Abstract
Clara cell protein (CC16) is an anti-inflammatory protein, which is expressed in the airway epithelium. It is involved in the development of airway inflammatory diseases, including chronic obstructive pulmonary disease and asthma. However, the exact molecular mechanism underlying its anti-inflammatory action remains to be fully elucidated. The aim of the present study was to define the protein profiles of the anti-inflammatory effect of CC16 in lipopolysaccharide (LPS)-treated rat tracheal epithelial (RTE) cells using shotgun proteomics. Protein extracts were obtained from control RTE cells, RTE cells treated with LPS and RTE cells treated with LPS and recombinant CC16 (rCC16). Subsequent label-free quantification and bioinformatics analyses identified 12 proteins that were differentially expressed in the three treatment groups as a cluster of five distinct groups according to their molecular functions. Five of the twelve proteins were revealed to be associated with the cytoskeleton: Matrix metalloproteinase-9, myosin heavy chain 10, actin-related protein-3 homolog, elongation factor 1-α-1 (EF-1-α-1), and acidic ribosomal phosphoprotein P0. Five of the twelve proteins were associated with cellular proliferation: DNA-dependent protein kinase catalytic subunit, EF-1-α-1, tyrosine 3-monooxygenase, caspase recruitment domain (CARD) protein 12 and adenosylhomocysteinase (SAHH) 3. Three proteins were associated with gene regulation: EF-1-α-1, SAHH 3 and acidic ribosomal phosphoprotein P0. Three proteins were associated with inflammation: Tyrosine 3-monooxygenase, CARD protein 12 and statin-related protein. ATPase (H+-transporting, V1 subunit A, isoform 1) was revealed to be associated with energy metabolism, and uridine diphosphate glycosyltransferase 1 family polypeptide A8 with drug metabolism and detoxification. The identified proteins were further validated using reverse transcription-quantitative polymerase chain reaction. These protein profiles, and their interacting protein network, may facilitate the elucidation of the molecular mechanisms underlying the anti-inflammatory effects of CC16.
Keywords: Clara cell protein, lipopolysaccharide, proteome, airway inflammation, label-free quantitation
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation, which is usually progressive and typically involves clinical or pathological presentations (chronic bronchitis, emphysema and small airway disease) (1). Accumulating evidence over the past decade has demonstrated that the pathology of COPD, in addition to bronchoconstriction, may be attributed to inflammation of the airways (2). Inflammation occurring in airway epithelium is a defense mechanism to remove the injurious stimuli, which ensures the healing of tissues and cells. However, prolonged inflammation often causes progressive damage to the airway structure and function.
Clara cell protein (CC16), an anti-inflammatory protein secreted by epithelial Clara cells of the airways, is involved in the development of airway inflammatory diseases, including COPD and asthma (3). Reduced levels of CC16 in the bronchial epithelium of COPD patients and reduction of CC16-positive epithelial cells in the small airways of asthmatics contribute to aggravation of inflammatory responses in chronic lung inflammation (4,5). Induction of CC16 expression by gene transfection inhibits interleukin (IL)-1β-induced IL-8 expression in BEAS-2B bronchial epithelial cells by suppressing the transcriptional activity of nuclear factor (NF)-κB (6). Our previous study demonstrated that recombinant rat CC16 suppressed lipopolysaccharide (LPS)-mediated inflammatory matrix metalloproteinase (MMP)-9 production through inactivation of nuclear factor κB (NF-κB) and p38 mitogen-activated protein kinase signaling pathways in rat tracheal epithelial (RTE) cells (7). The anti-inflammatory properties of CC16 may render it a useful strategy for treatment of inflammatory respiratory disorders; This may be verified by the elucidation of the underlying mechanism via protein profile analysis of CC16-treated cells. Proteomics is an efficient method to identify the associated proteins that are downregulated or overexpressed in complex biological processes.
Mass spectrometry (MS)-based proteomics technologies are powerful tools used for large-scale protein identification and quantitation (8). Among these techniques, label-free shotgun proteomics is highly effective for the identification of peptides and, subsequently, to obtain a global protein profile of a sample. Label-free shotgun proteomics provides a unique opportunity to measure peptides present in a sample and subsequently determine the abundance of the proteins across various samples (9). Notably, label-free shotgun proteomics is suitable for applications in complex biological systems and generates faster, cleaner and simpler results (10). Consequently, numerous researchers are employing label-free shotgun proteomics techniques for discovery studies (11,12). In the present study, label-free quantitative shotgun proteomics was combined with MS to compare the protein expression profiles in RTE cells cultured in the absence or presence of LPS and recombinant CC16 (rCC16), to examine the molecular mechanisms underlying the anti-inflammatory action of rCC16.
Materials and methods
Cell culture and drug treatment
RTE cells were purchased from the Cell Culture Center of the Chinese Academy of Medical Sciences (Beijing, China) and cultured in Minimal Essential Medium with Earle's Balanced Salts (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal calf serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml streptomycin in a 5% CO2 humidified atmosphere at 37°C. rCC16 was prepared as previously described (7) and stored at −70°C until use.
RTE cells were cultured in 12.5-cm diameter dishes at 1×108 cells/dish, and divided into three groups. The cells were washed with phosphate-buffered saline (PBS) and cultured in serum-free media (control group), serum-free media supplemented with 0.1 µg/ml LPS (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 24 h (LPS treatment group) or pretreated with 2.0 µg/ml rCC16 in serum-free media for 2 h prior to 0.1 µg/ml LPS treatment for a further 24 h (rCC16 treatment group) (7).
Sample collection and protein extraction
Following drug treatment, RTE cells were trypsinized with 0.25% trypsin-EDTA (Thermo Fisher Scientific, Inc.) and cellular proteins were extracted according to standard protocols (13). Briefly, all samples were resuspended in 50 µl PBS, transferred to a 1.5-ml screw-capped tube and centrifuged at 10,000 × g for 30 min at 4°C. Next, 100 µl lysis buffer (7 M urea and 2 M thiourea) was added into each of the samples, which were then sonicated to extract total proteins. Proteins were precipitated with trichloroacetic acid for 30 min on ice and centrifuged at 40,000 × g for 30 min. Protein concentrations were determined using the Qubit Protein assay kit (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol.
The protein extracts were diluted with 50 mM NH4HCO3 to a final concentration 0.5 mg/ml. Following the addition of 100 mmol/l dithiothreitol to a final concentration of 10 mmol/l, the protein fractions were mixed at 56°C for 60 min, diluted 10X with 250 mmol/l 2-iodoacetamide and incubated in the dark for 60 min. Finally, the samples were digested with trypsin (substrate to enzyme mass to mass ratio, 50:1) at 37°C for 12 h. Digested supernatant fractions were stored at −80°C prior to MS analysis.
