Abstract
Serotonin is implicated in many aspects of behavioral regulation. Theoretical attempts to unify the multiple roles assigned to serotonin proposed that it regulates the impact of costs, such as delay or punishment, on action selection. Here, we show that serotonin also regulates other types of action costs such as effort. We compared behavioral performance in 58 healthy humans treated during 8 weeks with either placebo or the selective serotonin reuptake inhibitor escitalopram. The task involved trading handgrip force production against monetary benefits. Participants in the escitalopram group produced more effort and thereby achieved a higher payoff. Crucially, our computational analysis showed that this effect was underpinned by a specific reduction of effort cost, and not by any change in the weight of monetary incentives. This specific computational effect sheds new light on the physiological role of serotonin in behavioral regulation and on the clinical effect of drugs for depression.
Clinical trial Registration: ISRCTN75872983
DOI: http://dx.doi.org/10.7554/eLife.17282.001
Research Organism: Human
eLife digest
Neuromodulators are chemicals released in the brain that affect the activity of brain cells. Serotonin is a neuromodulator with the most complicated role: it is released in most brain regions and affects behavior in diverse ways. Serotonin is implicated in the regulation of mood, anxiety, impulsivity and learning. Moreover, most medications for depression target serotonin. A lack of motivation is an important symptom of depression, but exactly how serotonin affects motivation still remains unclear.
Meyniel et al. studied how increasing the amount of serotonin in the brain affects motivation in healthy people. The volunteers in the experiments squeezed a handgrip: the longer they squeezed, the more money they got as a reward. Before the experiment, some of the volunteers received an antidepressant drug that increases the amount of serotonin surrounding their brain cells, while others received a placebo.
The experiments revealed that, compared to the people who had the placebo, those who received the drug put in more effort to get a reward. More serotonin could increase motivation by reducing the perceived cost of putting in more effort, or by making people value the reward more. A mathematical model of the results showed that the increased motivation in the antidepressant group was more consistent with serotonin reducing the cost of putting in an effort, rather than increasing how much the reward was valued.
Combined with previous findings, these results suggest that serotonin affects the processing of cost associated with tasks – be that the amount of effort required, delays in getting a reward, or a punishment. Further experiments are now required to understand if the same mechanism operates in people with depression, and if so, whether it can be altered to promote recovery. It will also be important to better understand the interaction between serotonin and other neuromodulators such as dopamine.
Introduction
Selective serotonin reuptake inhibitors (SSRI) are the most prescribed medications for major depressive episodes (Bauer et al., 2008). The effects of SSRI on improving mood and reducing anxiety have been well documented (Trivedi et al., 2006; Stahl, 2008; Cipriani et al., 2009) and many experimental studies showed that serotonin modulates emotional processing even in healthy volunteers (Harmer et al., 2009; Serretti et al., 2010). Loss of interest or pleasure in daily activities is also a key symptom of depression (American Psychiatric Association, 2013). However, the impact of SSRI on the motivation deficit (apathy) remains rather controversial (Papakostas et al., 2006; Weber et al., 2009).
Indeed, several studies reported that SSRI treatments do not reduce apathy as much as other symptoms (Fava et al., 2014) or can even induce apathy in patients (Barnhart et al., 2004; Sansone and Sansone, 2010; Padala et al., 2012). However, other studies also reported that SSRI treatments increase motivation or at least the sensitivity to reward (Tang et al., 2009; Stoy et al., 2012; Yuen et al., 2014). The related animal literature is also contradictory: some studies on cost/benefit trade-off showed reduced effort expenditure with SSRIs (Yohn et al., 2016) or no effect with serotonin blockers (Denk et al., 2005), while other studies reported increased motor activity induced by SSRI (Weber et al., 2009) or optogenetic stimulation of the dorsal raphe nucleus (Warden et al., 2012).
One issue with clinical studies is that SSRI effects may be confounded by an interaction with the pathological state: an SSRI treatment was reported to decrease apathy in late-life depression (Yuen et al., 2014) and to increase it in Parkinson’s disease (Zahodne et al., 2012). Another issue is that apathy may not be a simple construct. It is typically captured as a loss of behavioral activation (Cléry-Melin et al., 2011; Treadway et al., 2012), which could arise from different causes at the cognitive level: for instance a diminished sensitivity to reward attractiveness, or alternatively an exacerbated sensitivity to action cost. The ambition of the present study therefore was two-fold: we aimed (1) to clarify the effects of an SSRI on the motivation of effortful behavior in healthy volunteers, unconfounded by psychopathology, and (2) to reconcile these effects with a more general role of serotonin in the sensitivity to action costs vs. benefits.
Although experimental findings seem diverse at first sight, they might suggest a general role for serotonin in the behavioral adaptation to action costs. Previous studies suggested that serotonin is implicated in the motor aspects of action production and also the valuation processes that motivate the action. Notably, serotonin was found to condition impulse control and the capacity for behavioral inhibition (Cools et al., 2005; Crockett et al., 2009; Warden et al., 2012; Guitart-Masip et al., 2014) and to promote patience for delayed rewards (Schweighofer et al., 2008; Miyazaki et al., 2014; Worbe et al., 2014). Serotonin also impacts the determinants of actions, for instance, how positive and negative outcomes guide learning in humans (Chamberlain et al., 2006; Crockett et al., 2009; Faulkner and Deakin, 2014), monkeys (Clarke et al., 2004) and rodents (Bari et al., 2010). Several attempts have been made previously to capture serotonin function in a coherent computational theory. Key ideas are that serotonin (1) regulates the impact of action costs such as punishments (Daw et al., 2002; Niv et al., 2007) or delay (Niv et al., 2007; Cools et al., 2011; Miyazaki et al., 2014) and (2) adjusts the propensity to activate versus inhibit the action (Boureau and Dayan, 2011; Cools et al., 2011).
Borrowing from these models, we retain the general working hypothesis that serotonin regulates behavioral activation by modulating the weight of action costs rather than benefits.
We further suggest that the notion of action cost could be more general than initially envisaged. Indeed, in the literature quoted above, actions are mostly implemented as binary responses (e.g., approach vs. avoidance) and their costs or benefits are manipulated through the valence of their outcome (e.g., monetary gain or loss). Such tasks therefore over-simplify the real-life situation, where actions may often require more or less effort, depending on their intensity and duration.
Here, we tested the specific hypothesis that serotonin regulates the weight of effort cost in action production, as opposed to the weight of expected benefit. To assess the impact of changing cerebral serotonin levels and how it unfolds over time, we compared different groups of healthy subjects treated under double blind conditions with escitalopram (an SSRI) or placebo during eight weeks. Participants performed a previously published task (Meyniel et al., 2013), during which they allocated physical effort over time in order to maximize a monetary payoff that increased with effort duration (see Figure 1A). The task thus involves trading a physical effort cost against distinct levels of monetary incentives. Although changes in cost and benefit were independently manipulated in the task, they may result in intricate effects at the behavioral level. To disentangle between potential effects of serotonin on cost or benefit estimates, or both, we used a formal model of how decisions are generated by a hidden level of computations in our task, as previously described (Meyniel et al., 2013, 2014). Based on this computational analysis of the behavior, we could pinpoint the specific effect of serotonin on cost-related parameters, and track the predicted effects of such a computational change onto the experimental measures.
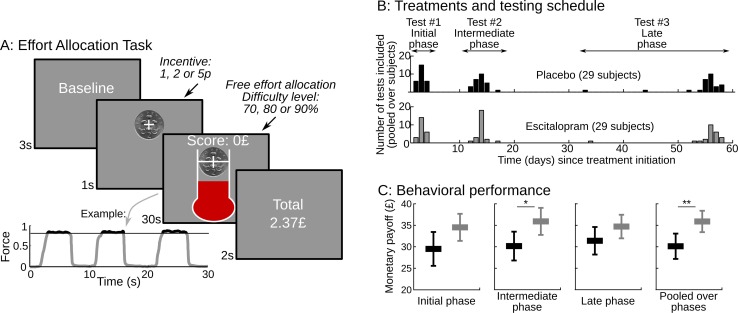
Figure 1. Task design and behavioral performance.
