ABSTRACT
Freshwater lakes emit large amounts of methane, some of which is produced in oxic surface waters. Two potential pathways for aerobic methane production exist: methanogenesis in oxygenated water, which has been observed in some lakes, and demethylation of small organic molecules. Although methane is produced via demethylation in oxic marine environments, this mechanism of methane release has not yet been demonstrated in freshwater systems. Genes related to the C-P lyase pathway, which cleaves C-P bonds in phosphonate compounds, were found in a metagenomic survey of the surface water of Lake Matano, which is chronically P starved and methane rich. We demonstrate that four bacterial isolates from Lake Matano obtain P from methylphosphonate and release methane and that this activity is repressed by phosphate. We further demonstrate that expression of phnJ, which encodes the enzyme that releases methane, is higher in the presence of methylphosphonate and lower when both methylphosphonate and phosphate are added. This gene is also found in most of the metagenomic data sets from freshwater environments. These experiments link methylphosphonate degradation and methane production with gene expression and phosphate availability in freshwater organisms and suggest that some of the excess methane in the Lake Matano surface water, and in other methane-rich lakes, may be produced by P-starved bacteria.
IMPORTANCE Methane is an important greenhouse gas and contributes substantially to global warming. Although freshwater environments are known to release methane into the atmosphere, estimates of the amount of methane emitted by freshwater lakes vary from 8 to 73 Tg per year. Methane emissions are difficult to predict in part because the source of the methane can vary: it is the end product of the energy-conserving pathway in methanogenic archaea, which live predominantly in anoxic sediments or waters but have also been identified in some oxic freshwater environments. More recently, methane release from small organic molecules has been observed in oxic marine environments. Here we show that demethylation of methylphosphonate may also contribute to methane release from lakes and that phosphate can repress this activity. Since lakes are typically phosphorus limited, some methane release in these environments may be a by-product of phosphorus metabolism rather than carbon or energy metabolism. Methane emissions from lakes are currently predicted using primary production, eutrophication status, extent of anoxia, and the shape and size of the lake; to improve prediction of methane emissions, phosphorus availability and sources may also need to be included in these models.
INTRODUCTION
Freshwater lakes may be responsible for as much as 20% of total annual methane emissions from natural sources (1–3), and supersaturation of methane in oxic water columns has been observed in many lakes (4–8). Some surface water methane is produced by methanogenesis in anoxic bottom waters, followed by upward diffusive transport or ebullition (9). Methanogenesis may also occur in oxic surface waters, either in anoxic microenvironments such as biofilms on phytoplankton or inside animal guts (5, 10) or potentially in oxygen-tolerant methanogens (11). Aerobic demethylation of organic compounds such as methylphosphonate (MPn) could also contribute to surface water methane release; however, this pathway has been observed only in marine systems (12–16). Because lakes may release as much methane to the atmosphere as the entire global ocean (8) and since the pathways for surface water methane production are poorly understood (17), a comprehensive characterization of the biological pathways responsible for methane production in these environments is critical to more-accurate predictions of global freshwater methane emissions (8, 9, 18–21).
Low-molecular-weight (LMW) phosphonates such as MPn, ethylphosphonate, and 2-aminoethylphosphonate (2-AEP) originate from the headgroups of phosphonolipids or are linked to exopolysaccharides and are produced in all three domains of life (22–25). Phosphonate biosynthetic pathways are encoded in ∼5% of microbial genomes in freshwater environments (24), suggesting that phosphonate substrates could be available in these environments. Because breaking the C-P bond in phosphonates has a higher activation energy than breaking the equivalent phosphoester bond, phosphonate compounds tend to be chemically stable (26, 27). Organisms capable of cleaving the C-P bond could potentially utilize either the organic functional group or the phosphorus, and indeed some pathogenic and enteric bacteria (28, 29), as well as bacteria isolated from soil (reviewed in references 26 and 27) and wastewater (30), are capable of using both the C and P moieties of phosphonate compounds.
Freshwater lakes are typically P limited (31), so in these environments, phosphonates would be more likely to provide P than C to organisms capable of phosphonate degradation. The organic functional group would then be released as a by-product. Thus, consumption of LMW phosphonates may result in the release of soluble organic compounds that can be used as C or energy sources (32) or of gaseous compounds that may escape consumption, such as ethane or methane (15, 33).