Liquid chromatography (LC)-tandem MS/MS analysis
The digested peptide mixtures were pressure-loaded onto a fused silica capillary column packed with 3-µm dionex C18 material [reversed phase (RP); Phenomenex, Torrance, CA, USA]. The RP sections of 100 Å were 15 cm long, and the column was washed with buffer A (water; 0.1% formic acid) and buffer B (can; 0.1% formic acid). Following desalting, a 5-mm, 300-µm C18 capture tip was placed in line with an Agilent 1100 quaternary HPLC (Agilent Technologies, Inc., Santa Clara, CA, USA) and analyzed using a 12-step separation.
The first step consisted of a 5-min gradient from 0 to 2% buffer B, followed by a 45-min gradient to 40% buffer B. Next, the buffer B flowed by 3-min gradient from 40 to 80% and 10-min at 80% buffer B. Following a 2-min buffer B gradient from 80 to 2%, ~20 µg tryptic peptide mixture was loaded onto the column and separated at a flow rate of 2 µl/min using a linear gradient. As peptides were eluted from the microcapillary column, they were electrosprayed directly into a micrOTOF-Q II™ mass spectrometer (Bruker Scientific Technology Co., Ltd., Beijing, China) with the application of a distal 180°C source temperature. The mass spectrometer was operated in the MS/MS (auto) mode. Survey MS scans were acquired in the time of flight (TOF)-Q II with the resolution set to a value of 20,000. Each survey scan (50~2,500) was followed by five data-dependent tandem MS/MS scans at 2 Hz normalized scan speed.
Bioinformatics analysis
Tandem mass spectra were searched against the mascot local host rat protein database version 2.1 (Matrix Science, Inc., Boston, MA, USA). The search results were then filtered using a cutoff of 1% as the peptide false identification rate. Peptides with a Z score <4 or Delta-Mass >5 ppm were rejected. The minimum number of peptides to identify a protein was set to 1. The default parameters for the quantification software, ProfileAnalysis software version 2.0, were used throughout the analysis (Bruker Scientific Technology Co., Ltd.).
Bioinformatics analysis was performed to categorize proteins based on the biological process, cellular component and molecular function using annotations in Protein Analysis Through Evolutionary Relationships (PANTHER) database version 6.1 (www.pantherdb.org) (14), which is in compliance with gene ontology (GO) standards. Signaling pathway analysis was performed with the tools on the Kyoto Encyclopedia of Genes and Genome (KEGG) database (www.genome.jp/kegg/pathway.html).
The identified proteins in the various groups were analyzed for their molecular function, biological process or pathway terms in PANTHER using the binomial test (15). Protein-protein interactions were obtained from the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database version 9.0 (string-db.org/), which contains known and predicted physical and functional protein-protein interactions (16). STRING in protein mode was used, and only interactions based on experimental protein-protein interactions and curated databases with confidence levels >0.5- were retained.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
To verify the differential patterns of protein expression obtained by LC-MS/MS analysis from cells treated with rCC16 and LPS or LPS only, or untreated control cells, total RNA was extracted from each of the groups of cells using TRIzol® reagent (CWBIO, Beijing, China). cDNA was synthesized using the SuperRT cDNA Synthesis Kit (cat. no. CW0741M; CWBIO, Beijing, China). Briefly, 20 µl reverse transcription mixture containing 4 µl deoxynucleotide triphosphate (dNTP) mix (2.5 mM each dNTP), 2 µl primer mix, 4 µl SuperRT buffer (5X), 1 µl SuperRT (200 U/µl), 1 µg RNA template and RNase-free water was prepared. The reaction conditions were as follows: Incubation at 42°C for 50 min followed by 85°C for 5 min using the PTC-100 Peltier Thermal Cycler (MJ Research, Inc., Waltham, MA, USA). qPCR was performed using an Applied Biosystems® Real-Time PCR Instrument (Thermo Fisher Scientific, Inc.) and a SYBR® Premix Ex Taq™ kit (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's protocol. qPCR was conducted in a 20 µl reaction mixture containing 1 µl cDNA under the following conditions: Denaturation at 95°C for 1 min, followed by 40 cycles of denaturation at 95°C for 10 sec and annealing at 60°C for 40 sec in triplicates. The 2-ΔΔCq method (17) was used to calculate the relative levels of target gene expression, and GAPDH expression was used as an internal control. The sequences of the primers used for qPCR are listed in Table I.
Table I.
Quantitative profiles of the differentially expressed proteins involved in label-free quantitative proteomics analysis and primer sequences.
| Sequences (5′-3′) | ||||||
|---|---|---|---|---|---|---|
| NCBI number | Protein | Unit peptides | Protein ratio LPS:RTE | Protein ratio rCC16+LPS:LPS | Forward | Reverse |
| gi|6756041 | Tyrosine 3-monooxygenase | 8 | 1.961 | 0.854 | CGAGCGATACGACGAAAT | GCTGATTATTCTCCAGGATGC |
| gi|206440 | Statin-related protein | 5 | 0.540 | 1.384 | GAAGAATGTGTCTGTCAAGGAC | GGGTGGTTCAGGATGATAAC |
| gi|40849846 | UDP glycosyltransferase 1 family polypeptide A8 | 4 | 0.813 | 3.431 | ATGGTCTACATTGGTGGGA | CGCTTTCTTCTCTGGAATCT |
| gi|13928704 | Myosin heavy chain 10, non-muscle | 4 | 5.167 | 0.539 | CAGATTCCTCTCCAACGG | GCAGCACTGAAGACACGA |
| gi|34852966 | Elongation factor 1-α-1 | 3 | 0.533 | 1.617 | TTGTTGCTGCTGGTGTTG | TGGTGACTCAGTGAAATCCA |
| gi|34879484 | Actin-related protein 3 homolog | 4 | 1.517 | 0.648 | GCATCCTGGACCTCAAGA | CAGCGATTGGAATGTGTTT |
| gi|34862480 | Caspase recruitment domain protein 12 | 4 | 0.826 | 1.782 | CCACGGAGGATGAGCAGTA | GGAGGTTCTTCAGATTACCCA |
| gi|34870011 | DNA-dependent protein kinase catalytic subunit | 3 | 0.709 | 1.304 | AGTGTTAGGTGAGGTTCATCCT | CAGTCCCTTTAGACAGCCA |
| gi|34852713 | ATPase, H+-transporting, V1 subunit A, isoform 1 | 1 | 0.793 | 1.368 | GAGAAGCCTCCATTTACACTG | GGTATGCTGGGTATCCACTAA |
| gi|34855075 | Putative adenosylhomocysteinase 3 | 2 | 0.613 | 1.417 | CACTAAGGAATGGCTGGAGTT | CATCAACCTCAGAATCGCA |
| gi|57708 | Acidic ribosomal phosphoprotein P0 | 4 | 1.326 | 0.741 | GACTACACTTTCCCACTGGC | TCCTCTGACTCTTCCTTTGC |
| GAPDH | GTGCCAGCCTCGTCTCATAG | CTTTGTCACAAGAGAAGGCAG | ||||
NCBI, National Center for Biotechnology Information; LPS, lipopolysaccharide; RTE; rat tracheal epithelial cell; CC16, Clara cell protein; UDP, uridine diphosphate; ATPase, adenosine triphosphatase.
