Abstract
The silencing of the tumor suppressor gene O-6-methylguanine-DNA methyltransferase (MGMT) by promoter methylation commonly occurs in human cancers. The relationship between MGMT promoter methylation and gastric cancer (GC) remains inconsistent. This study aimed to evaluate the potential value of MGMT promoter methylation in GC patients. Electronic databases were searched to identify eligible studies. The pooled odds ratio (OR) and the corresponding 95% confidence interval (95% CI) were used to evaluate the effects of MGMT methylation on GC risk and clinicopathological characteristics. In total, 31 eligible studies including 2988 GC patients and 2189 nonmalignant controls were involved in meta-analysis. In the pooled analysis, MGMT promoter methylation was significantly associated with GC risk (OR = 3.34, P < 0.001) and substantial heterogeneity (P < 0.001). Meta-regression and subgroup analyses based on the testing method, sample material and ethnicity failed to explain the sources of heterogeneity. Interestingly, MGMT methylation showed a trend associated with gender, and methylation is lower in males compared with females (OR = 0.76, 95% CI = 0.56–1.03). We did not find a significant association in relation to tumor types, clinical stage, age status or H. pylori status in cancer (all P > 0.1). MGMT promoter methylation may be correlated with the prognosis of GCs in disease free survival (DFS) or overall survival (OS) for univariate analysis. MGMT promoter methylation may play a crucial role in the carcinogenesis and prognosis of GC. MGMT methylation was not correlated with tumor types, clinical stage, age status, H. pylori status. However, the result of the association of MGMT methylation and gender should be considered with caution.
Introduction
As one of the most common malignant diseases, gastric cancer (GC) is the third leading cause of cancer-related deaths worldwide. According to global cancer statistics, approximately 951,600 new cases of gastric cancer were diagnosed in 2012, leading to an estimated 723,100 deaths worldwide [1]. Helicobacter pylori (H. pylori) infection affects more than 50% of the adult population in the world and accounts for 75% of all gastric cancer cases [2]. Therefore, H. pylori infection is a strong risk factor for GC, increasing the risk of developing gastric cancer. GC is divided into two main histological subtypes based on Lauren’s classification: intestinal and diffuse-type gastric cancer [3]. For both types, a strong association with H. pylori-correlated inflammation exists [4].
Epigenetic alterations are significantly associated with cancer [5]. DNA methylation is a common epigenetic alteration that plays a crucial role in the development of cancer [6, 7]. Accumulative evidence has demonstrated that GC involves a multistep progression process of gastric lesions with complex molecular changes, including DNA methylation [8, 9]. Located on 10q26, O6-methylguanine-DNA-methyltransferase (MGMT) encodes a DNA repair protein that counteracts the effect of treatment via removing alkyl adducts from the O6-position of guanine [10]. O6-Alkylated guanine leads to base mismatching and double-strand breaks, thereby inducing apoptosis and cell death [11]. Loss of MGMT expression by promoter methylation has been reported in many tumor types [10], including gastric cancer [12]. Therefore, we hypothesized that MGMT promoter methylation status might play a role in the development of gastric cancer.
The association between MGMT promoter methylation and GC risk remains controversial. Noreikienė et al. reported that the methylation rate of MGMT promoter was lower in GC than in non-tumor tissues [13]. Some studies showed that the methylation frequency of MGMT promoter was higher in GC than in nonmalignant samples [12, 14]. Therefore, we conducted a meta-analysis to assess the relationship between MGMT promoter methylation and GC by comparing cancer cases with nonmalignant controls. Moreover, we also evaluated the correlation between MGMT promoter methylation and gender, age status, tumor stage, tumor types and H. pylori status in cancer.
Materials and Methods
Literature search strategy and inclusion criteria
The relevant studies were identified by a systematic search of PubMed, Embase, Cochrane Library and EBSCO databases up to December 25, 2015, without language restrictions. The following key words and search terms were used: (O-6-methylguanine-DNA methyltransferase OR MGMT) AND (stomach OR gastric) AND (cancer OR tumor OR neoplasm OR carcinoma) AND (methylation OR epigene*). Moreover, a manual reference search for relevant articles was also performed to identify the potential additional studies.
Eligible studies had to meet the following inclusion criteria: 1) the study had a diagnosis of primary gastric cancer based on histopathological examination; 2) the study involved MGMT promoter methylation frequency in gastric cancer; 3) that study had sufficient data to evaluate the relationship between MGMT promoter methylation and gastric cancer; and 4) to avoid duplicated publications, the study selected was the most recent publication or the most complete paper if a series of studies existed. The studies excluded did not meet the inclusion criteria described above.
Data extraction and quality assessment
The following data were collected for eligible studies: the first author’s name, year of publication, country of origin, ethnicity, sample types, testing method, the number of gastric cancer patients, the number of control group, the number of methylation positive, expression information, clinicopathological parameters (i.e., tumor stage, tumor histotype, age status, sex status and Helicobacter pylori (H. pylori) infection status. Tumor stages 1–2 were defined as early stage, and tumor stages 3–4 were defined as advanced stage. Our study was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (S1 Table). Moreover, two reviewers independently estimated the quality of eligible studies according to Newcastle–Ottawa Scale (NOS) for case–control or cohort studies [15, 16], including three parameters of quality: selection (0–4), comparability (0–2), and outcome or exposure assessment (0–3). In this study, NOS scores ranged from 0 to 9 for each study, the study with 6 or more scores was considered as high quality, and a NOS score of less than 6 was considered as low quality [15].
