Abstract
Brazilian flora biodiversity has been widely investigated to identify effective and safe phytotherapeutic compounds. Among the investigated plant species, the Byrsonima genus exhibits promising biological activities. This study aimed at evaluating the cytotoxicity of B. correifolia, B. verbascifolia, B. fagifolia and B. intermedia extracts using different assays in two cell lines (primary gastric and HepG2 cells). The different extract concentrations effects on cell viability were assayed using the MTT, aquabluer, neutral red and LDH assays. Non-cytotoxic concentrations were selected to generate cell proliferation curves and to assess cell cycle kinetics by flow cytometry. Byrsonima extracts differentially affected cell viability depending on the metabolic cellular state and the biological parameter evaluated. B. fagifolia and B. intermedia extracts exhibited lower cytotoxic effects than B. correifolia and B. verbascifolia in all assays. The results obtained with LDH and flow cytometry assays were more reliable, suggesting that they can be useful in the screening for herbal medicine and to further characterize these extracts as phytotherapeutic compounds.
Keywords: Antioxidant, Byrsonima sp., Cytotoxicity, Cytostatic, Flow cytometry, Herbal medicine
Introduction
Plants are an important source of natural active compounds, many of which can be used as models for the new drugs synthesis, because they exhibit an almost inexhaustible range of diversity structure and physical–chemical and biological properties. Approximately 25 % of drugs prescribed worldwide are estimated to be derived from plants (Rates 2001). More than 50 % of the drugs that were approved over the last 30 years come directly or indirectly from natural products. In the cancer area, out of the 175 molecular drugs introduced between 1940s and 2010, 85 are natural products or derived from natural products (Newman and Cragg 2012).
Brazilian flora biodiversity has been widely investigated with respect to its therapeutic potential and has led to the identification of new phytotherapeutic compounds with demonstrated efficacy (Brasil 2006). Among the promising plant species, those belonging to the Byrsonima genus are noteworthy. This genus belongs to the family Malpighiaceae and contains approximately 150 species widely distributed throughout tropical America. Brazil is home to approximately 50 % of the Byrsonima species, which are found primarily in the North, Northeast and Central regions but may also be found in the Southeast region of the country, in “cerrado” areas (Brazilian savanna). In Brazil, these species are popularly known as “muricis” and, depending on the color of their flowers and fruits or place of occurrence, are differentiated as “murici da várzea”, “murici da mata”, or “murici-amarelo”, among others (revised by Guilhon-Simplicio and Pereira 2011).
Some Byrsonima species had already been characterized phytochemically such as Byrsonima verbascifolia (Dosseh et al. 1980), B. intermedia (Sannomiya et al. 2007) and B. fagifolia (Lima et al. 2008; Sannomiya et al. 2007a, b). All of them contain several constituents in common, like amentoflavone, (+)-catechin, (−)-epicatechin, and quercetin derivatives, but the amount present of these compounds differs (Sannomiya et al. 2005a, b, c; Rinaldo et al. 2010).
Some species exhibit high nutritional value and can be consumed as raw fruits or in the form of jams, sweets or ice cream. Furthermore, ethnopharmacological studies have demonstrated the popular use of Byrsonima plants for the treatment of diarrhea, fever, asthma, and skin infections (Guilhon-Simplicio and Pereira 2011). Research has confirmed the biological activities of these plants, especially with respect to topical and systemic anti-inflammatory activity (Maldini et al. 2009; Moreira et al. 2011), antioxidant (Pereira et al. 2015) and antimicrobial activities (Michelin et al. 2008).
Lima et al. (2008) and Santos et al. (2012), studying, respectively, B. fagifolia and B. intermedia extracts, observed gastroprotective and antidiarrheal activities, supporting the traditional use of this genus to treat gastrointestinal disorders. B. verbascifolia was identified as an antiviral agent (Cecilio et al. 2012). Data compiled from biological activities of Byrsonima species showed an incipient number of studies about B. correifolia.
Espanha et al. (2014) demonstrated that B. correifolia, B. verbascifolia, B. fagifolia and B. intermedia exhibit no mutagenic properties using the Ames test. Moreover, the authors have observed antimutagenic activity of all standardized extracts from these species. However, there are no data concerning the influence of these extracts on cell viability or any determination of their non-cytotoxic concentrations in human cell lines for the treatment of different diseases.
According to Weyermann et al. (2005) it is not advisable to conclude about cytotoxicity of a compound on the results obtained from only one method. Therefore, the aim of this study was to evaluate, using several assays, cell viability and non-cytotoxic concentrations of four extracts from different species of Byrsonima, B. correifolia, B. verbascifolia, B. fagifolia and B. intermedia and choose the most appropriate test to determine the cytotoxicity, measure the non-cytotoxic concentrations of extracts as a precondition for the use of these species as phytotherapeutic compounds and to evaluate their new biological activities.
Materials and methods
Plant material and extraction
The leaves of each species were collected in different locations in Brazil: B. correifolia A. Juss. (Voucher specimen number: 27151, deposited in Graziella Barroso Herbarium, at Federal University of Piauí) in Piauí (Northern region); B. verbascifolia (L.) DC. (Voucher specimen number: 481, deposited in Herbarium of the Federal University of Tocantins), B. fagifolia Nied. (Voucher specimen number: 743, deposited in Herbarium of the Federal University of Tocantins) in Tocantins (Midwest region) and B. intermedia A. Juss. (Voucher specimen number: 1426, deposited in UNICAMP Herbarium, in São Paulo (Southeast region)). The extraction process and preparation was the same as that described by Espanha et al. (2014).
Cell lines and cell culture
Two human cell lines were employed: primary normal gastric epithelium (GAS) and hepatocellular carcinoma (HepG2). The first cell line was provided by Dr. Rommel Rodríguez Burbano from the Federal University of Pará (UFPA) and was cultivated in Dulbecco’s Modified Eagle’s Medium (DMEM—Gibco, Grand Island, NY, USA) high glucose, supplemented with 10 % fetal bovine serum (FBS—CAS: 12657-029, Gibco) and with 0.06 g/L penicillin (CAS: 113-98-4, Sigma-Aldrich, St. Louis, MO, USA), 0.10 g/L streptomycin (CAS: 3810-74-0, Sigma-Aldrich) and 0.024 % sodium bicarbonate (CAS: 1444-55-8, Sigma-Aldrich). The second cell line was provided by Dra. Lusânia Maria Greggi Antunes from the Faculdade de Ciências Farmacêuticas—Universidade de São Paulo and was cultivated in DMEM supplemented with 15 % FBS, antibiotic–antimycotic solution (CAS: 15240-062, Gibco) and sodium bicarbonate. Cultures were maintained at 37 °C in a 5 % CO2 atmosphere and 95 % relative humidity.
Chemical agents
Benzo[a]pyrene—B[a]P (3,4-Benzo[a]pyrene—CAS: 50-32-8, Sigma-Aldrich), is an environmental pollutant carcinogen used in experimental models for cancer research (SIGMA-ALDRICH 2012). B[a]P is a polycyclic aromatic hydrocarbon (PAH) and was used as a DNA damage inducer at a concentration of 5.04 µg/mL. The B[a]P metabolites generated by the CYP1A1 enzyme acts as DNA intercalating agents and causes bases dissociation leading to the apurinic sites generation in the nucleic acid molecule (Uno et al. 2006).
Doxorubicin (DXR) (Adriamycin—CAS: 23214-92-8, Sigma-Aldrich) acts as a DNA intercalating agent in mammalian cells. DXR blocks DNA and RNA synthesis, inhibits DNA topoisomerase II and has clastogenic and aneugenic effects, causing single-strand breaks and membranes damage (Dhawan et al. 2003). This drug was used at a final concentration of 0.2 µg/mL.
Cytotoxicity assays
The four cytotoxicity assays used in this study were as follows: (1) MTT; (2) neutral red; (3) lactate dehydrogenase and (4) aquabluer.
Before treatments, the cells were sub-cultivated, washed with phosphate buffered saline (PBS) (pH 7.4) and trypsinized (trypsin 0.05 %—CAS: 25300-054—Gibco).