Statistical analysis
Data are presented as mean ± standard deviation for three independent experiments. Comparisons between groups were performed with the Student's t-test. Statistical analyses were performed in GraphPad Prism software version 5 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Proteomic analysis
Protein samples obtained from the LPS-treated, rCC16 + LPS-treated and control RTE cells were subjected to quantitative proteomic analysis using label-free shotgun proteomics involving RP fractionation and high-resolution Fourier transform MS. The resulting mass spectra were searched against the rat RefSeq protein database of 25,050 protein sequence inputs using the ProfileAnalysis search algorithm. In total, 613 non-redundant proteins were identified based on the identification of one or more unique peptides, and 122 of these proteins were revealed to be differentially expressed in the three treatment groups. Among the differentially expressed proteins, 87 were upregulated and 23 downregulated in LPS-treated RTE cells compared with control cells; and 75 were upregulated and 17 downregulated in cells treated with LPS + rCC16 compared with those treated with LPS alone.
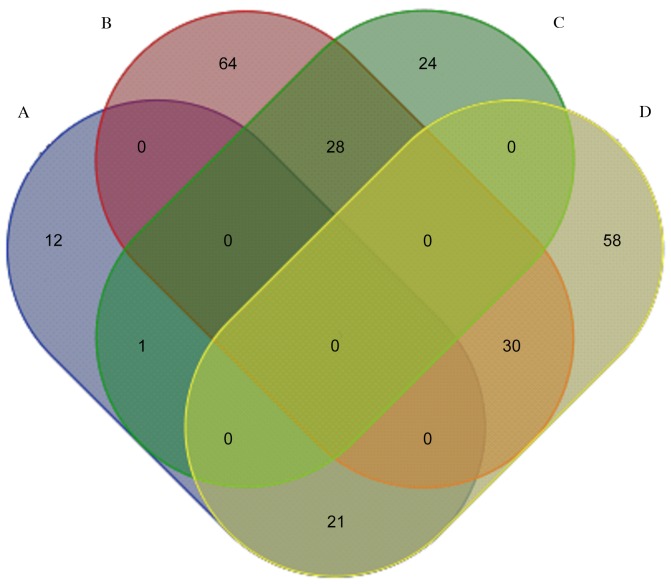
Fig. 1 presents a pairwise comparison of the identified proteins obtained from LPS-treated, rCC16 + LPS-treated and control RTE cells. A total of 28 proteins highly expressed in the LPS-treated group were downregulated in the rCC16 + LPS-treated group, and 21 proteins with low expression in the LPS-treated group were upregulated in the rCC16 + LPS-treated group. Of these 49 proteins, seven were downregulated in LPS-treated RTE cells, but upregulated in rCC16 + LPS-treated RTE cells, including uridine diphosphate (UDP) glycosyltransferase 1 family polypeptide A8 (UGT1A8), statin-related protein, elongation factor 1-α 1 (EF-1-α-1), adenosine triphosphatase (ATPase, H+-transporting, V1 subunit A, isoform 1; Atp6v1a1), DNA-dependent protein kinase catalytic subunit (DNA-PKcs), putative adenosylhomocysteinase (SAHH) 3 and caspase recruitment domain (CARD) protein 12. In addition, five proteins were observed to be upregulated in LPS-treated RTE cells and downregulated in rCC16 + LPS-treated RTE cells: Matrix metalloproteinase 9 (MMP-9), actin-related protein 3 homolog (Arp3), acidic ribosomal phosphoprotein P0, myosin heavy chain (MHC) type 10 (non-muscle) and tyrosine 3-monooxygenase (Fig. 2).
Figure 1.
Venn diagram presenting the differential expression patterns of the identified proteins. Comparisons of the differential expression patterns of the identified proteins were made as follows: (A) LPS vs. control, low expression in LPS-treated cells; (B) LPS vs. control, high expression in LPS-treated cells; (C) rCC16 + LPS vs. LPS, low expression in rCC16 + LPS-treated cells; and (D) rCC16 + LPS vs. LPS, high expression in rCC16 + LPS-treated cells. LPS, lipopolysaccharide; rCC16, recombinant Clara cell protein.
Figure 2.
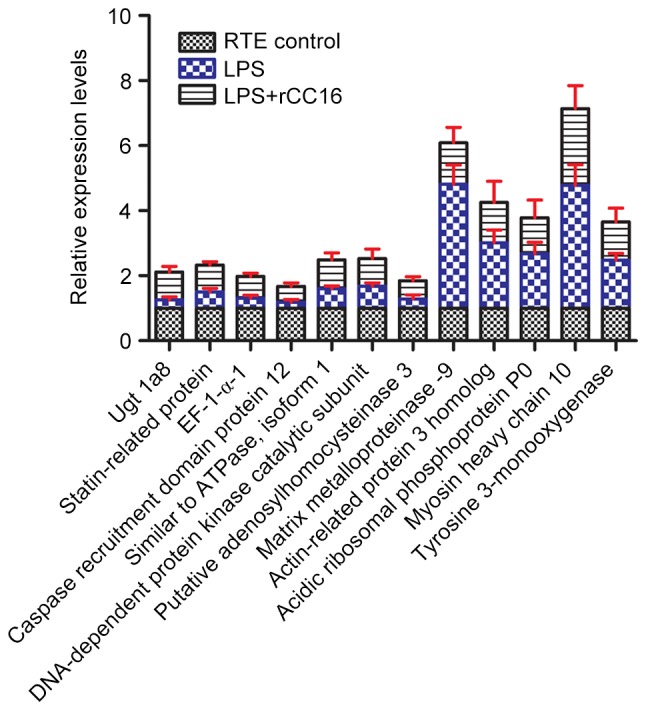
Protein expression profiles of RTE cells cultured in the presence or absence of LPS and rCC16. Seven proteins were downregulated by LPS only (LPS) and upregulated by additional rCC16 treatment (LPS+rCC16), and five proteins were upregulated by LPS only (LPS) and downregulated by further rCC16 treatment (LPS+rCC16). Data are presented as fold changes (≤0.83 for downregulated proteins or ≥1.2 for upregulated proteins) averaged from three independent experiments. RTE, rat tracheal epithelial; LPS, lipopolysaccharide; rCC16, recombinant Clara cell protein. Ugt 1a8, uridine diphosphate glycosyltransferase 1 family polypeptide A8; EF-1-α-1, elongation factor 1-α 1.
