High-light acclimation strategies of Chlamydomonas reinhardtii cells grown in different carbon supply regimes show the high plasticity of the photosynthetic apparatus.
Abstract
Photosynthetic organisms are exposed to drastic changes in light conditions, which can affect their photosynthetic efficiency and induce photodamage. To face these changes, they have developed a series of acclimation mechanisms. In this work, we have studied the acclimation strategies of Chlamydomonas reinhardtii, a model green alga that can grow using various carbon sources and is thus an excellent system in which to study photosynthesis. Like other photosynthetic algae, it has evolved inducible mechanisms to adapt to conditions where carbon supply is limiting. We have analyzed how the carbon availability influences the composition and organization of the photosynthetic apparatus and the capacity of the cells to acclimate to different light conditions. Using electron microscopy, biochemical, and fluorescence measurements, we show that differences in CO2 availability not only have a strong effect on the induction of the carbon-concentrating mechanisms but also change the acclimation strategy of the cells to light. For example, while cells in limiting CO2 maintain a large antenna even in high light and switch on energy-dissipative mechanisms, cells in high CO2 reduce the amount of pigments per cell and the antenna size. Our results show the high plasticity of the photosynthetic apparatus of C. reinhardtii. This alga is able to use various photoacclimation strategies, and the choice of which to activate strongly depends on the carbon availability.
Light sustains virtually all life on Earth through the process of photosynthesis. However, light can be very harmful for oxygenic photosynthetic organisms, as excess absorption can lead to the production of reactive oxygen species. In order to survive and grow, these organisms have developed various photoacclimation mechanisms operating on different time scales that protect the cell from photodamage. In the green alga Chlamydomonas reinhardtii, these mechanisms vary from negative phototaxis and multicomponent nonphotochemical quenching (NPQ) to a number of physiological and biochemical changes (Erickson et al., 2015). C. reinhardtii cells are around 10 μm in diameter, and a large part of their total volume is occupied by a single horseshoe-shaped chloroplast (Sager and Palade, 1957). The photosynthetic machinery responsible for the light reactions is located in thylakoid membranes and contains four major components: PSII, cytochrome b6f, PSI, and ATP synthase. Both photosystems bind chlorophyll (Chl) and carotenoid (Car) and are composed of a core and several outer antennae pigment-protein complexes, the main function of which is light harvesting and its conversion into chemical energy. The PSII core is composed of D1, D2, CP43, and CP47 pigment-protein complexes and several smaller subunits, the number of which varies between organisms (Shi et al., 2012). The outer antenna contains the light-harvesting complex II (LHCII), which in C. reinhardtii is encoded by nine LHCBM genes, and the minor antennae CP26 and CP29 (Nield et al., 2000; Teramoto et al., 2001; Natali and Croce, 2015). These complexes are assembled together to form PSII-LHCII supercomplexes (Tokutsu et al., 2012; Drop et al., 2014). The PSI core is composed of a PSAA-PSAB heterodimer and a number of smaller subunits (Jensen et al., 2007), and in C. reinhardtii the LHCI antenna consists of nine LHCA proteins (Mozzo et al., 2010) that are associated with the core to form the PSI-LHCI complex (Stauber et al., 2009; Drop et al., 2011).
The composition and organization of the thylakoid membrane is light dependent. The gene expression of different LHCs has been reported to be affected by light acclimation (Teramoto et al., 2002; Durnford et al., 2003; Yamano et al., 2008) and to be NAB1 regulated (Mussgnug et al., 2005). It has been observed that long-term high-light exposure of C. reinhardtii cells leads to a 50% decrease of Chl content (Neale and Melis, 1986; Bonente et al., 2012) and to changes in Chl-to-Car ratio (Niyogi et al., 1997a; Baroli et al., 2003; Bonente et al., 2012), suggesting reduction of the antenna size (Neale and Melis, 1986), although, in a more recent report (Bonente et al., 2012), it was concluded that the antenna size is not modulated by light in this alga. Recently, a dependence of the antenna components on the carbon availability also was reported. It was shown that, when cells grown in acetate are shifted from high to low CO2 concentration, the functional antenna size of PSII decreases and a down-regulation of LHCBM6/8 occurs (Berger et al., 2014).
In the short term, the main response to high light is the dissipation of energy absorbed in excess heat in a process called qE, or energy-dependent quenching, which is the fastest component of NPQ. In land plants, the main player in this process is the protein PsbS (Li et al., 2002, 2004), while in C. reinhardtii, the process is centered around LHCSR1 and LHCSR3 (Peers et al., 2009; Dinc et al., 2016). LHCSR3, the most studied of the two, is a pigment-protein complex that is expressed within 1 h of high-light exposure (Allorent et al., 2013) in combination with CO2 limitation (Yamano et al., 2008; Maruyama et al., 2014). The qE onset is triggered by lumen acidification sensed by LHCSR3/1 (Bonente et al., 2011; Liguori et al., 2013; Tokutsu and Minagawa, 2013; Dinc et al., 2016).
Cars are well known to be involved in photoprotection. They quench triplet Chl and scavenge singlet oxygen (1O2; Frank and Cogdell, 1996). In C. reinhardtii, the antioxidant role of xanthophylls is well illustrated by the mutant npq1 lor1 lacking lutein and zeaxanthin (Niyogi et al., 1997b). This mutant is deficient in qE, but compared with other qE-deficient mutants like npq4 (Peers et al., 2009) and npq5 (Elrad et al., 2002), which are LHCSR3 and LHCBM1 knockouts, respectively, it is extremely light sensitive, due to the absence of quenching of triplet Chl and 1O2 by zeaxanthin and lutein.
Aquatic oxygenic photosynthetic organisms meet several challenges in CO2 fixation (Moroney and Ynalvez, 2007). First, the diffusion of CO2 in water is 10,000 times slower than in air. Second, the CO2-fixing enzyme Rubisco is not selective for CO2 and also binds oxygen, resulting in the process of photorespiration. Third, the form of inorganic carbon depends on the pH (i.e. in alkaline pH, it is HCO3−, while in acidic pH, it is CO2; Beardall, 1981; Gehl et al., 1987). This diminishes even further the availability of CO2 in the cell. In order to overcome these CO2 fixation barriers, algae have developed carbon-concentrating mechanisms (CCMs; Moroney and Ynalvez, 2007). The essence of these processes lies in the active pumping of inorganic carbon in the cell via a number of transporters that concentrate it in the pyrenoid, a ball-like structure containing Rubisco, Rubisco activase, and intrapyrenoid thylakoids and surrounded by a starch sheath. In the pyrenoid, HCO3− is converted to CO2 by CARBONIC ANHYDRASE3 (CAH3; Blanco-Rivero et al., 2012; Sinetova et al., 2012) and then fixed by Rubisco in the Calvin-Benson-Bassham cycle. CAH3 also is suggested to provide HCO3− in the proximity of the oxygen-evolving complex, where it may function as a proton carrier, removing H+ from water splitting to avoid photoinhibition (Villarejo et al., 2002; Shutova et al., 2008).
C. reinhardtii also can grow mixotrophically using alternative organic carbon sources present in its environment. For example, it can take up acetate, which is then incorporated into the citric cycle, producing reducing equivalents and CO2 (Johnson and Alric, 2012), and into the glyoxylate cycle, producing malate (Lauersen et al., 2016). In the presence of acetate, it has been reported that CO2 uptake and oxygen evolution were decreased by half under saturating CO2 and light intensities without affecting PSII efficiency, respiration, and cell growth (Heifetz et al., 2000). In addition, reactions of the oxidative pentose phosphate and glycolysis pathways, inactive under phototrophic conditions, show substantial flux under mixotrophic conditions (Chapman et al., 2015). Furthermore, acetate can replace PSII-associated HCO3−, reducing 1O2 formation and, therefore, acting as a photoprotector during high-light acclimation (Roach et al., 2013).
In short, high-light acclimation is a complex, multicomponent process that happens on different time scales. Furthermore, it is embedded in the overall metabolic network and is potentially influenced by different nutrients and metabolic states. A thorough understanding of this process and its regulation is crucial for fundamental research and applications. To determine if different carbon supply conditions trigger different light acclimation strategies and photoprotective responses, we systematically studied C. reinhardtii cells grown in mixotrophic, photoautotrophic, and high-CO2 photoautotrophic conditions in different light intensities.
We show that C. reinhardtii cells use different strategies to acclimate to high light depending on the carbon availability and trophic status. These results underline the strong connection between metabolism and light acclimation responses and reconcile the data from various reports. Furthermore, our study demonstrates how, in a dynamic system such as C. reinhardtii, a single change in growth conditions has large effects at multiple levels.
