Abstract
Background
Dioscorea is a widely distributed and highly diversified genus in tropical regions where it is represented by ten main clades, one of which diversified exclusively in Africa. In southern Africa it is characterised by a distinct group of species with a pachycaul or “elephant’s foot” structure that is partially to fully exposed above the substrate. In contrast to African representatives of the genus from other clades, occurring mainly in forest or woodland, the pachycaul taxa and their southern African relatives occur in diverse habitats ranging from woodland to open vegetation. Here we investigate patterns of diversification in the African clade, time of transition from forest to more open habitat, and morphological traits associated with each habitat and evaluate if such transitions have led to modification of reproductive organs and mode of dispersal.
Results
The Africa clade originated in the Oligocene and comprises four subclades. The Dioscorea buchananii subclade (southeastern tropical Africa and South Africa) is sister to the East African subclade, which is respectively sister to the recently evolved sister South African (e. g., Cape and Pachycaul) subclades. The Cape and Pachycaul subclades diversified in the east of the Cape Peninsula in the mid Miocene, in an area with complex geomorphology and climate, where the fynbos, thicket, succulent karoo and forest biomes meet.
Conclusions
Diversification out of forest is associated with major shifts in morphology of the perennial tuber (specifically an increase in size and orientation which presumably led them to become pachycaul) and rotation of stem (from twining to non-twining). The iconic elephant's foot morphology, observed in grasslands and thicket biomes, where its corky bark may offer protection against fire and herbivory, evolved since mid Miocene. A shift in pollination trait is observed within the forest, but entry into open habitat does not show association with reproductive morphology, except in the seed wing, which has switched from winged all round the seed margin to just at the base or at the apex of it, or has been even replaced by an elaiosome.
Electronic supplementary material
The online version of this article (doi:10.1186/s12862-016-0812-z) contains supplementary material, which is available to authorized users.
Keywords: Biogeography, Dioscoreales, “elephant’s foot”, Fire adaptation, Habitat transition, Pachycaul, Southern Africa, Yams
Background
Dioscorea L. is a monocotyledonous plant genus that is highly diverse in many tropical regions of the world, with comparatively few taxa found in temperate latitudes. It comprises over 600 species, almost all of which have perennating organs (rhizome and/or tuber). These organs give rise to herbaceous, usually twining stems bearing leaves with basal and apical petiolar pulvinii and campylodromous venation. Most species are dioecious, with relatively small, typically monocotyledonous trimerous flowers in spicate or racemose (partial) inflorescences, with female plants usually containing up to six (usually) winged seeds in each inferior ovary. The highest species diversity per unit area is found in tropical areas, for example, southern Brazil, parts of Mexico, the Greater Antilles, western Madagascar and Asia from southern China to the Isthmus of Kra in Thailand [1–5]. These are largely areas with seasonal climates supporting open, deciduous forests that allow these light-demanding plants to thrive.
Wilkin et al. [6] established the broad phylogenetic outline of Dioscorea, which comprises 10 main clades. The same tree topology has been supported through significantly increased sampling and a further plastid marker [7] as well as was with additional data from the nuclear region Xdh, (Viruel, personal communication). The first branching group, the Stenophora clade (Fig. 3), is rhizomatous, with its highest diversity in subtropical Asia, followed by two large clades endemic to the Neotropics. The remaining clades comprise smaller units of diversity from the Mediterranean and Africa plus the principal reservoirs of species numbers in the Caribbean, Madagascar and the palaeotropics as a whole. Thus the focus of research in this genus has now shifted to species forming these 10 major clades.
Fig. 3.

Bayesian 50 % MR consensus tree of African Dioscorea lineages with Bootstrap (BP) and Posterior Probabilities (PP) values located above and below branches, respectively. Branches that collapse in a polytomy in the MP strict consensus tree are illustrated by a •. “Clade” names (in bold on the figure) follow Viruel et al. [7]. Within the Africa clade, subclade names are proposed terminology used in this publication
Of those 10 major clades, three are distributed in sub-Saharan Africa. One of these is the first Dioscorea clade to be studied here via a species level phylogeny, the Africa clade of Viruel et al. [7]. It is also the only clade to have diversified exclusively in Africa and comprises 13 species, as listed in [1] with minor taxonomic changes made in [8]. Nine are South African (sub)endemic species, two extend from South Africa into southern tropical Africa (D. buchananii Benth. and D. sylvatica Ecklon) and two are disjunct in northeastern tropical Africa (D. gillettii Milne-Redh. and D. kituiensis Wilkin & Muasya). In contrast, the remaining Dioscorea clades found in sub-Saharan Africa sensu [7] are poorly represented in South Africa, with only one species in the Enantiophyllum clade (D. cotinifolia Kunth; Fig. 3) and three in the Compound Leaved clade (CL; Fig. 3). This contrasts with the substantial tropical African diversity in these lineages.
The species of the Africa clade (Fig. 3) of Viruel et al. [7] possess a number of distinctive or unusual morphological traits. They include perennial tubers, some of which are large, “elephant’s foot” pachycaul structures that are partially or wholly exposed from the substrate (Fig. 1; [9, 10]). Similar structures also occur infrequently in neotropical species such as D. mexicana Scheidw.; two of the main neotropical lineages of Dioscorea also possess perennial tubers. Stems are usually sinistrorse (climbing towards the left hand, as viewed externally) but in some taxa they are non-twining [10, 11]. This trait is also encountered elsewhere, for example in the Mediterranean clade (D. pyrenaica Bubani & Bordère ex Gren. and D. chouardii Gaussen; Fig. 3) from Pyrenean France and Spain, in the Epipetrum group from Chile [12], and D. hexagona Baker from Madagascar [13]. Leaves are always alternate and blades are entire to deeply palmately lobed. Stamen number is reduced from 6 to 3 in one species [14]. Seeds in the Africa clade vary from possessing a wing all round the margin of the seed with a longer and shorter axis to being winged just at the apex [10, 15]. This is correlated with a capsular fruit that is longer than broad. Dioscorea gillettii and D. kituiensis have seeds that are wingless but possess an aril-like structure [16].
Fig. 1.
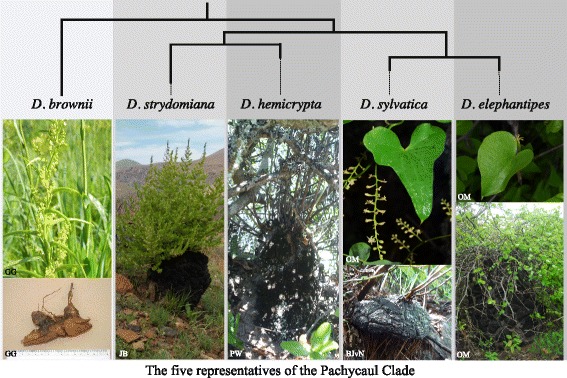
Mapping of habit, tuber and leaf traits on five South Africa yam lineages of the Pachycaul clade. Photographs: BJvN = Brian J van Niekerk; GG = Graham Grieve; JB = John Burrows; OM = Olivier Maurin; PW = Paul Wilkin
Among Dioscorea species occurring in Africa, the Africa clade is the richest source of steroidal saponins [17, 18]. Dioscorea sylvatica in particular was extracted from the wild in South Africa in the 1950s to produce synthetic human hormones for contraceptive purposes and other steroidal drugs. In contrast, some taxa of the CL clade have alkaloid chemistry [18] which is the basis of the use of D. dregeana in South African traditional medicine (e.g. [19]). The principal use of Enantiophyllum clade species is as a starch source that feeds at least 60 million people in tropical Africa [20].
The species of the CL and the Enantiophyllum clades are typical of the genus as a whole in that they mainly inhabit forest or woodland biomes, often those that are seasonal in climate. However, the Africa clade occupies an unusually broad range of vegetation types for the genus, including not only afromontane forests or forest margins and savannah woodlands but also the fynbos heathlands, succulent karoo and thicket. This observation reinforces three key questions that this research sets out to investigate. First, what are the patterns and timing of diversification in the Africa clade, especially in relation to transitions from forest to more open habitats such as thickets and karoo? Second, how are the traits associated with forest or woodland habitats modified in taxa inhabiting more open biomes, especially vegetative traits of perennating organs, stems and leaves, including their size and shape? Finally, are floral and fruit reproductive traits similarly affected by these biome shifts in addition to vegetative traits?
Methods
Taxon sampling
Representatives of all known African perennial-tubered Dioscorea (Dioscoreaceae) were sampled (Table 1). These included five pachycaul species, three Cape species, three species of the D. buchananii subclade (as defined by Wilkin and Muasya [8]), two species of the southern African members of the CL clade, and two species from the Enantiophyllum clade as well as two species from the East Africa subclade. We also included representatives from all known Dioscorea lineages [6]: four from the Mediterranean clade, D. tentaculigera and D. prazeri from South-East Asia and two New World taxa (D. brachybotrya and D. galeottiana), respectively belonging to the New World I (NWI) and II clades (NWII); and Tacca and Stenomeris (Dioscoreaceae) were selected as outgroups. Voucher specimen information and GenBank accession numbers are listed in Table 1 and trace files and sequences are available on the Barcode of Life Data System (BOLD; www.boldsystems.org).
