The Scots pine heartwood formation takes place in the summer and is marked by programmed cell death.
Abstract
Scots pine (Pinus sylvestris L.) wood is desired in woodworking industries due to its favorable timber characteristics and natural durability that is contributed by heartwood extractives. It has been discussed whether the Scots pine heartwood extractives (mainly stilbenes and resin acids) are synthesized in the cells of the transition zone between sapwood and heartwood, or if they are transported from the sapwood. Timing of heartwood formation during the yearly cycle has also not been unambiguously defined. We measured steady-state mRNA levels in Scots pine transition zone and sapwood using RNA sequencing. Year-round expression profiles of selected transcripts were further investigated by quantitative RT-PCR. Differentially accumulating transcripts suggest that, of the Scots pine heartwood extractives, stilbenes are synthesized in situ in the transition zone and gain their carbon-skeletons from Suc and triglycerides. Resin acids, on the other hand, are synthesized early in the spring mainly in the sapwood, meaning that they must be transported to the heartwood transition zone. Heartwood formation is marked by programmed cell death that occurs during the summer months in the transition zone.
Wood biosynthesis is a complex process that involves several developmental activities such as cell division, cell expansion, cell wall thickening, and programmed cell death (Plomion et al., 2001). In many, but not all tree species, the final stage in wood development is formation of heartwood (HW). HW is traditionally defined as “the inner layers of wood, which, in the growing tree, have ceased to contain living cells, and in which the reserve materials (e.g. starch) have been removed or converted into heartwood substances”, while sapwood (SW) is defined as “the portion of the wood that in the living tree contains living cells and reserve materials” (IAWA Committee, 1964). Heartwood can also be defined as the region where extractives have accumulated and by this definition it can be metabolically active (Beekwilder et al., 2014; Celedon et al., 2016), although all changes associated with HW formation may not have yet occurred (Taylor et al., 2002).
The biological role of HW is still unclear, but it may be important in long-term resistance against pathogens (Venäläinen et al., 2004). Nevertheless, HW has a clear economic importance due to the natural decay resistance of HW timber in many tree species (Singh and Singh, 2011) and due to the increasing pharmaceutical and biocide applications of HW substances (Kampe and Magel, 2013).
Heartwood can be found in both angiosperm (e.g. Acacia, Catalpa, Juglans, and Robinia) and gymnosperm (e.g. Cryptomeria, Larix, Picea, and Pinus) tree species. It is physiologically inactive, drier compared with the SW, and filled with HW extractives (Taylor et al., 2002). SW, on the other hand, is physiologically active, has high moisture content, and functions in water, nutrient, and sugar transport among the roots and foliage (Taylor et al., 2002).
As the tree grows, SW is converted to HW. This takes place in the narrow transition zone (TZ), the living cells in which reserve materials like starch are consumed. TZ is drier than SW, but not as dry as HW (Bergström, 2003; Hillis, 1987). The width of the TZ varies both within and between tree species and could be influenced by seasonal as well as other environmental aspects. In general, the TZ is about one to three annual rings wide (Hillis, 1987); in Scots pine (Pinus sylvestris L.), it is usually one to two annual rings wide (Gustafsson, 2001).
It is still unclear when and how the initiation of HW formation occurs. Involvement of the plant hormones ethylene and auxin in regulation of the HW formation have been suggested (Nilsson et al., 2002; Shain and Hillis, 1973; Hillis, 1968). Spicer (2005) described HW formation as a form of programmed cell death (PCD), where the organelles of ray parenchyma cells in the TZ are gradually disintegrated. Several studies have shown that the cell wall structure of ray parenchyma cells change and the cells gradually die toward HW at the boundary between SW and HW (Yang et al., 2003; Nakaba et al., 2008; Magel et al., 2001; Nakada and Fukatsu, 2012; von Arx et al., 2015). In addition to plant hormones, Nakada and Fukatsu (2012) suggested that desiccation at the boundary between SW and HW initiates HW formation.
The specific timing of the HW formation is unclear and may certainly be species-specific. Different studies have described the process taking place at different times of the year. HW formation in black locust (Robinia pseudoacacia) took place in autumn, while in radiata pine (Pinus radiata) and black walnut (Juglans nigra), it occurred during the dormancy period in winter (Taylor et al., 2002; Kampe and Magel, 2013). Bergström et al. (1999) concluded that there is no specific time of the year for HW formation in Scots pine. Indeed, the timing of HW formation is not clearly understood nor has been satisfactorily described to date.
Two types of HW formation were distinguished by Magel (2000). In type I, or Robinia-type of HW formation, HW extractives are biosynthesized in situ and accumulate in the TZ. Precursors to the extractives are not present in the aging SW. In type II, or Juglans-type of HW formation, HW extractives are formed through transformation of phenolic precursors by hydrolysis, oxidation, and polymerization in the TZ. In type II HW formation, HW extractive precursors are gradually accumulating in the aging SW (Magel, 2000; Kampe and Magel, 2013). Deposition of HW extractives and PCD marks the end of the HW formation (Spicer, 2005). Many HW extractives are synthesized also in living tissues when trees are under biotic or abiotic stress and thus contribute to the active defense of SW and other living tissues (Chiron et al., 2000b).
In Scots pine, HW extractives consist of the stilbenes pinosylvin (PS) and pinosylvin monomethyl ether (PSME), resin acids, and free fatty acids (Saranpää and Nyberg, 1987). Variation of Scots pine HW phenolic extractive content between tree individuals is wide and strongly correlates with decay resistance of HW (Harju and Venäläinen, 2006; Leinonen et al., 2008). Although extractive content is influenced by the environment (Magel, 2000), it is highly inherited (Fries et al., 2000; Partanen et al., 2011).
It is still unclear whether Scots pine HW extractives are synthesized in the cells of TZ between SW and HW or transported from the SW. Furthermore, the timing of HW formation during the yearly cycle has not been unambiguously defined. In this work we used a transcriptomic approach to characterize changes in gene expression during HW formation, i.e. in TZ compared to SW. Using a set of indicator genes, defined through transcriptomics, we further addressed the question of timing of HW formation by quantitating gene expression in SW and TZ throughout the year in two Scots pine individuals.
RESULTS
Localization of the Transition Zone and Transcript Sampling
Increment cores were pencil-marked in the field for the border between the wet and dry zones and photographed under UV illumination in the laboratory to localize the fluorescing stilbenes. Fluorescence was observed in the whole of the dry region, except for the outermost, approximately one annual-ring-wide, zone (Fig. 1A).
Figure 1.
Localization of TZ from SW to HW in Scots pine. A, The border between wet and dry parts was marked with a pencil upon collection of the increment cores. Stilbenes pinosylvin and its monomethyl ether give strong fluorescence under UV light (above) allowing straightforward visualization of HW extractives. The bright blue stilbene fluorescence was observed in the whole of the dry part except for the outermost approximately 1-year-ring-wide zone next to the pencil mark. To determine the TZ in molecular terms, eight annual ring pairs around the pencil mark (zones 1–8, shown below under ambient light) were sampled and total RNA was extracted. B, RNA yield (calculated based on A260) was comparable from zones 5 to 8, less RNA was obtained from zone 4, and practically none from zones 1 to 3, as expected because the inner part does not contain living cells. C, Semiquantitative RT-PCR for the pine STS transcript was performed for each sample. The outermost dry zone (zone 4) showed strongest STS expression and, thus, was designated as the TZ (yellow triangle). The SW sampling position for RNA-Seq was specified as 4 to 5 annual rings outwards from the TZ (white triangle). Data shown in the figure are from tree 20, sampled in June 2010. See Supplemental Table S1 for other similar samples.
To determine the TZ in molecular terms, increment cores from two trees, collected in June 2010, were sectioned per year ring around the pencil mark (Fig. 1A) and total RNA was extracted. The RNA yield, based on A260, was comparable from zones 5 to 8 and somewhat less from zone 4 (Fig. 1B and Supplemental Table S1). Zones 1 to 3 yielded much less material absorbing at 260 nm, except for the June sample of tree 11 (Supplemental Table S1). These samples were analyzed using a Bioanalyzer and RT-PCR with primers designed for a Scots pine actin gene. Results indicated that the absorbance was not due to RNA (Supplemental Fig. S1), in line with the observations by Nakaba et al. (2008) and with the definition of HW (IAWA Committee, 1964). Semiquantitative RT-PCR for the pine stilbene synthase (STS) encoding transcript was performed for each sample. The outermost dry zone (zone 4) showed strongest STS expression in all sampled series (Supplemental Fig. S2) and was designated as the TZ, well in accordance of the definition by Bergström (2003) and Hillis (1987). The SW sampling position was specified as four or five annual rings outwards from the TZ (Fig. 1A).
To choose an ideal sampling time, we harvested increment cores from two trees during the summer months June, July, and August of 2010. Semiquantitative RT-PCR for the STS transcript was carried out from SW and TZ (Supplemental Fig. S2). The pattern was consistent through these months, showing STS activity in TZ but not in SW. As the earliest sampling month (June) tended to show stronger activity than samples toward the end of summer, it was chosen as the full-scale transcriptome sampling month for the following year.
Scots Pine Transcriptomes
Approximately 30 increment cores (per tree) were drilled from four 46-year-old unrelated Scots pine trees at breast height at the Leppävirta progeny trial stand in June 2011. SW and TZ samples from each tree were pooled for RNA extraction, tested for STS expression (Supplemental Fig. S3), and sequenced using the SOLiD platform. A quantity of 12 million to 17 million paired-end reads were obtained from each RNA sample. All transcriptome libraries were mapped against the Pinus EST collection version 9.0 (c Databases, 2014), the mapping rate being 64% to 66% for each library (Supplemental Table S2). The Pinus EST collection is a combined collection of expressed sequence tags from 36 pine species (P. taeda, P. sylvestris, P. radiata etc.). Sequences from different species show high similarity and their assembly into tentative consensus (TC) sequences has been done as if they originated from a single species (Quackenbush et al., 1999). Therefore, the collection of 77 326 TC sequences in the Pinus EST collection is not an indicator of the pine gene number in any sense, nor do they represent necessarily assemblies that are species-specific, although many assemblies match one-to-one with cDNA molecules isolated from individual species. For example, while TC154538 is 98% identical to the Scots pine STS mRNA sequence S50350, there are altogether 25 STS-encoding TCs matching S50350 for 10% to 100% in length and 88% to 98% for sequence identity. By the nature of the Pinus EST collection, the number of similar TC sequences sharing differential expression has no meaning (only their annotation does), although they may sometimes represent gene family members in Scots pine.
