Abstract
Lactoferrin (LF) and retinoic acid (RA) are enriched in colostrum, milk, and mucosal tissues. We recently showed that LF-induced IgA class switching through binding to betaglycan (transforming growth factor-beta receptor III, TβRIII) and activation of canonical TGF-β signaling. We investigated the combined effect of LF and RA on the overall IgA response. An increase in IgA production by LF was further augmented by RA. This combination effect was also evident in Ig germ-line α (GLα) transcription and GLα promoter activity, indicating that LF in cooperation with RA increased IgA isotype switching. We subsequently found that RA enhanced TβRIII expression and that this increase contributed to LF-stimulated IgA production. In addition to the IgA response, LF and RA in combination also enhanced the expression of the gut-homing molecules C-C chemokine receptor 9 (CCR9) and α4β7 on B cells. Finally, peroral administration of LF and RA enhanced the frequency of CCR9+IgA+ plasma cells in the lamina propria. Taken together, these results suggest that LF in cooperation with RA can contribute to the establishment of gut IgA responses.
Keywords: gut homing molecule, IgA, lactoferrin, retinoic acid, TGF-β receptor
Introduction
IgA is the most important antibody that protects mucosal surfaces against pathogens. Numerous IgA-producing B cells are found in the gut lamina propria (LP).1 Gut-tropic B cells robustly express C-C chemokine receptor 9 (CCR9) and α4β7, which play a role in the mucosal trafficking of these cells.2,3,4
Ig class switch recombination (CSR) allows the recombined variable region gene segment (VDJ) to be expressed with a new downstream heavy chain constant region (CH) gene. CSR is achieved by deletional recombination between switch (S) region sequences placed upstream of each of the CH genes, with the exception of Cδ.5 CSR is directed to a particular CH gene by cytokines that stimulate transcription from germ-line (GL) CH genes prior to CSR to the same CH gene. It is well established that transforming growth factor-beta 1 (TGF-β1) induces IgA CSR.6,7,8
Retinoic acid (RA) is a natural bioactive metabolite of vitamin A that regulates a broad range of biological processes by binding to specific nuclear retinoid receptors.9 Moreover, RA can cause IgA isotype switching,10,11 and GALT-DC-derived RA enhances the expression of the gut-homing molecules CCR9 and α4β7 on B cells.12 We recently demonstrated that RA selectively activated IgA isotype switching and synergized with TGF-β1 to promote IgA production and the expression of gut-homing molecules in mice.13
Lactoferrin (LF) is found at high concentrations on many mucosal surfaces.14 LF induces the activation, proliferation, and differentiation of immune cells. This activity has been related to a direct effect of LF on the cells through specific LF receptors, including LF receptor (LFR), low-density lipoprotein receptor-related protein (LRP)-1, nucleolin, and proteoglycan.15,16 Among these receptors, betaglycan (TGFβRIII, TβRIII) is a type of proteoglycan that was originally identified as a non-signaling coreceptor in TGF-β signaling.17,18 We recently showed that LF caused IgA and IgG2b isotype switching by binding to TβRIII and activating canonical TGF-β signaling.19
Although LF and RA are abundant inmucosal secretions and are known to cause IgA isotype switching, their combined effect on the overall gut IgA response has not been characterized. In this study, we found that LF and RA synergized to enhance IgA isotype switching and the expression of gut-homing molecules on B cells. Moreover, RA increased the expression of TβRIII, which was the LFR and resulted in a robust IgA response.
Methods
Animals
BALB/c mice were obtained from DaehanBiolink. Co. Ltd (Chungcheongbuk-do, Korea). The animals were fed Purina Laboratory Rodent Chow 5001 ad libitum. Mice that were 8–12 weeks of age were used in this study. Animal care was performed in accordance with the institutional guidelines set forth by Kangwon National University.
Cell preparation and reagents
Mouse splenic B-cell suspensions were prepared as previously described.7 Briefly, T cells were depleted from the cell suspensions by treatment with a cocktail of anti-Thy1.2, anti-Lyt2.2, and anti-L3T4 monoclonal antibodies and low-tox rabbit complement (Cedarlane, Ontario, Canada). As a result, B cells constituted more than 90% of the residual population as assessed by the presence of surface Ig using flow cytometric analysis. Subsequently, resting splenic CD43− B cells were negatively sorted using 10 μL of anti-CD43 (Ly-48) microbeads per 107 cells and a magnetic column (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer's instructions. The murine B-cell lymphoma cell line CH12F3-2A (surface μ+) was provided by Dr. T. Honjo (Kyoto University, Kyoto, Japan).