Statistical analysis
Stata software (version 12.0, Stata Corporation, College Station, TX, USA) was used for statistical analysis. The overall odds ratio (OR) and the corresponding 95% confidence interval (95% CI) were calculated to evaluate the association between MGMT promoter methylation and GC risk. In addition, the association of MGMT promoter methylation and clinicopathological features was also assessed via the pooled OR with 95% CI. Statistical heterogeneity was examined using the chi-square test and Q statistics [17]. If heterogeneity was significant (I2 ≥ 50% or p < 0.1), the random-effects model was used. Meta-regression analyses and subgroup analyses were performed to further evaluate the sources of heterogeneity. Otherwise, a fixed-effects model was used [18, 19]. A sensitivity analysis was also conducted to assess the influence and stability of an individual study on the pooled OR by deleting one study [20]. The publication bias was detected using Egger’s test for the analysis with greater 9 studies [21]. We also conducted a cumulative meta-analysis by precision method to evaluate the possible publication bias for the result with less than 10 studies [22].
Results
Study characteristics
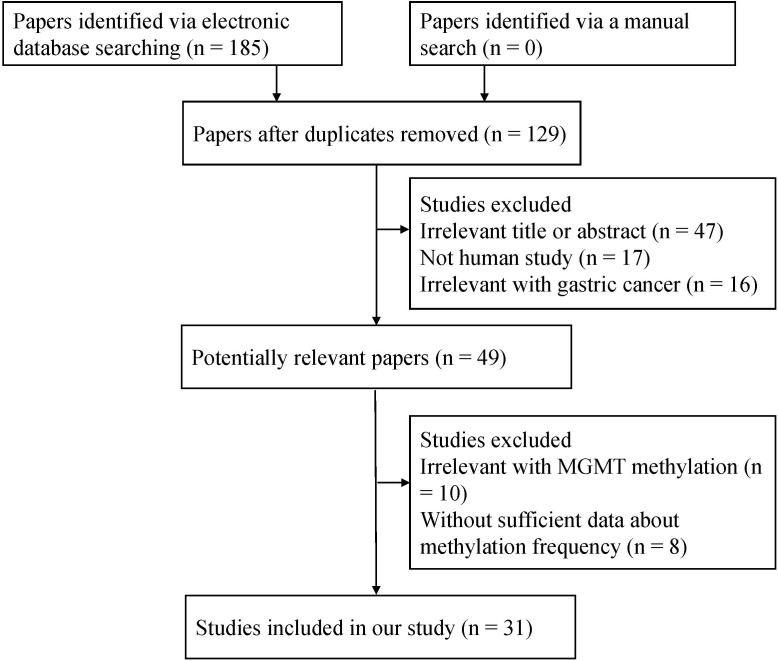
Initially, a total of 185 studies were identified by searching electronic databases. Based on the inclusion criteria described above, 31 studies [12–14, 23–49] [50] that reported the sufficient data were ultimately included in the current meta-analysis (Fig 1), including a total of 2988 GC patients and 2189 nonmalignant controls. Of these studies, 20 studies reporting 2120 cases and 2189 nonmalignant controls were calculated to assess the association between MGMT methylation and GC risk, and 17 studies reporting 1299 male GC patients and 775 female GC patients were used to evaluate the association between MGMT methylation and gender. Furthermore, 11 studies, including 464 patients with intestinal gastric cancer and 416 patients with diffuse gastric cancer, evaluated the association between MGMT methylation and tumor type; 10 studies including 221 stage 1–2 patients and 469 stage 3–4 patients evaluated the association between MGMT methylation and tumor stage; 9 studies assessed the correlation between MGMT promoter methylation and age status (more than or equal to 60 years: 387 GC patients, less than or equal to 60 years: 315 GC patients); and 3 studies involving 139 H. pylori-positive patients and 147 H. pylori-negative patients explored the association between MGMT methylation and H. pylori infection status. 2 studies with 198 GC patients reported survival. The basic characteristics of included studies were presented in S2 Table.
Fig 1. Flow chart of the literature search strategy.
MGMT gene methylation and risk of GC
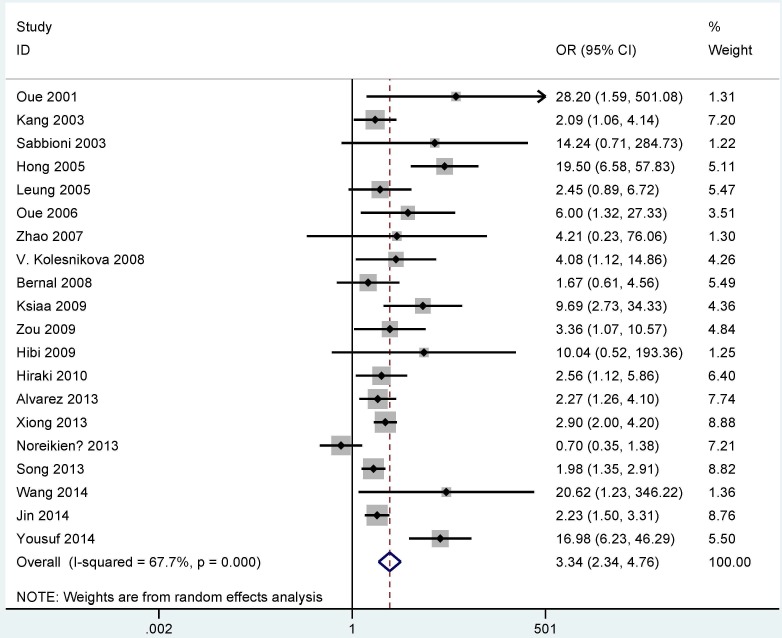
In the comparison of GC and control groups, substantial heterogeneity was obvious (I2 = 67.7% and P < 0.001); thus, a random-effects model was used. The result showed that the overall OR for MGMT promoter methylation in cancer cases compared with nonmalignant controls was 3.34 (95% CI = 2.34–4.76, P < 0.001) (Fig 2).