We also mapped the reads to an annotated Trinity (Haas et al., 2013) assembly generated from the RNA sequencing (RNA-Seq) reads themselves after converting them first to nucleotides. While the mapping rate was often higher and fold changes for differentially expressed transcripts in some cases larger, the expression data obtained was very similar to that obtained with Pinus EST collection as reference. We present here results with the Pinus EST collection because it includes transcripts that are not expressed in our samples (e.g. transcripts for resin acid biosynthesis, see below) and the assemblies (TCs) are longer. Although the full genome of Loblolly pine (Pinus taeda) was recently published with draft gene models (Wegrzyn et al., 2014), it performed very poorly as a target for mapping our reads and the mapping rate was only approximately 6% for each library (Supplemental Table S2).
Differential expression analysis was carried out using edgeR (see “Materials and Methods”). A total of 1673 transcripts had statistically significant (false discovery rate, FDR < 0.05) differential expression in TZ compared to SW, 1021 were up-regulated (Supplemental Table S3), and 652 were down-regulated (Supplemental Table S4). Gene ontology (GO) functional enrichment analysis (Supplemental Table S5) indicated that GO categories like biosynthetic and secondary metabolic processes and response to stimulus were significantly overrepresented in the TZ. We focused on pinosylvin biosynthesis (secondary metabolism) and on its upstream pathways, then extended our interest to other processes such as plant hormonal signaling, dehydration, programmed cell death, transcription factors, cell wall modification, and defense.
Metabolic Processes Activated during Heartwood Formation
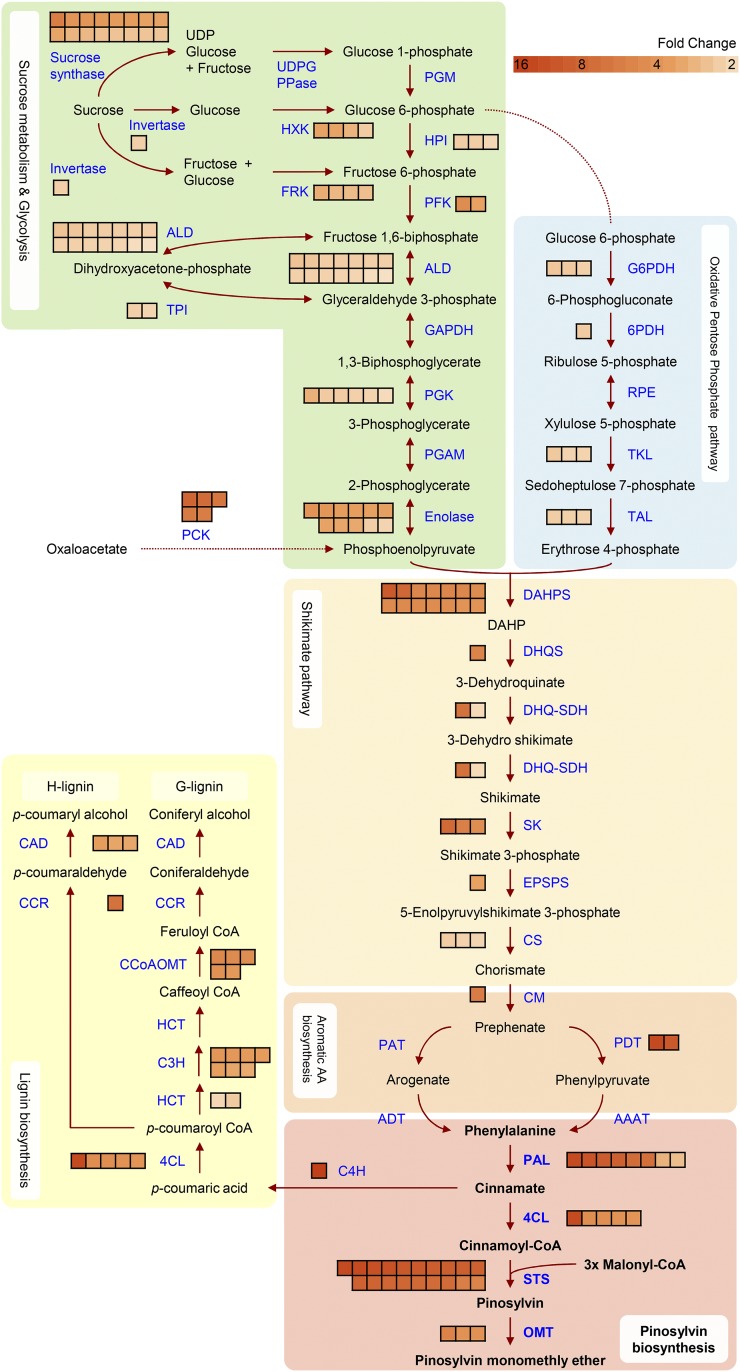
The biosynthesis of stilbenes is initiated by conversion of Phe to cinnamic acid, catalyzed by the enzyme Phe ammonia lyase (PAL). STS converts the activated cinnamic acid (cinnamoyl-CoA) into pinosylvin. We defined the TZ as the zone where STS encoding transcripts are most highly expressed. Congruently, we could observe 5- to 13-fold up-regulation of STS-encoding TC sequences in TZ compared to SW. Our transcriptome data also showed that PAL-encoding transcripts were 7- to 12-fold up-regulated in the TZ. The next enzyme, presumably an acyl-CoA ligase acting on cinnamate, has not been characterized from pine, but some 4-coumarate:CoA ligase (4CL) transcripts were up-regulated with a 3- to 13-fold change. Lignin biosynthesis-related transcripts (including 4CL) were also up-regulated in TZ. In the end of the pathway, pinosylvin is in part methylated to PSME. Chiron et al. (2000a) characterized in Scots pine an O-methyl transferase catalyzing this reaction; however, TC180420 that matches the published PMT sequence (99% identity) was not up-regulated (in fact, very few reads mapped to it). Instead, another O-methyltransferase-encoding TC (TC166778) was up-regulated with a 5-fold change in TZ compared to SW (Fig. 2, Supplemental Table S6).
Figure 2.
Schematic diagram of primary and secondary metabolism pathways activated in the TZ. The arrows in the figure represent enzymatic reactions. Individual boxes next to the arrows represent transcripts encoding the corresponding enzymes, up-regulated in TZ compared to SW. During HW formation, the source of the carbon skeleton is delivered by breaking down mainly Suc, channeled downstream to the shikimate pathway, and finally to the pinosylvin biosynthesis pathway. The upstream and downstream pathways of the pinosylvin biosynthesis were highly up-regulated during HW formation, suggesting that the HW extractive was synthesized in the TZ. In addition, lignin biosynthesis-related transcripts are expressed during HW formation, indicating that lignification of cell wall ray parenchyma cells was taking place in the TZ. UDPG PPase, UDP Glc pyrophosphorylase; HXK, hexokinase; FRK, fructokinase; PGM, phosphoglucomutase; HPI, hexose P isomerase; PFK, phosphofructokinase; ALD, aldolase; TPI, triose P isomerase; GAPDH, glyceraldehyde 3-P dehydrogenase; PGK, phosphoglycerate kinase; PGAM, phosphoglycerate mutase; PCK, phosphoenolpyruvate carboxykinase; G6PDH, Glc 6-P dehydrogenase; 6PDH, 6-phospogluconate dehydrogenase; RPE, ribulose 5-P epimerase; TKL, transketolase; TAL, transladolase; DAHP, 3-Deoxy-d-arabinoheptulosonate-7-P; DAHPS, DAHP synthase; DHQS, 3-dehydroquinate synthase; DHQ-SDH, dehydroquinate dehydratase-shikimate dehydrogenase; SK, shikimate kinase; EPSPS, 5-enolpyruvylshikimate 3-P synthase; CS, chorismate synthase; CM, chorismate mutase; AA, amino acid; PAT, prephenate aminotransferase; ADT, arogenate dehydratase; PDT, prephenate dehydratase; AAAT, aromatic amino acid aminotransferase; PAL, Phe ammonia lyase; 4CL, 4-coumarate:CoA ligase; STS, stilbene synthase; OMT, O-methyltransferase; C4H, transcinnamate 4-monooxygenase; HCT, hydroxycinnamoyl-CoA:shikimate/quinate hydroxycinnamoyltransferase; C3H, p-coumaroyl shikimate/quinate 3′-hydroxylase; CCoAOMT, caffeoyl-CoA O-methyltransferase; CCR, cinnamoyl-CoA reductase; CAD, cinnamyl-alcohol dehydrogenase.
Upstream of stilbene pathway is the shikimate pathway that provides Phe for stilbene biosynthesis, as well as for the phenylpropanoid pathway in general (including lignin and lignan biosynthesis) and for protein synthesis. The shikimate pathway starts with the conversion of phosphoenolpyruvate and erythrose 4-P to 3-deoxy-d-arabino-heptulosonate-7-P (DAHP) by the enzyme DAHP synthase (DAHPS). DAHPS is thought to be the rate-controlling enzyme of the shikimate pathway (Tzin et al., 2012), and we observed 4- to 7-fold up-regulation in TZ compared to SW for TC sequences encoding DAHPS. Several other TC sequences encoding enzymes of the shikimate pathway were up-regulated in TZ, including those for 3-dehydroquinate synthase (4-fold) and shikimate kinase (4- to 7-fold) and finally chorismate mutase and prephenate dehydratase, 5- and 10- to 13-fold, respectively (Fig. 2 and Supplemental Table S6).