Bovine LF was supplied by Morinaga Milk Co., Ltd. (Zama, Japan); the preparation contained less than 5.0 pg mg−1 of lipopolysaccharide (LPS) (endotoxin).20 Anti-bovine LF antiserum was purchased from Bethyl Laboratories, Inc. (Montgomery, TX, USA). RA and LPS (Escherichia coli O111:B4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Recombinant human TGF-β1 and the anti-TGFβRIII antibody (Ab) were purchased from R&D Systems (Minneapolis, MN, USA). Goat serum was purchased from Invitrogen (Carlsbad, CA, USA). BMS614 was purchased from Tocris (Bristol, UK). The antibodies used in the ELISA were purchased from Southern Biotechnology (Birmingham, AL, USA). The GLα promoter reporter for −448 to +72 (M-GLα) was described previously.21
Isotype-specific ELISA
Isotype-specific ELISAs were performed as described previously.22 The absorbance of the reaction products was measured at 415 nm with an ELISA reader (VERSAMAX reader, Molecular Devices, Sunnyvale, CA, USA).
RNA preparation and RT-PCR
RNA preparation, reverse transcription, and PCR were performed as described previously.22 The following PCR primers were synthesized by Bioneer (Seoul, Korea): germ-line transcript (GLTα) sense, 5′-CAA GAA GGA GAA GGT GAT TCA G-3′ and antisense, 5′-GAG CTG GTG GGA GTG TCA GTG-3′ GLTγ1 sense, 5′-CAG CCT GGT G TC AAC TAG-3′ and antisense, 5′-CTG TAC ATA TGC AAG GCT-3′ GLTγ2a sense, 5′-GCT GAT GTA CCT ACC TGA GAG A-3′ and antisense, 5′-GCT GGG CCA GGT GCT CGA GGT T-3′ GLTγ2b sense, 5′-GGG AGA GCA CTG GGC CTT-3′ and antisense, 5′-AGT CAC TGA CTC AGG GAA-3′ GLTγ3 sense, 5′-CAA GTG GAT CTG AAC ACA-3′ and antisense, 5′-GGC TCC ATA GTT CCA TT-3′ PSTα sense, 5′- GAG CTG GTG GGA GTG TCA GTG-3′ and antisense, 5′- CTC TGG CCC TGC TTA TTG TTG-3′ CTα sense, 5′- CTA CCA TAG GGA AGA TAG CCT -3′ and antisense, 5′- TCT GAA CCT TCA AGG ATG CTC TGG -3′ AID sense, 5′-TGC TAC GTG AAG AGG AG-3′ and antisense, 5′- TCC CAG TCT GAG ATG TAG CG-3′ and β-actin sense, 5′-CAT GTT TGA GAC CTT CAA CAC CCC-3′ and antisense, 5′-GCC ATC TCC TGC TCG AAG TCT AG-3′. All of the reagents for RT-PCR were purchased from Promega (Madison, WI, USA). PCR reactions for β-actin were performed in parallel to normalize cDNA concentrations within each set of samples. Band intensities were quantified using the Scion Image software (Scion Corp., Frederick, MD, USA).
Carboxyfluorescein succinimidyl ester (CFSE) labeling and flow cytometric analysis
Isolated spleen B cells were labeled with a CFSE kit (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. Dilution of CFSE was measured by counting 30,000 viable cells with a FACSCalibur (BD Biosciences, San Diego, CA, USA). The cells were stained with anti-mouse CD45R/B220-biotin (clone RA3-6B2; BD Pharmingen, San Diego, CA, USA), anti-mouse CCR9-PE (clone 242503; R&D Systems), anti-mouse LPAM1 (α4β7)-biotin (clone DATK32; Southern Biotechnology), anti-mouse CD138-biotin (clone 281–2; BD Pharmingen), anti-mouse TGF-βRIII (R&D Systems), goat anti-rabbit IgG-FITC (Abcam), streptavidin–allophycocyanin (eBioscience, San Diego, CA, USA), and streptavidin–FITC (Southern Biotech). For the detection of intracellular IgA, cultured cells were permeabilized with 0.1% saponin for 30 min and stained with FITC-labeled goat anti-mouse IgA Ab (Southern Biotechnology).
Transfection and luciferase assays
Transfections were performed by electroporation with a Gene PulserII (Bio-Rad, Hercules, CA, USA) as previously described.22,23 Reporter plasmids were cotransfected with the expression plasmids and pCMV-βgal (Stratagene, La Jolla, CA, USA), and luciferase and β-gal assays were performed as previously described.22,23
Preparation of intestinal LP lymphocytes
Mice were killed using a CO2 box. The intestines were removed and transferred to a beaker containing cold phosphate-buffered saline (PBS). To remove mucus and intraepithelial lymphocytes, fat-free intestines were incubated at room temperature with 1 mM dithiothreitol in PBS and 30 mM ethylene diamine tetraacetic acid (Bio-Rad, Hercules, CA, USA) in PBS. Next, the intestines were digested with 0.05% collagenase solution (Worthington Biochemical Corp. Lakewood, NJ, USA for 90 min at 37 °C. Percoll (GE healthcare, Bio-Sciences AB, Uppsala, Sweden) gradient density centrifugation was performed to isolate LP lymphocytes.
Statistical analysis
Significant differences between the experimental groups were determined by analysis of variance. Values of P < 0.05 by unpaired two-tailed Student's t-tests were considered significant.
