotypic tissue culture | Keros et al. (2007); Pietzak et al. (2015) | |||
| 0.7 M DMSO + 0.1 M surcorse + 10% HSA | Start: 0°C, hold 9 min, −0.5°C/min to −8°C, hold 5 min + seeding, hold 15 min, −0.5°C/min to −40°C, hold 10 min, −7°C/min to −80°C, LN2 | Maintenance of tissue integrity and activity in xenografts | Wyns et al. 2007, (2008); Curaba et al. (2011a); Poels et al. (2013, 2014) | |||
| Uncontrolled | Mouse | Immature | 1.5 M DMSO + 0.07 M sucrose | Insulated container in −80°C, LN2 | Spz in allografts | Goossens et al. (2008), Baert et al. (2012) |
| Cell Banker 1 | Vial in −80°C, LN2 | Offspring using spz from organotypic culture | Yokonishi et al. (2014) | |||
| Sheep | Immature | 3 M DMSO + 20% FBS | Insulated container in −80°C, LN2 | Spz after xenografting | Pukazhenthi et al. (2015) | |
| Deer | Immature | 1.5 M DMSO + 80% FBS | Insulated container in −80°C, LN2 | Spc in xenografts | Pothana et al. (2015) | |
| Bovine | Mature | 1.4 M DMSO + 0–20% NCS | Insulated container in −80°C, LN2 | Preservation of SSCs | Wu et al. (2011, 2014) | |
| Immature | 2.8 M DMSO + 20% FBS | Insulated container in −80°C, LN2 | Maintenance of tissue integrity and activity in organotypic tissue culture | Devi et al. (2014) | ||
| 1.5 M DMSO + 0.2 M trehalose + 10% FBS | 2 h in −20°C, −80°C, LN2 | Preservation of testicular cells | Zhang et al. (2015) | |||
| Monkey | Immature | 1.4 M DMSO + 10% FCS | In −20°C, seeding at −13°C, to −20°C, LN2 | Spz in autografts | Jahnukainen et al. 2007, (2012) | |
| Human | Mature | 1.5 M DMSO + 0.15 M sucrose + 10% HSA | Insulated container in −80°C, LN2 | Maintenance of tissue integrity, preservation of cell dynamics in propagation culture | Baert et al. (2013), (2015) | |
| 1.28 M DMSO + 25% FBS | Insulated container in −80°C, LN2 | Preservation of SSCs | Yango et al. (2014) | |||
| Vitrification | Mouse | Immature | 2.8 M DMSO + 2.8 M EG + 25% HSA | Straw, LN2 | Maintenance of tissue integrity and activity in organotypic tissue culture | Curaba et al. (2011b) |
| 6.77 M EG + 0.6 M sucrose | Straw-in-straw, LN2 | Preservation of SSCs | Gouk et al. (2011) | |||
| 2.1 M DMSO + 2.7 M EG + 0.5 M sucrose + 20% FBS | SSV | Spz in allografts, Spz in organotypic culture | Baert et al. (2012), Dumont et al. (2015) | |||
| 2.1 M DMSO + 2.7 M EG + 20% FBS + 0.5 M sucrose | Vial, LN2 | Preservation of SSCs | Gholami et al. (2013) | |||
| Stem Cell Keep | Vial, LN2 | Offspring using spz from organotypic culture | Yokonishi et al. (2014) | |||
| Porcine | Immature | 2.1 M DMSO + 2.7 M EG + 0.5 M sucrose + 20% FCS | SSV | Spz in xenografts | Abrishami et al. (2010) | |
| 35% EG + 5% PVP + 0.3 M trehalose | SSV | Offspring using spz from xenografts | Kaneko et al. (2013) | |||
| Monkey | Immature | 2.1 M DMSO + 2.7 M EG + 0.5 M sucrose + 25% HSA | Straw, LN2 | Maintenance of tissue integrity and activity in xenografts | Poels et al. (2012) | |
| Human | Immature | 2.8 M DMSO + 2.8 M EG + 25% HSA | Straw, LN2 | Maintenance of tissue integrity and activity in organotypic tissue culture | Curaba et al. (2011a) | |
| 2.1 M DMSO + 2.7 M EG + 0.5 M sucrose + 25% HSA | Straw, LN2 | Maintenance of tissue integrity and activity in xenografts | Poels et al. (2013) |
DMSO, dimethyl sulfoxide; EG, ethylene glycol; FBS, fetal bovine serum; HSA, human serum albumin; LN2, liquid nitrogen; NCS, newborn calf serum; spz, spermatozoa; SSC, spermatogonial stem cell; SSV, solid-surface vitrification.
An overview of the most compelling mammalian TT cryopreservation protocols is given in Table I and include mouse, rat, pig, bovine, monkey and human studies. Some of these present interesting and promising results on post-thaw cell and tissue integrity, and show the ability to purify viable SSCs for further use (Milazzo et al., 2008; Gouk et al., 2011; Wu et al., 2011, 2014; Unni et al., 2012; Gholami et al., 2013; Pacchiarotti et al., 2013; Yango et al., 2014; Zhang et al., 2015). However, these evaluations are merely indicative for the quality of the cryopreservation protocol. Testicular cell activity assessment by in vitro culture or transplantation assays should be included in TT cryopreservation studies, as cell and tissue integrity do not necessarily correctly predict the functionality of the testicular cells, hence it may lead to false positive or negative discrimination (Jahnukainen et al., 2007; Zeng et al., 2009; Baert et al., 2012). Using animal models, several protocols have proven to preserve immature TT. In rodents, maintenance of tissue integrity and activity in organotypic cultures was reported using slow freezing and vitrification (Travers et al., 2011; Curaba et al., 2011b). Complete germ cell differentiation was observed by in vitro maturation of vitrified-thawed TT fragments (Dumont et al., 2015), as well as after grafting slow-frozen mouse and rat samples and vitrified-thawed mouse samples (Schlatt et al., 2002; Milazzo et al., 2010; Baert et al., 2012; Yildiz et al., 2013). Importantly, true validation of slow freezing as a means to preserve TT functionality came with the publication from Shinohara et al. (2002) in which they reported birth of mouse offspring from sperm retrieved from samples grafted after thawing. Moreover, healthy offspring were produced with sperm grown from slow-frozen or vitrified-thawed immature mouse TT in an organotypic culture setup (Yokonishi et al., 2014). These achievements from the rodent model have been confirmed in higher mammals. Slow frozen bovine, sheep and deer TT tissue gave promising results in an organotypic culture or xenografting approach after thawing (Devi et al., 2014; Pothana et al., 2015; Pukazhenthi et al., 2015). Grafted immature porcine and non-human primate immature TT pieces that were cryopreserved by slow freezing or vitrification were able to sustain spermatogenesis (Jahnukainen et al., 2007, 2012; Abrishami et al., 2010; Poels et al., 2012). Furthermore, normal reproductive development of pigs produced from sperm that was retrieved from vitrified-thawed xenotransplanted immature TT was reported (Kaneko et al., 2013, 2014). For human tissue, studies focusing on slow freezing have resulted in well-established protocols using programmable freezers that are able to keep the tissue integrity and activity in organotypic cultures or long-term xenografts (Kvist et al., 2006; Keros et al., 2007; Wyns et al., 2007, 2008; Curaba et al., 2011a; Poels et al., 2013, 2014). To maximize the quality and integrity of human TT during cryopreservation, there is a continuous search to improve or replace current protocols. In recent years, faster and cheaper alternatives are on the rise. Similar results to programmed slow freezing were observed with vitrified-thawed immature human TT (Curaba et al., 2011a; Poels et al., 2013). In addition, our group has published an uncontrolled slow freezing protocol able to protect the integrity of adult human TT and maintain cell dynamics in long-term propagation culture, with results similar to fresh controls (Baert et al., 2013, 2015). However, confirmation with immature TT is still warranted.
Upon removal from storage, CPAs that have reached the internal compartments of cells must diffuse back through numerous membranes in the tissue regardless of whether ice crystal formation occurred during freezing or was circumvented by vitrification. Earlier studies pointed out that consistent cooling and warming rates, for instance slow cooling followed by slow thawing, or fast cooling followed by fast thawing, can improve cell/tissue survival after cryopreservation. However, as mentioned before, cell damage in tissues can occur during warming due to the heat and mass transfer limitation (CPA cytotoxicity or recrystallization/devitrification) (Mazur, 1970; Karlsson and Toner, 1996). Therefore, optimal procedures for thawing and CPA removal are also critical for tissue survival after cryopreservation. However, there has been very limited research addressing these issues. Tissues are generally removed from storage by rapid warming and gradual removal of loaded CPAs. The ambient temperature for warming can have great impact as thawing of adult bovine tissue at 37°C and 97–100°C resulted in better cell viability and spermatogonial survival compared to that at 4°C (Wu et al., 2011).
Storage vessels
Different TT freezing protocols have been developed over the years using either straws or vials to store cryopreserved TT. The choice of the vessel was mostly made based on previous literature and the logistic situation. To our knowledge, only the group of Rives performed a comparative study in which they found that the morphology of immature rat TT was better protected using vials compared to straws (Travers et al., 2011).