Further upstream, transcripts encoding phosphoenolpyruvate carboxykinase were up-regulated 5- to 8-fold in TZ. This enzyme converts oxaloacetate to phosphoenolpyruvate in gluconeogenesis. TC sequences for enzymes involved in glycolysis and the oxidative pentose phosphate pathway also showed up-regulation and finally transcripts for Suc synthase were induced with a 2- to 5-fold change (Fig. 2 and Supplemental Table S6). Activity of Suc synthase is often taken as indication of metabolic activity (Winter and Huber, 2000), showing that metabolite production is taking place in the TZ before it is programmed for cell death (see below). It further indicates that breakdown of sugars is then channeled to the downstream processes like stilbene biosynthesis both for building the carbon backbone and for providing metabolic energy for the process. Our transcriptome data show that stilbene biosynthesis definitely takes place in situ in the TZ.
Plant Hormones in the Transition Zone
The plant hormones ethylene and auxin have been suggested to be involved in HW formation, with their ratio being particularly important (Hillis, 1968; Shain and Hillis, 1973). Indeed, for TCs encoding 1-aminocyclopropane-1-carboxylate oxidase (ACO), we observed the highest differential expression ratio between SW and TZ, an induction of 36- to 61-fold (Supplemental Table S6). ACC synthase (ACS) was not induced, but S-adenosyl-Met (SAM) synthase was expressed 3 times to 9 times more strongly in TZ than in SW. Note that SAM synthase provides substrate also for methylation of pinosylvin. Transcripts relating to auxin biosynthesis or transport were not up- or down-regulated in TZ, but a group of auxin response and auxin related or -induced transcripts were down-regulated with an average of 2-fold change between TZ and SW (Supplemental Table S6). Gibberellins have not been suggested to be involved in HW formation. Beauchamp (2011) was able to detect the inactive precursor GA24 in both SW and TZ, but not the active forms or the 2-hydroxylated inactive forms. Still, one of the induced transcripts encoding GA 2-oxidase (TC171916) was 16 times up-regulated in TZ compared to SW.
Dehydration and Programmed Cell Death during Heartwood Formation
Moisture content in the TZ is low but still higher than in the HW. We observed that a group of desiccation-related (DPR) transcripts was up-regulated with a fold change of 23 to 31 in TZ (Supplemental Table S6). DPR transcripts have been suggested to be involved in tolerance of desiccation in plants (Zha et al., 2013; Piatkowski et al., 1990). A group of aquaporin-like transcripts, on the other hand, were down-regulated (Supplemental Table S6). Aquaporins are involved in regulation of cell-to-cell water transport and in maintaining water homeostasis in plants (Bienert and Chaumont, 2011; Hachez et al., 2006). Up-regulation of the DPR and down-regulation of aquaporin transcripts may relate to the fact that water is withdrawn from the TZ during HW formation. Transcripts encoding an S1-like nuclease and a bifunctional nuclease (BFN) were highly induced in TZ with 13- and 20-fold change (Supplemental Table S6) compared to SW. Both nucleases have been described to be involved in PCD (Bollhöner et al., 2012; Farage-Barhom et al., 2008). Up-regulation of these transcripts indicated that PCD was taking place in TZ.
Cell Wall Modification during Heartwood Formation
Cell wall modification and lignification-related enzymes were up-regulated during HW formation. Lignification has been shown to be part of the HW formation process in Scots pine (Bergström, 2003). Transcripts encoding enzymes involved in lignification were up-regulated in TZ at an average of 4- to 16-fold (Fig. 2, Supplemental Table S6). Transcripts relating to cell wall carbohydrate modification were expressed at an average 12-fold higher in TZ than in SW (Supplemental Table S6).
Transcription Factors in the Transition Zone
Two TC sequences encoding transcription factors, a MYB-like transcription factor and a NAC domain-containing protein, were expressed 12- and 6-fold higher, respectively, in TZ compared to SW (Supplemental Table S6). Transcription factors of the MYB family are often key regulators of secondary metabolite pathways (Dubos et al., 2010). NAC domain proteins are involved in secondary cell wall biosynthesis and PCD (Nakano et al., 2015; Bollhöner et al., 2012).
Resin Acids and Plant Defense
Resin acids are conifer defense barriers to biotic challenges. They can be found in the bark, in SW and in HW. According to Harju et al. (2003), HW contains four times more resin acids on a volume basis than SW. It was therefore surprising that transcripts involved in terpenoid biosynthesis and its upstream pathways were neither up- nor down-regulated in the June transcriptome data. In fact, very few reads mapped to these TC sequences, with an average 95%, and up to 99%, identity with the published diterpene synthase (Ro and Bohlmann, 2006) and abietadienol/abietadienal oxidase (CYP720B; Geisler et al., 2016) genes, indicating that constitutive synthesis of resin acids was not taking place either (Supplemental Table S7). On the other hand, a set of pathogenesis-related transcripts were up-regulated 5- to 20-fold (Supplemental Table S6).
A set of unknown transcripts was also up- and down-regulated in our transcriptome data, sometimes up to 12-fold (Supplemental Table S6).
Year-round Expression Profiles
The Scots pine RNA-Seq data collected in June shed light on the metabolic activities in the TZ during active growth of the tree. It did not answer the question of when, during the yearly cycle, HW formation is most active, and left completely open both the time and place of resin acid biosynthesis. Based on the June results, we selected transcripts up-regulated in TZ and apparently related to HW formation as well as to resin acid biosynthesis for a year-round study using quantitative RT-PCR (qPCR; Supplemental Table S8).
We studied the expression profiles in the SW and TZ of increment cores of two trees, harvested monthly in Punkaharju from March 2011 to March 2012. The January 2012 increment cores were lost due to fragmentation of the frozen tissue upon removal from the drill core.
PCR efficiency was determined for each primer pair used in the analysis, so the qPCR results also reflect relative transcript levels between different genes. Total RNA yields of the test trees varied, but not systematically throughout the year (Supplemental Table S9). Level of actin and histone transcripts compared to rRNA (i.e. total RNA amount) in the extracted RNA samples varied remarkably little (Supplemental Table S10). Level of each selected transcript was expressed relative to the geometric mean of the actin and histone transcripts, and is shown throughout the year in Figures 3 and 4.
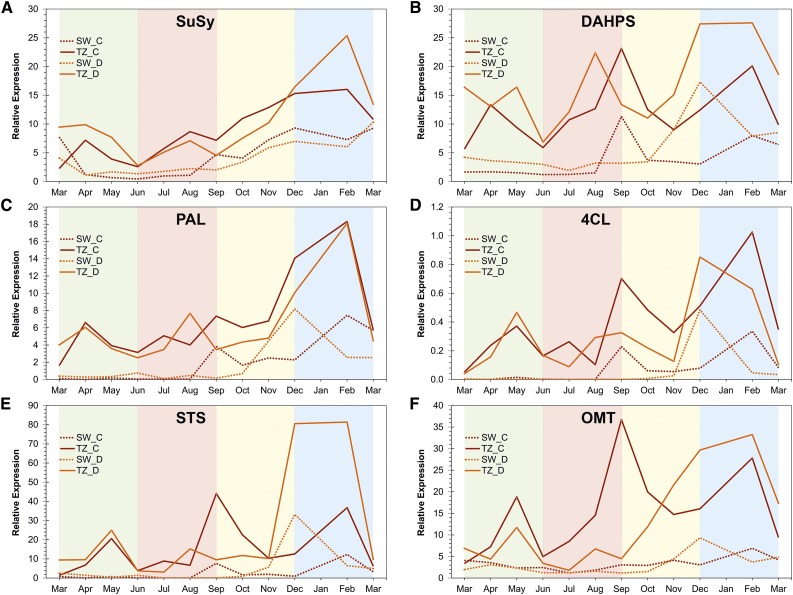
Figure 3.
Expression profile of A, Suc synthase; B, DAHP synthase; C, Phe ammonia lyase; D, 4-coumarate:CoA ligase; E, stilbene synthase; and F, O-methyltransferase transcripts in the TZ and SW of trees C and D from March 2011 to March 2012. Metabolic activities were initiated in the spring, showed a trough in June, and recovered during the summer months. In general, transcripts related to pine stilbene biosynthesis and its upstream pathways shared a similar profile throughout the year. The green, red, yellow, and blue backgrounds indicate the spring, summer, autumn, and winter months, respectively. The y axis is the expression relative to the geometric mean of actin and histone transcripts.
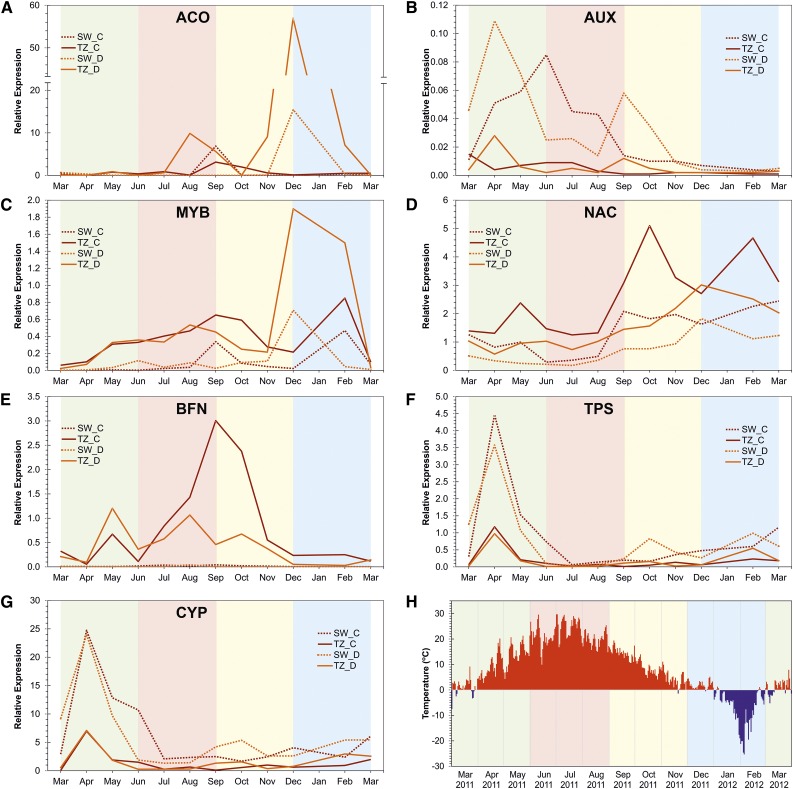
Figure 4.