Table 1.
Table of material and Genbank accession. Collectors references acronyms are Olivier Maurin (OM), Muthama Muasya (AMM), Sebsebe Demissew (SD), Pilar Catalán (PC), Ernesto Pérez-Collazos (EP). Genbank accessions in plain text are new to this study, accessions in bold were retrieved from www.ncbi.nlm.nih.gov
| Taxon | Voucher (Museum) | Distribution | Habitat | Habit | rbcL | matK | trnL-F | trnH-psbA | PsaA-Ycf3 | rpl32-trnL |
|---|---|---|---|---|---|---|---|---|---|---|
| Dioscorea brachybotrya Poepp. | Rudall P. s.n (K) | C. & S. Chile to Argentina | At medium altitude up to the timber line, and at low altitude in interior valleys | Shrub climber, 2 m in height | AF307469 | AY956482 | KM878027 | KR086979 | KR087124 | KR088291 |
| Dioscorea brownii Schinz | Grieve 53 (K) | South Africa - KwaZulu-Natal | Grassland | Pachycaul slightly emerging with annual solitary stems reaching 1 m in height | KR087028 | KR086942 | KR070855 | KR086980 | KR087125 | KR088292 |
| Dioscorea buchananii Benth. | Bingham10290 (K) | Tanzania, Angola, Malawi, Mozambique, Zambia, Zimbabwe, DRC | Combretum thickets and at proximity of woody outcrop | Twining wine with perennial tuber and stems up to 9 m long | KR087029 | KR086943 | KR070856 | KR086981 | KR087126 | KR088293 |
| Dioscorea bulbifera L. | OM3576 (BNRH) | Native in Tropical and subtropical region from Africa, Asia and Australasia. Introduced elsewhere | Forest and woodlands | Twining wine with perennial tuber and stems up to 12 m long | KR087030 | KR086944 | KR070857 | KR086982 | KR087127 | KR088294 |
| Dioscorea burchellii Baker | AMM6650 (BOL) | South Africa - Eastern Cape | At medium to high Altitude in dense fynbos vegetation | Tuberous perennial, shoot to 1 m height | KR087032 | KR086946 | KR070859 | KR086984 | KR087129 | KR088296 |
| Dioscorea burchellii Baker | AMM6704A (BOL) | - | - | - | KR087031 | KR086945 | KR070858 | KR086983 | KR087128 | KR088295 |
| Dioscorea chouardii Gaussen | PC334 (JACA) | Spain | Limestone rock-crevices | Tuberous perennial, shoot to 1 m height | KM877855 | KM877907 | KR070860 | KR086985 | KR087130 | KR088297 |
| Dioscorea communis (L.) Caddick & Wilkin | Chase536 (K) | Europe, North African and temperate Asia | Woodland and woodland hedges | Herbaceous with climbing stem, up to 4 m in height | KR087033 | KR086947 | KR070871 | KR086996 | KR087141 | KR088308 |
| Dioscorea cotinifolia Kunth | AMM6112 (BOL) | Mozambique, South Africa and Swaziland | Open dry forest, forest margins, scrubby vegetation and rocky places | Tuberous plant with vigorous annual twining stems reaching up to 10 m height | KR087034 | KR086948 | KR070862 | KR086987 | KR087132 | KR088299 |
| Dioscorea cotinifolia Kunth | AMM6158 (BOL) | - | - | - | KR087035 | KR086949 | KR070863 | KR086988 | KR087133 | KR088300 |
| Dioscorea cotinifolia Kunth | OM1458 (BNRH) | - | - | - | KR087036 | KR086950 | KR070864 | KR086989 | KR087134 | KR088301 |
| Dioscorea dregeana (Kunth) T. Durand & Schinz | AMM6104 (BOL) | Mozambique and South Africa | Forest, woodlands found among rocks and ravine | Tuberous plants with annual to persistent stems reaching up to 12 m height | KR087038 | KR086952 | KR070866 | KR086991 | KR087136 | KR088303 |
| Dioscorea dregeana (Kunth) T. Durand & Schinz | AMM6166 (BOL) | - | - | - | KR087039 | KR086953 | KR070868 | KR086993 | KR087138 | KR088305 |
| Dioscorea dregeana (Kunth) T. Durand & Schinz | OM1465 (BNRH) | - | - | - | JQ025042 | JQ024957 | KR070867 | KR086992 | KR087137 | KR088304 |
| Dioscorea dregeana (Kunth) T. Durand & Schinz | OM2247 (BNRH) | - | - | - | KR087037 | KR086951 | KR070865 | KR086990 | KR087135 | KR088302 |
| Dioscorea dumetorum (Kunth) Pax | OM2315 (BNRH) | Sub-Saharan Africa, excluding parts of Southern Africa | Forest and woodlands and along riverbanks, generally at low altitude | Tuberous plants with annual to persistent stems reaching up to 5 m height | KR087040 | KR086954 | KR070869 | KR086994 | KR087139 | KR088306 |
| Dioscorea dumetorum (Kunth) Pax | OM3953 (BNRH) | - | - | - | KR087041 | KR086955 | KR070870 | KR086995 | KR087140 | KR088307 |
| Dioscorea elephantipes (L’Hér.) Engl. | AMM5225 (BOL) | Namibia, South Africa - All Capes provinces | From Medium to high altitude, in thorny and succulent vegetation (e.g. Thickets) | Pachycaul up to 80 cm in diameter, with shoot up to 1 m height | KR087042 | KR086956 | KR070872 | KR086997 | KR087142 | KR088309 |
| Dioscorea elephantipes (L’Hér.) Engl. | AMM5226a (BOL) | - | - | - | KR087043 | KR086957 | KR070873 | KR086998 | KR087143 | KR088310 |
| Dioscorea elephantipes (L’Hér.) Engl. | AMM6713 (BOL) | - | - | - | KR087044 | KR086958 | KR070874 | KR086999 | KR087144 | KR088311 |
| Dioscorea galeottiana Kunth | Telez13090 (MEXU) | Centre America, Mexico | Tropical Dry Forest | Perennial tuber, climber | AY904796 | AY956499 | KM878046 | KR087000 | KR087145 | KR088312 |
| Dioscorea gillettii Milne-Redh. | SD7051 (ETH) | Southern Ethiopia | Dry woodland vegetation | Perennial tuber with twining stems up to 1.5 m height | KR087046 | KR086960 | KR070876 | KR087002 | KR087147 | KR088314 |
| Dioscorea gillettii Milne-Redh. | SD7052 (ETH) | - | - | - | KR087045 | KR086959 | KR070875 | KR087001 | KR087146 | KR088313 |
| Dioscorea hemicrypta Burkill | AMM5800 (BOL) | South Africa - Western Cape | From Medium to high altitude, in thorny and succulent vegetation (e.g. Thickets) | Pachycaul, partially emerged with annual shoot emerging from the crown | KR087050 | KR086964 | KR070880 | KR087006 | KR087151 | KR088318 |
| Dioscorea hemicrypta Burkill | AMM6633 (BOL) | - | - | - | KR087049 | KR086963 | KR070879 | KR087005 | KR087150 | KR088317 |
| Dioscorea hemicrypta Burkill | AMM6886a (BOL) | - | - | - | KR087047 | KR086961 | KR070877 | KR087003 | KR087148 | KR088315 |
| Dioscorea hemicrypta Burkill | AMM6697 (BOL) | - | - | - | KR087048 | KR086962 | KR070878 | KR087004 | KR087149 | KR088316 |
| Dioscorea kituiensis Wilkin & Muasya | Mwachala 949a (K) | Eastern Kenya | Rocky area in dry woodlands | Tuberous perennial with twining stems reaching 1.5 m height | KR087051 | KR086965 | KR070881 | KR087007 | KR087152 | KR088319 |
| Dioscorea multiloba Kunth | AMM6167 (BOL) | South Africa - Eastern Cape, KwaZulu-Natal and Swaziland | High altitude forests vegetation. | Tuberous perennial with twining stems reaching 2 m in height | KR087052 | KR086966 | KR070882 | KR087008 | KR087153 | KR088320 |
| Dioscorea mundii Baker | AMM6641 (BOL) | South Africa - Western Cape | Coastal forest vegetation | Perennial with underground tuber from which stems arise (or from the bases of old stems) that climb to at least 5 m in height in surrounding vegetation | KR087054 | KR086968 | KR070884 | KR087010 | KR087155 | KR088322 |
| Dioscorea mundii Baker | AMM6642 (BOL) | - | - | - | KR087053 | KR086967 | KR070883 | KR087009 | KR087154 | KR088321 |
| Dioscorea orientalis (J. Thiébaut) Caddick & Wilkin | Tori 1 (HUJ) | Temperate western Asia | Mediterranean Woodlands and Shrublands | Geophyte, climber | KM877858 | KM877911 | KM878066 | KR087011 | KR087156 | KR088323 |
| Dioscorea prazeri Prain & Burkill | Wilkin1075 (K) | Asia | Open vegetation in mixed forests | Rhizome, with climbing stem up to 5 m height | AY973485 | KM877871 | KM878019 | KR087012 | KR087157 | KR088324 |
| Dioscorea pyrenaica Bubani & Bordère ex Gren. | EP1038 (JACA) | France, Spain (Pyrenees mountain range) | On limestone rocks | Tuberculous plant with short annual stems reaching 40 cm height | KM877859 | KM877912 | KM878067 | KR087013 | KR087158 | n.a. |
| Dioscorea rupicola Kunth | AMM3676 (BOL) | South Africa - Eastern Cape, KwaZulu-Natal | Occurring in open areas at high altitude in shady temperate and humid forest | Perennial tuber with twining stems growing on surrounding vegetation | KR087055 | KR086969 | KR070885 | KR087014 | KR087159 | KR088325 |