Categorization of the 12 differentially expressed proteins based on GO annotation
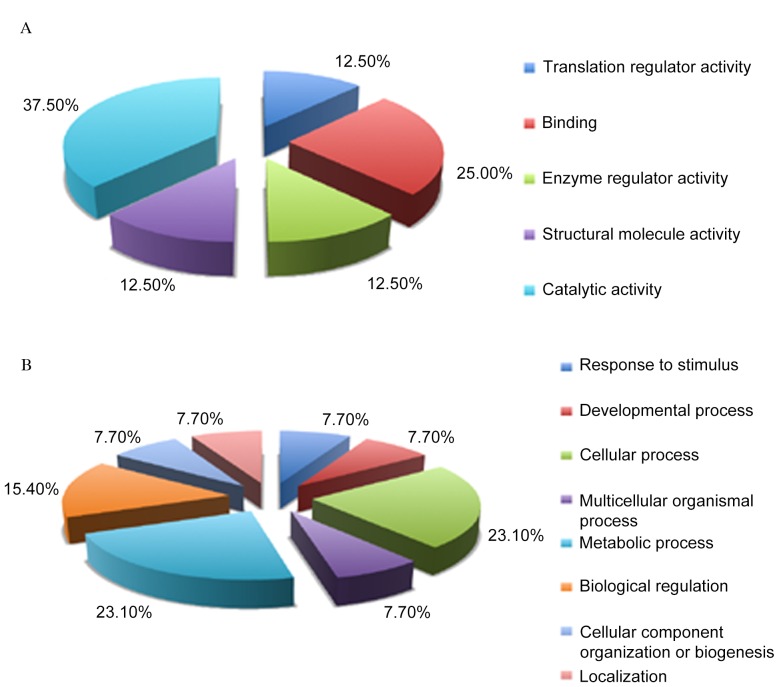
Differentially expressed proteins were categorized as those that were downregulated in LPS-treated RTE cells, but upregulated in rCC16+LPS-treated RTE cells, or those which were upregulated in LPS-treated RTE cells but downregulated in rCC16+LPS-treated RTE cells (Fig. 2). To further understand these differentially expressed proteins, a GO analysis was performed with the PANTHER classification system to determine the molecular functions and associated biological processes of these 12 proteins. Based on their molecular functions, these differentially expressed proteins may be classified into five groups: Translation regulator activity (12.5%), binding (25%), enzyme regular activity (12.5%), structural molecule activity (12.5%) and catalytic activity (37.5%; Fig. 3A). Accordingly, these proteins were classified into eight groups of biological processes: Response to stimulus (7.7%), developmental process (7.7%), cellular process (23.1%), multicellular organismal process (7.7%), metabolic process (23.1%), biological regulation (15.4%), cellular component organization or biogenesis (7.7%) and localization (7.7%; Fig. 3B). These data suggest the possible molecular components and pathways involved in the transcription and energy metabolism activities of LPS and rCC16-responsive RTE cells.
Figure 3.
Gene ontology analysis of differentially expressed proteins based on (A) their molecular functions and (B) their associated biological processes, using PANTHER classification. Over-representation statistics were performed using the hypergeometric analysis and Benjamini & Hochberg False Discovery Rate correction.
STRING protein-protein analysis of differentially expressed proteins
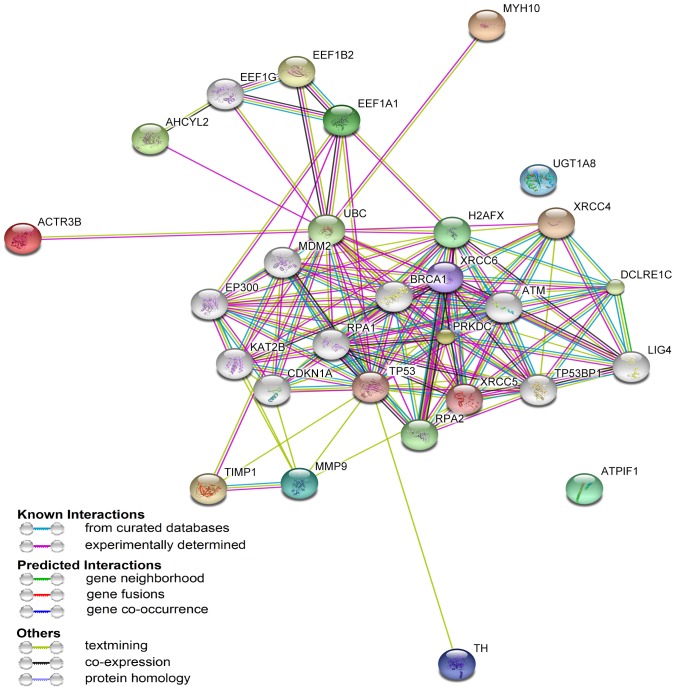
STRING is a database program for protein-protein interaction analysis to generate a network of interactions from a variety of sources, including various interaction databases, text mining, genetic interactions and shared pathway interactions. To further characterize the functions associated with the marked differences in the expression of the 12 proteins, these proteins were analyzed using STRING software version 9.0- Known and Predicted Protein-Protein Interactions. As presented in Fig. 4, the 12 differentially expressed proteins were connected with numerous proteins associated with inflammation, including tumor protein p53 (TP53) and ataxia telangiectasia-mutated kinase (ATM) (18). These data indicate that an interacting network of proteins is associated with the molecular mechanisms underlying the anti-inflammatory activity of CC16.
Figure 4.
A network of interacting proteins associated with anti-inflammatory action of rCC16, as revealed by Search Tool for the Retrieval of Interacting Genes/Proteins analysis. rCC16, recombinant Clara cell protein.
RT-qPCR analysis of differentially expressed proteins
To further determine the differential expression patterns of these proteins in RTE cells cultured in the absence or presence of LPS and rCC16, RT-qPCR was performed for transcriptional analyses of the differentially expressed proteins at the mRNA level, (with the exception of MMP-9, the expression of which has been previously described by our laboratory) (7). As presented in Fig. 5, all 11 genes exhibited similar patterns of expression to their corresponding proteins, including tyrosine 3-monooxygenase, statin-related protein, UGT1A8, MHC type 10, EF-1-α-1, Arp3, CARD protein 12, acidic ribosomal phosphoprotein P0, DNA-PKcs, Atp6v1a1 and putative SAHH 3. Certain variations may exist between the levels of qPCR-determined gene expression and their proteins, including, for example, EF-1-α-1, CARD protein 12 and DNA-PKcs. These variations between protein and mRNA expression levels may be due to alterations in the regulation of protein translation and degradation (19). In addition, specific processes that occur prior to translation may influence the mRNA translation efficiency. For example, the poly (A) tail length can affect transcript stability and therefore the abundance of a specific mRNA sequence (20).
Figure 5.