Results
LF in combination with RA specifically increases IgA secretion
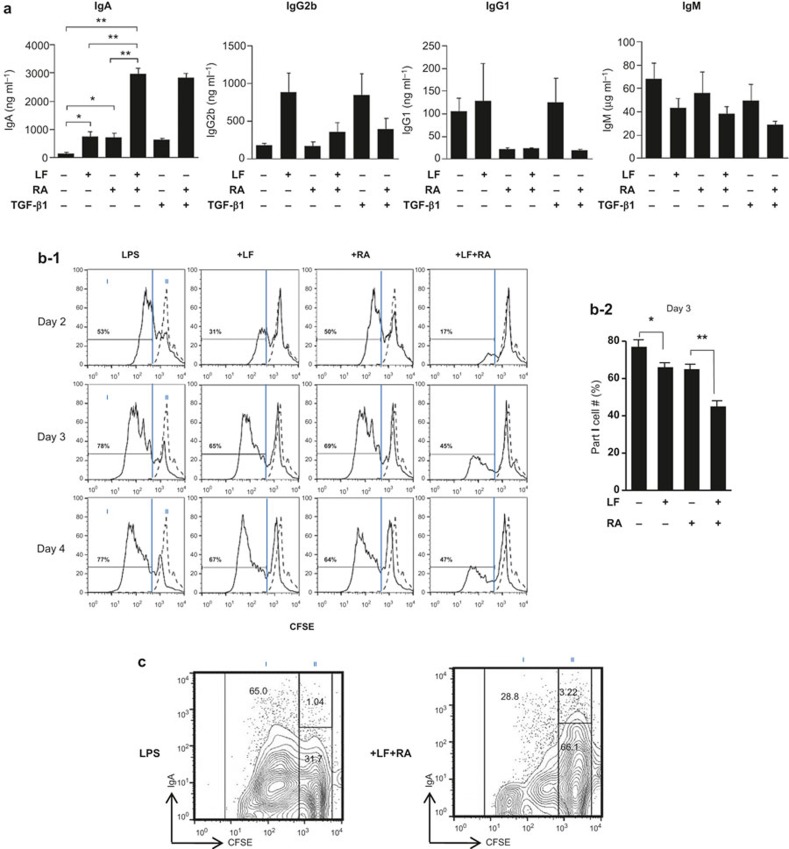
We recently demonstrated that administering RA and TGF-β1 in combination markedly increases IgA secretion.13 Additionally, LF induces IgA and IgG2b CSR through TGF-β signaling.19 Therefore, we assessed the effect of LF and RA on Ig expression. Similar to TGF-β1, LF increased IgA and IgG2b production by mouse splenic B cells. In this experiment, the IgA increased by LF was further augmented by RA (P < 0.01) (Figure 1a). In contrast, the combination effect was not seen with the other isotypes (IgG2b, IgG1, and IgM).
Figure 1.
Effect of LF and RA on Ig secretion and B cell proliferation. (a) Mouse splenic B cells were cultured with LPS (12.5 μg mL−1), LF (60 μg mL−1), RA (25 nM), and TGF-β1 (0.2 ng mL−1). After 7 days of culture, supernatants were harvested, and the levels of Ig secretion were determined by ELISA. The data represent the mean of triplicate samples ± SEM. (b-1) Isolated splenic B cells were labeled with a CFSE kit and cultured as in panel (a). B-cell proliferation was assessed after 48, 72, and 96 h by analyzing the CFSE dilution in the same number of viable cells by flow cytometric analysis. The dotted line indicates CFSE-labeled B cells on day 0. (b-2) B-cell proliferation was assessed after 72 h. The data represent the mean of triplicate samples ± SEM. (c) Splenic B cells were labeled with a CFSE kit on day 0, and mIgA was stained on day 4. *P < 0.05; **P < 0.01. LF, lactoferrin; RA, retinoic acid; LPS, lipopolysaccharide; TGF, transforming growth factor.
Our previous study revealed that the IgA enhancing activity of TGF-β1 was related in part to its ability to inhibit cell growth.24 Therefore, it was necessary to determine the effect of LF and RA on B-cell growth. All concentrations of LF and RA that increased IgA secretion inhibited B cell proliferation (Figure 1b). Moreover, the combination of LF and RA suppressed B-cell proliferation to a greater extent than either LF or RA alone. The suppression of cell proliferation by LF and RA was also noticeable in Figure 1C, where these two molecules decreased population I (more dividing cells) by 2-fold (from 65.0% to 28.8%). Additionally, mIgA expression was apparent within population II (fewer dividing cells) and was increased from 1.14% to 3.02% by LF plus RA. These results indicate that LF in combination with RA specifically increases IgA secretion, at least partially through the combination's anti-proliferative action on B cells.