RESULTS
Induction of CCMs
C. reinhardtii wild-type strain CC-124 was grown mixotrophically (M) in the presence of acetate (Tris-acetate-phosphate [TAP] medium), photoautotrophically (P) in ambient air (approximately 400 µL L−1 CO2), and photoautotrophically in ambient air supplemented with 5% CO2 (CO2). To study high-light acclimation, the cells were grown in 500 µE m−2 s−1 white continuous light for more than six generations, while the control cells were kept in 50 µE m−2 s−1. To study possible morphological differences between the cells in the various conditions, we analyzed them by transmission electron microscopy. The images are presented in Figure 1.
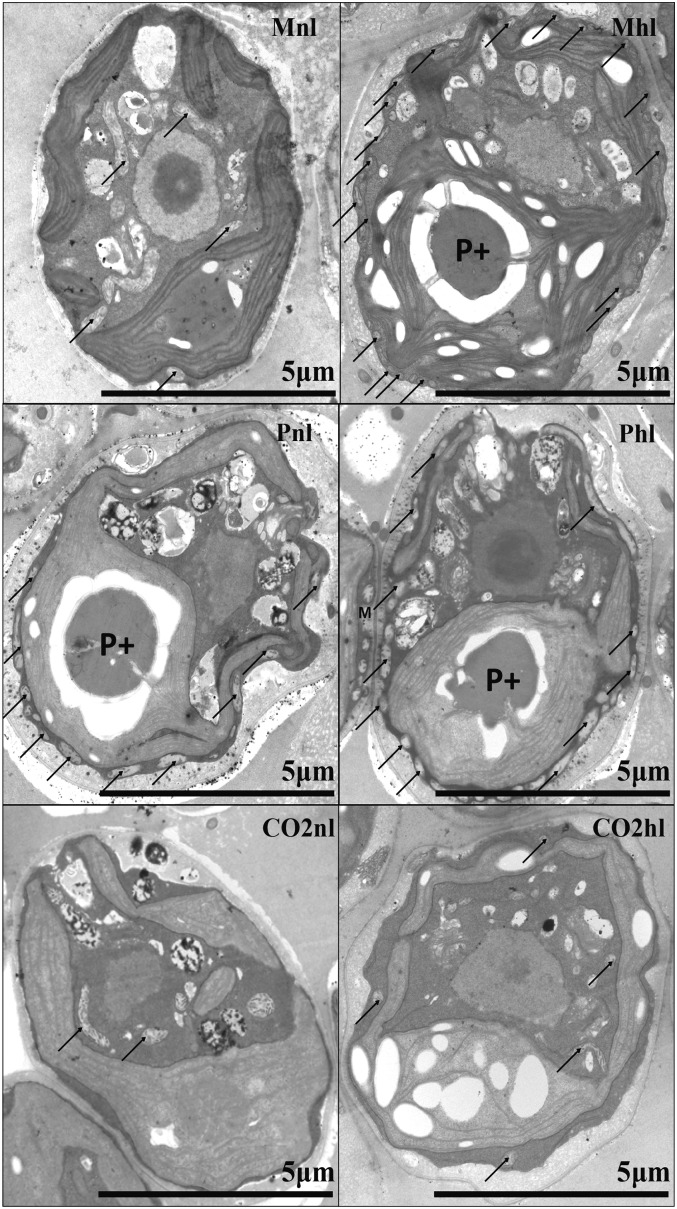
Figure 1.
Transmission electron microscopy images of C. reinhardtii cells grown in different carbon and light regimes. P+ shows a well-formed pyrenoid, and arrows indicate mitochondria.
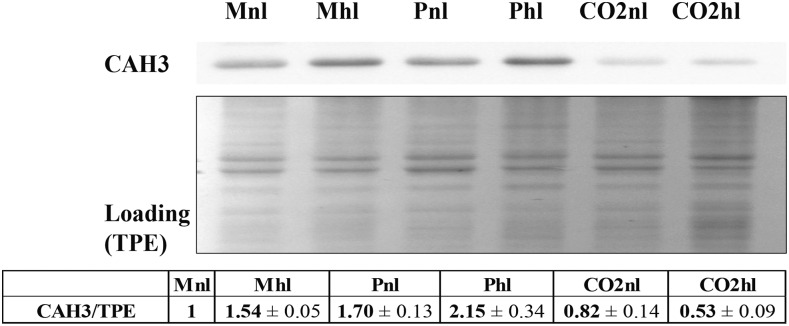
The pyrenoid was not formed in the presence of high CO2 (Fig. 1), while a clearly formed pyrenoid (Fig. 1, P+) was observed in mixotrophic with high light (Mhl), photoautotrophic with normal light (Pnl), and photoautotrophic with high light (Phl) conditions. Interestingly, a well-formed pyrenoid was absent in mixotrophic with normal light (Mnl) conditions, as can be inferred by the lack of the starch sheath that usually surrounds it. Starch sheaths around the pyrenoid were shown to become thicker under low-CO2 conditions (Kuchitsu et al., 1988; Ramazanov et al., 1994) and, together with a low-carbon-induced (LCI) LCIB-LCIC complex layer, are hypothesized to prevent CO2 leakage from the pyrenoid (Yamano et al., 2010). Another interesting observation is that most mitochondria were in a central position in photoautotrophic in ambient air supplemented with 5% CO2 with normal light (CO2nl), photoautotrophic in ambient air supplemented with 5% CO2 with high light (CO2hl), and Mnl, but in Mhl, Pnl, and Phl they were located at the periphery of the cell in close contact with the chloroplast. It has been observed previously that, in low CO2, the mitochondria migrate from the central part of the cell to its periphery, and it was suggested that this migration happens to provide ATP for the active transport of HCO3− inside the cell (Geraghty and Spalding, 1996). Thus, these results suggest that CCM is induced in Mhl, Pnl, and Phl but not in Mnl, a condition in which the cells are apparently not strongly CO2 limited. This conclusion is supported by the increase of CAH3 expression level in Mhl, Pnl, and Phl, by 50%, 70%, and more than 100%, respectively, compared with Mnl (Fig. 2). In the CO2-enriched condition, the CAH3 level was even lower than in Mnl.
Figure 2.
Expression of CAH3. CAH3 expression was determined on total protein extract (TPE) from cells. Ten micrograms of proteins was loaded in each well. For quantitative densitometry analysis, CAH3 signal was normalized to total protein loading to correct for possible loading errors. For digital analysis, Gel-Pro software was used. The quantitative data were obtained from a minimum of two biological replicates and three technical repetitions.
Architecture of C. reinhardtii Thylakoid Membranes
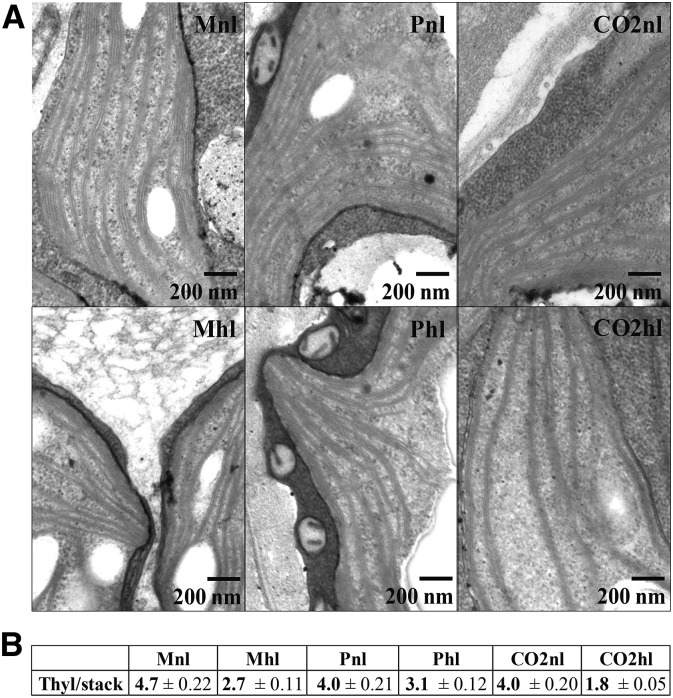
In land plants, the changes in membrane organization upon long-term acclimation to different light conditions are well documented (Anderson et al., 2012; Kirchhoff, 2013; Pribil et al., 2014), while little is known about how algae adapt to light conditions with respect to membrane organization. We used transmission electron microscopy to visualize the structural changes that C. reinhardtii cells undergo in various carbon supply and light conditions (Fig. 3). In all cells, the thylakoids form stacks that run throughout the chloroplast, while very few interconnecting regions (stroma lamellae) are observed (Fig. 3A), unlike in land plants, where stacks of thylakoid discs (grana) and stroma lamellae regions are visible (Shimoni et al., 2005). The number of thylakoids (with enclosed lumen) per stack ranges from two to 12 (Fig. 3B). This is in agreement with previous reports from C. reinhardtii (Engel et al., 2015). Interestingly, we observed that the number of thylakoids per stack decreases in high light, unlike in land plants (Anderson et al., 2012), and is influenced by the carbon supply regime. In normal light, there are, on average, five thylakoid double membranes in mixotrophically grown cells and photoautotrophically grown cells, independent of the CO2 concentration. In high light, the number of membranes per stack decreased differently: 43% for Mhl, 23% for Phl, and 55% for CO2hl.