In permanently stratified lakes with deep chemoclines, where the anoxic bottom waters are essentially disconnected from the air-water interface, methane accumulation is more likely to be due to the production of methane in the surface water than to upward diffusion, since the rate of diffusion should be lower than the rate of methane consumption (8, 34). Lake Matano, Indonesia, is a deep, permanently stratified, chronically P-limited lake. Methane is supersaturated in Lake Matano surface water (7, 35), even though most of the methane produced in the anoxic sediments and bottom waters is oxidized in the chemocline (7) and diffusion of methane upward through the 120-m epilimnion should be slow enough to allow complete aerobic consumption of methane produced below the chemocline. Because of the degree of P limitation in Lake Matano (36–38), we hypothesize that additional methane may be produced in the surface water by P-starved organisms acquiring P from methylphosphonate. Here we use metagenomic analysis, physiological experiments, and gene expression analysis to test the hypothesis that P acquisition by aerobic heterotrophs in Lake Matano may account for some of the excess methane in Lake Matano surface water. We show that genes encoding phosphonate degradation are enriched in metagenomic data from surface water and widespread in other freshwater metagenomic data sets, that four isolates produce methane from MPn, and that both methane production and expression of phnJ, which encodes a subunit of the C-P lyase complex (27, 39), change in response to phosphate availability.
MATERIALS AND METHODS
Sample collection and sequencing.
Surface water was collected from Lake Matano, Indonesia, for DNA extraction and sequencing from January to March 2009. Water (100 liters) was pumped through 0.22-μm Steripak filters, which were frozen on-site and kept frozen until processing. DNA was extracted and sequenced using a 454 Life Sciences GS-FLX instrument with Titanium series reagents, as described earlier for water collected from the Lake Matano chemocline (40).
Analysis of Lake Matano metagenomic data.
The raw reads were compared to the Clusters of Orthologous Groups (COG) database (41) using a BLASTx search with an E value cutoff of 10−10 and a minimum bit score of 50. The COG category of the best BLASTx hit was assigned to the raw reads. Odds ratios were calculated for each COG category identified in the metagenomic data set by calculating the ratio (A/B)/(C/D), where A is the number of reads in the metagenome that are in a given COG category, B is the number of reads in the metagenome that are in all of the other COG categories, C is the number of proteins in the COG database that are in a given COG category, and D is the number of proteins in the COG database that are in all of the other COG categories. COG categories with odds ratios of >1 were considered enriched (42), meaning that they are more highly represented in the metagenomic data than in the protein database. For comparison, odds ratios were calculated for the same COG categories in the metagenomic data from the chemocline in Lake Matano (40), where phosphate is not limiting (37).
Metagenomic reads were assembled into contigs using Newbler GS De Novo Assembler (software version 2.5.3) with the default settings for genomic projects. Contigs potentially including phosphonate degradation genes were identified by blasting the C-P lyase amino acid sequences from Escherichia coli (GenBank accession number NC_000913.3, locus tags b4092 to b4106 [https://www.ncbi.nlm.nih.gov/gene?LinkName=pubmed_gene&from_uid=1335942]) against the contigs. Contigs with at least three C-P lyase genes were analyzed using the open reading frame (ORF) finder (NCBI), BLAST (Basic Local Alignment Search Tool; NCBI), and CD-search (Conserved Domains; NCBI) to predict and annotate the genes on the contigs.
Analysis of other metagenomic data sets.
The PhnJ amino acid sequences from Pseudomonas stutzeri (NCBI accession no. AAR91738) and E. coli (NCBI accession no. AAC77059.1) were used as BLAST queries against freshwater metagenomic data sets publicly available at the Joint Genome Institute's Integrated Microbial Genomes website (https://img.jgi.doe.gov/cgi-bin/mer/main.cgi) and at the European Nucleotide Archive (http://www.ebi.ac.uk/ena) (43). Data sets were scored as positive for phnJ if they had at least one hit with an E value of less than 1 × 10−10.
Strains and growth conditions.