(A) The screenshots depict a trial as it was presented to subjects.Subjects were free to allocate their effort as they wished over the 30s corresponding to the trial duration. They were instructed that their monetary payoff would be proportional to both the monetary incentive and the effort duration, i.e. the time spent squeezing a handgrip harder than a target force level, which varied with task difficulty. Subjects were provided with on-line feedback on the payoff accumulated in the trial (score on the top) and on the instantaneous pressure exerted on the grip (fluid level in the thermometer). The force time series of an example trial is shown below the screenshots, revealing 3 effort periods, with rewarded effort (force above target) plotted in black (not gray). Two factors were manipulated across trials: (i) the incentive level, shown as a coin image (1, 2 or 5p) and (ii) the difficulty level, corresponding to the same white bar in the thermometer reached with different target force levels (70%, 80% or 90% of the maximal force). The last screen summarized the payoff cumulated over preceding trials. (B) Using a double-blind procedure, healthy subjects were assigned to one of the two treatment groups, corresponding to a daily intake of either placebo or escitalopram (10 mg during the initial phase, 20 mg during the intermediate and late phase) during 9 weeks. Each subject completed the effort allocation task three times at distinct treatment phases (initial, intermediate and late). Numbers of subjects and visits correspond to data sets included in the analysis after compliance and quality checks. (C) The three left-most graphs show task performance (as reflected in monetary payoff) sorted by treatment group (black: placebo; gray: escitalopram) and time since treatment onset. Statistical significance was assessed with two-sample, two-sided t-tests. On the right-most plot, payoff was averaged over visits at the subject level. Statistical significance was assessed with ANOVAs including treatments as between-subject factors and test phase (initial, intermediate or late) as a within-subject factor. *p<0.05; **p<0.005. Error bars indicate Student's 95% confidence intervals.
DOI: http://dx.doi.org/10.7554/eLife.17282.003
Results
Escitalopram improves performance in the effort allocation task
58 subjects were included in the analysis, see Table 1. We took the cumulated payoff as the primary measure of performance in the task and compared it between groups and visits (see Figure 1C). We found a significant effect of treatment group: performance was significantly improved in the escitalopram group (F1, 58.4=9.37, p=0.003) as compared to the placebo group. This difference was stable over time: there was no significant interaction between groups and visits (F2, 91=0.22, p=0.8). The average payoff per visit was £35.9±1.21 s.e.m. and £30.1±1.44 s.e.m. in the escitalopram and placebo groups respectively.
Table 1.
Details on participants N corresponds to the number of subjects per treatment type and phase. A few datasets were not available due technical problems and late withdrawals. Based on criteria specific to the present task (and not to the clinical trial), some subjects were excluded from the analysis ('excluded'). We report the age and sex of participants included in the analysis and the exact time of their test since the treatment onset.
| Treatment type | Treatment phase | N not available | N excluded | N after exclusion | Sex (Male / female) | Age (years) ± SD of included subjects | Time since treatment onset (days) ± SD for included subjects |
|---|---|---|---|---|---|---|---|
Placebo |
Initial |
0 |
5 |
27 |
14/13 |
23.4 ± 4.35 |
3.0 ± 0.68 |
Placebo |
Intermediate |
0 |
6 |
26 |
14/12 |
23.2 ± 4.33 |
13.8 ± 1.13 |
Placebo |
Late |
0 |
4 |
28 |
15/13 |
23.4 ± 4.27 |
54.7 ± 4.98 |
Escitalopram |
Initial |
1 |
8 |
23 |
11/12 |
24.5 ± 4.71 |
3.1 ± 0.63 |
Escitalopram |
Intermediate |
1 |
6 |
25 |
12/13 |
24.5 ± 4.51 |
14.0 ± 0.87 |
Escitalopram |
Late |
2 |
6 |
24 |
10/14 |
24.6 ± 4.61 |
55.3 ± 4.69 |
Escitalopram has a specific computational effect on effort cost
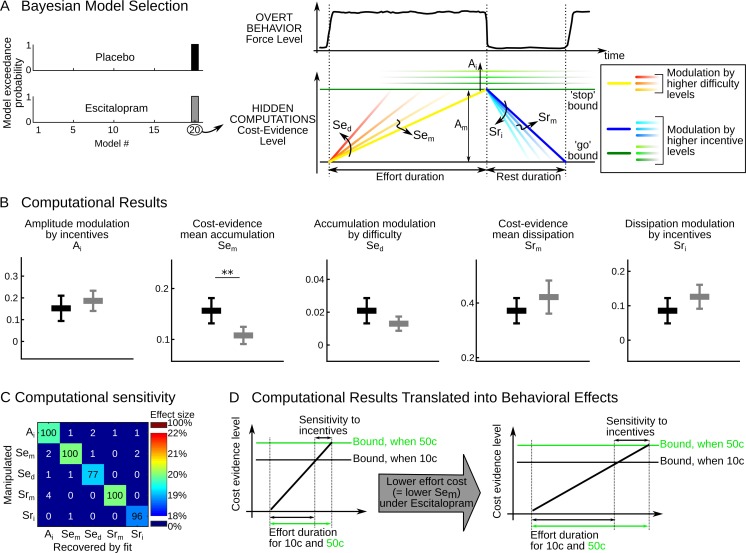
The better performance observed in the escitalopram group could be underpinned by different mechanisms: alleviation of effort costs or inflation of incentive values, or both. To disentangle between the two mechanisms, we relied on a computational model of effort allocation that was previously proposed (Meyniel et al., 2013; Meyniel et al., 2014). This model assumes that a single computational variable, termed cost evidence, accounts for the decision to stop and resume effort exertion. Cost evidence waxes during effort (with slope Se) until reaching an upper bound where effort is stopped, and it wanes during rest (with slope Sr) until reaching a lower bound where effort is resumed. The distance between bounds is the cost-evidence amplitude (denoted A). Effort and rest durations are determined by the ratios of amplitude and slopes (see Figure 2A) so that performance depends on the value of latent parameters (A, Se, Sr) and its potential modulation by task factors (monetary incentive and effort difficulty). We used a Bayesian Model Selection procedure previously validated (Meyniel et al., 2013, 2014) to pinpoint the effect of the task factors onto these latent parameters (see Materials and methods).
Figure 2. Computational results.
(A) The cost-evidence accumulation model assumes that effort and rest durations are respectively determined by the accumulation (mean slope Sem) and dissipation (mean slope Srm) of cost evidence between bounds (mean amplitude Am). Possible modulations of these parameters by incentive and difficulty levels were implemented in 20 distinct models. In the best model identified (#20) by Bayesian selection, increasing effort difficulty shortens effort duration by steepening the accumulation slope (a parametric effect controlled by parameter Sed and illustrated with colors from yellow to red). Increasing the incentive level has two effects: first, it shortens rest duration by speeding up the dissipation (parametric effect of Sri, illustrated by colors from dark to light blue); second, it lengthens effort duration by pushing back the bounds (parametric effect of Ai, illustrated by green scaling). (B) Plots show inter-subject means and Student's 95% confidence intervals obtained for the fitted values of model parameters (which were averaged over visits at the subject level). To facilitate visual comparison, scales and offsets were adjusted so that mean and error bars are visually equal across plots in the placebo group. Statistical significance corresponds to ANOVAs including treatment group (escitalopram vs. placebo) as a between-subject factor and treatment phase as a within-subject factor (initial, intermediate or late); **p<0.005. (C) Data in the placebo group served as a baseline to simulate effort and rest durations after imposing a 20% increase in computational parameters. In the table, each row corresponds to a simulated change in one single parameter. Colors denote the effect sizes recovered by model fitting for each parameter, as percent of change compared to baseline. Numbers indicate the percentage of 'hit' (on the diagonal) and 'false alarm' (off-diagonal) in detecting a significant change in parameter values with a paired t-test thresholded at p<0.01. (D) The graph illustrates why the effect of escitalopram, characterized at the computational level as a reduced accumulation slope of cost-evidence during effort (Sem), should translate at the behavioral level into both a longer effort duration and an increased sensitivity of effort duration to incentive level.