In contrast to freezing, studies dealing with vitrification carefully consider which vessel to be used during cryopreservation: an open or closed device. Open devices are devices allowing direct contact of the sample with the cooling solution, typically liquid nirogen (LN2). Using open devices, the cooling rates achieved are in general approximately 20,000–30,000°C/min which favors good vitrification of the sample (Scholz, 2012). Indeed, open vitrification systems, i.e. open straw, have been successfully employed to preserve the integrity and activity in organotypic culture and long-term xenografting of immature mouse, monkey and human TT after cryopreservation (Curaba et al., 2011b; Poels et al., 2012, 2013). The problem is that direct contact with the cooling solution introduces a risk of pathogen transmission to the sample during cooling and a high risk of cross contamination in the container. In closed systems on the other hand, the sample is not in contact with the cooling solution during freezing or storage, thereby, tackling the problem of contamination. One drawback of a closed system is that the cooling rate is much lower and therefore requires higher concentrations of CPAs to prevent ice crystal formation. This makes the protocols potentially more dangerous for cells due to the cytotoxicity of CPAs (Scholz, 2012). However, two closed vitrification systems have been described for TT cryopreservation with exciting results. Firstly, vitrification of immature mouse TT was successfully performed by simply plunging the vial containing the CPA-submerged samples in LN2 (Gholami et al., 2013; Yokonishi et al., 2014). Secondly, vitrification was achieved by solid-surface vitrification. This procedure includes exposing TT samples to a vitrification solution before placing them on a sterile aluminum boat floating on LN2, and transferring the samples into precooled vials followed by plunging them into LN2 (Abrishami et al., 2010). Using this approach, the functionality of immature mouse and porcine TT could be maintained during cryopreservation (Baert et al., 2012; Kaneko et al., 2013, 2014; Dumont et al., 2015). Our group studied the feasibility of vitrifying mature human tissue with a protocol proven to preserve the functionality of immature porcine TT upon xenografting (Abrishami et al., 2010). However, we observed signs of cryoinjury to the human TT and therefore solid-surface vitrification needs further optimization for human applications (Baert et al., 2013).
Testicular cell suspension cryopreservation
Since the introduction of the SSCT technique in 1994 (Brinster and Zimmermann, 1994), cryobanking of TCSs has been proposed as a plausible solution for fertility preservation in prepubertal boys (Avarbock et al., 1996; Frederickx et al., 2004). Indeed, TCS cryopreservation might facilitate SSC survival bypassing the hurdles of heat and mass exchange encountered with tissue cryopreservation (Wyns et al., 2010). However, the handling and cryopreservation of TCSs, requires exposition to high concentrations of digestive and cryoprotective solutions which may interfere with cell recovery, viability and functionality. Yet, the ability of TCSs to endure long-term cryopreservation (>14 years) was evaluated by Wu et al. (2012). Maintenance of viability and the ability to colonize and reestablish spermatogenesis was shown in recipient seminiferous tubules after SSCT. Importantly, in non-human primates, successful regeneration of spermatogenesis has been reported in the rhesus. Sperm obtained after SSCT with cryopreserved TCSs permitted the generation of embryos by in vitro fertilization (Hermann et al., 2012).
Tissue preparation
In contrast to cryopreservation of tissues, isolation of single cell suspensions requires enzymatic digestion which might influence germ cell viability and change the biophysical properties of the cells, increasing cell sensitivity to the cryopreservation process (Karlsson and Toner, 1996). Isolation of mouse testicular cell populations rely on a two-step enzymatic digestion based on the activity of collagenase and trypsin (Bellvé et al., 1977). Evidence of cell stress is the reduced viability and functionality of cell suspensions after digestion and re-suspension (Griswold, 1998; Brook et al., 2001; Joyce et al., 2001). Mechanical tissue disaggregation is an efficient and time-effective alternative to enzymatic digestion. High cell numbers, viability and spermatogonial enrichment were reported after mechanical disaggregation of TT (Schneider et al., 2015). However, the cell viability in the samples was highly variable among suspensions, suggesting the need for further optimization of this method.
Cooling, cryoprotection and warming after storage
Regardless the disaggregation method, TCSs contain (immature) Sertoli cells, Leydig cells, myoid cells and only a small percentage of SSCs. Similar to other tissue-specific stem cells, SSCs are rare and represent only 0.03% of all germ cells in the rodent testes (Phillips et al., 2010). This heterogeneous cell population varies in function, size, water content, and membrane permeability. Together with the scarcity of SSCs, this renders the development of an efficient cryopreservation procedure a real challenge. Literature reports a post-thaw viability ranging from 29 to 68%, depending on the cryopreservation method used and the type of cell concentration and viability assessment. An overview of these studies is shown in Table II.
Table II.
Overview of experimental cryopreservation protocols for mammalian testicular cell suspensions.
| Protocol | Species | (Im)mature | Cryoprotectant | Freezing rate | Viability after thawing (%) ± STDV | Main outcome | Reference |
|---|---|---|---|---|---|---|---|
| Controlled | Mouse | Immature | 1.5 M DMSO | Start: room temp, −5°C/min to −7°C, hold 15 min + seeding, −0.3°C/min to −80°C, LN2 | 48 ± 6 | Donor derived spermatogenesis | Frederickx et al. (2004) |
| Start: room temp, −5°C/min to −7°C, hold 15 min + seeding, −0.3°C/min to −40°C, LN2 | 56 ± 2 | ||||||
| 1.5 M EG | Start: room temp, −5°C/min to −7°C, hold 15 min + seeding, −0.3°C/min to −40°C, LN2 | 60 ± 8 | |||||
| Bovine | Immature | 1.4 M glycerol (10% FCS) | Start: 5°C, −1°C/min to −80°C, −50°C/min to −120°C, LN2 | 34 ± 5 | Recipient testicular colonization | Izadyar et al. (2002a,b) | |
| Start: 5°C, −1°C/min to −80°C, −50°C/min to −120°C, LN2 | 29 ± 4 | ||||||
| 1.4 M DMSO (10% FCS) | Start: 5°C, −1°C/min to −80°C, −50°C/min to −120°C, LN2 | 55 ± 4 | |||||
| Start: 5°C, −5°C/min to −80°C, −50°C/min to −120°C, LN2 | 39 ± 4 | ||||||
| Human | Adulta | 10% HAS, 10% DMSO, 1% Dextran | Start: room temperature, 4°C for 10 minutes, −1°C/min to −80°C, −50°C/min to −120°C, LN2 | 33 ± 5 | No functional assay performed | Pacchiarotti et al. (2013) | |
| Uncontrolled | Mouse | Mature | 3 M DMSO | Insulated container −70°C, LN2 | 3 ± 4 | Donor derived spermatogenesis resulting in offspring | Wu et al. (2012) |
| DMSO | Insulated container −80°C, LN2 | 67 ± 6 | Donor derived spermatogenesis resulting in offspring | Kanatsu-Shinohara et al. (2003) | |||
| Immature | 1.5 M DMSO | Insulated container −80°C, LN2 | 36 ± 2 | Donor derived spermatogenesis | Frederickx et al. (2004) | ||
| 1.5 M DMSO (10% FCS) 200 mM dissacharide (trehalose) | Insulated container −80°C, LN2 | N/A | Donor derived spermatogenesis | Lee et al. (2014b) | |||
| 3 M DMSO | Insulated container −70°C, LN2 | 12 ± 4 | Donor derived spermatogenesis resulting in offspring | Wu et al. (2012) | |||
| Rat | Mature | 3 M DMSO | Insulated container −70°C, LN2 | 2 ± 2 | N/A | Wu et al. (2012) | |
| Hamster | Mature | 1.5 M DMSO | Insulated container −70°C, LN2 | 43 ± 8 | xenogeneic spermatogenesis | Ogawa et al. (1999) | |
| Rabbit | Mature | 3 M DMSO | Insulated container −70°C, LN2 | 13 | N/A | Wu et al. (2012) | |
| Rabbit | Mature | 1.5 M DMSO | Insulated container −70°C, LN2 | 63 ± 22 | Recipient testicular colonization | Dobrinski et al. (1999a,b) | |
| Immature | |||||||
| Dog | Mature | 1.5 M DMSO | Insulated container −70°C, LN2 | 63 ± 22 | Recipient testicular colonization | Dobrinski et al. (1999a,b) | |
| Immature | |||||||
| Boar | Immature | 1.5 M DMSO (10% FCS) 200 mM dissacharide (trehalose) | Insulated container −80°C, LN2 | 62 ± 3 | Recipient testicular colonization | Lee et al. (2014a) | |
| Bovine | Immature | No cryoprotectant (10% FCS) | Insulated container −80°C, LN2 | 36 ± 3 | Recipient testicular colonization | Izadyar et al. (2002a,b) | |
| 1.4 M DMSO (10% FCS) | Insulated container −80°C, LN2 | 49 ± 5 | |||||
| 60 ± 5 | |||||||
| 1.4 M DMSO (20% FCS) | Insulated container −80°C, LN2 | 49 ± 5 | |||||
| 1.4 M DMSO, 0.07 M sucrose | 68 ± 3 | ||||||
| 1.4 M DMSO, 0.14 M sucrose | 67 ± 3 | ||||||
| 1.4 M DMSO, 0.21 M sucrose | 66 ± 4 | ||||||
| Baboon | Mature | 3 M DMSO | Insulated container −70°C, LN2 | 13 | N/A | Wu et al. (2012) | |
| Rhesus monkey | Mature | 10% DMSO | Insulated container −80°C, LN2 | 58 ± 4 | Recipient testicular colonization | Hermann et al. (2007) | |
| Vitrification | Human | Adult | (1)1.1 M DMSO,1.34 M EG, 10%HSA | open pulled straw (OPS) vitrification | 56 ± 24 | No functional assay performed | Sá et al. (2012) |
| (2)0.67 M S,2.3 M DMSO, 3.0 M EG, 10%HSA | |||||||
| N/A | Human | Adult | 1.28 M DMSO 25% FCS | −1°C/min, LN2 | 75 ± 4 | No functional assay performed | Yango et al. (2014) |
| Fetal | 67 ± 4 |
DMSO: dimethyl sulfoxide, EG: ethylene glycol, FCS: fetal bovine serum, HSA: human serum albumin, N/A: information not available, LN2: liquid nitrogen.