| Dioscorea sansibarensis Pax | OM2421 (BNRH) | Sub-Saharan Africa, including Madagascar but excluding Southern Africa | Humid forest, low altitude, along riverine | Large tuberous species with vigorous stems up to 30 m in length climbing and trailing on surrounding vegetation | KR087056 | KR086970 | KR070886 | KR087015 | KR087160 | KR088326 |
| Dioscorea schimperana Hochst. ex Kunth | OM2372 (BNRH) | Tropical Africa, including Zambia, Zimbabwe, Malawi and Mozambique | Open vegetation, on Rocks, termites’ mounts and along riverbanks | Annual tuber, producing vigorous shoots reaching 8 m height | KR087058 | KR086972 | KR070888 | KR087017 | KR087162 | KR088328 |
| Dioscorea schimperana Hochst. ex Kunth | OM3532 (BNRH) | - | - | - | KR087057 | KR086971 | KR070887 | KR087016 | KR087161 | KR088327 |
| Dioscorea stipulosa Uline ex R. Knuth | AMM6748 (BOL) | South Africa - Eastern Cape | In fynbos, in moist rich soils | Perennial tuber with annual shoot reaching up to 3 m in length | KR087060 | KR086974 | KR070890 | KR087019 | KR087164 | KR088330 |
| Dioscorea stipulosa Uline ex R. Knuth | AMM6800 (BOL) | - | - | - | KR087059 | KR086973 | KR070889 | KR087018 | KR087163 | KR088329 |
| Dioscorea strydomiana Wilkin | AMM6124 (BOL) | South Africa, Mpumalanga | Open woodland | Pachycaul up to 80 cm in diameter, with shoot up to 90 cm height | KF147467 | KF147390 | KR070892 | KR087021 | KR087166 | KR088332 |
| Dioscorea strydomiana Wilkin | Burrows 10627 (BNRH) | - | - | - | KR087061 | KR086975 | KR070891 | KR087020 | KR087165 | KR088331 |
| Dioscorea sylvatica Eckl. | Burrows 12487 (BNRH) | Southern Africa | Found from low to high altitude in a variable range of vegetation from Dunes, to rocky outcrop and open woodland vegetation. | Perennial tuber, with Herbaceous annual stem reaching 4 m in length | KR087062 | KR086976 | KR070893 | KR087022 | KR087167 | KR088333 |
| Dioscorea sylvatica Eckl. | OM1433 (BNRH) | - | - | - | KR087063 | KR086977 | KR070894 | KR087023 | KR087168 | KR088334 |
| Dioscorea sylvatica Eckl. f. glauca | Burrows 12477 (BNRH) | - | - | - | KR087064 | KR086978 | KR070895 | KR087024 | KR087169 | KR088335 |
| Dioscorea tentaculigera Prain & Burkill | Thapyai 436 | South Central Chine, Myanmar and Thailand | Evergreen forest from medium to high altitude | Perennial tuber, with climbing stems up to 4 m in length | AY972828 | AY939886 | KM878070 | KR087025 | KR087170 | KR088336 |
|
Tamus edulis Lowe (combination in Dioscorea pending) |
Chase 3425 (K) | Mediterranean region | Woodland and woodland hedges | Herbaceous with climbing stem | AY939891 | AY973843 | KR070861 | KR086986 | KR087131 | KR088298 |
| Outgroups | ||||||||||
| Stenomeris borneensis Oliv. | Brun19174 (K) | Tropical Asia | - | - | AF307475 | AY973836 | - | - | - | - |
| Tacca plantaginea (Hance) Drenth | ZL002 (n.a.) | Tropical Asia | - | - | JF944619 | JF956650 | - | - | - | - |
DNA extraction, amplification, sequencing and alignment
DNA was extracted from 0.3 g silica gel dried leaves [21] using 2x CTAB method [22] with the addition of 2 % polyvinyl pyrrolidone (PVP) to reduce the effects of high polysaccharide concentration in the samples. In order to avoid problems of PCR inhibition, DNA was precipitated in 2.5 volume ethanol and purified using QIAquick PCR Purification Kit according to manufacturer’s protocol (QiIAgen Inc., Hilden, Germany). All PCR reactions were carried out using Thermo Scientific Master Mix (Thermo Fischer Scientific, Waltham, Massachusetts, USA).
Amplification of rbcLa was carried out using the primers rbcLa-F and rbcLa-R described respectively by Levin et al. [23] and Kress and Erickson [24]. For matK, the following primers were used MatK-1R-Kim-F and MatK-3 F-Kim-R (Kim, unpublished; [25]). Amplification of trnL-F was carried out using primers c and f of Taberlet et al. [26], but the internal primers d and e were also used for several taxa due to difficulty in amplifying the region as a single piece. The trnH-psbA spacer was amplified using primers 1 F and 2R [27]. The psaA-ycf3 spacer was amplified using the PG1f and PG2r primers [28]. Finally the rpl32-trnL (UAG) intergenic spacer was amplified according to Shaw et al. [29]. Amplified products were purified using QIAquick columns (QIAgen, Germany) following the manufacturer’s protocol.
PCR amplification primers were also used as cycle sequencing primers. Cycle sequencing reactions were carried out using BigDye© V3.1 Terminator Mix (Applied Biosystems, Inc., ABI, Warrington, Cheshire, UK) and cleaned using the EtOH-NaCl method provided by ABI; they were then sequenced on an ABI 3130xl genetic analyser. Complementary strands were assembled and edited using Sequencher version 5.1 (Gene Codes Corp., Ann Arbor, Michigan, USA) and sequences were aligned manually in PAUP* (version 4.0b1; [30]) without difficulty due to low levels of insertions/deletions.
Phylogenetic analyses: parsimony and Bayesian approaches
Maximum parsimony (MP) using PAUP* version 4.0b1 [30] was performed on the individual and combined datasets. Tree searches were conducted using 1,000 replicates of random taxon addition, retaining 10 trees at each step, with tree-bisection-reconnection (TBR) branch swapping and MulTrees in effect (saving multiple equally parsimonious trees). Support for clades in all analyses was estimated using bootstrap analysis [31] with 1000 replicates, simple sequence addition, TBR swapping, with MulTrees in effect but saving a maximum of 10 trees per replicate. Delayed transformation character optimization (DELTRAN) was used to calculate branch lengths, due to reported errors http://paup.sc.fsu.edu/paupfaq/paupans.html with accelerated transformation optimization (ACCTRAN) in PAUP v.4.0b1. Bootstrap support (BP) was classified as high (85–100 %), moderate (75–84 %) or low (50–74 %). Bootstrap values are provided in Fig. 3. All data sets were analyzed separately, and the individual bootstrap consensus trees examined by eye to identify topological conflicts, i.e. moderate to high support for different placement of taxa. In order to test for significant conflicts between the independent DNA data matrices, a partition homogeneity test was performed [32–34]. The Incongruence Length Difference (ILD) test of Farris et al. [32] implemented in PAUP* 4.0 b10 [30] was performed through 1000 random-order-entry replicates to estimate if the six datasets were significantly different from random partitions of the same size. Non-significant results indicated that the six data sets were not heterogeneous. Highly congruent contrasted topologies (see Results) also supported the merging of the four data matrices into a single concatenated data set that was used for subsequent phylogenetic analyses.
Bayesian analysis (BI; [35, 36]) was performed using MRBAYES v. 3.1.2. For each matrix rbcLa, matK, trnL-F, trnH-psbA, psaA-ycf3 and rpl32-trnL the most appropriate model was selected using MODELTEST v. 3.06 [37]. For matK and trnL-F the model TVM + G was selected, then for rbcLa, trnH-psbA, psaA-ycf3 and rpl32-trnL, the following model were selected, respectively TVM + I, HKY + G, HKY + I + G and GTR + G. The analysis was run on the CIPRES cluster [38] using a MCMC of 10 million generations with a sample frequency of 500, imposing the closest nst = 6 rates = gamma model available in the program. The resulting trees were plotted against their likelihoods to determine the point where likelihoods converged on a maximum value, and all the trees before the convergence were discarded as ‘burn-in’ (5000 trees). All remaining trees were imported into PAUP 4.0b10, and a majority-rule consensus tree was produced showing frequencies (i.e. posterior probabilities or PP) of all observed bi-partitions. The following scale was used to evaluate the PPs values: below 0.95, weakly supported; 0.95-1.00, well supported.