For all cytotoxicity assays, 1.0 × 104 HepG2 or GAS cells were seeded in 96-well culture plates 24 h prior to the treatments. After this period, the culture medium was removed and the cells were washed with PBS. Subsequently, in each well 200 µL of serum-free medium was added containing 1 of 10 different concentrations of plant extracts based on the maximal dilution obtained in the solvent (SV—7 H2O: 3 MeOH). The final concentrations of the extracts in the cell cultures were 0.08; 0.15; 0.31; 0.61; 1.2; 2.4; 4.8; 9.75; 19.5 and 39 µg/mL for B. correifolia, and 0.15; 0.31; 0.61; 1.2; 2.4; 4.8; 9.75; 19.5; 39 and 78 µg/mL for B. verbascifolia, B. fagifolia and B. intermedia. These concentrations were obtained diluting the extracts in a stock solution of solvent concentrated 100×. From this, an aliquot was used to treat cells in a final methanol concentration in culture of 0.3 %. Cells treated only with PBS were used as negative controls, and another group was exposed to extract solvent (SV) for 24 h in order to prove that the solvent used is not cytotoxic. The EC50 was calculated using the MTT and neutral red assays to determine the dose-dependent effect on cell lethality using the software CalcuSyn (Biosoft 1996–2005). Three non-cytotoxic concentrations were chosen for the cell proliferation and flow cytometry analyses. All experiments were performed in triplicate (three cultures/treatment).
MTT assay
The MTT assay described by Mosmann (1983) has been widely used as a measure of cytotoxicity and cell viability after the exposure of living cells to toxic substances. The mitochondrial capacity and integrity are related to cell viability (Bernhard et al. 2003). In this assay, the tetrazolium salt (-3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide—CAS: 298-93-1, Sigma-Aldrich) is yellow in color and is easily incorporated by viable cells and reduced by the mitochondrial enzyme succinate dehydrogenase to the formazan compound, which is purple in color.
The technique was performed as described by Serpeloni et al. (2015) and Ribeiro et al. (2016). The results are expressed as the percentage of viable cells concerning the negative control group (PBS), which was considered to represent 100 % viability. Finally, the viability was obtained using the following formula:
AquaBluer
The cell viability was also assessed using the aquabluer assay (also known as Alamar Blue). This assay monitors the reducing environment of the living cell. Viable cells with active metabolism can reduce resazurin into the resorufin product that is pink and fluorescent (Rampersad 2012). The assay was carried out according to the manufacturer’s recommendations (MultiTarget Pharmaceuticals LLC, Salt Lake City, UT, USA). The first step was to seed 1.0 × 103 of HepG2 cells in 96-well culture plates 24 h prior of the treatments. After this period, the culture medium was removed and the cells were washed with phosphate buffer saline (PBS). Subsequently, in each well 200 µL of complete medium was added containing the same concentrations of plant extracts described in cytotoxicity assays section. Cells treated only with PBS were used as negative controls, and another group was exposed to extract solvent (SV) for 24 h. The plates were read in a fluorescence plate reader with intensity of 540ex/590em. The results are expressed as the percentage of viable cells with respect to the negative control group (PBS), which was considered to represent 100 % of viability; the cell viability was obtained using the same formula shown in “MTT assay” section.
Neutral red
The cell viability assessment using the neutral red assay was performed according to Repetto et al. (2008) with some modifications. The neutral red assay is based on the ability of viable cells to bind and incorporate the supravital dye neutral red (CAS: 553-24-2—Sigma-Aldrich).
This dye is weakly cationic and penetrates cell membranes by passive diffusion. The dye accumulates intracellularly in lysosomes, where it binds to anionic and/or phosphate groups of the lysosomal matrix. The quantity of dye incorporated into cells is measured by spectrometry and is directly proportional to the cell number with intact membranes.
After treatment, 100 µL of neutral red solution (33 μg/mL of neutral red powder diluted in PBS) was added per well, and the cells were incubated for 3 h at 37 °C. Subsequently, 0.2 mL of 1 % acetic acid in 50 % ethanol was added to each well, and the absorbance was read at 540 nm in a spectrophotometer.
The results are expressed as a percentage of viable cells concerning the negative control group (PBS), which was considered to exhibit 100 % viability; the cell viability was obtained using the same formula as shown in “MTT assay” section.
Lactate dehydrogenase assay
Cytosolic lactate dehydrogenase (LDH—CAS: 86-2-30—Labtest, Lagoa Santa, MG, BR) catalyzes the conversion of pyruvate to lactate in the presence of NADH. Cells with damaged membranes release the LDH enzyme into the extracellular medium. The increase in absorbance at 340 nm due to the oxidation of NADH is proportional to LDH activity in the sample. To determine total LDH activity, cells from the positive control group were treated with 1 % Triton 100×. After treatment, samples were analyzed using a commercial LDH kit following the manufacturer’s recommendations, and LDH activity was expressed as U/mL.
Cell proliferation curves
Cell growth curves were generated by seeding 2.5 × 104 HepG2 or GAS cells per well in 24-well culture plate in a final volume of 2 mL of complete culture medium. Three concentrations of each extract were tested, and positive controls (B[a]P—5.04 µg/mL for HepG2 cells and DXR—0.2 µg/mL for GAS cells), a negative control (PBS) and a solvent control (SV—0.3 % of methanol in culture) were included. All treatments were performed with independent cultures in triplicate. The different concentrations of each extract were evaluated at 24, 48, 72, 96 and 120 h of exposure. After each exposure time, cells were trypsinized, and 20 µL was collected to count the number of cells.
A proliferation curve based on the determination of the total protein content was also performed using the technique proposed by Da Costa Lopes et al. (2000), where the proteins were dyed with 1 % bromophenol blue. The steps were the same as performed by studies of Cilião et al. (2015) and Serpeloni et al. (2015).
Flow cytometry
The cell cycle analysis was performed as described by Ribeiro et al. (2016) and Serpeloni et al. (2015), 3.0 × 105 cells were seeded in complete culture medium in a final volume of 2.5 mL in 12-well plates and were treated according to the protocol described before. Control groups were also included (positive DXR 0.2 µg/mL for GAS cells and B[a]P 5.04 µg/mL for HepG2 cells), negative (PBS) and solvent (SV).
At the time of harvest, the medium was removed and reserved, and the cells were washed with PBS, trypsinized and resuspended with the medium previously reserved. Cell suspensions were centrifuged at 700g for 5 min. At this time, the supernatant was removed, and the pellet was washed with PBS, following further centrifugation. Subsequently, PBS was removed, and the pellet was resuspended in 2 mL of cold 70 % ethanol and kept at −20 °C until analysis.
Before reading, the sample was stored at −20 °C and it was centrifuged (700g, 4 °C for 5 min), and 70 % ethanol was replaced by 1 mL of ice cold PBS, followed by additional centrifugation. After centrifugation the PBS was removed, and the cells were resuspended in 200 μL of propidium iodide solution (10 mL PBS: 10 μL Triton: RNase 100 μL: 40 μL propidium iodide) and kept at room temperature for 30 min. 5000 cells were analyzed using a FACSVantage (Becton–Dickinson, Franklin Lakes, NJ, USA) flow cytometer. Data were analyzed using Cell Quest software (Becton–Dickinson), and cell cycle profiles were designed using the Flow Jo software (Tree Star Incorporation, Ashland, OR, USA). The results are expressed as the percentage of cells in different phases of the cell cycle: subG1 (cell death), G1, S and G2/M.
Data analysis
All results were evaluated using the GraphPad Prism 6® software system (La Jolla, CA, USA). Statistical analyses were conducted using one-way variance analysis (ANOVA). The samples homogeneity was tested using Bartlett’s test, followed by Tukey’s test. All data are presented as the mean ± SD, with a significance level of p ≤ 0.05.