aSexual reassignment
In a first attempt to cryopreserve mouse SSCs, simple cryopreservation procedures for cultured somatic cells were extrapolated to TCSs (Avarbock et al., 1996). This same cryopreservation method was used for rabbit and dog TCSs (Dobrinski et al., 1999a). After freezing and thawing, cells were transplanted into recipient mice. These donor cells were able to colonize mouse testes but did not differentiate beyond the stage of spermatogonia as a consequence of the phylogenetic distance between donor and recipient animals. Yet, this provided strong evidence that SSCs of many mammalian species could be preserved for long periods using a similar cryopreservation protocol (Avarbock et al., 1996; Ogawa et al., 1999; Dobrinski et al., 1999a, 2000). This was unexpected as cryopreservation methods for mature spermatozoa differ between species. Subsequently, a first attempt to establish a freezing protocol for crude human TCSs was reported by Brook et al. (2001). Human TCSs were isolated and cryopreserved through a controlled-rate freezing method with glycerol as CPA. Yet, an overall low cell viability was observed. Furthermore, no clinical-grade requirements nor functional assays were included in this study.
Additionally, information on the optimal cooling rate specific for SSCs remains scant. SSCs are presumed to have a comparable size and nucleus/cytoplasm ratio to lymphocytes, for which an optimal cooling rate of 10°C/min has already been defined (Thorpe et al., 1976; Frederickx et al., 2004). However, development of a cryopreservation method specific for purified bovine type A spermatogonia resulted in enhanced survival rates when using DMSO and an uncontrolled cooling rate of 1°C/min, which is the optimal rate for hematopoietic stem cells (Grilli et al., 1980; Donaldson et al., 1996; Izadyar et al., 2002b). This study highlighted the advantage of an uncontrolled-rate freezing to the non-linear cooling rate (Izadyar et al., 2002b). Indeed, up to the point of ice formation (Fig. 2), high cooling rates increase the risk of ‘fast cooling damage’ (Izadyar et al., 2002b). After that point, uncontrolled freezing increases the freezing rate approaching −80°C/min at temperatures between −40°C and −60°C (Liu et al., 2000) minimizing the risk of damage. In addition, frozen-thawed type A spermatogonia retained their ability to colonize the testis of a recipient mouse demonstrating that long-term preservation of type A spermatogonia (including SSCs) is possible without apparent harmful effects to their function. To circumvent the risk of ‘fast cooling damage’, Frederickx et al. (2004) reported controlled-freezing of TCSs at a rate below 1°C/min. On the one hand, slow controlled-freezing leads to better cell dehydration and reduced intracellular ice formation (Mazur, 1990), but on the other, this causes an increase in intracellular and extracellular solute concentrations, stressing the cell membrane by extreme shrinkage (Mazur and Rigopoulos, 1983). After thawing and transplantation, cell survival was acceptable and reinstallation of spermatogenesis was achieved but the efficiency could be improved.
Given the low viability achieved with controlled and uncontrolled methods, intracellular ice crystal formation could still be the main cause for cell damage. Indeed, achieving optimal freezing rates for SSCs is a real hurdle. The rate has to be slow enough to prevent production of intracellular ice and yet rapid enough to minimize the time that cells are exposed to solution effects (Mazur, 1984). Thus, other options need to be considered. An elegant study by Sá et al. (2012) showed 55.6 ± 23.8 % viability for human adult diploid testicular cell suspensions after performing vitrification in open pulled straw (OPSs). To this date, this is the best recovery rate reported in the human model. Its advantage was due to the benefits of evading ice formation through vitrification. However, OPS vitrification requires direct contact between LN2 and the medium containing SSCs. This contact carries a potential hazard for transmission of infective agents, rendering this method difficult to implement in the clinical practice (Martino et al., 1996; Vajta et al., 1998).
DMSO, a widely used CPA, has been found to promote cell survival during cryopreservation. DMSO cryoprotective properties are observed at a concentration of 1.5 M (Table II) (Frederickx et al., 2004). DMSO is a slow permeating molecule that penetrates rather freely across cell membranes and prevents intracellular ice crystal formation (Akkök et al., 2011). Such concentrations have proven to allow supercooled cells to freeze well above −40°C thereby shortening the time window of possible damage during cryopreservation (Mazur, 1984; Karlsson et al., 1993). Yet, due to its toxicity, the high concentration and long exposure of DMSO could have several consequences when used in a clinical application (Brayton, 1986; Zambelli et al., 1998). Pacchiarotti et al. (2013) prompted an investigation to develop an effective clinical-grade procedure for the cryopreservation of human testicular cells and/or tissue under current Good Tissue Practice and Manufacturing practices regulations. DMSO, dextran and human serum albumin (HSA) were the components of the cryopreservation medium. This study reported DMSO to be suitable for safe and effective handling of human testicular cells and tissues, as it has been validated in human cord blood (Rubinstein et al., 1995), bone marrow (Odavic et al., 1980) and peripheral blood stem cell freezing (Körbling and Freireich, 2011) and other clinical applications (Pacchiarotti et al., 2013). Yet, this study used TT and cells from sexual reassignment patients, hence, further studies are needed to determine whether these findings obtained from hormone-treated patients can be generalized to other patients. Studies aiming to reduce, replace or combine DMSO with less toxic CPAs have not found a better substitute for DMSO in terms of recovery, survival and functionality of frozen-thawed cells (Table II) (Izadyar et al., 2002b; Frederickx et al., 2004; Pacchiarotti et al., 2013; Lee et al., 2013a, 2014b).
Addition of polymeric non-penetrant CPAs is believed to protect the cell by forming a viscous shell stabilizing cell membranes during dehydration and rehydration (Anchordoguy et al., 1987; Sum et al., 2003; Lee et al., 2014b). Supplementing cryoprotective media with different sugar molecules (glucose, fructose, mannose, galactose, trehalose) increased post-thaw viability in mouse SSCs (Izadyar et al., 2002b; Lee et al., 2013b, 2014b). Uncontrolled freezing with a basic medium supplemented with fetal calf serum, DMSO and 50 mM trehalose (a high molecular weight sugar) resulted in a significantly increased post-thaw cell viability and preserved the functional capacities of mouse SSCs during one week of culture compared to cells frozen using the same freezing method without trehalose (Lee et al., 2013b). The use of a higher concentration of trehalose (200 mM) in a serum-free cryopreservation medium confirmed its effectiveness and revealed a concentration-dependent increase of the proliferation capacity of the frozen-thawed cells. Additionally, employing a similar cryopreservation medium has proven to be effective in the cryopreservation of porcine SSCs, thus demonstrating effectiveness in a higher animal model (Lee et al., 2014a). Yet, no studies have observed the influence of different CPA sugar molecules on human TCS.
Storage at −196°C is favored for TCSs. In theory, at this temperature, cryopreserved cells and tissues can endure storage for centuries (Mazur, 1984; Swain and Smith, 2010). Yet, to this date, the sole proof for effectively storing functional SSCs for a long-term was given by the birth of fertile offspring from mouse SSCs which had been stored for approximately 14 years (Wu et al., 2012).
In the same way as freezing, thawing can cause cellular and extracellular damage although in an inversed sequence (crystal formation, ice formation and rehydration). Cryopreserved TCSs are often thawed by immersion in a 37°C water bath, which was found to be better than thawing in ice water (Frederickx et al., 2004). This corroborated the hypothesis that rapid warming avoids growth of ice crystals, which may have occurred during rapid cooling (Mazur, 1970). CPA removal procedures are also critical factors for cell survival after cryopreservation. However, thawing is often poorly controlled. Clinical grade cryopreservation and banking require standardization, automation and safety assessment of all the technological steps including thawing. In view of the implementation of a clinical procedure, controlled thawing is still an unexplored alternative (Gurina et al., 2015).
Storage vessels
To the best of our knowledge there are no studies that compare different vessels (e.g straws, vials) and it is therefore difficult to make recommendations. Yet, Saragusty et al. (2009) described how volume and cell concentration affect cell viability after cryopreservation. With ice growth, the intra-vessel pressure increases, causing the cells to pack in the unfrozen part of the medium and provokes cell destruction by a ‘pack effect’. The larger ‘surface-area-to-volume ratio’ in vials compared to straws might reduce this ‘pack effect’ allowing easier extracellular ice expansion (Saragusty et al., 2009).