Seven heterotrophic bacteria isolated from the surface water of Lake Matano (Table 1) (38) were screened for their ability to acquire P from MPn. The base medium was the National Botanical Research Institute phosphate growth medium (NBRIP) without added P [per liter: MgCl2·6H2O, 5 g; MgSO4·7H2O, 0.25 g; KCl, 0.2 g; (NH4)2SO4, 0.1 g; glucose, 10 g] (44). To test for MPn utilization, strains were grown in NBRIP base medium with 0.2 mM MPn as the only P source. Production of methane from MPn was characterized in the four strains (LM-1, LM-5, LM6-1, and LM-Y) capable of using MPn as their sole source of P. MPn, K2HPO4, or both were added to the P-free NBRIP medium as P sources. The pH of the medium was adjusted to 7.0 with 1 mM solutions of NaHCO3 or acetic acid for the medium with added K2HPO4 or 1 mM solutions of NaHCO3 or NaOH for medium with added MPn, as appropriate. Prior to measuring growth curves, cells were grown in NBRIP with K2HPO4 as the only P source. Serum bottles (60 ml) containing 30 ml medium and air in the headspace were inoculated with cells to an optical density at 660 nm (OD660) of ∼0.05 and incubated horizontally at 30°C with shaking at ∼150 rpm. Growth was monitored by measuring the OD660.
TABLE 1.
Bacterial strains used in this studya
| Strain designation | Closest related cultivated species | Ability to use: |
|
|---|---|---|---|
| MPn | 2-AEP | ||
| LM-2 | Microbacterium testaceum | − | − |
| LM-1 | Agrobacterium tumefaciens | + | + |
| LM-5 | Rhizobium sp. | + | + |
| LM6-1 | Agrobacterium tumefaciens | + | + |
| LM-P | Methylobacterium podarium | − | − |
| 2-LM-22 | Acinetobacter sp. | − | − |
| LM-Y | Pantoea ananatis | + | + |
All strains listed were isolated from water collected from Lake Matano, Indonesia, as described previously (38). The ability to use methylphosphonate (MPn) or 2-aminoethylphosphonate (2-AEP) was assessed by monitoring growth in NBRIP medium with MPn or 2-AEP supplied as the sole phosphorus source at a concentration of 0.2 mM. The 2-AEP results were originally reported in reference 38.
For the initial growth experiments, MPn, KH2PO4, or both were added to concentrations of 0.2 mM each. For experiments characterizing methane release with different concentrations of MPn, no phosphate was added to the medium, and MPn was added to concentrations of 5 μM, 10 μM, 50 μM, 100 μM, or 200 μM. To determine the effect of phosphate addition on MPn degradation, the initial MPn concentration was 50 μM and the starting K2HPO4 concentration was 0 μM, 4 μM, 10 μM, 50 μM, or 100 μM.
To calculate the total methane production, we assumed it to be in equilibrium between the gas and aqueous phases. In serum bottles with a headspace-to-liquid ratio of 30 ml/30 ml, the percentage of methane in the gas phase (fg) is 96.87% at 30°C based on equation 1 (45), using a value of 0.0013 mol liter−1 atm−1 for Henry's law constant, KH (35):
| (1) |
where Vg and Vw are the volumes of the gas and aqueous phases, respectively, and R is the gas constant. The amount of methane produced was calculated by dividing the quantity of methane in the headspace by fg.
Percent repression of methane production by phosphate was calculated as follows:
| (2) |
where n = 4, 10, 50, or 100 μM.
Measurements of CH4.
Methane in the culture headspace was measured by gas chromatography coupled with a flame ionization detector (GC-FID), Agilent 7890A (G3440A). Gas was sampled from the culture headspace (25 μl for the LM-Y initial growth experiment; 100 μl for all other experiments) using a gas-tight syringe and injected into the GC-FID at an inlet temperature of 250°C. The carrier gas was helium, at a rate of 25 ml min−1. The oven temperature was 90°C for 4 min, and then the temperature was increased by 120°C min−1 for 1.25 min, until a final temperature of 240°C was reached. The temperature was maintained at 240°C for 1.5 min to remove any sample residue in the column. The detector temperature was 200°C, with hydrogen and airflows of 35 and 350 ml min−1, respectively. MSDChem was used to integrate the peaks. Methane standards with 99 ppm methane in nitrogen gas (item no. 01-04-212; Scotty) and >99.9% methane (item no. GMT10015TC; Matheson) were used to prepare a standard curve of peak area and concentration (49.5 ppm, 99 ppm, 999 ppm, and 1,996.01 ppm). The methane concentration obtained based on the standard curves was then converted from parts per million to micromolar using the molar gas volume 24.87 liters mol−1 at 30°C for ideal gas at 1 atm of pressure.
