Abstract
Aerobic methane oxidation (AMO) is one of the primary biologic pathways regulating the amount of methane (CH4) released into the environment. AMO acts as a sink of CH4, converting it into carbon dioxide before it reaches the atmosphere. It is of interest for (paleo)climate and carbon cycling studies to identify lipid biomarkers that can be used to trace AMO events, especially at times when the role of methane in the carbon cycle was more pronounced than today. AMO bacteria are known to synthesise bacteriohopanepolyol (BHP) lipids. Preliminary evidence pointed towards 35-aminobacteriohopane-30,31,32,33,34-pentol (aminopentol) being a characteristic biomarker for Type I methanotrophs. Here, the BHP compositions were examined for species of the recently described novel Type I methanotroph bacterial genera Methylomarinum and Methylomarinovum, as well as for a novel species of a Type I Methylomicrobium. Aminopentol was the most abundant BHP only in Methylomarinovum caldicuralii, while Methylomicrobium did not produce aminopentol at all. In addition to the expected regular aminotriol and aminotetrol BHPs, novel structures tentatively identified as methylcarbamate lipids related to C-35 amino-BHPs (MC-BHPs) were found to be synthesised in significant amounts by some AMO cultures. Subsequently, sediments and authigenic carbonates from methane-influenced marine environments were analysed. Most samples also did not contain significant amounts of aminopentol, indicating that aminopentol is not a useful biomarker for marine aerobic methanotophic bacteria. However, the BHP composition of the marine samples do point toward the novel MC-BHPs components being potential new biomarkers for AMO.
Introduction
Methane (CH4) is a potent greenhouse gas, and its atmospheric concentration has tripled since pre-industrial times (e.g. [1,2]). Global oceans hold large subsurface reservoirs of CH4 in the form of gas hydrates. These stores are precariously dependent on temperature and pressure. A rapid destabilisation of gas hydrates has been proposed to have caused vast releases of marine CH4 in the past [3]. Increased input of CH4 into the atmosphere has been interpreted through records of excursions of significant δ13C depletion in the geological record, such as in the Palaeocene-Eocene Thermal Maximum (PETM) [4–7].
CH4 release into the atmosphere is regulated by methanotrophy, which converts CH4 into CO2, thereby playing a key role in the carbon biogeochemical cycle [8]. Although traditionally anaerobic archaea have been the most studied methanotrophs (e.g., AMNE-1 and ANME-2; cf. [9]), recent observations have highlighted the importance of bacteria performing aerobic CH4 oxidation (AMO) in marine, estuarine, and riverine fan environments (e.g., [10–14]). For example, pelagic AMO activity rose significantly after the Macondo oil well blowout in 2010 [15]. However, this activity was short-lived, highlighting the complexity of natural community interactions in response to increased CH4 [16]. It is thus important to recognise and trace methanotrophy during past extreme events in order to understand its potential to mitigate future CH4 release.
AMO bacteria belong to two phyla, Proteobacteria and Verrucomicrobia. Most isolates of Verrucomicrobia are thermoacidophilic [17–20], and have been found primarily in acidic, geothermal environments [21]. Aerobic methanotrophic members of Proteobacteria belong to two distinct classes, separated based on their carbon assimilation pathways [8]. Type II methanotrophs, members of the Alphaproteobacteria, are associated with terrestrial settings ([8], and references therein), whereas Type I methanotrophs members of the Gammaproteobacteria are widespread in aquatic systems, although they are also found in terrestrial systems. Both Type I and Type II methanotrophs are known to synthesise bacteriohopanepolyol (BHP) lipids [22]. BHPs are precursors to hopanes, which are the most ubiquitous lipids in the geological record [23,24]. Therefore, being able to trace AMO using hopanoid biomarkers is of value to the study of the carbon cycle in the past.
Previous work exploring hopanoids as biomarker lipids found that methylation at the C-3 position (3-Me-BHPs; Fig 1, I3Me, II3Me, III3Me) was a possible indication of methanotrophic origin [25,26]. However, attributing C-3 methylation to AMO was challenged by the revelation that bacteria other than methanotrophs have the genes to methylate at C-3 [27]. C-3 methylation is more likely a requirement for cell survival in late stationary phase [27]. Moreover, not all methanotrophic bacteria have the gene to methylate at this position, [27,28], nor are 3-Me BHP precursors found in all CH4-influenced environments (Table 1). However, AMO bacteria are often considered to be the most likely source of 3-Me hopanoids in marine sediments due to their depleted carbon isotope signatures (e.g., [29]) and because they are frequently accompanied by 4-methylated steroids, which are also known as biomarkers of methanotrophic bacteria (e.g., [30,31]). In other studies, diplopterol and diploptene have been interpreted as biomarkers for methanotrophy, notably in anoxic environments [32–35]. However, neither diplopterol nor diploptene is source-specific to AMO [36], and these studies also relied on very negative δ13C lipid values (e.g., ca. -61‰ for diplopterol; -61 to -74‰ for diploptene) as an indication of CH4 being the carbon source for the organism producing these lipids. Yet, recent work has emphasised that not all AMO-derived carbon shows a depleted isotope signature, especially in terrestrial systems where Type II methanotrophs tend to dominate. For example, only limited depletion in 13C, with values between -25‰ and -40‰, have been reported for hopanoids with an inferred methanotrophic origin from modern peat bogs [37,38] as well as other ancient lignite deposits [39]. Also, BHPs in Congo deep sea fan sediments, originating from low-latitude wetlands [40], had higher 13C values than expected (i.e., C30 hopanol ~ -41‰; [14]). In marine CH4 seep carbonates from the Gulf of Mexico, BHPs, hopanoic acids, and 4-methylated sterols of aerobic methanotrophs were found with similar δ13C values as the CH4 source [41]. These observations can be the result of dilution from other heterotrophic bacterial sources that make it difficult to identify subordinate methanotroph contributions [37]. The metabolic pathways used by the AMO bacteria for CH4 assimilation can also have a profound effect on the level of isotopic depletion, with values for serine pathway methanotrophs (Type II) ranging from 12‰ depleted to 10‰ enriched relative to the CH4 substrate [42]. Furthermore, to analyse δ13C of intact BHPs, these must first be converted into primary alcohols by periodic acid/sodium borohydride cleavage [43]. For example, the δ13C value of 35-aminobacteriohopane-30,31,32,33,34-pentol (aminopentol; Fig 1, I) is measured on the C30-hopanol product, which includes all converted hexa-functionalised BHPs (i.e., not only aminopentol), as well as any free-hopanols that are present in a sample before BHP conversion. While the contamination of free-hopanols can be circumnavigated by column separations (e.g., [41]), measuring the δ13C values of intact BHPs is not currently possible.
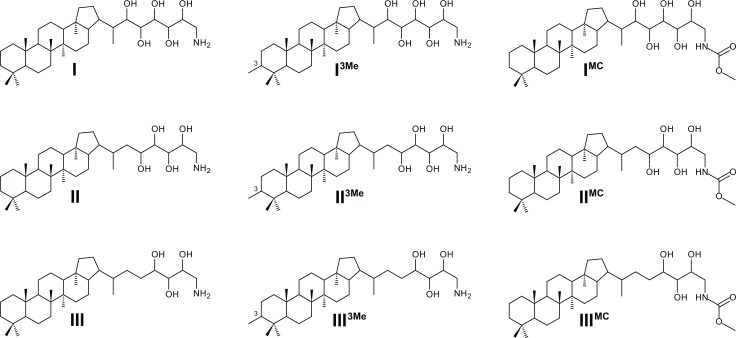
Fig 1. Chemical structures of bacteriohopanepolyol lipids.
I, aminopentol; I3Me, 3-methyl-aminopentol; IMC, methylcarbamate-aminopentol; II, aminotetrol; II3Me, 3-methyl-aminotetrol; IIMC, methylcarbamate-aminotetrol; III, aminotriol; III3Me, 3-methyl-aminotriol; IIMC, methylcarbamate-aminotriol. The proposed structure of methylcarbamate(MC)-aminopentol (IMC), MC-aminotetrol (IIMC), and MC-aminotriol (IIIMC) are tentatively based on mass spectral identification (S1 File).
Table 1. Presence and absence of aminopentol and related methylated and unsaturated homologues in previously investigated environmental settings.
| aminopentol | aminotetrol | aminotriol | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | I | ΔI | I3Me | II | II3Me | III | III3Me | Reference |
| Soils | ||||||||
| Pasture [manured] (UK) | + | - | - | + | - | + | + | [112] |
| Pasture [not manured] (UK) | - | - | - | + | - | + | + | [112] |
| Rice Paddy (Vietnam) | + | - | - | + | - | + | - | [112] |
| Woodland (North East England) | + | - | - | + | - | + | + | [112] |
| South West France | + | - | - | + | - | + | - | [116] |
| Amazon | + | - | - | + | - | + | - | [109] |
| Congo | + | + | - | + | - | + | + | [40] |
| Lean Delta Peamafrost [ice complex] | + | - | - | + | - | + | - | [110] |
| Human Sewage [treated] | - | - | - | + | - | + | - | [117] |
| Forest, Grassland Soils (Alberta, Canada) | - | - | - | + | - | + | - | [118] |
| Peat and Lignite | ||||||||
| River Tet Catchment (France) | + | - | - | + | - | + | - | [116] |
| Moorhouse (UK) | + | - | - | + | - | + | - | [46] |
| Misten Bog (Belgium) | + | - | - | + | - | + | - | [119] |
| Bisendorfer Moor (Germany) | + | - | - | + | - | + | + | [111] |
| The Cobham Lignite (UK) | + | - | - | + | - | + | - | [49] |
| Geothermal Environments | + | |||||||
| Cyanobacterial mat (Surprise Valley, Nevada, USA) | + | - | - | + | - | + | - | [120] |
| Silica Sinter (Orakie Korako, Taupo Volcanic Zone, New Zealand) | + | - | + | + | - | + | - | [113] |
| Silica Sinter (Champagne Pool, Taupo Volcanic Zone, New Zealand) | - | - | - | - | - | + | - | [121] |
| Orange mat (Yellowstone, USA) | - | - | - | - | - | + | - | [122] |
| Other Microbial mats | ||||||||
| Mars Oasic (Antarctica) | - | - | - | - | - | + | - | [48] |
| Hypolith (Devon Island, Arctic) | - | - | - | - | - | + | - | [48] |
| Cyanobacterial Mat (Christmas Island, Kiribati) | - | - | - | - | - | + | - | [123] |
| Lake Sediments | ||||||||
| Lake Windermere (UK) | + | - | - | + | - | + | - | ¶ |
| Lake Cadagno (Switzerland) | + | - | - | + | - | + | - | [26] |
| Lake Holzmaar (Germany) | + | - | - | + | - | + | - | [26] |
| Lake Nkunga (Kenya) | + | - | - | + | - | + | - | [45] |
| Priest Pot (England) | + | - | - | + | - | + | - | [26,45] |
| La Piscina de Yuriria (Mexico) | + | - | + | + | + | + | - | [26,45] |
| Laguna de Zempoala (Mexico) | + | - | - | + | - | + | - | [26] |
| Loch Ness (UK) | + | - | - | + | - | + | - | [26,45] |
| Lake Druzhby (Antarctica) | + | - | - | + | - | + | - | [26,45] |
| Sombre Lake (Signy Island, Southern Atlantic Ocean) | + | - | - | + | - | + | - | [26] |
| Heywood Lake (Signy Island, Southern Atlantic Ocean) | + | - | - | + | - | + | - | [26] |
| Ace Lake [Freshwater unit] | + | - | - | + | - | + | - | [104] |
| Ace Lake [meromictic unit] | - | - | - | + | - | + | - | [104] |
| Ace Lake [marine unit] | - | - | - | - | - | + | - | [104] |
| Wetlands | ||||||||
| Amazon | + | + | - | + | - | + | - | [109] |
| Congo | + | + | - | + | - | + | - | [40] |
| Stream/River/Estuary Sediments | ||||||||
| Arctic Rivers (Indigirka, Kolyma, Lena, Ob, Yenisei, Yukon, Mackenzie) | + | - | - | + | - | + | - | [124] |
| Glacial Outflow Stream (Svalbard) | + | - | - | + | - | + | - | [125] |
| Kalix River [surface sediment transect] | + | - | - | + | - | + | - | [126] |
| Kolyma River [surface sediment transect] | + | - | - | + | - | + | - | [127] |
| Yenisei River [mouth, and surrounding area] | + | - | - | + | - | + | - | [128] |
| Yangtze River [estuary, inner shelf] | + | - | - | + | - | + | - | [82,129] |
| Congo River [estuary] | + | + | - | + | - | + | - | [14,40] |
| Water Column | ||||||||
| Priest Pot [pond] (UK) | + | - | - | + | - | + | - | ¶ |
| River Water (Panama) | + | - | - | + | - | + | - | [130] |
| Yenisei River | + | - | - | + | - | + | - | [128] |
| Black Sea [oxic/anoxic transition zone] | + | - | + | + | - | + | - | [12] |
| Black Sea [oxic/anoxic transition zone] | + | - | - | + | - | + | - | [108] |
| Gotland Deep, Baltic Sea | + | - | - | + | - | + | - | [107] |
| Marine Water (off Panama coast) | - | - | - | - | - | + | - | [130] |
| Pelagic [sub-oxic and anoxic] (Arabian Sea, Peru Margin, Cariaco Basin) | - | - | - | - | - | + | - | [90] |
| Cariaco Basin | - | - | - | - | - | + | - | [131] |
| California Current | - | - | - | - | - | + | - | [91] |
| Modern/Recent Marine Sediments | ||||||||
| Gotland Deep, Baltic Sea | + | - | - | + | - | + | - | [107] |
| Congo River Deep-Sea Fan | + | + | - | + | - | + | - | [14] |
| Amazon Shelf and Fan | + | - | - | + | - | + | - | [109] |
| East Siberian Arctic Shelf | + | - | - | + | - | + | - | [79,126] |
| Black Sea | - | - | - | + | - | + | - | [88] |
| Southwest African Coast | - | - | - | + | - | + | (+) | [89] |
| Chukchi Sea | - | - | - | + | - | + | - | [132] |
| Alaskan Beaufort Sea | - | - | - | - | - | + | - | [132] |
| Baltic Sea | - | - | - | + | - | + | - | [133] |
| Marine Carbonates | ||||||||
| Carbonate, Gulf of Mexico | - | - | - | + | - | + | - | [72] |
| Authigenic carbonates, Gulf of Mexico | - | - | - | + | - | + | - | [41] |
| Seep Carbonate, Arabian Sea | - | - | - | + | - | + | - | [106] |
| Other samples | ||||||||
| Membrane Foulant [river water] | + | - | - | - | - | + | - | [134] |
| Membrane Foulant [brackish water] | + | - | - | + | - | + | - | [134] |
| Membrane Foulants [seawater] | - | - | - | - | - | + | - | [134] |
| Mussel Gill Tissue | - | - | - | + | - | + | - | [103] |
+, detected in at least one of the samples; -, not detected in any of the samples
(+) indicates a methylated aminotriol was detected but the position of methylation was not identified
¶Talbot and Farrimond, unpublished data
Aminopentol is thought to be the most diagnostic BHP for AMO (see review in [14]). Aminopentol and its methylated and unsaturated homologues (i.e., I3Me, ΔI) have been found almost exclusively in Type I aerobic methanotrophs [22,44–46]. Moreover, aminopentol (I) has been found in a wide range of environments, which indicates potential as a biomarker for AMO (Table 1). Additionally, 35-aminobacteriohopane-31,32,33,34-tetrol (aminotetrol, II) and 35-aminobacteriohopane-32,33,34-triol (aminotriol, III) are also synthesised by Type I and Type II AMO bacteria. However, II and III are less source-specific as both are synthesised by some species of sulfate reducing bacteria (SRB) of the genus Desulfovibrio [47], and III is synthesised by many other aerobic bacteria ([48], and references therein).