Fig 2. Forest plot of the correlation between MGMT methylation and GC.
Subgroup analyses of MGMT promoter methylation
The subgroup analyses were conducted based on the methylation detection method (MSP, MethyLight or Pyrosequencing), sample material (fresh frozen tissue, formalin-fixed paraffin-embedded tissue or blood) and race (Caucasians, Asians or mixed population) (Table 1). In the subgroup analysis of the testing method, the pooled OR was 3.56 (95% CI = 2.30–5.51, P < 0.001) for the MSP subgroup among 15 studies, 3.62 (95% CI = 2.01–6.53, P < 0.001) for the MethyLight subgroup among 4 studies, and 2.27 (95% CI = 1.26–4.10, P = 0.006) for the Pyrosequencing subgroup in 1 study. In the subgroup analysis of the sample material, the OR value for the fresh frozen (FF) tissue subgroup was 3.86 (95% CI = 2.24–6.63, P < 0.001) among 11 studies. The OR for the formalin-fixed paraffin-embedded (FFPE) tissue subgroup was 2.68 (95% CI = 1.87–3.82, P < 0.001) among 6 studies, and the OR for the blood sample subgroup was 2.97 (95% CI = 1.35–6.57, P = 0.007) among 2 studies. The result by subgroup analysis of race revealed that MGMT methylation was significantly associated with GC risk in Asian and Caucasian populations (OR = 3.80, 95% CI = 2.56–5.64, P < 0.001; OR = 2.91, 95% CI = 1.07–7.89, P = 0.036; respectively) among 14 studies and 5 studies, respectively, but not in the mixed population in one study (P = 0.316).
Table 1. The summary of OR in cancer vs. control.
| Studies | Overall OR (95 CI %) | I2; p | P-value | Cases | Controls | p (Egger's test) | |
|---|---|---|---|---|---|---|---|
| Total | 20 | 3.34 (2.34–4.76) | 67.7; < 0.001 | < 0.001 | 2120 | 2189 | 0.021 |
| Subgroup | |||||||
| Method | |||||||
| MSP | 15 | 3.56 (2.30–5.51) | 74.8%; < 0.001 | < 0.001 | 1747 | 1937 | 0.063 |
| MethyLight | 4 | 3.62 (2.01–6.53) | 5.2%; 0.367 | < 0.001 | 281 | 155 | NA |
| PSQ | 1 | 2.27 (1.26–4.10) | NA; NA | 0.006 | 92 | 97 | NA |
| Material | |||||||
| FFT | 11 | 3.86 (2.24–6.63) | 80.5%; < 0.001 | < 0.001 | 1615 | 1645 | 0.087 |
| FFPE | 6 | 2.68 (1.87–3.82) | 14.6%; 0.320 | < 0.001 | 404 | 494 | NA |
| Blood | 2 | 2.97 (1.35–6.57) | 0.0%; 0.541 | 0.007 | 80 | 44 | NA |
| Race | |||||||
| Caucasians | 5 | 2.91 (1.07–7.89) | 78.2%; 0.001 | 0.036 | 270 | 247 | NA |
| Mix | 1 | 1.67 (0.61–4.56) | NA; NA | 0.316 | 47 | 47 | NA |
| Asians | 14 | 3.80 (2.56–5.64) | 64.8%; < 0.001 | < 0.001 | 1803 | 1895 | 0.016 |
Mix: mixed population; PSQ: Pyrosequencing; FFT: fresh frozen tissue; FFPE: formalin-fixed and paraffin-embedded tissue; MSP: methylation-specific polymerase chain reaction; NA: not applicable; OR: odds ratio; 95% CI: 95% confidence interval.
Meta-regression and subgroup analyses in the GC and control group
According to the methylation detection method (MSP, MethyLight or Pyrosequencing), sample material (fresh frozen tissue, formalin-fixed paraffin-embedded tissue or blood) and race (Caucasians, Asians or mixed population), subgroup analysis (Table 1) and meta-regression analysis (Table 2) were performed to explore the potential sources of heterogeneity. Heterogeneity based on subgroup analysis of the detection method revealed significant differences (MSP subgroup: I2 = 74.8%, P < 0.001; MethyLight subgroup: I2 = 5.2%, P = 0.367). Significantly different evidence of heterogeneity was noted in different sample material subgroups (FF tissue subgroup: I2 = 80.5%, P < 0.001; FFPE tissue subgroup: I2 = 14.6%, P = 0.320; blood sample subgroup: I2 = 0.0%, P = 0.541). Heterogeneity was observed within different ethnicity subgroups (Caucasian population subgroup: I2 = 78.2%, P = 0.001; Asian population subgroup: I2 = 64.8%, P < 0.001). The result revealed that subgroup analyses did not identify the sources of heterogeneity.
Table 2. Meta-regression analysis in cancer vs. control.
| Subgroup | Coefficient (95% CI) | t | P value |
|---|---|---|---|
| Sample material | 0.81 | ||
| FFPE | -0.056 (-1.791, 1.680) | -0.07 | 0.947 |
| FFT | 0.263 (-1.374, 1.899) | 0.34 | 0.738 |
| Ethnicity | 0.51 | ||
| Asians | 0.919 (-1.108, 2.945) | 0.96 | 0.352 |
| Caucasians | 0.496 (-1.662, 2.653) | 0.48 | 0.634 |
| Testing method | 0.857 | ||
| PSQ | -0.516 (-2.715, 1.684) | -0.49 | 0.627 |
| MSP | -0.018 (-1.358, 1.323) | -0.03 | 0.978 |
PSQ: Pyrosequencing; FFT: fresh frozen tissue; FFPE: formalin-fixed and paraffin-embedded tissue; MSP: methylation-specific polymerase chain reaction; 95% CI: 95% confidence interval.