Divergence time estimation
Divergence times were estimated using a Bayesian MCMC approach implemented in BEAST (v. 1.4.8; [39]), which allows simultaneous estimation of the topology, substitution rates and node ages [39]. The GTR + I + G implemented model of sequence evolution for each partition based on the Akaike information criterion (AIC) scores for substitution models evaluated using MrModeltest (version 2.3; [40]) with a gamma-distribution with four rate categories. A speciation model following a Yule process was selected as the tree prior, with an uncorrelated lognormal (UCLN) model for rate variation among branches. For this analysis, we used a single representative per species since the Yule speciation model forces the analysis to “create” speciation events at every node and therefore makes the estimation of splits older within a species.
First, the Bayesian consensus tree topology was used as a starting tree and adjusted so that branch lengths satisfied all fossil prior constraints, using PATHd8 v.1.0 [41]. Fossil dates or calibration points were used to constrain specific nodes to minimum, maximum or fixed ages. The crown node age of Dioscoreaceae was calibrated at 80 mya according to Jansen & Bremer [42]. A first fossil, Dioscorea lyelli (Wat.) Fritel, was used to calibrate the node of Dioscorea prazeri Prain & Burkill, (representative of the Stenophora clade). The fossil was discovered in the Cuisian stage of the Ypresian age at the Paris basin [43] and provided a minimum constraint of 48.2 ± 1.0 mya (LogNormal Prior mean = 48.2, SD 0.008) for the stem node of Stenophora. A second fossil, D. wilkinii Pan, attributed to the node of the Compound Leaved clade that comprises D. dregeana - D. dumetorum, provided a minimum constraint of 27.2 ± 0.1 mya (LogNormal Prior mean = 27.2, SD 0.002) for that node [44]. We performed four independent runs of MCMC, each for 100 million generations, sampling every 1000 generations. We assessed the MCMC log files for convergence using the effective sample size (ESS) statistics in Tracer v.1.5 [39]. The BEAST analysis reported ESS values > 200, indicating that the posterior estimates were not unduly influenced by autocorrelation. The resulting tree files from the four runs were then combined using LogCombiner v.1.7.5 [39], discarding the first 25 % trees as burn-in. The maximum clade credibility consensus tree, with means and 95 % highest posterior density (HPD) intervals, was generated with TreeAnnotator v.1.7.5 [39].
Map preparation
Distribution maps illustrated on Fig. 2 were prepared using occurrence data downloaded from http://newposa.sanbi.org and http://www.gbif.org. Distribution ranges were drawn on Adobe ® Illustrator ® CS6. Figure 2a represents the occurrence of the three major subclades occurring in South Africa, the Pachycaul, Cape and D. buchananii subclades, while Fig. 2b displays the distribution of all species belonging to the Pachycaul subclade.
Fig. 2.
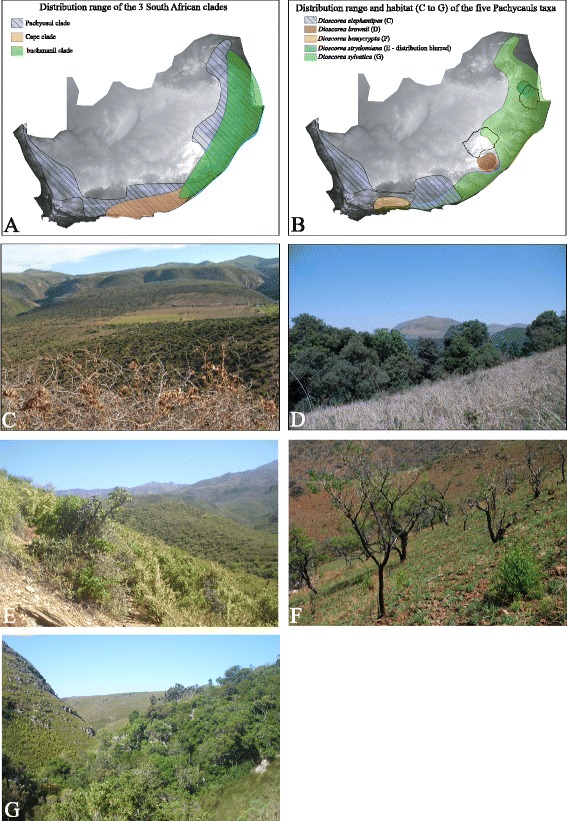
Distribution maps and habitat images of the southern African Dioscorea taxa. a Distribution map of the three South African subclades of the Africa clade: Pachycaul, Cape and D. buchananii. b Distribution map of the five Pachycaul subclade species in South Africa. Note that the distribution of D. elephantipes extends slightly into Namibia, and D. sylvatica extends into Mozambique, Zambia and Zimbabwe. From c to g: in order, habitat of D. elephantipes, Dioscorea brownii, D. hemycrypta, D. strydomiana, and D. sylvatica. The habitat image of D. elephantipes (c) displays in the foreground shoots and fruits of this taxa; the habitat image for D. strydomiana has an immature or damaged specimen in the bottom right corner. All other images only show the habitat and individuals of the species are not visible. Photographs: c-g: Paul Wilkin
Results
Statistics for MP analysis for the six plastid markers and combined dataset are presented in Table 2. Of all the genes used, matK and rpl32-trnL had a significantly higher number of variable sites (27.85 % and 25.17 % respectively) compared to the other regions than display percentages below 10 % (see Table 2). The number of potentially informative characters is higher for matK (12.07 %) than rpl32-trnL (10.01 %), however contribution to total of parsimony informative character (PIC) is lower for matK (26.35 %) than for rpl32-trnL (30.84 %; Table 2).
Table 2.
Maximum parsimony statistics from the analyses of the separate and combined data sets
| rbcLa | matK | trnL-F | trnH-psbA | psaA-ycf3 | rpl32-trnL | Combined | |
|---|---|---|---|---|---|---|---|
| No. of taxa | 49 | 49 | 47 | 47 | 47 | 47 | 49 |
| No. of included characters (= aligned length) |
529 | 729 | 738 | 409 | 709 | 1029 | 4143 |
| No. of constant characters | 472 | 526 | 613 | 361 | 602 | 770 | 3344 |
| No. of variable sites | 57 | 203 | 125 | 48 | 107 | 259 | 799 |
| (10.77 %) | (27.85 %) | (16.94 %) | (11.74 %) | (15.09 %) | (25.17 %) | (19.29 %) | |
| No. of parsimony informative character (PIC) | 32 | 88 | 47 | 16 | 48 | 103 | 334 |
| (6.05 %) | (12.07 %) | (6.37 %) | (3.91 %) | (6.77 %) | (10.01 %) | (8.07 %) | |
| Contribution to total number of PIC | 9.58 % | 26.35 % | 14.07 % | 4.79 % | 14.37 % | 30.84 % | 100 % |
| No. of most parsimonious trees | 1 | 6154 | 7520 | 10000 | 3300 | 9960 | 72 |
| Tree Length | 77 | 275 | 163 | 59 | 153 | 340 | 1102 |
| CI | 0.81 | 0.84 | 0.84 | 0.92 | 0.8 | 0.86 | 0.82 |
| RI | 0.87 | 0.87 | 0.85 | 0.91 | 0.86 | 0.88 | 0.85 |
| Average number of changes per variable site (number of steps/number of variable sites) | 1.35 | 1.35 | 1.3 | 1.23 | 1.43 | 1.31 | 1.38 |
Maximum parsimony analyses
MP analysis of each of the six regions resulted in trees that were similar in topology (Additional files 1, 2, 3, 4, 5 and 6), and were thus combined and treated as a single dataset. ILD test results provide support for congruence (p > 0.05). The psaA-ycf3 region is significantly different from rbcLa, matK, trnL-F, trnH-psbA, and rpl32-trnL and probably caused by the psaA-ycf3 sequence of D. galeottiana. However, the observed congruence between the trees obtained for each region separately and the ILD results (Table 3) support combining these regions. The statistics for the MP analysis for the combined data is presented in Table 2. From the heuristic search, we found 72 most parsimonious trees of which one is presented in the supplementary Additional file 7. The combined MP tree is largely congruent with that obtained from Bayesian analysis and therefore bootstrap values recovered in the MP analysis are plotted onto the Bayesian consensus tree (Fig. 3).
Table 3.
Incongruence Length Difference (Farris test)
| rbcLa | matK | trnL-F | trnH-psbA | psaA-ycf3 | rpl32-trnL | |
|---|---|---|---|---|---|---|
| rbcLa | - | |||||
| matK | 0.362 | - | ||||
| trnL-F | 0.711 | 0.175 | - | |||
| trnH-psbA | 0.679 | 0.023 | 0.474 | - | ||
| psaA-ycf3 | 0.047 | 0.001 | 0.001 | 0.757 | - | |
| rpl32-trnL | 0.922 | 0.750 | 0.748 | 0.549 | 0.001 | - |
Values in bold identify partitions significantly incongruent at p= 0.05
Bayesian analysis
The Bayesian majority-rule consensus tree is presented in Fig. 3. Generally, the Bayesian analysis generated a better-supported topology than the MP analysis, resolving some polytomies observed in the MP results (see • in Additional file 7). Dioscorea is strongly supported as monophyletic (100 Bootstrap Percentage, BP; 1.0 Posterior Probabilities, PP). Within Dioscorea the topology is congruent with [6] and [7]. Three major clades are retrieved (Africa, Enantiophyllum/CL, Mediterranean), with the Central-American D. galeottiana (NWII clade), the Chilean D. brachybotrya (NWI clade) and the Asian D. prazeri, successively sister (99 BP/1.0 PP; 100 BP/1.0 PP; 100 BP/1.0 PP respectively) to these three core clades.