DOI: http://dx.doi.org/10.7554/eLife.17282.005
Replicating our previous studies, the best model showed that incentives impacted the amplitude (A) and dissipation slope (Sr), whereas effort difficulty impacted the accumulation slope (Se), as illustrated in Figure 2A, right. To capture all the effects, the cost-evidence accumulation model therefore necessitates five free parameters: the mean slope of cost-evidence accumulation (Sem) and its steepening for higher difficulty levels (Sed); the mean slope of cost-evidence dissipation during rest (Srm) and its steepening for higher incentives (Sri); and the expansion of the cost-evidence amplitude as higher incentives push the bounds back (Ai). The fact that the same best model was found independently in each treatment group with high confidence levels (exceedance probabilities xp>98% in each group) indicates that all subjects can be characterized within this common computational framework. We computed for each subject the best-fitting values of the model parameters (Ai, Sem, Sed, Srm, Sri). Since the interaction between treatments (placebo vs. escitalopram) and treatment phase was not significant for any parameter (all F2, 91<2.03, p>0.14), we provide fitted values pooled over treatment phases in Figure 2B.
Only one model parameter was significantly different between the placebo and escitalopram groups: the cost-evidence accumulation slope (Sem, F1, 58.0=9.88, p=0.003). This parameter captures the average value of effort cost across conditions: there was a 31% decrease in the escitalopram group compared to the placebo group (on average with ± s.e.m., placebo: 0.16±0.012, escitalopram: 0.11±0.008); the difference was actually significant at each visit (all p<0.015; two-sided t-test).
The difference between escitalopram and placebo never reached significance for the other model parameters (all p>0.08, see Table 2; on average with ± s.e.m. for placebo vs. escitalopram, Ai: 0.15±0.028 vs. 0.19±0.022, Sed: 0.021±0.004 vs. 0.013±0.002, Srm: 0.37±0.022 vs. 0.42±0.029, Sri: 0.086±0.018 vs. 0.126±0.016). We checked the sensitivity and specificity of our model fitting procedure in detecting a treatment effect through simulations. A 20% change in any given parameter was reliably detected and the difference recovered by model fitting was significant in a 96% of simulations at least for Ai, Sem, Srm and Sri and in a 77% of simulations for Sed. Importantly, a change in one single parameter was recovered without propagating to other parameters and the false alarm rate was below 5% for all parameters (Figure 2C).
Table 2.
Treatment effect on computational parameters and behavioral measures.
All numbers are p-values obtained from ANOVAs. p-values lower than 0.05/5=0.01 (computational parameters) and 0.05/6=0.008 (behavioral measures) appears in bold to show significant effects that survive correction for multiple comparisons.
| Variable | Treatment | Visit | Interaction |
|---|---|---|---|
Ai |
0.388 |
0.197 |
0.406 |
Sem |
0.003 |
0.039 |
0.260 |
Sed |
0.0797 |
0.543 |
0.137 |
Srm |
0.186 |
0.778 |
0.612 |
Sri |
0.130 |
0.196 |
0.557 |
Effort duration – mean |
0.002 |
0.199 |
0.875 |
Effort duration – sensitivity to incentive |
0.023 |
0.187 |
0.115 |
Effort duration – sensitivity to difficulty |
0.247 |
0.813 |
0.318 |
Rest duration – mean |
0.213 |
0.482 |
0.531 |
Rest duration – sensitivity to incentive |
0.162 |
0.937 |
0.807 |
Rest duration – sensitivity to difficulty |
0.115 |
0.423 |
0.681 |
To further test the specificity of SSRI effect on cost-evidence accumulation, we performed another Bayesian model selection that contrasted the two groups of subjects. Parameters of the cost-evidence accumulation model were fitted on the placebo group data to serve as a reference. In the escitalopram group, they were fixed to these reference values and only one or two modulations were permitted to capture the treatment effects. Models including a modulation of Sem outperformed the others (log Bayes Factor, all Δ>147.9), see Table 3. A version with modulations of both Sem and Sed was slightly more likely than a version with only a modulation of Sem (Δ=3) and much more likely than any other combination (Δ>5.9).
Table 3.
Model comparison assessing the specificity of treatment effect.
Data in the escitalopram group were fitted with the cost-evidence accumulation model. The parameters were fixed to the values fitted onto the placebo group, excepted when a modulation was permitted. The first row contains models that permit the modulation of one single parameter, whereas the remaining rows correspond to models that permit a combination of two modulations. Each cell gives log Bayes Factor (i.e. log model evidence) relative to the null model. Higher values denote better models.
Sem |
Sed |
Srm |
Sri |
Ai |
|
|---|---|---|---|---|---|
Only one modulation |
173.6 |
19.9 |
−4.2 |
−3.8 |
3.7 |
Also includes Sem |
176.6 |
169.6 |
169.7 |
170.7 |
|
Also includes Sed |
15.7 |
16.1 |
21.7 |
||
Also includes Srm |
−7.3 |
−1.1 |
|||
Also includes Sri |
1.2 |
Thus, the specific effect of SSRI on cost-related parameters supports our hypothesis that serotonin is involved in the estimation of action cost, rather than benefits.
Computational effects of escitalopram translate into behavioral effects
Reducing the effort cost (Sem) only should have two effects at the behavioral level (see Figure 2D). The first is straightforward: the duration of effort epochs should be longer. The second is less trivial: the sensitivity of effort duration to incentive level should be increased. This is because in our model, incentive level modulates the amplitude between bounds (as captured by parameter Ai). Thus, the effect of incentive level on effort duration is proportional to the accumulation slope Sem: if the accumulation is slower, then a given displacement of the upper bound will have a larger effect on effort duration.
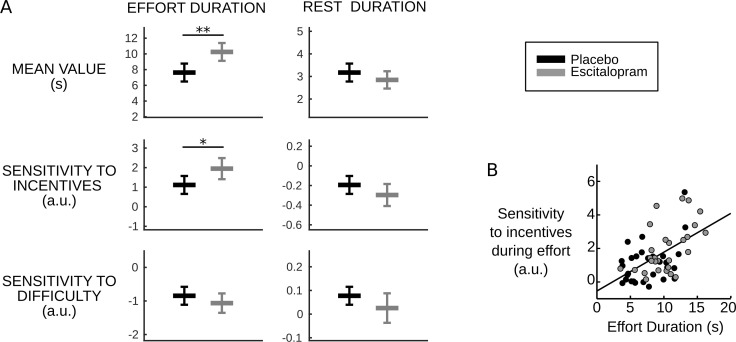
Fulfilling these predictions, we found both a longer effort duration per se (F1, 58.1=10.72, p=0.002, on average placebo: 7.63s±0.56, escitalopram: 10.2s±0.55, with s.e.m.) and a higher sensitivity of effort duration to incentive level (F1, 58.5=5.46, p=0.023, on average with ± s.e.m., placebo: 1.11±0.22, escitalopram: 1.95±0.26) in the escitalopram group compared to placebo (see Figure 3A). The difference between the two treatment groups never reached significance for the other behavioral variables (all p>0.11, on average with ± s.e.m., for placebo vs. escitalopram, effort sensitivity to difficulty: −0.84±0.13 vs. −1.06±0.14, rest duration: 3.17s±0.19 vs 2.85s±0.19, rest sensitivity to incentives: −0.19±0.04 vs. −0.30±0.05, rest sensitivity to difficulty: 0.077±0.018 vs. 0.025±0.03).