Results
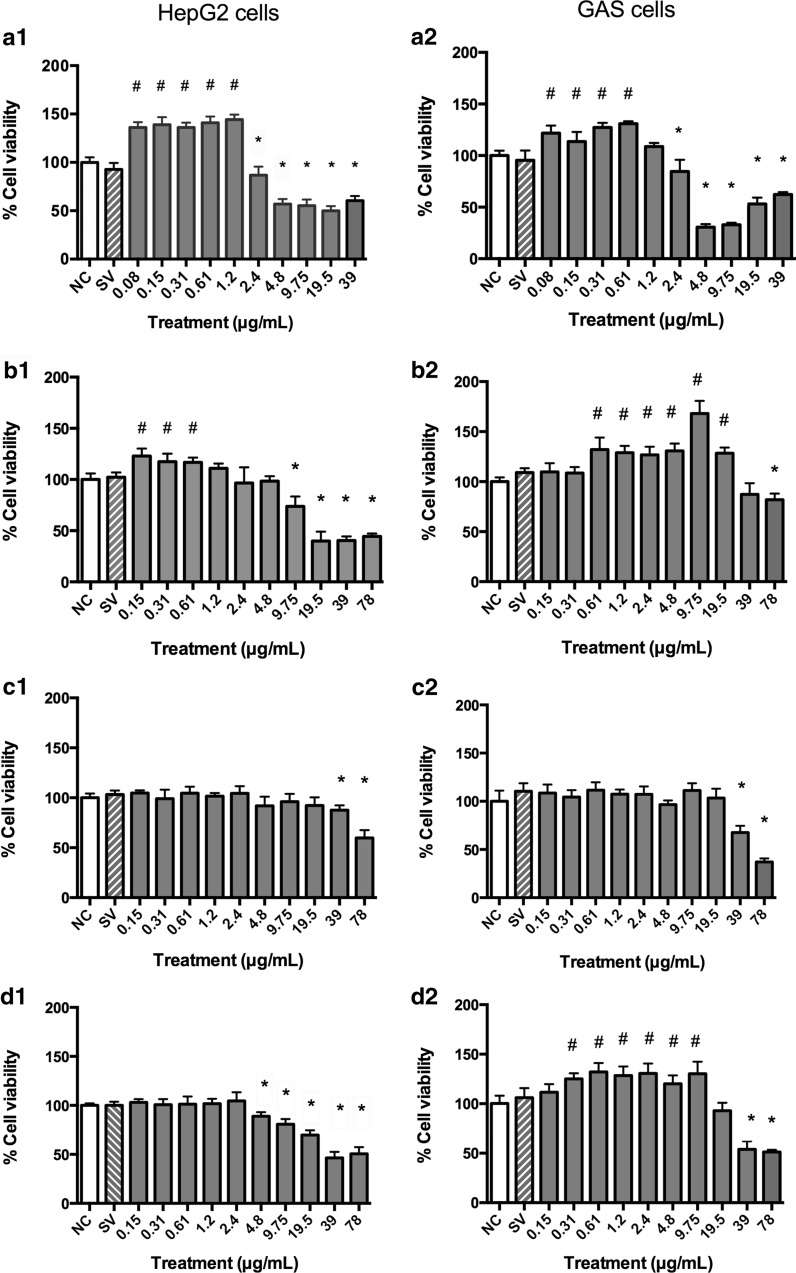
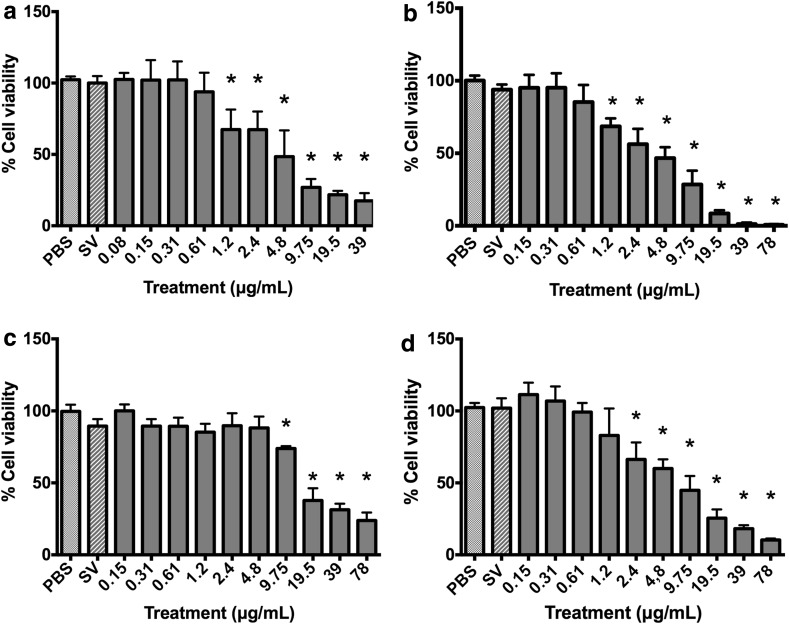
In the MTT assay, the B. correifolia, B. verbascifolia and B. intermedia species exhibited similar characteristics. The lowest concentrations of these extracts resulted in an increase in the viability rate to both cell lines analyzed (Fig. 1). This result prompted us to conduct the following experiments: (1) extracts incubation with tetrazolium salt in the cells absence; (2) cells incubation with extracts for 23 h followed by washing and lysis for 1 h and (3) use of the aqua bluer assay in HepG2 cells to assess if the redox state of the cells was responsible for the apparent cell viability increase (Fig. 2).
Fig. 1.
Percentage of viable HepG2 (1) and GAS (2) cells after 24 h treatment with different concentrations of extracts (MTT assay): a B. correifolia; b B. verbascifolia; c B. fagifolia; and d B. intermedia; negative control—NC (PBS) and solvent (SV) groups. The results represent mean ± SD of independent cultures in triplicate. NC represents 100 % viability. PBS phosphate buffered saline. Values significantly differing from the negative control group by ANOVA and Tukey test: hash symbol increase and asterisk decrease
Fig. 2.