The following meta-regression analysis was used. However, the result of meta-regression analysis showed that the methylation detection method, sample material and ethnicity failed to identify the source of heterogeneity (P > 0.1). This result was consistent with the subgroup analysis.
The association between MGMT methylation and clinicopathological features
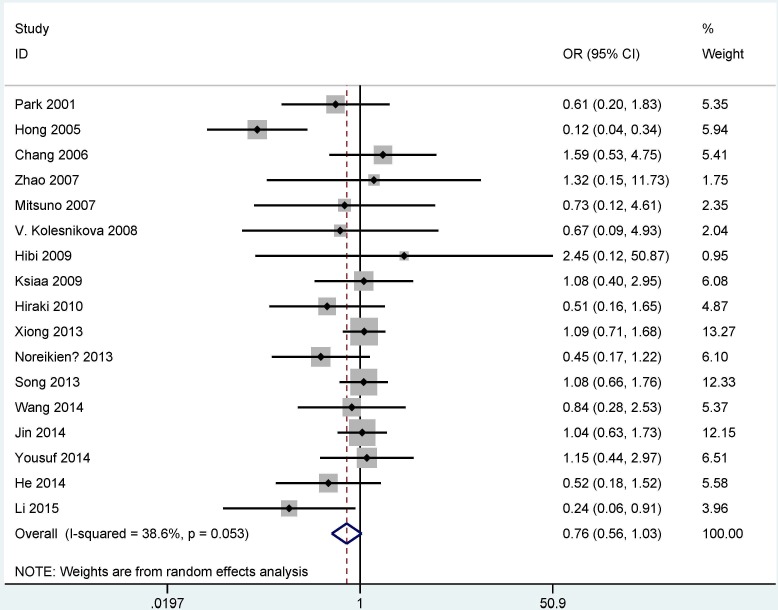
Table 3 showed the relationship between MGMT methylation and clinicopathological features. The analyses of the correlation of MGMT methylation, gender, tumor types, and tumor stage used the random-effects model (all P < 0.1), but a fixed-effects model was used for H. pylori infection status and age status (P > 0.1). The result suggested that MGMT methylation had a trend toward less frequency in male gastric cancer patients compared with female gastric cancer patients (OR = 0.76, 95% CI = 0.56–1.03, P = 0.077) (Fig 3). No significant differences in MGMT methylation were noted in relation to tumor type, tumor stage, age status and H. pylori infection status in GC (all P > 0.1) (Table 3).
Table 3. The correlation of MGMT promoter methylation and clinicopathological features.
| Studies | Overall OR (95CI %) | I2; p | P value | GC patients | p (Egger's test) | ||
|---|---|---|---|---|---|---|---|
| Gender | Male | Female | |||||
| 17 | 0.76 (0.56–1.03) | 38.6%; 0.053 | 0.077 | 1299 | 775 | 0.167 | |
| Tumor stage | Stage 1–2 | Stage 3–4 | |||||
| 10 | 0.65 (0.33–1.26) | 46.4%; 0.052 | 0.205 | 221 | 469 | 0.97 | |
| Tumor types | Intestinal | Diffuse | |||||
| 11 | 1.09 (0.66–1.78) | 50.3%; 0.028 | 0.74 | 464 | 416 | 0.105 | |
| Age | >/ = 60 years | </ = 60 years | |||||
| 9 | 1.03 (0.71–1.49) | 8.9%; 0.361 | 0.897 | 387 | 315 | NA | |
| H. pylori | Positive | Negative | |||||
| 3 | 1.19 (0.66–2.15) | 0.0%; 0.823 | 0.564 | 139 | 147 | NA | |
NA: not applicable; OR: odds ratio; 95% CI: 95% confidence interval.
Fig 3. Forest plot of the correlation between MGMT methylation and gender.
MGMT promoter methylation as a prognostic factor for GC
Two studies reported the prognosis of MGMT promoter methylation [48, 50]. Park et al. reported that there was significant association between MGMT promoter methylation and 5-year disease free survival (DFS) for univariate analysis (P < 0.02) [48]. Shi et al. reported that MGMT promoter methylation was correlated with overall survival (OS) of GCs using univariate analysis [50].
Sensitivity analysis
To assess the stability of the overall OR and the change of heterogeneity based on the omission of single study, sensitivity analyses were conducted in cancer cases vs. nonmalignant controls and male cancer cases vs. female cancer cases. In the comparison of cancer cases and controls, when Noreikienė 2013 et al. ([13], Lithuania), Yousuf 2014 et al. ([12], China) and Hong 2005 et al. ([43], Korea) were successively removed, heterogeneity was significantly decreased (P-values were 0.001, 0.021 and 0.364, respectively); however, the pooled OR was not significantly changed (ORs were 3.62, 3.11 and 2.58, respectively). The overall OR between MGMT methylation and gender in cancer was substantially changed based on omission of Hong 2005 et al. ([43], Korea), with a change from 0.76 (95% CI = 0.56–1.03) to 0.93 (95% CI = 0.75–1.15) and no heterogeneity (P = 0.704).