LF and RA in combination cause IgA isotype switching at the molecular level
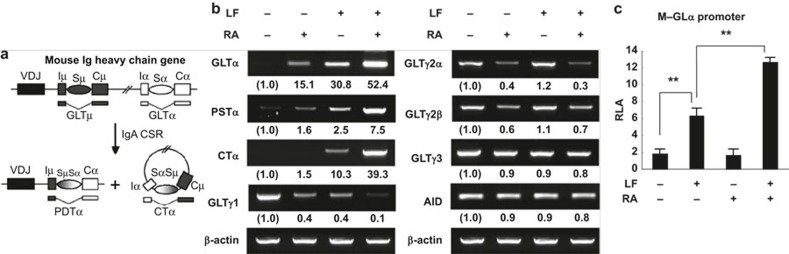
Because LF and RA selectively increase IgA secretion, we sought to discover whether LF and RA enhanced the expression of transcripts associated with IgA CSR. As shown in the diagram in Figure 2a, once CSR to IgA occurs, the transcription of unrearranged CH genes produces the corresponding GLTs and continues to be active, thereby generating post-switch transcripts (PSTs) and circle transcripts (CTs).25,26 Thus, the expression of GLTs, PSTs, and CTs can be used to indicate active Ig CSR. We recently demonstrated that RA substantially enhanced GLTα expression in mice and humans.13,27 Paralleling the Ig secretion pattern, the expression of GLTα, PSTα, and CTα were enhanced by both LF and RA alone, and this increase was enhanced by combining LF and RA (Figure 2b). In contrast, the expression of other GLTs (GLTγ1, GLTγ2a, GLTγ2b, and GLTγ3) was reduced under the same conditions. Neither LF nor RA enhanced the expression of AID, an essential enzyme involved in Ig CSR, in this experiment. Subsequently, we assessed the effect of LF and RA on GLα promoter activity using an M-GLα reporter.21 As shown in Figure 2c, the promoter activity was increased by both LF and RA alone and further augmented by LF and RA in combination. Taken together, these results suggest that LF and RA in combination selectively cause IgA isotype switching at the molecular level.
Figure 2.
Effect of LF and RA on Ig germ-line transcript expression and activity of the GLα promoter. (a) Diagram of DNA recombination occurring while switching to IgA. (b) Normal splenic B cells were cultured with LPS (12.5 μg mL−1), LF (60 μg mL−1), and RA (25 nM). After 2 days of culture, total RNA was isolated and the GLT, PSTα, CTα, AID, and β-actin levels were measured by RT-PCR. (c) CH12F3-2A B lymphoma cells were transfected with the M-GLα-luc reporter (10 μg) prior to treatment with LF (60 μg mL−1) and RA (10 μM) for 18 h. Promoter activity was determined by luciferase activity. The data represent the mean of triplicate samples ± SEM. GLTs, germ-line transcripts; LF, lactoferrin; RA, retinoic acid; LPS, lipopolysaccharide; PSTα, α post-switch transcript; RLA, relative luciferase activity. **P < 0.01.
RA induces the expression of betaglycan (TβRIII), resulting in increased IgA production
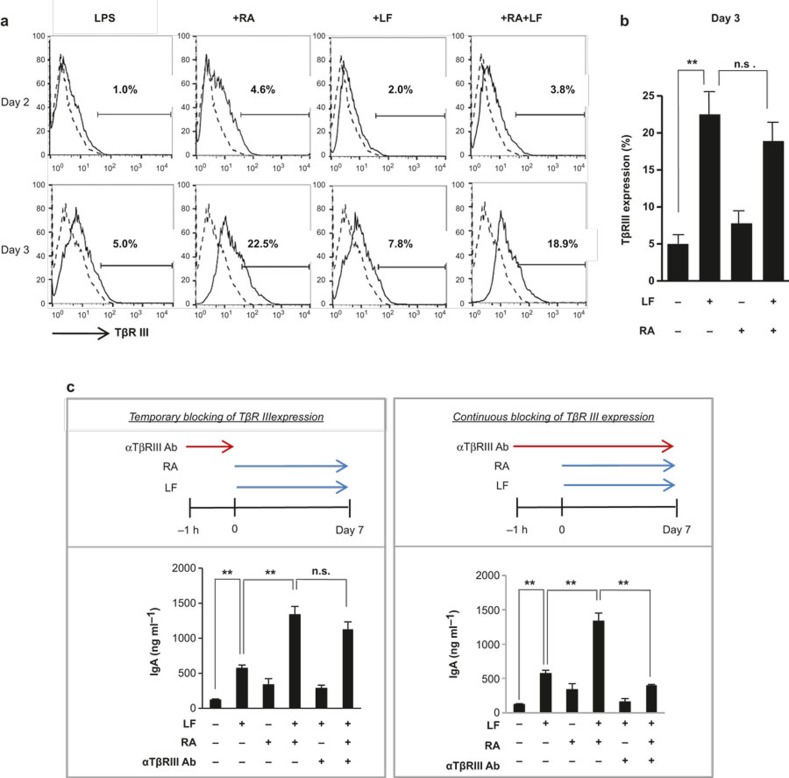
RA has been demonstrated to increase TβRIII expression during myogenesis.28 Because LF induces IgA production by binding to TβRIII,19 it was important to investigate whether RA increased TβRIII expression in mouse B cells and whether this increase affected LF-dependent IgA production. RA markedly increased the expression of TβRIII on the surface of mouse splenic B cells (Figure 3a) in a dose-dependent manner (Supplementary Figure 1). In this study, TβRIII expression was increased by day 3 without the addition of any stimulants. LF marginally improved the expression (Figure 3a). The expression level of TβRIII in the presence of RA and LF was found to be almost the same as expression in the presence of RA alone. These results indicate that LF does not induce TβRIII expression. Taken together, our results strongly suggest that RA enhances LF-mediated IgA production through the induction of TβRIII. This possibility was tested by neutralizing the activity of TβRIII. We compared IgA and IgG2b production between conditions that resulted in the temporary and continuous blockade of TβRIII expression (Figure 3b and Supplementary Figure 2). When TβRIII expression was transiently neutralized, the combination effect of LF and RA on IgA production was still apparent (Figure 3b, left panel). In contrast, when TβRIII expression was continuously neutralized, the combination effect was no longer observed (P < 0.01) (Figure 3b, right panel). These results reveal that one of the IgA enhancing effects of RA is attributable to its induction of TβRIII expression during culture.