Expression profile of A, ACC oxidase; B, auxin responsive protein; C, a MYB-like transcription factor; D, a NAC domain transcription factor; E, the PCD marker BFN; F, diterpene synthase; G, abietadienol/abietadienal oxidase transcripts in the TZ and SW of tree C and D; and H, the daily maximum temperature in Punkaharju where sampling took place from March 2011 to March 2012. The year-round qPCR result showed that ACC oxidase very strongly reacted to as yet undefined stimuli at different times during the year. On the other hand, the expression level of the auxin responsive transcript in the TZ was generally low and ceased in the winter. Expression profile of the MYB-like and NAC domain protein-encoding transcripts was higher in TZ in both trees, increased gradually through the summer months, and further accumulated in the winter. The expression level of BFN transcript of both trees showed an increase in May in the TZ, was maintained over the summer months, and then gradually ceased in late autumn. BFN transcript was not expressed in SW at any time. The transcription level of diterpene synthase and abietadienol/abietadienal oxidase was always greater in the SW than in the TZ in both trees. Both transcripts (in SW and TZ) started to rise in mid-spring, dropped in late spring, decreased throughout the summer, and ceased in winter. The green, red, yellow, and blue backgrounds indicate the spring, summer, autumn, and winter months, respectively. The y axis is the expression relative to the geometric mean of actin and histone transcripts.
We determined by qPCR expression levels of the selected transcripts also in the SW and TZ RNA samples of the four trees from which the RNA-Seq data were generated and differential expression was calculated. The two data sets are in good agreement (Supplemental Table S11).
Pinosylvin Biosynthesis Pathway Transcripts Showed a Concerted Expression Profile
The year-round expression profiles of the two analyzed trees varied, so they were not averaged but are shown separately. Still, many processes important for HW formation shared correlated patterns (Figs. 3 and 4). The expression of the stilbene biosynthesis pathway transcripts (PAL, 4CL, STS, OMT) showed statistically significant correlation (Fig. 5). Shikimate pathway (DAHPS) followed the stilbene pathway closely, as did Suc synthase transcript levels. Transcript levels for all these markers in TZ increased in the spring (Fig. 3). Subsequently, they all dropped in June and showed recovery during the summer months. PAL, 4CL, STS, and OMT transcripts were expressed at very low level in the SW throughout the summer months. The expression level of these transcripts differed between the two trees in autumn months and, interestingly, the SW followed the pattern although at a several-fold lower level. The level of all of these transcripts showed increase during the winter months in both the TZ and the SW, even when the temperature was constantly below the freezing point (Fig. 4H).
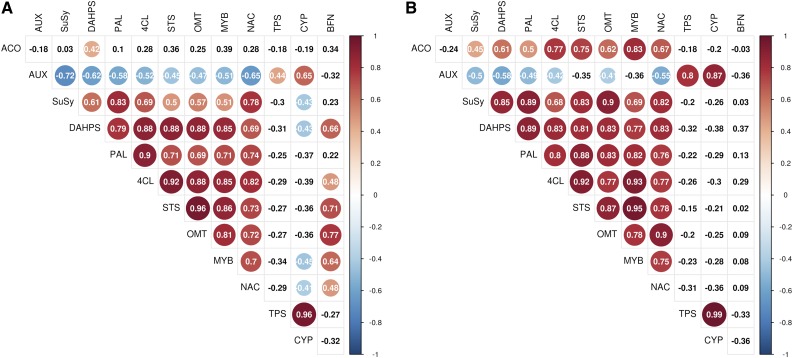
Figure 5.
Pearson’s correlation matrix between the selected transcripts in the year-round qPCR expression study of (A) tree C and (B) tree D. Statically significant (P < 0.05) Pearson’s r values are highlighted. The shikimate and stilbene pathways show high correlation. The MYB-like and NAC domain transcription factors are strongly correlated with the stilbene pathway. Diterpene synthase and abietadienol/abietadienal oxidase (CYP720B) are strongly correlated to each other.
Expression Profiles of Plant Hormone-Related Transcripts Varied
Many studies have concluded that ethylene would play an important role in HW formation. In the June 2011 RNA-Seq data we observed that ACO was the number-one differentially expressed transcript in TZ, compared to SW. The year-round qPCR data showed that ACO very strongly reacted to as yet undefined stimuli throughout the sampling scenes, sometimes more strongly in SW and very differently in the two trees sampled (Fig. 4A). The observed differential expression of ACO in the RNA-Seq samples seems not to be a typical pattern during HW formation.
The expression level of the auxin-responsive (AUX) transcript in the TZ was generally very low, and ceased completely in the winter (Fig. 4B). This transcript was chosen because it was down-regulated in TZ in the RNA-Seq data, and it indeed showed lower expression in TZ throughout the year.
The Two Transcription Factors Shared a Similar Profile
Expression of the MYB-like and NAC domain-containing protein-encoding transcripts were higher in TZ in both trees throughout the year (Fig. 4, C and D). In both trees their expression pattern correlated very clearly with the stilbene synthase pathway transcripts as well as those for DAHPS and Suc synthase (Fig. 5).
Programmed Cell Death
A very clear feature of the PCD-related BFN transcript was that it was absent in SW. In both trees, the transcript in TZ showed increased accumulation in May, a trough in June, and gradual increase through the late summer, in synchrony with the stilbene pathway. BFN was not following the increased stilbene biosynthesis peaks in late autumn and winter, suggesting that different signals were inducing the stilbene pathway at that time (Fig. 4E).
Expression Profiles of Resin Acid Biosynthesis Genes
Transcripts involved in terpenoid biosynthesis were expressed at low level in the June transcriptome data, both in SW and TZ. Excitingly, we noticed that the transcript levels of diterpene synthase and abietadienol/abietadienal oxidase (CYP720B) encoding transcripts (Fig. 4, F and G) in both SW and TZ started to rise in early spring, dropped in late spring or early summer, and remained low during the summer, autumn, and winter. The expression level for these transcripts was greater in the SW than in the TZ. The pattern was similar in both trees.
Correlation between Months of the Year
We also calculated correlations between each sampling month for the expression pattern of the selected genes (Supplemental Fig. S4). We expected that highest correlation would be observed between successive months, and the TZ samples of both trees followed this pattern roughly. On the contrary, the SW samples showed strong correlations across the months, i.e. between months that are far apart in the yearly cycle (Supplemental Fig. S4). Interestingly, this means that the TZ follows seasonal changes for its gene expression patterns much more strongly than the SW does.
DISCUSSION
Scots pine wood is extensively used as sawn timber because of its strength, straightness, and visual appearance. The HW of Scots pine is particularly valued for properties such as its dimensional stability and natural durability. Extractives of HW give, e.g. color and low hygroscopicity to HW timber and are key determinants of the passive defense, i.e. resistance against degradation of wood by microorganisms (Nakada and Fukatsu, 2012; Harju and Venäläinen, 2006; Leinonen et al., 2008). Sampling of Scots pine transcriptomes in the SW and TZ to HW by RNA sequencing in June and qPCR throughout the year allowed us to identify the place and time during the yearly cycle when these important components are synthesized and HW is formed.
From Carbon Skeletons to the Heartwood Phenolics
During HW formation, reserves of the SW are consumed, in Scots pine mainly starch and triglycerides (Magel, 2000; Bergström, 2003; Nakada and Fukatsu, 2012). Magel (2000) concluded that the most important contributor for Suc cleavage was Suc synthase followed by invertase. Up-regulation of transcripts in TZ for both of these enzymes was observed in our data. In addition, transcripts relating both to glycolysis and oxidative pentose P pathway were expressed at an average 2-fold more in TZ than SW, suggesting that the breakdown of sugars is channeled downstream for synthesis of precursors to the shikimate pathway, and finally to the pinosylvin pathway.
Phosphoenolpyruvate carboxykinase, an enzyme that converts oxaloacetate to phosphoenolpyruvate (Bowsher et al., 2008), was found up-regulated strongly in TZ. In plants, oxaloacetate is generated from malate by malate dehydrogenase in the cytosol. Malate is synthesized from breakdown of plant triglycerides via the glyoxylate pathway in plant fat storage tissues (glyoxysomes). Study of Saranpää and Höll (1989) and Bergström (2003) showed that amount of free fatty acids was increased in the TZ and HW but not in SW. In addition, lipophilic droplets were observed in cells of TZ associated with HW formation (Taylor et al., 2002; Kampe and Magel, 2013). Up-regulation of phosphoenolpyruvate carboxykinase may indicate that triglycerides are used not only as a direct source of HW extractives (free fatty acids), but also as an energy and carbon source for stilbenes.
During HW formation, the whole shikimate pathway is expressed at an average of 2-fold higher in TZ and the rate-limiting enzyme DAHPS up to 7-fold higher. This pathway connects the primary metabolism (aromatic amino acid biosynthesis) to the phenylpropanoid pathway, including the stilbene and lignin biosynthesis pathways. Both of these branches are active in TZ, the stilbene pathway being responsible for generation of the pine-specific HW extractives pinosylvin and its monomethyl ether. Pinosylvin lacks hydroxylation of the Phe-derived aromatic ring, similar to cinnamic acid. Furthermore, the Scots pine STS prefers cinnamoyl-CoA to 4-coumaroyl-CoA (Fliegmann et al., 1992; Kodan et al., 2002). Only a few plant 4CLs activate cinnamic acid (Kumar and Ellis, 2003), and in some plant species the activity of enzymes with a more distant relationship to 4CL have been shown to be involved in cinnamoyl-CoA formation (Gaid et al., 2012; Klempien et al., 2012). We observed a possible 4CL-like candidate from our transcriptome data that might be specific to pine pinosylvin biosynthesis during HW formation. Nevertheless, characterization of its enzymatic specificity is needed to address this hypothesis.