Aminopentol made up a very minor proportion of the BHP composition in a SRB culture (<0.1% of total BHPs in Desulfovibrio salexigens). The ratio of aminopentol to the more ubiquitous aminotriol was 1:1352 [47]. We can therefore discount SRB as the source of aminopentol in an environmental sample with a high ratio of aminopentol:aminotriol. A similar approach was recently used by [49] based on the ratio of aminotetrol:aminotriol which has been found in the range 1:20–100 in some species of Desulfovibrio SRB [47,50,51]. Interestingly, some of the species of SRB cultures analysed by Blumenberg et al. [47,50,51] also synthesised diplopterol and diploptene, which could explain the enhanced presence of these lipids in CH4-influenced anoxic sites.
Representatives from only a small number of Type I methanotroph genera have been tested for BHP production (e.g., [22,36,46,52,53]. Many more recently described genera, including novel genera from marine and other (hyper)saline environments, have yet to be explored (e.g., [54–56]). Moreover, relatively few studies of BHP distributions have targeted marine environments (Table 1). It is important, therefore, to determine whether aminopentol, which is seen as a diagnostic marker for Type I methanotrophs, is present in methanotrophs isolated from marine and other saline environments, and whether we can find aminopentol in CH4-influenced marine sediments. This knowledge will have implications for the use of aminopentol as a biomarker to trace AMO in modern and ancient marine environments.
In this study, our goal was to develop an appropriate biomarker approach for AMO, which will allow high throughput analysis of sediment without the requirement for laborious chemical conversion steps prior to compound specific isotope analysis. To this end, we screened the BHP distributions of three genera of aerobic methanotrophs (i.e., Methylomicrobium, Methylomarinum and Methylomarinovum). Methylomarinum and Methylomarinovum have not previously been investigated for BHPs. Two species of Methylomicrobium have been described previously [52,53], however, we include an additional species Methylomicrobium kenyense. These data are combined with literature BHP distributions of other AMO genera, including the recently reported Type I genus, Methylobacter [28], in order to facilitate interpretation of BHP distributions in six selected modern marine sediments from CH4-influenced systems. Two marine sites not influenced by CH4 were also investigated as controls for background marine BHP signatures.
Methods
Methanotroph pure cultures
Cultivation of Methylomarinum vadi IT-4
Previously described Methylomarinum vadi IT-4 was isolated from a microbial mat sample (in-situ temperature 30–40°C) collected at a shallow marine hydrothermal system (depth, ~23 m) in a coral reef off Taketomi Island, Okinawa, Japan [56,57]. Cultivation of this strain was performed at JAMSTEC, Japan, using MJmet medium at pH 6.6 at 37°C. A detailed site description and the enrichment and isolation procedures can be found in [57].
Cultivation of Methylomarinovum spp
Previously described Methylomarinovum caldicuralii IT-9 was isolated from the hot vent fluid (52°C) collected at the main vent site (depth, 23 m) in the shallow marine hydrothermal system where Methylomarinum vadi IT-4 was isolated [55,57]. Methylomarinovum sp. IN45 is a new isolate from a deep-sea hydrothermal field in Okinawa Trough, Japan (H. Hirayama, pers. comm.). The cultivation of strains IT-9 and IN45 was performed at JAMSTEC, Japan, using MJmet medium at 45°C, and at pH 6.2 and 6.6, respectively.
Cultivation of Methylomicrobium spp
Methylomicrobium alcaliphilum and Methylomicrobium kenyense were first isolated from highly alkaline soda lakes in Russia and Kenya, respectively [58]. Both M. alcaliphilum (DSM-number 19304) and M. kenyense (DSM-number 19305) are from the Leibniz Institute DSMZ (German Collection of Microorganisms and Cell Cultures). The cultivation of both strains was done at the Center for Applied Geosciences at the University of Tübingen, Germany, at pH 9.1 and at 28°C with a high salt NMS medium (1.5% NaCl).
Marine sediment and carbonate samples
Håkon Mosby Mud Volcano (HMMV)
HMMV has been extensively studied for both aerobic and anaerobic methanotrophy [13,59]. The flow of CH4 in the center of the HMMV is restricted, and AMO was observed to be the most dominant process within the centre of the crater, performed by Type I methanotrophs [60]. Sediments from the HMMV were collected aboard the RV Polarstern (cruise ARK XXIV/2; 2009) and the RV Maria S Merian (cruise MSM 16/2; 2010) [61].
Barents Sea carbonate crust (BSCC)
The Barents Sea is a well-studied area of active CH4 seepage. The seafloor geology is marked by pockmarks [62], gas hydrates, gas flares [63], and patches of carbonate crusts [64], indicating active CH4 seepage [10,64]. We analysed eight depths (each 2–3 cm thick) from a push core (P120020 PR4) of a cold seep carbonate crust sampled at Loppa High/Polheim Sub-Platform area in the SW Barents Sea (72° 34’02.07”N, 20° 52’05.96”E). The core penetrated to a depth of 19 cm below sea floor (cmbsf). These samples were taken aboard the RV Fugro Meridian in September 2012 by Lundin Petroleum Norway.
Amon Mud Volcano (AMV)
The Amon mud volcano (AMV) is located in the Nile deep-sea fan, in the Eastern Basin of the Mediterranean Sea. Hydrocarbons, muds, and fluids are transported to the surface via one main feeding channel from the deep subsurface, creating a stark thermal gradient in the sediment. Oxidation of CH4 in the water column directly above AMV has been attributed to AMO using 13C and 2H isotopic values [65]. Sediments from AMV were collected aboard the RV Meteor (cruise M70/2, BIONIL; 2006) and the RV Maria S Merian (cruise MSM 13/3; 2009) [66].
New Zealand Seeps (NZS)
The Hikurangi continental margin, east of New Zealand’s North Island has been described as a biogeographically “new” cold seep province, characterised by endemic faunal communities [67]. Surface (0–2 cmbsf) and subsurface (10–12 cmbsf) sediment samples from three New Zealand seep (NZS) sites were collected aboard the RV Sonne (cruise So-191; 2007) [68]. These were dominated by distinct biota: Frenulata (Omakere Ridge), Ampharetidae (Wairarapa Takahae), and sulfur-oxidising bacteria (Wairarapa Takahae).
Gulfo Dulce (GD) surface sediments
A sill at 60 m water depth physically cuts off Golfo Dulce from the Pacific Ocean, which promotes anoxic conditions within the basin. Recently, GD was shown to contain authigenic carbonate formations at shallow (ca. 10 m) water depth [69]. GD sediments were collected along a transect from 10 to 140 m water depth in March 2008, as described in [70].
Gulf of Mexico (GoM) cold seeps
The Gulf of Mexico (GoM) holds an abundance of thermogenic gas. The venting of this gas from deep subsurface forms gas hydrates, free CH4, which are the sources of energy for microbial chemosynthetic communities, and authigenic carbonates [71]. Pancost et al., [72] described the BHP composition of material from five carbonate rock and nodule sites in GoM. Two of the carbonates were shown to contain low quantities of aminopentol. Here, we reinvestigated the BHP signatures of these sediments.
GoM sediments
The GoM also houses the outflow of the Mississippi River Delta, transporting terrestrial material into the Gulf. Three GoM sites to the best of our knowledge not influenced by CH4 (27°30'N, 87°20'W; 28°20'N, 89°38'W; 26°50'N, 92°40'W; two sediment depths at each site) were investigated in this study [73].
Peru Margin (PM)
An intense upwelling regimes fertilises surface water productivity on the Peru Margin (PM). This lends to oxygen utilisation in the water column, causing the Eastern South Pacific Oxygen Minimum Zone (ESP OMZ) [74]. Three PM sediments were analysed (10–15, 20–25 and 40–45 cmbsf) from a core taken within the ESP OMZ, at 100 m water depth [73,75].
Lipid extraction
Total lipid extraction
All freeze-dried bacterial cells and marine sediments, except the Barents Sea samples extracted at GFZ Potsdam, were extracted using a modified Bligh-Dyer method [76,77]. Briefly, freeze-dried material was extracted in 19 mL of a 10:5:4 (v:v:v) mixture of MeOH:chloroform:H2O in a 50 mLTeflon tube. This mixture was sonicated for 15 min at 40°C, and centrifuged for 10 min. The supernatant was transferred to a second tube, and the residue re-extracted twice more. The chloroform in the supernatant was separated from the aqueous phase by adding water until the H2O:MeOH ratio was 1:1 (v:v), and collected. This procedure was repeated for the subsequent extractions. The collected chloroform total lipid extract (TLE) was dried by rotary evaporation in a round-bottom flask. The extraction protocol at GFZ Potsdam was similar but used a mixture of MeOH:DCM:ammonium acetate buffer [78].
Solid Phase Extraction
In-house comparisons have shown that amino-BHPs are better detected after solid phase extraction (SPE). An aliquot of the TLE was separated over a 1 mg NH2 solid phase extraction cartridge, as described in [79]. Briefly, the aliquot was dissolved and loaded onto a hexane-rinsed cartridge using 200 μL chloroform. Six mL of a 98:2 (v:v) diethyl ether:acetic acid solution was eluted. Residual material was dissolved with 200 μL 2:1 (v:v) chloroform:MeOH and loaded onto the cartridge, followed by elution with 10 mL of MeOH. BHPs were isolated from the MeOH fraction.
Lipid analyses
BHP preparation and HPLC/APCI–MSn analyses
A known amount (ca. 5–10 μg/g dry sediment) of internal standard (5α-pregnane-3β,20β-diol) was added to SPE extracts of the TLE for BHP analysis. Samples were acetylated in 0.5 mL of a 1:1 (v:v) mixture of acetic anhydride and pyridine at 50°C for 1 h, then left to stand overnight at room temperature [80]. Solvent was dried under a stream of N2 on a 50°C heating block. BHP samples were dissolved in MeOH:propan-2-ol (3:2; v:v), and filtered on 0.2 μm PTFE filters.
BHPs were analysed by high performance liquid chromatography coupled to positive ion atmospheric pressure chemical ionization mass spectrometry (HPLC/APCI-MS), using a data-dependent scan mode (2 events) on an HPLC system equipped with an ion trap MS, as described in [46,81]. Further structural information for novel BHPs was obtained by way of MS3 spectra. BHP concentrations were (semi) quantitatively estimated based on the response factor of authentic standards (M. Rohmer; Strasbourg, France and [46,77]) relative to the internal standard.
Results
In this study we investigated the BHP distributions in species of three AMO marine genera, and of eight marine environments, six of which were CH4-influenced.
Novel nitrogen-containing BHP components
In addition to the ‘regular amino-BHPs’ (e.g., I, II, and III; Figs 1 and 2), a suite of novel compounds were found in the methanotrophs and screened marine samples. Identification of these compounds is described in detail in the Supplementary Information (S1 File). Briefly, these components were related to the 35-amino-BHPs but differ in their terminal groups at C-35, which are tentatively proposed to comprise a methylcarbamate rather than a simple amine on the basis of interpretation of their APCI MS2 and MS3 spectra. In each case, the novel compounds (IMC, IMC’, IIMC, IIIMC; Figs 1 and 2) elute after their ‘regular’ amino-BHP analogues (i.e., I, I’, II, III; Fig 2). This indicates that the tentatively-assigned terminal group structures are less polar than the regular terminal amines (after acetylation). The novel structures include: 35-methylcarbamate-bacteriohopane-32,33,34-triol (MC-triol herein; IIIMC), 35-methylcarbamate-bacteriohopane-31,32,33,34-tetrol (MC-tetrol herein; IIMC), 35-methylcarbamate-bacteriohopane-30,31,32,33,34-pentol (MC-pentol herein; IMC) and an isomer of IMC (IMC’) akin to the early-eluting aminopentol isomer (I’), which was found, based on mass spectra, in a culture of Methylovulum-like strain M200 [46].