The Mediterranean clade is well-supported in both analyses (100 % BP; 0.97 PP), containing taxa from Spain and the south of France. Within this clade two well-supported lineages are identified, a “Spanish-Pyrenees mountain range” clade (100 BP/1.0 PP), and a more geographically dispersed taxa clade showing a wider distribution from Europe to the eastern Mediterranean and the Canary Islands (99BP/1.0 PP). The Mediterranean clade is weakly supported (0.73 PP) in the BI analysis as sister to a large clade comprising (1) the Enantiophyllum and the CL clades (including Dioscorea sansibarensis) and (2) the Africa clade. (1) comprises a combination of the weakly supported (BP < 50 %, 0.79 PP) D. tentaculigera sister to the Enantiophyllum clade and the CL clade, with D. bulbifera and D. sansibarensis successively sister to the CL clade. The large clade comprising (1) and (2) received no support in the MP analyses however it was weakly supported (0.73 PP) in BI.
The Africa clade is weakly supported in MP while strongly supported in the BI analyses (64 BP/1.0 PP); it includes the D. buchananii, East Africa, Cape and Pachycaul subclades. The Pachycaul subclade is strongly supported as monophyletic (96BP/1.0 PP) with D. brownii sister to all other pachycauls. Dioscorea brownii is a taxon restricted to montane grassland of KwaZulu-Natal (Fig. 2b and d) displaying a horizontal tuber a few centimetres in diameter with non-twining erect stems arising from vertical lobes (Fig. 1). Within the pachycaul group two sister lineages can be identified: 1) D. hemicrypta and D. strydomiana (0.99 PP). Both are characterised by a pachycaul tuber partially to wholly protruding above the substrate (Fig. 1), which reaches ca. 1 m in height and diameter in the latter. Dioscorea hemicrypta is endemic to the Little Karoo area South of the Swartberg Mountains in the Western Cape (Fig. 2b) while D. strydomiana has a single locality in Barberton area of Mpumalanga Province (Fig. 2), South Africa. 2) D. elephantipes and D. sylvatica have wide distribution ranges in South Africa (Fig. 2b), and are well supported as monophyletic in the BI analysis (0.97 PP) although it received weak support in the MP analyses (51 BP). These two taxa have well-developed pachycauls (Fig. 1), though that of D. sylvatica is usually below the substrate. The pachycaul of D. elephantipes can also reach ca. 1 m in height and diameter. Successively sister to the Pachycaul subclade are the Cape and the East Africa subclades (98 BP/1.0 PP and 100 BP/1.0 PP, respectively). The D. buchananii subclade of African Dioscorea, sister to the others, is resolved as the first branching lineage (100 BP/1.0 PP) within the Africa clade.
Dating analysis
The results of the dating analysis using BEAST are shown in Fig. 4. The topology retrieved is similar to that from BI. Results suggest an origin of the genus Dioscorea around 80.95 Ma and radiation from around 48.83 Ma. The first three diverging lineages of Dioscorea, the SE Asian D. prazeri (Stenophora clade) and the two New World taxa included in this study, D. brachybotrya (NWI) and D. galeottiana (NWII), split around 48.83 Ma, 42.92 Ma and 38.76 Ma respectively. Two successive splits at 36.09 Ma and 35.66 Ma were inferred for the ancestors of the Mediterranean clade and its sister lineage and for CL/Enantiophyllum and the Africa clade, respectively. The Mediterranean clade was estimated to have diversified at 29.51 Ma, and the ancestors of the South East-Asian D. tentaculigera and its sister group, the Enantiophyllum clade, and the CL clade at 34.78 Ma, 28.93 Ma and 29.74 Ma, respectively.
Fig. 4.
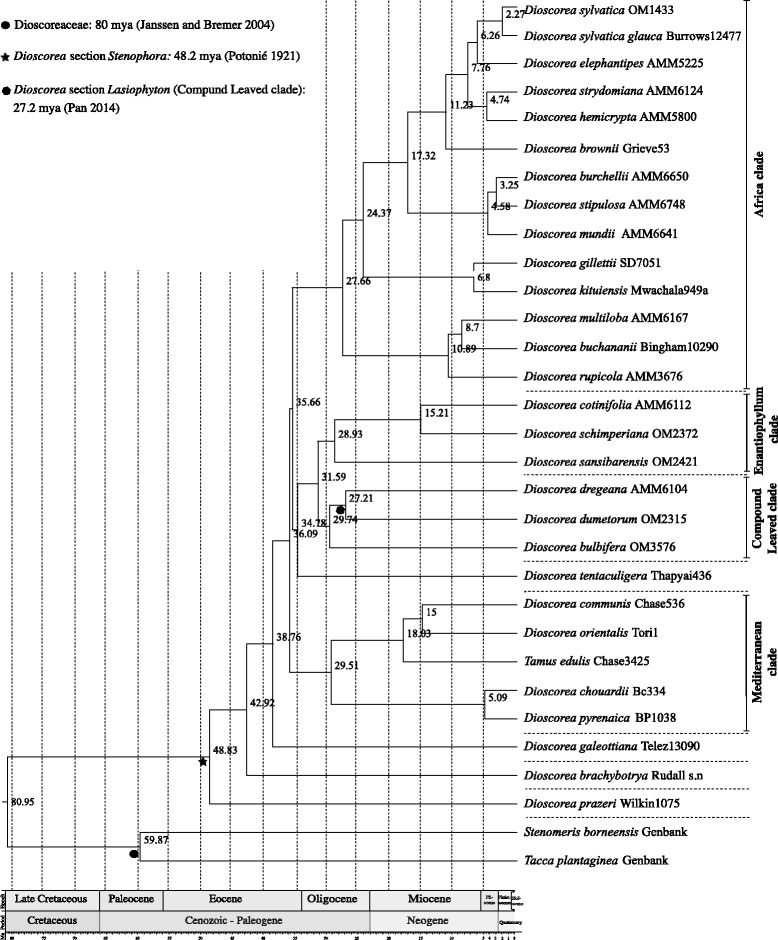
Beast chronogram of the African yam lineages dated using three calibrations points (● Dioscoreaceae: 80 mya [42], ★Dioscorea Stenophora clade crown node 48.2 mya [43] and  Dioscorea dregeana - D. dumetorum clade node in Counpound Leaved clade 27.2 mya [44]). Only the four major clades are displayed in the figure. For subclade information, refer to Fig. 3
Dioscorea dregeana - D. dumetorum clade node in Counpound Leaved clade 27.2 mya [44]). Only the four major clades are displayed in the figure. For subclade information, refer to Fig. 3
The successive splits of the Africa clade, the East Africa and sister lineage, and the core Cape and Pachycaul group were inferred to have occurred split at 27.66 Ma, 24.37 Ma and 17.32 Ma, respectively. Within the Pachycaul subclade a latter split of KwaZulu-Natal D. brownii and its sister lineage was dated at 11.23 Ma.
Discussion
Evolution of African yams
Data generated in this study produced a well-resolved dated phylogeny thus improving our understanding of the relationships within the Africa clade and more specifically within southern African Dioscorea. The current evolutionary study of yams focuses on southern African lineages, but representative taxa from other lineages were included to cover morphological and phylogenetic diversity of Dioscorea. The inferred phylogeny is congruent with previous studies (e.g. [6, 7]), though more largely sampled. Taxa occurring in southern Africa are nested within a strongly supported predominantly Old World clade (Mediterranean, Enantiophyllum, CL and Africa clades; Fig. 3 and 4), which likely originated in the Eocene. The Africa clade is further resolved into four subclades (Fig. 4) which are forest twiners with basally lobed leaves (D. buchananii subclade); savannah twiners (East Africa subclade); twiners in Cape forest and fynbos habitats (Cape subclade); and the diverse Pachycaul subclade comprising the open habitat elephant-foot yams with large, vertically oriented partially to wholly exposed tubers and stems with reduced to absent twining as well as a forest twiner (usually with similar but buried tubers) and an erect montane grassland taxon with a narrow horizontal tuber from which non-twining erect stems arise from vertical lobes.
Our analyses support that the Africa clade has four main subclades, (1) D. buchananii (2) East Africa, (3) Cape and (4) Pachycaul. Phylogenetic reconstruction placed the Cape subclade as sister to the Pachycaul and the Eastern African subclade sister to it. For the Pachycaul subclade, which was the main focus in this study, we found that D. brownii with a horizontal woody underground tuber from montane grassland in KwaZulu-Natal to be the earliest deriving taxon, sister to all four other pachycaul species (represented in two clades). The first include two taxa with restricted distribution (D. hemicrypta and D. strydomiana, respectively from the Little Karoo in the Western Cape and from a single locality in the Mpumalanga province) and displaying pachycauls located partially to completely above the substrate. The second group (D. elephantipes and D. sylvatica) has a much wider distribution. Both D. elephantipes and D. strydomiana possess pachycauls that can grow ca. 1 m in height and diameter.