Amplification and sequencing of phnJ in isolates.
Initially, the primers PhnJoc1 and PhnJoc2 (Table 2) were used to amplify the phnJ genes from isolates (46, 47). Each PCR volume (25 μl) included 1× Mg-free buffer (item number D4545; Sigma-Aldrich), 1.5 mM MgCl2, 0.25 μM each primer, 0.2 mM each deoxynucleoside triphosphate (dNTP), 0.75 U SigmaTaq (Sigma-Aldrich item number D1806), and 0.5 μl (7 to 30 ng) template DNA. PCR conditions were 94°C for 3 min, followed by 35 cycles of 94°C for 30 s, 52.5°C for 30 s, and 72°C for 1 min, with a final extension at 72°C for 5 min.
TABLE 2.
Primers used for phnJ sequencing and RT-PCR analysisa
| Target (strain[s]) | Application | Forward primer (sequence) | Reverse primer (sequence) | Source or references |
|---|---|---|---|---|
| phnJ | PCR | PhnJoc1 (AARGTRATMGAYCARGG) | PhnJoc2 (CATYTTYGGATTRTCRAA) | 46, 47 |
| phnJ1 (LM-1, LM-5, and LM6-1) | RT-PCR | phnJ-F1 (ACCATCATCCAGACGCGGCA) | phnJ-R1 (AGCTTGACGTGCATCAGGCC) | This study |
| phnJ2 (LM-Y) | RT-PCR | phnJ-F2 (TCAGACGCGTCACCGTATT) | phnJ-R2 (CTTCGTACAGTTTGACCTGC) | This study |
Degenerate primers were used to amplify and sequence phnJ from all isolates; specific primers were designed based on the sequences to use for qPCR. Strains LM-1, LM-5, and LM6-1 are members of Alphaproteobacteria that are closely related to each other, and the same primer pair was used for all three. Primers for LM-Y were slightly different but were complementary to the same region of the gene and generated a product of the same size.
PCR products (∼400 bp) were extracted from agarose gels and sequenced by Sanger sequencing at the University of Delaware Sequencing and Genotyping Center. Primers for RT-PCR were designed based on these sequences (phnJ-F1/phnJ-R1 for strains LM-1, LM-5, and LM6-1 and phnJ-F2/phnJ-R2 for strain LM-Y; see Table 2 for sequences).
Expression of phnJ during growth on different P sources.
Liquid subsamples (1.5 or 3.0 ml) were removed from all cultures during the mid-exponential and stationary phases of growth on each P source (MPn, K2HPO4, or both) and stored at −20°C in RNALater until processing. For RNA extraction, cells were stabilized in RNA Protect Reagent (catalog number 76506; Qiagen) and then digested with lysozyme (15 mg ml−1) and 20 μl proteinase K (800 U ml−1, catalog no. P8107S; New England BioLabs) at room temperature for 15 min. RNA was then purified from the bacterial lysate using the Qiagen RNEasy minikit (catalog no. 74104; Qiagen) according to the manufacturer's instructions. Genomic DNA contamination was removed by Turbo DNase treatment (catalog no. AM1907; Ambion). RNA was used as the template for reverse transcription using the RETROscript kit (catalog no. AM1710; Ambion). RNA (∼1 μg) and random decamers (2 μl, 50 μM) were denatured at 75°C for 3 min and then immediately placed on ice. RT buffer (2 μl, 10×), dNTP mix (4 μl, 2.5 mM each), RNase inhibitor (1 μl, 10 U μl−1), and Moloney murine leukemia virus reverse transcriptase (MMLV-RT; 1 μl, 100 U μl−1) were added to the mixture to a volume of 20 μl. This solution was incubated at 44°C for 1 h and then at 92°C for 10 min to inactivate the reverse transcriptase (MMLV-RT). The single-stranded cDNA product was used as the template for quantitative PCR (qPCR).