Figure 3.
Changes of thylakoid stacking in C. reinhardtii cells grown in different carbon supply and light regimes. A, Electron microscopy images, recorded under 60K magnification. B, Thylakoid stack numbers (double layers with enclosed lumen), counted from a minimum of 50 different stacks from at least five different C. reinhardtii cells.
Doubling Time, Pigment Content, and Maximal PSII Quantum Efficiency
The cell division time of C. reinhardtii depends on carbon source and light intensity (Table I). For Mnl and CO2nl cells, we observed a 9-h cell doubling time, in agreement with the literature (Harris, 2008). Pnl and Phl cells divided around once per day, which is similar to a previous report as well (Bonente et al., 2012), while CO2hl cells divided every 5 h. In summary, cell doubling time is faster in high light, especially in CO2hl, where growth is sustained by higher carbon fixation rate in the presence of elevated CO2 levels. In Table I, the Fv/Fm is reported. The highest values in this condition were observed for CO2nl and CO2hl cells, while a lower value for Phl cells suggests an overall higher stress.
Table I. Properties of C. reinhardtii acclimated to different light and carbon supply regimes.
Changes in C. reinhardtii phenotype upon acclimation to different light and carbon supply regimes are shown. Data columns from left to right show cell number doubling time; Chl content per cell; Chl a/b ratio, calculated from the fitting of absorption spectra of 80% acetone-extracted pigments from cells; PSI/PSII ratio, measured based on the electrochromic shift (ECS) signal; PSI/PSII ratio, obtained by immunoblot (WB) quantification of PSAA and CP43; LHCII/PSII monomer calculations based on Chl a/b and PSI/PSII data, as described in “Materials and Methods”; and maximal quantum efficiency of PSII (Fv/Fm). The data are averages and se derived from a minimum of two biological replicates, each with three technical replicates.
| Condition | Doubling Time | Chl Content | Chl a/b | PSI/PSII ECS | PSI/PSII WB | LHCII/PSII | Fv/Fm |
|---|---|---|---|---|---|---|---|
| h | pg | ||||||
| Mnl | 9.0 ± 0.0 | 3.9 ± 0.21 | 2.57 ± 0.00 | 1.05 ± 0.34 | 1.00 ± 0.00 | 4.9 | 0.71 ± 0.01 |
| Mhl | 7.3 ± 0.4 | 2.0 ± 0.11 | 2.87 ± 0.03 | 1.13 ± 0.02 | 1.35 ± 0.30 | 4.3 | 0.70 ± 0.02 |
| Pnl | 25.0 ± 1.4 | 2.6 ± 0.02 | 2.55 ± 0.03 | 1.67 ± 0.11 | 1.95 ± 0.31 | 8 | 0.68 ± 0.01 |
| Phl | 22.5 ± 2.1 | 1.3 ± 0.02 | 2.57 ± 0.02 | 1.54 ± 0.58 | 1.65 ± 0.28 | 7.5 | 0.59 ± 0.00 |
| CO2nl | 8.8 ± 0.4 | 2.1 ± 0.12 | 2.56 ± 0.05 | 0.96 ± 0.04 | 1.04 ± 0.17 | 4.9 | 0.75 ± 0.01 |
| CO2hl | 4.6 ± 0.5 | 0.6 ± 0.02 | 3.00 ± 0.05 | 0.62 ± 0.04 | 0.66 ± 0.13 | 2.2 | 0.76 ± 0.01 |
On the one hand, Chl content per cell decreased to half in high light in M and P and in CO2 to one-third compared with cells grown in normal light in the same carbon condition (Table I). On the other hand, even in normal light, the Chl content differed depending on carbon supply, with the highest in Mnl (3.9 pg cell−1), then Pnl (2.6 pg cell−1), and the lowest in CO2nl (2.1 pg cell−1). Therefore, our data show that the amount of Chl per cell varies not only depending on light intensity, as shown before (Neale and Melis, 1986; Bonente et al., 2011), but also depending on trophic state and CO2 availability.
The Chl a/b ratio is indicative of changes in the composition of the photosynthetic apparatus, as Chl b is present only in the outer antennae of the two photosystems. In normal light, the Chl a/b ratio was 2.55 to 2.57 in all growth conditions (Table I). High-light acclimation affected the Chl a/b ratio in a different way depending on the carbon supply. While the Chl a/b ratio was the same (2.55–2.57) in Pnl and Phl, in agreement with previous data (Bonente et al., 2012), it increased in Mhl and CO2hl (2.9–3) compared with normal light, suggesting that in these conditions the antenna size decreases as part of the strategy of high-light acclimation. However, changes in Chl a/b ratio can reflect changes in both the antenna size of the photosystems and their ratio. To disentangle these effects, PSI/PSII was estimated based on the ECS of the Car’s absorption (Bailleul et al., 2010; Table I). In Mnl cells, the ratio between PSI and PSII reaction centers was close to 1, as reported before (Allorent et al., 2013). In Mhl, the PSI/PSII ratio was very similar (1.1), while it increased in P cells (normal light, 1.7; high light, 1.5). In CO2nl cells, the PSI/PSII ratio was similar to that in M cells (1) while it decreased in high light (0.6). It is interesting that the changes in PSI/PSII ratio are far larger than what was reported for plants (Anderson et al., 1995; Ballottari et al., 2007; Wientjes et al., 2013b), indicating that, in C. reinhardtii, the photosynthetic apparatus is very flexible and can change drastically in response to the metabolic state of the cell.
To evaluate if the observed functional changes in PSI/PSII ratio are due to a change in the relative content of the photosystems or only to a change in their functionality, we performed immunoblotting on the total protein extracts using antibodies against PSAA (a subunit of PSI) and CP43 (a subunit of PSII). The protein data show the same trend as the functional data, indicating that the changes in the PSI/PSII ratio are largely due to changes in the protein content (Fig. 4).
Figure 4.
PSI/PSII ratio changes in C. reinhardtii cells grown in different carbon supply and light regimes. PSI/PSII ratio was obtained by immunoblot quantification of PSAA and CP43. Ten micrograms of proteins was loaded in each well. For digital analysis, Gel-Pro software was used. The data are averages and se derived from two biological replicates, each with three technical replicates.
Combining Chl a/b and PSI/PSII, the number of LHCII trimers per PSII core monomer can be estimated (Table I). According to our calculation (see “Materials and Methods”), in Mnl and CO2nl, the LHCII/PSII ratio was around 5. A decrease in this ratio was observed upon high-light acclimation, although to a different extent: by less than one LHCII trimer in M (LHCII/PSII in Mhl = 4.3) and by three in CO2 (LHCII/PSII in CO2hl = 2.2). On the contrary, in Pnl and Phl, the LHCII antenna was even larger and did not change much under high-light acclimation, eight and 7.5 LHCII/PSII, respectively. Drop et al. (2014) showed that the PSII monomer of C. reinhardtii can directly coordinate a maximum of three LHCIIs, leaving then many extra LHCIIs. This is especially true in Pnl and Phl conditions.
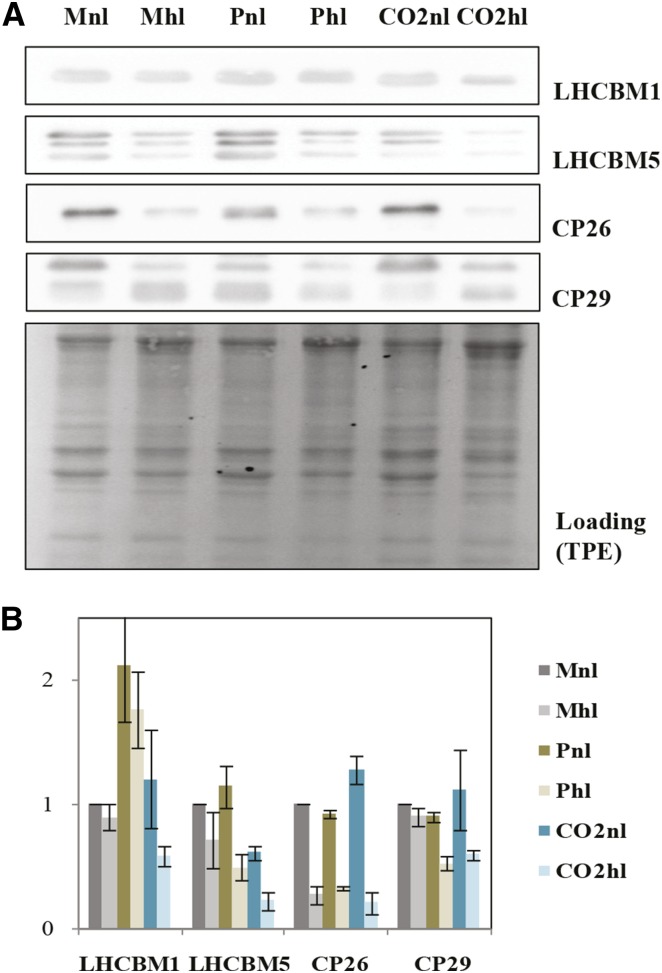
To support our calculations, we performed immunoblot analyses using antibodies against LHC subunits, namely LHCBM1, LHCBM5, CP26, and CP29 (Fig. 5A). We present quantitative analyses of these proteins in Figure 5B. First, we observed that the amount of all four antenna proteins depends on both light and carbon availability. In Mhl, the decrease of the antennae compared with normal light was very small for all the proteins except CP26. On the contrary, in high CO2, the amount of all antenna complexes decreased strongly in high light. The situation was again different in P, which showed far higher levels of LHCBM1 in normal light compared with all other conditions. In high light, no change in this complex was observed, while the amount of all other antennas decreased.