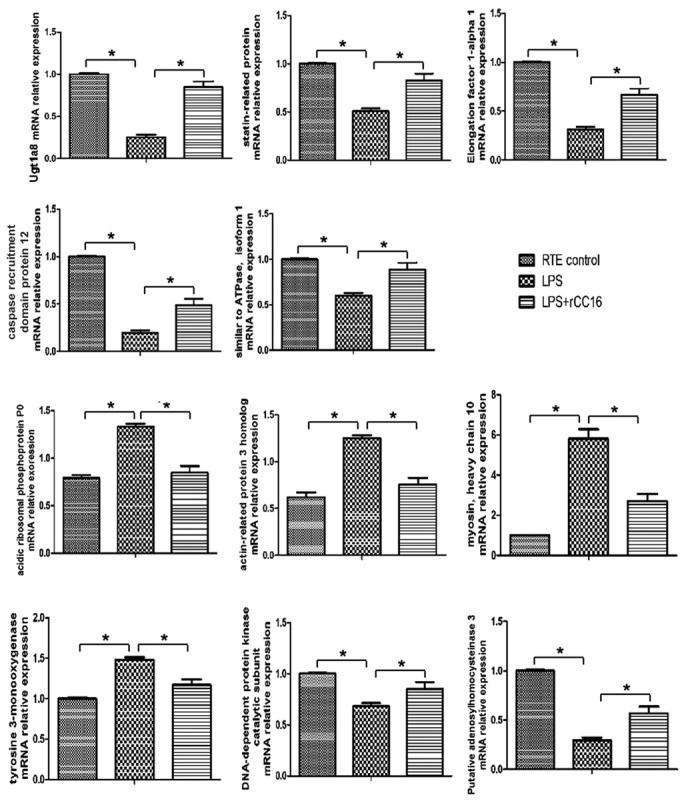
Reverse transcription-quantitative polymerase chain reaction analysis of genes coding for the differentially expressed proteins. mRNA expression levels followed a similar pattern to protein expression levels. *P<0.05. Ugt 1a8, uridine diphosphate glycosyltransferase 1 family polypeptide A8.
Discussion
CC16 is an anti-inflammatory protein involved in the development of airway inflammatory diseases, including COPD. The present study has identified a differential pattern of protein expressions in RTE cells cultured in the presence or absence of LPS and rCC16 using label-free shotgun proteomics, and verified this at the mRNA level by RT-qPCR. The differentially expressed proteins of various molecular functions and associated biological processes are connected with numerous proteins involved in inflammation in a unique protein-protein interacting network. The present study provides a basis for the further understanding of the role of CC16 and its anti-inflammation activity in the development of airway diseases, including COPD.
Inflammation is one of mechanisms underlying the development of COPD. Therefore, reducing the inflammatory response is regarded as the primary interference measure for COPD patients. It is well-known that CC16 is a secretory protein with anti-inflammatory characteristics (21). Reduced levels of CC16 in the bronchial epithelium or sputum supernatants of COPD patients may be a contributing factor in airway inflammations (4,22). Consequently, administration of CC16 may be an effective treatment for COPD. Our previous study demonstrated that rCC16 inhibited the expression of COPD-contributing MMP-9 in LPS-treated RTE cells via the attenuation of NF-κB activation (7). The present study has identified a network of proteins involved in the mechanisms underlying the anti-inflammatory activity of rCC16.
For example, exposure of RTE cells to LPS increased MHC type 10 expression that was abolished by further rCC16 treatment. Systemic muscle inflammation is known to be a major factor in COPD, particularly following exacerbations (23). The changes of MHC perturb the function and structure of muscle (24) and types IIx, IIa and I of MHC isoforms are expressed in human skeletal muscle (25). The downregulation of the type I isoform, and upregulation of the type IIa isoform, has been demonstrated in patients with COPD (26). In addition, reduction of MHC IIa fibers has been reported in LPS-treated fetal membranes (chorioamnionitis) (27). While the reason for rCC16-reduced MHC type 10 expression remains unclear, carbonylation of MHC is well known to be degraded by the ubiquitin-proteasome pathway (24). Therefore, rCC16 may be involved in the process of carbonylation of MHC type 10. In addition, the cytoskeletal protein Arp3 is important in airway smooth muscle contraction as a subunit of the Arp2/3 complex that initiates the branching of actin filaments (28). A previous study revealed that the carbachol-stimulated cytoskeletal recruitment of Arp3 was upregulated at short muscle lengths and downregulated at long muscle lengths (29). In the present study, Arp3 was increased in LPS-treated RTE cells, and decreased by further rCC16 treatment. Similarly, rCC16 reduced the levels of LPS-induced expression of tyrosine 3-monooxygenase, which encodes the protein, 14-3-3 η. The latter belongs to the 14-3-3 family of proteins that mediate signal transduction and are required for mitotic progression. Upregulation of 14-3-3 η has been evident in sporadic Creutzfeldt-Jakob disease, joint inflammation and head-and-neck squamous cell carcinoma (30–32). In accordance with this, the results of the present study demonstrated that rCC16 enhanced the expression of DNA-PKcs, which is crucial for DNA double-strand break repair and normal cell cycle progression (33). High-dose CC16 has been revealed to delay the growth of immortalized bronchial epithelial cells and inhibit platelet-derived growth factor-induced airway smooth muscle cell proliferation (34,35). CC16 knockdown in vivo results in augmented inflammation due to polymorphonuclear leukocyte recruitment to the air space in mice exposed to LPS (36). Therefore, rCC16 may target the signaling molecules involved in cytoskeletal recruitment and mitotic progressions of bronchial epithelial cells and inflammatory cells.
In the present study, a number of proteins were identified that were downregulated by LPS and upregulated by rCC16 in RTE cells, including statin-related protein, CARD protein 12, eukaryotic translation EF-1-α-1, SAHH and acidic ribosomal phosphoprotein P0. Statin-related protein is a 57 kDa nuclear protein present in non-dividing cells (37) and involved in the process of myopathy (38). Although the functions of statin-related protein remain to be fully elucidated, an anti-inflammatory activity via tumor necrosis factor-α reduction has been reported for the statin-related rosuvastatin protein (39). CARD proteins are scaffold proteins that function as key regulators of NF-κB signaling by providing a link between membrane receptors and NF-κB transcriptional subunits (40). CARD11 and CARD14-mediated alterations in NF-κB signaling are thought to be an early event in the development of cutaneous squamous cell carcinoma and psoriasis, respectively (40,41). CARD9 in macrophages mediates necrotic smooth muscle cell-induced inflammation by activating NF-κB (42). Other CARD proteins, including CARD16, CARD17 and CARD18, negatively regulate inflammation by inhibiting the activation of caspase-1, which may induce the expression of IL-1β without altering NF-κB signaling (43,44). EF-1-α-1 is a ubiquitously expressed GTPase that couples the hydrolysis of guanosine triphosphate (GTP) to guanosine diphosphate (GDP), with the delivery of aminoacyl transfer RNAs to the ribosome during protein translation (45,46). In addition, it is involved in cell proliferation, cytoskeletal organization and protein degradation (47–49). High levels of EF-1-α-1 have been associated with survival (50) and downregulation of EF-1-α-1 expression resulted in cell death (51). SAHH 3 is an NAD+-dependent tetrameric enzyme that catalyzes the breakdown of S-adenosylhomocysteine to adenosine and homocysteine, and is therefore important in cell growth and regulation of gene expression (52). Loss of SAHH function may result in global inhibition of cellular methyltransferase enzymes due to S-adenosylhomocysteine accumulation (52). Acidic ribosomal phosphoprotein P0 is a structural component of the ribosome of all organisms. It interacts with trans-acting factor Mrt4 during ribosome assembly and is important for protein synthesis (53). These data together suggest that rCC16 may function as an anti-inflammatory effector by altering the expression of tyrosine 3-monooxygenase, statin-related protein, CARD, eEF1A1, SAHH and acidic ribosomal phosphoprotein P0. The effect of CC16 on these proteins requires further study.