Publication bias
As shown in Tables 1 and 3, slight publication bias was detected by Egger’s test only in the comparison of cancer samples and control samples and in the Asian population subgroup (P = 0.021 and P = 0.016, respectively). When cancer was compared to controls, we removed two studies with low quality [34, 44], and re-calculated the pooled OR (OR = 3.26, 95% CI = 2.26–4.71, P < 0.001), with a slight publication bias (P = 0.039). Obvious publication bias was not noted in other analyses for the result with more than 9 studies (all P > 0.05). For the analysis with less than 10 studies, a cumulative meta-analysis by precision method did not find obvious evidence of publication bias (S3 Table).
Discussion
The hypermethylation of tumor suppressor genes and hypomethylation of oncogenes are two essential molecular mechanisms of epigenomic regulation, which play key roles in the initiation and progression of cancer [51–53]. MGMT has been reported as a tumor suppressor gene in colorectal cancer [54]. The methylation status of the MGMT promoter has been observed in some cancers, such as non-small cell lung cancer [55], glioblastoma [56], and breast cancer [57]. Several studies showed that significant association was found between MGMT promoter methylation and its expression in GC, with loss of MGMT expression [12, 32, 40, 49]. In addition, the methylation frequency of the MGMT promoter was inconsistent in gastric cancer, with a range from 7% [38] to 70% [34]. Noreikienė et al. reported that the methylation level of MGMT promoter was 36.2% in GC samples, and 44.9% in non-tumor tissues [13]. Some studies reported that MGMT promoter methylation frequency was higher in GC than in non-tumor samples [12, 14, 33]. Therefore, we performed a meta-analysis to evaluate the correlation between MGMT promoter methylation and GC. In the current study, the methylation frequency of MGMT promoter was inconsistent in GC, subgroup analysis of DNA methylation testing method revealed that MGMT promoter methylation had a similar frequency in different methods. Thus, the possible reason of inconsistent methylation frequency of the MGMT may be different CpG sites of the promoter.
Our findings showed that the MGMT methylation status was significantly associated with the risk of GC (OR = 3.34, 95% CI = 2.34–4.76, P < 0.001), suggesting that MGMT methylation can be crucial for the carcinogenesis of gastric cancer.
Further subgroup analyses were conducted according to the methylation detection method (MSP, MethyLight or Pyrosequencing), sample material (fresh frozen tissue, formalin-fixed paraffin-embedded tissue or blood) and race (Caucasians, Asians or mixed population). The results showed that the association between MGMT methylation and GC was correlated with different detection methods and different sample materials. Subgroup analysis based on ethnicity demonstrated that MGMT methylation was significantly associated with GC in the Asian (OR = 3.80, P < 0.001) and Caucasian populations (OR = 2.91, P = 0.036) but not in a mixed population (P = 0.316). However, the results should be carefully considered as only one study or two studies with small sample sizes were included in the Pyrosequencing, blood sample, and mixed population subgroups.
Significant heterogeneity existed in cancer cases compared with controls (P < 0.001). Therefore, we performed meta-regression and subgroup analyses to explain the sources of heterogeneity. The results of subgroup analyses and meta-regression analyses were consistent but were unable to identify the sources of heterogeneity. The following sensitivity analysis was conducted to identify the stability of the overall OR by deleting individual studies. Three studies (Noreikienė 2013 et al., Yousuf 2014 et al. and Hong 2005 et al.) were successively removed, and the pooled OR (OR = 2.58, 95% CI = 2.12–3.14, P < 0.001) remained significant with no evidence of heterogeneity (P = 0.364). However, the value was slightly smaller than that in the current meta-analysis (OR = 3.34, 95% CI = 2.34–4.76, P < 0.001), suggesting that a significant association existed between MGMT methylation and GC. Therefore, our result was stable and reliable.
We further analyzed the clinicopathological significance of MGMT promoter methylation in GC patients. For gender status, the overall OR was 0.76 (95% CI = 0.56–1.03) in 1299 male GC patients and 775 female GC patients, indicating that the MGMT methylation status had a trend associated with gender status. The result showed that methylated MGMT may be a susceptible gene for female GC patients. Based on the existence of heterogeneity (I2 = 38.6% and P = 0.053), the result of sensitivity analysis by omitting a single study (Hong 2005 et al.: 64 male patients and 36 female patients) showed that the summary OR was 0.93 (95% CI = 0.75–1.15), suggesting that MGMT methylation was not correlated with gender status, with no evidence of heterogeneity (P = 0.704). This result should be applied with caution. In addition, only two studies with small sample sizes (136 male GC patients and 66 female GC patients) reported that MGMT promoter methylation rate was significantly lower in male than in female [25, 43]. Although the present study was shown to be methylated in the promoter, the included studies did not state specific location of CpG sites of the MGMT promoter. Therefore, the above analysis of MGMT promoter methylation with gender status may be still required to confirm the result in detail in the future. Other clinicopathological features were also analyzed, including tumor stage (OR = 0.65, 95% CI = 0.33–1.26), tumor type (OR = 1.09, 95% CI = 0.66–1.78), age status (OR = 1.03, 95% CI = 0.71–1.49), and H. pylori infection status (OR = 1.19, 95% CI = 0.66–2.15). The results suggested that MGMT methylation was not associated with tumor stage, tumor type, age status or H. pylori infection status.