Figure 5.
PSII antennae size alteration during C. reinhardtii light acclimation in different carbon supply regimes. A, Immunoblot data of PSII antennae proteins LHCBM1, LHCBM5, CP26, and CP29. Ten micrograms of total protein extract (TPE) was loaded per well. B, Densitometry analysis of LHCBM1, LHCBM5, CP26, and CP29, normalized to loading. The data are averages se derived from two biological replicates, with three repetitions each. For digital analysis, Gel-Pro software was used.
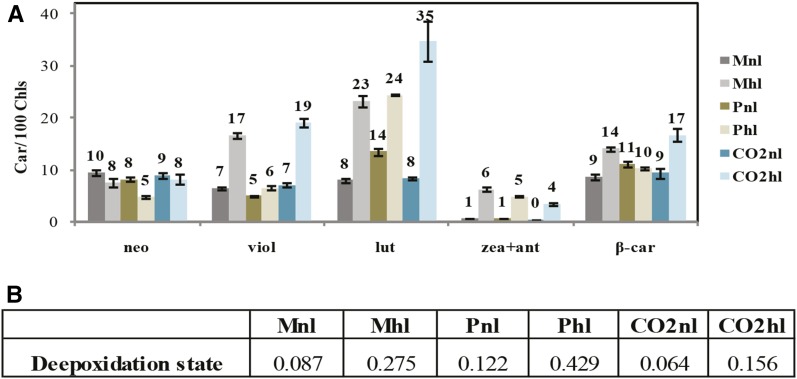
Car Composition Dependency on Light and Carbon Availability
To determine the Car involvement in high-light acclimation in different carbon supply conditions, we analyzed the pigment extracts (Fig. 6). In normal light, the Car-to-Chl ratio was independent of the carbon source and availability, with the exception of a slightly higher lutein and β-carotene content in Pnl. In high light, the Car-to-Chl ratio increased in all conditions due to a relative increase in lutein and violaxanthin + antheraxanthin + zeaxanthin. This increase was particularly remarkable in CO2hl cells (approximately 4-fold increase), despite a strong reduction of the antenna size, which might indicate the presence of free xanthophylls in the membrane (also visible in the relative increase in β-carotene). Finally, the deepoxidation state, calculated as the ratio between antheraxanthin + zeaxanthin and violaxanthin + antheraxanthin + zeaxanthin, was higher in high light, with the highest deepoxidation state (approximately 0.4) being observed in Phl (Fig. 6B). Because deepoxidized forms of violaxanthin participate directly in photoprotection, this suggests a higher photoprotective capacity in Phl.
Figure 6.
Car content changes within C. reinhardtii cells acclimated to different carbon and light supply regimes. A, Amount of individual Cars normalized to 100 Chls, based on HPLC analysis. Error bars indicate se. B, Deepoxidation state.
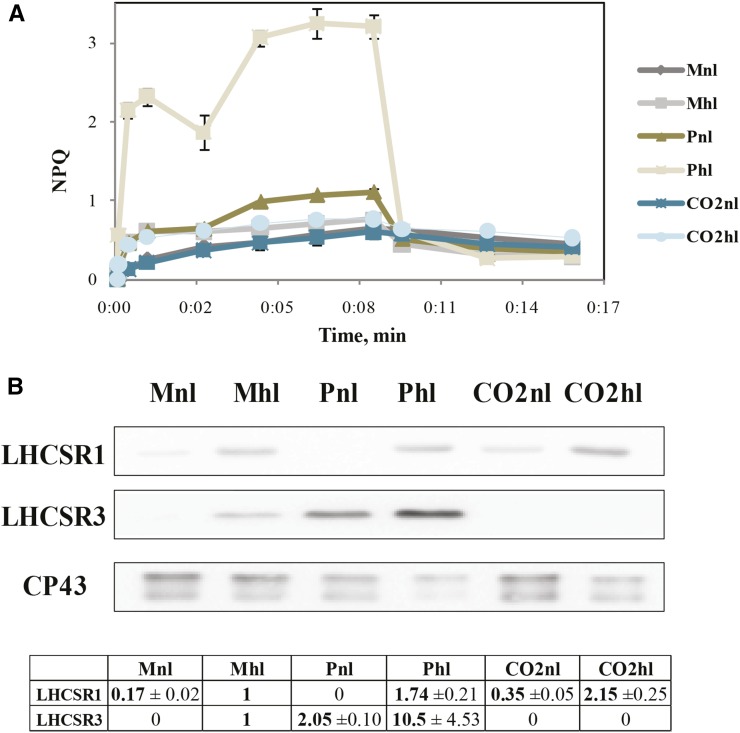
NPQ Capacity
The above results show differences in long-term high-light acclimation strategies in different carbon supply conditions, suggesting possible differences in short-term high-light responses, namely NPQ. In order to systematically study the dependence of the heat dissipation capacity of C. reinhardtii cells on the growth conditions, we measured NPQ induction and relaxation (Fig. 7A). We observed that NPQ induction also depends on both light and carbon supply conditions. The highest quenching is observed for Phl cells, which is indeed the condition in which NPQ is normally monitored (Peers et al., 2009) and where the highest deepoxidation state of xanthophylls was observed. However, we also observed a buildup of NPQ in Pnl. In all other conditions, NPQ was below 1 and did not fully relax in the dark, suggesting the presence of long-term quenching or photoinhibition. It has been shown that the fast rise of NPQ (qE) depends on LHCSR3, and this protein is expressed in high light and low CO2 (Peers et al., 2009; Allorent et al., 2013; Maruyama et al., 2014). The LHCSR3 protein expression was then checked by immunoblotting (Fig. 7B). LHCSR3 was highly expressed in Phl, but it was also present in Pnl and Mhl cells, while it was not present in high CO2, even in high light.
Figure 7.
Changes in C. reinhardtii NPQ capacity upon different acclimations. A, NPQ measured on a Dual PAM-100. B, Immunoblot detection of LHCSR1, LHCSR3, and CP43. Ten micrograms per well of total protein extract from different cell samples was loaded. For digital analysis, Gel-Pro software was used. In the table, LHCSR1/CP43 and LHCSR3/CP43 estimations are presented.
LHCSR1 is another protein that was suggested to participate to NPQ (Peers et al., 2009; Berteotti et al., 2016) and was shown to induce a pH-dependent quenching in LHCII (Dinc et al., 2016). It has been shown at the RNA level that LHCSR1 increases in medium light in high CO2 and especially in high light and high CO2 (Yamano et al., 2008). Our data show that LHCSR1 is expressed in all conditions except Pnl and might be responsible for the quenching observed in the absence of LHCSR3 (CO2hl; Fig. 7).
DISCUSSION
C. reinhardtii is a model organism that can grow on different carbon sources. This metabolic flexibility is certainly an advantage for the study of photosynthesis because it permits the study of mutants that cannot perform photosynthesis (Harris, 2008). However, the carbon metabolism might affect the composition and functionality of the photosynthetic apparatus, making difficult the comparison of results obtained with cells grown in the presence of different carbon sources. To understand how the carbon supply influences the light reactions, we then studied long-term (photosynthetic machinery remodeling) acclimation responses to light and the capacity of NPQ induction of C. reinhardtii cells grown mixotrophically in acetate and photoautotrophically in ambient CO2 or CO2-enriched conditions. In the following, we first discuss the activation of the CCM as a response to low CO2 and how it is affected in the presence of acetate. Then, we compare the composition of the photosynthetic apparatus in the three different conditions under normal light, and finally, we discuss the differences in the short-term and long-term responses to high light.