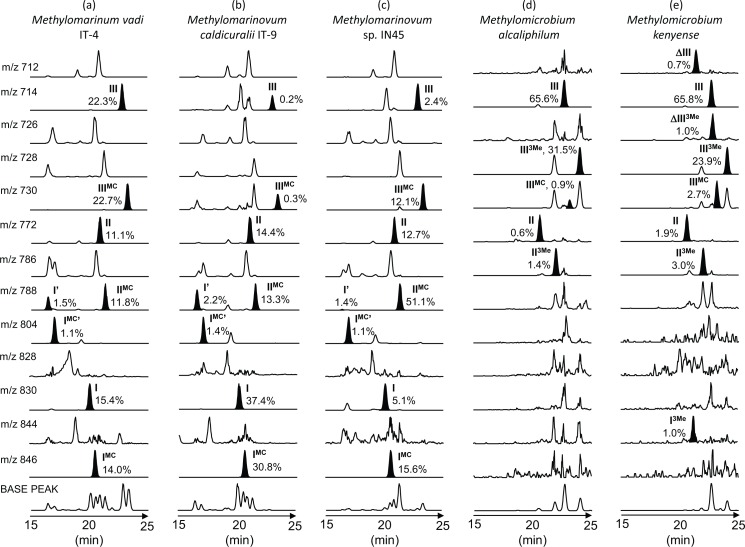
Fig 2. Distribution of nitrogen-containing BHPs in novel Type I methanotroph cultures.
Partial mass chromatograms (15–25 min) showing relative abundances (%) of BHPs (shaded peaks) in the acetylated total lipid extracts of (a) Methylomarinum vadi IT-4, (b) Methylomarinovum caldicuralii IT-9, (c) Methylomarinovum sp. IN45, (d) Methylomicrobium alcaliphilum, and (e) Methylomicrobium kenyense.
Methanotroph BHP signatures
Four previously untested methanotrophs isolated from marine or saline, alkaline lacustrine environments, belonging to the three genera Methylomarinum, Methylomarinovum, and Methylomicrobium, were analysed for their BHP composition. An additional species Methylomicrobium alcaliphilum, the partial BHP composition of which was recently reported in [53], is also shown here in full for comparison with Methylomicrobium kenyense. All of the methanotroph cultures investigated only synthesised BHPs with a nitrogen atom at C-35 position (nitrogen-containing BHPs herein). The relative abundances of BHPs are indicated as the percentage of total BHPs in acetylated extracts, and are presented in Fig 2. The low starting mass of some of the dry cell material led to uncertainty in the calculations of absolute concentrations, which are therefore not reported.
BHP inventory of Methylomarinum vadi IT-4
The most abundant BHPs in Methylomarinum vadi IT-4 were aminotriol (III), 22.3% and MC-triol (IIIMC), 22.7% (Fig 2A). Aminopentol (I), MC-pentol (IMC), aminotetrol (II), and MC-aminotetrol (IIMC) made up 15.4%, 14.0%, 11.1%, and 11.8% of total BHPs, respectively, with lower levels of the aminopentol isomers (I’ and IMC’; Fig 2A). No C-3 methylated or unsaturated equivalents of aminotriol, aminotetrol and aminopentol were found in Methylomarinum vadi IT-4.
BHP inventory of Methylomarinovum spp
The most abundant BHP in Methylomarinovum caldicuralii IT-9 was aminopentol (I), 37.4% (Fig 2B). However, Methylomarinovum sp. IN45 had only 5.1% aminopentol (Fig 2C). M. caldicuralii IT-9 had 30.8% MC-pentol, 14.4% aminotetrol, and 13.3% MC-tetrol, and lower abundances of aminopentol isomer, MC-pentol isomer, aminotriol, and MC-triol (Fig 2B). The most abundant BHP in Methylomarinovum sp. IN45 was MC-tetrol (IIMC), making up 51.1%. Methylomarinovum sp. IN45 also contained 12.7% aminotetrol, 15.6% MC-pentol, 12.1% MC-triol and lower levels of aminotriol, and the aminopentol isomers (Fig 2C). No C-3 methylated nor unsaturated equivalents of aminotriol, aminotetrol and aminopentol were present in either strains.
BHP inventory of Methylomicrobium spp
The Methylomicrobium alcaliphilum and Methylomicrobium kenyense cultures did not contain aminopentol (I) above detection limit (Fig 2D and 2E) although M. kenyense was found to contain minor abundance of 3-Me-aminopentol (I3Me; 1.0%; Fig 2E). The most abundant BHP in both Methylomicrobium cultures was aminotriol (III), making up ca. 65% of all BHPs in both species. The second most abundant BHP was 3-Me-aminotriol (III3Me) at 31.5% in M. alcaliphilum and slightly less in M. kenyense (23.9%). Both species also contained lower levels of aminotetrol (II) and 3-Me-aminotetrol (II3Me). M. kenyense also contained unsaturated compounds (ΔIII and ΔIII3Me; Fig 2E). The only MC compound identified in either Methylomicrobium sp. was MC-triol and then only at low levels (<3%).
Marine sediment and carbonate BHP signatures
Eight marine settings were studied for their BHP signatures (Table 2). Six of these were known to be influenced by CH4 (i.e., HHMV, BSCC, AMV, NZS, GoM cold seeps, GD) and two were used as comparison background marine levels (i.e., GoM sediments, PM).
Table 2. Concentrations (μg/g sediment) of amino-BHPs in marine sediment samples.
| Amino-BHPs | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | ΔI | IMC | I3Me | II | IIMC | II3Me | III | ΔIII | IIIMC | III3Me | ΔIIIMC | ΔIII3Me | Total nitrogen- containing BHPs (ug/g sediment) | ||
| Base Peaka | 830 | 828 | 846 | 844 | 772 | 788 | 786 | 714 | 712 | 730 | 728 | 728 | 726 | ||
| Håkon Mosby Mud Volcano (HMMV) | |||||||||||||||
| new mud flow 0–1 cm | PS74/2 169–1 PUC3 229 | bdl | bdl | bdl | bdl | 0.02 | 0.01 | bdl | 0.17 | 0.02 | 0.14 | bdl | 0.01 | bdl | 0.38 |
| aged flow 0–1 cm | ARK XXIV-2 PS74 172–1 237 | bdl | bdl | bdl | bdl | 0.01 | 0.01 | bdl | 0.29 | 0.05 | 0.81 | bdl | 0.13 | bdl | 1.3 |
| center 0–1 cm | MSM16/2 847–1 MUC 53 | bdl | bdl | bdl | bdl | 0.01 | 0.01 | bdl | 0.63 | 0.09 | 1.26 | bdl | 0.19 | bdl | 2.19 |
| center 10–12 cm | MSM16/2 HMMV 63 | bdl | bdl | bdl | bdl | 0.01 | 0.02 | bdl | 0.17 | 0.02 | 0.17 | bdl | 0.02 | bdl | 0.41 |
| new mud flow 0–1 cm | MSM16/2 838–1 MUC 33 | bdl | bdl | bdl | bdl | 0.02 | 0.01 | bdl | 1.07 | 0.09 | 0.82 | bdl | 0.06 | bdl | 2.07 |
| newer mud flow 0–1 cm | MSM16/2 855–1 MUC 73 | bdl | bdl | bdl | bdl | 0.01 | 0.01 | bdl | 1.1 | 0.13 | 1.24 | bdl | 0.15 | bdl | 2.64 |
| newer mud flow 10–12 cm | MSM16/2 HMMV 83 | bdl | bdl | bdl | bdl | 0.02 | 0.01 | bdl | 0.17 | 0.02 | 0.11 | bdl | 0.01 | bdl | 0.33 |
| aged flow 0–1 cm | MSM16/2 823–1 MUC 19 | bdl | bdl | bdl | bdl | 0.02 | 0.02 | bdl | 0.56 | 0.05 | 0.88 | bdl | 0.07 | bdl | 1.61 |
| aged flow 10–12 cm | MSM16/2 HMMV 28 | bdl | bdl | bdl | bdl | 0.01 | 0.01 | bdl | 0.15 | 0.01 | 0.11 | bdl | 0.01 | bdl | 0.29 |
| Barents Sea Carbonate Crusts (BSCC) depth profile | |||||||||||||||
| 0–2 cmbsf | bdl | bdl | bdl | bdl | 1.36 | bdl | bdl | 4.45 | 0.12 | bdl | bdl | bdl | bdl | 5.93 | |
| 2–4 cmbsf | bdl | bdl | bdl | bdl | 0.21 | bdl | bdl | 0.93 | 0.06 | bdl | bdl | bdl | bdl | 1.2 | |
| 4–6 cmbsf | bdl | bdl | bdl | bdl | 0.04 | bdl | bdl | 0.27 | 0.03 | 0.02 | bdl | bdl | bdl | 0.36 | |
| 6–8 cmbsf | bdl | bdl | bdl | bdl | 0.03 | bdl | bdl | 0.19 | 0.02 | 0.02 | bdl | bdl | bdl | 0.26 | |
| 8–10 cmbsf | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.07 | bdl | 0.02 | bdl | bdl | bdl | 0.09 | |
| 10–13 cmbsf | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.03 | bdl | 0.01 | bdl | bdl | bdl | 0.04 | |
| 13–16 cmbsf | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.08 | bdl | bdl | bdl | bdl | bdl | 0.08 | |
| 16–19 cmbsf | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.11 | bdl | bdl | bdl | bdl | bdl | 0.11 | |
| Amon Mud Volcano (AMV) | |||||||||||||||
| central dome 0–1 cm | M70/2a 760 PUC33 24 | bdl | bdl | bdl | bdl | 0.02 | 0.07 | bdl | 0.42 | 0.13 | 2.67 | bdl | 0.96 | bdl | 4.27 |
| sulfur band 0–1 cm | M70/2a 765 PUC49+50 71 | 0.18 | bdl | 0.12 | bdl | 0.38 | 0.27 | bdl | 1.24 | 0.81 | 1.33 | bdl | 1.02 | bdl | 5.35 |
| sulfur band 10–12 cm | M70/2 81 | 0.36 | 0.04 | 0.11 | bdl | 0.3 | 0.11 | bdl | 1.07 | 1.09 | 0.48 | bdl | 0.5 | bdl | 4.06 |
| sulfur band 0–1 cm | M70/2a 790 PUC68 172 | 0.36 | bdl | 0.11 | bdl | 1.49 | 0.49 | bdl | 3.82 | 1.29 | 1.49 | bdl | 0.61 | bdl | 9.67 |
| sulfur band, white mat | MSM13/3 947–1 PUC31 73D | 0.09 | bdl | 0.11 | bdl | 0.31 | 0.24 | bdl | 0.64 | 0.36 | 0.71 | bdl | 0.44 | bdl | 2.9 |
| sulfur band 0–1 cm | MSM13/3 968–1 PUC15 122 | 0.08 | bdl | 0.06 | bdl | 0.75 | 0.49 | bdl | 0.73 | 0.46 | 0.77 | bdl | 0.28 | bdl | 3.62 |
| bacterial mats 0–1 cm | MSM13/3 929–1 PUC22 45D | 0.05 | bdl | 0.01 | bdl | 1.13 | 0.17 | bdl | 1.11 | 0.43 | 0.5 | bdl | 0.16 | bdl | 3.56 |
| bacterial mats 10–12 cm | MSM13/3 50D | bdl | bdl | bdl | bdl | 0.01 | bdl | bdl | 0.03 | bdl | 0.02 | bdl | bdl | bdl | 0.07 |
| gassy centre, 10–12 cm | MSM13/3 61D | bdl | bdl | bdl | bdl | 0.01 | bdl | bdl | 0.04 | 0.01 | 0.02 | bdl | 0.01 | bdl | 0.08 |
| New Zealand Seeps (NZS) | |||||||||||||||
| 0–2 cmbsf | Frenulata 45 | bdl | bdl | bdl | bdl | 0.09 | 0.06 | bdl | 0.21 | 0.03 | 0.29 | bdl | 0.05 | bdl | 0.73 |
| 10–12 cmbsf | Frenulata 45 | bdl | bdl | bdl | bdl | 0.13 | 0.03 | bdl | 0.26 | 0.04 | 0.15 | bdl | 0.02 | bdl | 0.63 |
| 0–2 cmbsf | Ampharetidae 309 | 0.06 | bdl | bdl | bdl | 1.11 | 0.17 | bdl | 1.32 | 1.17 | 2.59 | bdl | 0.26 | bdl | 6.67 |
| 10–12 cmbsf | Ampharetidae 309 | bdl | bdl | bdl | bdl | 0.01 | 0.02 | bdl | 0.08 | 0.02 | 0.13 | bdl | 0.02 | bdl | 0.28 |
| 0–2 cmbsf | Sulfur-oxidising bacteria 315 | 0.02 | bdl | bdl | bdl | 0.66 | 0.32 | bdl | 2.24 | 0.23 | 2.13 | bdl | 0.21 | bdl | 5.8 |
| 10–12 cmbsf | Sulfur-oxidising bacteria 315 | bdl | bdl | bdl | bdl | 0.03 | bdl | bdl | 0.14 | 0.02 | 0.1 | bdl | bdl | bdl | 0.28 |
| Golfo Dulce (GD) Surface Sediment | |||||||||||||||
| 10 m water depth | SG 1 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.01 | bdl | bdl | bdl | bdl | bdl | 0.01 |
| 24 m water depth | SG 2 | 0.16 | bdl | bdl | bdl | 0.03 | bdl | bdl | 0.36 | bdl | 0.02 | bdl | bdl | bdl | 0.57 |
| 77 m water depth | SG 4 | 0.02 | bdl | bdl | bdl | 0.01 | bdl | bdl | 0.15 | 0.03 | 0.01 | bdl | bdl | bdl | 0.23 |
| 90 m water depth | SG 5 | 0.04 | bdl | bdl | bdl | 0.02 | bdl | bdl | 0.17 | 0.05 | 0.01 | bdl | bdl | bdl | 0.29 |
| 110 m water depth | SG 6 | bdl | bdl | bdl | bdl | 0.01 | bdl | bdl | 0.08 | 0.07 | 0.02 | bdl | bdl | bdl | 0.18 |
| 120 m water depth | SG 7 | 0.04 | bdl | bdl | bdl | 0.02 | bdl | bdl | 0.36 | 0.2 | 0.02 | bdl | bdl | bdl | 0.63 |
| 140 m water depth | SG 8 | 0.01 | bdl | bdl | bdl | <0.01 | bdl | bdl | 0.11 | 0.08 | 0.01 | bdl | bdl | bdl | 0.21 |
| Gulf of Mexico (GoM) Cold Seeps | |||||||||||||||
| GC 234 CNSTS 4434 | bdl | bdl | bdl | bdl | 0.01 | bdl | bdl | 0.09 | bdl | bdl | bdl | bdl | bdl | 0.1 | |
| 234 4436 | bdl | bdl | bdl | bdl | <0.01 | bdl | bdl | 0.04 | bdl | bdl | bdl | bdl | bdl | 0.04 | |
| GC 234 tube worm roots 4435 | bdl | bdl | bdl | bdl | <0.01 | bdl | bdl | 0.02 | bdl | bdl | bdl | bdl | bdl | 0.02 | |
| GC 232 | bdl | bdl | bdl | bdl | 0.01 | bdl | bdl | 0.01 | bdl | bdl | bdl | bdl | bdl | 0.02 | |
| GC 185 | bdl | bdl | bdl | bdl | 0.76 | bdl | bdl | 0.2 | bdl | bdl | bdl | bdl | bdl | 0.95 | |
| GoM Sediments | |||||||||||||||
| West Gulf | WG2-099/6 | bdl | bdl | bdl | bdl | <0.01 | bdl | bdl | 0.01 | bdl | bdl | bdl | bdl | bdl | 0.01 |
| 26°50'N, 92°40'W | WG2-099/9 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | <0.01 | bdl | <0.01 | bdl | bdl | bdl | 0.01 |