In addition, the present study demonstrated that rCC16 promoted expression of Atp6v1a1 and UGT1A8. Atp6v1a1 belongs to the respiratory electron transport chain (ETC) of oxidative phosphorylation in energy metabolism. Downregulated expression of Atp6v1a1 inhibits the efficiency of ETC and ATP synthesis (54). This is consistent with the knowledge that the disorder of skeletal muscle energy metabolism is important in COPD, and that systemic inflammation alters the state of energy metabolism in peripheral muscles, causing them to dysfunction (55). While UGTs are well known for their drug-metabolizing and detoxification potentials in drug toxicity (56), UGT1A8 has in addition been associated with metabolism of mycophenolic acid (57), raloxifene (58) and piceatannol, which is a dietary polyphenol present in grapes and wine and may possess anticancer and anti-inflammatory activities (59). Therefore, the anti-inflammatory action of rCC16 may be associated with the activities of skeletal muscle energy metabolism and UGT1A8.
The differentially expressed proteins of diverse functions and biological processes were linked to numerous other inflammation-associated proteins, including TP53 and ATM by protein-protein interaction analysis using STRING software. Expression of inflammatory cytokine genes may be directly induced by TP53- and ATM-dependent mechanisms upon radiation exposure in human monocytes and macrophages (18). In addition, activation of pro-inflammatory pathways during COPD involves enhanced expression of TP53 and TP21 in lung fibroblasts (60). However, ATM deficiency in mice delays the acute inflammatory response following myocardial infarction (61). While TP53 and ATM pathways are always integrated with NF-κB signaling in inflammation (62–64), their role in the anti-inflammatory effect of CC16 remains to be determined.
In conclusion, the results of the present study demonstrate that label-free differential proteomics is able to reveal the protein profiles of the anti-inflammatory action of CC16 on RTE cells. The differential proteins and their interacting network of proteins identified in the present study may provide a basis for elucidating the molecular mechanisms underlying the anti-inflammatory effects of CC16. Further studies into the role of these proteins in COPD pathogenesis and its cellular and tissue models may lead to the development of novel diagnostic and therapeutic strategies for this disease.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81200032), the Research Project Supported by Shanxi Scholarship Council of China (grant no. 2015-101), the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (grant no. 2016-097) and the Research Fund for Doctoral Program of Shanxi Medical University (grant no. 03201539).
References
- 1.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen LC, Zhang Z, Myers AC, Huang SK. Cutting edge: Altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10-kDa protein. J Immunol. 2001;167:3025–3028. doi: 10.4049/jimmunol.167.6.3025. [DOI] [PubMed] [Google Scholar]
- 4.Pilette C, Godding V, Kiss R, Delos M, Verbeken E, Decaestecker C, De Paepe K, Vaerman JP, Decramer M, Sibille Y. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:185–194. doi: 10.1164/ajrccm.163.1.9912137. [DOI] [PubMed] [Google Scholar]
- 5.Shijubo N, Itoh Y, Yamaguchi T, Imada A, Hirasawa M, Yamada T, Kawai T, Abe S. Clara cell protein-positive epithelial cells are reduced in small airways of asthmatics. Am J Respir Crit Care Med. 1999;160:930–933. doi: 10.1164/ajrccm.160.3.9803113. [DOI] [PubMed] [Google Scholar]
- 6.Long XB, Hu S, Wang N, Zhen HT, Cui YH, Liu Z. Clara cell 10-kDa protein gene transfection inhibits NF-κB activity in airway epithelial cells. PLoS One. 2012;7:e35960. doi: 10.1371/journal.pone.0035960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang M, Wang H, Bai JZ, Cao D, Jiang Y, Zhang C, Liu Z, Zhang X, Hu X, Xu J, Du Y. Recombinant rat CC16 protein inhibits LPS-induced MMP-9 expression via NF-κB pathway in rat tracheal epithelial cells. Exp Biol Med (Maywood) 2015;240:1266–1278. doi: 10.1177/1535370215570202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cravatt BF, Simon GM, Yates JR., III The biological impact of mass-spectrometry-based proteomics. Nature. 2007;450:991–1000. doi: 10.1038/nature06525. [DOI] [PubMed] [Google Scholar]
- 9.Domon B, Aebersold R. Options and considerations when selecting a quantitative proteomics strategy. Nat Biotechnol. 2010;28:710–721. doi: 10.1038/nbt.1661. [DOI] [PubMed] [Google Scholar]
- 10.Bauer KM, Lambert PA, Hummon AB. Comparative label-free LC-MS/MS analysis of colorectal adenocarcinoma and metastatic cells treated with 5-fluorouracil. Proteomics. 2012;12:1928–1937. doi: 10.1002/pmic.201200041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clough T, Thaminy S, Ragg S, Aebersold R, Vitek O. Statistical protein quantification and significance analysis in label-free LC-MS experiments with complex designs. BMC Bioinformatics. 2012;13:S6. doi: 10.1186/1471-2105-13-S16-S6. (Suppl 16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niehl A, Zhang ZJ, Kuiper M, Peck SC, Heinlein M. Label-free quantitative proteomic analysis of systemic responses to local wounding and virus infection in Arabidopsis thaliana. J Proteome Res. 2013;12:2491–2503. doi: 10.1021/pr3010698. [DOI] [PubMed] [Google Scholar]
- 13.Guerrera IC, Quetier I, Fetouchi R, Moreau F, Vauloup-Fellous C, Lekbaby B, Rousselot C, Chhuon C, Edelman A, Lefevre M, et al. Regulation of interleukin-6 in head and neck squamous cell carcinoma is related to papillomavirus infection. J Proteome Res. 2014;13:1002–1011. doi: 10.1021/pr401009f. [DOI] [PubMed] [Google Scholar]
- 14.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho RJ, Campbell MJ. Transcription, genomes, function. Trends Genet. 2000;16:409–415. doi: 10.1016/S0168-9525(00)02065-5. [DOI] [PubMed] [Google Scholar]