When GC was compared to nonmalignant specimens, a slight publication bias was observed (P = 0.021). We determined whether these studies excluded with low quality contributed to reduce the potential publication bias. When two studies were deleted [34, 44], we found that the combined OR was not significantly changed (OR = 3.26, P < 0.001), a slight publication bias was also detected in the remaining 18 studies (P = 0.039), which suggested that poor-quality studies did not mainly impact the risk of bias. In addition, we deleted two studies with high quality [31, 47], no evidence of publication bias was observed in the remaining 18 studies (P = 0.081 > 0.05), indicating the stability of our analyses. For the result with fewer than 10 studies, a cumulative meta-analysis was analyzed in our study. The result showed that no significant publication bias was found in relation to age status and H. pylori infection status etc. (n < 10). Based on the smaller studies or sample sizes, further well-designed, large-scale studies are very essential to validate our results in the future.
This study had several limitations. First, the PubMed, Embase, Cochrane Library and EBSCO databases were used to minimize publication bias. However, publication bias was detected based on Egger’s test in cancer case vs. controls (P = 0.021) and in the Asian population subgroup (P = 0.016). The papers with positive results are more often published than papers with negative results. Articles with other styles, such as unpublished studies and conference abstracts, were excluded due to insufficient data. Second, the main ethnic populations were Asians and Caucasians, and other ethnicities, such as Africans, were limited. Therefore, the association between MGMT methylation and other ethnicities was not evaluated based on insufficient data. Third, the sample size of some subgroup analyses, such as blood sample and mixed population, were smaller. Fourth, one study with 79 patients reported that MGMT promoter methylation was notably correlated with 5-year disease free survival (DFS) in univariate analysis. One study with 119 patients reported that significant correlation was found between MGMT promoter methylation and OS for univariate analysis. These results should be carefully considered, and more studies with large sample size should be performed in the future.
In conclusion, the results showed that MGMT methylation may play a key role in GC initiation. It may be correlated with DFS and OS of GC patients in univariate analysis. In addition, we did not find that MGMT promoter methylation was associated with tumor histology, tumor stage, age status, H. pylori status in GC patients. The result of the correlation between MGMT methylation and gender was not stable, which should be conservatively considered.
Supporting Information
(DOCX)
(DOC)
(DOC)
(DOC)
Acknowledgments
We sincerely thank all authors who provided published data for our study.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. 10.3322/caac.21262 . [DOI] [PubMed] [Google Scholar]
- 2.Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci. 2014;59(8):1698–709. 10.1007/s10620-014-3063-0 . [DOI] [PubMed] [Google Scholar]
- 3.Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3(8):592–600. 10.1038/nrc1141 . [DOI] [PubMed] [Google Scholar]
- 4.Handa Y, Saitoh T, Kawaguchi M, Misaka R, Ohno H, Tsai CR, et al. Association of Helicobacter pylori and diffuse type gastric cancer. J Gastroenterol. 1996;31 Suppl 9:29–32. . [PubMed] [Google Scholar]
- 5.Khan SA, Reddy D, Gupta S. Global histone post-translational modifications and cancer: Biomarkers for diagnosis, prognosis and treatment? World J Biol Chem. 2015;6(4):333–45. 10.4331/wjbc.v6.i4.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karsli-Ceppioglu S, Dagdemir A, Judes G, Ngollo M, Penault-Llorca F, Pajon A, et al. Epigenetic mechanisms of breast cancer: an update of the current knowledge. Epigenomics. 2014;6(6):651–64. 10.2217/epi.14.59 . [DOI] [PubMed] [Google Scholar]
- 7.Suijkerbuijk KP, van der Wall E, van Laar T, Vooijs M, van Diest PJ. [Epigenetic processes in malignant transformation: the role of DNA methylation in cancer development]. Ned Tijdschr Geneeskd. 2007;151(16):907–13. . [PubMed] [Google Scholar]
- 8.Ushijima T, Nakajima T, Maekita T. DNA methylation as a marker for the past and future. J Gastroenterol. 2006;41(5):401–7. 10.1007/s00535-006-1846-6 . [DOI] [PubMed] [Google Scholar]
- 9.Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5(2):121–5. . [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Salehi F, Scheithauer BW, Rotondo F, Syro LV, Kovacs K. Role of MGMT in tumor development, progression, diagnosis, treatment and prognosis. Anticancer Res. 2009;29(10):3759–68. . [PubMed] [Google Scholar]
- 11.Karran P, Bignami M. DNA damage tolerance, mismatch repair and genome instability. Bioessays. 1994;16(11):833–9. 10.1002/bies.950161110 . [DOI] [PubMed] [Google Scholar]
- 12.Yousuf A, Bhat MY, Pandith AA, Afroze D, Khan NP, Alam K, et al. MGMT gene silencing by promoter hypermethylation in gastric cancer in a high incidence area. Cell Oncol (Dordr). 2014;37(4):245–52. 10.1007/s13402-014-0179-3 . [DOI] [PubMed] [Google Scholar]
