Abstract
Exposure to microgravity during space flight affects almost all human physiological systems. Migration, proliferation, and differentiation of stem cells are crucial for tissues repair and regeneration. However, the effect of microgravity on the migration potentials of bone marrow mesenchymal stem cells (BMSCs) is unclear, which are important progenitor and supporting cells. Here, we utilized a clinostat to model simulated microgravity (SMG) and found that SMG obviously inhibited migration of rat BMSCs. We detected significant reorganization of F-actin filaments and increased Young’s modulus of BMSCs after exposure to SMG. Moreover, Y-27632 (a specific inhibitor of ROCK) abrogated the inhibited migration capacity and polymerized F-actin filament of BMSCs under SMG. Interestingly, we found that transferring BMSCs to normal gravity also attenuated the polymerized F-actin filament and Young’s modulus of BMSCs induced by SMG, but could not recover migration capacity of BMSCs inhibited by SMG. Taken together, we propose that SMG inhibits migration of BMSCs through remodeling F-actin and increasing cell stiffness.
Keywords: Simulated microgravity, Mesenchymal stem cells, Migration, Cytoskeleton, Cell stiffness
Introduction
Space flights can result in physiological and pathological changes within the human body. Several notable effects include cardiovascular deconditioning, muscle atrophy, immune dysfunction and bone loss (Zayzafoon et al. 2005). These physiological and pathological changes take place at the cellular level and microgravity strongly affects morphology, proliferation, differentiation, and signal transduction in cells (Zhang et al. 2015a, b). Recently, increasing evidence revealed that abnormal regulation or function of cells under microgravity may cause numerous diseases (Wehland et al. 2013; Paulsen et al. 2015). BMSCs are important progenitor and supporting cells that have the intrinsic ability to self-renew and differentiate into multiple types of cells (See et al. 2010). BMSCs have been shown to be able to repair damaged organisms or tissues in both human and animal studies (See et al. 2010; Li et al. 2015). This approach relies on the their migration to the site of injury (Li et al. 2015). Although the fact that microgravity significantly inhibits proliferation and alters differentiation fates of BMSCs has been demonstrated, it is still unclear about the effect of microgravity on migration of BMSCs (Wang et al. 2014; Yan et al. 2015). A better understanding of this effect may provide a novel sight on mechanisms of physiological changes caused by microgravity.
Cell migration is a dynamic and highly regulated process, which can be divided into four steps: extension of lamellipodia, formation of focal adhesions, contraction of the cells body, and detachment of the tail (Hopkins et al. 2006; Wang et al. 2013). At the molecular level, cell migration critically depends on the actin system. The remodeling of actin cytoskeleton provides a driving force to push membrane forward at the leading edge and a traction force to move the cell body (Callan-Jones and Voituriez 2016). Generally, the reorganization of actin cytoskeleton is mediated by Rho-associated kinase (Rock), a major downstream target of Rho. Rock phosphorylates LIM kinase, which then phosphorylates confilin, resulting in actin cytoskeleton stabilization (Nakashima and Lazo 2010). It has been reported that Rho-GTPases act as key sensors in cell adaptation to microgravity and mediates the reorganization of actin cytoskeleton under microgravity (Louis et al. 2015). But the role of Rho signaling in cell migration under microgravity is still unclear.
To elucidate the effect of microgravity on cell migration of BMSCs, we utilized a clinostat to model SMG and studied the roles of Rock and actin cytoskeleton in this process. We demonstrated that SMG significantly inhibits migration of BMSCs, mainly through Rock-F-actin pathway.
Materials and methods
Cell isolation and culture
All animal experiments in this study were approved by the Local Committee of Animal Use and Protection of Chongqing University. Rat MSCs were isolated by the Percoll density gradient centrifugation method, as described previously (Zhang et al. 2015a, b). Briefly, femur and tibia from Sprague–Dawley rats were sawn, and gelatinous marrow was extracted under sterile condition. MSCs were separated by density gradient centrifugation using Percoll (Sigma, St. Louis, MO, USA. d = 1.037 g/mL) for 30 min at 2500 rpm. BMSCs were then enriched in the intermediate zone and were cultured in Dulbecco’s modified Eagle’s medium (DEME, Hyclone, Logan, UT, USA) with 10 % fetal bovine serum (FBS, GIBCO, Grand Island, NY, USA), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C with 5 % CO2. In this study, all cells used were between passages 3–5. For Rock inhibition, 5 μM Y-27632 (Sigma) was added to the medium.