- 16.Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, Muller J, Doerks T, Julien P, Roth A, Simonovic M, et al. STRING 8-a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37:D412–D416. doi: 10.1093/nar/gkn760. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Candeias SM, Testard I. The many interactions between the innate immune system and the response to radiation. Cancer Lett. 2015;368:173–178. doi: 10.1016/j.canlet.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 19.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lackner DH, Beilharz TH, Marguerat S, Mata J, Watt S, Schubert F, Preiss T, Bähler J. A network of multiple regulatory layers shapes gene expression in fission yeast. Mol Cell. 2007;26:145–155. doi: 10.1016/j.molcel.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irander K, Palm JP, Borres MP, Ghafouri B. Clara cell protein in nasal lavage fluid and nasal nitric oxide-biomarkers with anti-inflammatory properties in allergic rhinitis. Clin Mol Allergy. 2012;10:4. doi: 10.1186/1476-7961-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lensmar C, Nord M, Gudmundsson GH, Roquet A, Andersson O, Jörnvall H, Eklund A, Grunewald J, Agerberth B. Decreased pulmonary levels of the anti-inflammatory Clara cell 16 kDa protein after induction of airway inflammation in asthmatics. Cell Mol Life Sci. 2000;57:976–981. doi: 10.1007/PL00000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spruit MA, Gosselink R, Troosters T, Kasran A, Gayan-Ramirez G, Bogaerts P, Bouillon R, Decramer M. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58:752–756. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada T, Mishima T, Sakamoto M, Sugiyama M, Matsunaga S, Wada M. Oxidation of myosin heavy chain and reduction in force production in hyperthyroid rat soleus. J Appl Physiol (1985) 2006;100:1520–1526. doi: 10.1152/japplphysiol.01456.2005. [DOI] [PubMed] [Google Scholar]
- 25.Serrano AL, Pérez M, Lucía A, Chicharro JL, Quiroz-Rothe E, Rivero JL. Immunolabelling, histochemistry and in situ hybridisation in human skeletal muscle fibres to detect myosin heavy chain expression at the protein and mRNA level. J Anat. 2001;199:329–337. doi: 10.1046/j.1469-7580.2001.19930329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maltais F, Sullivan MJ, LeBlanc P, Schachat FH, Simard C, Blank JM, Jobin J. Altered expression of myosin heavy chain in the vastus lateralis muscle in patients with COPD. Eur Respir J. 1999;13:850–854. doi: 10.1034/j.1399-3003.1999.13d26.x. [DOI] [PubMed] [Google Scholar]
- 27.Song Y, Karisnan K, Noble PB, Berry CA, Lavin T, Moss TJ, Bakker AJ, Pinniger GJ, Pillow JJ. In utero LPS exposure impairs preterm diaphragm contractility. Am J Respir Cell Mol Biol. 2013;49:866–874. doi: 10.1165/rcmb.2013-0107OC. [DOI] [PubMed] [Google Scholar]
- 28.Goley ED, Welch MD. The ARP2/3 complex: An actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 29.Kim HR, Liu K, Roberts TJ, Hai CM. Length-dependent modulation of cytoskeletal remodeling and mechanical energetics in airway smooth muscle. Am J Respir Cell Mol Biol. 2011;44:888–897. doi: 10.1165/rcmb.2010-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schubert KO, Focking M, Cotter DR. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling and glucose metabolism: Potential roles in GABAergic interneuron pathology. Schizophr Res. 2015;167:64–72. doi: 10.1016/j.schres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Yun J, Jeong BH, Kim HJ, Park YJ, Lee YJ, Choi EK, Carp RI, Kim YS. A polymorphism in the YWHAH gene encoding 14-3-3 eta that is not associated with sporadic Creutzfeldt-Jakob disease (CJD) Mol Biol Rep. 2012;39:3619–3625. doi: 10.1007/s11033-011-1136-0. [DOI] [PubMed] [Google Scholar]
- 32.Kilani RT, Maksymowych WP, Aitken A, Boire G, St-Pierre Y, Li Y, Ghahary A. Detection of high levels of 2 specific isoforms of 14-3-3 proteins in synovial fluid from patients with joint inflammation. J Rheumatol. 2007;34:1650–1657. [PubMed] [Google Scholar]
- 33.Davis AJ, Chen BP, Chen DJ. DNA-PK: A dynamic enzyme in a versatile DSB repair pathway. DNA Repair (Amst) 2014;17:21–29. doi: 10.1016/j.dnarep.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y, Xu YD, Yin LM, Wang Y, Ran J, Liu Q, Ma ZF, Liu YY, Yang YQ. Recombinant rat CC10 protein inhibits PDGF-induced airway smooth muscle cells proliferation and migration. Biomed Res Int. 2013;2013:690937. doi: 10.1155/2013/690937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linnoila RI, Szabo E, DeMayo F, Witschi H, Sabourin C, Malkinson A. The role of CC10 in pulmonary carcinogenesis: From a marker to tumor suppression. Ann N Y Acad Sci. 2000;923:249–267. doi: 10.1111/j.1749-6632.2000.tb05534.x. [DOI] [PubMed] [Google Scholar]
- 36.Snyder JC, Reynolds SD, Hollingsworth JW, Li Z, Kaminski N, Stripp BR. Clara cells attenuate the inflammatory response through regulation of macrophage behavior. Am J Respir Cell Mol Biol. 2010;42:161–171. doi: 10.1165/rcmb.2008-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ann DK, Wechsler A, Lin HH, Wang E. Isoproterenol downregulation of statin-related gene expression in the rat parotid gland. J Cell Sci. 1991;100:641–647. doi: 10.1242/jcs.100.3.641. [DOI] [PubMed] [Google Scholar]
- 38.Apostolopoulou M, Corsini A, Roden M. The role of mitochondria in statin-induced myopathy. Eur J Clin Invest. 2015;45:745–754. doi: 10.1111/eci.12461. [DOI] [PubMed] [Google Scholar]
- 39.McGuire TR, Kalil AC, Dobesh PP, Klepser DG, Olsen KM. Anti-inflammatory effects of rosuvastatin in healthy subjects: A prospective longitudinal study. Curr Pharm Des. 2014;20:1156–1160. doi: 10.2174/1381612820666140127163313. [DOI] [PubMed] [Google Scholar]
- 40.Watt SA, Purdie KJ, den Breems NY, Dimon M, Arron ST, McHugh AT, Xue DJ, Dayal JH, Proby CM, Harwood CA, et al. Novel CARD11 mutations in human cutaneous squamous cell carcinoma lead to aberrant NF-κB regulation. Am J Pathol. 2015;185:2354–2363. doi: 10.1016/j.ajpath.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harden JL, Lewis SM, Pierson KC, Suárez-Fariñas M, Lentini T, Ortenzio FS, Zaba LC, Goldbach-Mansky R, Bowcock AM, Lowes MA. CARD14 expression in dermal endothelial cells in psoriasis. PLoS One. 2014;9:e111255. doi: 10.1371/journal.pone.0111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Wang Y, Shi H, Jia L, Cheng J, Cui W, Li H, Li P, Du J. CARD9 mediates necrotic smooth muscle cell-induced inflammation in macrophages contributing to neointima formation of vein grafts. Cardiovasc Res. 2015;108:148–158. doi: 10.1093/cvr/cvv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Druilhe A, Srinivasula SM, Razmara M, Ahmad M, Alnemri ES. Regulation of IL-1beta generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ. 2001;8:649–657. doi: 10.1038/sj.cdd.4400881. [DOI] [PubMed] [Google Scholar]
