Abstract
Post-traumatic osteoarthritis (PTOA) develops as a result of traumatic loading that causes tears of the soft tissues in the knee. A modified transection model, where the anterior cruciate ligament (ACL) and both menisci were transected, was used on skeletally mature Flemish Giant rabbits. Gross morphological assessments, elastic moduli, and glycosaminoglycan (GAG) coverage of the menisci were determined to quantify the amount of tissue damage 12 weeks post injury. This study is one of the first to monitor meniscal changes after inducing combined meniscal and ACL transections. A decrease in elastic moduli as well as a decrease in GAG coverage was seen.
Introduction
PTOA is a debilitating joint disease resulting from joint trauma. A number of injuries can lead to PTOA of the knee, but it is most commonly seen following sports injuries where ACL and meniscal injuries have occurred [1]. Because the menisci have been shown to bear 45–75% of the load in the knee joint [2], meniscal damage is thought to be associated with the development of OA and merits further investigation [3].
In order to study the chronic advancement of PTOA, numerous in vivo animal models have used a surgical ACL transection (ACLT) model to mimic damage to injured joints [4–11]. These models destabilized the knee joint surgically by transecting the ACL, and then monitor joint degradation over time. Driven by results of ACLT models, current clinical practice includes repair of the torn ACL in an effort to arrest or slow the development of OA following a traumatic event that tears the ACL. Reconstruction of the ACL is usually accomplished by the use of either autograft or allograft tissue [12]. Unfortunately, surgical reconstruction of the injured joint does not appear to prevent PTOA. Many patients who undergo ACL reconstruction exhibit early radiographic evidence of OA [13,14]. For example, regardless of acute ACL reconstruction, 24% of patients still presented radiographic evidence of OA after 11 years. Researchers have prospectively evaluated articular cartilage health using arthroscopy in 105 patients at the time of and following ACL reconstruction [15]. Their findings document progressive degeneration of articular cartilage in the repaired knee joint in all patients. The current literature thus indicates that the occurrence of post-traumatic joint OA does not seem to depend on whether or not the ACL is reconstructed. Others have reported the prevalence of OA to be 78% in both reconstructed and nonreconstructed patients after 14 years [16].
While the ACLT model has shown injuries to the ACL can lead to the classical characteristics associated with human OA, it fails to account for accompanying acute meniscal damages that are often associated with the human trauma. With meniscal damage occurring in as high as 82% of knees with acute ligament tears [17], it is important to understand how this damage affects the mechanical and morphological properties of menisci in the subsequent chronic setting. Because of the important load distribution properties of the menisci, meniscal tissue is likely not benign in the development of OA [3,18]. This is supported clinically by cartilage degeneration following meniscectomies [19–21]. Water flow through meniscal tissue is regulated in part by aggrecan, a proteoglycan with GAG side chains, which contributes to the viscoelastic nature of the tissue [22]. GAG depletion in meniscal tissue has been shown to reduce the coefficient of viscosity and reduce the equilibrium modulus of the tissue [23]. Additionally, the tissue is limited in its healing capacity due to the primary avascular nature of the meniscus. Clinically, it has been shown that when left untreated, a combined ACL and meniscal tear will become more severe over time [24–26].
Given the high incidence of OA following tears of the ACL and meniscus, additional research is still necessary to understand this pathological state. Additionally, no previous models have considered the classic case of ACL tear combined with meniscus tears. The majority of previous studies have been either isolated ACL tears [6–10,27–33] or meniscectomy models [33–37]. Thus, there is a need to develop a model that can accurately represent this combined case. To this end, a modified ACL transection (mACLT) model has been developed. In the mACLT model, the ACL is fully transected, and both the medial and lateral menisci are partially transected. To our knowledge, this is the first combined ACL and meniscal transection study that investigates chronic changes in the menisci. There have been previous studies that combine an ACL transection with a partial or total meniscectomy [4,38,39], but the focus of these studies was on articular cartilage and bone changes. Partial transections will be performed in both the medial and lateral hemijoints as literature has shown them to occur almost equally (44% medially and 56% laterally) [24] in acute ACL injuries.
The objective of this study was to investigate changes to meniscal tissue 12 weeks after joint trauma in the form of ACL and meniscal transections. This mACLT model will characterize meniscal damage that has been previously unaccounted for in the traditional ACLT model. An indentation relaxation test will be used to determine the instantaneous and equilibrium compressive elastic moduli of the menisci. Histological analysis will be performed to monitor changes in GAG coverage. It is hypothesized that both mechanical properties and GAG coverage will be decreased in the mACLT joint compared with the contralateral control 12 weeks post trauma.
Methods
Modified ACL Transection Model.
Six skeletally mature Flemish Giant rabbits (5.3 ± 0.6 kg) were used in the study. All animals were housed in individual cages (60 × 60 × 14 in.) for the duration of the study, which was approved by Michigan State University and Colorado State University All-University Committees on Animal Use and Care. Animals were placed under anesthesia and the right limb of each animal underwent an ACL transection, as well as meniscal transections to both the medial and lateral menisci. The left limb was left unaffected and served as a control. A licensed veterinary technician monitored the rabbits for pain and buprenorphine (0.3 ml/kg BW) was given every 8 h for 72 h following surgery. At 12 weeks post injury, the animals were euthanized.
Animals were anesthetized with 0.01 mg/kg glycopyrrolate subcutaneously, 2 mg/kg xylazine (20 mg/ml) intramuscularly, and 15 mg/kg ketamine hydrochloride intramuscularly as a pre-anesthetic medication. Animals were administered warmed subcutaneous fluids peri-operatively. Each animal was maintained with 1–4% isoflurane and oxygen, delivered by mask using a precision vaporizer and a bains nonrebreathing system. A full scavenger system on the bains circuit was used. The right limb of each animal was shaved and prepared using a 70% povidone-iodine scrub and 70% alcohol. With the animal in dorsal recumbency, a parapatellar 2 cm skin incision was made over the medial aspect of the knee joint. The skin incision was followed by a small (∼1 cm) medial arthrotomy. The fat pad was gently retracted cranially to expose the cranial cruciate ligament. Using a #11 scalpel blade, the cruciate ligament was transected between its origin and insertion. The medial meniscus then received a radial transection in the white zone of the central region with a longitudinal transection extending through the main body. The lateral meniscus was transected radially in the white zone of the central region and with a minor longitudinal cut extending anteriorly (Fig. 1). The initial goal with the modified model was to create the same complex posterior tears in both the medial and lateral compartment. Due to the tightness of the lateral posterior compartment it was not possible to make the longitudinal cut posteriorly as was done on the medial meniscus. In an effort to not disturb additional tissues in the joint to gain access to this lateral posterior compartment, different tears were created. It should be noted however that complex tears of the posterior compartment of the menisci are very common and seen in both the medial and lateral hemijoints [9,24,40,41]. The joint capsule was sutured immediately after transection using 3/0 polydioxanone monofilament synthetic absorbable suture (PDS). The subcuticular layer and skin were closed in sequence using 4-0 PDS and subcutaneous.
Fig. 1.