Clinorotation to simulated microgravity
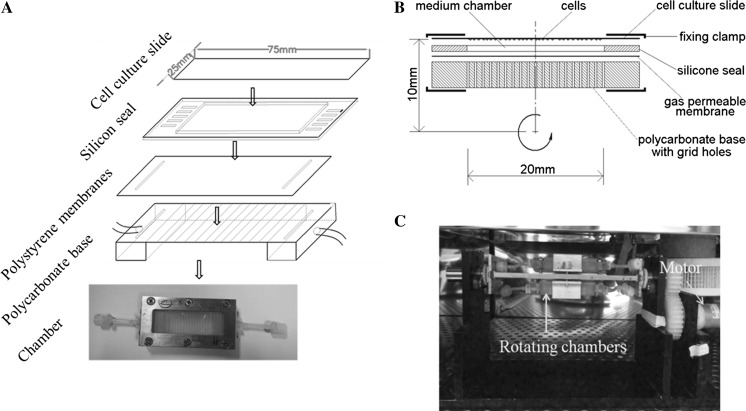
The 2D-clinostat device utilized in this study was constructed by the National Microgravity Laboratory, Institute of Mechanics, Chinese Academy of Sciences, China. The clinostat is an effective, ground-based tool used to simulate certain effects under microgravity on the principle that continuous rotation constantly changed cell orientation with respect to gravity. The clinostat used in this experiment is a device used for providing a vector-averaged reduction in the apparent gravity on cells and the average gravitational force acting on the cells generated by the clinostat is reduced to approximately 10−3 g when the clinostat rotates at 10 rpm, compared with normal gravity (1 g). The chamber was constructed using a cell culture slide (25 × 75 mm, Thermo Scientific, Waltham, MA, USA), a silicon seal, a gas-permeable polystyrene membrane, and a polycarbonate base (Fig. 1a). Briefly, BMSCs were injected into a chamber at a total count of 3 × 105 cells and all air bubbles were carefully removed to prevent fluid shear stress. One side of the chamber is a cell culture slide, the opposite side is a gas-permeable membrane adhered to a polycarbonate base with grid holes, and the surrounding wall of the chamber is a 1-mm-high silicone seal. Two fixing clamps are fastened by bolts to keep the chamber liquid-protective. The distance from the center of the cell culture slide to rotation axis is 10 mm (Fig. 1b). After cells attach to the slide for 24 h, the chambers were fixed to the clinostat and the effect of simulated microgravity was achieved by rotation around the horizontal axis at 10 rpm for 24 h in an incubator at 37 °C (Fig. 1c). As controls, static cells were cultured in a chamber under the same conditions, but were not subjected to any rotation.
Fig. 1.
Photograph of the microgravity simulator device employed in the present study to simulate microgravity conditions. a The chamber was constructed mainly by a cell culture slide (25 × 75 mm, Permanox, NUNS, Rosklide, Denmark), a silica gel plate, a gas-permeable polystyrene membranes and a polycarbonate base. b Schematic of the cross section along the length direction of the flat chamber. c Photograph of the clinostat employed in the present study to model simulated microgravity
Trans-membrane migration assay
At the end of gravity or microgravity exposure, cells cultured in microscope slides were washed with PBS three times and digested by 0.25 % trypsin-0.02 % EDTA and then were collected through centrifugation at 1000 rpm for 5 min to perform trans-membrane migration assay by transwell chambers (8 µm, Millipore, Billerica, MA, USA) in 24-well plate. Approximate single cell suspension with 2 × 104 cells in 100 µL serum-free medium were added in upper well, and 600 µL DMEM medium with 1 % serum were added in the bottom well. Cells were allowed to migrate in a standard incubator (a humidified atmosphere of 5 % CO2 and 95 % air at 37 °C) for 6 h. Cells on the upper membrane of transwell chamber were removed with cotton-tipped swabs. The migrated cells were fixed and stained with 0.05 % crystal violet in PBS for 15 min. Chambers were gently washed with PBS to remove additional crystal violent and then were exposed at air condition to dry for overnight. Images were taken from four fields randomly selected from each chamber for counting migration cells. The number of cells in 4 fields in the same group was averaged as the number of migration cells.