CCM Is Not Induced in Cells Growing in Acetate in Normal Light
We observed that the activation of CCM is lower in cells grown in the presence of acetate in low light (Mnl) than in cells grown in ambient CO2 in low light (Pnl), high light (Phl), or high light in the presence of acetate (Mhl). Indeed, CAH3 expression and pyrenoid formation in Mnl, although somewhat increased compared with high-CO2 conditions, were developed to a significantly lower extent than in Mhl, Pnl, and Phl. Similarly, mitochondria migration toward the cell wall was observed in Pnl, Phl, and Mhl but not in Mnl. Altogether, these observations suggests that the cells in Mnl are not CO2 limited. As the light intensity as well as the air ambient CO2 concentration are the same in Pnl and Mnl, this absence of CO2 limitation in Mnl cells is probably due to the increased CO2 production by Krebs cycle activity in the mitochondria in the presence of acetate (Johnson and Alric, 2012), which in turn can be used directly by Rubisco in the chloroplast. On the other hand, in high light, the amount of CO2 produced in the mitochondria in M is insufficient to sustain photosynthesis as the cells switch on CCM.
The Composition of the Photosynthetic Apparatus Depends on the Carbon Availability
The composition of the photosynthetic apparatus in normal light for cells that are not CO2 limited (meaning M and CO2) is virtually identical in terms of antenna size, pigment composition, PSI/PSII ratio, and NPQ capacity. The main difference is the amount of Chl per cell, which is much higher in M than in P and CO2. The higher number of thylakoids per stack in Mnl might partly account for this difference. It also has been reported that C. reinhardtii cells grown at 120 μE m−2 s−1 are smaller in high-salt medium (HSM; P) than in TAP medium (M) or HSM + CO2 (CO2; Fischer et al., 2006). In this respect, we cannot exclude the possibility that the cell volume is slightly different in our conditions, as a 20% increase in cell diameter is enough to explain the difference in Chl content. In addition, it has been reported that, in mixotrophic conditions, PSII is more photoprotected than in photoautotrophic conditions showing less 1O2 production (Roach et al., 2013). The decrease of 1O2 under mixotrophic growth might be responsible to some extent for the overall higher Chl/cell content in mixotrophic compared with photoautotrophic conditions, as an accumulation of 1O2 is one of the main triggers for the down-regulation of the photosynthetic genes (Erickson et al., 2015).
Cells grown in P at ambient CO2 instead showed a different composition of the photosynthetic apparatus. In particular, the relative amount of PSII to PSI was reduced strongly compared with the other conditions, while the antenna increased. It is plausible that this difference is related to the presence of CCM that results in a higher need for ATP, which is supported by a change in the ratio between linear and cyclic electron transport explaining the higher relative amount of PSI. Indeed, a 33% increased cyclic electron flow was observed for cells grown in low CO2 compared with high CO2 (Lucker and Kramer, 2013), and it was also shown that the intrapyrenoid thylakoids are enriched in PSI (Gunning and Schwartz, 2000; Blanco-Rivero et al., 2012).
The higher amount of LHCII compared with the PSII core in P at ambient CO2 indicates a larger antenna size for PSII in these conditions, although we cannot exclude that part of LHCII acts as an antenna of PSI, as observed in plants (Wientjes et al., 2013a). Interestingly, the higher amount of LHCII is due mainly to an increased amount of LHCBM1. The increase of LHCBM1 in those conditions is particularly interesting because this gene product was reported to be important for the process of NPQ (Elrad et al., 2002; Ferrante et al., 2012). Indeed, Pnl cells also express LHCSR3, which is another protein essential for NPQ (Peers et al., 2009). These cells are able to develop NPQ, although at a lower level than high-light-grown cells. Notably, the same light intensity does not lead to the expression of LHCSR3 in M and CO2 conditions. This suggests that, despite the presence of CCM in Mhl, the cells are already light saturated.
The Acclimation Responses to High Light
For C. reinhardtii cells grown photoautotrophically, a decrease in Chl content upon exposure to high light was reported before (Neale and Melis, 1986; Durnford et al., 2003; Bonente et al., 2011). Here, we show that this decrease occurs in all conditions upon exposure to high light, independent of the carbon availability. This effect is very large in M and P, where the amount of Chl is reduced to half of that in normal light, but even more extreme in CO2, where only 30% of the Chls remain upon exposure to high light.
While the reduction of Chl content seems to be a common strategy, the way this reduction is achieved differs in the different conditions. It was shown previously that no reduction of the antenna proteins occurs upon high-light acclimation (Bonente et al., 2012), while another report (Neale and Melis, 1986) showed a 40% reduction of the functional PSII antennae size in high light. Here, we show that there is no disagreement between these results, as the LHCII reduction depends on the growth conditions. Indeed, we did not observe a reduction of the antenna size in cells grown photoautotrophically at ambient CO2, which is the condition used by Bonente et al. (2012), while a large reduction occurred in photoautotrophic growth at high CO2, which is the condition used by Neale and Melis (1986). Upon exposure to high light, a reduction of the antenna size, although smaller, also was observed in the presence of acetate.
CO2 cells also show a strong reduction in PSI/PSII ratio under high light, while this value decreases only slightly in P at ambient CO2 and even increases in M cells. These results seem to correlate with the presence of CCM and CO2 availability. CO2 cells, which are not limited by CO2 and do not need investments for CO2 concentration, acclimate to high light by strongly modulating the amount of proteins and the composition of the photosynthetic apparatus. This allows them to optimize the light usage, and the result is a very fast growth and a high PSII efficiency (Fv/Fm).
Cells in M and P instead show a much limited capacity of redesigning the photosynthetic apparatus in response to light. In particular, the CO2 limitation in photoautotrophic cells seems to lead to a different high-light acclimation strategy: even in high light, Phl cells maintain a very large antenna, which, in principle, is harmful because it can easily lead to overexcitation, but at the same time it express LHCSR3, thus activating dissipative processes. It is possible that the large antenna is needed to support the relatively low amount of PSII and the high energetic needs of the cells to sustain CCM. In these conditions, it would be more effective for the cell to maintain a large antenna and to activate NPQ when needed. This is in agreement with the increase in P cells of LHCBM1, the LHCBM subunit that was shown to be involved in NPQ (Elrad et al., 2002)
The high-light acclimation strategy of the cells growing in the presence of acetate also differs. In M conditions, the relative amount of PSI increases, probably to support CCM, which was not active in normal light. The antenna size decreases only slightly, and NPQ is activated via the expression of LHCSR3, although at a lower level than in P cells.
Interestingly, a strong increase in the amount of xanthophylls with respect to Chls is observed in all conditions upon exposure to high light, not only in photoautotrophic conditions at ambient CO2 concentration, as shown before (Niyogi et al., 1997a; Baroli et al., 2003; Bonente et al., 2012), but also at high CO2 and in the presence of acetate. This also means that the increase in xanthophylls is independent of the changes in antenna size and, indeed, is even more pronounced in CO2 cells, where the antenna is strongly reduced. This suggests that there is a large pool of xanthophylls free in the membrane, probably acting as 1O2 scavengers (Frank and Cogdell, 1996). The need for it seems to be higher in CO2hl cells, which perform a very low NPQ.
NPQ and the Expression of LHCSR1 and LHCSR3
NPQ in C. reinhardtii was shown to depend on the LHCSR genes (Peers et al., 2009). Two LHCSR gene products are present in C. reinhardtii cells, LHCSR1 and LHCSR3. These two proteins have a high sequence identity, but their RNA transcript levels were shown to be controlled differently: LHCSR3 mRNA was shown to be expressed in high light in low CO2, while LHCSR1 mRNA was expressed in medium light in high CO2 and especially in high light and high CO2 (Yamano et al., 2008). Here, we show, on the one hand, that LHCSR1 seems to be constitutively expressed (with the exception of Pnl), although its expression level increases in high light, as reported previously at the RNA level (Maruyama et al., 2014). On the other hand, the LHCSR3 protein is present in photoautotrophic conditions in ambient CO2 but also in the presence of acetate when the cells are in CO2 limitation (Mhl), a condition where CCM is induced. The amount of NPQ correlates directly with the amount of LHCSR3 in the cells, which follows the trend Phl > Pnl > Mhl. Conversely, LHCSR3 is absent in Mnl, where there is no CO2 limitation. Interestingly, unlike its RNA (Yamano et al., 2008), the LHCSR3 protein is absent in CO2hl. The data at the protein level then show that the expression of LHCSR3 is not triggered directly by light but rather by limitation in energy usage.