Figure 3.
RA increases LF-induced IgA expression through the induction of betaglycan (TβRIII) expression. (a) Normal splenic B cells were cultured with LPS (12.5 μg mL−1), LF (120 μg mL−1), and RA (100 nM) for 2 and 3 days. TβRIII expression was analyzed by fluorescence-activated cell sorting (FACS). The dotted line indicates surface TβRIII expression on day 0. (b) TβRIII expression was analyzed by FACS after culture for 3 days. The data represent the mean of triplicate samples ± SEM. (c) Culture conditions were the same as in panel (a). An anti-betaglycan Ab (5 μg mL−1) was added for the indicated periods. After 7 days of culture, the IgA level was determined by ELISA. The data represent the mean of triplicate samples ± SEM. **P < 0.01; n.s., not significant. LF, lactoferrin; RA, retinoic acid; LPS, lipopolysaccharide.
LF and RA synergize to induce the gut IgA response
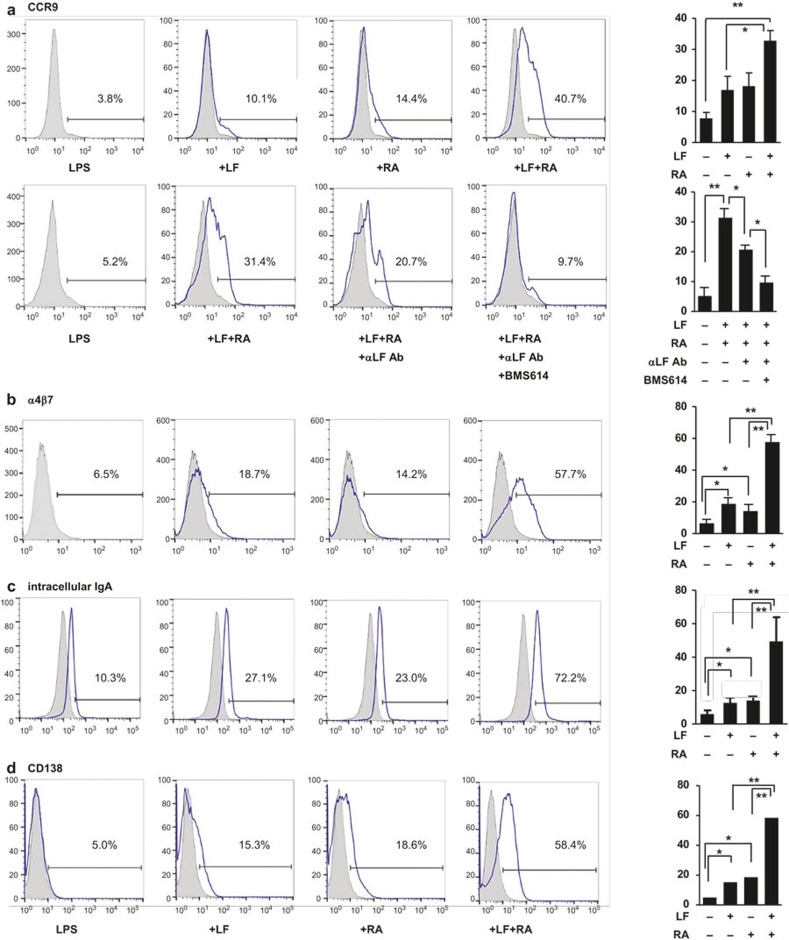
It is well established that RA plays a critical role in deploying lymphocytes into gut tissue.12,29 Because TGF-β further augments the expression of the gut-homing molecules induced by RA13, we assessed whether LF affected the expression of gut-homing molecules and plasma cell differentiation. Similar to the results obtained for the IgA response, LF increased the expression of the gut-homing receptor CCR9; this increase was enhanced by RA (Figure 4a, upper panel and Supplementary Figure 3). The combined effect of LF and RA on CCR9 expression was virtually abolished by treatment with an anti-LF Ab and BMS614 (retinoic acid receptor alpha (RARα) antagonist) (Figure 4a, lower panel), indicating that the LF and RA used in this study were not contaminated with any substance and that the expression-increasing activity of RA primarily involves the RARα mediator. Moreover, LF also increased the expression of another gut-homing molecule (α4β7), intracellular IgA, and CD138 (Figure 4b–4d). These results indicate that LF in association with RA can contribute to the gut IgA response via several mechanisms, including IgA isotype switching, the expression of gut-homing molecules, and the differentiation of plasma cells.