Wound healing assay
BMSCs were inoculated on microscope slides as mentioned above. After 24 h, microscope slides with adhered cells were extracted from the culture chamber and a linear wound of about 200 µm was scratched in the BMSCs monolayer using a plastic cell scraper (Corning, NY, USA). Images of wounds were taken under an inverted optical microscopy before and after microgravity or gravity exposure. Wound closure rate measured by Image J software was quantified by the ratio of the closure area to the initial wound areas. The equation of calculation was: ×100 [%], where Wn represents the percentage of wound closure, An represents the residual wound area at the measuring time, and A0 represents the initial wound area.
Immunofluorescence staining assay
BMSCs were seeded on sterilized 22 mm glass slide and cultured in the same way as mentioned above. Cells were cultured under gravity or SMG for 24 h. To block the rock signal, the cells were incubated with Y-27632 (5 μM), an inhibitor of rock, for 30 min at 37 °C before SMG exposure. In gravity recovery experiment, the cells were subjected to SMG for 24 h and subsequently cultured in normal gravity for 24 h. After that, the cells were fixed with 4 % paraformaldehyde for 30 min, permeabilized with 0.5 % Triton X-100 for 20 min and blocked with 1 % BSA (Sigma) for 1 h. 1 mL 1: 200 diluted rhodamine-phalloidin (Sigma-Aldrich) was added to evenly cover slides at 4 °C overnight. Nuclei were stained with DAPI (Sigma-Aldrich) for 10 min at room temperature before taking images. Fluorescence images were taken by confocal laser scanning microscopy (Olympus, Tokyo, Japan).
Atomic force microscopy analysis
BMSCs were seeded on sterilized 24 mm glass slides and cultured in the same way as mentioned above. Young’s Modulus of single cell on a microscopy slide was measured by atomic force microscopy (JPK, Berlin, Germany). Soft silicon nitride quadratic pyramid tip (0.02 N/m) with angle 17.5° was used in this experiment. Single cell with normal morphology was identified under optical microscopy and then AFM cantilever probe was positioned on perinuclear regions of cells. Each location was mechanically probed with AFM and the force curve was obtained by measuring cantilever deflection at vertical z-position of cantilever as it approached and indented cells. Cantilever descended toward cell at a velocity of 2 µm/s until a trigger force of 2 nN was reached. Young’s modulus of cells was calculated by force-distance curves collected from AFM system.
Statistical analysis
All values are expressed as the mean ± standard deviation (SD). A minimum of three independent experiments was performed for each assay. Statistical analysis was performed using a two-sided unpaired Student’s t test between two groups. For multiple groups, the statistical significance of two groups was calculated using the Bonferroni post hoc test. A level of p < 0.05 was accepted as statistically significant.
Results
SMG remodels F-actin and inhibits the migration of BMSCs
Trans-well results showed that SMG suppressed the trans-membrane migration capacity of BMSCs exposure to SMG for 24 h (Fig. 2a). The results of wound healing also revealed the negative effect of SMG on migration capacity of BMSCs (Fig. 2c). The actin cytoskeleton is sensitive to microgravity. After culturing in SMG for 24 h, F-actin filament of BMSCs reorganized, showing a polymerized and thicker actin network (Fig. 2e). Cell stiffness plays a vital role in migration capacity, and facilitates migration behaviors requiring proper cell stiffness, which is characterized by Young’s modulus. Then we assessed the Young’s modulus of BMSCs with or without SMG exposure for 24 h and found that the Young’s modulus of BMSCs exposed to SMG was markedly increased (Fig. 2f). Taken together, we demonstrated the inhibitory effect of SMG on BMSCs migration, and abnormal reorganization and cell stiffness may limit the deformability of BMSCs and repress BMSCs migration.
Fig. 2.