The end product of pine stilbene biosynthesis is PSME, catalyzed by PMT. A Scots pine PMT was purified and partially characterized by Chiron et al. (2000a). However, no transcript encoding this PMT was up-regulated in our transcriptome data, but instead another O-methyltransferase-encoding transcript was strongly induced in TZ. This finding suggests that the PMT characterized by Chiron et al. (2000a) might not be the correct Scots pine PMT for pine HW extractive biosynthesis.
The strong up-regulation of transcripts encoding enzymes leading to stilbene biosynthesis in TZ indicates that these HW extractives are synthesized in situ in the TZ, gaining the carbon skeleton from Suc. In this respect, Scots pine HW formation is of type I, or the Robinia-type (Magel, 2000).
Biosynthesis of Heartwood Resin Acids
In addition to stilbenes and free fatty acids, Scots pine HW contains, abundantly, resin acids (Harju et al., 2002; Fries et al., 2000). Contrary to expectations, resin acid biosynthesis was neither up- nor down-regulated in TZ compared to SW. Lorio (1986) proposed the concept of a balance between growth and differentiation for resin acid biosynthesis in southern pines. He suggested that during the growing season, the sink of photosynthates is preferentially the growth process, and only after the growing period are the resources available for resin acid production. The year-round expression profile of transcripts encoding diterpene synthase and abietadienol/abietadienal oxidase (CYP720B) showed very low expression during summer months but a strong peak in early spring. Throughout the year, resin acid biosynthesis-related transcripts accumulated more in SW than in TZ, although resin acids accumulate four times higher in HW than in SW (Venäläinen et al., 2003). Resin acids do not seem to be biosynthesized in situ in the TZ as stilbenes are, but instead they get loaded from SW to HW during HW formation. Therefore, from the resin acid biosynthesis point of view, Scots pine HW is of type II, or the Juglans type (Magel, 2000).
Balance between Ethylene and Auxin
The plant hormones ethylene and auxin have been suggested to be closely related to HW formation. A recent study by Beauchamp (2011) reported that auxin concentration was either reduced or below level of detection in the TZ, in line with the description of Hillis (1968) that depletion of auxin content is associated with HW formation. Shain and Hillis (1973) suggested that the balance between ethylene and auxin during HW formation is important. Indeed, transcripts encoding ACO were most strongly differentially expressed in TZ compared to SW. On the other hand, transcripts for ACS, the rate-limiting enzyme for ethylene biosynthesis in most plants (Woeste et al., 1999; Hudgins et al., 2006), were not induced. This may be a reflection of the fact that ACS regulation predominantly takes place at protein level (Chae and Kieber, 2005), which we did not address in our experiments. Transcripts for SAM synthase, the second substrate for ACS (Yoon and Kieber, 2013), were induced in TZ, but SAM is also needed for pinosylvin biosynthesis. The importance of ethylene in HW formation, at least as a sufficient inducer, can, however, be questioned because ACO was expressed erratically with little correlation between trees or zones. Although we designed repeated sampling of the trees to avoid wound induction (Supplemental Fig. S5), we cannot exclude that this caused a reaction in ACO. Transcripts related to auxin biosynthesis were not differentially expressed between SW and TZ. Again, regulation of auxin levels prominently takes place after biosynthesis of the hormone (Woodward and Bartel, 2005). Nevertheless, several auxin response transcription factors were down-regulated in TZ.
Transcriptional Control in the Transition Zone
Two transcription factor-encoding transcripts, for a NAC domain and a MYB-like protein, were found to be significantly up-regulated in the TZ and their expression correlated with the stilbene pathway transcripts throughout the year in both sampled trees. NAC domain transcription factors have been reported to be involved in developmental processes, for example, in secondary cell wall biosynthesis as well as biotic and abiotic stresses, acting as master switches to regulate downstream genes (Nakano et al., 2015). The up-regulated NAC domain protein, however, is not similar to the white spruce (Picea glauca) secondary cell wall biosynthesis NAC transcription factors, encoded by PgNAC4 and PgNAC7 (Duval et al., 2014). The MYB-like transcription factor is also not particularly similar to the loblolly pine (Pinus taeda) secondary cell wall biogenesis-related MYB transcription factors that were isolated from differentiating xylem, PtMYB1 (Patzlaff et al., 2003b) and PtMYB4 (Patzlaff et al., 2003a). Still, as MYB transcription factors are in many cases main regulators of secondary metabolism in plants, involvement of the up-regulated MYB transcript as well as the NAC transcript in stilbene or lignin biosynthesis deserves a closer look.
Cells in the Transition Zone Prepare for Death
Pine HW is dead tissue, and during HW formation the cells in the TZ are not only responsible for filling the HW with extractives, but also prepare for their own death.
Moisture content in the TZ is lower than in SW (Hillis, 1987; Bergström, 2003). The up-regulation of transcripts encoding desiccation-related proteins in TZ could result from the fact that water is withdrawn from the cells. The role of plant DPR proteins may help the cells to tolerate the dehydration condition and allow them to continue with the still necessary physiological processes during HW formation. Down-regulation of a group of aquaporin-like proteins may also relate to dehydration in the TZ.
Transcripts encoding enzymes necessary for lignification, as well as groups of cell wall polysaccharide modifying enzymes, were expressed in the TZ during HW formation. The cell walls of ray parenchyma cells in the TZ are indeed undergoing lignification, as observed by Bergström (2003) and Yamamoto (1982).
A chitinase and set of plant pathogenesis-related transcripts were up-regulated in the TZ. In general, these plant defense-related proteins are induced in response to biotic and abiotic stresses (van Loon, 1997). Chitinases have been reported to be involved not only in plant defense but also in developmental processes, such as secondary cell wall lignification, and they are induced by plant hormones (Grover, 2012). Yoshida et al. (2012) and Yang et al. (2004) also reported that a set of pathogenesis-related proteins are highly expressed in the TZ of Cryptomeria and Robinia, respectively.
The storage compounds in the TZ are depleted and consumed, and the parenchyma cell walls are undergoing modification. At the end of the process, all physiological processes will eventually cease.
Programmed Cell Death Is the Key for Heartwood Formation
HW formation can be seen as a form of tissue senescence (Spicer, 2005; Kampe and Magel, 2013). The BFN is an endonuclease similar to S1-like nucleases (Farage-Barhom et al., 2008), is induced during senescence, and is involved in plant developmental PCD (Farage-Barhom et al., 2008; Bollhöner et al., 2012). Farage-Barhom et al. (2008) demonstrated that the Arabidopsis BFN1 moves to the nucleus during leaf senescence and the protein was detected from fragmented nuclei in the late stage of leaf senescence (Bollhöner et al., 2012). Degradation of nuclei of ray parenchyma cells in the TZ has been observed in past studies (Taylor et al., 2002; Spicer, 2005; Nakada and Fukatsu, 2012). Thus, the up-regulation of the BFN transcript in TZ, compared to SW, is in line with the fact that PCD is involved in HW formation. Expression of the PCD transcript BFN in TZ appears to be a key marker to distinguish developmentally programmed HW formation in Scots pine from stress-induced extractive biosynthesis.
Timing of Heartwood Formation in Scots Pine
The year-round expression profiles of selected transcripts related to primary and secondary metabolic pathways, the pinosylvin biosynthesis pathway, plant hormones, transcription factors, programmed cell death, and resin acid biosynthesis pathway were investigated with the qPCR approach to understand whether there is a preferential time during the year when HW is formed.
As a general observation, we noticed that the expression levels in each tested trees varied in the autumn and in the winter, but shared a pattern during the summer months. It is apparent that the trees are responding both to shared developmental programs (e.g. HW formation), as well as to local challenges that may be different for each tree individual, responded to by different genotypes. The two trees analyzed were growing close to each other, but were not related.
The year-round expression profiles of the two transcripts related to resin acid biosynthesis were well in synchrony. The genes were expressed and, presumably, synthesis proceeded in early spring and was practically shut down for the summer months, in accordance to the concept suggested by Lorio (1986). Resin acid biosynthesis transcripts were expressed in both SW and TZ, but more strongly in SW, indicating that the compounds loaded in HW are not mainly synthesized in situ in the TZ.
The ACO transcripts, strongly differentially expressed in TZ compared to SW and related to the suggested ethylene involvement in HW formation, were expressed at very high levels in both SW and TZ in one of the two trees. This may reflect a challenge that this tree met in the late summer. Usually, however, regulation of ethylene synthesis is addressed to protein level control of ACS, not ACO. The auxin responsive transcript was expressed in the spring and summer in SW, with low levels in TZ throughout the year.
Transcripts responsible for stilbene biosynthesis, our signature pathway for HW formation, were always more strongly present in TZ than in SW. There seems to be a shared peak of activity in early summer, somewhat later than that for resin acid biosynthesis. In late summer and in winter the two trees were behaving nonsynchronously, but the pathway, including its upstream components of sugar metabolism and aromatic acid biosynthesis, were reacting in concert. Expression of the two transcription factor transcripts investigated strongly correlated with expression of the stilbene pathway genes. An interesting peculiarity is that transcripts for the stilbene pathway enzymes, and for the two transcription factors, accumulated during the winter months even when the maximum daily temperature kept well below zero for nearly two months (Figs. 3 and 4H). This does not seem to be an artifact; other transcripts did not show this, and RNA yields from the tissues do not explain this phenomenon, nor does the fraction of actin or histone transcripts compared to total RNA and used as reference.
In summary, we believe that we can answer the question when HW formation takes place in Scots pine: resin acids are formed early in the spring, followed by stilbenes, whose synthesis is carried out through the summer. Programmed cell death of TZ cells, i.e. their transformation into HW, takes place in autumn, when growth has ceased. In November, everything is over and the outermost dry zone of the tree trunk contains cells that prepare for the final active period in their life, taking place the next summer.