| Eastern Gulf | 88-C-1 DCS 128/17 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.01 | bdl | 0.01 | bdl | bdl | bdl | 0.02 |
| 27°30'N, 87°20'W | DCS 128/13 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | <0.01 | bdl | <0.01 | bdl | bdl | bdl | 0.01 |
| DCS 128/21 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | <0.01 | bdl | 0.01 | bdl | bdl | bdl | 0.01 | |
| Central Gulf | CGD-136/17 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.01 | bdl | 0.01 | bdl | bdl | bdl | 0.01 |
| 28°20'N, 89°38'W | CGD-136/13 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | <0.01 | bdl | 0.01 | bdl | bdl | bdl | 0.01 |
| Peru Sediment | |||||||||||||||
| 10–15 cmbsf | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.09 | bdl | bdl | bdl | bdl | bdl | 0.09 | |
| 20–25 cmbsf | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.02 | bdl | bdl | bdl | bdl | bdl | 0.02 | |
| 40–45 cmbsf | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.09 | bdl | bdl | bdl | bdl | bdl | 0.09 | |
abase peak = [M + H–CH3COOH]+
bdl–below detection limit
Håkon Mosby Mud Volcano (HMMV)
None of the HMMV sediment samples contained aminopentol (Table 2). The most abundant amino- and MC-BHPs in HMMV samples was either aminotriol (III) or MC-triol (IIICME), making up 22–52% and 32–62% of total nitrogen-containing BHPs, respectively. Unsaturated aminotriol, unsaturated MC-triol, aminotetrol, and MC-tetrol all made up <10% of total nitrogen-containing BHPs. Some HMMV samples contained relatively high concentration of anhydrobacteriohopanetetrol (anhydro-BHT). Minor abundances of BHT and BHT isomer, BHT-cyclitol ether (BHT-CE), and BHT-glucosamine (BHT-G) were detected in some samples (S1 Table).
Barents Sea carbonate crust (BSCC)
The 19 cm BSCC core contained a majority of aminotriol (III; 72–100% of nitrogen-containing BHPs; Table 2). MC-triol (IIIMC; 0–23%) peaked between 4 and 13 cm. Aminotetrol (II; 0–23%) was detected in the upper 8 cm. Minor contribution of unsaturated aminotriol (ΔIII; 0–7%) was detected in the upper sediments. Other BHPs detected included BHT, and low concentrations of anhydroBHT, BHT isomer, 2-methyl-BHT (2-Me-BHT), adenosylhopane, BHT-CE, and BHT-G (S1 Table).
Amon Mud Volcano (AMV)
The most abundant nitrogen-containing BHPs in sediments from the Amon mud volcano were aminotriol (III; 9.8–48.9%) and MC-triol (IIIMC;11.8–62.5%) (Table 2). Other nitrogen-containing BHPs in these sediments were aminotetrol (II; 0.5–31.8%), unsaturated aminotriol (ΔIII; 3.0–27.0%) and unsaturated MC-triol (ΔIIIMC; 0–22.5%). Minor abundances of aminopentol (I; 0–8.9%), and MC-pentol (IMC; 0–3.8%) were found in some AMV sediments. Other BHPs in the AMV sediments were BHT, anhydroBHT, 2-Me-BHT, 3-Me-BHT, BHT-CE, BHT-G, BHT isomer, and adenosylhopane (S1 Table).
New Zealand Seeps (NZS)
The most abundant nitrogen-containing BHP in the sediments from NZS were aminotriol (III; 28.5–72.7%), MC-triol (IIIMC; 13.2–46.4%) and aminotetrol (II; 5.1–20.6%) (Table 2). Unsaturated aminotriol (ΔIII), unsaturated MC-triol (ΔIIIMC), and MC-tetrol (IIMC) all made up <10%. Two sediment samples contained aminopentol, albeit in low abundance (<1% of nitrogen-containing BHPs). Both of these aminopentol positive samples also contained soil marker BHPs [77,82]. Other BHPs found in NZS samples were BHT, anhydroBHT, 2-methyl-BHT, 3-methyl-BHT, BHT isomer, adenosylhopane, and BHT-CE (S1 Table).
Gulfo Dulce (GD)
The most abundant nitrogen-containing BHP in GD surface sediments was aminotriol (III; 44.3–100%; Table 2). Unsaturated aminotriol (ΔIII) made up 0–41.3%. MC-triol (IIIMC; 0–11.4%) and aminotetrol (II; 0–6.0%) were found in most samples. Aminopentol was found in all but two surface samples, one of which was the shallowest site. Aminopentol abundance decreased with increasing water depth with the highest abundance at 24 m water depth (27.6% of nitrogen-containing BHPs). BHT, anhydroBHT, BHT isomer, and 2-Me-BHT were also found in GD (S1 Table).
Gulf of Mexico (GoM) cold seeps
GoM cold seeps only contained aminotriol (III; 20.5–100%) and aminotetrol (II; 0–79.5%) nitrogen-containing BHPs (Table 2). Other BHPs included BHT, soil marker BHPs, 2-Me-BHT, BHT isomer, and BHT-CE (S1 Table).
GoM sediments
GoM sediments from near the outflow of Mississippi River Delta showed an abundance of aminotriol (III; 26.6–80.7% of total nitrogen-containing BHPs), MC-triol (IIIMC; 0–73.4%) (Table 2). One sample from the Western Basin had 19.3% aminotetrol (II) relative to total BHPs; however, the concentration of aminotetrol was low (<0.01 μg/g sediment). Other BHPs included BHT, BHT isomer, anhydroBHT, 2-methyl-BHT, BHT-CE, and soil marker BHPs (S1 Table).
Peru Margin (PM)
The only nitrogen-containing BHP detected in sediments from the PM was aminotriol (III). Other BHPs found at this site included BHT, BHT isomer, anhydroBHT, 2-Me-BHT, adenosylhopane, and BHT-CE (S1 Table).
Discussion
BHP distributions in aerobic methanotrophs
Previously reported BHP distributions in AMO bacteria
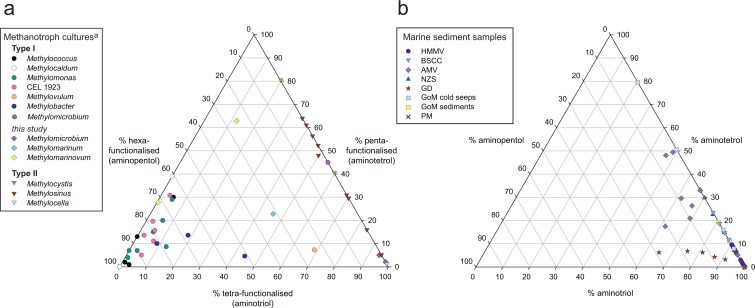
Traditionally, Type I and Type II AMO bacteria had been distinguished by their different BHP signatures (e.g. [52]; see also review in [14]). Prior to the investigation of BHPs in the Methylovulum-like strain M200 [46], most screened Type I methanotrophs synthesised a high percentage of aminopentol (I) and lower contributions from aminotetrol (II) and in some cases aminotriol (III), and clustered in the left-hand corner of the amino-BHP ternary plot (Fig 3A). In contrast, Type II methanotrophs did not contain aminopentol, had varying contributions from II and III, and clustered along the right-hand axis of Fig 3A. The high relative abundance of III observed in Methylovulum-like strain M200 was, therefore, originally seen as an outlier [46]. Similarly, [52] showed that a culture of Methylomicrobium album did not contain aminopentol. At the time this was presumed to be a contaminated culture, however, [53] also did not report I synthesis in cultures of Methylomicrobium alcaliphilum. All of the recently analysed Methylobacter spp. [28] join the more typical Type I methanotrophs in the left-hand corner of the plot, however, Methylobacter sp. BB5.1 increased the spread of the cluster with almost 40% III content.
Fig 3. Relative composition (%) of ‘regular’ amino-BHPs.
Distributions of aminotriol, aminotetrol and aminopentol, including their C-3 methylated homologues, where present, are shown for (a) methanotroph cultures including literature data (circles) and data from new cultures of Methylomicrobium, Methylomarinum, and Methylomarinovum (diamonds; this study) and (b) sediments and microbial mats from methane-rich marine settings (HMMV = Håkon Mosby mud volcano; BSCC = Barents Sea carbonate crust; AMV = Amon mud volcano; NZ = New Zealand; GD Golfo Dulce; GoM = Gulf of Mexico; PM = Peru Margin). Methanotroph literature data from [36,42,46,52,83]. aLiterature data from [36,42,83] was based on GC-MS hopanol quantifications after periodic acid treatment. Therefore, it is not possible to distinguish between amino- and non-amino-BHPs with the same number of functional groups. This is especially significant for the Type II aerobic methanotrophic bacteria that are known to synthesise bacteriohopanetetrol. For this reason, the axes of plot (a) are shown as functionality of the BHP-side chain.
Amino-BHP distributions in previously untested Type I AMO bacterial cultures
It was assumed that the screened species of AMO Type I bacteria investigated in this study would display similar BHP distributions as those of previously reported Type I bacteria. All three bacterial genera screened do indeed only contain amino-BHPs (Fig 2). However, the relative distribution of specific nitrogen-containing BHPs varies between genera, as well as between species belonging to the same genus. To allow for a more accurate comparison with data from the literature (Fig 3A, circles), only aminopentol, aminotetrol, aminotriol, and their methylated equivalents were considered when producing the ternary plot of ‘regular’ nitrogen-containing BHPs of the novel Type I cultures (Fig 3A, diamonds).
Aminopentol is the most abundant BHP in the novel species Methylomarinovum cadicuralii IT-9, which is in agreement with literature BHP compositions of most other Type I methanotrophs (Fig 3A, circles), e.g., Methylococcus capsulatus [44,52], Methylomonas sp. [42,46,52], psychrotolerant isolate CEL 1923 [42], and Methylocaldum tepidum [22]. However, a species in the same genus (Methylomarinovum sp. IN45) has a much lower abundance of aminopentol (5.0% of total amino-BHPs; Fig 2C). Methylomarinum vadi IT-4 shows relatively high proportions of aminopentol, but it is not the most abundant BHP (Fig 2A). Moreover, in our screening of two species of Methylomicrobium spp., aminopentol was not detected, similar to reported cultures of Methylomicrobium album and Methylomicrobium alcaliphilum [52,53]. Our results seem to confirm the near-absence of aminopentol in all screened Methylomicrobium spp., which are the first Type I methanotrophs apparently unable to synthesise aminopentol. However, changes in BHP composition can occur at different growth stages and under different conditions (e.g. [27,83,84]), so further studies would be required to fully confirm this. It appears that the BHP distributions of Methylomicrobium and Methylovulum, which do not synthesise high amounts of aminopentol, should also no longer be considered outliers given the low levels of aminopentol in M. vadi IT-4 and Methylomarinovum spp. (Fig 2). This suggests a greater variance in the BHPs of Type I methanotrophs than previously thought. Furthermore, as Methylomicrobium has been isolated from a diverse range of marine environments [85–87], the absence of aminopentol in this genus might greatly affect its application as a marine aerobic methanotrophy biomarker. This, however, does not invalidate the use of aminopentol as a biomarker for methanotrophy.