Divergence estimation analyses, which were in broad agreement with those of Viruel et al. [7] suggest Dioscorea originated around 78 mya with a diversification around 48 mya. In the Old World clade, the Mediterranean taxa split from the African clade around 32.06 mya, with the latter diversifying around 26.74 mya. The East African taxa then diversify separately around 5.72 mya. The D. buchananii subclade diverges at 21.83 mya, and the split between the Cape subclade and the Pachycaul subclade is observed around 13.88 mya. These results confirm that the Africa clade forms part of a predominantly Old World clade, which originated during the Eocene. During the Oligocene the African continent was covered with dense and humid forest and characterized the period of development for thinner, underground perennial tuber and twining shoots displaying marginally winged (gliding) seeds, which favor their dispersal under canopy under low wind conditions. Through the Miocene climatic changes drove habitat opening with the appearance of grasslands in eastern Africa and Mediterranean climate in South African and in the Cape flora. Such changes, particularly the prominence of fire, influenced the development of an erect woody type of stem, below ground or partially to fully above ground, with corky bark as protection. Seed morphology also adapted to climatic and environmental conditions through the development of basally and apically winged seeds, which are more efficient when released at low height but needing higher wind speeds for efficient dispersal. In east Africa, seeds are wingless suggesting ants may be the mode of dispersal.
Adaptation/colonization of yams to African biomes?
During the early Miocene, southern Africa was covered in forests [45–47], but increased edaphic heterogeneity due to uplifts [48] and increase in aridity and shifts in rainfall patterns after the formation of the Mediterranean climate resulted in the Cape flora (arid thickets, fire driven fynbos) and grasslands to the east [49]. The ancestor of the Africa clade would have thrived in open areas in forested habitats such as riverbanks as a twiner bearing perennial tubers. Colonization of non-forest habitats among Africa clade taxa involved shifts in stem and tuber morphology, most noticeable among the Pachycaul subclade, with large, long-lived tubers positioned fully or partially above sloping shale or rocky substrates (D. elephantipes, D. hemicrypta, D. strydomiana) versus fully below or at ground level [15] or sometimes fully exposed when occurring on/or between rocks (D. sylvatica). Erect, non-twining stems occur in taxa of frequently burned grasslands (D. brownii), or similarly burned open Acacia woodland with a strong grass understorey (D. strydomiana). The Pachycaul subclade taxa annually replace their photosynthetic tissues (stems and leaves) from the persistent tuber, a phenomenon observed in frequently burned habitats in southern Africa [50]. Only D. brownii and D. strydomiana occur within typical fire driven grassland habitats and their origin in late Miocene and Pliocene concurs with a similar time of origin of other southern African savannah flora [51]. Corky barks are observed covering the above-ground pachycauls both in fire prone grasslands and in (fire infrequent) thicket vegetation. Thick barks function to protect the plant from fire, an adaptation well documented in fire-prone areas [52–54], and may play an additional role of protection from herbivores. Damage to pachycauls, probably by porcupines, has been observed in populations of D. hemicrypta and D. strydomiana.
The Cape subclade comprises three species that occupy low elevation, high precipitation forested coastal habitats (D. mundii) or in middle to higher elevation fynbos heath vegetation (D. burchellii, D. stipulosa). All possess twining stems and have subterranean perennial tubers. Members of a clade occurring in both forest and fynbos habitats in the Cape flora is highly unusual, as adaptations for the forest environment (shade, no fire, richer soils) may not be advantageous in fynbos heath environment (open, frequent fires, nutrient poor). The habitats of D. mundii and D. burchellii are spatially separated by less than 10 km in the Eden district (that includes George and five surrounding municipalities) in the Western Cape. It has been noted that fynbos heath plants are more likely to disperse to similar environments occurring in distant lands (such as Australia) than to evolve adaptations to occupy a different (forest) biome nearby [55]. However, long distance dispersal is rare in Dioscorea, where wind dispersal is encountered almost without exception. The leaves of the two fynbos species are proportionally longer and narrower than those of D. mundii. We note that the Cape subclade is not sister to the Mediterranean subclade, the latter evolving independent traits observed in the Africa clade such as erect non-twining stems.
Within southern Africa Dioscorea, evolution into new non-forested biomes has occurred since the mid Miocene. The highest species diversity is in the east of the Cape (Fig. 2a; Eden, sensu Cowling & Pierce [56]), an area with complex geomorphology and climate, where several biomes (fynbos, forest, succulent karoo, thicket, grassland) are juxtapositioned. Speciation events accompanied evolution into the new biomes (e.g. grassland – D. brownii) or occurred subsequently in allopatry events (e.g. D. strydomiana/D. hemicrypta; D. gillettii/D. kituiensis; [10]).
The opening of vegetation during the Miocene in southern Africa had an important influence on seed morphology and therefore on their dispersal mode. In forest environments where yam species grow below the forest canopy and generally have a twining habit, lens-shaped seeds are characterized by flat papery wings all round the margin (Fig. 5A2 and A3), which allow them to glide effectively, even with low wind speeds. This is observed in all species of the D. buchananii subclade. According to Burkill [57] this is the optimal form for dispersal when seeds are released from greater height and in light winds, the conditions that pertain to forest climbers under a canopy. The two species of the East African subclade both possess wingless seeds but an aril (or elaiosome; Fig. 5B2 and B3) is present suggesting that myrmecochory may be its mode of dispersal [16]. However it remains confusing why such a trait evolved in habitats dominated by Acacia-Commiphora and Terminalia-Combretum, open savannah woodlands where wind dispersal is widespread, and where ant dispersal may not be dominant [58]. Contrarily, the two fynbos species are wind dispersed even though ant dispersal is thought to be prevalent in that habitat [59]. Dioscorea burchellii in particular is low growing and often concealed among fynbos shrubs. Loss of seed wings has arisen independently in the Mediterranean taxa D. pyrenaica and D. chouardii as well as in New World taxa such as D. sphaeroidea R. Couto & J.M.A. Braga, D. biloba (Phil.) Caddick & Wilkin and D. humilis Colla. It is likely to be linked to switches in dispersal mode. The Cape and Pachycaul subclade taxa have clearly evolved independently but display similar functional features, respectively basally and apically papery winged lens-shaped seeds (Fig. 5C2 and D2). Both of these seed wing traits allow the seeds to spin in flight in a similar manner to a samaroid fruit. It is likely that that basally winged seeds are easier to dislodge than apically winged seeds but both subclades still share convergent dispersal methods. Basally and apically winged seeds are features that have evolved on many occasions and have been observed in groups that generally produce fruits close to ground level. According to Burkill [57] such features are particularly efficient in open habitat where wind speeds are higher.
Fig. 5.
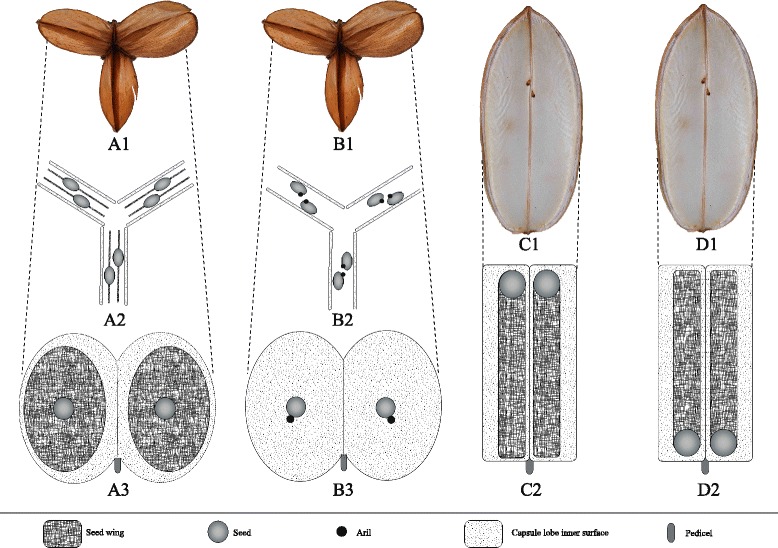
Capsular fruit forms with diagrammatic representations of seed wing shape, position and orientation within each capsule lobes in the Africa clade of Dioscorea. a Apical view of dehisced, empty capsule with rounded lobes (A1), diagrammatic cross-section of capsule through seeds and wings (A2) and diagrammatic side view of two opened lobes containing seeds winged all around the margin (A3): D. buchananii subclade. b Same views of similar capsule (B1) containing wingless, arillate seeds (B2, B3): East Africa subclade; c Side view of two opened oblong capsular lobes without seeds (C1) and diagrammatic side view of basally winged seeds (C2) associated with this capsule lobe shape: Cape subclade; d Same views of similar capsule lobes containing apically winged seeds (D1, D2); Pachycaul subclade
Interestingly, the only southern African species of the pan-palaeotropical Enantiophyllum clade, D. cotinifolia, differs from all other member of that clade by possessing an apically winged seed like that illustrated in Fig. 5D2; the rest have marginally winged (gliding) seeds (like those in Fig. 5A2 and 5A3). In South Africa, this taxon tends to occur in open seasonal woody vegetation (e.g.) that is less dense than the vegetation inhabited by tropical species, and its radiation at the end of the Miocene at similar time as South African taxa suggest parallel evolution in similar type of open environment.