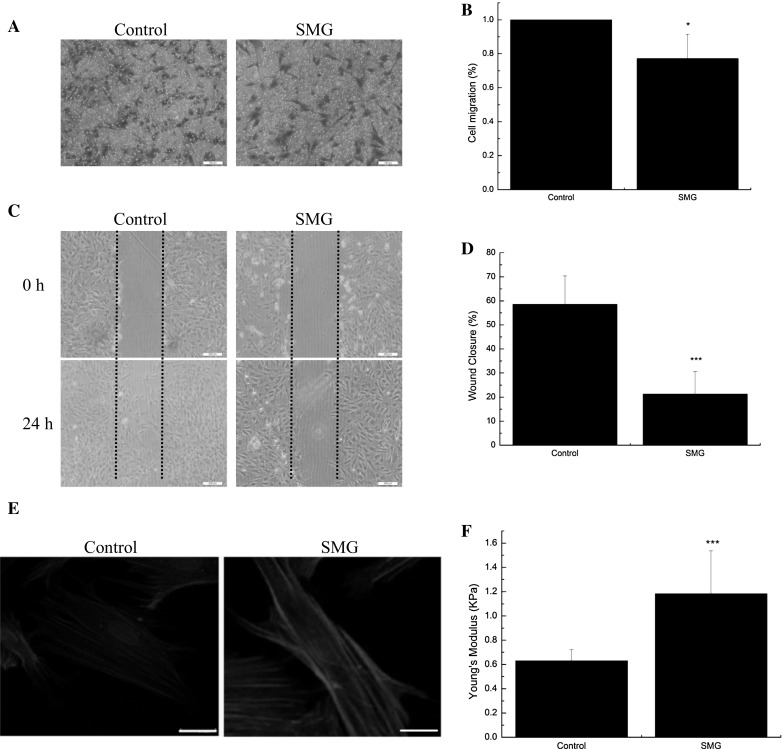
SMG inhibits the migration potentials of BMSCs and remodel F-actin. a Transwell experiments for the migration potentials of BMSCs after exposure to SMG for 24 h. b The results of statistical analysis. c Wound healing experiments for the migration potentials of BMSCs after exposure to SMG for 24 h. d The results of statistical analysis. e Fluorescence images of the F-actin cytoskeleton (red) and nucleus (blue) of BMSCs, cultured for 24 h under SMG. f Young’s modulus of BMSCs with or without SMG exposure for 24 h, n = 20 in each group. Bars in graph a, c and e are 200, 200 and 20 μm, respectively. The results are shown as the mean ± SD from three representative independent experiments. *p < 0.05 and ***p < 0.001. (Color figure online)
SMG inhibits migration of BMSCs through Rock-F-actin pathway
Rho-GTPases acts an important role in dynamics of actin cytoskeleton and they are probably very rapidly involved in the cell’s adaptation to microgravity-related condition. Thus, we tested whether GTPase proteins are involved in F-actin reorganization and migration of BMSCs under SMG. The F-actin staining and Young’s modulus results showed that Y-27632 obviously abrogated the reorganization of F-actin and increased cell stiffness caused by SMG (Fig. 3a, b). Moreover, there was a dramatic increase in migration capacity of BMSCs induced by Y-27632 under SMG (Fig. 3c, e). These results indicate that SMG inhibits migration capacity of BMSCs, primarily via the Rock-F-actin pathway.
Fig. 3.