There is also significant variation in the relative abundances of the other nitrogen-containing BHPs in Type I methanotrophs. A suite of novel BHPs identified as methylcarbamate (MC) BHPs are detected in all three genera screened in this study (Fig 2). These have not been reported in previous studies. Therefore, data available at Newcastle University from the analyses of Methylococcus capsulatus (Talbot et al., unpublished), Methylovulum-like strain M200, Methylomonas methanica, Methylomonas-like strain M5 [46], and Methylobacter spp. [28] were re-examined. IIIMC was identified retrospectively in Methylomonas methanica, and all species of Methylobacter. IIIMC constituted up 9.8% of total amino-BHPs in Methylobacter sp. BB 5.1. IIMC was present in low abundance (<0.2%) in two of the three species of Methylobacter (Methylobacter sp. BBA 5.1 and Methylobacter sp. BB 5.1), but absent in Methylobacter marinus A 45. IMC was absent in Methylococcus capsulatus and Methylovulum-like strain M200, but was present in all species of Methylobacter. IMC was also present in Methylomonas methanica but absent in a different species of this genus (i.e., Methylomonas-like strain M5; [46]). These results indicate that MC-BHPs are not universally present when their regular homologues are detected, and may be species specific and/or dependent on variations in growth conditions such as pH.
The two species of Methylomarinovum display significant variations in their BHP compositions (Fig 2B and 2C). Methylomarinovum sp. IN45 had a relatively low level of aminopentol. The most abundant BHP in Methylomarinovum sp. IN45 is IIMC. Although in lower abundances, IIIMC and IMC are also higher in comparison to their ‘regular’ homologues in this species compared to M. caldicuralii IT-9 (Fig 2B and 2C). In contrast, the most abundant BHP in Methylomarinovum caldicuralii IT-9 is aminopentol, followed by almost equal amounts of IMC. The different relative BHP distributions between the Methylomarinovum spp. highlight that there can be significant variations within a genus. Methylomarinovum sp. IN45 was isolated from a deep-sea hydrothermal field and perhaps the high levels of methylcarbamate components observed are the result of a physiological adaptation to higher pressure in this environment. This may explain why the relative abundances of components in Methylomarinovum caldicuralii IT-9, the same genus but isolated from a shallow submarine hydrothermal environment, are quite different. Perhaps the complex functionality of the terminal group of the methylcarbamate components is more effective at stabilising the cell membrane and decreasing fluidity under these conditions.
3-methylaminotriol (III3Me) was observed in both Methylomicrobium spp. (23.9–31.5% of total BHPs; Fig 2D and 2E) in agreement with a recent report in [53]. This compound was accompanied by low levels of 3-methylaminotetrol (II3Me) in both species and trace amounts of 3-methylaminopentol in M. kenyense (I3Me). The absence of C-3 methylated structures in the previously investigated Methylomicrobium album strain BG8 [52] may appear inconsistent with the organisms investigated here; however, genomic investigations have revealed that M. album is separated from halo(alkali)philic representatives of the Methylomicrobium genus such as M. alcaliphium and M. kenyense [58], and perhaps specific environmental conditions influence the BHP composition of Methylomicrobium spp. as they seemingly do within the Methylomarinovum genus.
No C-3 methylated equivalents of aminotriol (III3Me), aminotetrol (II3Me), nor aminopentol (I3Me) were present in Methylomarinum vadi IT-4 or the Methylomarinovum spp., adding to examples of Type I methanotroph species that contain amino-BHPs, but not their C-3 methylated equivalents (e.g., see review in [14]). The most abundant BHPs in Methylomarinum vadi IT-4 were aminotriol (III) and MC-triol (IIIMC), which were present in equal amounts (Fig 2A). Similar amounts of II and IIMC, and I and IMC were also observed in this culture. A high proportion of III is unusual for a Type I methanotroph, but has been observed before in the Methylovulum-like strain M200. (Fig 4A; [46]). The new data reiterate that aminopentol is not always the most abundant BHP in Type I methanotrophs, nor necessarily the most appropriate biomarker for AMO.
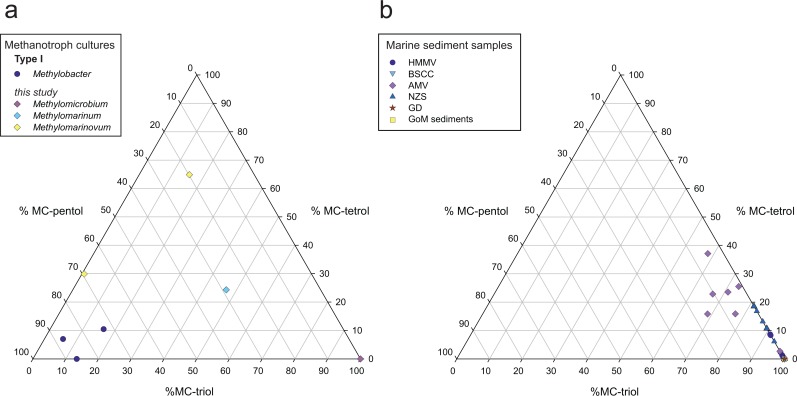
Fig 4. Relative composition (%) of methylcarbamate(MC)- BHPs.
Distributions of MC-triol, MC-tetrol and MC-pentol are shown for (a) methanotroph Type I cultures, Methylobacter, Methylomicrobium, Methylomarinum, and Methylomarinovum, and (b) marine sediments (HMMV = Håkon Mosby mud volcano; BSCC = Barents Sea carbonate crust; AMV = Amon mud volcano; NZ = New Zealand; GD Golfo Dulce; GoM = Gulf of Mexico). Methylobacter data from [28].
BHPs in marine environments
Lack of BHP diversity in marine environments
The screened marine sediments and authigenic carbonates do not show large diversity in their BHP signatures (S1 Table). The limited BHP distributions are also comparable to other reported marine sediment BHP signatures, all dominated by BHT and BHT isomer, from a number of locations including the Black Sea [88], the Benguela upwelling system [89], and the Arabian Sea [90]. More recently a similar pattern was also seen in water column samples from the California Current system, where the wide diversity observed in the gene responsible for hopane cyclisation (squalene-hopene cyclase) was not reflected by distinct BHP fingerprints related to this potential range of source-organisms [91]. However, genetic information is quickly lost, and we must strive to find lipid biomarkers to trace particular metabolisms in the geological record.
Non-nitrogen-containing BHP concentrations in the screened sediments do not show remarkable signatures (S1 Table). BHT and anhydro-BHT, thought to be a degradation product of BHT and other composite BHPs such as BHT cyclitol ether [92], were found at all sites. The presence of soil marker BHPs at some sites, particularly NZS, indicates that these sediments could be influenced by terrestrial input of organic matter (e.g., [77,82,93]). However, as adenosylhopane is an intermediate in the biosynthesis of all other side-chain extended BHPs [94], other sources cannot be entirely excluded. BHT isomer, a biomarker for anaerobic ammonium oxidation [70], was found in high concentrations in GD (previously reported in [70]), as well as in the PM sediments, which underlie the Peruvian OMZ, where anammox is known to be an important process [95], and where BHT isomer has previously been reported from the water column within the OMZ [90]. The most abundant of the three regular amino-BHPs in the CH4-influenced marine sediments was aminotriol, which is not source-specific (e.g., [48]).
Aminopentol in marine sediments
Although aminopentol was found in significant abundance in some of the reported and screened Type I methanotroph cultures (Fig 2), it was not found to be abundant in most of the CH4-influenced marine sites in this study (Table 2). In fact, it was only detected in AMV, GD surface sediments, and two NZS samples (Fig 3B; Table 2). The discrepancy between the distinct amino-BHP signatures of isolated Type I AMO bacteria and signatures of CH4-influenced marine sites is highlighted in the ternary plots of the relative composition of aminopentol, aminotetrol, and aminotriol (Fig 3A cf. Fig 3B). These differences could be due to the particular methanotrophic bacterial community responsible for methanotrophy in the CH4-influenced marine sediments. Ruff et al. [96] found that diversity in the global CH4 seep microbiome was controlled by environmental factors such as temperature and electron acceptor availability. Considering their findings, it is possible that the environmental conditions in most marine CH4-influenced sediments favour specific methanotroph communities. For example Methylomicrobium spp., found in saline environments [85–87] and saline, highly alkaline environments [58,97], and which do not produce aminopentol in significant amounts (Fig 2), could be present. However, the absence of C-3 methylated compounds is confounding for a Methylomicrobium source, pointing towards other methanotrophs that do not synthesise aminopentol. Yan et al. [98] found that 85% of the operational taxonomic units (OTUs) from the same sites as our GoM cold seeps did not group with known sequences of a subunit of particulate methane monooxygenase (pmoA). This would suggest the presence of novel methanotrophic species in GoM. In the same way, significant pmoA diversity has been observed in sediments from the North American margin [99], a shallow CH4 seep [100], a marine estuary [101], and hydrocarbon seeps [102]. pmoA OTUs from the NZS sediments grouped with methanotrophic endosymbionts [68], including Bathymodiolus spp., which have been shown to contain neither aminopentol nor methylated BHPs [103]. Nevertheless, the absence of methylated amino-BHPs in the screened marine sediments (Table 2) may suggest Methylomicrobium album, or a related species that also does not synthesise methylated amino-BHPs, being the dominant methanotroph in CH4-influenced marine environments.
These are not the first reports of marine CH4-influenced environments not containing aminopentol (Table 1). For example, using methods targeting the functional gene pmoA, which is produced by most methanotrophs, Type I methanotrophs were detected in all three units of Ace Lake sediments. However, aminopentol was only detected in sediments deposited under freshwater conditions (unit III) despite the fact that the modern meromictic water column, containing relict seawater left behind after the sea level fell around 9000 years ago, hosts the Type I methanotroph Methylosphaera hansonii [104]. No aminopentol was detected in the methanotrophic symbionts in the gill tissue of a cold-seep mussel, despite other lipid-based evidence suggesting the presence of a Type I methanotroph [103,105]. Similarly, CH4 seep carbonates from Alaminos Canyon, northern Gulf of Mexico [41] and the Northern Arabian Sea [106] were found to lack aminopentol. Conversely, aminopentol was detected in the water column of the Baltic Sea with supporting evidence for the presence of Type I methanotrophs from 13C-depleted PLFAs [107]. Aminopentol was also detected in the water column of the Black Sea in the oxic-anoxic water transition, but not in the underlying sediment [12,88,108].
The presence of aminopentol in sediments from the AMV, located on the Nile deep-sea fan, in the Eastern Basin of the Mediterranean Sea (Table 2) may be explained by Nile River outflow carrying terrestrial wetland methanotrophy signatures into the Mediterranean, as seen in the Amazon and Congo River fans [14,109]. This would appear to indicate that aminopentol is still an excellent biomarker for terrestrial AMO. The near-absence of soil-marker BHPs in AMV (Table 2) may still point towards in-situ marine production of aminopentol. However, the relative abundance of soil-markers in terrestrial settings has recently been found to be strongly influenced by environmental factors; higher temperatures and low pH (in peatlands) can both strongly reduce the relative proportion of soil marker BHPs as a proportion of total BHPs [40,110,111]. Aminopentol was found in NZS sediments that also contained soil marker BHPs (Table 2). Therefore, aminopentol in sediments from NZS may have originated from terrestrial sources. Aminopentol in GD surface sediments may be the result of a distinct AMO community living in the specific environment prone to carbonate formation in GD. Unfortunately, samples were not properly preserved to be able to determine AMO diversity using genetic-based analyses of the pmoA gene in these sediments. The cumulative results of the studied marine sites do, however, indicate that an absence of aminopentol is not necessarily evidence for the absence of methanotrophs or aerobic methane oxidation.
Alternative BHP biomarkers for AMO and implications for the marine sedimentary record of methanotrophy
Regular amino-BHPs
Screened Type I methanotrophs also produced varying amounts, depending on the genera, of aminotetrol (II) and aminotriol (III) (Fig 3A), both of which were found in CH4-influenced marine sediments (Fig 3B; Table 2). However, these two amino-BHPs are less source-specific to methanotrophic bacteria than aminopentol, and do not make ideal biomarker lipids for methanotrophy. Given that 3-Me-aminotriol (III3Me) made a significant contribution to the amino-BHP abundance in screened cultures of Methylomicrobium spp. (23.9 and 31.5% of total amino-BHPs; Fig 2D and 2E) and 9.8% in Methylobacter sp. BB5.1 [28], it was expected that III3Me would be an important amino-BHP in CH4-influenced marine sediments. However, III3Me was not found in any of the screened sediments (Table 2). Methylomicrobium alcaliphilum and Methylomicrobium kenyense are adapted to high alkalinity, but not necessarily to high salinity [58]. This distinct lack of III3Me in marine sediment samples would seem to indicate that the Methylomicrobium species we investigated are not the primary source of amino-BHPs in CH4-influenced marine environments.