Evaluation of viability of HepG2 cells treated with a B. correifolia; b B. verbascifolia; c B. fagifolia; and d B. intermedia; negative control—NC (PBS) and solvent (SV) in aquabluer assay. The results represent mean ± SD of independent cultures. NC represents 100 % viability. Asterisk values differ sigificantly from the negative control group by the ANOVA and Tukey test (p ≤ 0.05)
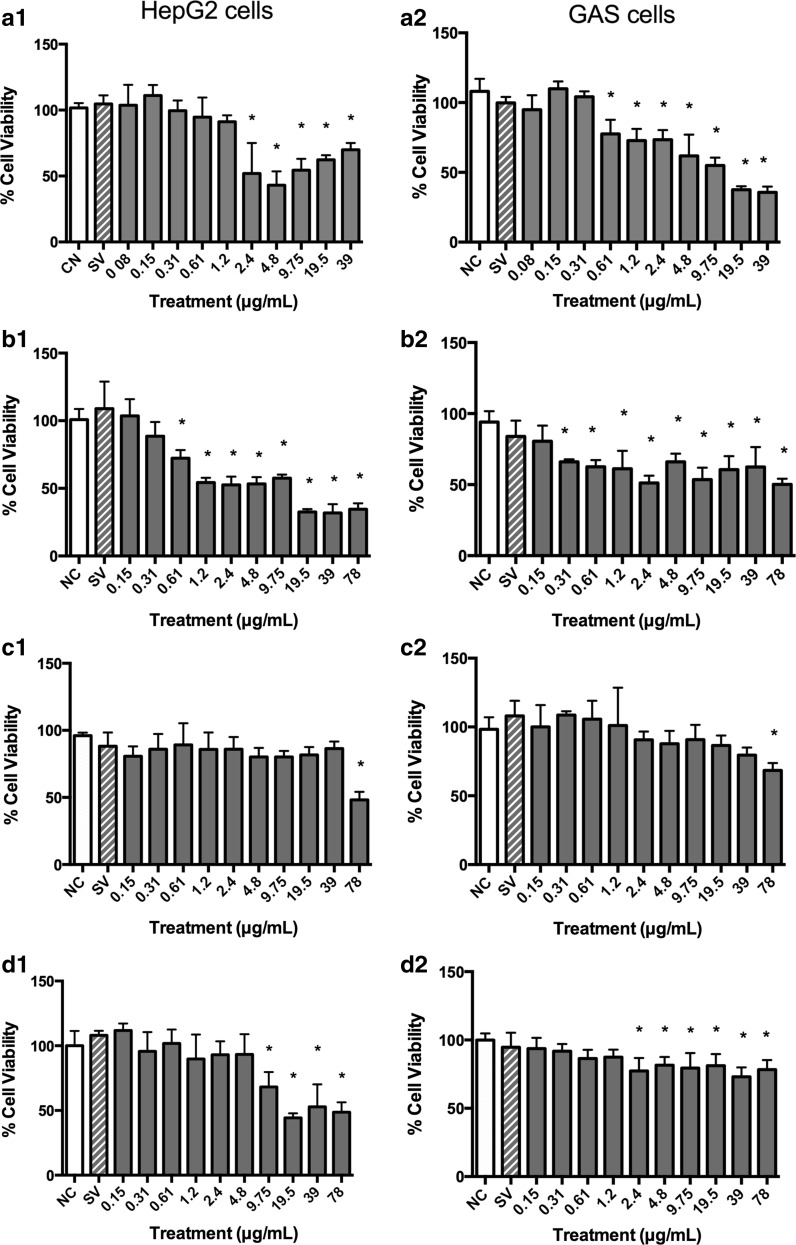
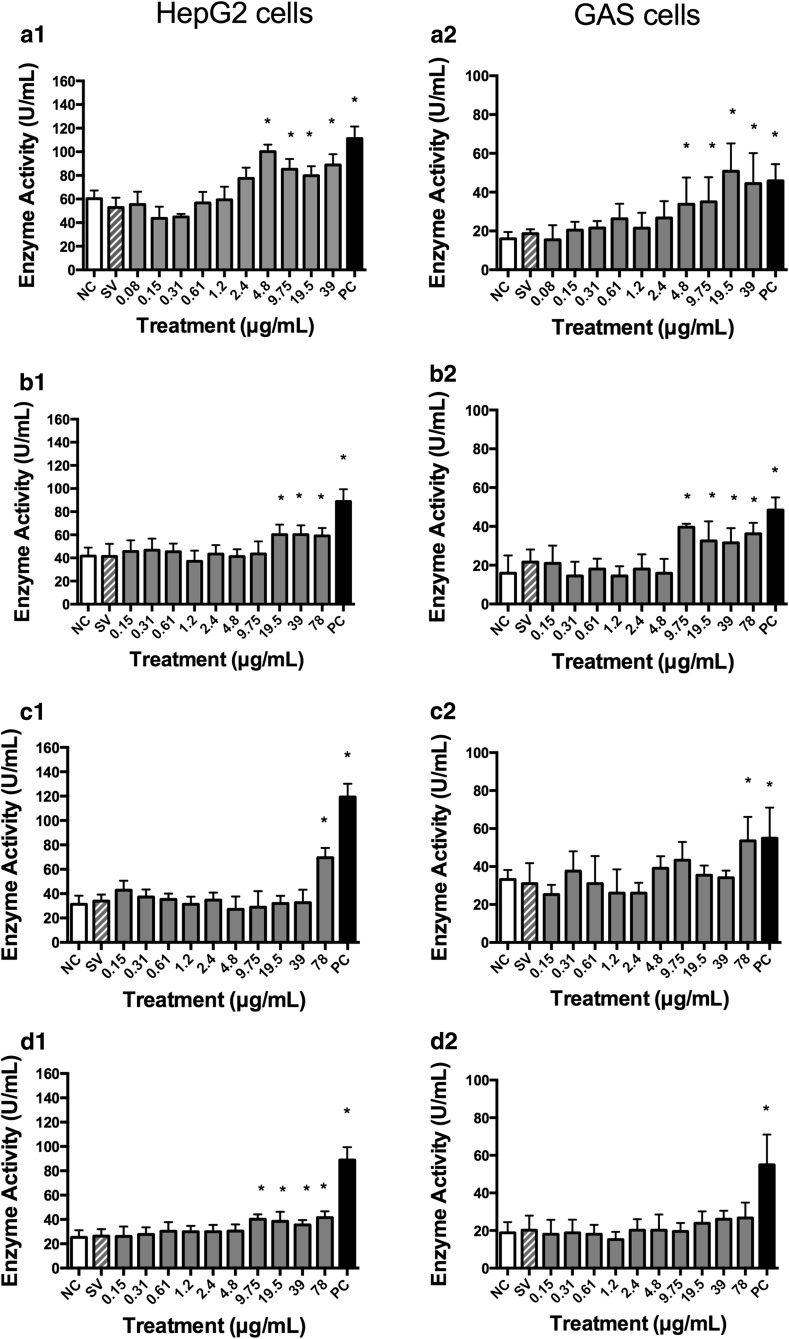
Concentrations equal to or greater than 1.2, 4.8 and 9.75 µg/mL of B. correifolia, B. verbascifolia and B. intermedia, respectively, reacted with the tetrazolium salt in absence of cells increasing the absorbance (data not showed) and the cellular lysates demonstrated that MTT reacted with B. correifolia, B. verbascifolia and B. intermedia at concentrations equal to and greater than 4.8; 19.5 and 78 µg/mL in HepG2 cells and 9.75; 39 and 78 µg/mL in GAS cells, respectively, also increasing the cell viability (data not showed). However, this increase in the cell viability at lower concentrations was not observed when the aquabluer assay was used (Fig. 2). Likewise, when the neutral red (Fig. 3) and LDH (Fig. 4) assays were used in both cell lines, no increase in cell viability was observed. These data show that the MTT assay is not recommended, at least not alone, to evaluate the cytotoxicity of plant extracts.
Fig. 3.
Cell viability of HepG2 (1) and GAS (2) cells 24 h after treatment with different extracts concentrations (Neutral Red assay): a B. correifolia; b B. verbascifolia; c B. fagifolia; and d B. intermedia; negative control—NC (PBS) and solvent (SV). The results represent mean ± SD of independent cultures in triplicate. NC represents 100 % viability. Asterisk values differ significantly from the negative control group by the ANOVA and Tukey test (p ≤ 0.05)
Fig. 4.
Lactate dehydrogenase enzyme activity of HepG2 (1) and GAS (2) cells after 24 h treatment with different concentrations of extracts (LDH assay): a B. correifolia; b B. verbascifolia; c B. fagifolia; d B. intermedia; negative control—NC (PBS); positive control—PC (1 % Triton 100×) and solvent (SV) groups. The results represent mean values ± SD of independent cultures in triplicate. Asterisk values differ significantly from the negative control group by ANOVA and Tukey test (p ≤ 0.05)
Byrsonimacorreifolia
The aquabluer assay results showed that the B. correifolia concentrations equal or greater than 1.2 µg/mL in HepG2 were cytotoxic (Fig. 2a). In the neutral red assay, concentrations equal to or greater than 2.4 or 0.61 µg/mL in HepG2 and GAS cells, respectively, resulted in cytotoxicity (Fig. 3a1, a2). In the LDH assay (Fig. 4) the cytotoxic concentrations were equal or higher than 4.8 µg/mL for both cell lines (Fig. 4a2). It is possible to note that regardless of the assay performed, the highest concentrations of the extracts were always cytotoxic.
The EC50 values calculated using neutral red assay data were 11.44 µg/mL (r = 0.721) and 16.11 µg/mL (r = 0.952) to HepG2 and GAS cells, respectively.
Concentrations of 0.08, 0.31 and 1.2 µg/mL were selected based on the results of the three cell viability assays described above for the evaluation of cell proliferation curves and flow cytometry analysis.