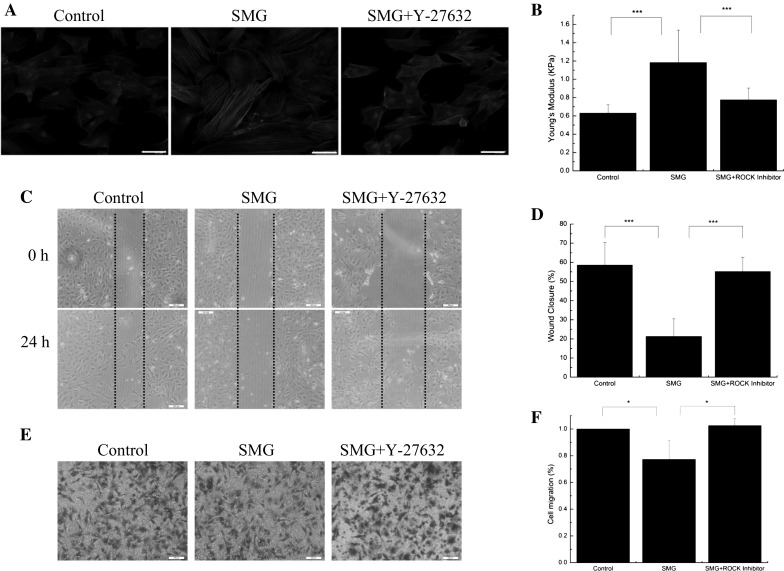
SMG inhibits the migration potentials of BMSCs via Rock-F-actin pathway. a Fluorescence images of the F-actin cytoskeleton (red) and nucleus (blue) of BMSCs, cultured for 24 h under SMG, with or without Y-27632 (5 μM). b Young’s modulus of BMSCs with or without SMG exposure for 24 h, n = 20 in each group. c Wound healing experiments for the migration potentials of BMSCs after exposure to SMG for 24 h. d The results of statistical analysis. e Transwell experiments for the migration potentials of BMSCs after exposure to SMG for 24 h. f The results of statistical analysis. Bars in graph a, b and c are 50, 200 and 200 μm, respectively. The results are shown as the mean ± SD from three representative independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001. (Color figure online)
Restoring gravity reversed abnormal F-actin cytoskeleton and cell stiffness
To further evaluate the role of gravity in the migration capacity and F-actin reorganization of BMSCs, we transferred BMSCs to normal gravity after exposure to SMG. Actin staining results revealed that normal gravity condition dramatically remodeled F-actin, which were thinner and contracted (Fig. 4a). Moreover, normal gravity also significantly reversed the increase in cell stiffness of BMSCs induced by SMG (Fig. 4b). However, we found that exposure to normal gravity for 24 h still could not recover the migration capacity of BMSCs, reflected by wound healing and transwell results (Fig. 4c, e). These results reconfirmed the influence of SMG on F-actin cytoskeleton and cell stiffness of BMSCs, and implied that SMG seriously inhibits migration potentials of BMSCs, even after restoring gravity condition.
Fig. 4.
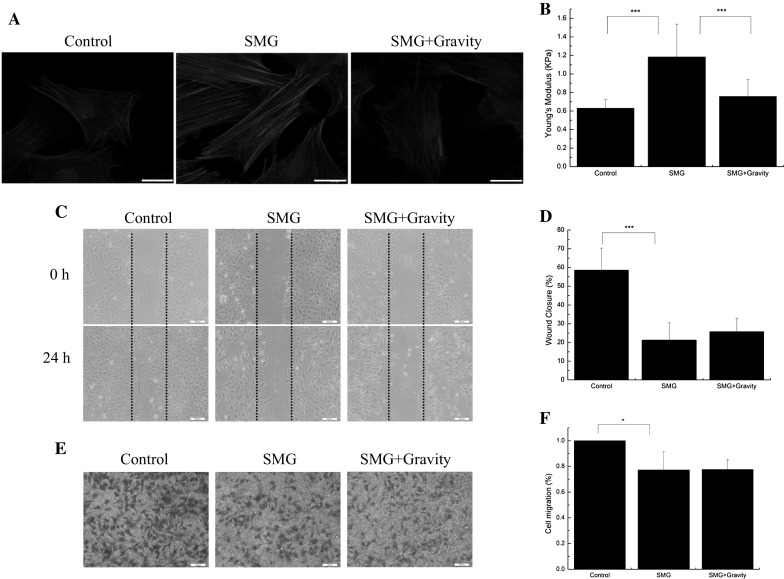
Restoring gravity reversed abnormal F-actin cytoskeleton and cell stiffness. a Fluorescence images of the F-actin cytoskeleton (red) and nucleus (blue) of BMSCs, cultured in SMG for 24 h, with or without restoring gravity for 24 h. b Young’s modulus of BMSCs, cultured in SMG for 24 h, with or without restoring gravity for 24 h, n = 20 in each group. c Wound healing experiments for the migration potentials of BMSCs. d The results of statistical analysis. e Transwell experiments for the migration potentials of BMSCs after exposure to SMG for 24 h. f The results of statistical analysis. Bars in graph a, b and c are 20, 200 and 200 μm, respectively. The results are shown as the mean ± SD from three representative independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001. (Color figure online)
Discussion
Increasing researches have confirmed that SMG had evident effects on cellular proliferation, apoptosis, migration and invasion, and gene expression (Jhala et al. 2014; Maier et al. 2015). Migration potentials are vital for BMSCs to repair damaged organisms or tissues (See et al. 2010; Li et al. 2015). However, it is not clear whether SMG has effects on migration capacity in BMSCs. In this study, we found that SMG for 24 h suppressed both wound healing and trans-membrane migration of BMSCs. Thus, SMG definitely has an inhibitory effect on the migration potentials of BMSCs. The effect of microgravity or simulated microgravity on cell migration is not consistent among different reports. The reduced cell migration induced by microgravity or simulated microgravity correlated with a decrease of F-actin (Plett et al. 2004; Li et al. 2009; Meloni et al. 2011), while extended expression of CXCR4 may mediate an increased BMSCs migration under simulated microgravity (Mitsuhara et al. 2013). Particularly, Siamwala et al. proposed that simulated microgravity perturbs actin polymerization to promote nitric oxide-associated migration of human Eahy926 