It remains a matter of debate, whether it is better to cryopreserve TT or TCSs (Wyns et al., 2010). Pacchiarotti et al. (2013) and Yango et al. (2014) stated that TT cryopreservation should be recommended. However, SSEA-4+ cells (a spermatogonial population) exhibited differential sensitivity to cryopreservation based on whether they were cryopreserved as TTs or as TCSs. Unfortunately, no functional proof was provided.
How to assay the functionality of SSCs?
It has been shown that cell viability does not necessarily correspond to the functional capacity of the SSCs (Frederickx et al., 2004; Jahnukainen et al., 2007; Zeng et al., 2009). In mammals, re-establishment of spermatogenesis is the only available system by which the stem cell function can be assessed. True validation of slow freezing and vitrification to preserve SSC functionality has been proven through TT tissue grafting and SSCT. In mouse (Shinohara et al., 2002; Frederickx et al., 2004; Wu et al., 2012) and higher mammals (Jahnukainen et al., 2007, 2012; Abrishami et al., 2010; Poels et al., 2012; Devi et al., 2014; Pothana et al., 2015; Pukazhenthi et al., 2015) spermatogenesis and offspring have been achieved after these procedures (Tables I and II). Functional frozen-thawed SSCs in suspension will generate colonies of donor-derived spermatogenesis after SSCT (Avarbock et al., 1996; Ogawa et al., 1999; Dobrinski et al., 1999b; Izadyar et al., 2002a,b; Frederickx et al., 2004; Hermann et al., 2007). The number of functional SSCs relates to the colony formation after transplantation (Nakagawa et al., 2007). Full proof for SSC functionality and fertility restoration is the production of donor-derived offspring (Kanatsu-Shinohara et al., 2003; Wu et al., 2012). SSCT with frozen-thawed cultured human SSCs to immunodeficient mice have demonstrated the ability of these cells to colonize the recipients tubules (Sadri-Ardekani et al., 2009).
Despite the promising results obtained in different models, the variability of methods used to assess cell viability and, more importantly, functionality hinders the translation to human SSCs. Additionally, the efficiency of the cryopreservation protocols can only be evaluated by transplantation assays, but this is not yet possible for human TT and TCSs. Thus, as long as there is no efficient way to evaluate the functionality for human SSCs after freezing, it is difficult to conclude which of the tested protocols is optimal.
Clinical, biological and genetic biosafety in cryopreservation of SSCs
Although fertility can be restored in mice having undergone SSCT or TTG, the question remains whether this process could be applied in a clinic in a safe and acceptable manner. So far, only a few reports have addressed safety issues related to the clinical implementation of male fertility restoration strategies using SSCs.
Cancer cell contamination
Many hematopoietic malignancies are capable of metastasizing through the blood. Testicular biopsies from cancer patients may contain malignant cells and thus, the risk for cancer relapse exists when transplanting these samples. For these patients, the preservation and transplantation of cancer-cell-free testicular cell suspensions is a requirement. Assessing the risks of transplanting carcinogenous cells into a cured patient is a critical step in the translation of the proposed fertility restoration techniques into a clinical application. In rats, transplanting only 20 leukemic cells to the testis, caused malignant relapse (Jahnukainen et al., 2001). In the human, the threshold number of malignant cells able to cause malignant relapse when transplanted to the testis is unknown. Therefore, it is of immense importance to detect contamination in the TT samples (Hou et al., 2007; Goossens et al., 2013). In case of contamination, the separation of SSCs from malignant cells before transplantation is necessary. Cell sorting techniques such as magnetic-activated and/or fluorescence-activated cell sorting, selective matrix adhesion, and selective cell culturing aiming to deplete cancer cells have been studied. Yet, in mice and human models the reported results are insufficient. The lack of specific SSC surface markers and the aggregation of germ and leukemic cells are limiting factors in positive selection of germ cells (Fujita et al., 2006; Geens et al., 2007, 2011; Dovey et al., 2013; Goossens et al., 2013; Valli et al., 2014). A pilot study by Sadri-Ardekani and Atala (2014b) reported a testicular cell culture system not only efficient for propagation of SSCs but also for eliminating contaminating acute lymphoblastic leukemai (ALL) cells. Culturing testicular cells in combination with ALL cells permitted the elimination of ALL cells after 26 days of culture. Anyhow, for patients at risk of malignant contamination, autologous TTG will not be possible, so the only option to restore fertility will be by transplanting cell suspensions or IVS.
Storage of TTs and TCSs in LN2 holds the risk that samples become infected with agents, especially viral agents, inadvertently released into storage tanks from other (infected) samples (Fuller and Paynter, 2004). Despite the fact that the LN2 liquid phase provides a more stable temperature for storage, storing in the vapor phase appears to be safer (Tedder et al., 1995; Fountain et al., 1997).
Genetics
Unfortunately, compared with the increasing body of evidence illustrating the effectiveness of SSCT and TTG in reproductive terms, only a few studies have addressed safety concerns regarding the influence on genetic and epigenetic modifications in germ cells. After SSCT and TTG with fresh samples, neither numerical chromosomal alterations, nor epigenetic modifications were detected in spermatozoa (Goossens et al., 2009, 2010, 2011). Extended cell manipulation and exposition to digestive and cryoprotective solutions, plus the hurdle of cryopreservation itself, may induce modifications in the germ line cells, e.g. changes in DNA methylation, or chromosomal abnormalities. Indeed, the disruption of SSCs from their niche might influence the establishment of correct epigenetic patterns and thus the development of spermatozoa arising from the possibly altered SSC (Goossens et al., 2011). Oocytes fertilized by spermatozoa arising from epigenetically altered SSCs develop abnormally. Only a few reports have addressed the influence of cryopreservation on genetic and epigenetic changes in SSCs. DMSO was found to induce modifications in the cell's epigenetic profile (Iwatani et al., 2006; Kawai et al., 2010). Wu et al. (2012) evaluated the ability of TCSs to endure long-term cryopreservation (>14 years). Cultured mouse and rat SSCs re-established complete spermatogenesis and produced fertile mouse progeny without apparent genetic or epigenetic errors. Also, genetic stability and spermatogenic function of adult mouse SSCs were reported after transplanting cultured and cryopreserved TCSs into germ cell-ablated recipients (Yuan et al., 2009). For TTs, cryopreservation has reported no deleterious effect on meiotic recombination and synapsis (Li et al., 2009). In non-human primates, sperm obtained after SSCT using cryopreserved TTCs was reported in the rhesus. The functional ability of donor-derived sperm to fertilize rhesus oocytes by in vitro fertilization and to stimulate early embryo development suggested that the sperm were functionally normal. Unfortunately, no surrogacy, thus, no proof of normal embryo development in the offspring was reported (Hermann et al., 2012). Further studies need to be conducted to confirm and examine the potential genetic or epigenetic changes in SSCs and offspring using frozen-thawed prepubertal TT or TCS samples.
Conclusion and future recommendations
Testicular toxicity is an inevitable long-term consequence of several therapeutic oncological regimens, leading to gonadal failure or sterility in many patients. Cryopreservation of TT is an option for fertility preservation when other techniques such as cryopreservation of ejaculated sperm are not available or applicable. Given the limited number of SSCs in the testes, an optimal cryopreservation protocol is a prerequisite for the successful clinical application of any fertility restoration strategy (Frederickx et al., 2004; Phillips et al., 2010).
Until now, the Univeristaire Ziekenhuis (UZ) Brussel has stored TT from more than 90 prepubertal boys. Recently, Picton et al. (2015) divulged the outcome of a survey distributed to 24 hospitals in Europe and Israel prior to December 2012, where they stated that more than 260 young patients had already undergone TT retrieval for fertility preservation. Of the responding hospitals, 50% actively offer TT cryobanking for fertility preservation in boys and adolescents and the remainder were seriously considering install a tissue-based fertility preservation program. However, a recent study of 23 French regional sperm and tissue banks recorded considerable inter-center variations in practices involving young patients seeking to preserve their fertility before cancer therapy, demonstrating that new fertility preservation strategies such as immature TT cryopreservation are still underused (Daudin et al., 2015). In consequence, germ cell banking is not universally practiced in pediatric oncology centers and practices differ between centers (Anderson et al., 2015). Furthermore, cryopreservation of human prepubertal testicular tissue or cells has not yet resulted in sperm production, neither in vivo nor in vitro, and therefore, albeit their clinical application, none of the proposed cryopreservation protocols have been fully validated.
Effective protocols for human TT are currently applied in the clinic (Keros et al., 2005; Kvist et al., 2006; Keros et al., 2007; Wyns et al., 2007; Baert et al., 2013). To date, controlled slow freezing protocols using 0.7 to 1.5 M DMSO, 0.1 M sucrose and 5 to 10% HSA for adult and prepubertal human TT have reported encouraging results, including the preservation of testicular cells, the maintenance of TT integrity and activity in organotypic tissue culture (Table I).