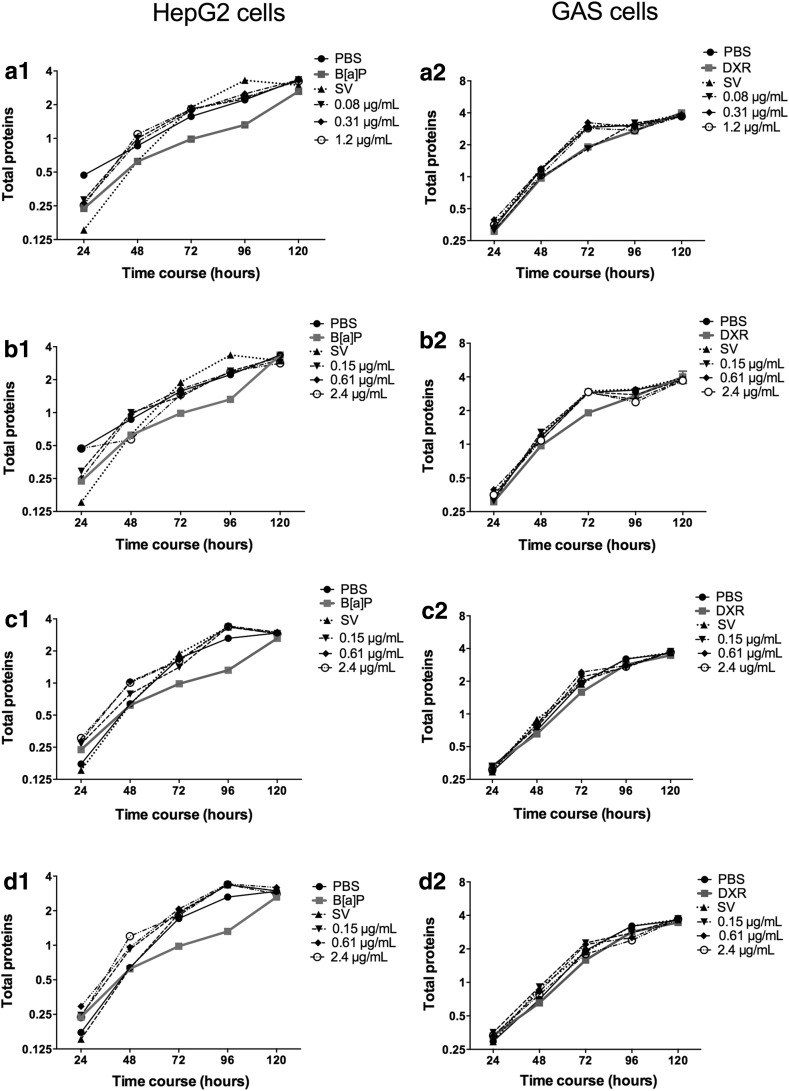
The proliferation curve results demonstrated that the three concentrations did not affect the cell viability or cell proliferation of either cell line even after 120 h of treatment (Fig. 5a).
Fig. 5.
Proliferation curves of HepG2 (1) and GAS (2) cells by total proteins quantification (log2 mean of absorbance) after treatment with extracts of a B. correifolia; b B. verbascifolia; c B. fagifolia; d B. intermedia; negative control (PBS); positive controls (B[a]P—5.04 µg/mL and DXR—0.2 µg/mL) and solvent (SV). The results represent mean ± SD of independent cultures in triplicate
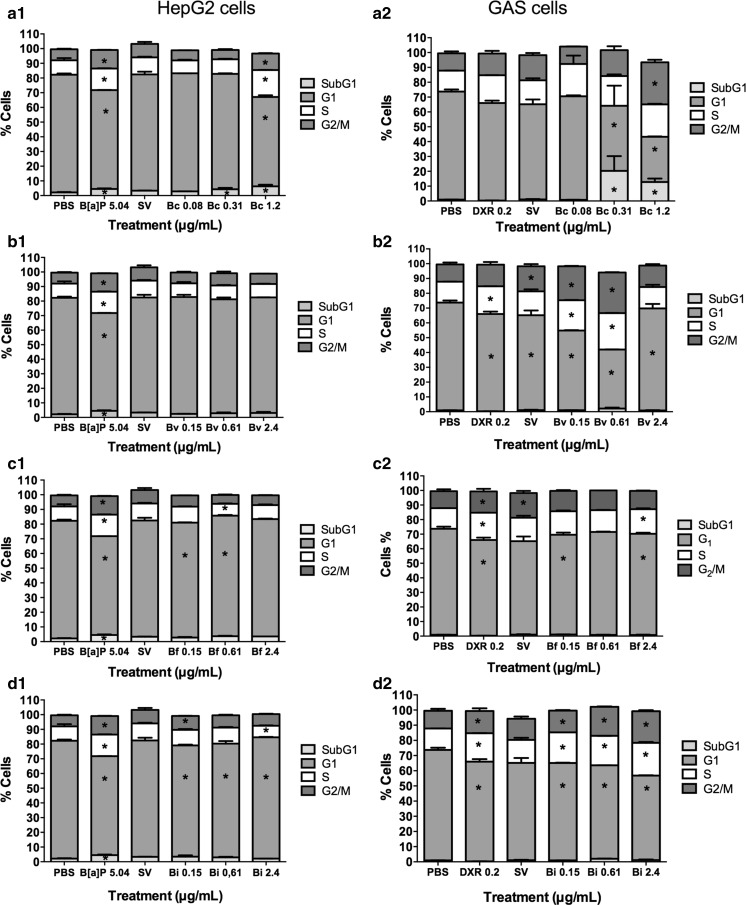
The cell cycle analysis in HepG2 cells (Fig. 6a1) revealed a decrease in the cell number in the G1 phase of the cell cycle at concentrations of 0.08 and 1.2 µg/mL relative to the negative control group. An increase in the cells number in the subG1 phase was also observed in the 0.31 and 1.2 µg/mL treatments. In GAS cells, treatment with 0.08 µg/mL resulted in no change in cell cycle distribution relative to the negative control (Fig. 6a2), whereas the concentrations of 0.31 and 1.2 µg/mL induced an increase in the cell number in subG1 and decrease in the cell number in G1. Furthermore, the 1.2 µg/mL concentration increased the cell number in G2 (Fig. 6a2).
Fig. 6.
Percentage of HepG2 (1) and GAS (2) cells in different phases of the cell cycle after treatment with extracts of a B. correifolia—Bc; b B. verbascifolia—Bv, c B. fagifolia—Bf; d B. intermedia—Bi; negative (PBS); positive (B[a]P—5.04 µg/mL or DXR 0.2 μg/mL) and solvent (SV) control groups. The cultures were exposed to the compounds for 24 h. The results represent mean values ± SD of independent cultures in triplicate. Asterisk values differ significantly from the negative control group as analyzed by the ANOVA and Tukey test
Byrsonimaverbascifolia
HepG2 and GAS cells treated with different concentrations of the B. verbascifolia extract exhibited different responses to the tests performed.
In aquabluer assay it is possible to note that the concentrations of B. verbascifolia equal or greater than 1.2 µg/mL presented cytotoxicity to HepG2 cells (Fig. 2b). In the neutral red assay (Fig. 3), a decrease in cell viability was observed at all concentrations, except for 0.15 and 0.31 µg/mL in HepG2 cells (Fig. 3b1) and 0.15 µg/mL in GAS cells (Fig. 3b2). However, in the LDH assay (Fig. 4), concentrations between 19.5 and 78 µg/mL (Fig. 4b1, b2) were cytotoxic for both cell lines, and 9.75 was also cytotoxic to GAS cells.
The EC50 values were calculated for HepG2 cells (5.41 µg/mL; r = 0.918) and for GAS cells (342.12 µg/mL; r = 0.73) using the neutral red assay.
Based on aquabluer, neutral red and LDH tests, the concentrations of 0.15; 0.61 and 2.4 µg/mL were selected for the evaluation of cell proliferation curves and flow cytometry. Through both counting cell numbers and measuring protein levels (Fig. 5b1, b2), we determined that HepG2 and GAS exhibited the same profile of proliferation in response to treatment.
The flow cytometry data of the HepG2 cells demonstrated that none of the concentrations interfered with the cell cycle distribution relative to the negative control (Fig. 6b1). Nevertheless, in GAS cells (Fig. 6b2), all concentrations reduced the cell number in the G1 phase, whereas the concentrations 0.15 and 0.61 µg/mL increased the cell number in the S and G2/M phases.