CONCLUSION
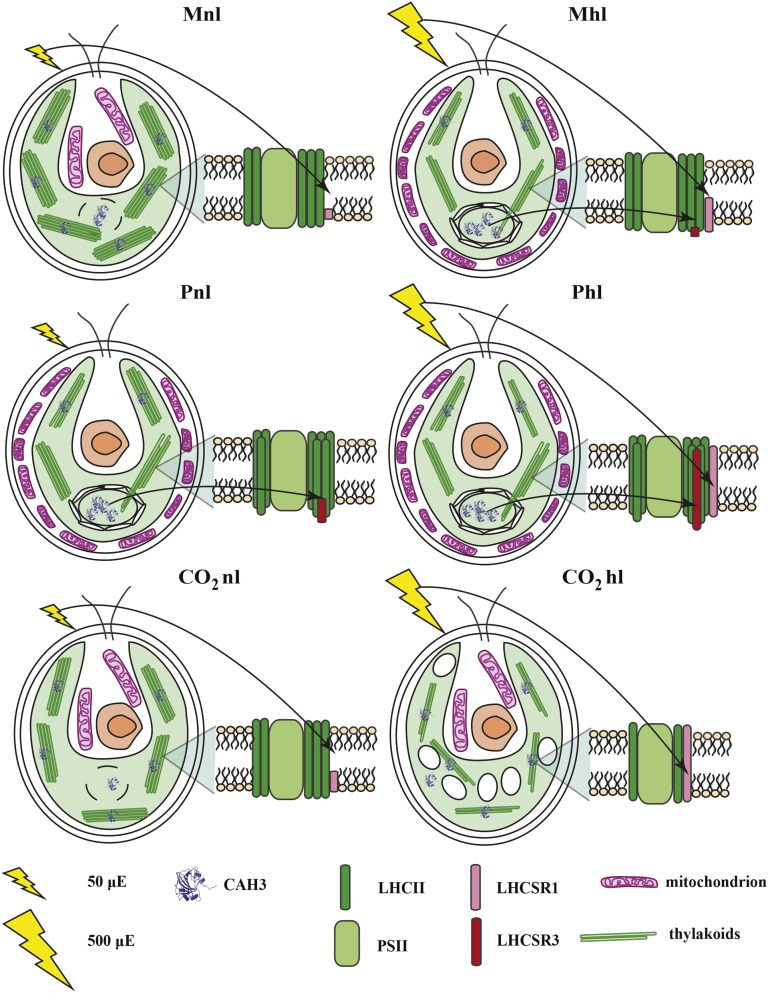
This work shows that C. reinhardtii cells can use a large set of strategies to acclimate to long- and short-term changes in light intensity. The choice of which set of responses is activated depends on the availability of carbon and the resources that need to be used for carbon concentration. This capacity makes C. reinhardtii a highly adaptable organism capable of growing in many different conditions. A summary of the different strategies is shown in Figure 8.
Figure 8.
Carbon supply and PSII photoacclimation cross talk model in C. reinhardtii.
It is particularly interesting to observe that the largest changes upon long-term acclimation occur in phototrophic conditions in the presence of high CO2 concentrations. In these conditions, the cells are able to optimize the light use efficiency by strongly reducing their absorption cross section while at the same time avoiding quenching mechanisms. This is the strategy that has been proposed to increase the photosynthetic efficiency for algae growing in photobioreactors, a strategy that apparently C. reinhardtii also evolved and one that can be optimized for maximal productivity.
MATERIALS AND METHODS
Cell Growth
Chlamydomonas reinhardtii wild-type cells (CC-124) were acclimated to high light (500 μE m−2 s−1) in M, P, and CO2 culture conditions and in normal light (50 μE m−2 s−1) as a control. For CO2 cells, pH was kept constant. The cells were grown for more than six generations in all conditions to reach an acclimated state. Prior to that, cells were grown mixotrophically in TAP medium in normal light and collected in their exponential growth phase at the concentration of 6 × 106 cells mL−1. Cells were pelleted down and resuspended in HSM in the case of photoautotrophic growth. In all conditions, cells were kept in exponential growth phase.
Dual PAM-100 Measurements
Fv/Fm was measured in parallel with NPQ using a Dual PAM-100 (Walz). Cells were dark adapted for 40 min before the measurements. TAP-grown cells were resuspended in HSM before dark adaptation in order to remove acetate, which keeps the plastoquinone pool reduced. NPQ measurements recorded actinic light-induced quenching of fluorescence during 9 and 7 min of quenching recovery after the actinic light was switched off (actinic light, 500 μE m−2 s−1; saturating pulse, 3,000 μE m−2 s−1; 250-ms pulse duration).
PSI/PSII Ratio Measurements
PSI/PSII ratio was measured as described Bailleul et al. (2010) using a JTS-10 pump-probe spectrophotometer. The measurement is based on the ECS of Car absorption caused by single-charge separation. The 520-nm values were corrected by subtracting the signal at 546 nm, to avoid absorption changes caused by energization of the membrane. ECS was first recorded with both PSII and PSI active. Then, PSII was inactivated by applying a saturating pulse in the presence of 200 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea and 1 mm hydroxylamine (inhibitors of PSII at acceptor [secondary electron-accepting plastoquinone of PSII site] and donor [oxygen-evolving complex] sides [Nugent et al., 2003], respectively). After that, the PSI response was recorded. To correct for the different electron turnover rates of PSII and PSI induced by a single flash of the xenon lamp, a correction factor of 1.6, obtained upon calibration of the xenon lamp with a laser, was used (Wientjes et al., 2011). Before the measurements, the cells were dark adapted, and TAP-grown cells were resuspended in HSM.
Pigment Analysis
Cells were pelleted down at 2,000g for 2 min at 4°C, the growth medium was removed, and the cells were resuspended in the same volume of 80% acetone to extract the pigments. The samples were vortexed, and the cell debris was pelleted down at 20,000g, 2 min, and 4°C. The pigment composition was analyzed by fitting the spectrum of the 80% acetone-extracted pigments with the spectra of the individual pigments, and HPLC was performed as described previously (Croce et al., 2002).
Total Protein Extract Preparation and Immunoblot Analyses
Total protein extracts were prepared as described by Ramundo et al. (2013). Western-blot analysis was performed as described by Dinc et al. (2014). The primary antibodies (Agrisera) were prepared in phosphate-buffered saline (PBS) salt solution containing 5% nonfat dry milk (Elk) and 0.1% Tween 20 in the following dilutions: CP43 (1:2,500), PSAA (1:1,000), LHCSR3 (1:1,000), LHCBM5 (1:5,000), LHCBM1 (1:2,000), CP29 (1:5,000), CP26 (1:5,000), and CAH3 (1:2,000). Ten micrograms of total protein extracts was loaded per well. To correct for loading errors, protein densities were normalized to the total protein-loading density of Ponceau Red-stained nitrocellulose membranes and analyzed using GelPro31 software.
Calculation of PSII Antenna Sizes
LHCII antennae size calculation is based on Chl a/b ratio values obtained from the fitting of absorption spectra of 80% acetone extracts from the cells and on the PSI/PSII ratio obtained by ECS measurements as described previously (Ünlü et al., 2014). We considered 35 Chl a per PSII monomeric core, 42 Chls (24 Chl a and 18 Chl b) for LHCII trimer (Liu et al., 2004), 226 Chls per PSI (Le Quiniou et al., 2015), 13 Chls per CP29 (nine Chl a and four Chl b; Pan et al., 2011), and 12 Chls per CP26 (eight Chl a and four Chl b) as for a monomeric LHCII. CP29 and CP26 are always in a 1:1 ratio with the core, and we have assumed that no changes in the PSI/LHCI ratio occur in the different conditions. Moreover, a decrease in Lhca content would not affect the LHCII/PSII ratio dramatically because of a much larger Chl content in LHCII than in Lhcas.
Electron Microscopy
C. reinhardtii cells were diluted in a solution containing 2% glutaraldehyde and 2% paraformaldehyde in 0.1 m PBS, pH 7.4, for 1 h at room temperature and then incubated at 4°C overnight. Then, 0.5% tannic acid was added to the cells and incubated for 1 h at room temperature. The cells were then washed five times in 0.1 m PBS buffer and postfixed in a solution of 1% OsO4 in PBS, pH 7.2 to 7.4. The combination of tannic acid/glutaraldehyde/paraformaldehyde followed by osmification increased the staining of the membranes. The samples were washed four times in sodium acetate buffer, pH 5.5, and block stained in 0.5% uranyl acetate in 0.1 m sodium acetate buffer, pH 5.5, for 12 h at 4°C. The samples were dehydrated in graded ethanol (50%, 75%, 95%, 100%, 100%, and 100%, 10 min each), passed through propylene oxide, and infiltrated in mixtures of Epon 812 and propylene oxide 1:1 and then 2:1, for 2 h each. The cells were infiltrated in pure Epon 812 overnight. Embedding was then performed in pure Epon 812, and curing was done in an oven at 60°C for 48 h. Sections of 60 nm thickness (gray interference color) were cut on an ultramicrotome (RMC MTX) using a diamond knife. The sections were deposited on single-hole grids coated with Formvar and carbon and double stained in aqueous solutions of 8% uranyl acetate for 25 min at 60°C and lead citrate for 3 min at room temperature. Thin sections were examined subsequently with a JEOL 100CX electron microscope at different magnifications.