Figure 3. Behavioral results.
(A) Plots show inter-subject means and Student's 95% confidence intervals obtained from linear regression.Regression coefficients were averaged over visits at the subject level. To facilitate visual comparison, scales and offsets were adjusted so that mean and s.e.m. are visually equal across plots in the placebo group. Statistical significance corresponds to ANOVAs including treatment group (escitalopram vs. placebo) as a between-subject factor and treatment phase as a within-subject factor (initial, intermediate or late); *p<0.05, **p<0.005. (B) As predicted by the cost-evidence accumulation model, effort duration and its sensitivity to incentive level are correlated across subjects (one dot corresponds to one subject; values were averaged across visits for each subject). The line shows the linear regression fit obtained when pooling the two treatment groups (ρ56=0.55, p<10–5).
DOI: http://dx.doi.org/10.7554/eLife.17282.010
Crucially, because they arise from a common cause, the model also predicts that the two effects should be correlated: the more Sem is reduced, the more effort duration and its sensitivity to incentive level should be increased. The Pearson correlation over subjects was indeed significantly positive in both groups (placebo: ρ27=0.43, p=0.02; escitalopram: ρ27=0.54, p=0.002, see Figure 3B).
Discussion
Our behavioral results in healthy volunteers show that the SSRI escitalopram improves global performance and hence payoff in a task that involves trading effort cost against monetary benefit. Taking advantage of the independent manipulation of cost and benefit, our computational analysis characterized the effect of escitalopram as a specific diminution of effort cost.
Together with previous findings, our results support the hypothesis that cost may be a general functional domain for serotonin (Boureau and Dayan, 2011; Dayan, 2012). For instance, interventions targeting serotonergic transmission during probabilistic and reversal learning paradigms in rodents, monkeys and humans suggested that serotonin impacts sensitivity to negative rather than positive feedback (Clarke et al., 2004; Chamberlain et al., 2006; Crockett et al., 2009; Bari et al., 2010; Cohen et al., 2015). The reduction of serotonergic transmission in humans following acute tryptophan depletion (for a review, see Faulkner and Deakin, 2014) reduced information sampling in a decision-making task, but crucially, only when sampling had a financial cost (Crockett et al., 2012). Serotonin may also impact moral costs, such as unfairness in social decision-making (Crockett et al., 2008). Furthermore, serotonin may control the cost of delay in reward delivery in rodents, such that a higher firing rate of serotonergic neurons correlates with an increased ability to wait for bigger rewards (Miyazaki et al., 2014), without affecting the sensitivity to the reward itself (Fonseca et al., 2015). Conversely, low serotonin levels, e.g. after acute tryptophan depletion, were suggested to exacerbate sensitivity to the cost of waiting, which could result in impulse control disorders (Crockett et al., 2009; Cools et al., 2011; Dayan, 2012). Thus, it is tempting to build a parsimonious computational theory on the idea that serotonin is involved in processing all kinds of action costs. This is compatible with the notion that different types of costs are processed by distinct neural circuits, since neuromodulators such as serotonin can affect many brain regions. However, such a generalization would necessitate assessing the putative role of each mono-amine and their interactions with different types of costs within the same study. Previous attempts in rodents have observed effects that are inconsistent with the present results: in cost/benefit trade-off tasks, serotonin blockers impacted choices only when costs were delays, and not physical efforts (Denk et al., 2005), whereas dopamine blockers affected both types of costs. Such discrepancies may call for caution when translating the results of pharmacological studies in rodents into medication effects in humans.
A general role for serotonin in cost processing also seems compatible with the effects on apathy and impulsivity reported in healthy and pathological conditions (Cools et al., 2005; Papakostas et al., 2006; Schweighofer et al., 2008; Crockett et al., 2009; Weber et al., 2009; Warden et al., 2012; Guitart-Masip et al., 2014; Miyazaki et al., 2014; Worbe et al., 2014). Indeed, according to our interpretation, SSRIs might reduce the perceived cost of performing actions, which would promote behavioral activation, hence the alleviation of apathy. As SSRIs might also reduce the perceived cost of delaying action, they would improve response control and reduce impulsivity (Cools et al., 2005; Cools et al., 2011; Crockett et al., 2009; Dayan, 2012; Bari and Robbins, 2013; Miyazaki et al., 2014; Fonseca et al., 2015).
However, it is important to note that the SSRI effects obtained here relate to effort cost and not to time discounting. Although time is central in our task, it departs from delay and waiting paradigms in several respects. First, the overall task duration was fixed and its pace was independent from how subjects allocated their effort within trials. Second, the reward was gained instantaneously, concomitantly to effort production, without delay. Therefore, the SSRI effect on effort duration (how long subjects sustain an effort) cannot be explained by a change in temporal discounting (how long subjects wait for a reward).
The direction of the effect obtained in the present study suggests that higher serotonin level alleviates the effort cost. This interpretation is based on the assumption that the primary effect of SSRI is to increase serotonin level in the synaptic cleft (Nutt et al., 1999; Stahl, 2008). However, the net effect of SSRI might not be so straightforward to interpret at the molecular level for several reasons. First, a high tonic concentration could have the paradoxical effect of reducing the sensitivity to phasic serotonergic signals (Faulkner and Deakin, 2014). Second, the ubiquitous negative feedback regulation by auto-receptors can initially revert the response expected from high extracellular levels of serotonin (Stahl, 2008; Fischer et al., 2014) and also produce non-linear dose-response functions for cognitive performance (Bari et al., 2010). Third, much evidence suggests that different serotonin projections to different forebrain systems mediate varying acute and chronic adaptive responses to aversive events (Hale et al., 2013; Faulkner and Deakin, 2014). The effect of serotonin also depends on the location in the brain and on the type of receptors, for instance, 5-HT1A and 5-HT2A receptors respectively inhibit and excite motoneurons (Jacobs and Azmitia, 1992). Thus, the superficial interpretation that serotonin helps overcoming effort costs will need to be refined by addressing the complexity of SSRI effects at the molecular level. We nevertheless note that the superficial interpretation is consistent with demonstrations that serotonin also helps overcoming other costs such as delay in humans (Schweighofer et al., 2008).
Refinement is also needed at the computational level. Our model does not specify how exactly serotonin could attenuate the impact of effort cost on action production. An indirect effect through an increase in muscular capacity can be excluded since there was no difference in maximal force between the placebo and SSRI groups at any visit. The computational analysis simply suggests that a slower accumulation of effort cost (lower Sem) under SSRI prolonged effort duration. It does not distinguish between down-regulation of the cost signal itself, or down-regulation of the weight this cost has in the decision to produce an effort. The former (perceptual) view would be in line with an analgesic effect of escitalopram, which is consistent with the findings that nociception and/or somatosensory perception are both modulated by serotonin at central levels (Jacobs and Azmitia, 1992), that serotonin is targeted by common drugs modulating pain like acetaminophen (Smith, 2009) and that serotonin modulates the inhibitory feedback loop that allows muscular fatigue to down-regulate the motor command (Gandevia, 2001; Cotel et al., 2013). The latter (decisional) view would be more generalizable to the capacity of overcoming other types of costs.