Byrsonimafagifolia
The aquabluer results showed that the B. fagifolia concentrations equal or greater than 9.75 µg/mL decreased HepG2 cells viability (Fig. 2c).
In the neutral red assay (Fig. 3c) and LDH assay (Fig. 4c), only the highest concentration tested (78 µg/mL) was cytotoxic for both cell lines. This extract exhibited low cytotoxicity in both cell lines, with EC50 values higher than the concentrations tested in neutral red assay (data not shown).
The concentrations of 0.15, 0.61 and 2.4 µg/mL were selected for the evaluation of cell proliferation curves and flow cytometry analysis. As with other extracts, the three concentrations of B. fagifolia evaluated using cell growth curves had no effect on the proliferation rate of either cell line tested (Fig. 5c).
The flow cytometry data (Fig. 6) revealed that the lowest concentration (0.15 µg/mL) decreased the number of HepG2 cells in the G1 phase, and the concentration of 0.61 µg/mL increased the cell number in the G1 and S phases relative to the negative control group (Fig. 6c1). With respect to GAS cell, at lower (0.15 µg/mL) and higher (2.4 µg/mL) concentrations, a decrease in the cell number in the G1 phase was observed, and an increase in the cell number in S phase was observed at higher concentrations (Fig. 6c2).
Byrsonimaintermedia
The results of aquabluer assay demonstrated that the concentrations of B. intermedia equal or greater than 2.4 µg/mL reduced the HepG2 cell viability (Fig. 2d).
In the neutral red assay (Fig. 3), concentrations between 9.75 and 78 µg/mL were cytotoxic to HepG2 cells, and concentrations between 2.4 and 78 µg/mL were cytotoxic to GAS cells (Fig. 3d1, d2). In the LDH assay (Fig. 4), concentrations between 9.75 and 78 µg/mL were cytotoxic in HepG2 cells (Fig. 4d1), and none of the concentrations were cytotoxic in GAS cells (Fig. 4d2). The EC50 values in the neutral red assay were 44.27 µg/mL (r = 0.913) for HepG2 cells and for GAS cells, the EC50 value fell outside the range of concentrations tested (data not shown). These data demonstrate different responses between the cell lines evaluated.
Concentrations of 0.15, 0.61 and 2.4 µg/mL were selected for cell proliferation curves evaluation (Fig. 5) and flow cytometry (Fig. 6). The proliferation curves data indicated treatment of HepG2 and GAS cells with the extract had no effect on the proliferation rate relative to untreated cells (Fig. 5d1, d2).
However, the cell cycle analysis for HepG2 cells (Fig. 6d1) revealed that 0.15 µg/mL extract reduced the cells number in the G1 phase and increased the cell number in the G2/M phase. The concentration of 0.61 µg/mL decreased the cell number in the G1 phase, and the highest concentration increased the cell number in the G1 phase and decreased the cell number in S phase (Fig. 6d1).
The three concentrations similarly interfered in the cell distribution of GAS cells by reducing the cell number in the G1 phase and increasing the cell number in the S and G2/M phases (Fig. 6d2).
Discussion
In Brazil, the Byrsonima genus is widely used in folk medicine for the treatment of many diseases (Guilhon-Simplicio and Pereira 2011). Species of this genus possess different pharmacological activities, which may be related to the presence of chemical compounds such as catechins and derivatives, gallic acid and its derivatives, and flavonoids (Sannomiya et al. 2005a, b, c, 2007a, b; Rinaldo et al. 2010; Guilhon-Simplicio and Pereira 2011).
To analyze the cell viability and cytotoxic properties of potential new phytotherapeutics, which are drugs with different chemical compounds, the best approach is to use several methods, thus analyzing different cellular parameters to reduce confounding factors and obtain reliable results (Weyermann et al. 2005; Abdullah et al. 2014). In the present study, different assays were employed to evaluate cytotoxicity to different cellular targets: respiratory chain activity (MTT), reducing cellular environment (Aquabluer), membrane integrity (LDH) and lysosomal activity (Neutral Red). The MTT assay is used in many areas, especially in the identification of new drugs; however, this assay has limitations. The disadvantages of this assay have been demonstrated since it was proposed by Mosmann (1983) and the limitations of this assay that interfere in the results are: presence of polyphenolic hydroxyl groups, pH and components of the medium, metabolism and cell concentrations (Scudiero et al. 1988; Plumb et al. 1989; Mei Han et al. 2010; Wang et al. 2011; Van Tonder et al. 2015).
The four Byrsonima extracts exhibited differential effects on cell viability depending on the methodology applied, and on the qualitative and quantitative differences in the chemical compounds, as related by Sannomiya et al. (2005a, b, c) and Rinaldo et al. (2010). The extracts assessed in the present study contain considerable levels of polyphenols, mainly quercetin (Guilhon-Simplicio and Pereira 2011).
The phytochemical studies reported in the literature with polar extracts of leaves of Byrsonima species such as B. crassa, B. intermedia and B. basiloba showed the occurrence of (+)-catequin, (−)-epicatechin, proanthocyanidins, glycosylated flavones, amentoflavone, gallic acid, methyl gallate and galloyl quinic acid derivatives (Sannomiya et al. 2005a, b, c, 2007a, b).
B. fagifolia differs from the other studied species from the point of chemical view. Four glycosylated flavones were isolated from the polar extract while twenty-one galloyl quinic acid derivatives and five flavonoids were identified from the infusion of the leaves (Sannomiya et al. 2007a, b; Lima et al. 2008). This fact can explain the low cytotoxicity of B. fagifolia relative to the others species (B. correifolia and B. verbascifolia) in the MTT, aquabluer, neutral red and LDH assays (Figs. 1c, 2c, 3c, 4c). As B. fagifolia, the extract of B. intermedia also exhibited a low cytotoxic effect (Figs. 1d, 2d, 3d, 4d) and amentoflavone is present in high concentrations in B. intermedia.
The in vitro cytotoxicity assays are the first parameters to evaluate the toxicity of a compound and determine non-cytotoxic concentrations of new drugs (OECD—Genetic Toxicology Test Guidelines 2015). The toxicity is the major cause for the high failure rate (40–50 %) of pharmaceutical drug (Sumantran 2011) but also false the positive results in mutagenicity tests (Kirkland et al. 2007). So, taking into account this and according to the importance of medicinal plants as a source of new drugs (Newman and Cragg 2012), the low cytotoxicity of B. fagifolia and B. intermedia, combined with the ethnopharmacological knowledge of these species (Guilhon-Simplicio and Pereira 2011) encourage future studies to investigate the therapeutic potential and action mechanism of B. fagifolia and B. intermedia species.
Contrary to what was obtained for B. fagifolia and B. intermedia, many cytotoxic concentrations were detected when HepG2 and GAS cells were treated with extracts of B. correifolia and B. verbascifolia; the EC50 values calculated for both extracts were among the 10 concentrations evaluated in the neutral red assays.
Given the evidence that molecules that have cytotoxic effect may also have anticancer activity, detecting compounds with cytostatic effect is extremely important for the identification of new anticancer agents (Sumantran 2011; Di Giorgio et al. 2011). Therefore, the population should carefully use B. correifolia and B. verbascifolia extracts, and investigations of these extracts should be restricted to therapeutic use in cancer treatment.
The assay based on the ability of viable cells to bind and incorporate the supravital dye neutral red exhibited a good sensitivity. However, some chemicals can induce irreversible precipitation of neutral red dye in fine crystals, resulting in an overestimation of toxic effects (Repetto et al. 2008). This effect may explain the apparent increase on toxicity observed in the present study for all concentrations (except 0.15 µg/mL) of B. verbascifolia in both cell lines (Fig. 2b). The data obtained with flow cytometry and proliferation curves confirmed that the concentrations of 0.61 and 2.4 µg/mL (Fig. 2b) did not increase the cell number in subG1 phase and did not change the cell cycle kinetics.
The loss of integrity or damage of cellular membranes due to cell death results in the release of cytosolic enzymes as LDH. Enzyme activity level correlates with the level of cell death/membrane damage and provides a precise measurement of cytotoxicity induced by chemical compounds (Korzeniewski and Callewaert 1983).