Acknowledgments
We thank Laura M. Roy for helpful suggestions.
Glossary
- NPQ
nonphotochemical quenching
- Chl
chlorophyll
- Car
carotenoid
- CCM
carbon-concentrating mechanism
- TAP
Tris-acetate-phosphate
- ECS
electrochromic shift
- HSM
high-salt medium
- PBS
phosphate-buffered saline
- M
mixotrophic
- P
photoautotrophic
- Mhl
mixotrophic with high light
- Mnl
mixotrophic with normal light
- Pnl
photoautotrophic with normal light
- Phl
photoautotrophic with high light
- CO2
photoautotrophic in ambient air supplemented with 5% CO2
- CO2nl
photoautotrophic in ambient air supplemented with 5% CO2 with normal light
- CO2hl
photoautotrophic in ambient air supplemented with 5% CO2 with high light
Footnotes
This work was supported by the BioSolar Cells open innovation consortium, supported by the Dutch Ministry of Economic Affairs, Agriculture, and Innovation, by the Foundation for Fundamental Research on Matter, by the European Research Council (grant no. ASAP 281341), and by the Netherlands Organization for Scientific Research (CW ECHO grant to R.C.).
Articles can be viewed without a subscription.
References
- Allorent G, Tokutsu R, Roach T, Peers G, Cardol P, Girard-Bascou J, Seigneurin-Berny D, Petroutsos D, Kuntz M, Breyton C, et al. (2013) A dual strategy to cope with high light in Chlamydomonas reinhardtii. Plant Cell 25: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Chow WS, Park YI (1995) The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46: 129–139 [DOI] [PubMed] [Google Scholar]
- Anderson JM, Horton P, Kim E (2012) Towards elucidation of dynamic structural changes of plant thylakoid architecture. Philos Trans R Soc Lond B Biol Sci 367: 3515–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B, Cardol P, Breyton C, Finazzi G (2010) Electrochromism: a useful probe to study algal photosynthesis. Photosynth Res 106: 179–189 [DOI] [PubMed] [Google Scholar]
- Ballottari M, Dall’Osto L, Morosinotto T, Bassi R (2007) Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J Biol Chem 282: 8947–8958 [DOI] [PubMed] [Google Scholar]
- Baroli I, Do AD, Yamane T, Niyogi KK (2003) Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell 15: 992–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardall J. (1981) CO2 accumulation by Chlorella saccharophila (Chlorophyceae) at low external pH: evidence for active transport of inorganic carbon at the chloroplast envelope. J Phycol 17: 371–373 [Google Scholar]
- Berger H, Blifernez-Klassen O, Ballottari M, Bassi R, Wobbe L, Kruse O (2014) Integration of carbon assimilation modes with photosynthetic light capture in the green alga Chlamydomonas reinhardtii. Mol Plant 7: 1545–1559 [DOI] [PubMed] [Google Scholar]
- Berteotti S, Ballottari M, Bassi B (2016) Increased biomass productivity in green algae by tuning non-photochemical quenching. Sci Rep 6: 21339. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Blanco-Rivero A, Shutova T, Román MJ, Villarejo A, Martinez F (2012) Phosphorylation controls the localization and activation of the lumenal carbonic anhydrase in Chlamydomonas reinhardtii. PLoS ONE 7: e49063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonente G, Ballottari M, Truong TB, Morosinotto T, Ahn TK, Fleming GR, Niyogi KK, Bassi R (2011) Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biol 9: e1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M (2012) Acclimation of Chlamydomonas reinhardtii to different growth irradiances. J Biol Chem 287: 5833–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SP, Paget CM, Johnson GN, Schwartz JM (2015) Flux balance analysis reveals acetate metabolism modulates cyclic electron flow and alternative glycolytic pathways in Chlamydomonas reinhardtii. Front Plant Sci 6: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce R, Canino G, Ros F, Bassi R (2002) Chromophore organization in the higher-plant photosystem II antenna protein CP26. Biochemistry 41: 7334–7343 [DOI] [PubMed] [Google Scholar]
- Dinc E, Ramundo S, Croce R, Rochaix JD (2014) Repressible chloroplast gene expression in Chlamydomonas: a new tool for the study of the photosynthetic apparatus. Biochim Biophys Acta 1837: 1548–1552 [DOI] [PubMed] [Google Scholar]
- Dinc E, Tian L, Roy LM, Roth R, Goodenough U, Croce R (2016) LHCSR1 induces a fast and reversible pH-dependent fluorescence quenching in LHCII in Chlamydomonas reinhardtii cells. Proc Natl Acad Sci USA 113: 7673–7678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drop B, Webber-Birungi M, Fusetti F, Kouřil R, Redding KE, Boekema EJ, Croce R (2011) Photosystem I of Chlamydomonas reinhardtii contains nine light-harvesting complexes (Lhca) located on one side of the core. J Biol Chem 286: 44878–44887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drop B, Webber-Birungi M, Yadav SKN, Filipowicz-Szymanska A, Fusetti F, Boekema EJ, Croce R (2014) Light-harvesting complex II (LHCII) and its supramolecular organization in Chlamydomonas reinhardtii. Biochim Biophys Acta 1837: 63–72 [DOI] [PubMed] [Google Scholar]
- Durnford DG, Price JA, McKim SM, Sarchfield ML (2003) Light-harvesting complex gene expression is controlled by both transcriptional and post-transcriptional mechanisms during photoacclimation in Chlamydomonas reinhardtii. Physiol Plant 118: 193–205 [Google Scholar]
- Elrad D, Niyogi KK, Grossman AR (2002) A major light-harvesting polypeptide of photosystem II functions in thermal dissipation. Plant Cell 14: 1801–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel BD, Schaffer M, Kuhn Cuellar L, Villa E, Plitzko JM, Baumeister W (2015) Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography. eLife 4: 1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson E, Wakao S, Niyogi KK (2015) Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J 82: 449–465 [DOI] [PubMed] [Google Scholar]
- Ferrante P, Ballottari M, Bonente G, Giuliano G, Bassi R (2012) LHCBM1 and LHCBM2/7 polypeptides, components of major LHCII complex, have distinct functional roles in photosynthetic antenna system of Chlamydomonas reinhardtii. J Biol Chem 287: 16276–16288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BB, Wiesendanger M, Eggen RI (2006) Growth condition-dependent sensitivity, photodamage and stress response of Chlamydomonas reinhardtii exposed to high light conditions. Plant Cell Physiol 47: 1135–1145 [DOI] [PubMed] [Google Scholar]
- Frank HA, Cogdell RJ (1996) Carotenoids in photosynthesis. Photochem Photobiol 63: 257–264 [DOI] [PubMed] [Google Scholar]
- Gehl KA, Cook CM, Colman B (1987) The effect of external pH on the apparent CO2 affinity of Chlorella saccharophila. J Exp Bot 38: 1203–1210 [Google Scholar]
- Geraghty AM, Spalding MH (1996) Molecular and structural changes in Chlamydomonas under limiting CO2: a possible mitochondrial role in adaptation. Plant Physiol 111: 1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BES, Schwartz OM (2000) Confocal microscopy of thylakoid autofluorescence in relation to origin of grana and phylogeny in the green algae. Aust J Plant Physiol 27: 645 [Google Scholar]
- Harris EH, (2008) Chlamydomonas in the laboratory. In EH Harris, DB Stern, GB Witman, Chlamydomonas Sourcebook, Ed 2, Vol 1. Academic Press, Elsevier, Oxford, UK, pp 241–302 [Google Scholar]
- Heifetz PB, Förster B, Osmond CB, Giles LJ, Boynton JE (2000) Effects of acetate on facultative autotrophy in Chlamydomonas reinhardtii assessed by photosynthetic measurements and stable isotope analyses. Plant Physiol 122: 1439–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PE, Bassi R, Boekema EJ, Dekker JP, Jansson S, Leister D, Robinson C, Scheller HV (2007) Structure, function and regulation of plant photosystem I. Biochim Biophys Acta 1767: 335–352 [DOI] [PubMed] [Google Scholar]
- Johnson X, Alric J (2012) Interaction between starch breakdown, acetate assimilation, and photosynthetic cyclic electron flow in Chlamydomonas reinhardtii. J Biol Chem 287: 26445–26452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H. (2013) Architectural switches in plant thylakoid membranes. Photosynth Res 116: 481–487 [DOI] [PubMed] [Google Scholar]
- Kuchitsu K, Tsuzuki M, Miyachi S (1988) Characterization of the pyrenoid isolated from unicellular green alga Chlamydomonas reinhardtii: particulate form of RuBisCO protein. Protoplasma 144: 17–24 [Google Scholar]
- Lauersen KJ, Willamme R, Coosemans N, Joris M, Kruse O, Remacle C (2016) Peroxisomal microbodies are at the crossroads of acetate assimilation in the green microalga Chlamydomonas reinhardtii. Algal Res 16: 266–274 [Google Scholar]