III3Me has only occasionally been reported from environmental samples including some soils [82,112] and most recently in a peat core from Germany [111], but only at very low levels (Table 1). Other C-3 methylated amino-BHPs are even less common (Table 1). I3Me was first reported from a neo-volcanic, eutrophic and saline lake sediment (La Piscina de Yuriria, Mexico; [45]), and subsequently from a geothermal silica sinter (Opaheke Pool hot spring, New Zealand; [113]). The pentafunctionalised II3Me was also present in the Mexican lake sediment. Both of these compounds were reported in one study on the Black Sea water column [12], but were absent at another site [108]. The apparent discrepancy between the very limited occurrence of C-3 methylated BHPs (as measured using the periodic acid cleavage technique which converts polyfunctionalised BHPs into GC-amenable primary alcohols; e.g., [36,75]) and their wider occurrence in the form of 3-Me hopanes in ancient rocks and oils was first identified in [114]. These authors found 3-Me-BHPs to be abundant only in a very limited number of settings, under quite specific conditions (i.e., some alkaline lakes). The occurrence of 3-Me-hopanes in marine authigenic carbonates [31,115], which form under highly alkaline conditions are also consistent with a Methylomicrobium source ([58], and references therein). It was further suggested that 3-Me-BHPs and hexafunctionalized BHPs appear to have different sources (possibly, but not necessarily restricted to, only Type I methanotrophs; [114]). Culture studies (on the moderately thermophilic Type I methanotroph Methylococcus capsulatus) have shown that production of C-3 methylated compounds may be related to growth stage. Higher relative proportions of methylated BHPs replaced the non-methylated equivalents during stationary phase growth [83], and appear to be necessary for maintaining intra-cellular membrane structures [27]. These important physiological roles for methylated BHPs are at odds with the very sparse occurrence of these compounds in modern settings (Table 1), and clearly our understanding of the factors controlling their biosynthesis and subsequent preservation in sediments is still limited, hampering interpretation of certain BHP signatures.
Methylcarbamate-BHPs
Most of the marine sediments influenced by CH4 contained at least MC-triol, albeit at relatively low abundances (Fig 4B; Table 2). The fact that the MC-BHPs were found in all strains of methanotrophs analysed, though not all components in the suite were present in every strain, shows the biomarker potential of these BHPs for AMO (Fig 3A). MC-tetrol (IIMC) was the most abundant component in Methylomarinovum sp. IN45, and MC-BHPs were found in higher abundance than the ‘regular’ 35-amino-BHP homologues, which may allow this particular hydrothermal vent species to be identified in environmental settings. Unsaturated MC-triol (ΔIIICME) was found in high abundance in AMV, HMMV, and NZS, but was not found in any of the methanotroph cultures. This is possibly because the BHP signatures in most CH4-influenced marine sediments are sourced from AMO bacteria that have no cultured relatives or at least none which have been tested for BHP production.
Given the small diversity in BHPs found in marine sediments and the need for an AMO biomarker, there appear to be few BHPs that meet the criteria of being source-specific and abundant. This has significant implications for the development of a proxy using aminopentol to trace AMO in marine settings. Applying MC-BHPs combined with the traditional suite of amino-BHPs (e.g. aminopentol, aminotetrol, and aminotriol) seems to be the most appropriate biomarker course for AMO.
Conclusions
Isolated methanotrophs from previously unexamined genera and species displayed marked differences in their relative abundances of amino-bacteriohopanepolyols (BHPs). Aminopentol (I) was the most abundant BHP in Methylomarinovum caldicuralii IT-9, which fits with the typical BHP signature of known Type I methanotrophs. However, the BHP signatures of Methylomarinovum sp. IN45 and Methylomarinum vadi IT-4 both did not show aminopentol as the most abundant BHP. Moreover, neither of the Methylomicrobium spp. contained aminopentol and only one contained a low level of 3-methyl-aminopentol showing that not all Type I methanotrophs synthesise aminopentol, agreeing with previous environmental studies. Considering Methylomicrobium can be prevalent in marine environments, this has implications for the use of aminopentol as a biomarker for marine methanotrophy. A suite of components related to amino-BHPs, but with methylcarbamate (MC) terminal groups, were detected for the first time, and were present in all Type I methanotroph strains tested. Marine sediments influenced by CH4 did not contain significant amount of aminopentol, but did contain MC-BHPs. This study highlights the relatively low BHP diversity within marine sediments, and indicates that the combined use of MC-BHPs and amino-BHPs might be preferential to trace aerobic methane oxidation (AMO) in marine settings.
Supporting Information
(DOCX)
Concentration (μg/g sediment) of other BHPs in marine sediments samples presented in this study.
(XLSX)
Acknowledgments
We are deeply thankful to A. Boetius for supplying the sediments from HMMV, AMV, and NZS. Additional information about these sites can be found on the Pangaea database (http://www.pangaea.de/).The PM sediments analysed in this study were originally provided by T. Eglinton and J. Repeta. R.D. Pancost is thanked for use of GoM cold seep material. The GoM sediments used in this study were provided by R. Sassen and S. Sweet of the Geochemical & Environmental Research Group (GERG) at Texas A&M University, U.S.A. We thank the captain and the crew of the RV Kaiyo, the operation team of the ROV Hyper-Dolphin, and the shipboard scientific party for the invaluable help in collecting deep-sea samples. We also thank M. Abe and K. Tanaka for their assistance in cultivation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was funded by a Starting Grant (No. 258734) awarded to HMT for project AMOPROX, from the European Research Council. KM and JCN thank Lundin Petroleum for financial support. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lelieveld J, Crutzen PJ, Dentener FJ (1998) Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B 50: 128–150. [Google Scholar]
- 2.IPCC (2007) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (Eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. [Google Scholar]
- 3.Dickens GR, O'Neil JR, Rea DK, Owen RM (1995) Dissociation of oceanic methane hydrate as a cause of the carbon isotope excursion at the end of the Paleocene. Paleoceanography 10: 965–971. [Google Scholar]
- 4.Dickens GR, Castillo MM, Walker JCG (1997) A blast of gas in the latest Paleocene: Simulating first-order effects of massive dissociation of oceanic methane hydrate. Geology 25: 259–262. [DOI] [PubMed] [Google Scholar]
- 5.Zachos JC, Rohl U, Schellenberg SA, Sluijs A, Hodell DA, Kelly DC, et al. (2005) Rapid acidification of the ocean during the Paleocene-Eocene thermal maximum. Science 308: 1611–1615. 10.1126/science.1109004 [DOI] [PubMed] [Google Scholar]
- 6.Handley L, Pearson PN, McMillan IK, Pancost RD (2008) Large terrestrial and marine carbon and hydrogen isotope excursions in a new Paleocene/Eocene boundary section from Tanzania. Earth Planet Sci Lett 275: 17–25. [Google Scholar]
- 7.McInerney FA, Wing SL (2011) The Paleocene-Eocene thermal maximum: a perturbation of carbon cycle, climate, and biosphere with implications for the future. Annu Rev Earth Planetary Sci 39: 489–516. [Google Scholar]
- 8.Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60: 439–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieburth JM, Johnson PW, Eberhardt MA, Sieracki ME, Lidstrom M, Laux D (1987) The first methane-oxidizing bacterium from the upper mixing layer of the deep ocean—Methylomonas pelagica sp. nov. Curr Microbiol 14: 285–293. [Google Scholar]
- 10.Hovland M, Svensen H, Forsberg CF, Johansen H, Fichler C, Fossa JH, et al. (2005) Complex pockmarks with carbonate-ridges off mid-Norway: Products of sediment degassing. Mar Geol 218: 191–206. [Google Scholar]
- 11.McDonald IR, Smith K, Lidstrom ME (2005) Methanotrophic populations in estuarine sediment from Newport Bay, California. FEMS Microbiol Lett 250: 287–293. 10.1016/j.femsle.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 12.Blumenberg M, Seifert R, Michaelis W (2007) Aerobic methanotrophy in the oxic-anoxic transition zone of the Black Sea water column. Org Geochem 38: 84–91. [Google Scholar]
- 13.Loesekann T, Knittel K, Nadalig T, Fuchs B, Niemann H, Boetius A, et al. (2007) Diversity and abundance of aerobic and anaerobic methane oxidizers at the Haakon Mosby mud volcano, Barents Sea. Appl Environ Microbiol 73: 3348–3362. 10.1128/AEM.00016-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talbot HM, Handley L, Spencer-Jones CL, Dinga BJ, Schefuß E, Mann PJ, et al. (2014) Variability in aerobic methane oxidation over the past 1.2 Myrs recorded in microbial biomarker signatures from Congo fan sediments. Geochim Cosmochim Acta 133: 387–401. [Google Scholar]
- 15.Kessler JD, Valentine DL, Redmond MC, Du M, Chan EW, Mendes SD, et al. (2011) A persistent oxygen anomaly reveals the fate of spilled methane in the deep Gulf of Mexico. Science 331: 312–315. 10.1126/science.1199697 [DOI] [PubMed] [Google Scholar]
- 16.Crespo-Medina M, Meile CD, Hunter KS, Diercks AR, Asper VL, Orphan VJ, et al. (2014) The rise and fall of methanotrophy following a deepwater oil-well blowout. Nat Geosci 7: 423–427. [Google Scholar]
- 17.Dunfield PF, Yuryev A, Senin P, Smirnova AV, Stott MB, Hou S, et al. (2007) Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia. Nature 450: 879–U18. 10.1038/nature06411 [DOI] [PubMed] [Google Scholar]
- 18.Pol A, Heijmans K, Harhangi HR, Tedesco D, Jetten MSM, op den Camp HJM (2007) Methanotrophy below pH1 by a new Verrucomicrobia species. Nature 450: 874–U17. 10.1038/nature06222 [DOI] [PubMed] [Google Scholar]
- 19.Islam T, Jensen S, Reigstad LJ, Larsen O, and Birkeland N- K (2008) Methane oxidation at 55 degrees C and pH 2 by a thermoacidophilic bacterium belonging to the Verrucomicrobia phylum. P Natl Acad Sci USA 105: 300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Teeseling MCF, Pol A, Harhangi HR, van der Zwart S, Jetten MSM, Op den Camp HJM (2014) Expanding the verrucomicrobial methanotrophic world: description of three novel species of Methylacidimicrobium gen. nov. Appl Environ Microbiol 80: 6782–6791. 10.1128/AEM.01838-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp CE, Smirnova AV, Graham JM, Stott MB, Khadka R, Moore TR, et al. (2014) Distribution and diversity of Verrucomicrobia methanotrophs in geothermal and acidic environments. Environ Microbiol 16: 1867–1878. 10.1111/1462-2920.12454 [DOI] [PubMed] [Google Scholar]
- 22.Cvejic JH, Bodrossy L, Kovacs KL, Rohmer M (2000) Bacterial triterpenoids of the hopane series from the methanotrophic bacteria Methylocaldum spp.: phylogenetic implications and first evidence for an unsaturated aminobacteriopanepolyol. FEMS Microbiol Lett 182: 361–365. [DOI] [PubMed] [Google Scholar]
- 23.Ourisson G, Albrecht P (1992) Hopanoids 1. Geohapanoids—the most abundant natural-products on Earth. Acc Chem Res 25: 398–402. [Google Scholar]
- 24.Farrimond P, Fox PA, Innes HE, Miskin IP, Head IM (1998) Bacterial sources of hopanoids in recent sediments: improving our understanding of ancient hopane biomarkers. Ancient Biomolecules 2: 147–166. [Google Scholar]
- 25.Zundel M, Rohmer M (1985) Prokaryotic triterpenoids. 1. 3-beta-methylhopanoids from Acetobacter species and Methylococcus-capsulatus. Eur J Biochem 150: 23–27. [DOI] [PubMed] [Google Scholar]
- 26.Talbot HM, Watson DF, Pearson EJ, Farrimond P (2003) Diverse biohopanoid compositions of non-marine sediments. Org Geochem 34: 1353–1371. [Google Scholar]
- 27.Welander PV Summons RE (2012) Discovery, taxonomic distribution, and phenotypic characterization of a gene required for 3-methylhopanoid production. P Natl Acad Sci USA 109: 12905–12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborne KA (2015) Environmental controls on bacteriohopanepolyol signatures in estuarine sediments. PhD Thesis. Newcastle University, UK.
- 29.Collister JW, Summons RE, Lichtfouse E, Hayes JM (1992) An isotopic biogeochemical study of the Green River oil-shale. Org Geochem 19: 265–276. [DOI] [PubMed] [Google Scholar]
- 30.Bouvier P, Rohmer M, Benveniste P, Ourisson G (1976) Δ8(14)-steroids in the bacterium Methylococcus capsulatus. Biochem J 159: 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birgel D, Peckmann J (2008) Aerobic methanotrophy at ancient marine methane seeps: A synthesis. Org Geochem 39: 1659–1667. [Google Scholar]
- 32.Elvert M, Suess E, Greinert J, Whiticar MJ (2000) Archaea mediating anaerobic methane oxidation in deep-sea sediments at cold seeps of the eastern Aleutian subduction zone. Org Geochem 31: 1175–1187. [Google Scholar]
- 33.Pancost RD, Sinninghe Damsté JS, de Lint S, van der Maarel M, Gottschal JC, Medinaut Shipboard Scientific Party (2000) Biomarker evidence for widespread anaerobic methane oxidation in Mediterranean sediments by a consortium of methanogenic archaea and bacteria. Appl Environ Microbiol 66: 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinrichs KU (2001) A molecular recorder of methane hydrate destabilization. Geochem Geophy Geosy 2: art. no.-2000GC000118.