For cancer patients, tissue could contain malignant cells and thus, the risk of cancer relapse exists when using these samples. Thus, cancer cell-free TCSs would be the best option to preserve and restore fertility for these patients (Sá et al., 2012). Currently, OPS vitrification, including high concentrations of DMSO (1.1 to 2.8 M), non-penetrant CPAs and 10% HSA has resulted in the highest cell viability. However, this technique is at risk of introducing infectious agents, thus, lacks the option of a clinical application. In contrast, a controlled slow freezing protocol, produced under effective clinical-grade guidelines for the cryopreservation of human testicular cells, although achieving a lower cell viability, was conferred by Pacchiarotti et al. (2013) as an alternative in view of a clinical application.
Recommendations for the future
Optimization of cryopreservation protocols requires the refinements to the freezing and thawing rates, the osmotic conditions, the choice and concentration of CPAs, and the equilibration times in the CPAss. Improvements in all these factors might result in better survival and functionality of human tissue and cell samples permitting successful fertility restoration. An optimal cryopreservation protocol for human TT and TCS should benefit from the experience gathered in different animal models. These results should be judiciously used for the optimization and standardization of a clinical-grade cryopreservation protocol. For instance, alternatives to reduce, replace or combine toxic CPAs with less toxic factors such as antioxidant reagents and antiapoptotic factors are still unexplored alternatives which should be prompted (Aliakbari et al., 2016). Additionally, the use of convenient and inexpensive techniques, such as uncontrolled freezing or vitrification, should be studied as an alternative to controlled freezing (Baert et al., 2012). More importantly, information regarding the efficiency of these protocols and the influence on genetic and epigenetic modifications in germ cells after the cryopreservation procedure must be carefully studied.
For cancer patients, several burdens regarding biosafety lead to the question whether it is better to cryopreserve cell suspensions or TT. Later, the subsequent functional restoration of cryopreserved samples is a challenging task. Indeed, male fertility strategies such as SSCT, TTG and IVS are all promising experimental approaches to restore fertility (Orwig and Schlatt, 2005; Hermann et al., 2012; Faes et al., 2013; Nickkholgh et al., 2014). Finally, since SSC cryopreservation has a major impact on the outcome of fertility restoration techniques, national guidelines and recommendations for good tissue banking would be helpful (Picton et al., 2015).
Authors’ roles
J.O. and Y.B. were involved in the conception and design of this review, drafting of the article and final approval. K.F. was involved in revision of the article and final approval. E.G. was involved in the conception and design of this review, revision of the article, and final approval.
Funding
This review paper was written with support from: European Commission Research and Innovation Marie Slodowska Actions (MSCA) – Seventh Framework program (FP7-People-2013-ITN-603568); the Agency for Innovation by Science and Technology (IWT); the Flemish League against Cancer-Public Utility Foundation; the Vrije Universiteit Brussel (Methusalem); and UZ Brussel (Wetenshappelijk Fonds Willy Gepts).
Conflict of interest
The authors declare that no competing interests exist.
References
- Abrishami M, Anzar M, Yang Y, Honaramooz A. Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology 2010;73:86–96. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Ranganathan P, Kattal N, Pasqualotto F, Hallak J, Khayal S, Mascha E. Fertility after cancer: a prospective review of assisted reproductive outcome with banked semen specimens. Fertil Steril 2004;81:342–348. [DOI] [PubMed] [Google Scholar]
- Akkök CA, Liseth K, Melve GK, Ersvær E, Hervig T, Bruserud O. Is there a scientific basis for a recommended standardization of collection and cryopreservation of peripheral blood stem cell grafts. Cytotherapy 2011;13:1013–1024. [DOI] [PubMed] [Google Scholar]
- Aksglaede L, Wikström AM, Rajpert-De Meyts E, Dunkel L, Skakkebaek NE, Juul A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update 2006;12:39–48. [DOI] [PubMed] [Google Scholar]
- Aliakbari F, Gilani M, Amidi F, Baazm M, Korouji M, Izadyar F, Yazdekhasti H. Improving the efficacy of cryopreservation of spermatogonia stem cells by antioxidant supplements. Cell Reprod 2016;18:87–95. [DOI] [PubMed] [Google Scholar]
- Anchordoguy TJ, Rudolph AS, Carpenter JF, Crowe JH. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology 1987;24:324–331. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WHB. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 2015;3:556–567. [DOI] [PubMed] [Google Scholar]
- Anserini P, Chiodi S, Spinelli S, Costa M, Conte N, Copello F, Bacigalupo A. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant 2002;30:447–451. [DOI] [PubMed] [Google Scholar]
- Arkoun B, Dumont L, Milazzo J-P, Rondanino C, Bironneau A, Wils J, Rives N. Does soaking temperature during controlled slow freezing of pre-pubertal mouse testes influence course of in vitro spermatogenesis. Cell Tissue Res 2015;364:661–674. [DOI] [PubMed] [Google Scholar]
- Ash P. The influence of radiation on fertility in man. Br J Radiol 1980;53:271–278. [DOI] [PubMed] [Google Scholar]
- Avarbock MR, Brinster CJ, Brinster RL. Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nat Med 1996;2:693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert Y, Braye A, Struijk RB, Pelt AMM, van Goossens E. Cryopreservation of testicular tissue before long-term testicular cell culture does not alter in vitro cell dynamics. Fertil Steril 2015;104:1244–1252. [DOI] [PubMed] [Google Scholar]
- Baert Y, Goossens E, van Saen D, Ning L, in't Veld P, Tournaye H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril 2012;97:1152–1157. [DOI] [PubMed] [Google Scholar]
- Baert Y, Van Saen D, Haentjens P, in't Veld P, Tournaye H, Goossens E. What is the best cryopreservation protocol for human testicular tissue banking . Hum Reprod 2013;28:1816–1826. [DOI] [PubMed] [Google Scholar]
- Bellvé AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol 1977;74:68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y, Vannier J-P, Yakouben K, Thuret I, Bordigoni P. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood 2007;110:2749–2756. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 2003;88:622–626. [DOI] [PubMed] [Google Scholar]
- Brayton CF. Dimethyl sulfoxide (DMSO): a review. Cornell Vet 1986;76:61–90. [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA 1994;91:11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG, Sc D. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil Steril 2001;75:269–274. [DOI] [PubMed] [Google Scholar]
- Chemes HE. Infancy is not a quiescent period of testicular development. Int J Androl 2001;24:2–7. [DOI] [PubMed] [Google Scholar]
- Crabbe E, Verheyen G, Tournaye H, Steirteghem A, Van. Freezing of testicular tissue as a minced suspension preserves sperm quality better than whole-biopsy freezing when glycerol is used as cryoprotectant. Int J Androl 1999;22:43–48. [DOI] [PubMed] [Google Scholar]
- Curaba M, Poels J, Langendonckt A, van, Donnez J, Wyns C. Can prepubertal human testicular tissue be cryopreserved by vitrification. Fertil Steril 2011. a;95:2123. [DOI] [PubMed] [Google Scholar]
- Curaba M, Verleysen M, Amorim CA, Dolmans M-M, Van Langendonckt A, Hovatta O, Wyns C, Donnez J. Cryopreservation of prepubertal mouse testicular tissue by vitrification. Fertil Steril 2011. b;95:1229–1234. [DOI] [PubMed] [Google Scholar]
- Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, Eustache F, Saïas-Magnan J, Papaxanthos-Roche A, Cabry-Goubet R. Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS). Fertil Steril 2015;103:478–486. [DOI] [PubMed] [Google Scholar]
- De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet 2014;384:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Dedeken L, Moffa F, Tsepelidis S, Brachet C, Delbaere A, Devreker F, Ferster A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod 2015;30:2107–2109. [DOI] [PubMed] [Google Scholar]
- Devi L, Makala H, Pothana L, Nirmalkar K. Comparative efficacies of six different media for cryopreservation of immature buffalo (Bubalus bubalis) calf testis. Reprod Fertil Dev 2014;28(7):872–885. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Avarbock MR, Brinster RL. Germ cell transplantation from large domestic animals into mouse testes. Mol Reprod Dev 2000;57:270–279. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod 1999. a;61:1331–1339. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Ogawa T, Avarbock MR, Brinster RL. Computer assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol Reprod Dev 1999. b;53:142–148. [DOI] [PubMed] [Google Scholar]
- Donaldson C, Armitage WJ, Denning-Kendall PA, Nicol AJ, Bradley BA, Hows JM. Optimal cryopreservation of human umbilical cord blood. Bone Marrow Transplant 1996;18:725–731. [PubMed] [Google Scholar]