As the serotonin and dopamine systems strongly interact (Dremencov et al., 2009; Cools et al., 2011; Schilström et al., 2011; Fischer et al., 2014), the SSRI effect observed here might also be at least in part mediated by dopamine. Indeed, the beneficial effect of SSRI shown here is reminiscent of previous reports about dopaminergic manipulations in mesolimbic structures, which affected the propensity to choose high reward – high effort over low reward – low effort options in rodents (Cousins et al., 1996; Salamone et al., 2007). However, if SSRIs antagonize dopamine release (Dremencov et al., 2009; Cools et al., 2011), escitalopram should have reduced effort production in our task (but see Schilström et al., 2011). Moreover, the fact that escitalopram did not modulate incentive effects on parameters such as the amplitude or dissipation slope argues against a participation of dopamine. Indeed dopaminergic manipulations in humans have been repeatedly shown to affect reward processing, not only in learning contexts (Frank et al., 2004; Pessiglione et al., 2006; Palminteri et al., 2009) but also in reward/effort trade-off paradigms (Wardle et al., 2011; Treadway et al., 2012; Le Bouc et al., 2016). It might be that serotonin and dopamine have complementary roles in promoting action production (and alleviating apathy): the former by reducing effort cost and the latter by enhancing the incentive value of potential rewards.
Previous studies suggest that we should distinguish between acute and chronic effects of SSRIs (Fischer et al., 2014). We did not find an interaction with time in our results but only a main effect of treatment group. Both parametric and non-parametric statistics showed that such a group difference is very unlikely to arise from chance in sampling the population (under the null hypothesis). General psychological assessment at baseline was also similar in the two groups. However, we acknowledge that our shortest time since treatment onset (3 days) may already depart from the acute regime, that ceiling effects in the SSRI group could in principle mask an interaction with time, and that our study may lack the statistical power to reveal such an effect. This absence of an interaction contrasts with the apparently delayed clinical effect of SSRIs in depressed patients, which usually take weeks to improve mood (Stahl, 2008). However, at the molecular level, serotonin release is boosted by SSRIs from treatment onset (Kobayashi et al., 2008) and SSRI effects on emotional processing also occur with little or no delay in healthy subjects (Harmer et al., 2003). In pathological conditions, time may be needed to adjust to the new, less negative perception of costs, as well as the reduced emotional bias, and convert these implicit changes into a conscious subjective improvement that can be reported to the practitioner (Harmer et al., 2009; Cools et al., 2011). This idea has been formalized in a recent study showing how positive and negative outcomes can shape mood on the long run (Eldar and Niv, 2015). Further studies in depressed patients are needed to assess whether an early detection of effort cost attenuation could be used to predict long-term treatment effects on clinical symptoms.
Finally, our results also indicate that non-trivial behavioral effects can be accounted for by a change in a single computational parameter (Sem): a specific modulation of cost can result in both longer efforts and an increased sensitivity of effort duration to potential benefits in our results. Intuitively, this effect on reward sensitivity corresponds to the idea that when perceived costs are too high, a change in reward prospect will have little effect. Thus, our analysis shows that the behavioral consequences of cost and benefit estimates are intricate but computationally tractable. Our paradigm and model could provide experimental and conceptual tools to refine the description of motivational disorders. Distinct dysfunctions, such as amplification of effort cost vs. flattening of reward prospects, might call for different treatments: if drugs modulating serotonin only affect cost estimates, then other drugs (possibly dopaminergic) should aim to impact the valuation of potential benefits.
Materials and methods
Participants
Healthy volunteers (18–45 years old) were recruited to the study by public advertisement after approval by the Ethics Committee of Berkshire, UK (protocol CL1-20098-81, on 28th Sept. 2011) and registration as a clinical trial, ISRCTN75872983. Participants gave their written informed consent prior to participation. Normal health was checked by clinical and psychiatric examinations including laboratory tests. Exclusions and withdrawals from the study were adjusted to include 64 participants (2 treatment groups of 32 participants, 16 men per group), tested at 3 separate visits, resulting in 192 completions of the task. Given that the clinical trial was exploratory and also included tests from other research groups, the sample size was not selected specifically for our study; however it appeared reasonable given typical studies in the field. Indeed, published between-subject comparisons of placebo and anti-depressant treatments were made on a lower sample size per group (e.g. N=14 in Harmer et al., 2004, N=20 in Chamberlain et al., 2006 and N=30 in Guitart-Masip et al., 2014), and a single visit per subject (while we have three). Due to technical problems or late withdrawals, 4 task completions were not fully acquired and therefore unusable. The remaining data were checked for quality by F.M. prior to unblinding: 11 were excluded due to mis-calibration of task difficulty; 9 due to hardware default or signal quality; 15 for non-compliance with task instructions. Subjects were asked to produce an effort in every trial. As there were eight trials per condition in the task, a given visit was excluded for non-compliance when the total number of effort (or rest) was lower than three in at least one condition. As a result, the number of participants per visit varied between 23 and 28 in each treatment group, for a total of 153 task completions. Note that a given participant may produce an interpretable dataset at a given visit and not at another. The total number of participants with at least one interpretable dataset across the three visits happened to be 29 in each treatment group. The finally included data set is summarized in Table 1.
Sex ratio (14/29 vs. 16/29, Z test for proportion: z= 0.53, p=0.6), ratio of excluded data sets (15/96 vs. 20/92, z=1.08, p=0.28) and age (mean with ± s.e.m., 23.2 ± 0.8 vs. 24.2 ± 0.8, p=0.38) were similar in the two groups. We also used psychological tests to assess differences between groups of subjects at baseline, before treatment. T-test comparison showed no significant difference in Mood Visual Analog Scales for the items 'happy' (p=0.75), 'sad' (p=0.96), 'hostile' (p=0.48), 'alert' (p=0.73), 'anxious' (p=0.51), 'calm' (p=0.14), nor in the Hospital Anxiety Depression score for anxiety (p=0.68) and depression (p=0.35). The difference in State-Trait Anxiety Inventory score was at p=0.04 at baseline but p=0.45 and p=0.83 after 7 and 55 days of treatment respectively. Therefore, psychological variables were not significantly different at baseline when correcting for multiple comparisons (at p=0.05/9) and the one passing the uncorrected threshold p=0.05 was not significantly different during the testing phase.
Drug and testing schedule
Two centers participated in the study (Oxford and Manchester, UK). Data were collected between January 2012 and July 2013. Participants were randomly assigned to one of the two parallel treatment groups following a double-blind procedure: placebo or escitalopram (10 mg during week #1 and #9; 20 mg from week #2 to #8). The randomisation list was constructed in blind, by the Institut de Recherches Internationales Servier, with stratification by gender and center (Oxford, Manchester). Treatments were conditioned by Les Laboratoires Servier Industrie so as to be visually indistinguishable and shipped in numbered containers to the investigators. The randomisation list was not made available to the investigators until the final data set had been checked for quality and locked by transfer to a Contract Research Organization (Biotrial). Participants took a daily oral capsule around 8 P.M. for 9 weeks and performed the Effort Allocation Task three times at distinct latencies (Figure 1B): initial (2–4 days after treatment onset), intermediate (12–17 days) and late (52–60 days for all subjects but 3, who were tested between the 33rd and 44th days before withdrawing from the study). The task was not performed on week #9. The clinical trial included other tests, not presented here, to assess emotional processing, sexual acceptability and learning abilities. Safety evaluations (adverse events collection, blood pressure/heart rate and laboratory test) were performed along with the study.
Experimental set-up
The Effort Allocation Task is schematized in Figure 1A and detailed in a previous publication (Meyniel et al., 2013). The only change was the adoption of the local currency (British pounds). On each trial, participants had 30 s that could be spent either resting or squeezing a handgrip. They were instructed that the payoff would be proportional to both the monetary incentive and the time spent above a force target corresponding to effort difficulty. The task lasted approximately one hour and was split into 8 blocks. The factor levels (monetary incentives: 1, 2 or 5p and effort difficulty: 70, 80, 90% of the maximal force) were manipulated independently and crossed, resulting in 9 conditions, each corresponding to a trial, presented in a randomized order in each block. Left and right hands were used alternatively over blocks. Participants were encouraged to maximize their payoff at each trial, and told that the cumulated payoff would be added to their financial compensation for participating in the study. Unbeknown to them, this payoff was rounded up to a fixed amount after the last visit such that all participants eventually received the same total. The task difficulty was adjusted to the subject's maximal force, which was measured at each visit before the test. The procedure for measuring the maximal force (sustained handgrip squeezing) is detailed in (Meyniel et al., 2013) and follows published guidelines (Gandevia, 2001). The maximal force was not affected by treatments (F3, 113.7=0.93, p=0.42), visits (F2, 180=1.34, p=0.26) or by the interaction of these two factors (F6, 180=0.27, p=0.95).
