Abstract
As of May 2016, 81 countries have introduced Rotarix or RotaTeq rotavirus vaccines into their national immunization program. Despite initially slow uptake in some countries and differences in vaccine effectiveness (VE) between high-, low- and middle-income countries, impact of the vaccines has been swift and striking in all settings, with good VE against vaccine-type and nonvaccine-type strains. Newly published research indicates poor nutrition is associated with decreased VE and breastfeeding at the time of vaccination does not affect vaccine response. Vaccines in development and proposed alternate schedules also promise to address limitations of the current vaccines and optimize rotavirus disease prevention.
Keywords: rotavirus, rotavirus vaccine, vaccines prophylactic
Current status
Two live attenuated oral rotavirus vaccines were licensed in 2006; RotaTeq (rotavirus vaccine [RV] 5, Merck & Co.) is a three-dose pentavalent bovine-human reassortant rotavirus vaccine and Rotarix (RV1, GSK Biologics) is a two-dose monovalent human rotavirus vaccine. In 2009, the WHO recommended implementation of rotavirus vaccines worldwide; rotavirus vaccine is recommended to be administered in infancy concurrently with polio, diphtheria-tetanus-pertussis (DTP) and pneumococcal (PCV) vaccines as early as 6 weeks of age [1].
Global use & coverage of rotavirus vaccines
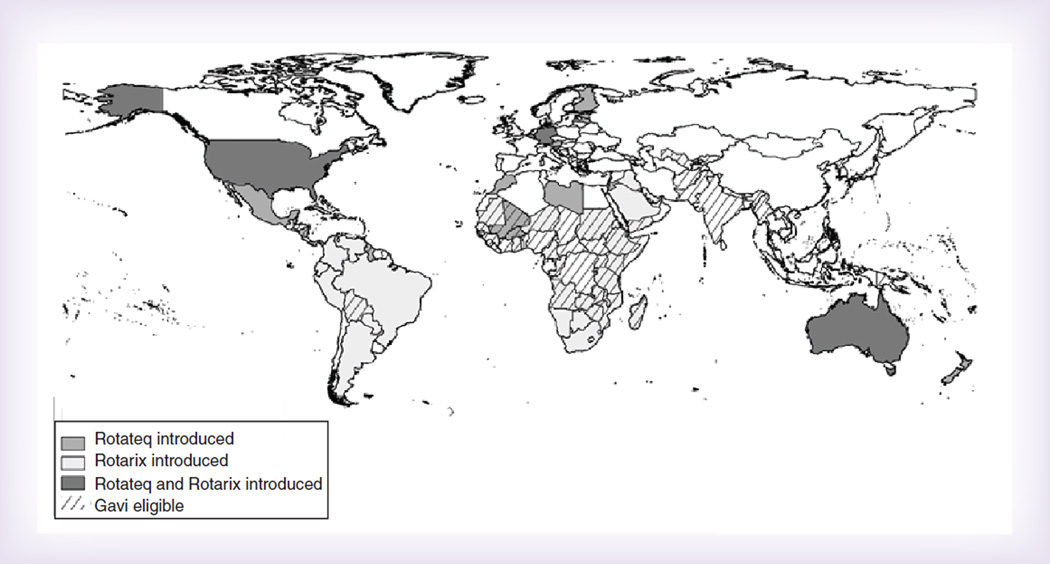
As of May 2016, 81 countries have introduced rotavirus vaccine into their national immunization program (Figure 1) [2]. WHO estimates that, globally, 23% of eligible infants received a full course of rotavirus vaccine in 2015, with coverage nearly fourfold less than the estimated global coverage for polio and DTP vaccines (86%) [3]. These substantial differences in coverage at the global level are attributable to some countries with large birth cohorts that have not introduced rotavirus vaccine nationally, including China, Democratic Republic of Congo and India (which began phased introduction in 2016), and at the national level to uneven coverage of routine vaccines within many countries. For example, in 2013 completion of the rotavirus vaccine series in the USA increased by nearly 30 percentage points over 5 years to 73%. However, despite increasing uptake rotavirus vaccine, coverage remained about 20 percentage points lower than DTP, polio and PCV vaccines [4]. Similarly, rotavirus vaccination coverage in other early introducing countries in the Americas lagged behind concurrently administered vaccines during the period immediately following rotavirus vaccine introduction [5]; this trend continued at least 3 years after introduction [6]. This difference has not been observed in early introducing African countries. For example rotavirus coverage and DTP coverage were 91% 1 year after rotavirus vaccine introduction in Burkina Faso and 98 and 99%, respectively, 2 years after rotavirus vaccine introduction in Rwanda [7].
Figure 1.
Rotavirus vaccines used in countries that have introduced rotavirus vaccine into their national immunization programs.