Overall, apart from seed wing form, reproductive morphology in the Africa clade has been less impacted by biome shifts than vegetative morphology. The only significant variation in floral form is found in D. rupicola, which has only 3 stamens and a discoid torus (as opposed to 6 and a thin, bowl-shaped torus); it also has narrower tepals than those in D. buchananii or D. multiloba and the flower is held pendent. However, these changes are probably linked to the shift to a different pollinator within the forest biome in which this species, principally found the Drakensberg and high elevations in the Eastern Cape [8].
Conclusions
Diversification out of forest is associated with a major increase in perennial tuber size and change in tuber orientation from horizontal to vertical, both of which presumably underlie the development of pachycauly. There is also a shift in stem habit, from twining on supporting vegetation to erect and self-supporting. This diversification does not show association with reproductive morphology, except in the seed wing, which has switched from being winged all round the seed margin (to promote gliding flight) to only on its basal or apical side (generating spinning flight). The wing has even been completely replaced by an elaiosome in two species. The single pollinator shift event is observed within the forest biome.
Although only D. brownii and D. strydomiana currently occur within typical fire driven grassland, the transition of the vegetation from closed habitat to savanna grasslands occurring during the Miocene and Pliocene, with an associated increase in fire regime and similar time of origin of other southern African savannah flora elements, suggests that this change has influenced the development of corky barks covering the above-ground pachycauls and therefore the origin of efficient fire and perhaps herbivory protection.
Acknowledgements
We thank the Government of Canada through Genome Canada and the Ontario Genomics Institute (2008-OGI-ICI-03), the International Development Research Centre (IDRC), Canada and the Central Analytical Facility of the Faculty of Science, University of Johannesburg (Spectrau), the Spanish Aragon Government and the European Social Fund (PC, Bioflora research grant), and the South African National Research Foundation for financial support towards fieldwork and sequencing. Plant samples were collected with permission of land owners and respective authorities, with special thanks for assistance going to Charles Stirton, Graham Grieves, Geoffrey Mwachala, John Burrows, Sebsebe Demissew, the late Tony Abbotts. The Royal Botanic Gardens, Kew, DNA Bank, in particularly Felix Forest, Laszlo Csiba and Rhina Duque-Thues, for DNA aliquots and Bezeng S. Bezeng for assistance with preparation of distribution maps.
Availability of data and materials
The trace files and sequences are available on the Barcode of Life Data System (BOLD; www.boldsystems.org). All trees and the combined data matrix are available on request from the authors (olive.maurin@gmail.com).
Authors’ contributions
OM, AMM, PW: designed research; OM, EZS, MvdB: performed research; MvdB: contributed new reagents/analytic tools; PC, JV: provided some data and guidance; OM, JV: analyzed data; OM, AMM, PW, MvdB, PC: wrote the paper. All authors read and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable for this study.
Abbreviations
- CL
Compound-Leaved
- CTAB
Hexadecyltrimethylammonium bromide
- ILD
Incongruence Length Difference
- NWI
New World I (clade)
- NWII
New World II (clade)
- Xdh (nuclear region Xdh)
Newly Sequenced Nuclear Gene
Additional files
rbcLa MP tree. One of the most equally parsimonious tree generated from the Maximum Parsimony (MP) analysis using rbcLa sequence dataset. Values above branches are number of steps and values below branches are reported percentage of Bootstrap support values. Collapsing branches from the strict consensus tree obtained in the combined Maximum Parsimony (MP) analysis are illustrated with a •. (PDF 210 kb)
matK MP tree. One of the most equally parsimonious tree generated from the Maximum Parsimony (MP) analysis using matK sequence dataset. Values above branches are number of steps and values below branches are reported percentage of Bootstrap support values. Collapsing branches from the strict consensus tree obtained in the combined Maximum Parsimony (MP) analysis are illustrated with a •. (PDF 230 kb)
trnL-F MP tree. One of the most equally parsimonious trees generated from the Maximum Parsimony (MP) analysis using trnL-F sequence dataset. Values above branches are number of steps and values below branches are reported percentage of Bootstrap support values. Collapsing branches from the strict consensus tree obtained in the combined Maximum Parsimony (MP) analysis are illustrated with a •. (PDF 221 kb)
trnH-psbA MP tree. One of the most equally parsimonious trees generated from the Maximum Parsimony (MP) analysis using trnH-psbA sequence dataset. Values above branches are number of steps and values below branches are reported percentage of Bootstrap support values. Collapsing branches from the strict consensus tree obtained in the combined Maximum Parsimony (MP) analysis are illustrated with a •. (PDF 206 kb)
psaA-ycf3 MP tree. One of the most equally parsimonious trees generated from the Maximum Parsimony (MP) analysis using psaA-ycf3 sequence dataset. Values above branches are number of steps and values below branches are reported percentage of Bootstrap support values. Collapsing branches from the strict consensus tree obtained in the combined Maximum Parsimony (MP) analysis are illustrated with a •. (PDF 220 kb)
rpl32-trnL MP tree. One of the most equally parsimonious trees generated from the Maximum Parsimony (MP) analysis using rpl32-trnL sequence dataset. Values above branches are number of steps and values below branches are reported percentage of Bootstrap support values. Collapsing branches from the strict consensus tree obtained in the combined Maximum Parsimony (MP) analysis are illustrated with a •. (PDF 224 kb)
Combined MP tree. One of the most equally parsimonious trees generated from the Maximum Parsimony (MP) analysis using the combined sequence dataset. Values above branches are number of steps and values below branches are reported percentage of Bootstrap support values. Collapsing branches from the strict consensus tree obtained in the combined Maximum Parsimony (MP) analysis are illustrated with a •. (PDF 264 kb)
References
- 1.eMonocot Team. http://emonocot.org. Accessed June 2015.
- 2.Kirizawa M, Xifreda CC, Couto R, Araújo D. Dioscoreaceae. In: Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. 2015. http://floradobrasil.jbrj.gov.br. Accessed June 2015.
- 3.Tellez VO, Schubert BG. Dioscoreaceae. In: Davidse G, Sousa MS, Chater AO, editors. Flora Mesoamericana. Mexico D.F: Universidad Nacional Autonoma de Mexico; 1994. pp. 54–5. [Google Scholar]
- 4.Ding Z, Gilbert MG. Dioscoreaceae. In: Wu Z, Raven PH, editors. Flora of China. St. Louis: Science Press: Beijing, and Missouri Botanical Garden Press; 2000. pp. 276–96. [Google Scholar]
- 5.Wilkin P, Thapyai C. Dioscoreaceae. In: Santsuk T, Larsen K, editors. Flora of Thailand. Bangkok: The Forest Herbarium, National Park, Wildlife and Plant Conservation Department; 2009. pp. 1–140. [Google Scholar]
- 6.Wilkin P, Schols P, Chase MW, Chayamarit K, Furness CA, Huysmans, et al.A plastid gene phylogeny of the Yam genus, Dioscorea: roots, fruits and Madagascar. Syst Bot. 2005;30:736–49.
- 7.Viruel J, Segarra-Moragues JG, Raz L Forest F, Wilkin P, Sanmartin I, et al. Late Cretaceous-Early Eocene origin of yams (Dioscorea, Dioscoreaceae) in the Laurasian Palearctic and their subsequent Oligocene-Miocene diversification. J Biogeography. 2016;43:750–762.
- 8.Wilkin P, Muasya AM. Clarifying the Dioscorea buchananii Benth. species complex: a new potentially extinct subspecies for South Africa. PhytoKeys. 2015;48:51–72. doi: 10.3897/phytokeys.48.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkill IH. Testudinaria as a section of the genus Dioscorea. S Afr J Bot. 1951;18:177–91. [Google Scholar]
- 10.Wilkin P, Burrows J, Burrows S, Muasya AM, van Wyk E. A critically endangered new species of yam (Dioscorea strydomiana Wilkin, Dioscoreaceae) from Mpumalanga. S Afr Kew Bull. 2010;65:421–33. doi: 10.1007/s12225-010-9227-y. [DOI] [Google Scholar]
- 11.Hilliard OM, Burtt BL. Notes on some plants of southern Africa chiefly from Natal. Notes from the Royal Botanic Garden, Edinburgh. 1976;34:283–5.