- 35.Thiel V, Peckmann J, Richnow HH, Luth U, Reitner J, Michaelis W (2001) Molecular signals for anaerobic methane oxidation in Black Sea seep carbonates and a microbial mat. Mar Chem 73: 97–112. [Google Scholar]
- 36.Rohmer M, Bouviernave P, Ourisson G (1984) Distribution of hopanoid triterpenes in prokaryotes. J Gen Microbiol 130: 1137–1150. [Google Scholar]
- 37.Pancost RD, van Geel B, Baas M, Sinninghe Damsté JS (2000) δ13C values and radiocarbon dates of microbial biomarkers as tracers for carbon cycling in peat deposits. Geology 28: 663–666. [Google Scholar]
- 38.van Winden JF, Kip N, Reichart G- J, Jetten MSM, Op den Camp HJM, Sinninghe Damsté JS (2010) Lipids of symbiotic methane-oxidizing bacteria in peat moss studied using stable carbon isotopic labelling. Org Geochem 41: 1040–1044. [Google Scholar]
- 39.Inglis GN, Collinson ME, Riegel W, Wilde V, Robson BE, Lenz OK, et al. (2015) Ecological and biogeochemical change in an early Paleogene peat-forming environment: Linking biomarkers and palynology. Palaeogeogr Palaeocl 438: 245–255. [Google Scholar]
- 40.Spencer-Jones CL, Wagner T, Dinga BJ, Schefuß E, Mann PJ, Poulsen J R, et al. (2015) Bacteriohopanepolyols in tropical soils and sediments from the Congo River catchment area.Org Geochem 89–90: 1–13. [Google Scholar]
- 41.Birgel D, Feng D, Roberts HH, Peckmann J (2011) Changing redox conditions at cold seeps as revealed by authigenic carbonates from Alaminos Canyon, northern Gulf of Mexico. Chem Geol 285; 82–96. [Google Scholar]
- 42.Jahnke LL, Summons RE, Hope JM, Des Marais DJ (1999) Carbon isotopic fractionation in lipids from methanotrophic bacteria II: the effects of physiology and environmental parameters on the biosynthesis and isotopic signatures of biomarkers. Geochim Cosmochim Acta 63: 79–93. [DOI] [PubMed] [Google Scholar]
- 43.Crossman ZM, McNamara N, Parekh N, Ineson P, Evershed RP (2001) A new method for identifying the origins of simple and complex hopanoids in sedimentary materials using stable isotope labelling with 13(CH4) and compound specific stable isotope analyses. Org Geochem 32: 359–364. [Google Scholar]
- 44.Neunlist S, Rohmer M (1985) Novel hopanoids from the methylotrophic bacteria Methylococcus capsulatus and Methylomonas methanica—(22S)-35-aminobacteriohopane-30,31,32,33,34-pentol and (22S)-35-amino-3-beta-methylbacteriohopane-30,31,32,33,34-pentol. Biochem J 231: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talbot HM, Farrimond P (2007) Bacterial populations recorded in diverse sedimentary biohopanoid distributions.Org Geochem 38: 1212–1225. [Google Scholar]
- 46.van Winden JF, Talbot HM, Kip N, Reichart GJ, Pol A, McNamara NP, et al. (2012) Bacteriohopanepolyol signatures as markers for methanotrophic bacteria in peat moss. Geochim Cosmochim Acta 77: 52–61. [Google Scholar]
- 47.Blumenberg M, Hoppert M, Krueger M, Dreier A, Thiel V (2012) Novel findings on hopanoid occurrences among sulfate reducing bacteria: Is there a direct link to nitrogen fixation? Org Geochem 49: 1–5. [Google Scholar]
- 48.Talbot HM, Summons RE, Jahnke LL, Cockell CS, Rohmer M, Farrimond P (2008) Cyanobacterial bacteriohopanepolyol signatures from cultures and natural environmental settings. Org Geochem 39: 232–263. [Google Scholar]
- 49.Talbot HM, Bischoff J, Inglis GN, Collinson ME, Pancost RD (2016) Polyfunctionalised bio- and geohopanoids in the Eocene Cobham Lignite. Org Geochem 96: 77–92. [Google Scholar]
- 50.Blumenberg M, Krüger M, Nauhaus K, Talbot HM, Oppermann BI, Seifert R, et al. (2006) Biosynthesis of hopanoids by sulfate-reducing bacteria (genus Desulfovibrio). Environ Microbiol 8: 1220–1227. 10.1111/j.1462-2920.2006.01014.x [DOI] [PubMed] [Google Scholar]
- 51.Blumenberg M, Oppermann BI, Guyoneaud R, Michaelis W (2009) Hopanoid production by Desulfovibrio bastinii isolated from oilfield formation water. FEMS Microbiol Lett 293: 73–78. 10.1111/j.1574-6968.2009.01520.x [DOI] [PubMed] [Google Scholar]
- 52.Talbot HM, Watson DF, Murrell JC, Carter JF, Farrimond P (2001) Analysis of intact bacteriohopanepolyols from methanotrophic bacteria by reversed-phase high-performance liquid chromatography-atmospheric pressure chemical ionisation mass spectrometry. J Chromatogr A 921: 175–185. [DOI] [PubMed] [Google Scholar]
- 53.Banta AB, Wei JH, Welander PV (2015) A distinct pathway for tetrahymanol synthesis in bacteria. P Natl Acad Sci USA 112: 13478–13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dedysh SN (2009) Exploring methanotroph diversity in acidic northern wetlands: Molecular and cultivation-based studies. Microbiology 78: 655–669. [Google Scholar]
- 55.Hirayama H, Abe M, Miyazaki M, Nunoura T, Furushima Y, Yamamoto H, et al. (2014) Methylomarinovum caldicuralii gen. nov., sp nov., a moderately thermophilic methanotroph isolated from a shallow submarine hydrothermal system, and proposal of the family Methylothermaceae fam. nov. Int J Syst Evol Microbiol 64: 989–999. 10.1099/ijs.0.058172-0 [DOI] [PubMed] [Google Scholar]
- 56.Hirayama H, Fuse H, Abe M, Miyazaki M, Nakamura T, Nunoura T, et al. (2013) Methylomarinum vadi gen. nov., sp nov., a methanotroph isolated from two distinct marine environments. Int J Syst Evol Microbiol 63: 1073–1082. 10.1099/ijs.0.040568-0 [DOI] [PubMed] [Google Scholar]
- 57.Hirayama H, Sunamura M, Takai K, Nunoura T, Noguchi T, Oida H, et al. (2007) Culture-dependent and -independent characterization of microbial communities associated with a shallow submarine hydrothermal system occurring within a coral reef off Taketomi Island, Japan. Appl Environ Microbiol 73: 7642–7656. 10.1128/AEM.01258-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalyuzhnaya MG, Khmelenina V, Eshinimaev B, Sorokin D, Fuse H, Lidstrom M, et al. (2008) Classification of halo(alkali)philic and halo(alkali)tolerant methanotrophs provisionally assigned to the genera Methylomicrobium and Methylobacter and emended description of the genus Methylomicrobium. Int J Syst Evol Microbiol 58: 591–596. 10.1099/ijs.0.65317-0 [DOI] [PubMed] [Google Scholar]
- 59.Elvert M, Niemann H (2008) Occurrence of unusual steroids and hopanoids derived from aerobic methanotrophs at an active marine mud volcano. Org Geochem 39: 167–177. [Google Scholar]
- 60.Niemann H, Losekann T, de Beer D, Elvert M, Nadalig T, Knittel K, Amann et al. (2006) Novel microbial communities of the Haakon Mosby mud volcano and their role as a methane sink. Nature 443: 854–858. 10.1038/nature05227 [DOI] [PubMed] [Google Scholar]
- 61.Felden J, Wenzhoefer F, Feseker T, and Boetius A (2010) Transport and consumption of oxygen and methane in different habitats of the Hakon Mosby Mud Volcano (HMMV). Limnol Oceanogr 55: 2366–2380. [Google Scholar]
- 62.Nickel JC, di Primio R, Mangelsdorf K, Stoddart D, Kallmeyer J (2012) Characterization of microbial activity in pockmark fields of the SW-Barents Sea. Mar Geol 332: 152–162. [Google Scholar]
- 63.Chand S, Thorsnes T, Rise L, Brunstad H, Stoddart D, Bøe R, et al. (2012) Multiple episodes of fluid flow in the SW Barents Sea (Loppa High) evidenced by gas flares, pockmarks and gas hydrate accumulation. Earth Planet Sci Lett 331: 305–314. [Google Scholar]
- 64.Crémière A, Lepland A, Chand S, Sahy D, Condon DJ, Noble SR, et al. (2016) Timescales of methane seepage on the Norwegian margin following collapse of the Scandinavian Ice Sheet. Nat Commun 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mastalerz V, de Lange GJ, Dählmann A (2009) Differential aerobic an anaeroic oxidation of hydrocarbon gases discharged at muc volcanoes in the Nile deep-sea fan. Geochim Cosmochim Acta 73: 3849–3863. [Google Scholar]
- 66.Felden J, Lichtschlag A, Wenzhoefer F, de Beer D, Feseker T, Ristova PP, et al. (2013) Limitations of microbial hydrocarbon degradation at the Amon mud volcano (Nile deep-sea fan). Biogeosciences 10: 3269–3283. [Google Scholar]
- 67.Baco AR, Rowden AA, Levin LA, Smith CR, Bowden DA (2010) Initial characterization of cold seep faunal communities on the New Zealand Hikurangi margin. Mar Geol 272: 251–259. [Google Scholar]
- 68.Ruff SE, Arnds J, Knittel K, Amann R, Wegener G, Ramette A, et al. (2013) Microbial Communities of Deep-Sea Methane Seeps at Hikurangi Continental Margin (New Zealand). PLOS ONE 8(9): e72627 10.1371/journal.pone.0072627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wild C, Rixen T, Sánchez-Noguera C, Merico A, Jiménez C, Cortés J, et al. (2015) A carbonate platform associated with shallow cold methane seeps in Golfo Dulce, Pacific Costa Rica. Galaxea, Journal of Coral Reef Studies 17: 13–14. [Google Scholar]
- 70.Rush D, Sinninghe Damsté JS, Poulton SW, Thamdrup B, Garside AL, Acuña González J, et al. (2014) Anaerobic ammonium-oxidising bacteria: A biological source of the bacteriohopanetetrol stereoisomer in marine sediments. Geochim Cosmochim Acta 140: 50–64. [Google Scholar]
- 71.Sassen R, Roberts HH, Carney R, Milkov AV, DeFreitas DA, Lanoil B, et al. (2004) Free hydrocarbon gas, gas hydrate, and authigenic minerals in chemosynthetic communities of the northern Gulf of Mexico continental slope: relation to microbial processes. Chem Geol 205: 195–217. [Google Scholar]
- 72.Pancost RD, Zhang CLL, Tavacoli J, Talbot HM, Farrimond P, Schouten S, et al. (2005) Lipid biomarkers preserved in hydrate-associated authigenic carbonate rocks of the Gulf of Mexico. Palaeogeogr Palaeocl 227: 48–66. [Google Scholar]
- 73.Watson DF (2002) Environmental distribution and sedimentary fate of hopanoid biological marker compounds. PhD Thesis. Newcastle University, UK.
- 74.Paulmier A, Ruiz-Pino D (2009) Oxygen minimum zones (OMZs) in the modern ocean. Prog Oceanogr 80: 113–128. [Google Scholar]
- 75.Farrimond P, Head IM, Innes HE (2000) Environmental influence on the biohopanoid composition of recent sediments. Geochim Cosmochim Acta 64: 2985–2992. [Google Scholar]
- 76.Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 77.Cooke MP, Talbot HM, Farrimond P (2008) Bacterial populations recorded in bacteriohopanepolyol distributions in soils from Northern England. Org Geochem 39: 1347–1358. [Google Scholar]
- 78.Zink K- G, Mangelsdorf K (2004) Efficient and rapid method for the extraction of intact phospholipids from sediments combined with molecular structure elucidation using LC-ESI-MS/MS analysis. Anal Bioanal Chem 380: 798–812. 10.1007/s00216-004-2828-2 [DOI] [PubMed] [Google Scholar]
- 79.Bischoff J, Sparkes RB, Doğrul Selver A, Spencer RGM, Gustafsson Ö, Semiletov IP et al. (2016) Source, transport and fate of soil organic matter inferred from microbial biomarker lipids on the East Siberian Arctic Shelf. Biogeosci Discuss: 10.5194/bg-2016-128 [DOI] [Google Scholar]
- 80.Spencer-Jones CL 2016 Novel concepts derived from microbial biomarkers in the Congo System: Implications for continental methane cycling. PhD Thesis. Newcastle University, UK.