- Dovey SL, Valli B, Hermann B. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J Clin Invest 2013;123:1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont L, Arkoun B, Jumeau F, Milazzo J-P, Bironneau A, Liot D, Wils J, Rondanino C, Rives N. Assessment of the optimal vitrification protocol for pre-pubertal mice testes leading to successful in vitro production of flagellated spermatozoa. Andrology 2015;3:611–625. [DOI] [PubMed] [Google Scholar]
- Faes K, Tournaye H, Goethals L, Lahoutte T, Hoorens A, Goossens E. Testicular cell transplantation into the human testes. Fertil Steril 2013;100:981–988. [DOI] [PubMed] [Google Scholar]
- Foresta C, Galeazzi C, Bettella A, Marin P, Rossato M, Garolla A, Ferlin A. Analysis of meiosis in intratesticular germ cells from subjects affected by classic Klinefelter's syndrome. J Clin Endocrinol Metab 1999;84:3807–3810. [DOI] [PubMed] [Google Scholar]
- Fountain D, Ralston M, Higgins N, Gorlin JB, Uhl L, Wheeler C, Antin JH, Churchill WH, Benjamin RJ. Liquid nitrogen freezers: a potential source of microbial contamination of hematopoietic stem cell components. Transfusion 1997;37:585–591. [DOI] [PubMed] [Google Scholar]
- Frederickx V, Michiels A, Goossens E, De Block G, VanSteirteghem AC, Tournaye H. Recovery, survival and functional evaluation by transplantation of frozen-thawed mouse germ cells. Hum Reprod 2004;19:948–953. [DOI] [PubMed] [Google Scholar]
- Fujita K, Tsujimura A, Miyagawa Y, Kiuchi H, Matsuoka Y, Takao T, Takada S, Nonomura N, Okuyama A. Isolation of germ cells from leukemia and lymphoma cells in a human in vitro model: potential clinical application for restoring human fertility after anticancer therapy. Cancer Res 2006;66:11166–11171. [DOI] [PubMed] [Google Scholar]
- Fuller B, Paynter S. Fundamentals of cryobiology in reproductive medicine. Reprod Biomed Online 2004;9:680–691. [DOI] [PubMed] [Google Scholar]
- Garg T, Larosa C, Strawn E, Robb P, Sandlow JI. Outcomes after testicular aspiration and testicular tissue cryopreservation for Obstructive Azoospermia and Ejaculatory Dysfunction 2008. JURO 2008;180:2577–2580. [DOI] [PubMed] [Google Scholar]
- Geens M, Goossens E, Tournaye H. Cell selection by selective matrix adhesion is not sufficiently efficient for complete malignant cell depletion from contaminated human testicular cell suspensions. Fertil Steril 2011;95:787–791. [DOI] [PubMed] [Google Scholar]
- Geens M, Van De Velde H, De Block G, Goossens E, Van Steirteghem A, Tournaye H. The efficiency of magnetic-activated cell sorting and fluorescence- activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Hum Reprod 2007;22:733–742. [DOI] [PubMed] [Google Scholar]
- Gholami M, Hemadi M, Saki G, Zendedel A, Khodadadi A, Mohammadi-Asi J. Does prepubertal testicular tissue vitrification influence spermatogonial stem cells (SSCs) viability. J Assist Reprod Genet 2013;30:1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gies I, De Schepper J, Goossens E, Van Saen D, Pennings G, Tournaye H. Spermatogonial stem cell preservation in boys with Klinefelter syndrome: To bank or not to bank, that's the question. Fertil Steril 2012. a;98:284–289. [DOI] [PubMed] [Google Scholar]
- Gies I, De Schepper J, Van Saen D, Anckaert E, Goossens E, Tournaye H. Failure of a combined clinical-and hormonal-based strategy to detect early spermatogenesis and retrieve spermatogonial stem cells in 47,XXY boys by single testicular biopsy. Hum Reprod 2012. b;27:998–1004. [DOI] [PubMed] [Google Scholar]
- Goossens E, Bilgec T, Van Saen D, Tournaye H. Mouse germ cells go through typical epigenetic modifications after intratesticular tissue grafting. Hum Reprod 2011;26:3388–3400. [DOI] [PubMed] [Google Scholar]
- Goossens E, De Block G, Tournaye H. Computer-assisted motility analysis of spermatozoa obtained after spermatogonial stem cell transplantation in the mouse. Fertil Steril 2008;90:1411–1416. [DOI] [PubMed] [Google Scholar]
- Goossens E, De Rycke M, Haentjens P, Tournaye H. DNA methylation patterns of spermatozoa and two generations of offspring obtained after murine spermatogonial stem cell transplantation. Hum Reprod 2009;24:2255–2263. [DOI] [PubMed] [Google Scholar]
- Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: from research to clinic. Hum Reprod 2013;28:897–907. [DOI] [PubMed] [Google Scholar]
- Goossens E, de Vos P, Tournaye H. Array comparative genomic hybridization analysis does not show genetic alterations in spermatozoa and offspring generated after spermatogonial stem cell transplantation in the mouse. Hum Reprod 2010;25:1836–1842. [DOI] [PubMed] [Google Scholar]
- Gouk SS, Loh YFJ, Kumar SD, Watson PF, Kuleshova LL. Cryopreservation of mouse testicular tissue: prospect for harvesting spermatogonial stem cells for fertility preservation. Fertil Steril 2011;95:2399–2403. [DOI] [PubMed] [Google Scholar]
- Grilli G, Porcellini A, Lucarelli G. Role of serum on cryopreservation and subsequent viability of mouse bone marrow hemopoietic stem cells. Cryobiology 1980;17:516–520. [DOI] [PubMed] [Google Scholar]
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol 1998;9:411–416. [DOI] [PubMed] [Google Scholar]
- Gurina TM, Pakhomov AV, Polyakova AL, Legach EI, Bozhok GA. The development of the cell cryopreservation protocol with controlled rate thawing. Cell Tissue Bank 2016;17:303. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Lin C-C, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells 2007;25:2330–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 2012;11:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Andersson M, Eksborg S, Söder O, Jahnukainen K. Xenotransplantation of testicular tissue into nude mice can be used for detecting leukemic cell contamination. Hum Reprod 2007;22:1899–1906. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Foudila T, Siegberg R, Johansson K, von Smitten K, Reima I. Pregnancy resulting from intracytoplasmic injection of spermatozoa from a frozen-thawed testicular biopsy specimen. Hum Reprod 1996;11:2472–2473. [DOI] [PubMed] [Google Scholar]
- Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr 2005;34:12–17. [DOI] [PubMed] [Google Scholar]
- Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol 2010;116:1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatani M, Ikegami K, Kremenska Y, Hattori N, Tanaka S, Yagi S, Shiota K. Dimethyl sulfoxide has an impact on epigenetic profile in mouse embryoid body. Stem Cells 2006;24:2549–2556. [DOI] [PubMed] [Google Scholar]
- Izadyar F, Den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG. Proliferation and differentiation of bovine Type A spermatogonia during long-term culture. Biol Reprod 2002. a;68:272–281. [DOI] [PubMed] [Google Scholar]
- Izadyar F, Matthijs-Rijsenbilt JJ, den Ouden K, Creemers LB, Woelders H, de Rooij DG. Development of a cryopreservation protocol for Type A spermatogonia. J Androl 2002. b;23:537–545. [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod 2007;22:1060–1067. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Hou M, Schlatt S. Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab 2011;25:287–302. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Nurmio M, Schlatt S. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res 2012;72:5174–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnukainen K, Hou M, Petersen C, Setchell B, Söder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res 2001;61:706–710. [PubMed] [Google Scholar]
- Joyce AD, Gosden RG, Sc D. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil Steril 2001;75:269–274. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Ogura A, Toyokuni S, Shinohara T. Restoration of fertility in infertile mice by transplantation of cryopreserved male germline stem cells. Hum Reprod 2003;18:2660–2667. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Kikuchi K, Nakai M, Somfai T, Noguchi J, Tanihara F, Ito J, Kashiwazaki N. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS One 2013;8:e70989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Kikuchi K, Tanihara F, Noguchi J, Nakai M, Ito J, Kashiwazaki N. Normal reproductive development of pigs produced using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. Theriogenology 2014;82:325–331. [DOI] [PubMed] [Google Scholar]
- Karlsson JO, Cravalho EG, Borel Rinkes IH, Tompkins RG, Yarmush ML, Toner M. Nucleation and growth of ice crystals inside cultured hepatocytes during freezing in the presence of dimethyl sulfoxide. Biophys J 1993;65:2524–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials 1996;17:243–256. [DOI] [PubMed] [Google Scholar]
- Kawai K, Li YS, Song MF, Kasai H. DNA methylation by dimethyl sulfoxide and methionine sulfoxide triggered by hydroxyl radical and implications for epigenetic modifications. Bioorganic Med Chem Lett 2010;20:260–265. [DOI] [PubMed] [Google Scholar]
- Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: Comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod 2005;20:1676–1687. [DOI] [PubMed] [Google Scholar]
- Keros V, Hultenby K, Borgstrom B, Fridstrom M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod 2007;22:1384–1395. [DOI] [PubMed] [Google Scholar]
- Körbling M, Freireich EJ. Twenty-five years of peripheral blood stem cell transplantation. Blood 2011;117:6411–6416. [DOI] [PubMed] [Google Scholar]
- Kvist K, Thorup J, Byskov AG, Hoyer PE, Mollgard K, Yding Andersen C. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum Reprod 2006;21:484–491. [DOI] [PubMed] [Google Scholar]
- Lee YA, Kim YH, Ha SJ, Kim KJ, Kim BGJ, Choi SH, Kim IC, Schmidt JA, Ryu BY, Kim BGJ. Cryopreservation of porcine spermatogonial stem cells by slow-freezing testis tissue in trehalose. J Anim Sci 2014. a;92:984–995. [DOI] [PubMed] [Google Scholar]