CONCLUSION
Past studies have suggested that ethylene plays an important role in HW formation. Up-regulation of ACO in TZ seemed first to support this, but because ACO was found to react strongly also in SW during the growth season, the evidence is not conclusive.
The carbon skeleton for the HW stilbene biosynthesis is obtained by breaking down Suc and is channeled to pinosylvin biosynthesis through the shikimate pathway. The result demonstrates that the Scots pine HW stilbenes are synthesized in situ in the TZ.
Two transcription factors closely follow phenylpropanoid biosynthesis in the TZ, leading not only to stilbenes but also to lignins. Finally, expression of a PCD regulator distinguishes developmentally regulated HW stilbene production from other activating signals (stilbenes are also stress metabolites).
Stilbene biosynthesis clearly takes place in TZ cells, but resin acids, another prominent group of HW extractives, are mainly synthesized in the SW, based on steady-state levels of transcripts encoding enzymes needed for their biosynthesis. Therefore, HW formation is of type I from the stilbene point of view, but type II from the resin acids point of view.
Our transcriptome data indicates that HW formation takes place during the growth period, with different timing for resin acid and stilbene biosynthesis.
MATERIALS AND METHODS
Plant Material for RNA Sequencing and qPCR Studies
Increment cores for RNA-Seq were sampled in June 2011 from four 46-years-old Scots pine (Pinus sylvestris L.) trees (T285, T328, T335, and T415) at Leppävirta progeny trial stand no. 30902 (62°25′N, 27°45′E), established by the Finnish Forest Research Institute, METLA. On average, 30 increment cores (5 mm in diameter) were randomly drilled through each tree trunk with a battery-driven increment core borer approximately at chest height level (130 cm), avoiding the knots. The border between wet and dry wood was marked with a pencil upon collection, after which the increment cores were frozen in dry ice and transferred to the −80°C laboratory freezer.
Increment cores for initial tests (including STS RT-PCR) throughout the summer months June to August in 2010 were sampled from 35-years-old Scots pine trees at Punkaharju progeny trial stand no. 62201 (61°49′N, 29°20′E) with the aforementioned method (trees 11 and 20). Increment cores for the year-round expression study were sampled from another two 35- to 36-years-old Scots pine trees (trees C and D) at the same progeny trial in Punkaharju from March 2011 to March 2012. Samples were harvested in the middle of each month. Four increment cores were drilled through the pith each month, approximately 1 cm distance apart. During each sampling date, the sampling position was moved laterally and longitudinally around the trunk (Supplemental Fig. S5). By this procedure we minimized the effects of the previous drills on the samples. The sampling took place from several interwhorls starting from approximately 180 cm downward. The increment cores were frozen in liquid nitrogen or N2 and stored at −80°C.
Localization of the Transition Zone
PS and PSME give strong fluorescence under UV excitation (Harju et al., 2009), allowing straightforward visualization of HW extractives. The frozen increment cores were illuminated at wavelength 365 nm and photographed. The TZ annual ring of each increment core was identified according to both the pencil mark made in the field and the fluorescence image made in the laboratory. We discarded increment cores where the pattern was not regular, for example when stilbenes were present outside of the dry zone in SW, indicating a reaction to a biotic or abiotic challenge.
Total RNA Extraction
Annual rings from TZ and SW were excised with a scalpel while keeping the increment core frozen. SW sample consisted of four to five pairs of annual rings located at approximately four or five annual rings away from the TZ. Samples were ground to fine powder in liquid nitrogen or N2 using, first, a mortar and pestle, and subsequently a milling machine (Oscillating Mill MM400; Retsch).
Total RNA isolation for SW and TZ samples was carried out as described by Chang et al. (1993) with minor modifications. After overnight lithium chloride precipitation, the RNA pellet was collected by centrifugation at 10,000g for 30 min at 4°C, washed with 70% cold ethanol, air-dried for 3 min, and dissolved in 50 µL nuclease-free Milli-Q water (Millipore). Genomic DNA was digested with DNase I, followed by purification with RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s protocol. The concentration and the quality of the genomic DNA-free total RNA was assessed with spectrophotometer (GeneQuant 1300; Eppendorf) and agarose gel electrophoresis.
Semiquantitative STS RT-PCR
Semiquantitative STS RT-PCR was performed for localization of the TZ and screening for an ideal of sampling time. The annual ring sampling and total RNA isolation were performed as described above. First-strand cDNA was synthesized using 50 ng of total RNA with SuperScript III reverse transcriptase (Invitrogen) with oligo(dT)20 primers according to manufacturer’s instructions in a 20 µL reaction volume. The STS RT-PCR was carried out with the program 94°C for 2 min, 24 cycles of 94°C for 30 s, 57°C for 1 min, and 72°C for 1 min and 72°C for 7 min. Primers (Supplemental Table S12) were designed to span an exon-intron boundary with Primer3 software (Untergasser et al., 2007).
Sample Preparation for RNA Sequencing
Eight micrograms of genomic DNA free total RNA was selectively depleted from rRNA according to the procedures described in RiboMinus Plant Kit for RNA-Seq (Invitrogen). A quantity of 500 ng of rRNA-depleted RNA was used for transcriptome library preparation. After the ligation of SOLiD adaptor mix and reverse transcription, size fractionation of the transcriptome library was performed with gel size selection of 150 to 200 bp cDNA, followed by amplification and purification according to the protocols described in SOLiD Total RNA-Seq Kit (Invitrogen). Next, paired-end sequencing was conducted with SOLiD 5500xl at the Institute of Biotechnology, University of Helsinki.
Mapping of Reads and Data Handling
The paired-end color space reads of SW and TZ RNA-Seq libraries were mapped against the Pinus EST collection, ver. 9.0 (The Gene Index Databases, 2014) using the SHRiMP2 alignment tool (David et al., 2011) with parameters as shown in Supplemental Protocol S1. Results were obtained in the Sequence Alignment/Map format (Li et al., 2009) and then converted to sorted Binary Alignment/Ma format, and duplicates were removed with Picard tools (Wysoker et al., 2013). The abovementioned tasks were carried out on the Finnish Grid Infrastructure clusters and supercluster servers at the CSC-IT Center for Science.
Differential Expression Analysis
Tables of mapped read counts for SW and TZ RNA-Seq libraries were imported to an edgeR R session to build a DGEList object for differential analysis (Robinson et al., 2010). Low-expression transcripts of Pinus ESTs collection were filtered; only transcripts that achieved at least eight counts per million in at least four libraries were kept. The trimmed mean of M-value normalization, dispersion estimation, and differential expression analysis was then carried out. A list of the statistically significant differentially expressed transcripts at FDR < 0.05 was then exported to a text file for downstream analysis.
The Pinus EST collection has annotations but as some of them are not informative or are missing, we compared the sequences to databases using the Blastx algorithm (Altschul et al., 1997) and obtained annotation updates for many cases. Original and updated annotations are shown in Supplemental Tables S3 and S4.
Gene Ontology Enrichment Analysis
GO terms of Pinus EST collection was obtained using Blast2GO (Conesa et al., 2005). GOSlim plant ontology mapping was then carried out to reduce complexity of obtained GO annotations. GO term enrichment analysis was performed for up-regulated and down-regulated transcripts with GO annotated Pinus EST collection as referral using Fisher's exact test with multiple testing correction of FDR function implemented in Blast2GO. General enriched GO terms were filtered from the result list at FDR < 0.05.
De Novo Assembly and Differential Expression Analysis
The paired-end color space reads of SW and TZ RNA-Seq libraries were converted first to nucleotides with an in-house tool and assembled using Trinity software (Haas et al., 2013). Minimum kmer threshold abundance (min_kmer_cov) was set as 2, library type parameter (SS_lib_type) as FR, and other assembly parameters were default. Color space reads of SW and TZ RNA-Seq libraries were then mapped against the Trinity assembly, data handling and differential expression analysis were performed as described above. Reads and the assembly have been submitted to the NCBI under Bioproject no. PRJNA311312, SRA data no. SRP069810.
Real-Time Quantitative RT-PCR
The expression levels of selected transcripts in the SW and TZ of trees C and D (March 2011 to March 2012) and trees sampled for RNA-Seq were determined by qPCR. The annual ring sampling, total RNA isolation, and genomic DNA digestion were carried out as described above. First-strand cDNA was synthesized with SuperScript III reverse transcriptase (Invitrogen) with oligo(dT)20 primers according to manufacturer’s instructions in 40 µL reaction volume using either 100 ng (trees C and D) or 350 ng (trees T285, T328, T335, and T415) of total RNA.
A PCR reaction mixture was prepared in 15 µL volume containing 7.5 µL of LightCycler 480 SYBR Green I Master mix (Life Technologies), 0.5 µM forward and reverse primers, and 5 µL of 14× diluted cDNA. The real-time quantitative RT-PCR was carried out in LightCycler 480 instrument (Roche) with the program 95°C for 10 min, 45 cycles of 95°C for 10 s, 57°C for 10 s, and 72°C for 10 s followed by melting curve analysis to check the specificity of each reaction.
Primers (Supplemental Table S12) were designed with Primer3 software (Untergasser et al., 2007). Amplification efficiencies were tested using a dilution series of pooled SW and TZ cDNA samples as a template. Primers were accepted if efficiency was at least 1.8 with se of less than 5%. In addition to no template controls, no reverse transcriptase controls were run to verify that samples did not carry genomic DNA contamination. Transcript-specific amplification efficiency (E) was determined using a module in the EasyqpcR R package (Sylvain, 2015).
The raw expression value of each transcript and two reference transcripts (encoding actin and histone) was calculated using the formula, ECq (E to the power of quantification cycle values, Cq). The relative expression of each transcript was obtained by dividing the raw expression value of each transcript with the geometric mean of raw expression value of two reference transcripts (Supplemental Protocol S2).