Behavioral analysis
The cumulated effort duration determined the payoff obtained at each trial. Yet there are many ways of chunking this cumulated duration depending on the duration of each effort and rest epochs. Effort and rest epochs were determined based on the force time series (Figure 1A provides an example) using an off-line algorithm (Meyniel et al., 2014). The offline detection algorithm was based on both the force signal normalized by the calibration maximal force, and its temporal derivative. Samples with positive derivative, exceeding one standard deviation of the derivative time series, and force level higher than 0.5 (half the maximum) were tentatively marked as effort onsets. Effort offsets were defined similarly for negative derivative values and force levels below 0.5. When multiple offsets were detected between two onsets, all but the last one were discarded. If multiple onsets were detected before an offset, the one with minimum force was kept. An offset was marked at the trial end if the effort was still sustained at that moment. Elapsed time between effort onsets and offsets determined effort and rest durations. The first rest duration was the elapsed time between trial onset and the first effort onset.
We performed a model-free analysis of the behavior, for each subject and visit, with multiple linear regressions done separately for effort and rest durations. The linear models included the factors of interest (incentive and difficulty) and temporal factors (block number; trial number within a block, effort or rest epoch number within a trial). Regressors (excepted the constant) were z-scored so that regression coefficients (beta estimates) correspond to standardized effect sizes. These beta values were then compared between treatments using ANOVA (see statistical analysis below).
Computational analysis
The cost-evidence accumulation framework
To account for the decision to stop and resume effort exertion in our task, we previously developed a computational model that was supported by both behavioral and neuroimaging data (Meyniel et al., 2013; Meyniel et al., 2014). The central assumption of our model is that the allocation of effort over time is underpinned by the variation of a single computational variable. This decision variable waxes during effort until reaching an upper bound, which triggers effort cessation, and wanes during rest until reaching a lower bound, which triggers effort resumption. The model thus has three latent parameters: the accumulation () and dissipation () slopes of cost evidence and the amplitude of these variations (A; the distance between bounds). The ratios of amplitude and slopes determine the effort and rest durations (see Figure 2A).
The computational analysis, detailed below, aimed at (1) identifying how the model latent parameters are modulated by the task factors (incentive and difficulty levels), (2) testing whether these modulations differ between treatment groups and (3) assessing the specificity and sensitivity of our fitting procedure in detecting potential differences between treatment groups.
Model fit and selection
In this section, we provide details about model fit and model selection, although we followed the exact same methods as reported in our previous publication (Meyniel et al., 2013).
In principle, each model latent parameter (accumulation slope , dissipation slope and amplitude of variations ) could be modulated by each task factor, which we formalized as linear effects:
| (1) |
where and are the mean durations of effort and rest epochs (fitted across experimental conditions); I and D are the z-scored incentive and difficulty levels; , , , , and capture potential modulations by task factors. The goal of model selection was to identify whether allowing fewer modulations, e.g. only , and , could still provide a reasonable goodness-of-fit. In total, there are 26=64 possible combinations of the six modulations by task factors. However, we noted in (Meyniel et al., 2013) that only 20 combinations can a priori reproduce the behavioral effects observed in the effort allocation task (and potentially other effects): increased with incentive, decreased with incentive, and decreased with difficulty. These 20 models correspond to four possible combinations of the incentive effect: or or or crossed with five possible combinations of the difficulty effect: or or or or . Besides these modulation terms, all models also included the mean accumulation and dissipation slopes, and , as free parameters. Since effort and rest durations are determined by the ratios of amplitude and slopes in Equation 1, the mean amplitude was not considered as a free parameter; it was fixed to one, so that each subject could be characterized by a unique set of best-fitting parameter values instead of an infinity of proportional solutions.
We used Bayesian model selection to identify a solution with the best tradeoff between goodness-of-fit and simplicity (number of modulation terms in the model). The 20 models were fitted for each subject and visit separately using a variational Bayesian procedure described in (Daunizeau et al., 2014). We used non-informative priors for the fitted parameter values. This variational procedure provides, for each visit and model, the posterior mean values of the latent parameters and the model evidence. The latter is a key summary statistic on the basis of which the best model is identified (Stephan et al., 2009; Daunizeau et al., 2014). In order to obtain a single summary statistic per subject, we took the joint (i.e., product of) model evidence over visits. The algorithm used for group-level, random-effect Bayesian Model Selection is described in (Stephan et al., 2009) and implemented in the Matlab toolbox SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; function spm_BMS.m).
Best computational model identified
In both treatment groups, Bayesian model selection (see Figure 2A) identified the following model as providing the best trade-off between simplicity and goodness-of-fit of the data:
| (2) |
This is the same best model as in our previous publication (Meyniel et al., 2013), although it is fitted on different subjects. Fitted parameter values were on average positive such that higher incentives expanded the bounds of cost-evidence variations and quickened its dissipation during rest and higher difficulty levels increased the accumulation rate of cost-evidence during effort (see Figure 2B).
This computational model can be interpreted as follows, for a more complete discussion see (Meyniel et al., 2013, 2014). The accumulation of cost evidence may reflect the build-up of physiological fatigue: the instantaneous effort cost increases as it becomes gradually more difficult to sustain a given force level. As expected from a physiological perspective, fatigue builds up more quickly when higher force levels are exerted, which is captured by . An upper bound on the accumulation process ensures that instantaneous cost remains within a certain range that is adjusted to the reward rate. Indeed, the bound is pushed back for higher incentives (which is captured by ), as if an extra cost is allowed when a higher benefit makes it worthy. The bounded accumulation mechanism therefore seems to control the balance between cost and benefit, which could be implemented as an opponency between brain systems (Daw et al., 2002; Balasubramani et al., 2015).
The dissipation of cost evidence may reflect that it takes time to recover full exercising capacity after a strenuous effort. Interestingly, effort was not resumed immediately after cessation, but only after a substantial decrease in cost evidence. Waiting until a lower bound is reached may ensure that effort is not systematically produced at maximal instantaneous cost. Rest may therefore contribute to optimizing the cost/benefit balance on the long run. Importantly, an exceedingly long rest would also be suboptimal since it would reduce the payoff. Indeed, only efforts are rewarded in the task: there is therefore an opportunity cost to rest (Niv et al., 2007). This opportunity cost seems to be taken into account since the cost-evidence dissipation is speeded up for higher incentives (which is captured by ), so that effort can be resumed more quickly.
Specificity and sensitivity of the fitting procedure
The fitted parameter values were then compared between treatment groups (see below). We used simulations to assess whether our fitting procedure can detect specific changes in the model parameters between groups. For each subject in the placebo group, we fitted the model and took the mean value over visits of a given parameter (henceforth the 'target'). To simulate a potential effect of the SSRI treatment on this target parameter, we increased this value by a 20%. Then we computed mean effort and rest durations in each experimental condition using Equation 2 and we corrupted these means with noise (we added the residuals of fitted data taken from another random subject in the placebo group). Last, we fitted again the model by adjusting a second set of parameters onto these simulated mean effort and rest durations. To assess statistical significance, we repeated this procedure 100 times for each subject and each target parameter. We compared the initial parameter values (fitted on actual data) and the new ones (fitted on simulated data) for each simulation with paired t-test across subjects and the threshold p<0.05/5 to correct for the 5 parameters tested. For 'target' parameters, a significant difference indicates that the fitting procedure correctly recovers the simulated increase (a 'hit'); for 'non-target' parameters, a significant difference indicates a lack of specificity (a 'false alarm'). We report the simulated changes identified by the fitting procedure in Figure 2C, as the median parameter change (across subjects), averaged over simulations.