- 81.Talbot HM, Rohmer M, Farrimond P (2007) Rapid structural elucidation of composite bacterial hopanoids by atmospheric pressure chemical ionisation liquid chromatography/ion trap mass spectrometry. Rapid Commun Mass Spectrom 21: 880–892. 10.1002/rcm.2911 [DOI] [PubMed] [Google Scholar]
- 82.Zhu C, Talbot HM, Wagner T, Pan J- M, Pancost RD (2011) Distribution of hopanoids along a land to sea transect: Implications for microbial ecology and the use of hopanoids in environmental studies. Limnol Oceanogr 56: 1850–1865. [Google Scholar]
- 83.Summons RE, Jahnke LL, Roksandic Z (1994) Carbon isotopic fractionation in lipids from methanotrophic bacteria: Relevance for interpretation of the geochemical record of biomarkers. Geochim Cosmochim Acta 58: 2853–286. [DOI] [PubMed] [Google Scholar]
- 84.Joyeux C, Fouchard S, Llopiz P, Neunlist S (2004) Influence of the temperature and the growth phase on the hopanoids and fatty acids content of Frateuria aurantia (DSMZ 6220). FEMS Microbiol Ecol 47: 371–379. 10.1016/S0168-6496(03)00302-7 [DOI] [PubMed] [Google Scholar]
- 85.Fuse H, Ohta M, Takimura O, Murakami K, Inoue H, Yamaoka Y, et al. (1998) Oxidation of trichloroethylene and dimethyl sulfide by a marine Methylomicrobium strain containing soluble methane monooxygenase. Biosci Biotech Bioch 62: 1925–1931. [DOI] [PubMed] [Google Scholar]
- 86.Jensen S, Neufeld JD, Birkeland N- K, Hovland M, Murrell JC (2008) Methane assimilation and trophic interactions with marine Methylomicrobium in deep-water coral reef sediment off the coast of Norway. FEMS Microbiol Ecol 66: 320–330. 10.1111/j.1574-6941.2008.00575.x [DOI] [PubMed] [Google Scholar]
- 87.Sauter LM, Latypova E, Smalley NE, Lidstrom ME, Hallam S, Kalyuzhnaya MG (2012) Methanotrophic communities of Saanich Inlet: A microcosm perspective. Syst Appl Microbiol 35: 198–203. 10.1016/j.syapm.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 88.Blumenberg M, Seifert R, Kasten S, Bahlmann E, Michaelis W (2009) Euphotic zone bacterioplankton sources major sedimentary bacteriohopanepolyols in the Holocene Black Sea. Geochim Cosmochim Acta 73: 750–766. [Google Scholar]
- 89.Blumenberg M, Mollenhauer G, Zabel M, Reimer A, Thiel V (2010) Decoupling of bio- and geohopanoids in sediments of the Benguela Upwelling System (BUS). Org Geochem 41: 1119–1129. [Google Scholar]
- 90.Sáenz JP, Wakeham SG, Eglinton TI, Summons RE (2011) New constraints on the provenance of hopanoids in the marine geologic record: Bacteriohopanepolyols in marine suboxic and anoxic environments. Org Geochem 42: 1351–1362. [Google Scholar]
- 91.Kharbush JJ, Ugalde JA, Hogle SL, Allen EE, Aluwihare LI (2013) Composite bacterial hopanoids and their microbial producers across oxygen gradients in the water column of the California Current. Appl Environ Microbiol 79: 7491–7501. 10.1128/AEM.02367-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schaeffer P, Schmitt G, Adam P, Rohmer M (2008) Acid-catalyzed formation of 32,35-anhydrobacteriohopanetetrol from bacteriohopanetetrol. Org Geochem 39: 1479–1482. [Google Scholar]
- 93.Cooke MP, Talbot HM, and Wagner T (2008) Tracking soil organic carbon transport to continental margin sediments using soil-specific hopanoid biomarkers: A case study from the Congo fan (ODP site 1075). Org Geochem 39: 965–971. [Google Scholar]
- 94.Bradley AS, Pearson A, Sáenz JP, Marx CJ (2010) Adenosylhopane: The first intermediate in hopanoid side chain biosynthesis. Org Geochem 41: 1075–1081. [Google Scholar]
- 95.Hamersley MR, Lavik G, Woebken D, Rattray J E, Lam P, Hopmans EC, et al. (2007) Anaerobic ammonium oxidation in the Peruvian oxygen minimum zone. Limnol Oceanogr 52: 923–933. [Google Scholar]
- 96.Ruff SE, Biddle JF, Teske AP, Knittel K, Boetius A, Ramette A (2015) Global dispersion and local diversification of the methane seep microbiome. P Natl Acad Sci USA 112: 4015–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lidstrom ME (1988) Isloation and characterization of marine methanotrophs. A Van Leeuw J Microb 54: 189–199. [DOI] [PubMed] [Google Scholar]
- 98.Yan T, Zhou J, Zhang CL (2006) Diversity of functional genes for methanotrophs in sediments associated with gas hydrates and hydrocarbon seeps in the Gulf of Mexico. FEMS Microbiol Ecol 57: 251–259. 10.1111/j.1574-6941.2006.00122.x [DOI] [PubMed] [Google Scholar]
- 99.Tavormina PL, Ussler W III, Orphan VJ (2008) Planktonic and sediment-associated aerobic methanotrophs in two seep systems along the North American margin. Appl Environ Microbiol 74: 3985–3995. 10.1128/AEM.00069-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wasmund K, Kurtböke DI, Burns KA, Bourne DG (2009) Microbial diversity in sediments associated with a shallow methane seep in the tropical Timor Sea of Australia reveals a novel aerobic methanotroph diversity. FEMS Microbiol Ecol 68: 142–151. 10.1111/j.1574-6941.2009.00667.x [DOI] [PubMed] [Google Scholar]
- 101.Moussard H, Stralis-Pavese N, Bodrossy L, Neufeld JD, Murrell JC (2009) Identification of active methylotrophic bacteria inhabiting surface sediment of a marine estuary. Environ Microbiol Rep 1: 424–433. 10.1111/j.1758-2229.2009.00063.x [DOI] [PubMed] [Google Scholar]
- 102.Redmond MC, Valentine DL, Sessions AL (2010) Identification of novel methane-, ethane-, and propane-osidizing bacteria at marine hydrocarbon seeps by stable isotope probing. Appl Environ Microbiol 76: 6412–6422. 10.1128/AEM.00271-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kellermann MY, Schubotz F, Elvert M, Lipp JS, Birgel D, Prieto-Mollar X, et al. (2012) Symbiont-host relationships in chemosynthetic mussels: A comprehensive lipid biomarker study. Org Geochem 43: 112–124. [Google Scholar]
- 104.Coolen MJL, Talbot HM, Abbas BA, Ward C, Schouten S, Volkman JK, et al. (2008) Sources for sedimentary bacteriohopanepolyols as revealed by 16S rDNA stratigraphy. Environ Microbiol 10: 1783–1803. 10.1111/j.1462-2920.2008.01601.x [DOI] [PubMed] [Google Scholar]
- 105.Jahnke LL, Summons RE, Dowling LM, Zahiralis KD (1995) Identification of methanotrophic lipid biomarkers in cold-seep mussel gills—chemical and isotopic analysis. Appl and Environ Microbiol 61: 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Himmler T, Birgel D, Bayon G, Pape T, Ge L, Bohrmann G, et al. (2015) Formation of seep carbonates along the Makran convergent margin, northern Arabian Sea and amolecular and isotopic approach to constrain the carbon isotopic composition of parent methane. Chem Geol 415: 102–117. [Google Scholar]
- 107.Berndmeyer C, Thiel V, Schmale O, Blumenberg M. (2013) Biomarkers for aerobic methanotrophy in the water column of the stratified Gotland Deep (Baltic Sea). Org Geochem 55: 103–111. [Google Scholar]
- 108.Wakeham SG, Amann R, Freeman KH, Hopmans EC, Jorgensen BB, Putnam IF, et al. (2007) Microbial ecology of the stratified water column of the Black Sea as revealed by a comprehensive biomarker study. Org Geochem 38: 2070–2097. [Google Scholar]
- 109.Wagner T, Kallweit W, Talbot HM, Mollenhauer G, Boom A, Zabel M (2014) Microbial biomarkers support organic carbon transport from methane-rich Amazon wetlands to the shelf and deep sea fan during recent and glacial climate conditions. Org Geochem 67: 85–98. [Google Scholar]
- 110.Höfle S, Kusch S, Talbot HM, Mollenhauer G, Zubrzycki S, Burghardt S, et al. (2015) Characterization of bacterial populations in Arctic permafrost soils using bacteriohopanepolyols. Org Geochem 88: 1–16. [Google Scholar]
- 111.Talbot HM, McClymont E, Inglis GN, Evershed R, Pancost RD (2016) Origin and preservation of bacteriohopanepolyol and geohopanoid signatures in Sphagnum peat from Bissendorfer Moor (Germany). Org Geochem 97: 95–110. [Google Scholar]
- 112.Cooke MP (2011) The role of bacteriohopanepolyols as biomarkers for soil bacterial communities and soil derived organic matter. PhD Thesis. Newcastle University, UK.
- 113.Gibson RA, Talbot HM, Kaur G, Pancost RD, Mountain B (2008) Bacteriohopanepolyol signatures of cyanobacterial and methanotrophic bacterial populations recorded in a geothermal vent sinter. Org Geochem 39: 1020–1023. [Google Scholar]
- 114.Farrimond P, Talbot HM, Watson DF, Schulz LK, Wilhelms A (2004) Methylhopanoids: Molecular indicators of ancient bacteria and a petroleum correlation tool. Geochim Cosmochim Acta 68: 3873–3882. [Google Scholar]
- 115.Birgel D, Meister P, Lundberg R, Horath TD, Bontognali TRR, Bahniuk AM, De Rezende CE, Vasconcelos C, McKenzie JA (2015) Methanogenesis produces strong 13C enrichment in stromatolites of Lagoa Salgada, Brazil: a modern analogue for Palaeo-/Neoproterozoic stromatolites? Geobiology 13: 245–266. 10.1111/gbi.12130 [DOI] [PubMed] [Google Scholar]
- 116.Kim J- H, Talbot HM, Zarzycka B, Bauersachs T, Wagner T (2011) Occurrence and abundance of soil-specific bacterial membrane lipid markers in the Tet watershed (southern France): Soil-specific BHPs and branched GDGTs. Geochem Geophy Geosy 12: Q02008, 10.1029/2010GC003364 [DOI] [Google Scholar]
- 117.Redshaw CH, Cooke MP, Talbot HM, McGrath S, Rowland SJ (2008) Low biodegradability of fluoxetine HCl, diazepam and their human metabolites in sewage sludge-amended soil. J Soil Sediment 8: 217–230. [Google Scholar]
- 118.Xu Y, Cooke MP, Talbot HM, Simpson MJ (2009) Bacteriohopanepolyol signatures of bacterial populations in Western Canadian soils. Org Geochem 40: 79–86. [Google Scholar]
- 119.van Winden JF, Talbot HM, De Vleeschouwer FD, Reichart G- J, Sinninghe Damsté JS (2012) Variation in methanotroph-related proxies in peat deposits from Misten Bog, Hautes-Fagnes, Belgium. Org Geochem 53: 73–79. [Google Scholar]
- 120.Zhang CL, Huang Z, Li Y- L, Romanek CS, Mills GL, Gibson RA, et al. (2007) Lipid biomarkers, carbon isotopes, and phylogenetic characterization of bacteria in California and Nevada hot springs. Geomicrobiol J 24: 519–534. [Google Scholar]
- 121.Gibson RA, Sherry A, Kaur G, Pancost RD, Talbot HM (2014) Bacteriohopanepolyols preserved in silica sinters from Champagne Pool (New Zealand) indicate a declining temperature gradient over the lifetime of the vent. Org Geochem 69: 61–69. [Google Scholar]
- 122.Ricci JN, Coleman ML, Welander PV, Session AL, Summons RE, Spear JR et al. (2014) Diverse capacity for 2-methylhopanoid production correlates with a specific ecological niche. ISME J 8:675–684. 10.1038/ismej.2013.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Blumenberg M, Arp G, Reitner J, Schneider D, Daniel R, Thiel V (2013) Bacteriohopanepolyols in a stratified cyanobacterial mat from Kiritimati (Christmas Island, Kiribati). Org Geochem 55: 55–62. [Google Scholar]
- 124.Cooke MP, van Dongen BE, Talbot HM, Semiletov I, Shakhova N, Guo L, et al. (2009) Bacteriohopanepolyol biomarker composition of organic matter exported to the Arctic Ocean by seven of the major Arctic rivers. Org Geochem 40: 1151–1159. [Google Scholar]
- 125.Rethemeyer J, Schubotz F, Talbot HM, Cooke MP, Hinrichs K- U, Mollenhauer G (2010) Distribution of polar membrane lipids in permafrost soils and sediments of a small high Arctic catchment. Org Geochem 41: 1130–1145. [Google Scholar]
- 126.Doğrul Selver A, Talbot HM, Gustafsson Ö, Boult S, van Dongen BE (2012) Soil organic matter transport along an sub-Arctic river–sea transect. Org Geochem 51: 63–72. [Google Scholar]
- 127.Doğrul Selver A, Sparkes RB, Bischoff J, Talbot HM, Gustafsson Ö, Semiletov IP, et al. (2015) Distributions of bacterial and archaeal membrane lipids in surface sediments reflect differences in input and loss of terrestrial organic carbon along a cross-shelf Arctic transect. Org Geochem 84: 16–26. [Google Scholar]
- 128.De Jonge C, Talbot HM, Bischoff J, Stadnitskaia A, Cherkashov G, Sinninghe Damsté JS (2016) Bacteriohopanepolyol distribution in Yenisei River and Kara Sea suspended particulate matter and sediments traces terrigenous organic matter input. Geochim Cosmochim Acta 174: 85–101. [Google Scholar]
- 129.Zhu C, Talbot HM, Wagner T, Pan JM, Pancost RD (2010) Intense aerobic methane oxidation in the Yangtze Estuary: A record from 35-aminobacteriohopanepolyols in surface sediments. Org Geochem 41: 1056–1059. [Google Scholar]
- 130.Sáenz JP, Eglinton TI, Summons RE (2011) Abundance and structural diversity of bacteriohopanepolyols in suspended particulate matter along a river to ocean transect. Org Geochem 42: 774–780. [Google Scholar]
- 131.Wakeham SG, Turich C, Schubotz F, Podlaska A, Li XN, Varela R, et al. (2012) Biomarkers, chemistry and microbiology show chemoautotrophy in a multilayer chemocline in the Cariaco Basin. Deep-Sea Res Pt I 63: 133–156. [Google Scholar]
- 132.Taylor KA, Harvey HR (2011) Bacterial hopanoids as tracers of organic carbon sources and processing across the western Arctic continental shelf. Org Geochem 42: 487–497. [Google Scholar]
- 133.Blumenberg M, Berndmeyer C, Moros M, Muschalla M, Schmale O, Thiel V (2013) Bacteriohopanepolyols record stratification, nitrogen fixation and other biogeochemical perturbations in Holocene sediments of the central Baltic Sea. Biogeosciences 10: 2725–2735. [Google Scholar]
- 134.Mondamert L, Labanowski J, N’Goye F, Talbot HM, Croué JP (2011) High pressure membrane foulants of seawater, brackish water and river water: origin assessed by sugar and bacteriohopanepolyol signatures. Biofouling 27: 21–32. 10.1080/08927014.2010.536614 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Concentration (μg/g sediment) of other BHPs in marine sediments samples presented in this study.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.