- 12.Viruel J, Segarra-Moragues JG, Pérez-Collazos E, Villar L, Catalán P. Systematic Revision of the Epipetrum Group of Dioscorea (Dioscoreaceae) Endemic to Chile. Syst Bot. 2010;35:40–63. doi: 10.1600/036364410790862579. [DOI] [Google Scholar]
- 13.Burkill IH, Pierrier De La Bathie H. 44e famille Dioscoréacées. In: Humbert H, editor. Flore de Madagascar et des Comores. Paris: Firmin-Didot et Cie; 1950. [Google Scholar]
- 14.Archibald EEA. The genus Dioscorea in the Cape Province west of East London. S Afr J Bot. 1967;33:1–46. [Google Scholar]
- 15.Von T, Logischen I, van der Schijff HP, Robbertse PJ. The genus Dioscorea L. in South Africa. Boissiera. 1975;24:215–24. [Google Scholar]
- 16.Wilkin P, Muasya AM, Banks H, Furness CA, Vollensen K, Weber O, Demissew S. A New species of Yam from Kenya, Dioscorea kituiensis: pollen morphology, conservation status, and speciation. Syst Bot. 2009;34:652–9.
- 17.Codd LE. Drugs from wild yams. Afr Wildl. 1960;14(Suppl 3):215–25. [Google Scholar]
- 18.Coursey DG. Yams: an account of the nature, origins, cultivation and utilisation of the useful members of the Dioscoreaceae. In tropical agricultural series. London: Longmans, Green and Co. Ltd., UK; 1967.
- 19.Kulkarni MG, Street RA, Van Staden J. Germination and seedling growth requirements for propagation of Dioscorea dregeana (Kunth) Dur. and Schinz — A tuberous medicinal plant. S Afr J Bot. 2007;73:131–7. doi: 10.1016/j.sajb.2006.09.002. [DOI] [Google Scholar]
- 20.Asiedu R, Sartie A. Crops that feed the World 1. Yams Food Secur. 2010;2:305–15. doi: 10.1007/s12571-010-0085-0. [DOI] [Google Scholar]
- 21.Chase MW, Hills HG. Silica gel: an ideal material for field preservation of leaf samples. Taxon. 1991;40:215–20. doi: 10.2307/1222975. [DOI] [Google Scholar]
- 22.Doyle JJ, Doyle JL. A rapid isolation procedure for small amounts of leaf tissue. Photochem Bull Bot Soc Am. 1987;19:11–5. [Google Scholar]
- 23.Levin RA, Wagner WL, Hoch PC, Baum DA, Katinas L, Zimmer EA, Sytsma KJ. Family-level relationships of Onagraceae based on chloroplast rbcL and ndhF data. Am J Bot. 2003;90:107–15. doi: 10.3732/ajb.90.1.107. [DOI] [PubMed] [Google Scholar]
- 24.Kress J, Erickson DL. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS One. 2007 doi: 10.1371/journal.pone.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plant Working Group. DNA Barcoding of Plants: matK primers for angiosperms. Royal Botanic Garden Edinburgh. 2012.
- 26.Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol. 1991;17:1105–9. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- 27.Sang T, Crawford DJ, Stuessy TF. Chloroplast DNA phylogeny, reticulate evolution and biogeography of Paeonia (Paeoniaceae) Am J Bot. 1997;84:1120–36. doi: 10.2307/2446155. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Shi S. Phylogenetics in the Lythraceae sensu lato: a preliminary analysis based on plastic rbcL and psaA-ycf3 spacer, and ITS nrDNA sequences. Int J Plant Sci. 2002;163:215–25. doi: 10.1086/338392. [DOI] [Google Scholar]
- 29.Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the Tortoise and the Hare III. Am J Bot. 2007;94:275–88. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- 30.Swofford DL. PAUP*: phylogenetic analysis using parsimony (* and other methods), version 4.0b10. Massachusetts: Sinauer Associates; 2002. [Google Scholar]
- 31.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. doi: 10.1086/284325. [DOI] [Google Scholar]
- 32.Farris JS, Källersjö M, Kluge AG, Bult C. Permutations. Cladistics. 1994;10:65–76. doi: 10.1006/clad.1994.1005. [DOI] [PubMed] [Google Scholar]
- 33.Farris JS, Källersjö M, Kluge AG, Bult C. Testing significance of incongruence. Cladistics. 1995;10:315–9. doi: 10.1111/j.1096-0031.1994.tb00181.x. [DOI] [Google Scholar]
- 34.Symonds VV, Lloyd AM. An analysis of microsatellite loci in Arabidopsis thaliana: Mutational dynamics and application. Genetics. 2003;165:1475–88. doi: 10.1093/genetics/165.3.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huelsenbeck JP, Ronquist F. MRBAYES, Bayesian inference of Phylogenetic trees. Bioinformatics. 2001;17:754–5. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 36.Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–4. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 37.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinform (application note) 1998;14:817–8. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 38.Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, Chan L, et al. The CIPRES portals. [WWW document] URL http://www.phylo.org/sub_sections/portal (2009) [Accessed Nov 2014].
- 39.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nylander JAA. Modeltest v2. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University; 2004. [Google Scholar]
- 41.Britton T, Anderson CL, Jacquet D, Lundqvist S, Bremer K. Estimating divergence times in large phylogenetic trees. Syst Biol. 2007;56:741–52. doi: 10.1080/10635150701613783. [DOI] [PubMed] [Google Scholar]
- 42.Janssen T, Bremer K. The age of major monocot groups inferred from 800+ rbcL sequences. Bot J Linn Soc. 2004;146:385–98.
- 43.Potonié H. Lehrbuch der Palaeobotanik. Berlin: Gebrüder Borntraeger; 1921. [Google Scholar]
- 44.Pan AD, Jacobs BF, Currano ED. Dioscoreaceae fossils from the late Oligocene and early Miocene of Ethiopia. Bot J Linn Soc. 2014;175:17–28. doi: 10.1111/boj.12150. [DOI] [Google Scholar]
- 45.Anderson JM, Anderson HM, Cleal CJ. A brief history of the gymnosperms: classification, biodiversity, phytogeography and ecology. Pretoria: South African Biodiversity Institute; 2007. [Google Scholar]
- 46.Linder HP, Verboom GA. The evolution of regional species richness: the history of the southern African flora. Annu Rev Ecol Evol Syst. 2015;46:393–412. doi: 10.1146/annurev-ecolsys-112414-054322. [DOI] [Google Scholar]
- 47.McRae C. Life Etched in Stone. Johannesburg: The Geological Society of South Africa; 1999. [Google Scholar]
- 48.Cowling RM, Proches S, Partridge TC. Explaining the uniqueness of the cape flora: incorporating geomorphic evolution as a factor for explaining its diversification. Mol Phylogenet Evol. 2009;51:64–74. doi: 10.1016/j.ympev.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 49.Burgoyne PM, Van Wyk AE, Anderson JM, Schrire BD. Phanerozoic evolution of plants on the African plate. J Afr Earth Sci. 2005;43:13–52. doi: 10.1016/j.jafrearsci.2005.07.015. [DOI] [Google Scholar]
- 50.White F. The underground forests of Africa: a preliminary review. Gardens Bull Singapore. 1976;24:57–71. [Google Scholar]
- 51.Maurin O, Davies TJ, Burrows JE, Daru BH, Yessoufou K, Muasya AM, et al. Savanna fire and the origins of the “underground forests” of Africa. New Phytol. 2014;204:201–14. doi: 10.1111/nph.12936. [DOI] [PubMed] [Google Scholar]
- 52.Coutinho LM. Ecological effects of fire in Brazilian cerrado. In: Huntley BJ, Walker BH, editors. Ecology of tropical savannas. Berlin: Springer; 1982. pp. 273–91. [Google Scholar]
- 53.Coutinho LM. Fire in the ecology of the Brazilian cerrado. In: Goldamer JG, editor. Fire in the tropical biota – ecosystem process and global challenges. Berlin: Springer; 1990. pp. 82–105. [Google Scholar]
- 54.Hoffmann WA, Geiger EL, Gotsch SG, Rossatto DR, Silva LCR, Lau OL, et al. Ecological thresholds at the savanna–forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol Lett. 2012;15:759–68. doi: 10.1111/j.1461-0248.2012.01789.x. [DOI] [PubMed] [Google Scholar]
- 55.Crisp MD, Arroyo MTK, Cook LG, Gandolfo MA, Jordan GJ, McGlone MS, et al. Phylogenetic biome conservatism on a global scale. Nature. 2009;458:754–6. doi: 10.1038/nature07764. [DOI] [PubMed] [Google Scholar]
- 56.Cowling RM, Pierce SM. East of the Cape: Conserving Eden. Simon's Town: Fernwood Press. 2009.
- 57.Burkill IH. The organography and the evolution of the Dioscoreaceae, the family of the yams. Bot J Linn Soc. 1960;56:319–412. doi: 10.1111/j.1095-8339.1960.tb02508.x. [DOI] [Google Scholar]
- 58.Garcia MB, Antor RJ, Espadaler X. Ant pollination of the palaeoendemic dioecious Borderea pyrenaica (Dioscoreaceae) Plant Syst Evol. 1995;198:17–27. doi: 10.1007/BF00985105. [DOI] [Google Scholar]
- 59.Bond WJ, Slingsby PJ. Seed dispersal by ants in shrublands ot the cape province and its evolutionary implications. S Afr J Sci. 1983;79:231–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The trace files and sequences are available on the Barcode of Life Data System (BOLD; www.boldsystems.org). All trees and the combined data matrix are available on request from the authors (olive.maurin@gmail.com).