Specificity of the treatment effect
We also used Bayesian model comparison to test the specificity of treatment effect on computational parameters. We performed this comparison at the group level since our design is between-subject and different subjects may have different parameter values irrespective of the treatment type. To this end, we concatenated across subjects the mean effort and rest durations per condition, separately for each group. The parameters of the accumulation model (Equation 2) were fitted onto the placebo group data. These fitted values served as reference: AiREF, SemREF, SedREF, SrmREF, SriREF. We then modeled the potential treatment effect in the escitalopram group as a modulation of the reference parameters: Ai = AiREF*(1+δAi), Sem= SemREF*(1+δSem), and so on for Sed, Srm or Sri. For each parameter, the sign and value of δ captures the direction and strength of the treatment effect with respect to the placebo group. We designed single-modulation variants of the accumulation model (Equation 2) by forcing δ to zero for all but one parameter, for which δ remained free. In other words, a modulation of only one single parameter was permitted. We also designed double-modulation models in which two modulations are permitted and modeled with distinct δs. All models were fitted with the variational Bayesian procedure by (Daunizeau et al., 2014). Values of log model evidence are reported in Table 3 for model comparison.
Statistical analysis
We characterized the effect of treatments on (1) the monetary payoff, (2) the fitted parameters of the best computational model (Equation 2), (3) the regression coefficients of the model-free analysis. We provide source data files for each of these three sets of variables. We used a repeated-measure ANOVA with subjects as a random factor, treatment group as a between-subject factor and time since treatment onset (initial, intermediate, late) as a within-subject factor. Interaction between the within-subject factors was included in all ANOVAs. We followed up the ANOVA results with two-sample two-sided t-tests at a given time since treatment onset. Significance levels corrected for comparing multiple variables are included in Table 2.
Given our large sample size (N=58 included subjects), these classical parametric statistics reliably quantify the likelihood that the observed differences between treatment groups reported in our study may be due to chance in sampling the population. Indeed, we confirmed these significance levels with non-parametric permutation tests, using 10,000 permutations of treatment labels between subjects to estimate the probability that an equal or more extreme statistic (F or T depending on the test) could occur by chance.
Funding Statement
The funders had no role in data collection, interpretation and publication.
Funding Information
This paper was supported by the following grants:
Institut de Recherche Internationales Servier Study No. CL1-20098-81 to Florent Meyniel, Guy M Goodwin, JF William Deakin, Corinna Klinge, Christine MacFadyen, Holly Milligan, Emma Mullings, Mathias Pessiglione, Raphaël Gaillard.
French Ministère de la Recherche PhD Fellowship to Florent Meyniel.
Seventh Framework Programme Postdoc fellowship (no. 604102) to Florent Meyniel.
Additional information
Competing interests
FM: Supported by The French Ministère de la Recherche and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 604102 (Human Brain Project).
GMG: Holds shares in P1vital Ltd and has served as consultant, advisor or CME speaker in the last 12 months for AstraZeneca, Cephalon/Teva, Convergence, Eli Lilly, GSK, Lundbeck, Medscape, Otsuka, Servier, Sunovion, Takeda.
JFWD: Currently advises or carries out research funded by Autifony, Sunovion, Lundbeck, AstraZeneca and Servier; all payment is to the University of Manchester; he has share options in P1vital Ltd.
RG: Received compensation as a member of the scientific advisory board of Janssen, Lundbeck, Roche, Takeda; he has served as consultant and/or speaker for Astra Zeneca, Pierre Fabre, Lilly, Otsuka, SANOFI, Servier and received compensation, and he has received research support from Servier.
The other authors declare that no competing interests exist.
Author contributions
FM, Conception and design, Analysis and interpretation of data, Drafting or revising the article.
GMG, Conception and design, Drafting or revising the article.
JFWD, Conception and design, Drafting or revising the article.
CK, Collected all data together with CMF from the Oxford center, Acquisition of data.
CM, Collected all data together with CK from the Oxford center, Acquisition of data.
HM, Collected all data together with EM from the Manchester center, Acquisition of data.
EM, Collected all data together with EM from the Manchester center, Acquisition of data.
MP, Interpretation of the result, Conception and design, Drafting or revising the article.
RG, Interpretation of the results, Conception and design, Drafting or revising the article.
Ethics
Clinical trial registration ISRCTN75872983, URL: http://www.isrctn.com.
Human subjects: The study was approved by the Ethics Committee of Berkshire, UK on 28th Sept. 2011 (protocol CL1-20098-81) and was registered as a clinical trial (ISRCTN75872983). Healthy participants gave their written informed consent prior to participation.
Additional files
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5) American Psychiatric Pub; 2013. [Google Scholar]
- Balasubramani PP, Chakravarthy VS, Ravindran B, Moustafa AA. A network model of basal ganglia for understanding the roles of dopamine and serotonin in reward-punishment-risk based decision making. Frontiers in Computational Neuroscience. 2015;9:e17282. doi: 10.3389/fncom.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. Journal of Psychiatric Practice. 2004;10:196–199. doi: 10.1097/00131746-200405000-00010. [DOI] [PubMed] [Google Scholar]
- Bauer M, Monz BU, Montejo AL, Quail D, Dantchev N, Demyttenaere K, Garcia-Cebrian A, Grassi L, Perahia DG, Reed C, Tylee A. Prescribing patterns of antidepressants in Europe: results from the Factors Influencing Depression Endpoints Research (FINDER) study. European Psychiatry. 2008;23:66–73. doi: 10.1016/j.eurpsy.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Boureau YL, Dayan P. Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology. 2011;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Müller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JPT, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. The Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Cléry-Melin ML, Schmidt L, Lafargue G, Baup N, Fossati P, Pessiglione M. Why don't you try harder? An investigation of effort production in major depression. PLoS One. 2011;6:e17282. doi: 10.1371/journal.pone.0023178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Amoroso MW, Uchida N. Serotonergic neurons signal reward and punishment on multiple timescales. eLife. 2015;4:e17282. doi: 10.7554/eLife.06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW. Tryptophan depletion disrupts the motivational guidance of goal-directed behavior as a function of trait impulsivity. Neuropsychopharmacology. 2005;30:1362–1373. doi: 10.1038/sj.npp.1300704. [DOI] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology. 2011;36:98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotel F, Exley R, Cragg SJ, Perrier JF. Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. PNAS. 2013;110:4774–4779. doi: 10.1073/pnas.1216150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behavioural Brain Research. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Robbins TW. Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. Journal of Neuroscience. 2009;29:11993–11999. doi: 10.1523/JNEUROSCI.2513-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Smillie LD, Robbins TW. The effects of acute tryptophan depletion on costly information sampling: impulsivity or aversive processing? Psychopharmacology. 2012;219:587–597. doi: 10.1007/s00213-011-2577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320:e17282. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunizeau J, Adam V, Rigoux L. VBA: a probabilistic treatment of nonlinear models for neurobiological and behavioural data. PLoS Computational Biology. 2014;10:e17282. doi: 10.1371/journal.pcbi.1003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Networks. 2002;15:603–616. doi: 10.1016/S0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Dayan P. Twenty-five lessons from computational neuromodulation. Neuron. 2012;76:240–256. doi: 10.1016/j.neuron.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Doya K. Metalearning and neuromodulation. Neural Networks. 2002;15:495–506. doi: 10.1016/S0893-6080(02)00044-8. [DOI] [PubMed] [Google Scholar]