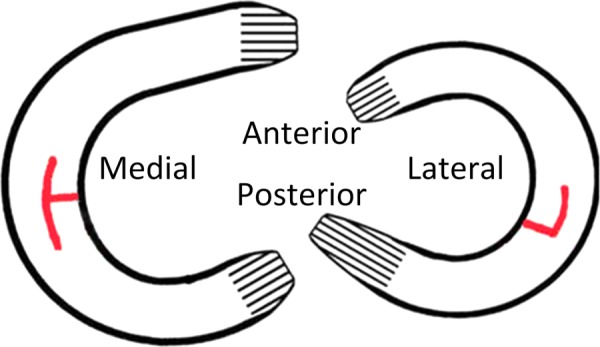
Diagram of meniscal transections
Previous ACLT models identified a range of time points where meniscal damage was observed (ranging from 1 to 32 weeks). At their earliest time of 12 weeks, a canine study by Smith et al. saw only 50% of the medial menisci was damaged and only 30% of the lateral, but at 32 weeks 91% of the medial and 57% of the lateral menisci were damaged [29]. Adams et al. looked at earlier time points of 1, 4, and 8 weeks in a canine ACLT model and documented damage occurring as early as week one and progressing to complete damage of both menisci by 8 weeks [32]. ACLT models using New Zealand White rabbits have shown a slightly quicker progression of damage with two studies showing complete damage by 8 weeks [8,33]. In a small Flemish Giant rabbit study by Killian et al., two of the three animals who received an ACL transection showed heavy damage to the medial menisci while only one of the three animals were seen to have lateral meniscal damage after 12 weeks [9]. Based on these previous works, a time point of 12 weeks was chosen to insure degradation of the joint and have a time point that was comparable with previous works.
Meniscus Harvesting and Preparation.
The menisci were harvested immediately following sacrifice and kept refrigerated (1.7–3.3 °C) until mechanically tested. The menisci were photographed and damage was quantified using a combination of previously established grading systems [31,42]. A morphological score from 0 to 4 was assigned to each region of both hemijoints with 0 = normal, 1 = surface damage, 2 = undisplaced tears, 3 = displaced tears, 4 = tissue maceration. Blind scoring was performed by four separate individuals and average numbers reported. Paired t-tests were performed to determine if significant (p < 0.05) morphological damage was present in the injured knees compared with their contralateral controls. An interclass correlation calculation was also used to identify the intergrader reliability.
Because mechanical and histological properties have been seen to be regionally dependent [22,43–45], each meniscus was sectioned into anterior, central, and posterior regions for testing. Specimens were kept hydrated with 0.9% phosphate buffered saline (PBS) solution before and during mechanical testing. Following mechanical testing, meniscal sections were fixed in 10% formalin for 14 days. Sections were then embedded in optimum cutting temperature medium (Pelco; Redding, CA) and flash frozen using liquid nitrogen. The face parallel to the cut surface of these regional sections was then cryosectioned using 6 μm slices and stained for GAG using safranin-O (Saf-O) and Fast Green (FCF).
Mechanical Evaluation.
Meniscal tissue was subjected to indentation, relaxation tests (Bionic Model 370.02 MTS Corp., Eden Praire, MN) in the anterior, central, and posterior regions, when enough intact tissue was present. The samples were tested in a PBS bath to prevent dehydration. The bath was attached to a two degree of freedom camera mount, and an x–y plate allowed for the indentation surface to be oriented normal to the indenter. A spherical tip with a diameter of 1.59 mm was used as an indenter, and loads were read using an 8.9 N load cell (Futek LSB200, Irvine, CA). Many biological tissues have been shown to behave viscoelastically and previous studies of meniscal tissue support that meniscus is as well [46]. It has been suggested that the flow of fluid through the tissue is at least partially responsible for this time dependent behavior [47]. Thus, both equilibrium and instantaneous moduli were computed.
Specimens were subjected to a preload of 20 mN before being indented 0.25 mm for 900 s. Similar to previous indentation testing on meniscal tissue of rabbits [48], a Hertzian contact equation (Eq. (1)) was applied and used to determine both the instantaneous and equilibrium moduli. The contact equation assumed contact between an elastic half space and a sphere, where F is the force, R is the radius of the indenter, d is the indentation depth, E 2 and υ 2 are the elastic modulus and Poisson's ratio of the indenter, and E 1 and υ 1 are the elastic modulus and Poisson's ratio of the tissue
| (1) |
Equation (1): Hertzian contact equation.
The elastic modulus and Poisson's ratio of the indenter tip were 210 GPa and 0.3, respectively. Based on a previous study [44], Poisson's ratio of the menisci was assumed to be 0.01 for all regions. A paired sample t-test was performed on the data and significance identified when p < 0.05. Data were evaluated using a custom matlab program. Instantaneous values were assessed at the peak force while equilibrium was assessed at 900 s.
GAG Staining.
GAG content was determined histologically using hematoxlyin, Saf-O, and FCF staining [49]. This process stains GAG red, nuclei black, and cytoplasm blue/green. Slides were imaged using an Olympus BH2 Microscope (Center Valley, PA) and MicroPublisher 5.0 RTV camera (QImaging, Surrey, BC, Canada). Staining intensity was graded similar to previous studies [42] with no Saf-O staining represented by a score of 0, slight staining = 1, moderate staining = 2, and strong staining = 3 (Fig. 2). Reported intensity grades were averaged from four separate graders.
Fig. 2.
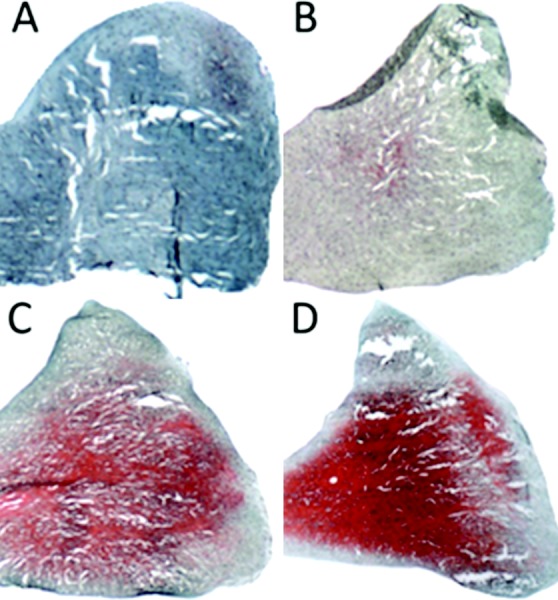
Safranin-O and Fast Green staining intensity: (a) no stain = 0, (b) slight staining = 1, (c) moderate staining = 2, and (d) strong staining = 3
Coverage was analyzed using Image J (NIH, Bethesda, MD) with FIJI package. Images were trimmed and converted to 8-bit images. Total area of the image was calculated using the Analyze Particles tool and summing particles. The area stained red, or the area associated with GAG coverage, was separated from the image using the Colour Deconvolution tool. Once separated, the area of GAG coverage was calculated by thresholding and analyzing particles (Fig. 3). Paired t-tests were performed on the control and transected limbs with significance determined when p < 0.05.
Fig. 3.
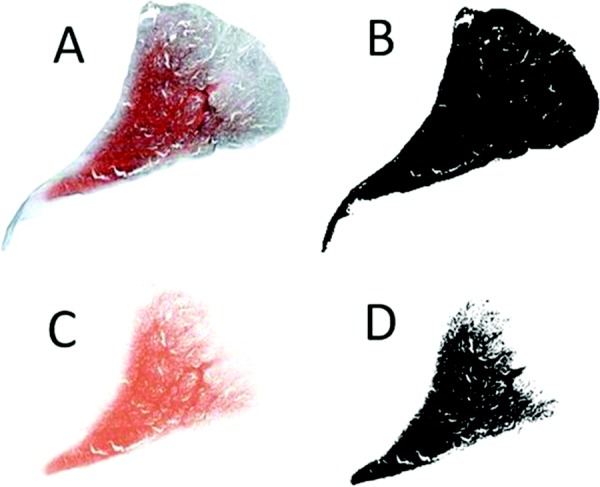
Representative histological images showing the image analysis for GAG coverage: (a) stitched and trimmed image was imported to scale, (b) analyze particles of whole menisci was used to determine overall area of meniscus, (c) colour deconvolution tool was used to remove area stained for GAG, and (d) analyze particles was used on the colour deconvoluted image to determine area of GAG coverage. Values from image (b) and (d) were used to determine % GAG coverage ((b − d)/b).
Results
Morphology.
Rabbits favored the contralateral limb for the first 1–3 days, but showed no signs of gait abnormality for the duration of the study. At time of euthanasia, all mACLT joints demonstrated osteoarthritic changes characterized by cartilage fibrillation, erosion, subchondral bone exposure, and osteophyte formation. Inflammation of the synovium and increased synovial fluid was present in all mACLT joints (Fig. 4 for mACLT and Fig. 5 for control images). Morphological scoring was highest in the lateral posterior region followed by the lateral central and medial central regions (Table 1). A paired t-test identified a significant increase in damage seen in the injured joints as compared with the contralateral control in all regions. An interclass correlation calculation was conducted to determine the reliability of scoring between graders. This correlation was found to be high (0.837) indicating good agreement between graders.
Fig. 4.
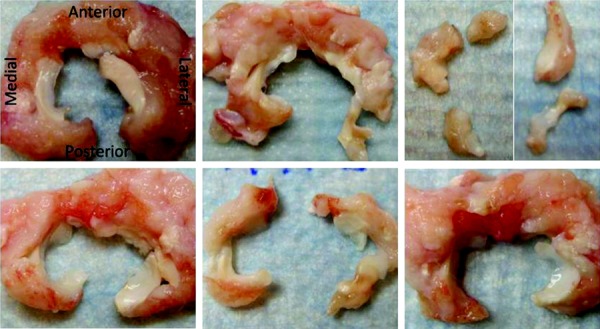
Menisci 12 week's postsurgery (animals 1–6 left to right and top to bottom, all specimens are oriented identical to the first image)
Fig. 5.
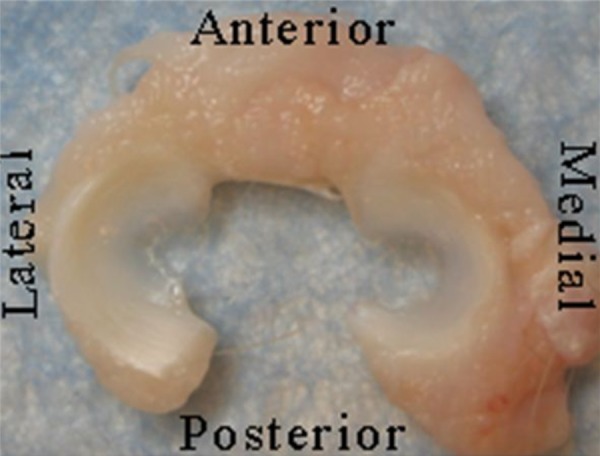
Control menisci
Table 1.
Meniscal morphological scores for injured joint (all contralateral regions were normal); * denotes significant differences between control and mACLT
|
Medial |
Lateral |
|||||
|---|---|---|---|---|---|---|
| Animal | A* | C* | P* | A* | C* | P* |
| 1 | 1 | 0 | 2 | 2 | 3 | 4 |
| 2 | 1 | 2 | 1 | 1 | 4 | 4 |
| 3 | 4 | 4 | 4 | 1 | 4 | 4 |
| 4 | 2 | 1 | 1 | 1 | 3 | 0 |
| 5 | 0 | 4 | 4 | 0 | 4 | 4 |
| 6 | 2 | 4 | 1 | 4 | 2 | 0 |
Mechanics.
Due to the state of tissue damage (Table 1), various regions of the menisci were unable to be mechanically tested, leading to varying sample sizes. The lateral meniscus had a sample size of n = 5 in the anterior region, with the more damaged central and posterior regions having sample sizes of n = 1 and n = 2, respectively. The medial meniscus had enough testable tissue for n = 5 in both the anterior and posterior regions, while the central region had n = 2. All regions of the contralateral (control) limb had a sample size of n = 6. Because mechanical data were lacking for specific regions, data from the anterior, central, and posterior regions were averaged for each animal and evaluations were made at the hemijoint level to allow for statistical analysis (Figs. 6(a) and 6(b)).
Fig. 6.
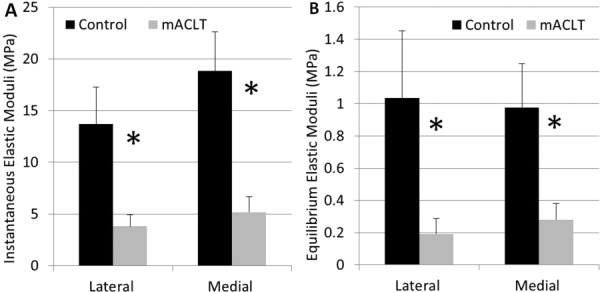
(a) Instantaneous elastic moduli by hemijoint (mean with standard error) and (b) equilibrium elastic moduli by hemijoint (mean with standard error); *denotes significant difference between control and mACLT
Mechanical results showed significant decreases (p < 0.05) in both the instantaneous and equilibrium elastic moduli between the control and transected limbs in both the medial and lateral hemijoints. Instantaneous elastic moduli decreased 72.1 ± 11.1% from control to transected limb in the lateral hemijoint and 72.5 ± 17% in the medial hemijoint. The equilibrium elastic moduli decreased 81.4±12.4% in the lateral hemijoint and 71.2 ± 35.8% in the medial hemijoint.
GAG.
Enough tissue was present in all regions for histological analysis allowing for n = 6 in all regions. Saf-O staining intensity was evaluated at the regional level and tended to be less intense in the injured limb compared with the control limb, indicating a decrease in GAG concentration (Fig. 7). Significant differences (p < 0.05) between the control and mACLT group for GAG concentration were found in the lateral anterior, lateral central, lateral posterior, and medial anterior regions.
Fig. 7.
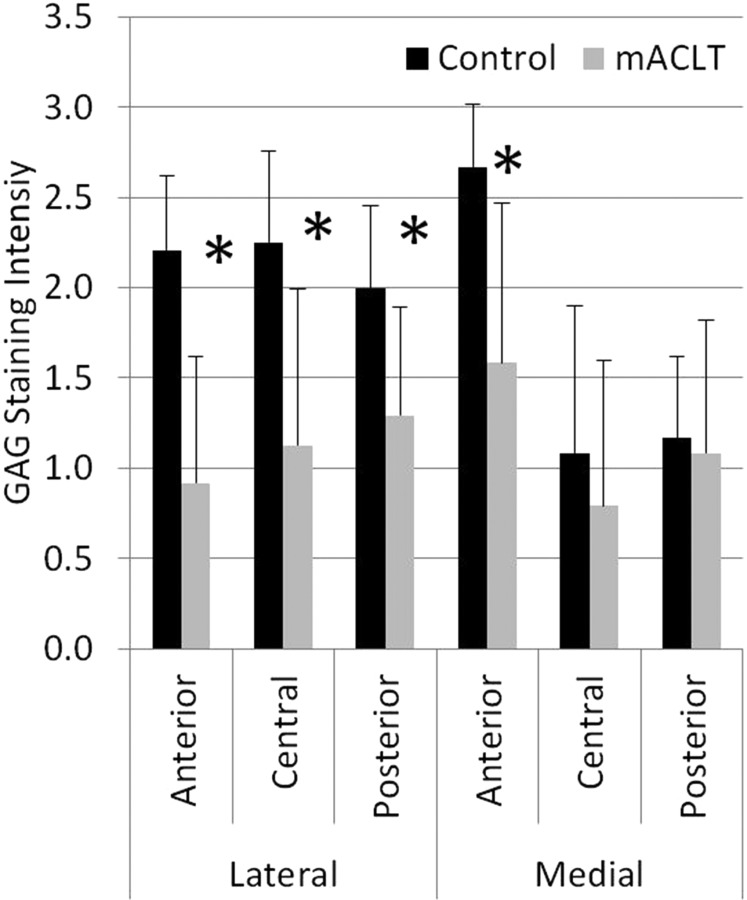
GAG intensity grading (mean with standard error); *denotes significant difference between control and mACLT
GAG coverage decreased from the control to transected limbs (Fig. 8). Percent GAG coverage was reduced in all regions in the mACLT model, and was significantly lower in all three lateral regions (p < 0.01) and in the medial anterior region (p < 0.05). When averaging regions, the lateral control menisci showed 30 ± 5.2% GAG coverage compared with only 10.1 ± 4% GAG coverage in the mACLT menisci. Similarly, in the medial menisci, GAG coverage was 20.2 ± 7.9% compared with only 10 ± 5.1% GAG coverage in the mACLT menisci.
Fig. 8.
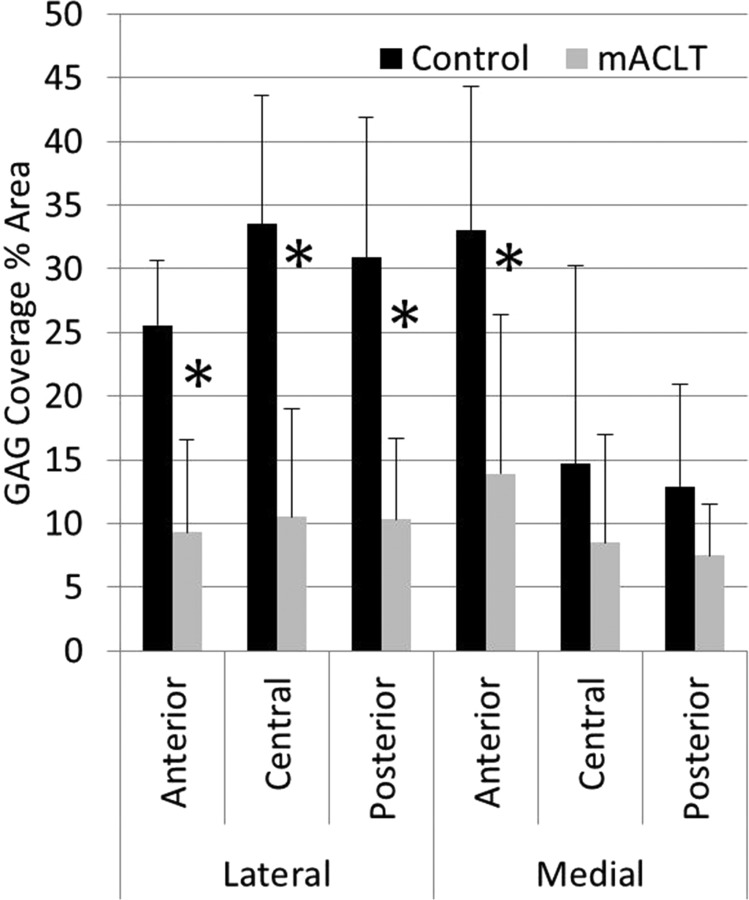
Percent area of GAG coverage by region and hemijoint (mean with standard error); *denotes significant difference between control and mACLT
Discussion
The findings of this study showed that ACL injury combined with meniscal damage result in a significant decrease in meniscal elastic modulus and significant decrease in GAG coverage over time. In general, surgical transection of the meniscus introduced undisplaced tears that eventually became displaced or lead to tissue degradation and loss. Morphological assessment done by visual inspection has been an effective and widely used method for assessing gross damage to osteoarthritic joints [6–9,27,28,30,31,42]. Damage occurred most often in the medial and lateral central regions and the lateral posterior region. This localization of damage was expected as these were the regions where initial damage was induced acutely, and chronic damage has been seen clinically [26,50,51].
Tissue maceration limited sample sizes in the central and posterior regions of the lateral meniscus and the central region of the medial meniscus. However, the remaining regions (medial anterior, medial posterior, and lateral anterior) showed significant decrease (p < 0.05) in both the instantaneous and equilibrium moduli between the control and mACLT menisci. Despite the inability to run statistical analysis on the lateral central, lateral posterior, and medial posterior, these regions were trending a decrease between control and transected limb for both the instantaneous elastic moduli and the equilibrium elastic moduli. It is hypothesized that this decrease would have been significant if a larger sample size was used.
A comparison of the mechanical property changes with other literature is challenging. The majority of previous PTOA models have used the traditional ACLT model and often times focus on cartilage and bone changes, without documenting changes in the material properties of the menisci. One previous study [44] did evaluate equilibrium moduli in the medial meniscus of healthy rabbits. A direct comparison of this data and our control data is difficult as sectioning and testing methods were different. However, the trending pattern of a higher modulus in the anterior region of the medial meniscus seen in the current study was consistent with this earlier study. We suspect that the dramatic changes in material properties that develop over 12 weeks would affect the ability of the meniscus to effectively distribute loads, whereby increasing stress on the underlying articular cartilage. The combined effect of a change in kinematics due to a torn ACL and meniscus, along with the change in meniscal material properties exacerbates development of OA.
Histological analyses indicated GAG coverage decreased 12 weeks after joint injury. Staining intensity tended to decrease from control to transected limb as well, with significant decrease in the lateral anterior, lateral central, lateral posterior, and medial anterior regions. All regions with a significant decrease in staining intensity also showed a significant decrease in GAG coverage. Data from the control limb are in agreement with a previous study of healthy lapine menisci [45] as well as to GAG coverage in human studies [52]. GAG coverage was found to be relatively uniform in the lateral hemijoint, while the medial anterior region had significantly more GAG than the medial central and posterior regions. This variation in GAG coverage may be responsible for differences in mechanical properties seen in the medial meniscus in the current and previous lapine studies [44].
There are conflicting reports as to whether or not GAG presence decreases in osteoarthritic knees. Some studies have shown an increase in GAG in osteoarthritic human knees [42,53]. However, a canine study using an ACLT model showed an initial decrease in GAG (1 week) with levels returning to normal or above normal over an extended period of time (3–18 months) [32]. Based on this, it is hypothesized that GAG likely decreases due to initial trauma, but if altered loading persists GAG production may rebound and increase in an attempt to compensate for damaged tissue.
It is very difficult to determine if this was “end-stage” OA. The other tissues in the joint (i.e., cartilage, bone) have not been analyzed to determine the integrity of these tissues. Only then can we label this as end-stage. It is interesting to note that in the healthy control limbs, the lateral menisci and the medial anterior meniscus tended to have elevated GAG staining and coverage; whereas the 12 weeks transected limbs had a much more uniform staining and coverage between the menisci. There is likely a change in load distribution in the transected limbs with the ACL and meniscal tearing, however this was not quantified. It is expected that the change in GAG coverage and intensity is a consequence of a change in loading. Developing a longitudinal study to look at time points before and after 12 weeks would help to better understand these post trauma changes in GAG content of the meniscal tissue.
Both elastic modulus and GAG coverage significantly decreased from the control to transected menisci at the hemijoint level. Looking at overall decrease from mean values, the lateral hemijoint experienced a larger decrease compared with the medial hemijoint. Unfortunately, due to tissue damage, regional comparisons between GAG and elastic moduli were not possible in the current study. It is believed that in articular cartilage, the equilibrium modulus is linearly related or closely associated with GAG content [54,55]. We however did not find a correlation between GAG content and the equilibrium elastic modulus in either the control or injured menisci (data not shown).
Despite a sample size of six animals, large statistical differences were detected for morphological as well as glycosaminoglycan staining and coverage. In an effort to isolate specific regional differences in material properties, an attempt was made to study anterior, central, and posterior regions of the meniscus for mechanical testing. Given the mechanical test procedure chosen for the study, it was not always possible to test all three regions for each meniscus in the mACLT limb. Tissue was however available from all six animals for mechanical testing, albeit not from all three regions of all animals. Thus, statistical analysis was only completed for the hemijoint averaged data. Given the extremely limited data available in the literature for material properties of meniscus, the regional data were presented for the control limb, as well as for the mACLT limb when available.
There were some limitations in the current study, including the determination of Saf-O coverage and intensity. While GAG intensity was obtained from qualitative analysis as done in past studies [42,56], an image analysis software was in fact utilized for quantifying GAG coverage changes. The possibility also exists that animal variability caused differences in transection location and size as the surgeries were performed by eyesight. However, this may have been limited by the use of a single surgeon (CD) for the surgeries. This study isolated a single tearing pattern, but it is recognized that tear type likely has an effect on degradation. A much more extensive study would be necessary to identify the effect of various tear types on the rate and magnitude of degradation. Finally, the effect of the isolated meniscal transections, without damaging the ACL, is yet unknown. For this reason, future studies looking at just the meniscal transections may prove helpful in this regard.
When compared with traditional transection models, the mACLT model showed a number of notable differences. Numerous studies of articular cartilage in ALCT lapine studies have shown more extensive damage in the medial hemijoint [6,7,10]. Similarly, ACLT models that have reported gross meniscal damage [6,8,29,32] have also shown damage to be more extensive in the medial meniscus. The theory behind the more highly worn medial compartment is that without the stability offered by an intact ACL, the medial meniscus acts to prevent tibial translation [57–60]. This in turn causes more wear in the medial as opposed to lateral hemijoint. With the mACLT model, chronic damage did not favor the medial compartment. In fact, the lateral compartment showed more gross damage, a greater decrease in equilibrium elastic moduli, and a greater decrease in GAG coverage. Most importantly, based on tear type and occurrence, the mACLT model also showed more extensive meniscal damage to both hemijoints than previously reported in studies using the ACLT model. For example, a 12-week ACLT study by Smith et al. resulted in medial severe tearing in 20% of the samples, mild tearing in 30%, and normal menisci in 50% of the samples [29]. Using the same metrics, our mACLT model demonstrated severe tearing in 67% of the medial samples and mild tearing in 33% of our samples. This difference between the two models is even more evident in the lateral hemijoint. Smith et al. reported only 10% of their samples having a tear in the lateral meniscus meanwhile 100% of the mACLT lateral menisci showed severe tearing. While this comparison is slightly biased since damage was introduced initially in the mACLT model, the current study suggested that the combined ACL and meniscal trauma in the mACLT model resulted in more severe degradation of meniscal tissue.
Elucidating the roles of other structures in the knee during the development of post-traumatic OA using this novel animal model will likely revolutionize modern clinical treatment of ACL/meniscal injury and provide a catapult for future longitudinal studies aimed at slowing or preventing OA. The data presented certainly show that meniscal tears, in combination with an ACL tear are certainly not benign and should be treated. What was previously unknown is how this altered loading environment affects the remaining meniscal tissue. Clearly, in just 12 weeks, the remaining meniscal tissue has a dramatic change in both material properties as well as biochemistry. This likely implies that following such a traumatic injury, not only should the ACL be repaired, and the damaged meniscus repaired when possible, an intervention will likely be necessary to arrest changes in the remaining meniscus that could exacerbate the development of OA. Future studies are certainly necessary with this model to understand how changes are affected by early reconstruction of the ACL and isolated or different meniscal damage.
Current studies are underway to document changes in articular cartilage, synovial fluid, and subchondral bone associated with this mACLT model. Combining all these data will allow correlations to be made between damage to the menisci, articular cartilage, and bone. Additionally, more time points will help to develop a longitudinal study that will document the changes in mechanical and histological properties of the menisci over time in order to more fully correlate property changes with the degree of meniscal tearing.
Acknowledgment
This work was supported by a grant from the National Institutes of Health (R21 AR060464)
Contributor Information
Kristine M. Fischenich, Department of Mechanical Engineering, , Colorado State University, , Campus Delivery 1374, , Fort Collins, CO 80523 , e-mail: kfischenich@gmail.com
Garrett A. Coatney, Department of Mechanical Engineering, , Colorado State University, , Campus Delivery 1374, , Fort Collins, CO 80523 , e-mail: gcoatney@rams.colostate.edu
John H. Haverkamp, Department of Mechanical Engineering, , Colorado State University, , Campus Delivery 1374, , Fort Collins, CO 80523 , e-mail: jhhaverk@rams.colostate.edu
Keith D. Button, Orthopaedic Biomechanics Laboratories, , College of Osteopathic Medicine, , Michigan State University, , East Fee Hall 965 Fee Road, Room A-439, , East Lansing, MI 48824 , e-mail: keithdbutton@gmail.com
Charlie DeCamp, Small Animal Clinical Sciences, , College of Veterinary, , Michigan State University, , 784 Wilson Road, Room G-100, , East Lansing, MI 48824 , e-mail: decampc@cvm.msu.edu.
Roger C. Haut, Orthopaedic Biomechanics Laboratories, , College of Osteopathic Medicine, , Michigan State University, , East Fee Hall 965 Fee Road, Room A-439, , East Lansing, MI 48824 , e-mail: haut@msu.edu
Tammy L. Haut Donahue, Department of Mechanical Engineering, , Colorado State University, , Campus Delivery 1374, , Fort Collins, CO 80523 , e-mail: tammy.donahue@colostate.edu.
References
- [1]. Felson, D. T. , 2004, “An Update on the Pathogenesis and Epidemiology of Osteoarthritis,” Radiol. Clin. North Am., 42(1), pp. 1–9. 10.1016/S0033-8389(03)00161-1 [DOI] [PubMed] [Google Scholar]
- [2]. Shrive, N. G. , O'Connor, J. J. , and Goodfellow, J. W. , 1978, “Load-Bearing in the Knee Joint,” Clin. Orthop. Relat. Res., 131, pp. 279–287. 10.1097/00003086-197803000-00046 [DOI] [PubMed] [Google Scholar]
- [3]. McGonagle, D. , Tan, A. L. , Carey, J. , and Benjamin, M. , 2010, “The Anatomical Basis for a Novel Classification of Osteoarthritis and Allied Disorders,” J. Anat., 216(3), pp. 279–291. 10.1111/j.1469-7580.2009.01186.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Intema, F. , Hazewinkel, H. A. W. , Gouwens, D. , Bijlsma, J. W. J. , Weinans, H. , Lafeber, F. P. J. G. , and Mastbergen, S. C. , 2010, “In Early OA, Thinning of the Subchondral Plate is Directly Related to Cartilage Damage: Results From a Canine ACLT-Meniscectomy Model,” Osteoarthritis Cartilage, 18(5), pp. 691–698. 10.1016/j.joca.2010.01.004 [DOI] [PubMed] [Google Scholar]
- [5]. Sniekers, Y. H. , Intema, F. , Lafeber, F. P. J. G. , van Osch, G. J. V. M. , van Leeuwen, J. P. T. M. , Weinans, H. , and Mastbergen, S. C. , 2008, “A Role for Subchondral Bone Changes in the Process of Osteoarthritis; A Micro-CT Study of Two Canine Models,” BMC Musculoskeletal Disord., 9, pp. 20–30. 10.1186/1471-2474-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Yoshioka, M. , Coutts, R. D. , Amiel, D. , and Hacker, S. A. , 1996, “Characterization of a Model of Osteoarthritis in the Rabbit Knee,” Osteoarthritis Cartilage, 4(2), pp. 87–98. 10.1016/S1063-4584(05)80318-8 [DOI] [PubMed] [Google Scholar]
- [7]. Chang, D. G. , Iverson, E. P. , Schinagl, R. M. , Sonoda, M. , Amiel, D. , Coutts, R. D. , and Sah, R. L. , 1997, “Quantitation and Localization of Cartilage Degeneration Following the Induction of Osteoarthritis in the Rabbit Knee,” Osteoarthritis Cartilage, 5(5), pp. 357–372. 10.1016/S1063-4584(97)80039-8 [DOI] [PubMed] [Google Scholar]
- [8]. Hellio Le Graverand, M. P. , Vignon, E. , Otterness, I. G. , and Hart, D. A. , 2001, “Early Changes in Lapine Menisci During Osteoarthritis Development: Part I: Cellular and Matrix Alterations,” Osteoarthritis Cartilage, 9(1), pp. 56–64. 10.1053/joca.2000.0350 [DOI] [PubMed] [Google Scholar]
- [9]. Killian, M. L. , Isaac, D. I. , Haut, R. C. , Déjardin, L. M. , Leetun, D. , and Donahue, T. L. H. , 2010, “Traumatic Anterior Cruciate Ligament Tear and Its Implications on Meniscal Degradation: A Preliminary Novel Lapine Osteoarthritis Model,” J. Surg. Res., 164(2), pp. 234–241. 10.1016/j.jss.2009.03.006 [DOI] [PubMed] [Google Scholar]
- [10]. Batiste, D. L. , Kirkley, A. , Laverty, S. , Thain, L. M. F. , Spouge, A. R. , and Holdsworth, D. W. , 2004, “Ex Vivo Characterization of Articular Cartilage and Bone Lesions in a Rabbit ACL Transection Model of Osteoarthritis Using MRI and Micro-CT,” Osteoarthritis Cartilage, 12(12), pp. 986–996. 10.1016/j.joca.2004.08.010 [DOI] [PubMed] [Google Scholar]
- [11]. McDevitt, C. A. , and Muir, H. , 1976, “Biochemical Changes in the Cartilage of the Knee in Experimental and Natural Osteoarthritis in the Dog,” J. Bone Joint Surg. Br., 58(1), pp. 94–101. [DOI] [PubMed] [Google Scholar]
- [12]. Mariscalco, M. W. , Magnussen, R. A. , Mehta, D. , Hewett, T. E. , Flanigan, D. C. , and Kaeding, C. C. , 2013, “Autograft Versus Nonirradiated Allograft Tissue for Anterior Cruciate Ligament Reconstruction: A Systematic Review,” Am. J. Sports Med., 42, pp. 492–499. 10.1177/0363546513497566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Kessler, M. A. , Behrend, H. , Henz, S. , Stutz, G. , Rukavina, A. , and Kuster, M. S. , 2008, “Function, Osteoarthritis and Activity After ACL-Rupture: 11 Years Follow-Up Results of Conservative Versus Reconstructive Treatment,” Knee Surg. Sports Traumatol., Arthroscopy, 16(5), pp. 442–448. 10.1007/s00167-008-0498-x [DOI] [PubMed] [Google Scholar]
- [14]. Oiestad, B. E. , Holm, I. , Engebretsen, L. , and Risberg, M. A. , 2011, “The Association Between Radiographic Knee Osteoarthritis and Knee Symptoms, Function and Quality of Life 10–15 Years After Anterior Cruciate Ligament Reconstruction,” Br. J. Sports Med., 45(7), pp. 583–588. 10.1136/bjsm.2010.073130 [DOI] [PubMed] [Google Scholar]
- [15]. Asano, H. , Muneta, T. , Ikeda, H. , Yagishita, K. , Kurihara, Y. , and Sekiya, I. , 2004, “Arthroscopic Evaluation of the Articular Cartilage After Anterior Cruciate Ligament Reconstruction: A Short-Term Prospective Study of 105 Patients,” Arthroscopy, 20(5), pp. 474–481. 10.1016/j.arthro.2004.03.006 [DOI] [PubMed] [Google Scholar]
- [16]. von Porat, A. , Roos, E. M. , and Roos, H. , 2004, “High Prevalence of Osteoarthritis 14 Years After an Anterior Cruciate Ligament Tear in Male Soccer Players: A Study of Radiographic and Patient Relevant Outcomes,” Ann. Rheum. Dis., 63(3), pp. 269–273. 10.1136/ard.2003.008136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. McDaniel, W. J. , and Dameron, T. B. , 1980, “Untreated Ruptures of the Anterior Cruciate Ligament. A Follow-Up Study,” J. Bone Joint Surg. Am., 62(5), pp. 696–705. [PubMed] [Google Scholar]
- [18]. Fairbank, T. J. , 1948, “Knee Joint Changes After Meniscectomy,” J. Bone Joint Surg. Br., 30B(4), pp. 664–670. [PubMed] [Google Scholar]
- [19]. Jackson, J. P. , 1968, “Degenerative Changes in the Knee After Meniscectomy,” Br. Med. J., 2(5604), pp. 525–527. 10.1136/bmj.2.5604.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Tapper, E. M. , and Hoover, N. W. , 1969, “Late Results After Meniscectomy,” J. Bone Joint Surg. Am., 51(3), pp. 517–526. [PubMed] [Google Scholar]
- [21]. Dandy, D. J. , and Jackson, R. W. , 1975, “The Diagnosis of Problems After Meniscectomy,” J. Bone Joint Surg. Br., 57(3), pp. 349–352. [PubMed] [Google Scholar]
- [22]. Fithian, D. C. , Kelly, M. A. , and Mow, V. C. , 1990, “Material Properties and Structure-Function Relationships in the Menisci,” Clin. Orthop. Relat. Res., 252, pp. 19–31. 10.1097/00003086-199003000-00004 [DOI] [PubMed] [Google Scholar]
- [23]. Sanchez-Adams, J. , Willard, V . P. , and Athanasiou, K. A. , 2011, “Regional Variation in the Mechanical Role of Knee Meniscus Glycosaminoglycans,” J. Appl. Physiol., 111(6), pp. 1590–1596. 10.1152/japplphysiol.00848.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Bellabarba, C. , Bush-Joseph, C. A. , and Bach, B. R. , 1997, “Patterns of Meniscal Injury in the Anterior Cruciate-Deficient Knee: A Review of the Literature,” Am. J. Orthop., 26(1), pp. 18–23. [PubMed] [Google Scholar]
- [25]. Wickiewicz, T. L. , 1990, “Meniscal Injuries in the Cruciate-Deficient Knee,” Clin. Sports Med., 9(3), pp. 681–694. [PubMed] [Google Scholar]
- [26]. Smith, J. P. , and Barrett, G. R. , 2001, “Medial and Lateral Meniscal Tear Patterns in Anterior Cruciate Ligament-Deficient Knees. A Prospective Analysis of 575 Tears,” Am. J. Sports Med., 29(4), pp. 415–419. [DOI] [PubMed] [Google Scholar]
- [27]. McDevitt, C. , Gilbertson, E. , and Muir, H. , 1977, “An Experimental Model of Osteoarthritis; Early Morphological and Biochemical Changes,” J. Bone Joint Surg. Br., 59(1), pp. 24–35. [DOI] [PubMed] [Google Scholar]
- [28]. Manicourt, D. H. , and Pita, J. C. , 1988, “Progressive Depletion of Hyaluronic Acid in Early Experimental Osteoarthritis in Dogs,” Arthritis Rheum., 31(4), pp. 538–544. 10.1002/art.1780310411 [DOI] [PubMed] [Google Scholar]
- [29]. Smith, G. N. , Mickler, E. A. , Albrecht, M. E. , Myers, S. L. , and Brandt, K. D. , 2002, “Severity of Medial Meniscus Damage in the Canine Knee After Anterior Cruciate Ligament Transection,” Osteoarthritis Cartilage, 10(4), pp. 321–326. 10.1053/joca.2002.0520 [DOI] [PubMed] [Google Scholar]
- [30]. Sandy, J. D. , Adams, M. E. , Billingham, M. E. , Plaas, A. , and Muir, H. , 1984, “in vivo and in vitro Stimulation of Chondrocyte Biosynthetic Activity in Early Experimental Osteoarthritis,” Arthritis Rheum., 27(4), pp. 388–397. 10.1002/art.1780270405 [DOI] [PubMed] [Google Scholar]
- [31]. Matyas, J. R. , Atley, L. , Ionescu, M. , Eyre, D. R. , and Poole, A. R. , 2004, “Analysis of Cartilage Biomarkers in the Early Phases of Canine Experimental Osteoarthritis,” Arthritis Rheum., 50(2), pp. 543–552. 10.1002/art.20027 [DOI] [PubMed] [Google Scholar]
- [32]. Adams, M. E. , Billingham, M. E. , and Muir, H. , 1983, “The Glycosaminoglycans in Menisci in Experimental and Natural Osteoarthritis,” Arthritis Rheum., 26(1), pp. 69–76. 10.1002/art.1780260111 [DOI] [PubMed] [Google Scholar]
- [33]. Wachsmuth, L. , Keiffer, R. , Juretschke, H.-P. , Raiss, R. X. , Kimmig, N. , and Lindhorst, E. , 2003, “In vivo Contrast-Enhanced Micro MR-Imaging of Experimental Osteoarthritis in the Rabbit Knee Joint at 7.1T1,” Osteoarthritis Cartilage, 11(12), pp. 891–902. 10.1016/j.joca.2003.08.008 [DOI] [PubMed] [Google Scholar]
- [34]. Beveridge, J. E. , Heard, B. J. , Shrive, N. G. , and Frank, C. B. , 2013, “Tibiofemoral Centroid Velocity Correlates More Consistently With Cartilage Damage Than Does Contact Path Length in Two Ovine Models of Stifle Injury,” J. Orthop. Res., 31, 1745–1756. 10.1002/jor.22429 [DOI] [PubMed] [Google Scholar]
- [35]. Lindhorst, E. , Wachsmuth, L. , Kimmig, N. , Raiss, R. , Aigner, T. , Atley, L. , and Eyre, D. , 2005, “Increase in Degraded Collagen Type II in Synovial Fluid Early in the Rabbit Meniscectomy Model of Osteoarthritis,” Osteoarthritis Cartilage, 13(2), pp. 139–145. 10.1016/j.joca.2004.10.017 [DOI] [PubMed] [Google Scholar]
- [36]. LeRoux, M. A. , Arokoski, J. , Vail, T. P. , Guilak, F. , Hyttinen, M. M. , Kiviranta, I . , and Setton, L. A. , 2000, “Simultaneous Changes in the Mechanical Properties, Quantitative Collagen Organization, and Proteoglycan Concentration of Articular Cartilage Following Canine Meniscectomy,” J. Orthop. Res., 18(3), pp. 383–392. 10.1002/jor.1100180309 [DOI] [PubMed] [Google Scholar]
- [37]. Messner, K. , Fahlgren, A. , Persliden, J. , and Andersson, B. M. , 2001, “Radiographic Joint Space Narrowing and Histologic Changes in a Rabbit Meniscectomy Model of Early Knee Osteoarthrosis,” Am. J. Sports Med., 29(2), pp. 151–160. [DOI] [PubMed] [Google Scholar]
- [38]. Hayami, T. , Pickarski, M. , Zhuo, Y. , Wesolowski, G. A. , Rodan, G. A. , and Duong, L. T. , 2006, “Characterization of Articular Cartilage and Subchondral Bone Changes in the Rat Anterior Cruciate Ligament Transection and Meniscectomized Models of Osteoarthritis,” Bone, 38(2), pp. 234–243. 10.1016/j.bone.2005.08.007 [DOI] [PubMed] [Google Scholar]
- [39]. Pickarski, M. , Hayami, T. , Zhuo, Y. , and Duong, L. T. , 2011, “Molecular Changes in Articular Cartilage and Subchondral Bone in the Rat Anterior Cruciate Ligament Transection and Meniscectomized Models of Osteoarthritis,” BMC Musculoskeletal Disord., 12(1), p. 197. 10.1186/1471-2474-12-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. De Smet, A. A. , and Graf, B. K. , 1994, “Meniscal Tears Missed on MR Imaging: Relationship to Meniscal Tear Patterns and Anterior Cruciate Ligament Tears,” Am. J. Roentgenol., 162(4), pp. 905–911. 10.2214/ajr.162.4.8141016 [DOI] [PubMed] [Google Scholar]