cells (Siamwala et al. 2010). Some possible factors may result in these discrepant results, including the types of cells, time of exposure to microgravity, the methods to simulate microgravity, and culture parameters. In general, cell migration is regarded as continuing circles of four concurrent and relative independent processes, including formation of pseudopodia by assembling free G-actin, active interaction of cell protrusions with extracellular matrix, tension between leading and trailing edges enforced by contraction of actin filaments mediated by myosin II, and tight bonds at leading part of cell and release of adhesive bonds at the cell rear (Friedl et al. 2011). During cell migration, dynamics reorganization of cytoskeleton in specific sites drive the cell to contraction and/or rotation. Hence, relatively loose cytoskeleton is beneficial for cell migration, either through reducing cell stiffness to easily pass through small holes or through quick degradation and assembly of cytoskeleton to lead cells stretch to crawl forward (Friedl et al. 2011; Lange and Fabry 2013). In this study, we found that SMG markedly inhibited BMSCs migration, accompanied with the augmented F-actin cytoskeleton formation and abnormal cell stiffness. These results implied that abnormal F-actin reorganization and cell stiffness may result in decreased migration potentials of BMSCs. However, it has been reported that cells exposed to microgravity had depolymerized, extenuated, and dispersely distributed actin filaments (Dai et al. 2007). Actually, the response of actin cytoskeleton to microgravity is complex and time dependent, and the reorganization of actin under simulated microgravity at different time points is not similar. Meyers et al. found that BMSCs cultured in simulated microgravity modeled by RCCS exhibited cortical actin formation as early as 3 h after initiation of simulated microgravity. After 7 days of culture in simulated microgravity, F-actin filaments are completely absent (Meyers et al. 2005). Besides, Wang et al. found that F-actin filaments were thin and dispersed under simulated microgravity modeled by a 2-D clinostat for 72 h, but partial restoration of F-actin occurred under simulated microgravity at 120 h (Wang et al. 2014). The reorganization of actin cytoskeleton is regulated by multiple factors, most notably Rho family GTPases. Rho, Cdc42 and Rac are recognized as the most important regulators of actin assembly (Lee and Dominguez 2010). During SMG, the reorganization of actin cytoskeleton may be attributed to Rho, which is reported to act as a sensor for microgravity (Louis et al. 2015). To clarify the mechanism of SMG remodeling F-actin, we utilized Y-27632, an inhibitor of Rock (a downstream factor of Rho), and found that Y-27632 significantly reversed the reorganization of F-actin induced by SMG. The increased cell stiffness and inhibitory migration potentials of BMSCs were also reversed by Y-27632. We demonstrated that Rho-Rock had a significant influence on migration potentials of BMSCs, which may mediate the decreased migration capacity and F-actin reorganization under SMG.
To further evaluate the effect of gravity on migration potentials of BMSCs, we transferred BMSCs to normal gravity for 24 h. Although this action partially reversed the abnormal cell stiffness and reorganization of BMSCs induced by SMG, it could not rescue the decreased migration potentials of BMSCs caused by SMG. These results reconfirmed that SMG contributed to the reorganization of F-actin and increased cell stiffiness. However, the results also implied that other factors may mediate the inhibited migration capacity of BMSCs, independently of Rock-F-actin-cell stiffiness pathway.