- Le Quiniou C, Tian L, Drop B, Wientjes E, van Stokkum IHM, van Oort B, Croce R (2015) PSI-LHCI of Chlamydomonas reinhardtii: increasing the absorption cross section without losing efficiency. Biochim Biophys Acta 1847: 458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279: 22866–22874 [DOI] [PubMed] [Google Scholar]
- Li XP, Muller-Moule P, Gilmore AM, Niyogi KK (2002) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99: 15222–15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori N, Roy LM, Opacic M, Croce R (2013) Regulation of light harvesting in the green alga Chlamydomonas reinhardtii: the C-terminus of LHCSR Is the knob of a dimmer switch. J Am Chem Soc 135: 18339–18342 [DOI] [PubMed] [Google Scholar]
- Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 428: 287–292 [DOI] [PubMed] [Google Scholar]
- Lucker B, Kramer DM (2013) Regulation of cyclic electron flow in Chlamydomonas reinhardtii under fluctuating carbon availability. Photosynth Res 117: 449–459 [DOI] [PubMed] [Google Scholar]
- Maruyama S, Tokutsu R, Minagawa J (2014) Transcriptional regulation of the stress-responsive light harvesting complex genes in Chlamydomonas reinhardtii. Plant Cell Physiol 55: 1304–1310 [DOI] [PubMed] [Google Scholar]
- Moroney JV, Ynalvez RA (2007) Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot Cell 6: 1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzo M, Mantelli M, Passarini F, Caffarri S, Croce R, Bassi R (2010) Functional analysis of photosystem I light-harvesting complexes (Lhca) gene products of Chlamydomonas reinhardtii. Biochim Biophys Acta 1797: 212–221 [DOI] [PubMed] [Google Scholar]
- Mussgnug JH, Wobbe L, Elles I, Claus C, Hamilton M, Fink A, Kahmann U, Kapazoglou A, Mullineaux CW, Hippler M, et al. (2005) NAB1 is an RNA binding protein involved in the light-regulated differential expression of the light-harvesting antenna of Chlamydomonas reinhardtii. Plant Cell 17: 3409–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali A, Croce R (2015) Characterization of the major light-harvesting complexes (LHCBM) of the green alga Chlamydomonas reinhardtii. PLoS ONE 10: e0119211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale P, Melis A (1986) Algal photosynthetic membrane complexes and the photosynthesis‐irradiance curve: a comparison of light‐adaptation responses in Chlamydomonas reinhardtii (Chlorophyta). J Phycol 638: 531–539 [Google Scholar]
- Nield J, Kruse O, Ruprecht J, Fonseca P, Büchel C (2000) Three-dimensional structure of Chlamydomonas reinhardtii and Synechococcus elongatus photosystem II complexes allows for comparison of their oxygen-evolving complex organization. J Biol Chem 275: 27940–27946 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Bjorkman O, Grossman AR (1997a) Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell 9: 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Björkman O, Grossman AR (1997b) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA 94: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent JHA, Muhiuddin IP, Evans MCW (2003) Effect of hydroxylamine on photosystem II: reinvestigation of electron paramagnetic resonance characteristics reveals possible S state intermediates. Biochemistry 42: 5500–5507 [DOI] [PubMed] [Google Scholar]
- Pan X, Li M, Wan T, Wang L, Jia C, Hou Z, Zhao X, Zhang J, Chang W (2011) Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat Struct Mol Biol 18: 309–315 [DOI] [PubMed] [Google Scholar]
- Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462: 518–521 [DOI] [PubMed] [Google Scholar]
- Pribil M, Labs M, Leister D (2014) Structure and dynamics of thylakoids in land plants. J Exp Bot 65: 1955–1972 [DOI] [PubMed] [Google Scholar]
- Ramazanov Z, Rawat M, Henk MC, Mason CB, Matthews SW, Moroney JV (1994) The induction of the CO2-concentrating mechanism is correlated with the formation of the starch sheath around the pyrenoid of Chlamydomonas reinhardtii. Planta 195: 210–216 [Google Scholar]
- Ramundo S, Rahire M, Schaad O, Rochaix JD (2013) Repression of essential chloroplast genes reveals new signaling pathways and regulatory feedback loops in Chlamydomonas. Plant Cell 25: 167–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach T, Sedoud A, Krieger-Liszkay A (2013) Acetate in mixotrophic growth medium affects photosystem II in Chlamydomonas reinhardtii and protects against photoinhibition. Biochim Biophys Acta 1827: 1183–1190 [DOI] [PubMed] [Google Scholar]
- Sager R, Palade GE (1957) Structure and development of the chloroplast in Chlamydomonas. I. The normal green cell. J Biophys Biochem Cytol 3: 463–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LX, Hall M, Funk C, Schröder WP (2012) Photosystem II, a growing complex: updates on newly discovered components and low molecular mass proteins. Biochim Biophys Acta 1817: 13–25 [DOI] [PubMed] [Google Scholar]
- Shimoni E, Rav-Hon O, Ohad I, Brumfeld V, Reich Z (2005) Three-dimensional organization of higher-plant chloroplast thylakoid membranes revealed by electron tomography. Plant Cell 17: 2580–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutova T, Kenneweg H, Buchta J, Nikitina J, Terentyev V, Chernyshov S, Andersson B, Allakhverdiev SI, Klimov VV, Dau H, et al. (2008) The photosystem II-associated Cah3 in Chlamydomonas enhances the O2 evolution rate by proton removal. EMBO J 27: 782–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinetova MA, Kupriyanova EV, Markelova AG, Allakhverdiev SI, Pronina NA (2012) Identification and functional role of the carbonic anhydrase Cah3 in thylakoid membranes of pyrenoid of Chlamydomonas reinhardtii. Biochim Biophys Acta 1817: 1248–1255 [DOI] [PubMed] [Google Scholar]
- Stauber EJ, Busch A, Naumann B, Svatos A, Hippler M (2009) Proteotypic profiling of LHCI from Chlamydomonas reinhardtii provides new insights into structure and function of the complex. Proteomics 9: 398–408 [DOI] [PubMed] [Google Scholar]
- Teramoto H, Nakamori A, Minagawa J, Ono TA (2002) Light-intensity-dependent expression of Lhc gene family encoding light-harvesting chlorophyll-a/b proteins of photosystem II in Chlamydomonas reinhardtii. Plant Physiol 130: 325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto H, Ono T, Minagawa J (2001) Identification of Lhcb gene family encoding the light-harvesting chlorophyll a / b proteins of photosystem II in Chlamydomonas reinhardtii. Plant Cell Physiol 42: 849–856 [DOI] [PubMed] [Google Scholar]
- Tokutsu R, Kato N, Bui KH, Ishikawa T, Minagawa J (2012) Revisiting the supramolecular organization of photosystem II in Chlamydomonas reinhardtii. J Biol Chem 287: 31574–31581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokutsu R, Minagawa J (2013) Energy-dissipative supercomplex of photosystem II associated with LHCSR3 in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 110: 10016–10021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ünlü C, Drop B, Croce R, van Amerongen H (2014) State transitions in Chlamydomonas reinhardtii strongly modulate the functional size of photosystem II but not of photosystem I. Proc Natl Acad Sci USA 111: 3460–3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarejo A, Shutova T, Moskvin O, Forssén M, Klimov VV, Samuelsson G (2002) A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J 21: 1930–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wientjes E, Van Amerongen H, Croce R (2013a) LHCII is an antenna of both photosystems after long-term acclimation. Biochim Biophys Acta 1827: 420–426 [DOI] [PubMed] [Google Scholar]
- Wientjes E, Van Amerongen H, Croce R (2013b) Quantum yield of charge separation in photosystem II: functional effect of changes in the antenna size upon light acclimation. J Phys Chem B 117: 11200–11208 [DOI] [PubMed] [Google Scholar]
- Wientjes E, van Stokkum IHM, van Amerongen H, Croce R (2011) Excitation-energy transfer dynamics of higher plant photosystem I light-harvesting complexes. Biophys J 100: 1372–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T, Miura K, Fukuzawa H (2008) Expression analysis of genes associated with the induction of the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol 147: 340–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T, Tsujikawa T, Hatano K, Ozawa S, Takahashi Y, Fukuzawa H (2010) Light and low-CO2-dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol 51: 1453–1468 [DOI] [PubMed] [Google Scholar]