Pearson Correlation Analysis
Pearson correlation coefficients of the expression levels of selected transcripts in the SW and TZ of trees C and D were calculated using Hmisc and cor R packages (Harrell, 2016; R Core Team, 2015). The correlogram was plotted with the corrplot R package (Wei and Simko, 2016). For calculation of correlation between sampling months, level for each transcript was normalized so that the average expression level throughout the months was 1.0.
Supplemental Data
The following supplemental materials are available.
Supplemental Protocol S1. Mapping parameters.
Supplemental Protocol S2. Calculation of qPCR-relative expression.
Supplemental Table S1. Yields and absorbance ratios of RNA extracted from increment core fractions.
Supplemental Table S2. The SW and TZ RNA sequencing library sizes and overall mapping rates of the reads.
Supplemental Table S3. The 1021 statistically significantly (FDR < 0.05) up-regulated transcripts in TZ compared to SW.
Supplemental Table S4. The 652 statistically significantly (FDR < 0.05) down-regulated transcripts in TZ compared to SW.
Supplemental Table S5. Functional enrichment of GO terms.
Supplemental Table S6. Transcripts of multiple metabolic processes differentially expressed (FDR < 0.05) in the TZ during HW formation.
Supplemental Table S7. Mapped read counts of diterpene synthase and abietadienol/abietadienal oxidase.
Supplemental Table S8. List of selected transcripts that were differentially expressed in the TZ or related to resin acid biosynthesis for qPCR of the year-round study.
Supplemental Table S9. Total RNA yields from SW and TZ throughout the year.
Supplemental Table S10. Expression level of actin and histone transcripts in the TZ and SW.
Supplemental Table S11. Comparison of the qPCR and RNA-Seq data for trees T285, T328, T335, and T415.
Supplemental Table S12. Primers used for STS RT-PCR and the year-round expression profile study.
Supplemental Figure S1. Assessment of RNA quality in the HW, TZ, and SW samples.
Supplemental Figure S2. STS expression through the summer months.
Supplemental Figure S3. Quality check for sequenced RNA samples.
Supplemental Figure S4. Pearson’s correlation matrix between sampling months.
Supplemental Figure S5. The drilling scheme of increment cores for qPCR study of trees C and D.
Supplementary Material
Acknowledgments
We acknowledge the CSC-IT Centre for Science Ltd. (Espoo, Finland) for generous computational resources for this work. We particularly thank Kimmo Mattila for his skilled assistance in computing. We acknowledge Jussi Tiainen and Heikki Kinnunen for harvesting the year-round increment core samples, and also thank our laboratory technicians Anu Rokkanen, Eija Takala, and Marja Huovila for their skilled technical assistance. Kirsi Lipponen and Eeva-Marja Turkki are acknowledged for the SOLiD sequencing, Tommaso Raffaello for discussions concerning qPCR data analysis, and Pekka Saranpää and Georg von Arx for discussions about whether Scots pine heartwood could contain living cells.
Footnotes
This work was funded by the National Technology Agency of Finland (Tekniikan Edistämiskeskus, TEKES; to K.K. and T.H.T.) and by the Finnish Forest Cluster.
Articles can be viewed without a subscription.
References
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp K. (2011) The biology of heartwood formation in Sitka spruce and Scots pine. PhD thesis. The University of Edinburgh, Edinburgh, Scotland, UK [Google Scholar]
- Beekwilder J, van Houwelingen A, Cankar K, van Dijk AD, de Jong RM, Stoopen G, Bouwmeester H, Achkar J, Sonke T, Bosch D (2014) Valencene synthase from the heartwood of Nootka cypress (Callitropsis nootkatensis) for biotechnological production of valencene. Plant Biotechnol J 12: 174–182 [DOI] [PubMed] [Google Scholar]
- Bergström B. (2003) Chemical and structural changes during heartwood formation in Pinus sylvestris. Forestry 76: 45–53 [Google Scholar]
- Bergström B, Gustafsson G, Gref R, Ericsson A (1999) Seasonal changes of pinosylvin distribution in the sapwood/heartwood boundary of Pinus sylvestris. Trees 14: 65–71 [Google Scholar]
- Bienert G, Chaumont F (2011) Plant Aquaporins: Roles in Water Homeostasis, Nutrition, and Signaling Processes. In Geisler M, Venema K, eds, Transporters and Pumps in Plant Signaling, Signaling and Communication in Plants, Vol 7 Springer, Berlin, Germany, pp 3–36 [Google Scholar]
- Bollhöner B, Prestele J, Tuominen H (2012) Xylem cell death: emerging understanding of regulation and function. J Exp Bot 63: 1081–1094 [DOI] [PubMed] [Google Scholar]
- Bowsher C, Steer MW, Tobin AK (2008) Plant Biochemistry. Garland Science, New York [Google Scholar]
- Celedon JM, Chiang A, Yuen MM, Diaz-Chavez ML, Madilao LL, Finnegan PM, Barbour EL, Bohlmann J (2016) Heartwood-specific transcriptome and metabolite signatures of tropical sandalwood (Santalum album) reveal the final step of (Z)-santalol fragrance biosynthesis. Plant J 86: 289–299 [DOI] [PubMed] [Google Scholar]
- Chae HS, Kieber JJ (2005) Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci 10: 291–296 [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Report 11: 113–116 [Google Scholar]
- Chiron H, Drouet A, Claudot AC, Eckerskorn C, Trost M, Heller W, Ernst D, Sandermann H Jr (2000a) Molecular cloning and functional expression of a stress-induced multifunctional o-methyltransferase with pinosylvin methyltransferase activity from Scots pine (Pinus sylvestris L.). Plant Mol Biol 44: 733–745 [DOI] [PubMed] [Google Scholar]
- Chiron H, Drouet A, Lieutier F, Payer HD, Ernst D, Sandermann H Jr (2000b) Gene induction of stilbene biosynthesis in Scots pine in response to ozone treatment, wounding, and fungal infection. Plant Physiol 124: 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676 [DOI] [PubMed] [Google Scholar]
- David M, Dzamba M, Lister D, Ilie L, Brudno M (2011) SHRiMP2: sensitive yet practical SHort Read Mapping. Bioinformatics 27: 1011–1012 [DOI] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15: 573–581 [DOI] [PubMed] [Google Scholar]
- Duval I, Lachance D, Giguère I, Bomal C, Morency MJ, Pelletier G, Boyle B, MacKay JJ, Séguin A (2014) Large-scale screening of transcription factor-promoter interactions in spruce reveals a transcriptional network involved in vascular development. J Exp Bot 65: 2319–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage-Barhom S, Burd S, Sonego L, Perl-Treves R, Lers A (2008) Expression analysis of the BFN1 nuclease gene promoter during senescence, abscission, and programmed cell death-related processes. J Exp Bot 59: 3247–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegmann J, Schröder G, Schanz S, Britsch L, Schröder J (1992) Molecular analysis of chalcone and dihydropinosylvin synthase from Scots pine (Pinus sylvestris), and differential regulation of these and related enzyme activities in stressed plants. Plant Mol Biol 18: 489–503 [DOI] [PubMed] [Google Scholar]
- Fries A, Ericsson T, Gref R (2000) High heritability of wood extractives in Pinus sylvestris progeny tests. Can J Res 30: 1707–1713 [Google Scholar]
- Gaid MM, Sircar D, Müller A, Beuerle T, Liu B, Ernst L, Hänsch R, Beerhues L (2012) Cinnamate:CoA ligase initiates the biosynthesis of a benzoate-derived xanthone phytoalexin in Hypericum calycinum cell cultures. Plant Physiol 160: 1267–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler K, Jensen NB, Yuen MMS, Madilao L, Bohlmann J (2016) Modularity of conifer diterpene resin acid biosynthesis: P450 enzymes of different CYP720B clades use alternative substrates and converge on the same products. Plant Physiol 171: 152–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A. (2012) Plant chitinases: genetic diversity and physiological roles. Crit Rev Plant Sci 31: 57–73 [Google Scholar]
- Gustafsson G. (2001) Heartwood and lightwood formation in Scots pine: a physiological approach. PhD thesis. Swedish University of Agricultural Sciences, Umeå, Sweden [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, Macmanes MD, Ott M, et al. (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8: 1494–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F (2006) Localization and quantification of plasma membrane aquaporin expression in maize primary root: a clue to understanding their role as cellular plumbers. Plant Mol Biol 62: 305–323 [DOI] [PubMed] [Google Scholar]
- Harju AM, Kainulainen P, Venäläinen M, Tiitta M, Viitanen H (2002) Differences in resin acid concentration between brown-rot resistant and susceptible Scots pine heartwood. Holzforschung 56: 479–486 [Google Scholar]
- Harju AM, Venäläinen M (2006) Measuring the decay resistance of Scots pine heartwood indirectly by the Folin-Ciocalteu assay. Can J For Res 36: 1797–1804 [Google Scholar]
- Harju A, Venäläinen M, Anttonen S, Viitanen H, Kainulainen P, Saranpää P, Vapaavuori E (2003) Chemical factors affecting the brown-rot decay resistance of Scots pine heartwood. Trees 17: 263–268 [Google Scholar]
- Harju AM, Venäläinen M, Laakso T, Saranpää P (2009) Wounding response in xylem of Scots pine seedlings shows wide genetic variation and connection with the constitutive defence of heartwood. Tree Physiol 29: 19–25 [DOI] [PubMed] [Google Scholar]
- Harrell FE., Jr with contributions from Charles Dupont and many others. (2016). Hmisc: Harrell Miscellaneous. R package version 3.17-4. https://CRAN.R-project.org/package=Hmisc
- Hillis WE. (1987) Heartwood and Tree Exudates. Springer, Berlin, Germany [Google Scholar]
- Hillis WE. (1968) Heartwood formation and its influence on utilization. Wood Sci Technol 2: 260–267 [Google Scholar]