In conclusion, our results firstly showed the inhibitory effect of SMG on migration potentials of BMSCs. This adverse effect is possibly due to the response of Rho to SMG, resulting in abnormal F-actin reorganization and cell stiffness. Interference of Rho pathway induced by Y-27632 rescued migration capacity of BMSCs, primarily through the Rock-F-actin-cell stiffness pathway. Thus, our results suggest that F-actin acts an important signaling mediator and a potential target to retain the migration potentials of BMSCs in SMG. Further study is required to detect the effect of microgravity on the migration potentials of BMSCs in vivo and evaluate the role of F-actin in this process.
Acknowledgments
We thank Prof. Long and Dr. Sun for crucial advice of the clinostat. This work was supported by Grants from the National Basic Research Program of China (2011CB710900), the Key Laboratory of Microgravity, Institute of Mechanics, Chinese Academy of Sciences (NML1516KFKT4-4), the National Natural Science Foundation of China (11272365, 11511140092, 11532004), the Fundamental Research Funds for the Central Universities (106112015CDJZR238807), the Research Fund for the Doctoral Program of Higher Education of China (20130191110029), and the Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (CQKLBST-2015-008).
References
- Callan-Jones AC, Voituriez R. Actin flows in cell migration: from locomotion and polarity to trajectories. Curr Opin Cell Biol. 2016;38:12–17. doi: 10.1016/j.ceb.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Dai ZQ, Wang R, Ling SK, Wan YM, Li YH. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif. 2007;40:671–684. doi: 10.1111/j.1365-2184.2007.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AM, Pineda AA, Winfree LM, Brown GT, Laukoetter MG, Nusrat A. Organized migration of epithelial cells requires control of adhesion and protrusion through Rho kinase effectors. Am J Physiol Gastrointest Liver Physiol. 2006;292:806–817. doi: 10.1152/ajpgi.00333.2006. [DOI] [PubMed] [Google Scholar]
- Jhala DV, Kale RK, Singh RP. Microgravity alters cancer growth and progression. Curr Cancer Drug Targets. 2014;14:394–406. doi: 10.2174/1568009614666140407113633. [DOI] [PubMed] [Google Scholar]
- Lange JR, Fabry B. Fabry: cell and tissue mechanics in cell migration. Exp Cell Res. 2013;319:2418–2423. doi: 10.1016/j.yexcr.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol Cells. 2010;29:311–325. doi: 10.1007/s10059-010-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang S, Chen J, Du T, Wang Y, Wang Z. Modeled microgravity causes changes in the cytoskeleton and focal adhesions, and decreases in migration in malignant human MCF-7 cells. Protoplasma. 2009;238:23–33. doi: 10.1007/s00709-009-0068-1. [DOI] [PubMed] [Google Scholar]
- Li J, Guo W, Xiong M, Han H, Chen J, Mao D, Tang B, Yu H, Zeng Y. Effect of SDF-1/CXCR4 axis on the migration of transplanted bone mesenchymal stem cells mobilized by erythropoietin toward lesion sites following spinal cord injury. Int J Mol Med. 2015;36:1205–1214. doi: 10.3892/ijmm.2015.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis F, Deroanne C, Nusgens B, Vico L, Guignandon A. RhoGTPases as key players in mammalian cell adaptation to microgravity. Biomed Res Int. 2015;2015:747693. doi: 10.1155/2015/747693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JA, Cialdai F, Monici M, Morbidelli L. The impact of microgravity and hypergravity on endothelial cells. Biomed Res Int. 2015;2015:434803. doi: 10.1155/2015/434803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni MA, Galleri G, Pani G, Saba A, Pippia P, Cogoli-Greuter M. Space flight affects motility and cytoskeletal structures in human monocyte cell line J-111. Cytoskeleton. 2011;68:125–137. doi: 10.1002/cm.20499. [DOI] [PubMed] [Google Scholar]