The abundance of the phnJ transcript was quantified in the cDNA products using qPCR. The qPCRs were performed on an Eppendorf real-time Mastercyclerep and processed in duplicates with 0.5 μl cDNA, 10 μl SYBR green FastMix for iQ (catalog no. 95071; Quanta), 0.25 μl each primer (25 mM), and 9 μl nuclease-free water. The reaction protocol was as follows: 95°C for 2 min 30 s, followed by 40 cycles of 95°C for 15 s, 62°C for 15 s, and 68°C for 30 s.
Accession number(s).
The metagenomic data obtained in this study have been deposited in the NCBI database with accession number SAMN03292416.
RESULTS AND DISCUSSION
Potential for phosphonate degradation in Lake Matano and other freshwater environments.
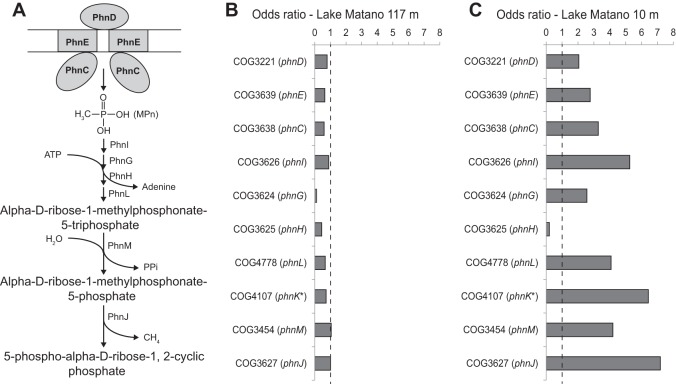
Heterotrophic bacteria isolated from Lake Matano were recently shown to utilize a diverse array of P sources, including 2-AEP (Table 1) and to make large changes in the RNA content and lipid composition of the cells when P starved (38). To assess the probability of methane production from MPn degradation in Lake Matano surface water, reads mapping to genes encoding the C-P lyase pathway (Fig. 1A) were quantified. All of the genes necessary for cleavage of MPn to methane and phosphate are present in the metagenomic data set from the surface water of Lake Matano (Fig. 1C). This pathway includes subunits of the phosphonate transporter (phnDEC), activation of the phosphonate by ATP (phnIGHL), release of diphosphate (phnM), and cleavage of the C-P bond, which results in release of methane (phnJ) (48).
FIG 1.
C-P lyase pathway in Lake Matano. (A) C-P lyase proteins that transport phosphonates (PhnCDE) and cleave the C-P bond (PhnGHIJKLM), based on data in references 48 and 66). PhnJ is responsible for the release of methane (or another organic group) from the intermediate. (B) Odds ratios of genes encoding proteins in the C-P lyase pathway in metagenomic data from the Lake Matano chemocline (40), where P is not limiting (37). Odds ratios were calculated for each COG category in the metagenomic data set by calculating the ratio (A/B)/(C/D), where A is the number of reads in the metagenome that are in a given COG category, B is the number of reads in the metagenome that are in all other COG categories, C is the number of proteins in the COG database that are in a given COG category, and D is the number of proteins in the COG database that are in all of the other COG categories. COG categories with odds ratios of <1 are not considered enriched; none of the genes involved in transport or degradation of phosphonates are enriched in the chemocline. (C) Odds ratios of genes encoding proteins in the C-P lyase pathway in metagenomic data from Lake Matano surface water, where P is limiting. COG categories with odds ratios of >1 are considered enriched in the Lake Matano data set compared to the COG database (42).
The odds ratios compare the frequency of each protein in the metagenomic data set to its frequency in the COG database (42). An odds ratio greater than 1 indicates that a predicted protein is present in the metagenomic data set at a higher frequency than expected based on its frequency in protein databases (42). Below the chemocline in Lake Matano, the phosphate concentration increases to ∼10 μM, making the microbes at this depth less P starved than those in the surface water (37, 49). Because the odds ratios for nearly all of the genes required for MPn transport and degradation are less than 1 in the metagenomic data from the P-replete chemocline (Fig. 1B) but greater than 1 in the P-limited surface water (Fig. 1C), we hypothesized that MPn could serve as a P source for some organisms in Lake Matano, which would release methane as a by-product.