- 13.Kupcinskaite-Noreikiene R, Skieceviciene J, Jonaitis L, Ugenskiene R, Kupcinskas J, Markelis R, et al. CpG island methylation of the MLH1, MGMT, DAPK, and CASP8 genes in cancerous and adjacent noncancerous stomach tissues. Medicina (Kaunas). 2013;49(8):361–6. . [PubMed] [Google Scholar]
- 14.Wang M, Li Y, Gao J, Li Y, Zhou J, Gu L, et al. p16 Methylation is associated with chemosensitivity to fluorouracil in patients with advanced gastric cancer. Med Oncol. 2014;31(6):988 10.1007/s12032-014-0988-2 . [DOI] [PubMed] [Google Scholar]
- 15.Athanasiou T, Al-Ruzzeh S, Kumar P, Crossman MC, Amrani M, Pepper JR, et al. Off-pump myocardial revascularization is associated with less incidence of stroke in elderly patients. Ann Thorac Surg. 2004;77(2):745–53. 10.1016/j.athoracsur.2003.07.002 . [DOI] [PubMed] [Google Scholar]
- 16.Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358(9285):870–5. 10.1016/S0140-6736(01)06069-X . [DOI] [PubMed] [Google Scholar]
- 17.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21(18):3672–3. 10.1093/bioinformatics/bti536 . [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R. Meta-analysis in the design and monitoring of clinical trials. Stat Med. 1996;15(12):1237–48; discussion 49–52. . [DOI] [PubMed] [Google Scholar]
- 20.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–6. . [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atakpo P, Vassar M. Publication bias in dermatology systematic reviews and meta-analyses. J Dermatol Sci. 2016;82(2):69–74. 10.1016/j.jdermsci.2016.02.005 . [DOI] [PubMed] [Google Scholar]
- 23.Guo H, Yan W, Yang Y, Guo M. [Promoter region methylation of DNA damage repair genes in human gastric cancer]. Zhonghua Yi Xue Za Zhi. 2014;94(28):2193–6. . [PubMed] [Google Scholar]
- 24.Jin J, Xie L, Xie CH, Zhou YF. Aberrant DNA methylation of MGMT and hMLH1 genes in prediction of gastric cancer. Genet Mol Res. 2014;13(2):4140–5. 10.4238/2014.May.30.9 . [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Yang Y, Lu Y, Herman JG, Brock MV, Zhao P, et al. Predictive value of CHFR and MLH1 methylation in human gastric cancer. Gastric Cancer. 2015;18(2):280–7. 10.1007/s10120-014-0370-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song B, Ai J, Kong X, Liu D, Li J. Aberrant DNA Methylation of P16, MGMT, and hMLH1 Genes in Combination with MTHFR C677T Genetic Polymorphism in gastric cancer. Pak J Med Sci. 2013;29(6):1338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong HL, Liu XQ, Sun AH, He Y, Li J, Xia Y. Aberrant DNA methylation of P16, MGMT, hMLH1 and hMSH2 genes in combination with the MTHFR C677T genetic polymorphism in gastric cancer. Asian Pac J Cancer Prev. 2013;14(5):3139–42. . [DOI] [PubMed] [Google Scholar]
- 28.Alvarez MC, Santos JC, Maniezzo N, Ladeira MS, da Silva AL, Scaletsky IC, et al. MGMT and MLH1 methylation in Helicobacter pylori-infected children and adults. World J Gastroenterol. 2013;19(20):3043–51. 10.3748/wjg.v19.i20.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider BG, Peng DF, Camargo MC, Piazuelo MB, Sicinschi LA, Mera R, et al. Promoter DNA hypermethylation in gastric biopsies from subjects at high and low risk for gastric cancer. Int J Cancer. 2010;127(11):2588–97. 10.1002/ijc.25274. 10.1002/ijc.25274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hibi K, Sakata M, Yokomizo K, Kitamura YH, Sakuraba K, Shirahata A, et al. Methylation of the MGMT gene is frequently detected in advanced gastric carcinoma. Anticancer Res. 2009;29(12):5053–5. . [PubMed] [Google Scholar]
- 31.Hiraki M, Kitajima Y, Sato S, Mitsuno M, Koga Y, Nakamura J, et al. Aberrant gene methylation in the lymph nodes provides a possible marker for diagnosing micrometastasis in gastric cancer. Ann Surg Oncol. 2010;17(4):1177–86. 10.1245/s10434-009-0815-8 . [DOI] [PubMed] [Google Scholar]
- 32.Zou XP, Zhang B, Zhang XQ, Chen M, Cao J, Liu WJ. Promoter hypermethylation of multiple genes in early gastric adenocarcinoma and precancerous lesions. Hum Pathol. 2009;40(11):1534–42. 10.1016/j.humpath.2009.01.029 . [DOI] [PubMed] [Google Scholar]
- 33.Ksiaa F, Ziadi S, Amara K, Korbi S, Trimeche M. Biological significance of promoter hypermethylation of tumor-related genes in patients with gastric carcinoma. Clin Chim Acta. 2009;404(2):128–33. 10.1016/j.cca.2009.03.044 . [DOI] [PubMed] [Google Scholar]
- 34.Kolesnikova EV, Tamkovich SN, Bryzgunova OE, Shelestyuk PI, Permyakova VI, Vlassov VV, et al. Circulating DNA in the blood of gastric cancer patients. Ann N Y Acad Sci. 2008;1137:226–31. 10.1196/annals.1448.009 . [DOI] [PubMed] [Google Scholar]
- 35.Bernal C, Vargas M, Ossandon F, Santibanez E, Urrutia J, Luengo V, et al. DNA methylation profile in diffuse type gastric cancer: evidence for hypermethylation of the BRCA1 promoter region in early-onset gastric carcinogenesis. Biol Res. 2008;41(3):303–15. doi: /S0716-97602008000300007 . [PubMed] [Google Scholar]
- 36.Cai JC, Liu D, Zhang HP, Zhong S, Xia NS. [Frequent promoter hypermethylation of several tumor suppressor genes in gastric carcinoma and foveolar epithelium]. Zhonghua Zhong Liu Za Zhi. 2007;29(7):510–3. . [PubMed] [Google Scholar]