- Meyers VE, Zayzafoon M, Douglas JT, McDonald JM. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res. 2005;20:1858–1866. doi: 10.1359/JBMR.050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhara T, Takeda M, Yamaguchi S, Manabe T, Matsumoto M, Kawahara Y, Yuge L, Kurisu K. Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury. Stem Cell Res Ther. 2013;4:35–45. doi: 10.1186/scrt184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Lazo JS. Phosphatase of regenerating liver-1 promotes cell migration and invasion and regulates filamentous actin dynamics. J Pharmacol Exp Ther. 2010;334:627–633. doi: 10.1124/jpet.110.167809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen K, Tauber S, Dumrese C, Bradacs G, Simmet DM, Golz N, Hauschild S, Raig C, Engeli S, Gutewort A, Hurlimann E, Biskup J, Unverdorben F, Rieder G, Hofmanner D, Mutschler L, Krammer S, Buttron I, Philpot C, Huge A, Lier H, Barz I, Engelmann F, Layer LE, Thiel CS, Ullrich O. Regulation of ICAM-1 in cells of the monocyte/macrophage system in microgravity. Biomed Res Int. 2015;2015:538786. doi: 10.1155/2015/538786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett PA, Abonour R, Frankovitz S, Orschell CM. Impact of modeled microgravity on migration, differentiation, and cell cycle control of primitive human hematopoietic progenitor cells. Exp Hematol. 2004;32:773–781. doi: 10.1016/j.exphem.2004.03.014. [DOI] [PubMed] [Google Scholar]
- See EY, Toh SL, Goh JC. Multilineage potential of bone-marrow-derived mesenchymal stem cell cell sheets: implications for tissue engineering. Tissue Eng Part A. 2010;16:1421–1431. doi: 10.1089/ten.tea.2009.0501. [DOI] [PubMed] [Google Scholar]
- Siamwala JH, Reddy SH, Majumder S, Kolluru GK, Muley A, Sinha S, Chatterjee S. Simulated microgravity perturbs actin polymerization to promote nitric oxide-associated migration in human immortalized Eahy926 cells. Protoplasma. 2010;242:3–12. doi: 10.1007/s00709-010-0114-z. [DOI] [PubMed] [Google Scholar]
- Wang R, Bi J, Ampah KK, Ba X, Liu W, Zeng X. Lipid rafts control human melanoma cell migration by regulating focal adhesion disassembly. Biochim Biophys Acta. 2013;1833:3195–3205. doi: 10.1016/j.bbamcr.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Wang N, Wang H, Chen J, Zhang X, Xie J, Li Z, Ma J, Wang W, Wang Z. The simulated microgravity enhances multipotential differentiation capacity of bone marrow mesenchymal stem cells. Cytotechnology. 2014;66:119–131. doi: 10.1007/s10616-013-9544-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland M, Ma X, Braun M, Hauslage J, Hemmersbach R, Bauer J, Grosse J, Infanger M, Grimm D. The impact of altered gravity and vibration on endothelial cells during a parabolic flight. Cell Physiol Biochem. 2013;31:432–451. doi: 10.1159/000343380. [DOI] [PubMed] [Google Scholar]
- Yan M, Wang Y, Yang M, Liu Y, Qu B, Ye Z, Liang W, Sun X, Luo Z. The effects and mechanisms of clinorotation on proliferation and differentiation in bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2015;460:327–332. doi: 10.1016/j.bbrc.2015.03.034. [DOI] [PubMed] [Google Scholar]
- Zayzafoon M, Meyers VE, McDonald JM. Microgravity: the immune response and bone. Immunol Rev. 2005;208:267–280. doi: 10.1111/j.0105-2896.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li L, Chen J, Wang J. Behavior of stem cells under outer-space microgravity and ground-based microgravity simulation. Cell Biol Int. 2015;39:647–656. doi: 10.1002/cbin.10452. [DOI] [PubMed] [Google Scholar]
- Zhang B, Luo Q, Chen Z, Sun J, Xu B, Ju Y, Song G. Cyclic mechanical stretching promotes migration but inhibits invasion of rat bone marrow stromal cells. Stem Cell Res. 2015;14:155–164. doi: 10.1016/j.scr.2015.01.001. [DOI] [PubMed] [Google Scholar]