Initially, RV1 and RV5 were the only infant vaccines licensed with upper age limits for administration (15 weeks for dose 1 and 8 months for the full vaccine series), which was identified as a partial explanation for coverage discrepancies with concurrently administered vaccines [1,8]. These upper age limits were imposed because of concerns about a possible increased risk of intussusception, a telescoping of the small intestine resulting in blockage that was associated with a previously available rotavirus vaccine [9]. This association with intussusception led to the withdrawal in 1999 of a rhesus-human reassortant rotavirus vaccine, Rotashield (RRV-TV, Wyeth), that was licensed and implemented in the USA in 1998. Rotashield caused an estimated one excess case of intussusception for every 10,000 children vaccinated, and some evidence suggested an increased risk with older age at vaccination [1,9–11]. In prelicensure trials for RV1 and RV5, no increased risk of intussusception was detected with either vaccine; however, postlicensure evaluations in Australia, Brazil, Mexico and the USA have found a risk of approximately 1–6 excess intussusception cases per 100,000 vaccinated infants [12–14]; comparable intussusception risk was documented in the UK, Singapore and in a meta-analysis of data from several countries [15–17].
In 2012, mathematical models showed RV1 and RV5 coverage could be improved by allowing for vaccination after the upper age limits of 15 weeks and 8 months, particularly in developing countries where delays in the timing of childhood vaccination are common. Furthermore, the benefits of preventing additional mortality due to severe diarrhea would outweigh the excess risk of intussusception potentially associated with expansion of the age of vaccination [8]. After reviewing these and other relevant data, WHO recommended that national immunization programs administer rotavirus vaccine without the upper age limits at the same time as DTP while continuing to promote timely vaccination. The updated recommendations allow rotavirus vaccination at any age up to 24 months [1].
Health benefits of rotavirus vaccination
Following rotavirus vaccine introduction, swift and striking declines in severe all-cause and rotavirus diarrhea have been demonstrated in many countries [18,19]. Early estimates of the reduction in all-cause diarrhea hospitalizations in high-income countries have ranged from 20 to 50% and from 17 to 55% in middle-income countries; the reduction in rotavirus hospitalizations has ranged from 49 to 92% in high-income countries, 54–59% in middle-income countries and 69–81% in low-income countries [19]. These reductions have been sustained for several years after introduction. For example, in the USA, during the first 4 years postvaccine introduction, the number of rotavirus positive tests declined by 86% in children <5 years of age [20]. Australian children <3 years of age experienced a 75% decrease in acute gastroenteritis hospitalizations in the 3 years following introduction [21]. In Mexico, there has been a 53% reduction in all-cause diarrhea mortality among children <5 years of age since rotavirus vaccine introduction in early 2007 [22].
Newly published articles have shown similar declines in many developing countries that have implemented rotavirus vaccination programs more recently. For example, three years after vaccine introduction, rotavirus hospitalizations in children <5 years of age declined by 69% in Armenia and 64% in Ghana, compared with their respective prevaccine rates [23,24]. Five years after introduction in South Africa, diarrhea hospitalizations in children <5 years of age decreased by 53%, with the most significant reductions in children <12 months and, to a lesser extent, children 12–23 months of age [25].
In addition to reductions in rotavirus and all-cause diarrhea among children who were eligible for vaccination, some countries have reported indirect benefits of vaccination in age groups not targeted for vaccination. Studies from the USA, Australia and Canada have reported declines in rotavirus and all-cause diarrhea in older children and adults [26–30]. Recent data from the UK showed that the number of hospitalizations prevented in older age groups following rotavirus vaccine introduction was higher than the number of hospitalizations prevented in children <1 year of age [31]. However indirect effects of rotavirus vaccination programs on age-ineligible children <5 years were not observed in Zambia and South Africa [20,32]. Long-term follow-up is needed in low- and middle-income countries to further assess indirect benefits of rotavirus vaccination.
In postlicensure evaluations, rotavirus vaccines have been shown to be effective in preventing severe rotavirus disease, though less so in low- and middle-income countries than high-income countries. Vaccine effectiveness (VE) in high-income countries has been estimated as 79–100% [19]. New data continues to document lower VE in low- and middle-income countries; recent point estimates for RV1 VE against hospitalization and other healthcare encounters have been as high as 79 and 62% in Moldova and Armenia, respectively, and between 50 and 60% in Bolivia, Botswana, Malawi and Zambia [24,33–37]. For RV5, VE was recently estimated to be 80% in Rwanda and 45% in Nicaragua [38,39]. Despite lower VE, countries with high burdens of disease have experienced important decreases in absolute numbers of rotavirus hospitalizations and deaths.
New information is also available about protection against vaccine and nonvaccine rotavirus genotypes. As RV1 and RV5 are derived from one and five strains of rotavirus, respectively, there has been concern that the vaccines would not protect against severe disease caused by nonvaccine rotavirus strains and vaccine use could select for these strains, as has been documented with pneumococcal and human papilloma-virus vaccines [18,40]. However, reassuringly, a meta-analysis using data from upper and middle income countries found that both vaccines protect against homotypic, partly heterotypic and fully heterotypic rotavirus strains causing severe disease (Table 1 & 2) [40]. Two smaller primary analyses in Nicaragua and Malawi assessed strain-specific VE and found promising but variable protection against a variety of strains; parallel impact evaluations found dramatic overall decreases in severe rotavirus disease and no selective pressure and no novel or breakthrough strains in these two studies [33,38]. As predominant strains of rotavirus vary from year to year and in different geographic locations, and since vaccine performance may differ in countries with different socioeconomic status, ongoing surveillance is important to assess vaccination impact against heterotypic rotavirus strains and monitor for selective pressure.
Table 1.
Vaccine effectiveness of RV1 against homotypic and heterotypic rotavirus genotypes.
| RV1 | Leshem et al. (2014) |
Bar-Zeev et al. (2016) Malawi % (95% CI) |
|
|---|---|---|---|
| High-income countries % 95% CI) | Middle-income countries % (95% CI) | ||
| Fully homotypic† | 94 (80–98) | 59 (36–73) | 82 (45–94) |
| Partially heterotypic‡ | 71 (39–86) | 72 (58–81) | 71 (20–89) |
| Fully heterotypic§ | 87 (76–93) | 47 (28–61) | 47 (−22–77) |
G1P (8).
G1 (any P type) or P (8) (any G type).
Any non-G1 and non-P (8) type.
RV: Rotavirus vaccine.
Table 2.
Vaccine effectiveness of RV5 against homotypic and heterotypic rotavirus genotypes.
| RV5 | Leshem et al. (2014) |
Patel et al. (2016) Nicaragua % (95% CI) |
|
|---|---|---|---|
| High-income countries % (95% CI) | Middle-income countries % (95% CI) | ||
| Fully homotypic† | 83 (78–87) | 35 (−1–58) | |
| Partially heterotypic‡ | 82 (70–89) | 37 (10–56) | 50 (26–66) |
G1–4P (8).
G1–4 (P other type) or P (8) (other G type).
RV: Rotavirus vaccine.
New developments
Improving rotavirus vaccine performance in developing countries
While rotavirus vaccines have shown substantial impact in developing countries, their modest VE in these settings leaves opportunity for further improvement of their performance. Consequently, hypotheses for lower VE in low-income countries and practical strategies to modify contributing factors are being explored.
In vitro studies suggested that breastfeeding at the time of administration of rotavirus vaccination might adversely affect rotavirus vaccine performance, as breastmilk contains antibody and other factors that could neutralize the vaccine virus [41]. Also, because levels of maternal antibodies against rotavirus are greater in developing countries, potential interference from breastfeeding could also be greater in these settings [42]. However, two recent randomized control trials (RCTs) found no statistically significant difference in antirotavirus immunoglobulin A seroconversion when breastfeeding was encouraged or withheld at the time of vaccination. A third RCT reported statistically significantly higher seroconversion among infants encouraged to breastfeed; the authors hypothesized breast milk may act as an additional buffer to neutralize stomach acid and improve vaccine take (Figure 2) [43–45].
Figure 2.
Antirotavirus immunoglobulin A seroconversion among infants after receiving two doses of Rotarix vaccine in three postlicensure randomized control trials.
†Statistically significant.
Previously, evidence has shown that concurrent live oral polio vaccine (OPV) administration reduces rotavirus vaccine replication in the gut, although decreased protection was not observed [46]; this was well documented with trivalent OPV, and recent studies have indicated similar interference with monovalent and bivalent OPV [47,48]. OPV is administered primarily in low-income countries and could partially explain lower VE in these settings. As part of the polio eradication ‘end-game’ strategy, it is anticipated that inactivated poliovirus vaccine will completely replace OPV after eradication [49]; this change is expected to improve rotavirus VE.
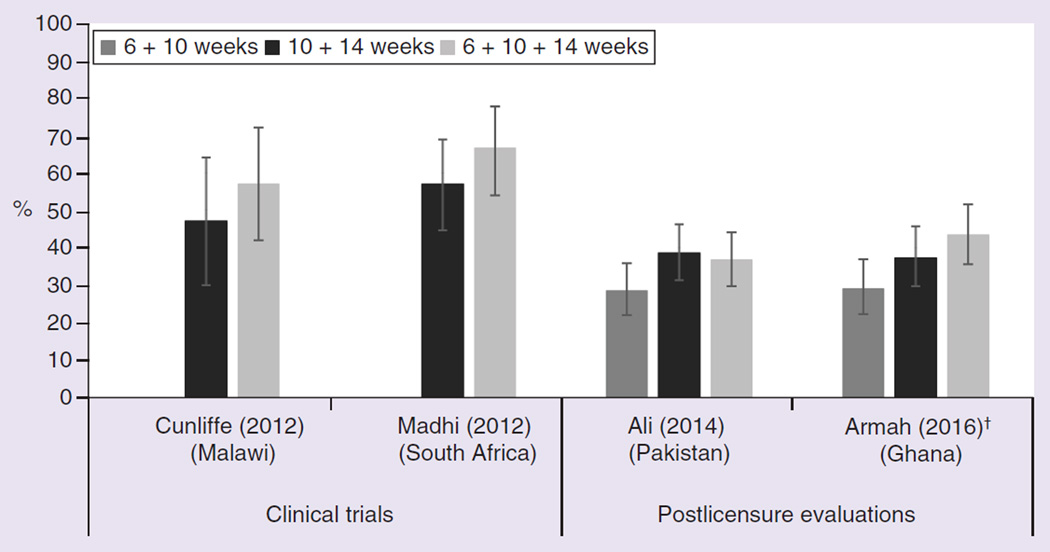
Adding a dose to the current two-dose RV1 or three-dose RV5 schedule has also been proposed to increase VE in low-income settings. One option is for a third primary dose of RV1; for many countries with doses recommended at 6 and 10 weeks, a third dose would be administered at 14 weeks of age. Programmatically, a three-dose schedule corresponds to the existing polio, DTP, Haemophilus influenzae b (Hib), hepatitis B and PCV infant schedule in many countries. A three-dose schedule was not found to significantly improve immunogenicity compared with a 10- and 14-week two-dose schedule during prelicensure trials in Malawi and South Africa (Figure 3) [25,50]; however in Ghana, statistically significantly higher anti-rotavirus IgA levels were observed after a three dose schedule versus a 6- and 10-week two-dose schedule in a postlicensure evaluation [23]. A similarly designed analysis from Pakistan did not show a difference [51].
Figure 3.
Antirotavirus immunoglobulin A seroconversion of infants after receiving two or three doses of Rotarix vaccine in two prelicensure and two postlicensure randomized control trials.
†Statistically significant.
An additional dose of RV1 at 9 months of age, when the first dose of measles-containing vaccine is recommended in many countries, is promising in mitigating waning VE in the second year of life. In Bangladesh, an RCT found an increase in antirotavirus IgG with a 9-month booster dose [52]. Results of an RCT in Mali evaluating a 9-month booster dose of RV5 have not been published to date [53]. Real world effectiveness of 9-month booster doses has not been evaluated and is needed before any recommendations can be made.
Suspicions that poor nutritional status reduces rotavirus VE have been supported by a recent observational study in Botswana where VE following two doses of RV1 was statistically significantly higher in children with no undernutrition as compared with children with moderate or severe undernutrition [35]; however, additional evidence is needed to fully understand the impact of malnutrition and to develop targeted interventions.
New rotavirus vaccines
Additional rotavirus vaccines are in development, including candidates with alternate schedules and routes of administration to improve protection (Table 3). Administration during the neonatal period could provide additional vaccine protection during the first 2 months of life and also may be less likely to pose a risk of vaccine-associated intussusception, since naturally occurring intussusception is rare in the first 3 months of life [10,54–55]. However, neonates have immature immune systems and higher levels of maternal antibodies against rotavirus, which may decrease VE [54,55]. Two new rotavirus vaccines had favorable results for a neonatal dose in stage II clinical trials. In New Zealand, one neonatal dose with two additional infant doses of RV3-BB, a monovalent human rotavirus vaccine, performed comparably to a three-dose infant schedule; however follow-up was limited to the first 6 months of life [54]. In Ghana, one neonatal dose with one infant dose of RRV-TV (Rotashield) had a vaccine efficacy of 64% [55].
Table 3.
Rotavirus vaccine that are regionally used, recently licensed or in development.
| Stage of development | Name/type | Composition | Route of administration |
Organization/company |
|---|---|---|---|---|
| Licensed for use in China | LLR | Live attenuated lamb rotavirus strain, G10P (12) | Oral | Lanzhou Institute of Biological Products, China |
| Licensed for use in India | ROTAVAC | Live attenuated neonatal rotavirus strain, G9P (11) (aka 116E) |
Oral | Bharat Biotech, India and PATH, USA |
| Licensed for use in Vietnam | Rotavin-M1 | Live attenuated human rotavirus strain, G1P (8) | Oral | POLYVAC, Vietnam |
| Phase III | LLR reassortants | Live attenuated lamb-human reassortant rotavirus strains, G2, G3, G4 |
Oral | Lanzhou Institute of Biological Products, China |
| Phase III | RotaShield | Live attenuated rhesus-human reassortant rotavirus strains, tetravalent |
Oral | International Medica Foundation and PATH, USA |
| Phase III | SII BRV-PV | Bovine-human reassortant rotavirus vaccine (G1, G2, G3, G4, G9) |
Oral | Serum Institute of India, India and PATH, USA |
| Phase II | RV3 | Live attenuated neonatal rotavirus strain, G3P (6) | Oral | Murdoch Children’s Research Institute, Australia and Biofarma, Indonesia |
| Phase II | Subunit | Truncated VP8 of P4, P6, P8 | Intramuscular | National Institutes of Health and PATH, USA |
| Phase II | UK reassortants | BRV tetravalent (G1–G4) | Oral | Shantha Biotech, India |
| Phase I | NF-R7 | Live attenuated lamb-human reassortant strain, G4 | Oral | Shenzhen Kangtai Biological Products Company, China |
| Phase I | UK reassortants | Bovine UK Human G1, G2, G3, G4, G9 reassortants 5V | Oral | Instituto Butantan, Brazil |
| Phase I | UK reassortants | Live attenuated bovine-human reassortant strains, tetravalent to hexavalent |
Oral | Wuhan Institute of Biological Products, China and PATH, USA |
| Preclinical | IRV | Inactivated G1P (8), G2P (4) | Intramuscular or intradermal |
US CDC |
| Preclinical | Subunit | VLPs: VP 2/6/7 and VP 2/4/6/7 | To be determined | Baylor College of Medicine, USA |
| Preclinical | Subunit | Truncated VP8 in norovirus P particles | To be determined | Cincinnati Children’s Hospital Medical Center, USA |
| Preclinical | UK reassortants | Live attenuated bovine-human reassortant strains, tetravalent to hexavalent |
Oral | Minhai Biotechnology Co., China |
| Research | Subunit | VP6 combined with norovirus G1 and GII VLPs | To be determined | University of Tampere School of Medicine, Finland |
BRV: Bovine rotavirus; IRV: Inactivated rotavirus vaccine; LLR: Lanzhou lamb rotavirus; PV: Pentavalent vaccine; VLP: Virus-like particle; VP: Viral protein.
Reproduced with permission from [56].
Three oral rotavirus vaccines are currently licensed in national markets: Lanzhou lamb rotavirus vaccine (LLR, Lanzhou Institute of Biological Products, China), Rotavin-M1 (POLYVAC, Vietnam) and ROTAVAC (Bharat Biotech, India) [56,57]. ROTAVAC is the first to be introduced into a public vaccination program as of April 2016 when it was introduced in four states in India, and postlicensure VE evaluations are ongoing [58]; LLR and Rotavin-M1 are only available on the private market. Several postlicensure case–control studies of LLR vaccine have documented effectiveness against diarrhea hospitalizations and laboratory-confirmed rotavirus disease [59,60]. Improved access to these locally manufactured vaccines would improve rotavirus disease prevention and vaccine coverage in countries with large populations and high burden of severe disease. Four additional live attenuated oral vaccines are in stage III clinical trials [56,57].
Nonreplicating rotavirus vaccines are in stage II clinical trials and earlier stages of development (Table 3). These injectable rotavirus vaccines offer several advantages over existing oral vaccines. An injectable vaccine would bypass poor absorption or interference in the gut, could be included in a combination product with concurrently administered injectable vaccines, which would increase coverage and may eliminate the risk of intussusception [61]. However, there are questions about how well these vaccines will generate immunity and mucosal immunity compared with the current live oral vaccines, since nonreplicating rotavirus vaccines contain either viral components or inactivated whole virus and will be administered parenterally.
Conclusion & future perspective
Rotavirus vaccines have been shown to dramatically reduce severe rotavirus disease caused by homotypic and heterotypic vaccine strains in a range of socioeconomic settings. The potential impact has not been fully realized as rotavirus vaccines have not been universally introduced into national immunization programs. To date, relatively few countries in Asia have introduced rotavirus vaccine, though several countries in the region are planning to add rotavirus vaccine to their national routine immunization schedules in the next 5 years. While VE is lower in low- and middle-income settings compared with high-income settings, research continues to demonstrate modifiable factors that may contribute to this disparity. Over the next decade, new rotavirus vaccines and vaccines currently in development may overcome some of the barriers to maximize the impact of current rotavirus vaccines.
EXECUTIVE SUMMARY.
Current status
Eighty-one countries have introduced RotaTeq (RV5, Merck & Co.), which is a three-dose pentavalent bovine-human reassortant rotavirus vaccine, and/or Rotarix (RV1, GSK Biologics), which is a two-dose monovalent human rotavirus vaccine, into their national vaccination schedule since 2006.
Large and sustained reductions in all-cause diarrhea and rotavirus hospitalizations have been reported in many early introducing countries.
Similar to data from prelicensure trails, postlicensure vaccine effectiveness point estimates are generally higher in high-income countries than low- and middle-income countries. There are several hypothesized, modifiable reasons for this difference.
Both vaccines have been shown to be effective in preventing severe rotavirus disease due to homotypic and heterotypic rotavirus vaccine strains, but long-term monitoring is needed to assess possible selection pressure.
New developments
Three recent randomized control trials (RCTs) found that breastfeeding immediately after oral rotavirus vaccine administration did not inhibit IgA seroconversion.
Alternate rotavirus vaccine schedules have been proposed as a way to improve vaccine effectiveness. A third primary dose at 14 weeks of age of RV1 had mixed impact on overall IgA seroconversion, resulting in significant improvement in Ghana but not in Pakistan. In an RCT in Bangladesh, administering an additional dose of RV1 at 9 months of age following a two-dose primary RV1 series did not affect the immune response to concomitantly administered measles and rubella vaccines and resulted in boosting of the immune response to rotavirus vaccine.
Two Phase II trials with neonatal dosing schedules have shown promising results.
There are three nationally licensed rotavirus vaccines, including one that was introduced into the infant vaccination schedule in four states in India earlier this year.
Several other rotavirus vaccines are currently in development, including nonreplicating rotavirus vaccines that are parenterally administered and have the potential to overcome the barriers to orally administered vaccines in developing countries.
Acknowledgments
This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•• of considerable interest
- 1.Rotavirus vaccines. WHO position paper – January 2013. Wkly Epidemiol. Rec. 2013;88(5):49–64. [PubMed] [Google Scholar]
- 2.PATH. Country introductions of rotavirus vaccines. http://sites.path.org/rotavirusvaccine. [Google Scholar]
- 3.WHO. Immunization coverage. www.who.int/mediacentre/factsheets.
- 4.Elam-Evans LD, Yankey D, Singleton JA, Kolasa M Centers for Disease C, Prevention. National, state, and selected local area vaccination coverage among children aged 19–35 months – United States, 2013. MMWR Morb. Mortal Wkly Rep. 2014;63(34):741–748. [PMC free article] [PubMed] [Google Scholar]
- 5.De Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev. Vaccines. 2008;7(3):345–353. doi: 10.1586/14760584.7.3.345. [DOI] [PubMed] [Google Scholar]
- 6.De Oliveira LH, Danovaro-Holliday MC, Sanwogou NJ, Ruiz-Matus C, Tambini G, Andrus JK. Progress in the introduction of the rotavirus vaccine in Latin America and the Caribbean: four years of accumulated experience. Pediatr. Infect. Dis. J. 2011;30(Suppl. 1):S61–S66. doi: 10.1097/INF.0b013e3181fefdd6. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Reported Estimate of Coverage. http://apps.who.int/immunization.
- 8.Patel MM, Clark AD, Sanderson CF, Tate J, Parashar UD. Removing the age restrictions for rotavirus vaccination: a benefit–risk modeling analysis. PLoS Med. 2012;9(10):e1001330. doi: 10.1371/journal.pmed.1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parashar UD, Alexander JP, Glass RI. Advisory Committee on Immunization Practices (ACIP); Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2006;55(RR-12):1–13. [PubMed] [Google Scholar]
- 10.Aliabadi N, Tate JE, Parashar UD. Potential safety issues and other factors that may affect the introduction and uptake of rotavirus vaccines. Clin. Microbiol. Infect. 2016 doi: 10.1016/j.cmi.2016.03.007. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peter G, Myers MG National Vaccine Advisory Committee; National Vaccine Program Office. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics. 2002;110(6):e67. doi: 10.1542/peds.110.6.e67. [DOI] [PubMed] [Google Scholar]
- 12.Carlin JB, Macartney KK, Lee KJ, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clin. Infect. Dis. 2013;57(10):1427–1434. doi: 10.1093/cid/cit520. [DOI] [PubMed] [Google Scholar]
- 13.Patel MM, Lopez-Collada VR, Bulhoes MM, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N. Engl. J. Med. 2011;364(24):2283–2292. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 14.Yih WK, Lieu TA, Kulldorff M, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N. Engl. J. Med. 2014;370(6):503–512. doi: 10.1056/NEJMoa1303164. [DOI] [PubMed] [Google Scholar]
- 15.Yung CF, Chan SP, Soh S, Tan A, Thoon KC. Intussusception and monovalent rotavirus vaccination in singapore: self-controlled case series and risk–benefit study. J. Pediatr. 2015;167(1):163–168. e161. doi: 10.1016/j.jpeds.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Escolano S, Hill C, Tubert-Bitter P. Intussusception risk after RotaTeq vaccination: evaluation from worldwide spontaneous reporting data using a self-controlled case series approach. Vaccine. 2015;33(8):1017–1020. doi: 10.1016/j.vaccine.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England: a self-controlled case-series evaluation. Vaccine. 2016;34(32):3684–3689. doi: 10.1016/j.vaccine.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 18.Patel MM, Glass R, Desai R, Tate JE, Parashar UD. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infect. Dis. 2012;12(7):561–570. doi: 10.1016/S1473-3099(12)70029-4. [DOI] [PubMed] [Google Scholar]
- 19.Tate JE, Parashar UD. Rotavirus vaccines in routine use. Clin. Infect. Dis. 2014;59(9):1291–1301. doi: 10.1093/cid/ciu564. [DOI] [PubMed] [Google Scholar]
- 20.Tate JE, Mutuc JD, Panozzo CA, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr. Infect. Dis. J. 2011;30(Suppl. 1):S30–S34. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 21.Buttery JP, Lambert SB, Grimwood K, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr. Infect. Dis. J. 2011;30(1 Suppl):S25–S29. doi: 10.1097/INF.0b013e3181fefdee. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Uribe E, Esparza-Aguilar M, Parashar UD, Richardson V. Sustained reduction of childhood diarrhea-related mortality and hospitalizations in mexico after rotavirus vaccine universalization. Clin. Infect. Dis. 2016;62(Suppl. 2):S133–S139. doi: 10.1093/cid/civ1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin. Infect. Dis. 2016;62(Suppl. 2):S200–S207. doi: 10.1093/cid/ciw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahakyan G, Grigoryan S, Wasley A, et al. Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Clin. Infect. Dis. 2016;62(Suppl. 2):S147–S154. doi: 10.1093/cid/ciw045. [DOI] [PubMed] [Google Scholar]
- 25.Groome MJ, Zell ER, Solomon F, et al. Temporal association of rotavirus vaccine introduction and reduction in all-cause childhood diarrheal hospitalizations in South Africa. Clin. Infect. Dis. 2016;62(Suppl. 2):S188–S195. doi: 10.1093/cid/civ1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke MF, Davidson GP, Gold MS, Marshall HS. Direct and indirect impact on rotavirus positive and all-cause gastroenteritis hospitalisations in South Australian children following the introduction of rotavirus vaccination. Vaccine. 2011;29(29–30):4663–4667. doi: 10.1016/j.vaccine.2011.04.109. [DOI] [PubMed] [Google Scholar]
- 27.Cortese MM, Dahl RM, Curns AT, Parashar UD. Protection against gastroenteritis in US households with children who received rotavirus vaccine. J. Infect. Dis. 2015;211(4):558–562. doi: 10.1093/infdis/jiu503. [DOI] [PubMed] [Google Scholar]
- 28.Gastanaduy PA, Curns AT, Parashar UD, Lopman BA. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA. 2013;310(8):851–853. doi: 10.1001/jama.2013.170800. [DOI] [PubMed] [Google Scholar]
- 29.Lambert SB, Faux CE, Hall L, et al. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med. J. Aust. 2009;191(3):157–160. doi: 10.5694/j.1326-5377.2009.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 30.Wilson SE, Rosella LC, Wang J, et al. Population-level impact of Ontario’s infant rotavirus immunization program: evidence of direct and indirect effects. PLoS ONE. 2016;11(5):e0154340. doi: 10.1371/journal.pone.0154340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atchison CJ, Stowe J, Andrews N, et al. Rapid declines in age group-specific rotavirus infection and acute gastroenteritis among vaccinated and unvaccinated individuals within 1 year of rotavirus vaccine introduction in England and Wales. J. Infect. Dis. 2016;213(2):243–249. doi: 10.1093/infdis/jiv398. [DOI] [PubMed] [Google Scholar]
- 32.Mpabalwani EM, Simwaka CJ, Mwenda JM, et al. Impact of rotavirus vaccination on diarrheal hospitalizations in children aged <5 years in Lusaka, Zambia. Clin. Infect. Dis. 2016;62(Suppl. 2):S183–S187. doi: 10.1093/cid/civ1027. [DOI] [PubMed] [Google Scholar]
- 33.Bar-Zeev N, Jere KC, Bennett A, et al. Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin. Infect. Dis. 2016;62(Suppl. 2):S213–S219. doi: 10.1093/cid/civ1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beres LK, Tate JE, Njobvu L, et al. A preliminary assessment of rotavirus vaccine effectiveness in Zambia. Clin. Infect. Dis. 2016;62(Suppl. 2):S175–S182. doi: 10.1093/cid/civ1206. [DOI] [PubMed] [Google Scholar]
- 35. Gastanaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case–control study. Clin. Infect. Dis. 2016;62(Suppl. 2):S161–S167. doi: 10.1093/cid/civ1207. •• This observational study presents rotavirus vaccine effectiveness against disease by nutritional status.
- 36.Gheorghita S, Birca L, Donos A, et al. Impact of rotavirus vaccine introduction and vaccine effectiveness in the Republic of Moldova. Clin. Infect. Dis. 2016;62(Suppl. 2):S140–S146. doi: 10.1093/cid/civ1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pringle KD, Patzi M, Tate JE, et al. Sustained effectiveness of rotavirus vaccine against very severe rotavirus disease through the second year of life, Bolivia 2013–2014. Clin. Infect. Dis. 2016;62(Suppl. 2):S115–S120. doi: 10.1093/cid/civ1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel M, Pedreira C, De Oliveira LH, et al. Effectiveness of pentavalent rotavirus vaccine against a diverse range of circulating strains in Nicaragua. Clin. Infect. Dis. 2016;62(Suppl. 2):S127–S132. doi: 10.1093/cid/civ1017. [DOI] [PubMed] [Google Scholar]
- 39.Tate JE, Ngabo F, Donnen P, et al. Effectiveness of pentavalent rotavirus vaccine under conditions of routine use in Rwanda. Clin. Infect. Dis. 2016;62(Suppl. 2):S208–S212. doi: 10.1093/cid/civ1016. [DOI] [PubMed] [Google Scholar]
- 40. Leshem E, Lopman B, Glass R, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect. Dis. 2014;14(9):847–856. doi: 10.1016/S1473-3099(14)70832-1. •• This meta-analysis summarizes rotavirus vaccine effectiveness against homotypic and heterotypic vaccine strains.
- 41.Moon SS, Wang Y, Shane AL, et al. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr. Infect. Dis. J. 2010;29(10):919–923. doi: 10.1097/INF.0b013e3181e232ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J. Infect. Dis. 2009;200(Suppl. 1):S39–S48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali A, Kazi AM, Cortese MM, et al. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine – a randomized trial. PLoS ONE. 2015;10(6):e0127622. doi: 10.1371/journal.pone.0127622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groome MJ, Moon SS, Velasquez D, et al. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull. World Health Organ. 2014;92(4):238–245. doi: 10.2471/BLT.13.128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rongsen-Chandola T, Strand TA, Goyal N, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine. 2014;32(Suppl. 1):A134–A139. doi: 10.1016/j.vaccine.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 46.Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine. 2012;30(Suppl. 1):A30–A35. doi: 10.1016/j.vaccine.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 47.Emperador DM, Velasquez DE, Estivariz CF, et al. Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent rotavirus vaccine immunogenicity in rural Bangladesh. Clin. Infect. Dis. 2016;62(2):150–156. doi: 10.1093/cid/civ807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramani S, Mamani N, Villena R, et al. Rotavirus serum IgA immune response in children receiving Rotarix® co-administered with bOPV or IPV. Pediatr. Infect. Dis. J. 2016 doi: 10.1097/INF.0000000000001253. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 49.WHO. Polio endgame strategic plan. www.who.int/immunization/diseases.
- 50.Madhi SA, Kirsten M, Louw C, et al. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine. 2012;30(Suppl. 1):A44–A51. doi: 10.1016/j.vaccine.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 51.Ali SA, Kazi AM, Cortese MM, et al. Impact of different dosing schedules on the immunogenicity of the human rotavirus vaccine in infants in Pakistan: a randomized trial. J. Infect. Dis. 2014;210(11):1772–1779. doi: 10.1093/infdis/jiu335. [DOI] [PubMed] [Google Scholar]
- 52. Zaman K, Fleming JA, Victor JC, et al. Noninterference of rotavirus vaccine with measles-rubella vaccine at 9 months of age and improvements in antirotavirus immunity: a randomized trial. J. Infect. Dis. 2016;213(11):1686–1693. doi: 10.1093/infdis/jiw024. •• Presents results from a randomized controlled trial showing potential improved immunogenicity following a booster dose of rotavirus vaccine.
- 53.PATH. Immune response to rotavirus vaccine after a supplemental dose given at 9 months of age with local EPI vaccines in Mali. https://clinicaltrials.gov.
- 54.Bines JE, Danchin M, Jackson P, et al. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015;15(12):1389–1397. doi: 10.1016/S1473-3099(15)00227-3. [DOI] [PubMed] [Google Scholar]
- 55.Armah GE, Kapikian AZ, Vesikari T, et al. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J. Infect. Dis. 2013;208(3):423–431. doi: 10.1093/infdis/jit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yen C, Tate JE, Hyde TB, et al. Rotavirus vaccines: current status and future considerations. Hum. Vaccin. Immunother. 2014;10(6):1436–1448. doi: 10.4161/hv.28857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steele D. The development of future vaccines against enteric diseases. www.fondation-merieux.org. [Google Scholar]
- 58.International Vaccine Access Center. VIEW-hub report: global vaccine introduction and implementation. 2016 www.jhsph.edu/research. [Google Scholar]
- 59.Fu C, Wang M, Liang J, He T, Wang D, Xu J. Effectiveness of Lanzhou lamb rotavirus vaccine against rotavirus gastroenteritis requiring hospitalization: a matched case–control study. Vaccine. 2007;25(52):8756–8761. doi: 10.1016/j.vaccine.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 60.Fu C, He Q, Xu J, et al. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine. 2012;31(1):154–158. doi: 10.1016/j.vaccine.2012.10.078. [DOI] [PubMed] [Google Scholar]
- 61.PATH. Exploring new, non-replicating rotavirus vaccines. http://sites.path.org/vaccinedevelopment. [Google Scholar]