- 44.Lamkanfi M, Denecker G, Kalai M, D'hondt K, Meeus A, Declercq W, Saelens X, Vandenabeele P. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1beta generation. J Biol Chem. 2004;279:51729–51738. doi: 10.1074/jbc.M407891200. [DOI] [PubMed] [Google Scholar]
- 45.Hershey JW. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- 46.Thornton S, Anand N, Purcell D, Lee J. Not just for housekeeping: Protein initiation and elongation factors in cell growth and tumorigenesis. J Mol Med (Berl) 2003;81:536–548. doi: 10.1007/s00109-003-0461-8. [DOI] [PubMed] [Google Scholar]
- 47.Lamberti A, Caraglia M, Longo O, Marra M, Abbruzzese A, Arcari P. The translation elongation factor 1A in tumorigenesis, signal transduction and apoptosis: Review article. Amino Acids. 2004;26:443–448. doi: 10.1007/s00726-004-0088-2. [DOI] [PubMed] [Google Scholar]
- 48.Gross SR, Kinzy TG. Translation elongation factor 1A is essential for regulation of the actin cytoskeleton and cell morphology. Nat Struct Mol Biol. 2005;12:772–778. doi: 10.1038/nsmb979. [DOI] [PubMed] [Google Scholar]
- 49.Chuang SM, Chen L, Lambertson D, Anand M, Kinzy TG, Madura K. Proteasome-mediated degradation of cotranslationally damaged proteins involves translation elongation factor 1A. Mol Cell Biol. 2005;25:403–413. doi: 10.1128/MCB.25.1.403-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamberti A, Longo O, Marra M, Tagliaferri P, Bismuto E, Fiengo A, Viscomi C, Budillon A, Rapp UR, Wang E, et al. C-Raf antagonizes apoptosis induced by IFN-alpha in human lung cancer cells by phosphorylation and increase of the intracellular content of elongation factor 1A. Cell Death Differ. 2007;14:952–962. doi: 10.1038/sj.cdd.4402102. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi Y, Yonehara S. Novel cell death by downregulation of eEF1A1 expression in tetraploids. Cell Death Differ. 2009;16:139–150. doi: 10.1038/cdd.2008.136. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Kavran JM, Chen Z, Karukurichi KR, Leahy DJ, Cole PA. Regulation of S-adenosylhomocysteine hydrolase by lysine acetylation. J Biol Chem. 2014;289:31361–31372. doi: 10.1074/jbc.M114.597153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodríguez-Mateos M, García-Gómez JJ, Francisco-Velilla R, Remacha M, de la Cruz J, Ballesta JP. Role and dynamics of the ribosomal protein P0 and its related trans-acting factor Mrt4 during ribosome assembly in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:7519–7532. doi: 10.1093/nar/gkp806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji B, La Y, Gao L, Zhu H, Tian N, Zhang M, Yang Y, Zhao X, Tang R, Ma G, et al. A comparative proteomics analysis of rat mitochondria from the cerebral cortex and hippocampus in response to antipsychotic medications. J Proteome Res. 2009;8:3633–3641. doi: 10.1021/pr800876z. [DOI] [PubMed] [Google Scholar]
- 55.Davidsen PK, Herbert JM, Antczak P, Clarke K, Ferrer E, Peinado VI, Gonzalez C, Roca J, Egginton S, Barberá JA, Falciani F. A systems biology approach reveals a link between systemic cytokines and skeletal muscle energy metabolism in a rodent smoking model and human COPD. Genome Med. 2014;6:59. doi: 10.1186/s13073-014-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meech R, Miners JO, Lewis BC, Mackenzie PI. The glycosidation of xenobiotics and endogenous compounds: Versatility and redundancy in the UDP glycosyltransferase superfamily. Pharmacol Ther. 2012;134:200–218. doi: 10.1016/j.pharmthera.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 57.Vu D, Tellez-Corrales E, Yang J, Qazi Y, Shah T, Naraghi R, Hutchinson IV, Min DI. Genetic polymorphisms of UGT1A8, UGT1A9 and HNF-1α and gastrointestinal symptoms in renal transplant recipients taking mycophenolic acid. Transpl Immunol. 2013;29:155–161. doi: 10.1016/j.trim.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Sun D, Jones NR, Manni A, Lazarus P. Characterization of raloxifene glucuronidation: Potential role of UGT1A8 genotype on raloxifene metabolism in vivo. Cancer Prev Res (Phila) 2013;6:719–730. doi: 10.1158/1940-6207.CAPR-12-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miksits M, Maier-Salamon A, Vo TP, Sulyok M, Schuhmacher R, Szekeres T, Jäger W. Glucuronidation of piceatannol by human liver microsomes: Major role of UGT1A1, UGT1A8 and UGT1A10. J Pharm Pharmacol. 2010;62:47–54. doi: 10.1211/jpp.62.01.0004. [DOI] [PubMed] [Google Scholar]
- 60.D'Anna C, Cigna D, Costanzo G, Ferraro M, Siena L, Vitulo P, Gjomarkaj M, Pace E. Cigarette smoke alters cell cycle and induces inflammation in lung fibroblasts. Life Sci. 2015;126:10–18. doi: 10.1016/j.lfs.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Daniel LL, Daniels CR, Harirforoosh S, Foster CR, Singh M, Singh K. Deficiency of ataxia telangiectasia mutated kinase delays inflammatory response in the heart following myocardial infarction. J Am Heart Assoc. 2014;3:e001286. doi: 10.1161/JAHA.114.001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 2015;5:1266–1283. doi: 10.3390/biom5031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pal S, Bhattacharjee A, Ali A, Mandal NC, Mandal SC, Pal M. Chronic inflammation and cancer: Potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J Inflamm (Lond) 2014;11:23. doi: 10.1186/1476-9255-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osorio FG, Bárcena C, Soria-Valles C, Ramsay AJ, de Carlos F, Cobo J, Fueyo A, Freije JM, López-Otín C. Nuclear lamina defects cause ATM-dependent NF-κB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 2012;26:2311–2324. doi: 10.1101/gad.197954.112. [DOI] [PMC free article] [PubMed] [Google Scholar]