Ulukaya et al. (2008) suggested caution in comparing cell viability assays because the MTT assay, depending on the drugs of choice, seem to give rise to higher viability levels than the ATP assay. Using plant extracts of Mangifera indica, Abdullah et al. (2014) detected a high correlation among the MTT, LDH and neutral red assays.
In the present study, after exposure to Byrsonima extracts, cytotoxicity was measured with the MTT, aquabluer, LDH, neutral red assays, and the different sensitivities of each assay were analyzed. The LDH assay demonstrated to be the most reliable once and no false positive or negative results were observed.
After performing the cytotoxicity assay, we selected three non-cytotoxic concentrations of each extract to identify possible therapeutic concentrations for the production of phytotherapeutic compounds. It is extremely important to evaluate how the concentrations of these extracts influence cell proliferation because it is possible to observe cytostatic effects independent of cytotoxic effects. To evaluate the cell proliferation, the best choice is to measure total cellular protein content, which is not dependent of cell metabolism (Skehan et al. 1990; Van Tonder et al. 2015). For this, sulforhodamine B (SRB) or bromophenol blue (Skehan et al. 1990; Cilião et al. 2015; Serpeloni et al. 2015) can be used as dye of cellular total protein content. The results obtained using the cell proliferation curves with bromophenol blue suggest that the three concentrations of all extracts evaluated did not alter the proliferative rate of either cell line in long-term treatment (Fig. 5).
Flow cytometry is a sensitive technique for cell cycle analysis. It evaluates individual cells by morphology, DNA content and cell viability. Concentrations of 0.31 and 1.2 mg/mL of B. correifolia extract induced cell death in flow cytometry analysis in both cell lines tested (Fig. 6) while the neutral red assay also detected decrease in cell viability but it was not significant (Fig. 3a1, a2). The cell death was not observed in the LDH assay because this assay detects the release of the enzyme lactate dehydrogenase by cells with damage, which does not occur during the apoptosis (Fig. 4a1, a2).
Regarding B. verbascifolia, it is clear that all concentrations of this extract did not affect the cell cycle of HepG2 cells, whereas in GAS cells the lowest concentrations decreased the cell number in G1 phase but increased the cell number in G2/M, which could indicate that the cell cycle arrest was due to DNA repair. These data are consistent with those of Zhang et al. (2009), who demonstrated that flavonols like quercetin were able to induce cell cycle arrest in the G2/M phase in the human esophageal squamous cell carcinoma cell line (KYSE-510). The cells accumulation in G2 may be due to DNA damage checkpoint (that may sense some of the persistent DNA lesions from the previous S phase as being inappropriately or not fully replicated DNA) (Kastan and Bartek 2004) and prevents the mitosis onset. Therefore, flow cytometry analysis was very useful to indicate how low and moderate cytotoxic concentrations of these extracts influence the cell cycle.
A comprehensive cytotoxic effect determination of plant extract requires methods that evaluate cell proliferation rate (growth curve) of cell lines exposed to short and long term treatments; and the use of various methodologies with different cellular targets, directed for detecting dead/damaged cells (LDH assay), and to detect a decrease in viable cell metabolism (MTT test, neutral red and aquabluer), avoiding false negative/positive and ensuring more reliable results (Weyermann et al. 2005; Abdullah et al. 2014).
Conclusion
The present study demonstrated that the most appropriate tests to determine the cytotoxicity and/or measure the non-cytotoxic concentrations of new phytotherapeutic compounds are the LDH assay, proliferation curve and cell cycle analysis by flow cytometry. B. correifolia and B. verbascifolia should be used carefully in light of their cytotoxic effects, whereas B. fagifolia and B. intermedia exhibit low cytotoxicity and should be further investigated for their use as chemopreventive agents. Although the Byrsonima genus is extensively used in Brazilian folk medicine, this study is the first to assess the effects of these extracts on human cell lines and contribute to characterize these species as herbal medicines.
Acknowledgments
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Process No. 2009/52237-9. The authors also thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship to Ana Flávia Leal Specian and PROAP; The Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq) for Grants to W. Vilegas, E.A. Varanda and I.M.S. Cólus.
Compliance with ethical standards
Conflict of interest
The authors have declared no conflicts of interest.
References
- Abdullah AH, Mohammed AS, AbdullaH R, Mirghani MES, Al-Qubaisi M. Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 andMDA-MB-231 cell lines) and bioactive constituents in the crude extract. J Altern Complement Med. 2014;14:1–10. doi: 10.1186/1472-6882-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard D, Schwaiger W, Crazzolara R, Tinhofer I, Kofler R, Csordas A. Enhanced MTT—reducing activity under growth inhibition by resveratrol in CEM-C7H2 lymphocytic leukemia cells. Cancer Lett. 2003;195:193–199. doi: 10.1016/S0304-3835(03)00157-5. [DOI] [PubMed] [Google Scholar]
- BRASIL (2006) Ministério da Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos Departamento de Assistência Farmacêutica. Política nacional de plantas medicinais e fitoterápicos, Brasília: Ministério da Saúde, 1–60
- Cecilio AB, De Faria DB, Oliveira PC, Caldas S, De Oliveira DA, Sobral MEG, Duarte MGR, Moreira CPS, Silva CG, De Almeida VL. Screening of Brazilian medicinal plants for antiviral activity against rotavirus. J Ethnopharmacol. 2012;141:975–981. doi: 10.1016/j.jep.2012.03.031. [DOI] [PubMed] [Google Scholar]
- Cilião HL, Ribeiro DL, Camargo-Godoy RB, Specian AF, Serpeloni JM, Cólus IM. Cytotoxic and genotoxic effects of high concentrations of the immunosuppressive drugs cyclosporine and tacrolimus in MRC-5 cells. Exp Toxicol Pathol. 2015;67:179–187. doi: 10.1016/j.etp.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Da Costa Lopes LC, Albano F, Laranja GAT, Alves LM, Silva LFM, Souza GP, Araujo IM, Nogueira-Neto JN, Felzenszwakb I, Kovary K. Toxicological evaluation by in vitro and in vivo assays of an aqueous extract prepared from Echinodorus macrophyllus leaves. Toxicol Lett. 2000;116:189–198. doi: 10.1016/S0378-4274(00)00220-4. [DOI] [PubMed] [Google Scholar]
- Dhawan A, Kayani MA, Parry JM, Parry E, Anderson D. Aneugenic and clastogenic effects of doxorubicin in human lymphocytes. Mutagenesis. 2003;18:487–490. doi: 10.1093/mutage/geg024. [DOI] [PubMed] [Google Scholar]
- Di Giorgio C, Benchabane Y, Boyer G, Piccerelle P, De Méo M. Evaluation of the mutagenic/clastogenic potential of 3, 6-di-substituted acridines targeted for anticancer chemotherapy. Food Chem Toxicol. 2011;49:2773–2779. doi: 10.1016/j.fct.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Dosseh C, Morreti C, Tessier AM, Delaveau P. Étude chimique des feuilles de Byrsonima verbascifolia rich. Ex Juss es Plantes Médicinales et Phytothérapie. 1980;14:136–142. [Google Scholar]
- Espanha LG, Resende FA, Lima Neto JS, Boldrin PK, Nogueira CH, Santoro de Camargo M, De Grandis RA, Campaner dos Santos L, Vilegas W, Varanda EA. Mutagenicity and antimutagenicity of six Brazilian Byrsonima species assessed by the Ames test. J Altern Complement Med. 2014;14:1–10. doi: 10.1186/1472-6882-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhon-Simplicio F, Pereira MM. Aspectos químicos e farmacológicos de Byrsonima (MALPIGHIACEAE) Quim Nova. 2011;34:1032–1041. doi: 10.1590/S0100-40422011000600021. [DOI] [Google Scholar]