- 37.Mitsuno M, Kitajima Y, Ide T, Ohtaka K, Tanaka M, Satoh S, et al. Aberrant methylation of p16 predicts candidates for 5-fluorouracil-based adjuvant therapy in gastric cancer patients. J Gastroenterol. 2007;42(11):866–73. 10.1007/s00535-007-2113-1 . [DOI] [PubMed] [Google Scholar]
- 38.Zhao YF, Zhang YG, Tian XX, Juan D, Jie Z. Aberrant methylation of multiple genes in gastric carcinomas. Int J Surg Pathol. 2007;15(3):242–51. 10.1177/1066896907302117 . [DOI] [PubMed] [Google Scholar]
- 39.Chang MS, Uozaki H, Chong JM, Ushiku T, Sakuma K, Ishikawa S, et al. CpG island methylation status in gastric carcinoma with and without infection of Epstein-Barr virus. Clin Cancer Res. 2006;12(10):2995–3002. 10.1158/1078-0432.CCR-05-1601 . [DOI] [PubMed] [Google Scholar]
- 40.Oue N, Mitani Y, Motoshita J, Matsumura S, Yoshida K, Kuniyasu H, et al. Accumulation of DNA methylation is associated with tumor stage in gastric cancer. Cancer. 2006;106(6):1250–9. 10.1002/cncr.21754 . [DOI] [PubMed] [Google Scholar]
- 41.Motoshita J, Oue N, Nakayama H, Kuraoka K, Aung PP, Taniyama K, et al. DNA methylation profiles of differentiated-type gastric carcinomas with distinct mucin phenotypes. Cancer Sci. 2005;96(8):474–9. 10.1111/j.1349-7006.2005.00074.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung WK, To KF, Chu ES, Chan MW, Bai AH, Ng EK, et al. Potential diagnostic and prognostic values of detecting promoter hypermethylation in the serum of patients with gastric cancer. Br J Cancer. 2005;92(12):2190–4. 10.1038/sj.bjc.6602636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong SH, Kim HG, Chung WB, Kim EY, Lee JY, Yoon SM, et al. DNA hypermethylation of tumor-related genes in gastric carcinoma. J Korean Med Sci. 2005;20(2):236–41. 10.3346/jkms.2005.20.2.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabbioni S, Miotto E, Veronese A, Sattin E, Gramantieri L, Bolondi L, et al. Multigene methylation analysis of gastrointestinal tumors: TPEF emerges as a frequent tumor-specific aberrantly methylated marker that can be detected in peripheral blood. Mol Diagn. 2003;7(3–4):201–7. . [DOI] [PubMed] [Google Scholar]
- 45.Oue N, Oshimo Y, Nakayama H, Ito R, Yoshida K, Matsusaki K, et al. DNA methylation of multiple genes in gastric carcinoma: association with histological type and CpG island methylator phenotype. Cancer Sci. 2003;94(10):901–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang GH, Lee S, Kim JS, Jung HY. Profile of aberrant CpG island methylation along multistep gastric carcinogenesis. Lab Invest. 2003;83(4):519–26. . [DOI] [PubMed] [Google Scholar]
- 47.Oue N, Sentani K, Yokozaki H, Kitadai Y, Ito R, Yasui W. Promoter methylation status of the DNA repair genes hMLH1 and MGMT in gastric carcinoma and metaplastic mucosa. Pathobiology. 2001;69(3):143–9. doi: 48769. . [DOI] [PubMed] [Google Scholar]
- 48.Park TJ, Han SU, Cho YK, Paik WK, Kim YB, Lim IK. Methylation of O(6)-methylguanine-DNA methyltransferase gene is associated significantly with K-ras mutation, lymph node invasion, tumor staging, and disease free survival in patients with gastric carcinoma. Cancer. 2001;92(11):2760–8. . [DOI] [PubMed] [Google Scholar]
- 49.Oue N, Shigeishi H, Kuniyasu H, Yokozaki H, Kuraoka K, Ito R, et al. Promoter hypermethylation of MGMT is associated with protein loss in gastric carcinoma. Int J Cancer. 2001;93(6):805–9. . [DOI] [PubMed] [Google Scholar]
- 50.Shi J, Zhang G, Yao D, Liu W, Wang N, Ji M, et al. Prognostic significance of aberrant gene methylation in gastric cancer. Am J Cancer Res. 2012;2(1):116–29. [PMC free article] [PubMed] [Google Scholar]
- 51.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266(1):6–11. 10.1016/j.canlet.2008.02.026 . [DOI] [PubMed] [Google Scholar]
- 52.Corson TW, Gallie BL. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer. 2007;46(7):617–34. 10.1002/gcc.20457 . [DOI] [PubMed] [Google Scholar]
- 53.Bodmer WF. 1998 Runme Shaw Memorial Lecture: somatic evolution of cancer. Ann Acad Med Singapore. 1999;28(3):323–9. . [PubMed] [Google Scholar]
- 54.Kondo Y, Shen L, Issa JP. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Mol Cell Biol. 2003;23(1):206–15. 10.1128/MCB.23.1.206-215.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang T, Chen X, Hong Q, Deng Z, Ma H, Xin Y, et al. Meta-analyses of gene methylation and smoking behavior in non-small cell lung cancer patients. Sci Rep. 2015;5:8897 10.1038/srep08897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Etcheverry A, Aubry M, Idbaih A, Vauleon E, Marie Y, Menei P, et al. DGKI methylation status modulates the prognostic value of MGMT in glioblastoma patients treated with combined radio-chemotherapy with temozolomide. PLoS One. 2014;9(9):e104455 10.1371/journal.pone.0104455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asiaf A, Ahmad ST, Malik AA, Aziz SA, Rasool Z, Masood A, et al. Protein expression and methylation of MGMT, a DNA repair gene and their correlation with clinicopathological parameters in invasive ductal carcinoma of the breast. Tumour Biol. 2015;36(8):6485–96. 10.1007/s13277-015-3339-9 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper.