Abstract
Abstract
Heavy metals are found naturally on Earth and exposure to them in the living environment is increasing as a consequence of human activity. The toxicity of six different metal oxide nanoparticles (NP) at different points in time was compared using resazurin assay. After incubating Caco2 and A549 cells with 100 μg/mL of Sb2O3, Mn3O4 and TiO2 nanoparticles (NPs) for 24 h no toxic effects were observed while Co3O4 and ZnO NPs had moderate effects and CuO NPs were toxic below 100 μg/mL (24 h EC25 = 11 for A549 and 71 μg/mL for Caco2). The long-term monitoring (up to 9 days) of cells to NPs revealed that the toxic effects of Mn3O4 and Sb2O3 NPs remarkably increased over time. The 9 day EC50 values for Sb2O3 NPs were 22 and 48 μg/mL for A549 and Caco2 cells; and for Mn3O4 NPs were 47 and 29 μg/mL for A549 and Caco2 cells, respectively. In general, the sensitivity of the cell lines in the resazurin assay was comparable. Trans epithelial electrical resistance (TEER) measurements were performed for both cell types exposed to Co3O4, Sb2O3 and CuO NPs. In TEER assay, the Caco2 cells were more susceptible to the toxic effects of these NPs than A549 cells, where the most toxic NPs were the Sb2O3 NPs: the permeability of the Caco2 cell layer exposed to 10 μg/mL Sb2O3 NPs already increased after 24 h of exposure.
Graphical Abstract
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-016-0032-9) contains supplementary material, which is available to authorized users.
Keywords: Metal oxide nanoparticles, Caco2 cells, A549 cells, Transepithelial electrical resistance (TEER), Long term viability, Non-monotonic dose–response (NMDR)
Introduction
Heavy metals are found naturally in the ground and their existence in the living environment is increasing as a consequence of human activity. Exposure to heavy metals is considered harmful for various species, including mammals. Currently, new materials—metal-based nanomaterials—are increasingly used in various applications such as medicines, electronics, consumer products, among others, making knowledge of the potential harmful effects of metal-based nanoparticles (NPs) on humans and the environment (Setua et al. 2014; Damoiseaux et al. 2011) even more important. Various studies in vivo and in vitro have demonstrated that ultrafine particles could be more toxic and immunogenic than coarser particles of the same material (WHO 2005; Feng and Yang 2012).
The mechanisms behind the toxic responses to NPs include reactive oxygen species generation, the induction of cellular oxidative stress, partial or complete NP dissolution with the release of toxic metal ions (Xia et al. 2008), protein damage or triggering of a protein unfolding response (Jung et al. 2007), membrane leakage, the generation of inflammation (including chronic granulomatous inflammation), induction of frustrated phagocytosis in phagocytic cells, fibrogenic responses, and the activation of cell signalling cascades (Damoiseaux et al. 2011).
At the same time, many trace elements are necessary for cells to function. For example, copper, zinc and cobalt are important for human health and a lack or excess of those microelements could cause serious health disorders. Copper is an essential trace element in the human body and is incorporated into various proteins and metalloenzymes (ceruloplasmin, copper/zinc superoxide dismutase, cytochrome c oxidase, etc.) behaving also as progenitor of free radicals (Brewer et al. 2007).
Cobalt, the essential trace element, is the active centre of the cobalamin (vitamin B12) corrinoid structure, which is similar to porphyrin in heme. Excessive exposure to cobalt is mostly reported as occupational exposure (factory workers) but elevated exposure could also result from prescribed cobalt therapies, and the use of cobalt-containing prosthetics (National Toxicology Program 2014). Cobalt compounds have been stated to be possible carcinogens for humans by the International Agency for Research on Cancer (IARC 2006).
Antimony has no known biological function in mammals. However, the use of antimony is increasing as a fire retardant in rubber, plastics, pigments, adhesives, textiles and paper; as a fining agent in glass manufacture; and has a long history of use as a medicine in the treatment of parasitic diseases (Sundar and Chakravarty 2010). Leaching antimony from polyvinylchloride (PVC) is enhanced at both low and high pH, by elevated temperature and by contact with body fluids (Cooper and Harrison 2009). Furthermore, recent findings prove that antimony used as polyester catalyst may leach from plastic polyethylene terephthalate (PET) bottles widely used for packaging drinking water or other beverages (Westerhoff et al. 2008; Fan et al. 2014). The IARC has stated that antimony trioxide could be a possible carcinogen for humans (IARC 1989).
Occupational exposure to various xenobiotics (e.g. NPs) is expected through inhalation, dermal contact or ingestion [via the gastrointestinal tract (GIT)]. Human studies have shown that inhaled NPs (radioactively-labeled <100 nm carbon particles were used) can pass into blood circulation within 1 min from inhalation (Nemmar et al. 2002).
Particles below 100 nm (even 150 nm) can be internalised by endocytosis. Considerably larger particles (200–500 nm) can enter cells by clathrin-or caveolae-mediated endocytic pathways (Keck and Müller 2013; Shang et al. 2014). Exposure to NPs may have an adverse effect on epithelial barriers and the information on the translocation of NPs via the epithelial barrier is important for the successful delivery of nanomedicines as well as for safety reasons. However, inflammation of the GIT caused by various toxicants may cause increased permeability of the epithelium, leading to a deterioration of the epithelial barrier function. Increased permeability of the GIT epithelium is more linked to a weakening of the tight junctions compared to adherent junctions, gap junctions, desmosomes or hemidesmosomes (Lerner and Matthias 2015).
Tight junctions establish a polarity of the epithelial cell layer by forming a seal between adjacent epithelial cells, thereby separating the luminal compartment surrounded by the apical surface of the cell layer from the interstitial compartment bordered with the basolateral surface (Schneeberg and Lynch 2004). To pass the physiological barrier formed by epithelial cells, molecules have to enter the cell by diffusion or active transport, and to be eliminated (exocytosed) from the basolateral side of the barrier (transcellular transport). Therefore, a decrease in the resistance of the epithelial cell layer may indicate loose barriers and the potential paracellular transport of NPs. In addition, there is evidence that epithelial barriers separate the endocrine growth factors expressed in the apical side of their receptors located in the basolateral side as shown in the case of heregulin by Vermeer et al. (2003). Leakage of the epithelial barrier might result in the autocrine stimulation of cell proliferation followed by neoplasmic formations.
As a rule, in vitro cytotoxicity tests are conducted using exposure times up to 24 h. Furthermore, the long-term tests are not common with NPs, even though long-term cytotoxicity tests are not rare (Wagner et al. 2009; Kasten et al. 2014; Müller et al. 2007). It has been discussed that there could be a linear correlation between short and long-term toxicity (Scheers et al. 2001). However, the possibility of exceptions due to various reasons, especially concerning NPs where the particles are covered with biocorona in physiological conditions, has been mentioned earlier (Sabella et al. 2014). The need to collect cytotoxicity data over time is derived also from the guidelines of the NICEATM summary of the multicenter evaluation of in vitro cytotoxicity (MEIC) (NICEATM 2001).
In this study, six MeO NPs (Co3O4, CuO, Mn3O4, Sb2O3, ZnO and TiO2 NPs) were tested for their toxic properties using Caco2 and A549 cells in vitro during prolonged exposure (up to 9 days). Three of these NPs (Co3O4, Sb2O3, CuO NPs) were additionally studied using a trans epithelial electrical resistance (TEER) assay, to evaluate the adverse effects of these NPs on the physiological membrane integrity of the epithelial cell layers of Caco2 and A549. Those three NPs were chosen for the TEER assay for different reasons: CuO NPs are well studied and known to be toxic at 10 μg/mL (Ivask et al. 2015; Jang et al. 2016). 100 μg/mL of Co3O4 or Sb2O3 NPs were not very toxic after 24 h for epithelial cells Caco2 and A549, but 6 μg/mL was lethal for murine fibroblasts Balb/c 3T3 after 7 days. Both Co3O4 and Sb2O3 NPs showed idiosyncratic effects in metabolic activity tests and had the highest absolute value of ζ-potential (above 20) in ultrapure water (MQ) (Titma T, Siigur J, Shimmo R, not published yet). Sb2O3 NPs are less studied but their possession of an unusual toxicity mechanism has already been mentioned before (Bregoli et al. 2009).
Materials and methods
Cell culture, reagents and chemicals
Immortalized epithelial cell lines were purchased from an American Type Culture Collection (ATCC, Manassas, Virginia, USA): Caco2 (ATCC HTB-37) cells initially originate from human colorectal adenocarcinoma and A549 cells (ATCC CCL-185) from human lung epithelial cells adenocarcinoma. Balb/c 3T3 clone A31 (ATCC CCL-163), the mouse fibroblast type cell line from embryonic tissue was obtained from the Finnish Centre for Alternative Methods (FICAM, Tampere, Finland).
The Caco2 cells were cultured in Minimum Essential Media (MEM) (Gibco, Life Technologies, Carlsbad, California, USA) supplemented with 15 % fetal bovine serum (FBS) (Biological Industries, Cromwell, CT, USA), 1 % non-essential amino acids (NEAA) (Gibco, Invitrogen, Carlsbad, California, USA), 1 % sodium pyruvate (Gibco, Invitrogen), 100 μg/mL and 100 U/mL of streptomycin-penicillin (PEST) (Naxo, Tartu, Estonia). The A549 cell complete cell medium (CCM) was Dulbecco’s Modified Eagle Medium (DMEM) with high glucose (Gibco, Life Technologies) supplemented with 10 % FBS and 1 % PEST. Balb/c 3T3 cells were cultured in DMEM (Gibco, Life Technologies) with 10 % Newborn Calf Serum (NBCS, Gibco, Life Technologies) and 100 μg/mL and 100 U/mL of streptomycin-penicillin. MEM without phenol red used in physicochemical measurements came from Life Technologies. A phosphate buffered saline (PBS) was from Lonza (Bazel, Switzerland). The resazurin used as a metabolic activity dye and sodium dodecyl sulfate (SDS), a positive control, were from Sigma-Aldrich (St.Louis, MO, USA). The cells were controlled for mycoplasma and endotoxin using MycoAlert (Lonza) and LAL (Lonza) tests, respectively.
The metal oxide nanoparticles (MeO NPs) were synthesized at the University of Bremen, Foundation Institute of Materials Science IWT (Bremen, Germany) using the flame pyrolysis method (George et al. 2009). The physicochemical characterization of NPs was performed as described in Ivask et al. (2015).
The NPs were suspended in autoclaved MilliQ (18 MΩ, Merck Millipore, Merck KGaA, Darmstadt, Germany) at a concentration of 400 μg metal/mL and sonicated for 4 min with 10 % amplitude (40 W, Branson probe sonicator, Branson Ultrasonics, Danbury, CT, USA). The suspension was sonicated again for 2.5 min before the NP suspension was prepared in the relevant cell culture media (100 μg metal/mL). Gentamicin sulfate (AppliChem GmbH, Darmstadt, Germany) (50 μg/mL) was added to the stock solution of the NPs (400 μg metal/mL) and kept in room conditions for 24 h before the working solutions were prepared to avoid contamination.
The time-dependent metabolic activity of Caco-2 and A549 cells
The cells were seeded to a 96-well plate in concentrations of 1 × 104 cells per well for Caco2 cells or 0.5 × 104 per well for A549 and Balb/c 3T3 cells. After 24 h of growth (95 % humidity, 37 °C, 5 % CO2) the cells were exposed to NPs using concentrations 3–100 μg metal/mL.
The metabolic activity of the cells was measured after a certain time interval of exposure using resazurin fluorescence assay. Resazurin is a cell permeable dye that is reduced by the cells with active metabolism to a highly fluorescent product resorufin. The fluorescent dye was added 2 h before the measurement timepoint. After 2 h, the supernatant was pipetted to 96-well plates and the resorufin was measured at a wavelength of 590 nm using an excitation wavelength of 560 nm with a fluorescence spectrometer LS 55 from PerkinElmer. The medium was replaced with CCM with no NPs added. The resazurin assay was repeated after every time interval followed with a change of CCM. The metabolic activity of the cell culture was monitored for at least 9 days. Tests were performed in three parallels with three replicates. Abiotic values (cell medium and NPs without cells) were always subtracted from the results to avoid an interference caused bias.
Chemicals for electron microscopy were of following origins: glutaraldehyde (50 % in H2O) was from Fluka (Buchs, Switzerland), OsO4 (4 % in H2O) agarose and propylene oxide (≥ 99.5 %) were from Sigma-Aldrich. Resin components: EPON 812, DDSA and DMP-30 were from SERVA (SERVA Electrophoresis GmbH, Heidelberg, Germany), MNA (~97 %) was from Fluka.
The cells were monitored using light microscopy (Olympus IMT-2, camera Olympus DP71, olympur, Tokyo, Japan). TEM observation and elemental analysis with SEM–FIB-EDX instrument (FEI Helios Nano-Lab 600, USA) using Energy-Dispersive X-ray Spectroscopy (EDX) function (Oxford Instruments, Abingdon, UK) was carried out as described in Ivask et al. (2015).
Transepithelial electrical resistance (TEER) assay
Co3O4, Sb2O3 and CuO NPs were studied using a TEER assay, to evaluate the adverse effects of these NPs on the epithelial resistance of Caco2 and A549 cells. The assay was performed on 24-well microplates. To each well of the microplate, a hanging insert (Merck Millipore, Cat# PIRP12R48) with pore size of 1.0 μm was placed. The cells were seeded at a density of 1.8 × 105 cells/cm2 in 0.2 mL to the apical side of the inserts. The CCM at a volume of 0.8 mL without cells covered the basolateral side of the inserts. The resistance was measured using an EMD Millipore Millicell-ERS2 Volt-Ohm Meter (Merck Millipore) every day. The medium was replaced every third day.
After approximately 7 days, when the resistance of the cell layer had risen to 2000–3000 Ω (660–990 Ω/cm2) for Caco2 and 700–800 Ω (230–260 Ω/cm2) for A549, and the cell layer was considered confluent, the medium on the apical side of the inserts was displaced to CCM supplemented with concentrations of 1, 10, 100 μg metal/mL (for Caco2 cells also 200 μg metal/mL) NPs. After the addition of the NPs, the resistance of the Caco2 cells was measured at 0, 24, 48 and 72 h and A549 cells were monitored for up to 12 days (288 h). A cell culture medium without NPs was always used as a negative control, sodium dodecyl sulphate (SDS) at 600 or 300 μg/mL was used as a positive control. Tests were performed in two parallels with three replicates. The TEER of inserts without cells was subtracted from all samples.
Balb/c 3T3 cell transformation assay (CTA)
CTA is reported in the list of accepted methods for REACH (Reg. EC 440/2008) and was carried out in accordance of the prevalidation report of ECVAM (2010). The assay is based on Balb/c 3T3 cell line ability to maintain a uniform contact-inhibited monolayer. The endpoint is the measurement of the Colony Forming Efficiency (CFE = [total number of colonies formed in the treatment dishes/total number of colonies formed in the control dishes] × 100).
The cells were seeded to a 6-well plate (Cellstar, Greiner, Frickenhausen, Germany) at concentrations of 0.5 × 104 cells per well. On the second day, the CCM was replaced with a dispersion of CCM with NPs at a concentration of 1 and 0.1 μg metal/mL. The dispersion of NPs was replaced with CCM without NPs after 48 h and thereafter the media were replaced every third day. The test was stopped at day seven after exposure. 0.7 % ethanol as an interlaboratory validated transformation agent for Balb/c 3T3 cells and IARC classified group 1 substance was used as positive control, as this substance had proven capability to transform the contact inhibition of Balb/c 3T3 cell. Cells without treatment served as a negative control. The cells were evaluated microscopically.
Statistical analysis
Data were analyzed using Microsoft Office Excel® 2007. Experimental results were presented as mean ± SD. EC10, EC25, and EC50 values were calculated using the program REGTOX: macro ExcelTM.
Results and discussion
In this study the long-term toxicity of six different metal oxide NPs has been studied using resazurin as a metabolic activity dye and three metal oxides from this set (Co3O4, Sb2O3 and CuO NPs) were additionally evaluated for their potential harmful effects using the TEER assay. Both assays were performed on two epithelial cell lines—Caco2 and A549.
Although long-term monitoring is inevitable in all preclinical in vivo and for now in most in vitro tests in drug design, it is not a common research method in basic research. However, if we are intending to look closely at the consequences of exposure to xenobiotics, the monitoring of long-term metabolic activity gives more accurate results for conclusions. Even if the test design for long-term effect evaluation is costly and time consuming the outcome is worth the expenditures. It especially concerns research into the safety of NPs due to scarce knowledge on the long-term effects.
Viability tests conducted using propidium iodide, MTT, or neutral red dye are not applicable for repeated testing, and therefore limit the data gathering contrary to dyes such as MTS, WST or resazurin responding to the metabolic activity of the cell. Long-term monitoring of cell metabolic activity when exposed to NPs has only been studied in recent years. Farcal et al. (2015) studied six oxide nanomaterials in twelve cellular models representing six target organs/systems using ten assays in nine laboratories. Long-term repeated dose exposure was performed for seven to 21 days using Caco2 cells. Pojo et al. (2013) studied the potential of carboxymethylchitosan/polyamidoamine NPs as a drug delivery system in glioblastoma cell models using long-term exposure over 7 days. MTS dye was used to evaluate the metabolic activity of the cells after their repeated exposure to NPs. In our study the exposure was performed only once followed by long-term monitoring of the exposed cells. The design was led from the knowledge that NPs are absorbed into the extracellular matrix and/or taken up by the cells (Grant et al. 2015; Ko et al. 2016). Therefore, the change in CCM removes only the unsettled part of NPs and leached ions as in real physiological conditions where the flow of extracellular fluid will remove free NPs and leached ions. The intention was to observe whether the impact of NPs, which were internalised into the cellular matrix and cells, changes over time. Repeated exposure of NPs will lead to a cumulative dosage because of the continuous internalisation of NPs by the cells and the extracellular matrix.
To assess the long-term metabolic activity of the cells we used resazurin. Resazurin, a non-fluorescent indicator dye, is converted to bright red fluorescent resorufin via the reduction reactions of metabolically active cells. Resazurin is not toxic to living cells and the results of the assay are in good correlation with those of MTS, ATP and LDH assays (Riss and Moravec 2004).
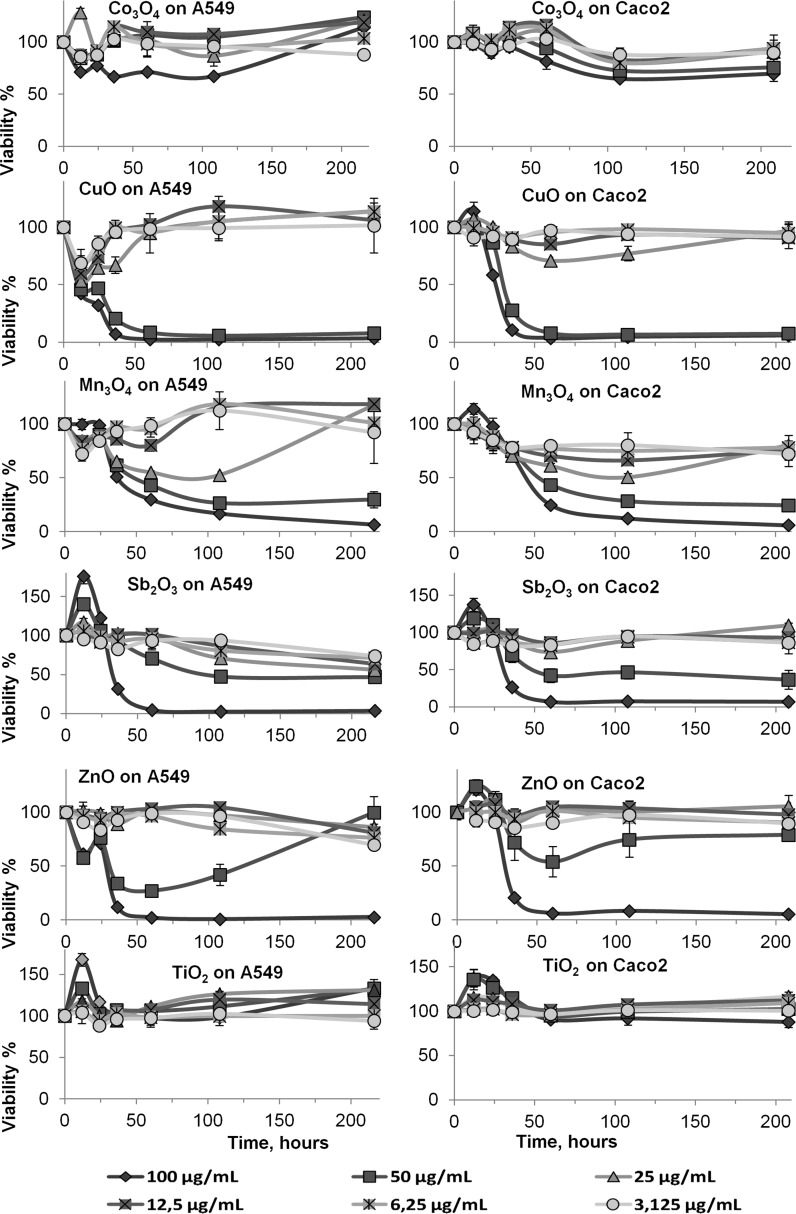
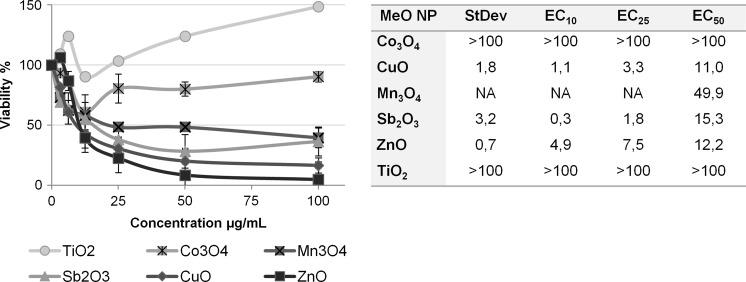
As found by various authors (Rotoli et al. 2012; Farcal et al. 2015) ZnO and CuO NPs were also toxic to the epithelial cells in our study (Fig. 1; Ivask et al. 2015). Interestingly, Sb2O3 and Mn3O4 NPs seemed to be as toxic as ZnO or even CuO NPs, although their toxicity emerged later. The long-term monitoring (for 9 days) of the toxicity of six NPs on two different cell types showed that for some NPs (Mn3O4, Co3O4 and Sb2O3 NPs) the toxicity emerged only after 24 h (Fig. 1; Table 1).
Fig. 1.
The 9 days (216 h) of metabolic activity in human lung epithelial cells A549 and human intestinal epithelial cells Caco2 cells after a single exposure to dispersions of MeO NPs at t = 0. Resazurin was used to measure metabolic activity. Concentrations of metal oxides are presented as μg metal/mL. The metabolic activity is presented as a % of the unexposed control cells. See also Table 1 for the respective EC values. The presented data are average values of three independent measurements ±SD
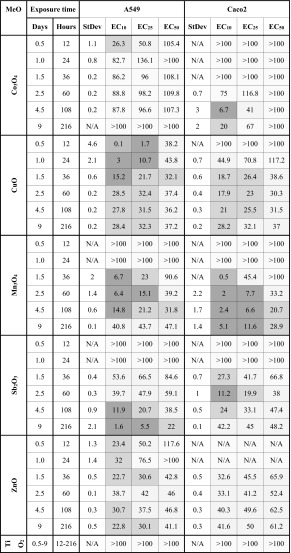
Table 1.
The EC10, EC25 and EC50 values (μg metal/mL) of the six nanoparticles (NPs) in two cellular systems (A549 and Caco2) calculated at different timepoints (12 h to 9 days)
The heat map shows an earlier toxicity of CuO and ZnO NPs. EC values are calculated from dose–response data presented in Fig. 1
N/A not applicable
In addition, the remarkable non-monotonic positive dose–response (NMDR) at 24 h on cell metabolic activity was observed in exposure of three NPs (Mn3O4, Sb2O3 and TiO2 NPs), which were negatively surface charged in MQ. We assume that cell proliferation is stimulated because of the activation of growth factor receptors followed by intracellular ROS generation (Bartłomiejczyk et al. 2013).
The surface charge of a NP is a function of solution pH, which is affected by the reactions that occur on the particle surface (Suttiponparnit et al. 2011). It has been shown that even if NPs have a negative surface charge in an MQ environment, the surface charge of NPs in a cell growth medium appears to be the same (negative) as the medium itself containing various negatively charged proteins (Ivask et al. 2015). Apparently, the exposure to MeO NPs of large hydrodynamic size, which depends on the surface charge and pH, results in a delayed harmful effect (Titma T, Siigur J, Shimmo R, not published yet; Fairhurst 2013).
Mn3O4 and Sb2O3 NPs showed no toxic effect at timepoint 24 h but the toxicity emerged later, which had not been mentioned before. Bregoli et al. (2009) found Sb2O3 NPs showing a specific type of toxicity towards erythroid precursor proliferation and Sb2O3 NPs were not found inside the damaged cells, but in close vicinity to the cell surface.
A comparison of EC10 and EC25 and EC50 (Table 1) reveals that there is a good correlation in the case of CuO, Mn3O4, ZnO NPs. Exposure to TiO2 NPs caused an increase in the metabolic activity of the cells with no toxicity during the test period as shown before (Rotoli et al. 2012). However, the exposure to Co3O4 NPs was accompanied by a small static decrease in metabolic activity. At 9 days most of the exposed cell layers showed the marks of recovery—EC50 was higher than at the fourth day. By contrast, the metabolic activity decreased with time in cells exposed to ZnO NPs and was remarkable when exposed to Sb2O3 NPs.
TEER measurements are used not only for epithelial barrier research but also for indications of the destruction of the extracellular matrix; for example, of neoplastic formations (Calabro et al. 2008; Buchert et al. 2012). To assess the effect of NPs on epithelial membrane integrity, we used the transepithelial resistance measurement of intact Caco2 and A549 epithelial cell monolayer. It is suggested that an enhancement of paracellular flux follows as a result of a toxic effect towards tight junctions caused by either direct interactions with tight junctional protein components, or by the changes in the second messenger systems, or from the direct or indirect effects on cytoskeletal proteins linked to the tight junctional components (Van Itallie and Anderson 2004).
The use of cell growth inserts mimics the natural epithelial layer with an apical and basolateral environment. The construction makes it possible to avoid the shortcomings of traditional cell monolayers growing in the bottom of the well, where the formation of an extracellular matrix and intracellular contact is low. The epithelial cells separating the luminal space from basolateral are sealed with tight junctions ensuring the polarity of the epithelial cell layer. Increased permeability of the epithelia is linked to a weakening of the tight junctions. Unregulated inflammation caused by various contaminants (e.g. chemicals) causes an increased permeability of the epithelium, leading to deterioration of the protective function of the epithelial barrier for the organism (Lodish et al. 2012).
The data obtained by Lin et al. (2011) who studied the effect of densely organic polymer-coated gold NPs on the integrity of the tight junctions of Caco2 cells stated that all NPs (5, 10 and 20 nm) in their study loosened the tight junctions but in most cases this effect was reversible. However, antimony trioxide NPs are the least investigated MeO NPs. There is no information on basic cytotoxicity but some evidence of pathological complications has been observed (Guildford et al. 2009; Bregoli et al. 2009).
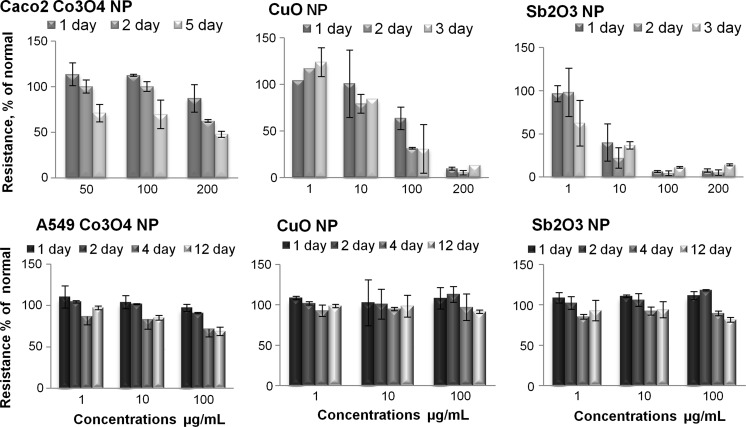
The changes in TEER in the intact Caco2 cell layer upon exposure to metal oxides were observed from 1 to 5 days and for A549 cells from 1 to 12 days. The exposure concentrations ranged from 1 to 200 μg/mL (Table ESM1, Fig. 2). According to the TEER measurements, the Caco2 cells were more susceptible than the A549 cells to the toxic effects of the studied NPs, especially towards Sb2O3 NPs (Fig. 2). The permeability of the Caco2 monolayer exposed to CuO NPs was less affected compared to the decrease in metabolic activity in the resazurin assay. The TEER measurements revealed that the permeability of the Caco2 cell layer exposed to 10 μg/mL Sb2O3 NPs decreased already at 12 h. The 24 h EC50 of TEER measurement was approximately 8 μg/mL (SD = 1.3) and at 48 h about 5 μg/mL (SD = 0.8). A similar decrease in the case of CuO NP in the same concentration emerged later. In both cases this effect was irreversible.
Fig. 2.
Time-dependent decrease of the resistance of the epithelial cell layer (% of control) of Caco2 (upper panels) and A549 cells (lower panels) after exposure to Co3O4, CuO and Sb2O3 NPs over 3 or 12 days (values of TEER measurements are shown in Table S1). The exposure time is indicated on the panels. The data presented are average values of the two independent measurements ±SD
We suggest that although the Sb2O3 NPs were toxic from the first contact with the proteins in the extracellular matrix, the internal toxicity was delayed because of the large corona of NPs, which are negatively charged in MQ (Titma T, Siigur J, Shimmo R, not published yet). The permeability of the Caco2 monolayer exposed a single time to 100 μg/mL of Sb2O3 NPs decreased to almost 0 % of the negative control and did not change much after 24 h, revealing the open access of foreign substances to the basal lamina. However, the Caco2 monolayer exposed to 50 μg/mL of Co3O4 NPs shows an obvious reduction in resistance when compared to the results from the resazurin tests.
The TEER of the A549 cell layer was monitored for 12 days due to the unexpectedly slight change in resistance. The behaviour of the lung epithelial model cells was apparently different. Whereas the Caco2 cell layer was severely affected, the A549 cell layer showed a moderate dose-dependent reduction in resistance. However, the toxicity did increase with time.
Studies observing ionic compounds indicate that cytotoxicity occurs before TEER becomes compromised (Konsoula and Barile 2005). Notably, the cytotoxicity of metallic NPs is frequently associated with leaching metal ions (Sabella et al. 2014; Zook et al. 2012; Setua et al. 2014). Rotoli et al. (2012) found that an increase in the permeability of a monolayer of Calu2 cells exposed to CuO, CeO2 or TiO2 NPs correlated with a decrease in metabolic activity on the seventh day. At the same time, it could be expected that at least at low doses the permeability of the monolayer will increase much earlier than the decrease in the metabolic activity of the cells as shown by Bender et al. (2011), and in our study on the Caco2 cell layer (Fig. 2 upper panel). Interestingly, the A549 cell layer exposed to CuO NPs, which of the three NPs decreased the metabolic activity the most, seemed to be in a healthy condition and showed a moderate decrease in permeability after 12 days.
This could be explained by the specialisation of lung epithelial tissue, designed to defend the airways against constant exposure of micro particles. As it is widely known, the formation of fibrotic granulomas is typical for lung tissue as a result of the granulomatous inflammatory process in the case of asbestosis, tuberculosis and even talcosis (Czul and Lascano 2011). Such granulomas are not known in GIT (but ulcerative colitis or Chron’s disease) due to the continuous self-renewal and migration kinetics of cells (James 2000; Clevers 2013). In addition, A549 cells have a shorter doubling time than Caco2 cells, and thereby allow the viable cells to overgrow damaged cells with endosomes/lysosomes full of NPs.
We observed, in addition, the effect of those MeO NPs on fibroblasts Balb/c 3T3 cell layer. Fibroblasts are the most common cells in connective tissue defending the organism’s general physiological barriers such as skin, and the epithelial tissue in the intestinal and respiratory tract. Therefore, it is understandable that fibroblasts do not form an integrated cell layer like a physiological membrane. In addition, Balb/c 3T3 cells are not embryonic human cells and not neoplasmic cells. This might be the cause of breaking the pattern. Furthermore, the effect of exposure on a single-layer cell culture could not extrapolate to the whole organism.
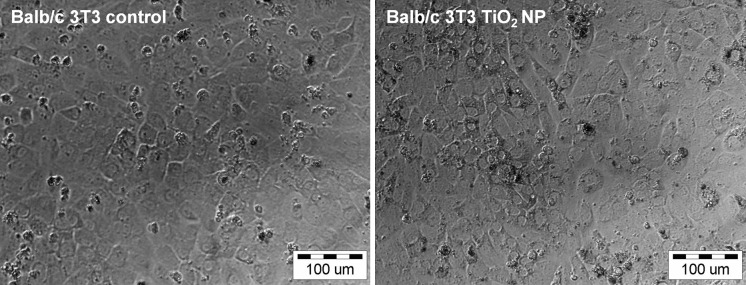
Fibroblasts even showed sensitivity to Co3O4 NPs, which were moderately toxic to human epithelial cells (Fig. 3). Surprisingly, the Balb/c 3T3 cells exposed to 6 μg/mL Co3O4, CuO, Mn3O4, Sb2O3, or ZnO NPs almost disappeared from the wells 7 days after a single exposure. The viability of Balb/c 3T3 cells exposed to Ti2O was similar to the negative control, where the characteristic contact inhibition of the cell line was altered (Figs. 3, 4).
Fig. 3.
The 24 h viability of Balb/c 3T3 cells exposed to MeO NP (resazurin assay). The presented values are at concentrations of μg metal/mL
Fig. 4.
Balb/c 3T3 cells 7 days after a single exposure to TiO2 at a concentration of 6 μg metal/mL. The cell layer exposed to TiO2 is similarly confluent to the normal negative control
The Balb/c 3T3 cell transformation assay (CTA) was performed to assess the potential carcinogenicity of the tested NPs. As the result no foci were observed following the exposure of Co3O4 or CuO or Sb2O3 at a concentration of 1 and 0.1 μg/mL. Nor were any changes in the morphology of the cells observed. Similar results have been previously shown by Guildford et al. (2009).
Various research methods are used for the closest observations and among them the visualization of the pharmacokinetics of NPs in in vitro cell cultures is one of the favourites. Using scanning or transmission electronic microscopy, the cell microstructure could be seen in detail.
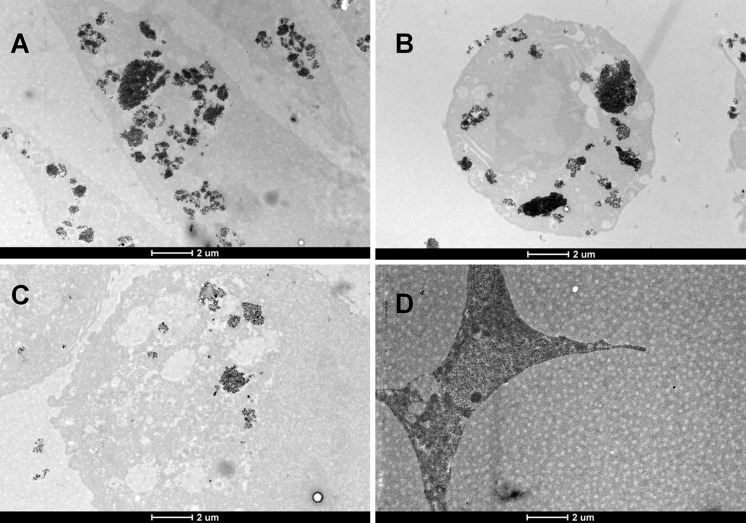
TEM images show the aggregated NPs within lysosomes and cell vesicles. The mitochondria and phago-/endo-/lysosomes as noted by Blanchette et al. (2009) are clearly seen in the image with Co3O4 exposure. It is proposed that in the acidic environment that exists in the lyzosomes, the NPs will be susceptible to enzymatic degradation (Leung and Wang 2010) or dissolution.
As seen in the image (Fig. 5a, b), remarkable amount of Co3O4 NPs aggregations had accumulated inside the cell vesicle. However, it could be concluded that not all MeO NP caused the evident loss of intestinal cell metabolic activity during the 24-h exposure. Still, the cytoplasmic structures are apparently disrupted in the case of Co3O4 and Sb2O3.
Fig. 5.
TEM images of cells exposed to MeO NPs for 24 h at concentration EC20. a Balb/c 3T3 cells exposed to Co3O4 NP at a concentration of 64.5 μg metal/mL; b A549 cells exposed to Co3O4 NP at a concentration of 56.1 μg metal/mL; c Caco2 cell exposed to Sb2O3 at a concentration of 44.7 μg metal/mL; d Caco2 cells exposed to CuO at a concentration of 3.1 μg metal/mL (photograph by M. Visnapuu)
The solubility of iron, zinc and copper oxide NPs is higher at low pH, and particles may dissolve in the acidic environment of the lysosomes (Fröhlich 2013). Similar behaviour is also reported for antimony trioxide found in the lysosomes (Fig. 5c). The effect of Sb2O3 and Co3O4 NPs is very similar to that described earlier by Bastian et al. (2009). Both Co3O4 and Sb2O3 enter the cell cytoplasm but not the nucleus; thereby, having cytotoxic effect at concentrations greater than 30 μg/mL.
The cytotoxicity of CuO NPs on Caco2 cells showed a number of similarities with the results observed by Cronholm et al. (2013). There are no clear aggregates on the TEM image (Fig. 5d) inside the CuO exposed Caco2 cells. This could be a consequence of the long exposure time (24 h) and the small concentration of CuO NPs (4 μg/mL), and mostly related to the increased dissolution of CuO NPs in the protein rich complete growth medium (41 %, see Ivask et al. 2015). Nevertheless, clear evidence of apoptotic markers could be seen. Some of the Caco2 cells showed a rhombic form expressing cell shrinkage in correlation with the light microscopy observations, where formulated invisible apoptotic bodies were expressed. Moreover, on the TEM image of the cell exposed to CuO NPs, chromatin condensation and DNA fragmentation inside the nucleus could be seen.
Conclusions
Our study has several novel aspects: (1) the long-term (exposure up to 9 days) harmful effects of six metal oxide NPs following a single exposure was studied; (2) for three metal oxide NPs the TEER assay was also used in parallel; (3) both of the assays were performed on two epithelial cell lines, Caco2 and A549; and (4) the effect of connective tissue on Balb/c 3T3 fibroblast juxtaposing the basolateral side of physiological barrier was studied.
CuO NPs were the most toxic for the epithelial cells at the 24-h timepoint as has been also reported before. It is remarkable that the decrease in metabolic activity in cells exposed to Sb2O3 and Mn3O4 NPs seemed to be as severe as those exposed to ZnO or even CuO NPs although the decrease emerged later. We found that epithelial cell layers of different origin showed a different response in terms of an increase in permeability to the exposure of MeO NPs despite similar results from the metabolic activity tests. It seems that conventional 24-h metabolic activity tests of at least some MeO NPs might lead to underestimating their toxicity and also to stimulated metabolic activity after 24 h. However, the immediate increase in permeability of the intact intestinal cell layer was obvious. Thus, we suggest using TEER measurements as part of basic toxicity tests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This research was supported by the EU FP7 Project MODERN under Grant Agreement No. 309314, SA Archimedes project Functional Food Ingredients, and Tallinn University Centre of Excellence “Natural sciences and sustainable development” and Estonian Institutional research funding project IUT 23-5. Thanks also go to Dr. Suman Pokhrel (University of Bremen, Germany) for providing the Co3O4, CuO, Mn3O4, Sb2O3, ZnO and TiO2 NPs. Thanks to Dr. Meeri Visnapuu (NICPB, Tartu University, Estonia) for TEM photographs. Thanks also go to Finnish Centre for Alternative Methods (FICAM, Tampere) for providing the Balb/c 3T3 cell line (ATCC CCL-163).
Complaince with ethical standard
Conflict of interest
The authors confirm that this article authorship or content has no conflict of interest.
References
- Bartłomiejczyk T, Lankoff A, Kruszewski M, Szumiel I. Silver nanoparticles—allies or adversaries? Ann Agric Environ Med. 2013;20:48–54. [PubMed] [Google Scholar]
- Bastian S, Busch W, Kühnel D, Springer A, Meissner T, Holke R, Scholz S, Iwe M, Pompe W, Gelinsky M, Potthoff A, Richter V, Ikonomidou C, Schirmer K. Toxicity of tungsten carbide and cobalt-doped tungsten carbide nanoparticles in mammalian cells in vitro. Environ Health Perspect. 2009;117:530–536. doi: 10.1289/ehp.0800121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CP, Hübner N-O, Weltman K-D, Scharf C, Kramer A. Tissue tolerable plasma and polihexanide: are synergistic effects possible to promote healing of chronic wounds? In vivo and in vitro results. In: Machala Z, editor. Plasma for bio-decontamination, medicine and food security. Berlin: Springer; 2011. pp. 324–325. [Google Scholar]
- Blanchette CD, Woo YH, Thomas C, Shen N, Sulchek TA, Hiddessen AL. Decoupling internalization, acidification and phagosomal-endosomal/lysosomal fusion during phagocytosis of InlA coated beads in epithelial cells. PLoS One. 2009;4:e6056. doi: 10.1371/journal.pone.0006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregoli L, Chiarini F, Gambarelli A, Sighinolfi G, Gatti AM, Santi P, Martelli AM, Cocco L. Toxicity of antimony trioxide nanoparticles on human hematopoietic progenitor cells and comparison to cell lines. Toxicology. 2009;262:121–129. doi: 10.1016/j.tox.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Harris ED, Askari FK. Textbook of hepatology: from basic science to clinical practice. 3. Oxford: Blackwell Publishing Ltd; 2007. Normal copper metabolism and lowering copper to subnormal levels for therapeutic purposes; pp. 226–232. [Google Scholar]
- Buchert M, Turksen K, Hollande F. Methods to examine tight junction physiology in cancer stem cells: TEER, paracellular permeability, and dilution potential measurements. Stem Cell Rev. 2012;8:1030–1034. doi: 10.1007/s12015-011-9334-7. [DOI] [PubMed] [Google Scholar]
- Calabro AR, Konsoula R, Barile FA. Evaluation of in vitro cytotoxicity and paracellular permeability of intact monolayers with mouse embryonic stem cells. Toxicol In Vitro. 2008;22:1273–1284. doi: 10.1016/j.tiv.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Cooper RG, Harrison AP. The exposure and health effects of antimony. Indian J Occup Environ Med. 2009;13:3–10. doi: 10.4103/0019-5278.50716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronholm P, Karlsson HL, Hedberg J, Lowe TA, Winnberg L, Elihn K, Wallinder IO, Möller L. Intracellular uptake and toxicity of Ag and CuO nanoparticles: a comparison between nanoparticles and their corresponding metal ions. Small. 2013;9:970–982. doi: 10.1002/smll.201201069. [DOI] [PubMed] [Google Scholar]
- Czul F, Lascano J. An uncommon hazard: pulmonary talcosis as a result of recurrent aspiration of baby powder. Respir Med CME. 2011;4:109–111. doi: 10.1016/j.rmedc.2011.02.001. [DOI] [Google Scholar]
- Damoiseaux R, George S, Li M, Pokhrel S, Ji Z, France B, Xia T, Suarez E, Rallo R, Mädler L, Cohen Y, Hoek EMV, Nel A. No time to lose—high throughput screening to assess nanomaterial safety. Nanoscale. 2011;3:1345–1360. doi: 10.1039/c0nr00618a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECVAM. Balb/c 3T3 Cell Transformation Assay Prevalidation study Report (2010). https://eurl-ecvam.jrc.ec.europa.eu/eurl-ecvam-recommendations/files-cta/ER2010-02_Balb.pdf. Accessed 10 May 2015
- Fairhurst D (2013) An overview of the zeta potential part 3: uses and applications. Am Pharmaceut Rev http://www.americanpharmaceuticalreview.com/Featured-Articles/139288-An-Overview-of-the-Zeta-Potential-Part-3-Uses-and-Applications. Accessed 12 June 2015
- Fan Y-Y, Zheng JL, Ren JH, Luo J, Cui XY, Ma LQ. Effects of storage temperature and duration on release of antimony and bisphenol A from polyethylene terephthalate drinking water bottles of China. Environ Pollut. 2014;192:113–120. doi: 10.1016/j.envpol.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Farcal L, Torres Andón F, Di Cristo L, Rotoli BM, Bussolati O, Bergamaschi E, Mech A, Hartmann NB, Rasmussen K, Riego-Sintes J, Ponti J, Kinsner-Ovaskainen A, Rossi F, Oomen A, Bos P, Chen R, Bai R, Chen C, Rocks L, Fulton N, Ross B, Hutchison G, Tran L, Mues S, Ossig R, Schnekenburger J, Campagnolo L, Vecchione L, Pietroiusti A, Fadeel B. Comprehensive in vitro toxicity testing of a panel of representative oxide nanomaterials: first steps towards an intelligent testing strategy. PLoS One. 2015;10:e0127174. doi: 10.1371/journal.pone.0127174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Yang W. Effects of particulate air pollution on cardiovascular health: a population health risk assessment. PLoS One. 2012;7:e33385. doi: 10.1371/journal.pone.0033385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich E. Cellular targets and mechanisms in the cytotoxic action of non-biodegradable engineered nanoparticles. Curr Drug Metab. 2013;14:976–988. doi: 10.2174/1389200211314090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Pokhrel S, Xia T, Gilbert B, Ji Z, Schowalter M, Rosenauer A, Damoiseaux R, Bradley KA, Mädler L, Nel AE. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano. 2009;4:15–29. doi: 10.1021/nn901503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DN, Cozad MJ, Grant DA, White RA, Grant SA. In vitro electromagnetic stimulation to enhance cell proliferation in extracellular matrix constructs with and without metallic nanoparticles. J Biomed Mater Res B Appl Biomater. 2015;103:1532–1540. doi: 10.1002/jbm.b.33338. [DOI] [PubMed] [Google Scholar]
- Guildford AL, Poletti T, Osbourne LH, Di Cerbo A, Gatti AM, Santin M. Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response. J R Soc Interface. 2009;6:1213–1221. doi: 10.1098/rsif.2009.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (1989) Antimony trioxide and antimony trisulfide. Some organic solvents, resin monomers and related compounds, pigments and occupational exposures in paint manufacture and painting. Monographs on the evaluation of carcinogenic risks to humans. IARC. WHO 47 [PMC free article] [PubMed]
- IARC . Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. Monographs on the evaluation of carcinogenic risks to humans. IARC. Geneva: WHO; 2006. p. 86. [PMC free article] [PubMed] [Google Scholar]
- Ivask A, Titma T, Visnapuu M, Vija H, Kakinen A, Sihtmae M, Pokhrel S, Madler L, Heinlaan M, Kisand V, Shimmo R, Kahru A. Toxicity of 11 metal oxide nanoparticles to three mammalian cell types in vitro. Curr Top Med Chem. 2015;15:1914–1929. doi: 10.2174/1568026615666150506150109. [DOI] [PubMed] [Google Scholar]
- James DG. A clinicopathological classification of granulomatous disorders. Postgrad Med J. 2000;76:457–465. doi: 10.1136/pmj.76.898.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S-J, Oh M-S, Yang SI, Cho E-M. Gene expression profiles of human neuroblastoma cells exposed to CuO nanoparticles and Cu ions. BioChip J. 2016;10:140–149. doi: 10.1007/s13206-016-0209-5. [DOI] [Google Scholar]
- Jung EJ, Avliyakulov NK, Boontheung P, Loo JA, Nel AE. Pro-oxidative DEP chemicals induce heat shock proteins and an unfolding protein response in a bronchial epithelial cell line as determined by DIGE analysis. Proteomics. 2007;7:3906–3918. doi: 10.1002/pmic.200700377. [DOI] [PubMed] [Google Scholar]
- Kasten A, Grüttner C, Kühn JP, Bader R, Pasold J, Frerich B. Comparative in vitro study on magnetic iron oxide nanoparticles for MRI tracking of adipose tissue-derived progenitor cells. PLoS One. 2014;9:e108055. doi: 10.1371/journal.pone.0108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck CM, Müller RH. Nanotoxicological classification system (NCS)—a guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur J Pharm Biopharm. 2013;84:445–448. doi: 10.1016/j.ejpb.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Ko H, Son S, Jeon J, Thambi T, Kwon S, Chae YS, Kang YM, Park JH. Tumor microenvironment-specific nanoparticles activatable by stepwise transformation. J Control Release. 2016;234:68–78. doi: 10.1016/j.jconrel.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Konsoula R, Barile FA. Correlation of in vitro cytotoxicity with paracellular permeability in Caco-2 cells. Toxicol In Vitro. 2005;19:675–684. doi: 10.1016/j.tiv.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015;14:479–489. doi: 10.1016/j.autrev.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Leung KC-F, Wang Y-XJ (2010) Mn–Fe nanowires towards cell labeling and magnetic resonance imaging. In: Nanowires science and technology. INTECH. doi:10.5772/39500
- Lin IC, Liang M, Liu TY, Ziora ZM, Monteiro MJ, Toth I. Interaction of densely polymer-coated gold nanoparticles with epithelial Caco-2 monolayers. Biomacromolecules. 2011;12:1339–1348. doi: 10.1021/bm200116z. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Kaiser CA, Krieger M, Bretscher A (2012) Transmembrane transport of ions and small molecules. In: Molecular cell biology, 7th edn. Freeman, W.H. & Company, New York, pp 473–516
- Müller K, Skepper JN, Posfai M, Trivedi R, Howarth S, Corot C, Lancelot E, Thompson PW, Brown AP, Gillard JH. Effect of ultrasmall superparamagnetic iron oxide nanoparticles (Ferumoxtran-10) on human monocyte-macrophages in vitro. Biomaterials. 2007;28:1629–1642. doi: 10.1016/j.biomaterials.2006.12.003. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (NTP) (2014) Report on carcinogens (roc) concept: cobalt, 13th edn. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service. http://ntp.niehs.nih.gov/pubhealth/roc/roc13/. Accessed 15 Mar 2015
- Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- NICEATM (2001) Report of the international workshop on in vitro methods for assessing acute systemic toxicity: results of an international workshop organized by the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) and the National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM). http://ntp.niehs.nih.gov/pubhealth/evalatm/test-method-evaluations/acute-systemic-tox/invitro/wksp-rpt-recs/index.html. Accessed 31 Aug 2015
- Pojo M, Cerqueira SR, Mota T, Xavier-Magalhães A, Ribeiro-Samy S, Mano JF, Oliveira JM, Reis RL, Sousa N, Costa BM, Salgado AJ. In vitro evaluation of the cytotoxicity and cellular uptake of CMCht/PAMAM dendrimer nanoparticles by glioblastoma cell models. J Nanopart Res. 2013;15:1621. doi: 10.1007/s11051-013-1621-6. [DOI] [Google Scholar]
- Riss TL, Moravec RA. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev Technol. 2004;2:51–62. doi: 10.1089/154065804322966315. [DOI] [PubMed] [Google Scholar]
- Rotoli BM, Bussolati O, Costa AL, Blosi M, Di Cristo L, Zanello PP, Bianchi MG, Visigalli R, Bergamaschi E. Comparative effects of metal oxide nanoparticles on human airway epithelial cells and macrophages. J Nanopart Res. 2012;14:1069. doi: 10.1007/s11051-012-1069-0. [DOI] [Google Scholar]
- Sabella S, Carney RP, Brunetti V, Malvindi MA, Al-Juffali N, Vecchio G, Janes SM, Bakr OM, Cingolani R, Stellacci F, Pompa PP. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale. 2014;6:7052–7061. doi: 10.1039/c4nr01234h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheers EM, Ekwall B, Dierickx PJ. In vitro long-term cytotoxicity testing of 27 MEIC chemicals on Hep G2 cells and comparison with acute human toxicity data. Toxicol In Vitro. 2001;15:153–161. doi: 10.1016/S0887-2333(00)00062-X. [DOI] [PubMed] [Google Scholar]
- Schneeberg EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Setua S, Ouberai M, Piccirillo SG, Watts C, Welland M. Cisplatin-tethered gold nanospheres for multimodal chemo-radiotherapy of glioblastoma. Nanoscale. 2014;6:10865–10873. doi: 10.1039/C4NR03693J. [DOI] [PubMed] [Google Scholar]
- Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnol. 2014;12:1. doi: 10.1186/1477-3155-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J. Antimony toxicity. Int J Environ Res Public Health. 2010;7:4267–4277. doi: 10.3390/ijerph7124267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttiponparnit K, Jiang J, Sahu E, Suvachittanont S, Charinpanitkul T, Biswas P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res Lett. 2011;6:1. doi: 10.1007/s11671-010-9772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie M, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;42:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- Wagner S, Munzer S, Behrens P, Scheper T, Bahnemann D, Kasper C. Cytotoxicity of titanium and silicon dioxide nanoparticles. J Phys Conf Ser. 2009;170:12022. doi: 10.1088/1742-6596/170/1/012022. [DOI] [Google Scholar]
- Westerhoff P, Prapaipong P, Shock E, Hillaireau A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008;42:551–556. doi: 10.1016/j.watres.2007.07.048. [DOI] [PubMed] [Google Scholar]
- WHO . Air quality guidelines: global update 2005: particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Geneva: World Health Organization; 2005. [PubMed] [Google Scholar]
- Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- Zook JM, Halter MD, Cleveland D, Long SE. Disentangling the effects of polymer coatings on silver nanoparticle agglomeration, dissolution, and toxicity to determine mechanisms of nanotoxicity. J Nanopart Res. 2012;14:1165. doi: 10.1007/s11051-012-1165-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.