- [41]. Cerabona, F. , Sherman, M. F. , Bonamo, J. R. , and Sklar, J. , 1988, “Patterns of Meniscal Injury With Acute Anterior Cruciate Ligament Tears,” Am. J. Sports Med., 16(6), pp. 603–609. 10.1177/036354658801600609 [DOI] [PubMed] [Google Scholar]
- [42]. Pauli, C. , Grogan, S. P. , Patil, S. , Otsuki, S. , Hasegawa, A. , Koziol, J. , Lotz, M. K. , and D'Lima, D. D. , 2011, “Macroscopic and Histopathologic Analysis of Human Knee Menisci in Aging and Osteoarthritis,” Osteoarthritis Cartilage, 19(9), pp. 1132–1141. 10.1016/j.joca.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Chia, H. N. , and Hull, M. L. , 2008, “Compressive Moduli of the Human Medial Meniscus in the Axial and Radial Directions at Equilibrium and at a Physiological Strain Rate,” J. Orthop. Res., 26(7), pp. 951–956. 10.1002/jor.20573 [DOI] [PubMed] [Google Scholar]
- [44]. Sweigart, M. A. , Zhu, C. F. , Burt, D. M. , DeHoll, P. D. , Agrawal, C. M. , Clanton, T. O. , and Athanasiou, K. A. , 2004, “Intraspecies and Interspecies Comparison of the Compressive Properties of the Medial Meniscus,” Ann. Biomed. Eng., 32(11), pp. 1569–1579. 10.1114/B:ABME.0000049040.70767.5c [DOI] [PubMed] [Google Scholar]
- [45]. Killian, M. L. , Lepinski, N. M. , Haut, R. C. , and Haut Donahue, T. L. , 2010, “Regional and Zonal Histo-Morphological Characteristics of the Lapine Menisci,” Anat. Rec., 293(12), pp. 1991–2000. 10.1002/ar.21296 [DOI] [PubMed] [Google Scholar]
- [46]. Zhu, W. , Chern, K. Y. , and Mow, V. C. , 1994, “Anisotropic Viscoelastic Shear Properties of Bovine Meniscus,” Clin. Orthop. Relat. Res., 306, pp. 34–45. [PubMed] [Google Scholar]
- [47]. Mow, V. C. , Arnoczky, S. P. , and Jackson, D. W. , 1992, Knee Meniscus: Basic and Clinical Foundations, Raven Press, New York. [Google Scholar]
- [48]. Li, G. , Moses, J. M. , Papannagari, R. , Pathare, N. P. , DeFrate, L. E. , and Gill, T. J. , 2006, “Anterior Cruciate Ligament Deficiency Alters the in vivo Motion of the Tibiofemoral Cartilage Contact Points in Both the Anteroposterior and Mediolateral Directions,” J. Bone Joint Surg. Am., 88(8), pp. 1826–1834. 10.2106/JBJS.E.00539 [DOI] [PubMed] [Google Scholar]
- [49]. Rosenberg, L. , 1971, “Chemical Basis for the Histological Use of Safranin O in the Study of Articular Cartilage,” J. Bone Joint Surg. Am., 53(1), pp. 69–82. [PubMed] [Google Scholar]
- [50]. Chan, W. P. , Lang, P. , Stevens, M. P. , Sack, K. , Majumdar, S. , Stoller, D. W. , Basch, C. , and Genant, H. K. , 1991, “Osteoarthritis of the Knee: Comparison of Radiography, CT, and MR Imaging to Assess Extent and Severity,” Am. J. Roentgenol., 157(4), pp. 799–806. 10.2214/ajr.157.4.1892040 [DOI] [PubMed] [Google Scholar]
- [51]. Greis, P. E. , Bardana, D. D. , Holmstrom, M. C. , and Burks, R. T. , 2002, “Meniscal Injury: I. Basic Science and Evaluation,” J. Am. Acad. Orthop. Surg., 10(3), pp. 168–176. [DOI] [PubMed] [Google Scholar]
- [52]. Bursac, P. , Arnoczky, S. , and York, A. , 2009, “Dynamic Compressive Behavior of Human Meniscus Correlates With Its Extra-Cellular Matrix Composition,” Biorheology, 46(3), pp. 227–237. [DOI] [PubMed] [Google Scholar]
- [53]. Sun, Y. , Mauerhan, D. R. , Kneisl, J. S. , James Norton, H. , Zinchenko, N. , Ingram, J. , Hanley, E. N. , and Gruber, H. E. , 2012, “Histological Examination of Collagen and Proteoglycan Changes in Osteoarthritic Menisci,” Open Rheum. J., 6(6), pp. 24–32. 10.2174/1874312901206010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Treppo, S. , Koepp, H. , Quan, E. C. , Cole, A. A. , Kuettner, K. E. , and Grodzinsky, A. J. , 2000, “Comparison of Biomechanical and Biochemical Properties of Cartilage From Human Knee and Ankle Pairs,” J. Orthop. Res., 18(5), pp. 739–748. 10.1002/jor.1100180510 [DOI] [PubMed] [Google Scholar]
- [55]. Bansal, P. N. , Joshi, N. S. , Entezari, V. , Grinstaff, M. W. , and Snyder, B. D. , 2010, “Contrast Enhanced Computed Tomography Can Predict the Glycosaminoglycan Content and Biomechanical Properties of Articular Cartilage,” Osteoarthritis Cartilage, 18(2), pp. 184–191. 10.1016/j.joca.2009.09.003 [DOI] [PubMed] [Google Scholar]
- [56]. Meister, K. , Indelicato, P. A. , Spanier, S. , Franklin, J. , and Batts, J. , 2004, “Histology of the Torn Meniscus: A Comparison of Histologic Differences in Meniscal Tissue Between Tears in Anterior Cruciate Ligament-Intact and Anterior Cruciate Ligament-Deficient Knees,” Am. J. Sports Med., 32(6), pp. 1479–1483. 10.1177/0363546503262182 [DOI] [PubMed] [Google Scholar]
- [57]. Allen, C. R. , Wong, E. K. , Livesay, G. A. , Sakane, M. , Fu, F. H. , and Woo, S. L. , 2000, “Importance of the Medial Meniscus in the Anterior Cruciate Ligament-Deficient Knee,” J. Orthop. Res., 18(1), pp. 109–115. 10.1002/jor.1100180116 [DOI] [PubMed] [Google Scholar]
- [58]. Shoemaker, S. C. , and Markolf, K. L. , 1986, “The Role of the Meniscus in the Anterior-Posterior Stability of the Loaded Anterior Cruciate-Deficient Knee. Effects of Partial Versus Total Excision,” J. Bone Joint Surg. Am., 68(1), pp. 71–79. [PubMed] [Google Scholar]
- [59]. Thompson, W. O. , and Fu, F. H. , 1993, “The Meniscus in the Cruciate-Deficient Knee,” Clin. Sports Med., 12(4), pp. 771–796. [PubMed] [Google Scholar]
- [60]. Warren, R. F. , and Levy, I. M. , 1983, “Meniscal Lesions Associated With Anterior Cruciate Ligament Injury,” Clin. Orthop. Relat. Res., 172, pp. 32–37. 10.1097/00003086-198301000-00008 [DOI] [PubMed] [Google Scholar]