- Lee YA, Kim YH, Ha SJ, Kim BJ, Kim KJ, Jung MS, Kim BG, Ryu BY. Effect of sugar molecules on the cryopreservation of mouse spermatogonial stem cells. Fertil Steril 2014. b;101:1165–1175. [DOI] [PubMed] [Google Scholar]
- Lee YA, Kim YH, Kim B, Jung MS, Auh J, Seo J, Park YS, Lee S, Ryu B. Cryopreservation of mouse spermatogonial stem cells in dimethylsulfoxide and polyethylene glycol. Biol Reprod 2013. a;89:109. [DOI] [PubMed] [Google Scholar]
- Lee YA, Kim YH, Kim BG, Kim KJ, Auh J, Schmidt JA, Ryu BY. Cryopreservation in trehalose preserves functional capacity of murine spermatogonial stem cells. PLoS One 2013. b;8:e54889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RL, Cravalho EG, Huggins CE. Water transport in a cluster of closely packed erythrocytes at subzero temperatures. Cryobiology 1977;14:549–558. [DOI] [PubMed] [Google Scholar]
- Li J, Leng M, Ma T, Yu D, Shi H, Shi Q. Cryopreservation has no effect on meiotic recombination and synapsis in testicular tissues. Fertil Steril 2009;91:1404–1407. [DOI] [PubMed] [Google Scholar]
- Liu J, Woods EJ, Agca Y, Critser ES, Critser JK. Cryobiology of rat embryos II: a theoretical model for the development of interrupted slow freezing procedures. Biol Reprod 2000;63:1303–1312. [DOI] [PubMed] [Google Scholar]
- Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukusa AK, Vermylen C, Vanabelle B, Curaba M, Brichard B, Chantrain C, Dupont S, Ferrant A, Wyns C. Bone marrow transplantation or hydroxyurea for sickle cell anemia: long-term effects on semen variables and hormone profiles. Pediatr Hematol Oncol 2009;26:186–194. [DOI] [PubMed] [Google Scholar]
- Martino A, Songsasen N, Leibo SP. Development into blastocysts of bovine oocytes cryopreserved by ultra-rapid cooling. Biol Reprod 1996;54:1059–1069. [DOI] [PubMed] [Google Scholar]
- Mazur P, Rigopoulos N. Contributions of unfrozen fraction and of salt concentration to the survival of slowly frozen human erythrocytes: influence of warming rate. Cryobiology 1983;20:274–289. [DOI] [PubMed] [Google Scholar]
- Mazur P. Cryobiology: the freezing of biological systems. Science 1970;168:939–949. [DOI] [PubMed] [Google Scholar]
- Mazur P. Equilibrium, quasi-equilibrium, and nonequilibrium freezing of mammalian embryos. Cell Biophys 1990;17:53–92. [DOI] [PubMed] [Google Scholar]
- Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol 1984;247:C125–C142. [DOI] [PubMed] [Google Scholar]
- Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977;272:251–272. [DOI] [PubMed] [Google Scholar]
- Milazzo JP, Travers A, Bironneau A, Safsaf A, Gruel E, Arnoult C, Macé B, Boyer O, Rives N. Rapid screening of cryopreservation protocols for murine prepubertal testicular tissue by histology and PCNA immunostaining. J Androl 2010;31:617–630. [DOI] [PubMed] [Google Scholar]
- Milazzo JP, Vaudreuil L, Cauliez B, Gruel E, Massé L, Mousset-Siméon N, Macé B, Rives N. Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum Reprod 2008;23:17–28. [DOI] [PubMed] [Google Scholar]
- Muldrew K, McGann LE. The osmotic rupture hypothesis of intracellular freezing injury. Biophys J 1994;66:532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell 2007;12:195–206. [DOI] [PubMed] [Google Scholar]
- Nickkholgh B, Mizrak SC, Korver CM, Van Daalen SKM, Meissner A, Repping S, Van Pelt AMM. Enrichment of spermatogonial stem cells from long-term cultured human testicular cells. Fertil Steril 2014;102:558–565. [DOI] [PubMed] [Google Scholar]
- Nugent D, Meirow D, Brook PF, Aubard Y, Gosden RG. Transplantation in reproductive medicine: previous experience, present knowledge and future prospects. Hum Reprod Update 1997;3:267–280. [DOI] [PubMed] [Google Scholar]
- Odavic R, Blom J, Beck EA, Bucher U. Cryoprotection of human bone marrow committed stem cells (CFU-c) by dextran, glycerol and dimethyl sulfoxide. Experientia 1980;36:1122–1124. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol Reprod 1999;60:515–521. [DOI] [PubMed] [Google Scholar]
- Oktay K, Oktem O. Fertility preservation medicine: a new field in the care of young cancer survivors. Pediatr Blood Cancer 2009;53:267–273. [DOI] [PubMed] [Google Scholar]
- Oosterhuis BE, Goodwin T, Kiernan M, Hudson MM, Dahl GV. Concerns about infertility risks among pediatric oncology patients and their parents. Pediatr Blood Cancer 2008;50:85–89. [DOI] [PubMed] [Google Scholar]
- Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr 2005: 51–56. [DOI] [PubMed] [Google Scholar]
- Pacchiarotti J, Ramos T, Howerton K, Greilach S, Zaragoza K, Olmstead M, Izadyar F. Developing a clinical-grade cryopreservation protocol for human testicular tissue and cells. Biomed Res Int 2013;2013:930–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:17–18. [DOI] [PubMed] [Google Scholar]
- Passweg JR, Rabusin M. Hematopoetic stem cell transplantation for immune thrombocytopenia and other refractory autoimmune cytopenias. Autoimmunity 2008;41:660–665. [DOI] [PubMed] [Google Scholar]
- Pfeifer S, Goldberg J, Lobo R, Pisarska M, Thomas M, Widra E, Sandlow J, Licht M, Rosen M, Vernon M et al. Ovarian tissue cryopreservation: a committee opinion. Fertil Steril 2014;101:1237–1243. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010;365:1663–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, Mitchell RT, Pennings G, Rives N, Tournaye H. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod 2015;30:2463–2475. [DOI] [PubMed] [Google Scholar]
- Pietzak EJ, Tasian GE, Tasian SK, Brinster RL, Carlson C, Ginsberg JP, Kolon TF. Histology of Testicular Biopsies Obtained for Experimental Fertility Preservation Protocol in Boys with Cancer. J Urol 2015;194:1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels J, Abou-Ghannam G, Herman S, Van Langendonckt A, Wese F-X, Wyns C. In search of better spermatogonial preservation by supplementation of cryopreserved human immature testicular tissue xenografts with N-acetylcysteine and testosterone. Front Surg 2014;1:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels J, Van Langendonckt A, Dehoux JP, Donnez J, Wyns C. Vitrification of non-human primate immature testicular tissue allows maintenance of proliferating spermatogonial cells after xenografting to recipient mice. Theriogenology 2012;77:1008–1013. [DOI] [PubMed] [Google Scholar]
- Poels J, Van Langendonckt A, Many M-C, Wese F-X, Wyns C. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod 2013;28:578–589. [DOI] [PubMed] [Google Scholar]
- Pothana L, Makala H, Devi L, Varma VP, Goel S. Germ cell differentiation in cryopreserved, immature, Indian spotted mouse deer (Moschiola indica) testes xenografted onto mice. Theriogenology 2015;83:625–633. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi BS, Nagashima J, Travis AJ, Costa GM, Escobar EN, França LR, Wildt DE. Slow Freezing, but Not Vitrification Supports Complete Spermatogenesis in Cryopreserved, Neonatal Sheep Testicular Xenografts. PLoS One 2015;10:e0123957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley MJ, Leach DR, Warner GA, Heller CG. Effect of graded doses of ionizing radiation on the human testis. Radiat Res 1974;59:665–678. [PubMed] [Google Scholar]
- Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, Taylor PE, Stevens CE. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA 1995;92:10119–10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruutiainen T, Miller S, Caplan A, Ginsberg JP. Expanding access to testicular tissue cryopreservation: an analysis by analogy. Am J Bioeth 2013;13:28–35. [DOI] [PubMed] [Google Scholar]
- Sá R, Cremades N, Malheiro I, Sousa M. Cryopreservation of human testicular diploid germ cell suspensions. Andrologia 2012;44:366–372. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Atala A. Testicular tissue cryopreservation and spermatogonial stem cell transplantation to restore fertility: from bench to bedside. Stem Cell Res Ther 2014. a;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Atala A. Eliminating acute lymphoblastic leukemia cells from human testicular cell cultures: A pilot study. Fertil Steril 2014. b;101:1072–1078. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Mizrak SC, Van Daalen SKM, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, De Reijke TM, De La Rosette JJMCH, Van Der Veen F. Propagation of human spermatogonial stem cells in vitro. JAMA 2009;302:2127–2134. [DOI] [PubMed] [Google Scholar]
- Saragusty J, Gacitua H, Rozenboim I, Arav A. Do physical forces contribute to cryodamage. Biotechnol Bioeng 2009;104:719–728. [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011;471:504–507. [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Kubota Y, Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat Protoc 2013;8:2098–3104. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction 2002;124:339–346. [DOI] [PubMed] [Google Scholar]
- Schneider F, Redmann K, Wistuba J, Schlatt S, Kliesch S, Neuhaus N. Comparison of enzymatic digestion and mechanical dissociation of human testicular tissues. Fertil Steril 2015;104:302–311. [DOI] [PubMed] [Google Scholar]
- Enrique Criado Scholz. (2012). The Problem of Contamination: Open vs. Closed vs. Semi-Closed Vitrification Systems, Current Frontiers in Cryopreservation, Prof. Igor Katkov (Ed.), InTech, DOI: 10.5772/36556. Available from: http://www.intechopen.com/books/current-frontiers-in-cryopreservation/the-problem-of-contamination-open-vs-close-vs-semi-close-vitrification-systems.
- Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer 1999;86:697–709. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Siegmund HI, Roesch W, Hadziselimovic F, Hofstaedter F. Male infertility: assessment of juvenile testicular dysfunction and risk for malignancy in cryptorchid boys based on resin section evaluation. Ultrastruct Pathol 2013;37:373–377. [DOI] [PubMed] [Google Scholar]
- Sciurano RB, Luna Hisano CV, Rahn MI, Brugo Olmedo S, Rey Valzacchi G, Coco R, Solari AJ. Focal spermatogenesis originates in euploid germ cells in classical Klinefelter patients. Hum Reprod 2009;24:2353–2360. [DOI] [PubMed] [Google Scholar]
- Shalet SM, Tsatsoulis A, Whitehead E, Read G. Vulnerability of the human Leydig cell to radiation damage is dependent upon age. J Endocrinol 1989;120:161–165. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, Kurome M, Nagashima H, Toyokuni S, Kogishi K. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in vitro microinsemination. Hum Reprod 2002;17:3039–3045. [DOI] [PubMed] [Google Scholar]
- Slavin S, Aker M, Shapira MY, Panigrahi S, Gabriel C, Or R. Non-myeloablative stem cell transplantation for the treatment of cancer and life-threatening non-malignant disorders; past accomplishments and future goals. Transfus Apher Sci 2002;27:159–166. [DOI] [PubMed] [Google Scholar]
- Sum AK, Faller R, de Pablo JJ. Molecular simulation study of phospholipid bilayers and insights of the interactions with disaccharides. Biophys J 2003;85:2830–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Smith GD. Fertility Cryopreservation. Fertil Cryopreserv 2010: 24–38. [Google Scholar]
- Tedder RS, Zuckerman MA, Goldstone AH, Hawkins AE, Fielding A, Briggs EM, Irwin D, Blair S, Gorman AM, Patterson KG. Hepatitis B transmission from contaminated cryopreservation tank. Lancet 1995;346:137–140. [DOI] [PubMed] [Google Scholar]
- Thomson AB, Campbell AJ, Irvine DC, Anderson RA, Kelnar CJH, Wallace WHB. Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: a case-control study. Lancet 2002;360:361–367. [DOI] [PubMed] [Google Scholar]
- Thorpe PE, Knight SC, Farrant J. Optimal conditions for the preservation of mouse lymph node cells in liquid nitrogen using cooling rate techniques. Cryobiology 1976;13:126–133. [DOI] [PubMed] [Google Scholar]
- Tournaye H, Dohle GR, Barratt CLR. Fertility preservation in men with cancer. Lancet 2014;384:1295–1301. [DOI] [PubMed] [Google Scholar]
- Tournaye H, Staessen C, Liebaers I, Van Assche E, Devroey P, Bonduelle M, Van Steirteghem A. Testicular sperm recovery in nine 47,XXY Klinefelter patients. Hum Reprod 1996;11:1644–9. [DOI] [PubMed] [Google Scholar]
- Travers A, Milazzo JP, Perdrix A, Metton C, Bironneau A, Macé B, Rives N. Assessment of freezing procedures for rat immature testicular tissue. Theriogenology 2011;76:981–990. [DOI] [PubMed] [Google Scholar]
- Trombetta C, Liguori G, Gianaroli L, Magli CM, Selman HA, Colpi G, Belgrano E, Vitali G, Ferraretti AP. Testicular sperm extraction combined with cryopreservation of testicular tissue in the treatment of azoospermia. Urol Int 2000: 15–20. [DOI] [PubMed] [Google Scholar]
- Unni S, Kasiviswanathan S, D'Souza S, Khavale S, Mukherjee S, Patwardhan S, Bhartiya D. Efficient cryopreservation of testicular tissue: effect of age, sample state, and concentration of cryoprotectant. Fertil Steril 2012;97:200–208. [DOI] [PubMed] [Google Scholar]
- Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H, Greve T, Callesen H. Open pulled straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev 1998;51:53–58. [DOI] [PubMed] [Google Scholar]
- Valli H, Sukhwani M, Dovey SL, Peters KA, Donohue J, Castro CA, Chu T, Marshall GR, Orwig KE. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril 2014;102:566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, Van Steirteghem A, Liebaers I. Cytogenetics of infertile men. Hum Reprod 1996;11:1–26. [DOI] [PubMed] [Google Scholar]
- Van Saen D, Gies I, De Schepper J, Tournaye H, Goossens E. Can pubertal boys with Klinefelter syndrome benefit from spermatogonial stem cell banking. Hum Reprod 2012. a;27:323–330. [DOI] [PubMed] [Google Scholar]
- Van Saen D, Tournaye H, Goossens E. Presence of spermatogonia in 47,XXY men with no spermatozoa recovered after testicular sperm extraction. Fertil Steril 2012. b;97:319–323. [DOI] [PubMed] [Google Scholar]
- Wallace WHB, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered. Lancet Oncol 2005;6:209–218. [DOI] [PubMed] [Google Scholar]
- Wikström AM, Hoei-Hansen CE, Dunkel L, Rajpert-De Meyts E. Immunoexpression of androgen receptor and nine markers of maturation in the testes of adolescent boys with Klinefelter syndrome: evidence for degeneration of germ cells at the onset of meiosis. J Clin Endocrinol Metab 2007;92:714–719. [DOI] [PubMed] [Google Scholar]
- Wu J, Hu T, Guo B, Yue Z, Yang Z. Cryopreservation of adult bovine testicular tissue for spermatogonia enrichment. Cryo Lett 2011;32:402–409. [PubMed] [Google Scholar]
- Wu JY, Sun YX, Wang AB, Che GY, Hu TJ, Zhang XM. Effect of newborn bovine serum on cryopreservation of adult bovine testicular tissue. Andrologia 2014;46:308–312. [DOI] [PubMed] [Google Scholar]
- Wu X, Goodyear SM, Abramowitz LK, Bartolomei MS, Tobias JW, Avarbock MR, Brinster RL. Fertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 years. Hum Reprod 2012;27:1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C, Curaba M, Martinez-Madrid B, Van Langendonckt A, François-Xavier W, Donnez J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum Reprod 2007;22:1603–1611. [DOI] [PubMed] [Google Scholar]
- Wyns C, Curaba M, Vanabelle B, Langendonckt A, van Donnez J. Options for fertility preservation in prepubertal boys. Hum Reprod Update 2010;16:312–328. [DOI] [PubMed] [Google Scholar]
- Wyns C, Van Langendonckt A, Wese F-X, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod 2008;23:2402–2414. [DOI] [PubMed] [Google Scholar]
- Yango P, Altman E, Smith JF, Klatsky PC, Tran ND. Optimizing cryopreservation of human spermatogonial stem cells: comparing the effectiveness of testicular tissue and single cell suspension cryopreservation. Fertil Steril 2014;102:1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavin S, Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology 2007;67:81–89. [DOI] [PubMed] [Google Scholar]
- Yildiz C, Mullen B, Jarvi K, McKerlie C, Lo KC. Effect of different cryoprotectant agents on spermatogenesis efficiency in cryopreserved and grafted neonatal mouse testicular tissue. Cryobiology 2013;67:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokonishi T, Sato T, Komeya M, Katagiri K, Kubota Y, Nakabayashi K, Hata K, Inoue K, Ogonuki N, Ogura A. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun 2014;5:4320. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Hou R, Wu J. Generation of mice by transplantation of an adult spermatogonial cell line after cryopreservation. Cell Prolif 2009;42:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambelli A, Poggi G, Da Prada G, Predrazzoli P, Cuomo A, Miotti D, Perotti C, Preti P, Robustelli della Cuna G. Clinical toxicity of cryopreserved circulating progenitor cells infusion. Anticancer Res 1998;18:4705–4708. [PubMed] [Google Scholar]
- Zeng W, Snedaker AK, Megee S, Rathi R, Chen F, Honaramooz A, Dobrinski IW. Preservation and transplantation of porcine testis tissue. Reprod Fertil Dev 2009;21:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-G, Wang Y-H, Han C, Hu S, Wang L-Q, Hu J-H. Effects of trehalose supplementation on cell viability and oxidative stress variables in frozen-thawed bovine calf testicular tissue. Cryobiology 2015;70:246–252. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, Xie M, Liu M, Guo X, Zheng Y. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell 2016;18:330–340. [DOI] [PubMed] [Google Scholar]