- Dremencov E, El Mansari M, Blier P. Effects of sustained serotonin reuptake inhibition on the firing of dopamine neurons in the rat ventral tegmental area. Journal of Psychiatry & Neuroscience. 2009;34:223–229. [PMC free article] [PubMed] [Google Scholar]
- Eldar E, Niv Y. Interaction between emotional state and learning underlies mood instability. Nature Communications. 2015;6:e17282. doi: 10.1038/ncomms7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P, Deakin JF. The role of serotonin in reward, punishment and behavioural inhibition in humans: insights from studies with acute tryptophan depletion. Neuroscience & Biobehavioral Reviews. 2014;46:365–378. doi: 10.1016/j.neubiorev.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Fava M, Ball S, Nelson JC, Sparks J, Konechnik T, Classi P, Dube S, Thase ME. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depression and Anxiety. 2014;31:250–257. doi: 10.1002/da.22199. [DOI] [PubMed] [Google Scholar]
- Fischer AG, Jocham G, Ullsperger M. Dual serotonergic signals: a key to understanding paradoxical effects? Trends in Cognitive Sciences. 2015;19:21–26. doi: 10.1016/j.tics.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Fonseca MS, Murakami M, Mainen ZF. Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing. Current Biology. 2015;25:306–315. doi: 10.1016/j.cub.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiological Reviews. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Economides M, Huys QJ, Frank MJ, Chowdhury R, Duzel E, Dayan P, Dolan RJ. Differential, but not opponent, effects of L -DOPA and citalopram on action learning with reward and punishment. Psychopharmacology. 2014;231:955–966. doi: 10.1007/s00213-013-3313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Raison CL, Lowry CA. Integrative physiology of depression and antidepressant drug action: implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacology & Therapeutics. 2013;137:108–118. doi: 10.1016/j.pharmthera.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Bhagwagar Z, Perrett DI, Völlm BA, Cowen PJ, Goodwin GM. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;28:148–152. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. The British Journal of Psychiatry. 2009;195:102–108. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiological Reviews. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hayashi E, Shimamura M, Kinoshita M, Murphy NP. Neurochemical responses to antidepressants in the prefrontal cortex of mice and their efficacy in preclinical models of anxiety-like and depression-like behavior: a comparative and correlational study. Psychopharmacology. 2008;197:567–580. doi: 10.1007/s00213-008-1070-6. [DOI] [PubMed] [Google Scholar]
- Le Bouc R, Rigoux L, Schmidt L, Degos B, Welter ML, Vidailhet M, Daunizeau J, Pessiglione M. Computational dissection of dopamine motor and motivational functions in humans. Journal of Neuroscience. 2016;36 doi: 10.1523/JNEUROSCI.3078-15.2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyniel F, Safra L, Pessiglione M. How the brain decides when to work and when to rest: dissociation of implicit-reactive from explicit-predictive computational processes. PLoS Computational Biology. 2014;10:e17282. doi: 10.1371/journal.pcbi.1003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyniel F, Sergent C, Rigoux L, Daunizeau J, Pessiglione M. Neurocomputational account of how the human brain decides when to have a break. PNAS. 2013;110:2641–2646. doi: 10.1073/pnas.1211925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Tanaka KF, Yamanaka A, Takahashi A, Tabuchi S, Doya K. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Current Biology. 2014;24:2033–2040. doi: 10.1016/j.cub.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology. 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Forshall S, Bell C, Rich A, Sandford J, Nash J, Argyropoulos S. Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. European Neuropsychopharmacology. 1999;9:S81–S86. doi: 10.1016/S0924-977X(99)00030-9. [DOI] [PubMed] [Google Scholar]
- Padala PR, Padala KP, Monga V, Ramirez DA, Sullivan DH. Reversal of SSRI-associated apathy syndrome by discontinuation of therapy. Annals of Pharmacotherapy. 2012;46:e17282. doi: 10.1345/aph.1Q656. [DOI] [PubMed] [Google Scholar]
- Palminteri S, Lebreton M, Worbe Y, Grabli D, Hartmann A, Pessiglione M. Pharmacological modulation of subliminal learning in Parkinson's and Tourette's syndromes. PNAS. 2009;106:19179–19184. doi: 10.1073/pnas.0904035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M. Resolution of sleepiness and fatigue in major depressive disorder: A comparison of bupropion and the selective serotonin reuptake inhibitors. Biological Psychiatry. 2006;60:1350–1355. doi: 10.1016/j.biopsych.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Sansone LA. SSRI-Induced Indifference. Psychiatry (Edgmont) 2010;7:14–18. [PMC free article] [PubMed] [Google Scholar]
- Schilström B, Konradsson-Geuken A, Ivanov V, Gertow J, Feltmann K, Marcus MM, Jardemark K, Svensson TH. Effects of S-citalopram, citalopram, and R-citalopram on the firing patterns of dopamine neurons in the ventral tegmental area, N-methyl-D-aspartate receptor-mediated transmission in the medial prefrontal cortex and cognitive function in the rat. Synapse. 2011;65:357–367. doi: 10.1002/syn.20853. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S, Doya K. Low-serotonin levels increase delayed reward discounting in humans. Journal of Neuroscience. 2008;28:4528–4532. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Calati R, Goracci A, Di Simplicio M, Castrogiovanni P, De Ronchi D. Antidepressants in healthy subjects: what are the psychotropic/psychological effects? European Neuropsychopharmacology. 2010;20:433–453. doi: 10.1016/j.euroneuro.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009;12:269–280. [PubMed] [Google Scholar]
- Stahl SM. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. (2nd ed) Cambridge University Press; 2008. Chapter 12 - Antidepressants. [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. NeuroImage. 2009;46:1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, Schmack K, Wrase J, Ricken R, Knutson B, Adli M, Bauer M, Heinz A, Ströhle A. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. Journal of Psychopharmacology. 2012;26:677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Tang TZ, DeRubeis RJ, Hollon SD, Amsterdam J, Shelton R, Schalet B. A placebo-controlled test of the effects of paroxetine and cognitive therapy on personality risk factors in depression. Arch Gen Psychiatry. 2009;66:1322–1330. doi: 10.1001/archgenpsychiatry.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. Journal of Abnormal Psychology. 2012;121:553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, STAR*D Study Team Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. American Journal of Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. Journal of Neuroscience. 2011;31:16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Talmon S, Schulze I, Boeddinghaus C, Gross G, Schoemaker H, Wicke KM. Running wheel activity is sensitive to acute treatment with selective inhibitors for either serotonin or norepinephrine reuptake. Psychopharmacology. 2009;203:753–762. doi: 10.1007/s00213-008-1420-4. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Savulich G, Voon V, Fernandez-Egea E, Robbins TW. Serotonin depletion induces 'waiting impulsivity' on the human four-choice serial reaction time task: cross-species translational significance. Neuropsychopharmacology. 2014;39:1519–1526. doi: 10.1038/npp.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn SE, Collins SL, Contreras-Mora HM, Errante EL, Rowland MA, Correa M, Salamone JD. Not all antidepressants are created equal: differential effects of monoamine uptake inhibitors on effort-related choice behavior. Neuropsychopharmacology. 2016;41:686–694. doi: 10.1038/npp.2015.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen GS, Gunning FM, Woods E, Klimstra SA, Hoptman MJ, Alexopoulos GS. Neuroanatomical correlates of apathy in late-life depression and antidepressant treatment response. Journal of Affective Disorders. 2014;166:179–186. doi: 10.1016/j.jad.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Bernal-Pacheco O, Bowers D, Ward H, Oyama G, Limotai N, Velez-Lago F, Rodriguez RL, Malaty I, McFarland NR, Okun MS. Are selective serotonin reuptake inhibitors associated with greater apathy in Parkinson’s disease? Journal of Neuropsychiatry and Clinical Neurosciences. 2012;24:326–330. doi: 10.1176/appi.neuropsych.11090210. [DOI] [PMC free article] [PubMed] [Google Scholar]