- Hudgins JW, Ralph SG, Franceschi VR, Bohlmann J (2006) Ethylene in induced conifer defense: cDNA cloning, protein expression, and cellular and subcellular localization of 1-aminocyclopropane-1-carboxylate oxidase in resin duct and phenolic parenchyma cells. Planta 224: 865–877 [DOI] [PubMed] [Google Scholar]
- IAWA Committee (1964) Multilingual glossary of terms used in wood anatomy. Committee on Nomenclature, International Association of Wood Anatomists (IAWA). Verlagsanstalt Buchdruckerei Konkordia Winterthur, Zürich, Switzerland [Google Scholar]
- Kampe A, Magel E (2013) New insights into heartwood and heartwood formation. In Fromm J, ed, Cellular Aspects of Wood Formation, Vol 20 Springer, Berlin, Germany, pp 71–95 [Google Scholar]
- Klempien A, Kaminaga Y, Qualley A, Nagegowda DA, Widhalm JR, Orlova I, Shasany AK, Taguchi G, Kish CM, Cooper BR, D’Auria JC, Rhodes D, et al. (2012) Contribution of CoA ligases to benzenoid biosynthesis in petunia flowers. Plant Cell 24: 2015–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodan A, Kuroda H, Sakai F (2002) A stilbene synthase from Japanese red pine (Pinus densiflora): implications for phytoalexin accumulation and down-regulation of flavonoid biosynthesis. Proc Natl Acad Sci USA 99: 3335–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Ellis BE (2003) 4-coumarate:CoA ligase gene family in Rubus idaeus: cDNA structures, evolution, and expression. Plant Mol Biol 51: 327–340 [DOI] [PubMed] [Google Scholar]
- Leinonen A, Harju AM, Venäläinen M, Saranpää P, Tapio L (2008) FT-NIR spectroscopy in predicting the decay resistance related characteristics of solid Scots pine (Pinus sylvestris L.) heartwood. Holzforschung 62: 284 [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorio JP. (1986) Growth-differentiation balance: a basis for understanding southern pine beetle-tree interactions. For Ecol Manage 14: 259–273 [Google Scholar]
- Magel EA. (2000) Biochemistry and physiology of heartwood formation. In Savidge R, Barnett JR, Napier R, eds, Cell and Molecular Biology of Wood Formation, Vol xvii, 530, 4 s. BIOS Scientific Publishers, Oxford, UK, pp 363–376 [Google Scholar]
- Magel EA, Hillinger C, Wagner T, Höll W (2001) Oxidative pentose phosphate pathway and pyridine nucleotides in relation to heartwood formation in Robinia pseudoacacia L. Phytochemistry 57: 1061–1068 [DOI] [PubMed] [Google Scholar]
- Nakaba S, Kubo T, Funada R (2008) Differences in patterns of cell death between ray parenchyma cells and ray tracheids in the conifers Pinus densiflora and Pinus rigida. Trees 22: 623–630 [Google Scholar]
- Nakada R, Fukatsu E (2012) Seasonal variation of heartwood formation in Larix kaempferi. Tree Physiol 32: 1497–1508 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Yamaguchi M, Endo H, Rejab NA, Ohtani M (2015) NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front Plant Sci 6: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Wikman S, Eklund L (2002) Induction of discolored wood in Scots pine (Pinus sylvestris). Tree Physiol. 22: 331–338 [DOI] [PubMed] [Google Scholar]
- Partanen J, Harju AM, Venäläinen M, Kärkkäinen K (2011) Highly heritable heartwood properties of Scots pine: possibilities for selective seed harvest in seed orchards. Can J Res 41: 1993–2000 [Google Scholar]
- Patzlaff A, McInnis S, Courtenay A, Surman C, Newman LJ, Smith C, Bevan MW, Mansfield S, Whetten RW, Sederoff RR, Campbell MM (2003a) Characterisation of a pine MYB that regulates lignification. Plant J 36: 743–754 [DOI] [PubMed] [Google Scholar]
- Patzlaff A, Newman LJ, Dubos C, Whetten RW, Smith C, McInnis S, Bevan MW, Sederoff RR, Campbell MM (2003b) Characterisation of Pt MYB1, an R2R3-MYB from pine xylem. Plant Mol Biol 53: 597–608 [DOI] [PubMed] [Google Scholar]
- Piatkowski D, Schneider K, Salamini F, Bartels D (1990) Characterization of five abscisic acid-responsive cDNA clones isolated from the desiccation-tolerant plant Craterostigma plantagineum and their relationship to other water-stress genes. Plant Physiol 94: 1682–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomion C, Leprovost G, Stokes A (2001) Wood formation in trees. Plant Physiol 127: 1513–1523 [PMC free article] [PubMed] [Google Scholar]
- Quackenbush J, Liang F, Holt I, Pertea G, Upton J (1999) The TIGR Gene Indices: reconstruction and representation of expressed gene sequences. Nucleic Acids Res 28: 141–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/ [Google Scholar]
- Ro DK, Bohlmann J (2006) Diterpene resin acid biosynthesis in loblolly pine (Pinus taeda): functional characterization of abietadiene/levopimaradiene synthase (PtTPS-LAS) cDNA and subcellular targeting of PtTPS-LAS and abietadienol/abietadienal oxidase (PtAO, CYP720B1). Phytochemistry 67: 1572–1578 [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranpää P, Höll W (1989) Soluble carbohydrates of Pinus sylvestris L. sapwood and heartwood. Trees 3: 138–143 [Google Scholar]
- Saranpää P, Nyberg H (1987) Lipids and sterols of Pinus sylvestris L. sapwood and heartwood. Trees 1: 82–87 [Google Scholar]
- Shain L, Hillis WE (1973) Ethylene production in xylem of Pinus radiata in relation to heartwood formation. Can J Bot 51: 1331–1335 [Google Scholar]
- Singh T, Singh AP (2011) A review on natural products as wood protectant. Wood Sci Technol 46: 851–870 [Google Scholar]
- Spicer R. (2005) Senescence in secondary xylem: heartwood formation as an active developmental program. In Holbrook NM, Zwieniecki M, eds, Vascular Transport in Plants. Elsevier/Academic Press, Oxford, UK, 457 [Google Scholar]
- Sylvain LP. (2015) EasyqpcR for easy analysis of real-time PCR data at IRTOMIT-INSERM U1082. IRTOMIT-INSERM U1082, sylvain.le.pape@univ-poitiers.fr, http://irtomit.labo.univ-poitiers.fr/
- Taylor A, Gartner B, Morrell J (2002) Heartwood formation and natural durability—a review. Wood Fiber Sci 34: 587–611 [Google Scholar]
- Dana-Farber Cancer Institute (2014) The Gene Index Databases. Harvard Medical School, Boston, MA, http://compbio.dfci.harvard.edu/tgi [Google Scholar]
- Tzin V, Malitsky S, Ben Zvi MM, Bedair M, Sumner L, Aharoni A, Galili G (2012) Expression of a bacterial feedback-insensitive 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase of the shikimate pathway in Arabidopsis elucidates potential metabolic bottlenecks between primary and secondary metabolism. New Phytol 194: 430–439 [DOI] [PubMed] [Google Scholar]
- Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35: W71–W74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC. (1997) Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol 103: 753–765 [Google Scholar]
- Venäläinen M, Harju AM, Kainulainen P, Viitanen H, Nikulainen H (2003) Variation in the decay resistance and its relationship with other wood characteristics in old Scots pines. [Variation de la résistance à la pourriture et relation avec les autres caractéristiques du bois dans les vieux pins sylvestres]. Ann Sci 60: 409–417 [Google Scholar]
- Venäläinen M, Harju A, Saranpää P, Kainulainen P, Tiitta M, Velling P (2004) The concentration of phenolics in brown-rot decay resistant and susceptible Scots pine heartwood. Wood Sci Technol 38: 109–118 [Google Scholar]
- von Arx G, Arzac A, Olano JM, Fonti P (2015) Assessing conifer ray parenchyma for ecological studies: pitfalls and guidelines. Front Plant Sci 6: 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyn JL, Liechty JD, Stevens KA, Wu LS, Loopstra CA, Vasquez-Gross HA, Dougherty WM, Lin BY, Zieve JJ, Martínez-García PJ, Holt C, Yandell M, et al. (2014) Unique features of the loblolly pine (Pinus taeda L.) megagenome revealed through sequence annotation. Genetics 196: 891–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei TY, Simko V (2016) Corrplot: Visualization of a Correlation Matrix. R package version 0.77. https://CRAN.R-project.org/package=corrplot
- Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Biochem Mol Biol 35: 253–289 [DOI] [PubMed] [Google Scholar]
- Woeste KE, Ye C, Kieber JJ (1999) Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol 119: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysoker A, Tibbetts K, Fennell T (2013) Picard, http://broadinstitute.github.io/picard
- Yamamoto K. (1982) Yearly and seasonal process of maturation of ray parenchyma cells in Pinus species. Res Bull Coll Experiment Forests Hokkaido University 39: 245–296 [Google Scholar]
- Yang J, Kamdem DP, Keathley DE, Han KH (2004) Seasonal changes in gene expression at the sapwood-heartwood transition zone of black locust (Robinia pseudoacacia) revealed by cDNA microarray analysis. Tree Physiol 24: 461–474 [DOI] [PubMed] [Google Scholar]
- Yang J, Park S, Kamdem DP, Keathley DE, Retzel E, Paule C, Kapur V, Han KH (2003) Novel gene expression profiles define the metabolic and physiological processes characteristic of wood and its extractive formation in a hardwood tree species, Robinia pseudoacacia. Plant Mol Biol 52: 935–956 [DOI] [PubMed] [Google Scholar]
- Yoon GM, Kieber JJ (2013) 1-Aminocyclopropane-1-carboxylic acid as a signalling molecule in plants. AoB Plants 5: 10.1093/aobpla/plt017 [Google Scholar]
- Yoshida K, Futamura N, Nishiguchi M (2012) Collection of expressed genes from the transition zone of Cryptomeria japonica in the dormant season. J Wood Sci 58: 89–103 [Google Scholar]
- Zha HG, Liu T, Zhou JJ, Sun H (2013) MS-desi, a desiccation-related protein in the floral nectar of the evergreen velvet bean (Mucuna sempervirens Hemsl): molecular identification and characterization. Planta 238: 77–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.