Figure 4.
LF and RA synergize to induce gut-homing molecules. Normal splenic CD43− B cells were cultured with LPS (12.5 μg mL−1), LF (60 μg mL−1), and RA (25 nM). After 6 days of culture, the cells were analyzed by FACS for the expression of surface CCR9 (a), α4β7 (b), intracellular IgA (c), and surface CD138 (d). In panel (a), anti-LF antiserum (1:8) was added to the LF 1 h prior to cell culture. Cells were pretreated with BMS614 (3 μM) 1 h prior to the addition of RA. The data represent the mean of triplicate samples ± SEM. *P < 0.05; **P < 0.01. The gray-shaded graph represents treatment with the isotype control. LF, lactoferrin; RA, retinoic acid; LPS, lipopolysaccharide.
Peroral administration of LF and RA increases the proportion of IgA-producing B cells in the intestinal LP
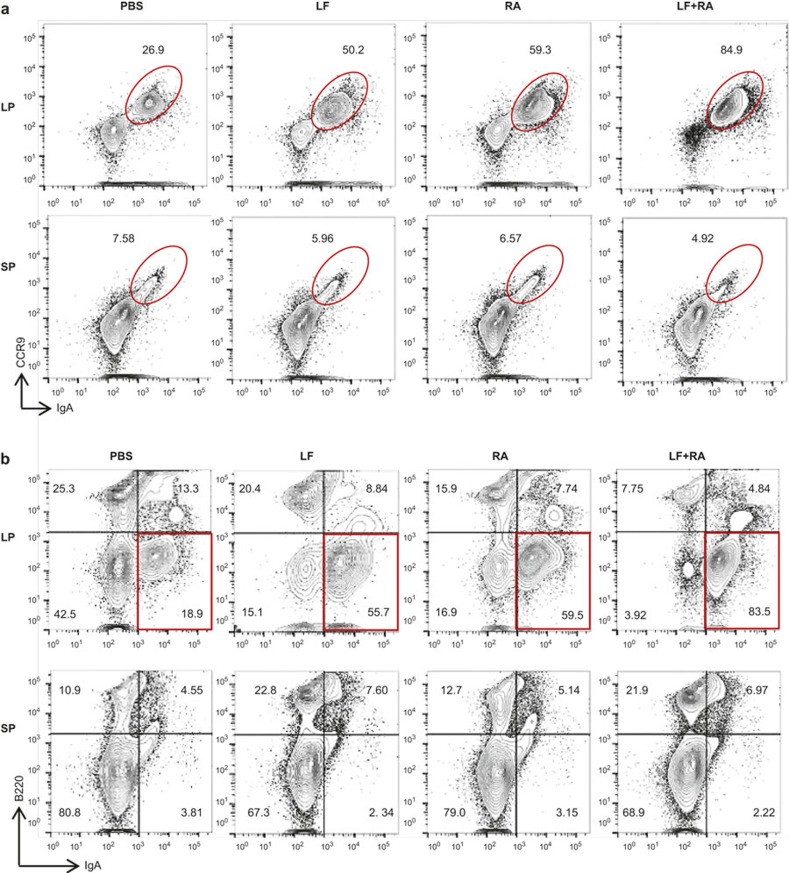
Because LF and RA increased IgA isotype switching and the expression of gut-homing molecules in vitro, we explored whether these molecules affected the gut IgA response in vivo. LF and RA were perorally administered 3 times each week for 15 weeks. The proportions of LP CCR9+IgA+ B cells and B220−IgA+ B cells (IgA-producing plasma cells) were markedly increased in mice treated with either LF or RA; these increases were enhanced in the presence of both LF and RA (Figure 5). In contrast, this enhancing effect of LF and RA was not observed in splenic B cells. These results suggest that perorally delivered LF and RA may contribute exclusively to local gut IgA synthesis and not to systemic IgA synthesis.
Figure 5.
In vivo effects of LF and RA on the gut IgA response. BALB/c mice were perorally administered LF (5 mg kg−1) and RA (5 mg kg−1) in PBS (100 μL) 3 times weekly for 15 weeks. Intestinal LP and spleen cells were isolated, analyzed for the expression of CCR9/intracellular IgA (a) and B220/intracellular IgA (b) by FACS. LF, lactoferrin; RA, retinoic acid; LP, lamina propria; SP, spleen.