- Han M, Li JF, Tan Q, Sun YY, Wang YY (2010) Limitations of the use of MTT assay for screening in drug discovery. J Chin Pharma Sci 9:195–200. http://118.145.16.238/Jwk_zgyxen/EN/Y2010/V19/I3/195
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kirkland D, Pfuhler S, Tweats D, Aardema M, Corvi R, Darroudi F, Elhajouji A, Glatt H, Hastwell P, Hayashi M, Kasper P, Kirchner S, Lynch S, Marzin D, Maurici D, Meunier JR, Müller L, Nohynek G, Parry J, Parry E, Thybaud V, Tice R, Benthem J, Vanparys P, White P. How to reduce false positive results when undertaking in vitro genotoxicity testing and thus avoid unnecessary follow-up animal tests: report of an ECVAM workshop. Mutat Res. 2007;628:31–55. doi: 10.1016/j.mrgentox.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Lima ZP, Santos RC, Torres TU. Byrsonima fagifolia: an integrative study to validate the gastroprotective, healing, antidiarrheal, antimicrobial and mutagenic action. J Ethnopharmacol. 2008;120:149–160. doi: 10.1016/j.jep.2008.07.047. [DOI] [PubMed] [Google Scholar]
- Maldini M, Sosa S, Montoro P, Giangaspero A, Balick MJ, Pizza C, Della Loggia R. Screening of the topical antiinflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifolia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcock. J Ethnopharmacol. 2009;122:430–433. doi: 10.1016/j.jep.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Michelin DC, Sannomiya M, Figueiredo ME, Rinaldo D, Santos LC, Souza-Brito ARM, Vilegas W, Salgado HRN. Antimicrobial activity of Byrsonima species (MALPIGHIACEAE) Rev Bras Farmacogn. 2008;18:690–695. doi: 10.1590/S0102-695X2008000500009. [DOI] [Google Scholar]
- Moreira LQ, Vilela FC, Orlandi L, Dias DF, Santos ALA, da Silva MA, Paiva R, Alves-da-Silva G, Giusti-Paiva A. Anti-inflammatory effect of extract and fractions from the leaves of Byrsonima intermedia A. Juss. in rats. J Ethnopharmacol. 2011;138:610–615. doi: 10.1016/j.jep.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural Products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (2015) Guidance document on revisions to OECD genetic toxicology test guidelines. Genetic toxicology Guidance Document, 31 Aug
- Pereira VV, Borel CR, Silva RR. Phytochemical screening, total phenolic content and antioxidant activity of Byrsonima species. Nat Prod Res. 2015 doi: 10.1080/14786419.2014.1002407. [DOI] [PubMed] [Google Scholar]
- Plumb JA, Milroy R, Kaye SB. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989;49:4435–4440. [PubMed] [Google Scholar]
- Rampersad SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012;12(9):12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rates SMK. Plants as source of drugs. Toxicon. 2001;39:603–613. doi: 10.1016/S0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Repetto G, Del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3:1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- Ribeiro DL, Cilião HL, Specian AFL, Serpeloni JM, Souza MF, Tangerina MMP, Vilegas W, Boldrin PK, Resende FA, Varanda EA, Vilegas W, Martínez-López W, Sannomiya M, Cólus IMS. Chemical and biological characterisation of Machaerium hirtum (Vell.) Stellfeld: absence of cytotoxicity and mutagenicity and possible chemopreventive potential. Mutagenesis. 2016;31:146–160. doi: 10.1093/mutage/gev066. [DOI] [PubMed] [Google Scholar]
- Rinaldo D, Batista JM, Jr, Rodrigues J, Benfatti AC, Rodrigues CM, Dos Santos LC, Furlan M, Vilegas W. Determination of catechin diastereomers from the leaves of Byrsonima species using chiral HPLC-PAD-CD. Chirality. 2010;22:726–733. doi: 10.1002/chir.20824. [DOI] [PubMed] [Google Scholar]
- Sannomiya M, Fonseca VB, Silva MA, Rocha LRM, Dos Santos LC, Hiruma-Lima CA, Souza Brito ARM, Vilegas W. Flavonoids and antiulcerogenic activity from Byrsonima crassa leaves extracts. J Ethnopharmacol. 2005;97:1–6. doi: 10.1016/j.jep.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Sannomiya M, Montoro P, Piacente S, Pizza C, Souza Brito ARM, Vilegas W. Application of liquid chromatography/electropsray ionization tandem mass spectrometry to the analysis of polyphenolic compounds from an infusion of Byrsonima crassa Niedenzu. Rapid Commun Mass Spectrom. 2005;19:2244–2250. doi: 10.1002/rcm.2053. [DOI] [PubMed] [Google Scholar]
- Sannomiya M, Silva MA, dos Santos LC, de Almeida LFR, Souza Brito ARM, Salgado HRN, Vilegas W. Avaliação química e da atividade antidiarréica das folhas de Byrsonima cinera DC. (Malpighiaceae) Braz J Pharm Sci. 2005;41:79–83. [Google Scholar]
- Sannomiya M, dos Santos LC, Carbone V, Napolitano A, Piacente S, Pizza C, Souza-Brito ARM, Vilegas W. Liquid chromatography/electrospray ionization tandem mass spectrometry profiling of compounds from the infusion of Byrsonima fagifolia Niedenzu. Rapid Commun Mass Spectrom. 2007;21:1393–1400. doi: 10.1002/rcm.2971. [DOI] [PubMed] [Google Scholar]
- Sannomiya M, Cardoso CRP, Figueiredo ME, Rodrigues CM, Dos Santos LC, Dos Santos FV, Serpeloni JM, Cólus IMS, Vilegas W, Varanda EA. Mutagenic evaluation and chemical investigation of Byrsonima intermedia A. Juss. leaf extracts. J Ethnopharmacol. 2007;112:319–326. doi: 10.1016/j.jep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Santos RC, Kushima H, Rodrigues CM, Sannomiya M, Rocha LRM, Bauab TM, Tamashiro J, Vilegas W, Hiruma-Lima CA. Byrsonima intermedia A. Juss.: gastric and duodenal anti-ulcer, antimicrobial and antidiarrheal effects in experimental rodent models. J Ethnopharmacol. 2012;140:203–212. doi: 10.1016/j.jep.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Scudiero DA, Shoemaker RH, Paul KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- Serpeloni JM, Specian AFL, Ribeiro DL, Tuttis K, Vilegas W, Martínez-López W, Dokkedal AL, Saldanha LL, Cólus IMS, Varanda EA. Antimutagenicity and induction of antioxidant defense by flavonoid rich extract of Myrcia bella Cambess. in normal and tumor gastric cells. J Ethnopharmacol. 2015;176:345–355. doi: 10.1016/j.jep.2015.11.003. [DOI] [PubMed] [Google Scholar]
- SIGMA-ALDRICH (2012) Benzo[a]pyrene. http://www.sigmaaldrich.com/catalog/product/sigma/b1760?lang=pt®ion=BR. Accessed 2012
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Sumantran Venil N. Cellular chemosensitivity assays: an overview. Cancer Cell Cult Methods Protoc. 2011;731:219–236. doi: 10.1007/978-1-61779-080-5_19. [DOI] [PubMed] [Google Scholar]
- Ulukaya E, Ozdikicioglu F, Oral AY, Demirci M. The MTT assay yields a relatively lower result of growth inhibition than the ATP assay depending on the chemotherapeutic drugs tested. Toxicol In Vitro. 2008;22:232–239. doi: 10.1016/j.tiv.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP. Oral Benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol. 2006;69:1103–1112. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- Van Tonder A, Joubert AM, Cromarty AD. Limitations of the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes. 2015;8:1. doi: 10.1186/s13104-015-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yu H, Wickliffe JK. Limitation of the MTT and XTT assays for measuring cell viability due to superoxide formation induced by nano-scale TiO2. Toxicol In Vitro. 2011;25:2147–2151. doi: 10.1016/j.tiv.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Weyermann J, Lochmann D, Zimmer A. A practical note on the use of cytotoxicity assays. Int J Pharm. 2005;288:369–376. doi: 10.1016/j.ijpharm.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao XH, Wang ZJ. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G 2/M arrest and apoptosis. Toxicol In Vitro. 2009;23:797–807. doi: 10.1016/j.tiv.2009.04.007. [DOI] [PubMed] [Google Scholar]