Discussion
We demonstrated that TGF-β1 and RA synergize to cause IgA CSR and the expression of gut-homing receptor such as CCR9 and α4β7.13 Additionally, we recently reported that LF enhanced IgA CSR by binding to TβRIII (betaglycan) and activating canonical TGF-β signaling.19 The present study extends these findings. Thus, similar to our findings with TGF-β1 plus RA, LF and RA synergized to increase IgA CSR. This LF/RA combination effect was expected to some extent because LF functions through canonical TGF-β signaling as noted above.19 Nevertheless, the mechanisms underlying IgA CSR caused by the combination of TGF-β1/RA and LF/RA will not be entirely identical because the role played by RA is believed to differ from the TGF-β1/RA and LF/RA combinations. Previously, we observed that RA could directly increase IgA CSR on its own and that the RA signal transduced through RAR.13,21 In addition to this direct effect of RA, RA enhanced the expression of TβRIII on B cells, which indirectly contributed to IgA CSR (Figure 3). Thus, it is apparent that RA in combination with LF enhances IgA CSR by at least two mechanisms: (i) direct effect by stimulating Ig GLα promoter activity and (ii) indirect effect by stimulating TβRIII promoter activity, as proposed in Supplementary Figure 4.
Our finding that LF enhances the expression of the gut-homing molecules CCR9 and α4β7 on B cells is novel. GALT-DC-derived RA induces CCR9 and α4β7 on T and B cells.12,29 Additionally, we recently showed that RA in combination with TGF-β1 enhanced CCR9 and α4β7 expression on B cells.13 Similarly, RA in combination with LF synergizes to increase the expression of two gut-homing molecules. This enhancing effect of RA and LF must be physiologically relevant to gut IgA responses because their peroral administration led to enhancement of the frequency of CCR9+IgA+ plasma cells in the LP (Figure 5). However, the mechanism underlying the CCR9 induction by LF and RA is unknown. The increase in CCR9 expression caused by LF and RA was completely abrogated by an anti-LF Ab and BMS614 (RARα antagonist). Therefore, it seems possible that RA increases CCR9 expression through RARα, as is the case for IgA CSR.13 However, we note that the present report does not address whether RA-induced TβRIII is associated with CCR9 expression, and if so, whether this association occurs through RARα. It remains to be determined whether LF and RA enhance both IgA CSR and the expression of gut-homing molecules via identical mechanisms.
Unexpectedly, LF and RA in combination synergized to enhance only IgA production, although LF alone increased IgA and IgG2b production. Indeed, RA diminished the LF-mediated IgG2b response. There are at least two possibilities to explain this finding. First, a highly specific activity of RA for IgA expression may override the combination effect of LF and RA. To address this issue, GLγ2b promoter activity under the influence of RA and/or LF must be analyzed. Second, the proliferation of IgA-producing B cells has been demonstrated to be slower than the proliferation of IgG2b-producing B cells.30 Therefore, it is apparent that the number of cell divisions affects CSR events. In this regard, the overwhelming anti-proliferative activity caused by LF plus RA must be detrimental to IgG2b CSR because this CSR occurs in multi-divided cells. Conversely, the same suppressive activity of the two reagents contributes to IgA CSR because this CSR occurs in less divided cells. The detailed kinetic relationship between B-cell proliferation and CSR warrants further examination.
In summary, we demonstrate that LF in cooperation with RA enhances the overall gut IgA response. The present data, along with our recent findings, indicate that LF enhances IgA CSR and CCR9 and α4β7 expression in B cells by binding to TβRIII (Supplementary Figure 4).19 In this study, RA is found to contribute to the expression of these genes by both binding directly to the target genes and indirectly increasing TβRIII expression. LF, retinoids, and TGF-β, which are abundant in colostrum, milk, and mucosal secretions,31 are thought to contribute to infant and adult gut IgA immunity. In this regard, TGF-β1, RA, and LF are potent candidates for mucosal adjuvants with improved safety because these molecules are endogenously produced.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2010-0012311 to Pyeung-Hyeun Kim and 2013R1A1A2057931 to Goo-Young Seo) and the second stage of the Brain Korea 21 program. Studies were conducted at the Institute of Bioscience and Biotechnology at Kangwon National University.
The authors declare no conflicts of interest.
Footnotes
Supplementary information accompanies the paper on Cellular & Molecular Immunology website: http://www.nature.com/cmi.
Supplementary Information
References
- Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol 1997; 51: 311–340. [DOI] [PubMed] [Google Scholar]
- Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol 1994; 152: 3282–3293. [PubMed] [Google Scholar]
- Pabst O, Ohl L, Wendland M, Wurbel MA, Kremmer E, Malissen B et al. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J Exp Med 2004; 199: 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 1996; 382: 366–370. [DOI] [PubMed] [Google Scholar]
- Stavnezer J. Molecular processes that regulate class switching. Curr Top Microbiol Immunol 2000; 245: 127–168. [DOI] [PubMed] [Google Scholar]
- Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med 1989; 170: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PH, Kagnoff MF. Transforming growth factor-beta 1 is a costimulator for IgA production. J Immunol 1990; 144: 3411–3416. [PubMed] [Google Scholar]
- Sonoda E, Matsumoto R, Hitoshi Y, Ishii T, Sugimoto M, Araki S et al. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med 1989; 170: 1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J 1996; 10: 940–954. [PubMed] [Google Scholar]
- Tokuyama Y, Tokuyama H. Retinoids as Ig isotype-switch modulators. The role of retinoids in directing isotype switching to IgA and IgG1 (IgE) in association with IL-4 and IL-5. Cell Immunol 1996; 170: 230–234. [DOI] [PubMed] [Google Scholar]
- Tokuyama H, Tokuyama Y. Retinoic acid induces the expression of germ-line C alpha transcript mainly by a TGF-beta-independent mechanism. Cell Immunol 1997; 176: 14–21. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 2006; 314: 1157–1160. [DOI] [PubMed] [Google Scholar]
- Seo GY, Jang YS, Kim HA, Lee MR, Park MH, Park SR et al. Retinoic acid, acting as a highly specific IgA isotype switch factor, cooperates with TGF-β1 to enhance the overall IgA response. J Leukoc Biol 2013; 94: 325–335. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care 2009; 12: 293–297. [DOI] [PubMed] [Google Scholar]
- Legrand D, Elass E, Carpentier M, Mazurier J. Interactions of lactoferrin with cells involved in immune function. Biochem Cell Biol 2006; 84: 282–290. [DOI] [PubMed] [Google Scholar]
- Puddu P, Valenti P, Gessani S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie 2009; 91: 11–18. [DOI] [PubMed] [Google Scholar]
- Bilandzic M, Stenvers KL. Betaglycan: a multifunctional accessory. Mol Cell Endocrinol 2011; 339: 180–189. [DOI] [PubMed] [Google Scholar]
- López-Casillas F, Wrana JL, Massagué J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell 1993; 73: 1435–1444. [DOI] [PubMed] [Google Scholar]
- Jang YS, Seo GY, Lee JM, Seo HY, Han HJ, Kim SJ et al. Lactoferrin causes IgA and IgG2b isotype switching through betaglycan binding and activation of canonical TGF-β signaling. Mucosal Immunol 2015; 8: 906–917. [DOI] [PubMed] [Google Scholar]
- Iigo M, Alexander DB, Long N, Xu J, Fukamachi K, Futakuchi M et al. Anticarcinogenesis pathways activated by bovine lactoferrin in the murine small intestine. Biochimie 2009; 91: 86–101. [DOI] [PubMed] [Google Scholar]
- Park M-H, Park S-R, Lee M-R, Kim Y-H, Kim P-H. Retinoic acid induces expression of Ig germ line α transcript, an IgA isotype switching indicative, through retinoic acid receptor. Genes Genomics 2011; 33: 83–88. [Google Scholar]
- Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol 2001; 31: 1706–1715. [DOI] [PubMed] [Google Scholar]
- Pardali E, Xie XQ, Tsapogas P, Itoh S, Arvanitidis K, Heldin CH et al. Smad and AML proteins synergistically confer transforming growth factor beta1 responsiveness to human germ-line IgA genes. J Biol Chem 2000; 275: 3552–3560. [DOI] [PubMed] [Google Scholar]
- Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol 1990; 145: 3773–3778. [PubMed] [Google Scholar]
- Li SC, Rothman PB, Zhang J, Chan C, Hirsh D, Alt FW. Expression of I mu-C gamma hybrid germline transcripts subsequent to immunoglobulin heavy chain class switching. Int Immunol 1994; 6: 491–497. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000; 102: 553–563. [DOI] [PubMed] [Google Scholar]
- Seo GY, Jang YS, Kim J, Choe J, Han HJ, Lee JM et al. Retinoic acid acts as a selective human IgA switch factor. Hum Immunol 2014; 75: 923–929. [DOI] [PubMed] [Google Scholar]
- Lopez-Casillas F, Riquelme C, Perez-Kato Y, Ponce-Castaneda MV, Osses N, Esparza-Lopez J et al. Betaglycan expression is transcriptionally up-regulated during skeletal muscle differentiation. Cloning of murine betaglycan gene promoter and its modulation by MyoD, retinoic acid, and transforming growth factor-beta. J Biol Chem 2003; 278: 382–390. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004; 21: 527–538. [DOI] [PubMed] [Google Scholar]
- Rivera-Munoz P, Soulas-Sprauel P, Le Guyader G, Abramowski V, Bruneau S, Fischer A et al. Reduced immunoglobulin class switch recombination in the absence of Artemis. Blood 2009; 114: 3601–3609. [DOI] [PubMed] [Google Scholar]
- Ballard KD, Mah E, Guo Y, Pei R, Volek JS, Bruno RS. Low-fat milk ingestion prevents postprandial hyperglycemia-mediated impairments in vascular endothelial function in obese individuals with metabolic syndrome. J Nutr 2013; 143: 1602–1610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.