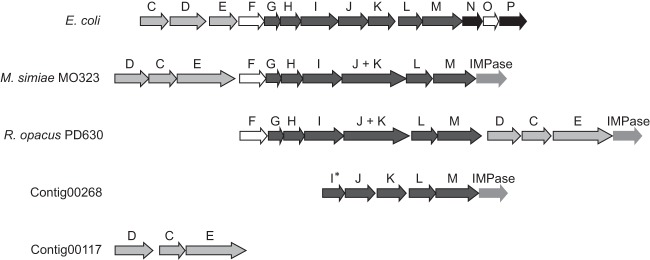
The Newbler GS De Novo Assembler was used to assemble the metagenomic reads from the Lake Matano surface water, and 530,076 reads (66.39% of the total number of reads) were assigned to 18,727 contigs. Amino acid sequences of C-P lyase proteins from E. coli (50) were used to query the contigs, and Contig00268 and contig00117 were chosen for further annotation. Contig00268 has 5,357 nucleotides and carries a partial phnI sequence, phnJKLM, a predicted inositol monophosphatase (IMPase), and a partial gene encoding the ATP-binding motif of an ABC transporter. Contig00117 has 7,056 nucleotides and carries the phosphonate transporter-encoding phnDCE, along with an alkaline phosphatase gene and a partial penicillin-binding protein-encoding gene (Fig. 2).
FIG 2.
Phosphonate-related gene clusters in Lake Matano metagenomic data. Contig00268 has high identity (66%) to the gene cluster encoding the multienzyme C-P lyase complex, phnIJKLM, from Rhodococcus opacus strains PD630 and R7 (only R. opacus strain PD630 is shown) and organization similar to that of the phn gene cluster from E. coli, whose C-P lyase pathway has been well studied (50, 67). Genes are colored based on their functions: transporter genes (phnCDE) are light gray, regulatory genes (phnFO) are white, phosphonate degradation genes (phnGHIJKLM) are dark gray, and the phnNP accessory genes are black.
Based on blastn analysis, Contig00268 has more than 83% coverage and 65% identity to gene clusters from three actinobacterial strains: Rhodococcus opacus strain PD630, R. opacus strain R7, and Mycobacterium simiae strain MO323. The three actinobacterial strains lack the accessory phnNP and the regulatory gene phnO that are part of the C-P lyase gene cluster in E. coli (50). The three actinobacterial strains all carry an inositol monophosphatase (IMPase)-encoding gene downstream of the C-P lyase gene clusters, and M. simiae encodes a penicillin-binding protein and an inositol 1-phosphate synthase upstream of the C-P lysase, while the R. opacus strains encode it downstream of the C-P lyase. In the two R. opacus strains, a predicted ABC transporter lies immediately downstream of the C-P lyase cluster (not shown). The best hit of blastn analysis for Contig00117 was also most similar to an Actinobacterium, Isoptericola dokdonensis DS-3, with 32% coverage and 71% identity. The predicted PhnE amino acid sequences in Contig00117, R. opacus, and M. simiae appear to have two PhnE domains (TIGR01097), in contrast to the E. coli PhnE, which has only one (Fig. 2). Several glyphosate- and phosphite-utilizing microbes have phn gene clusters with two copies of phnE (51, 52). In the gene cluster on Contig00117, these appear to be fused: no stop codon between the two phnE genes could be found, so we predict that they encode a single polypeptide.
The product of phnJ is responsible for cleavage of the C-P bond in phosphonate compounds and thus for release of methane from MPn (53), so the distribution of this gene in freshwater metagenomic data sets was assessed. Metagenomic data derived from freshwater samples and archived at the Integrated Microbial Genomes resource (https://img.jgi.doe.gov/) or the European Nucleotide Archive (http://www.ebi.ac.uk/ena) were queried for the presence of phnJ. Eighteen of the 23 data sets (78%) had homologs of phnJ (Table 3), suggesting that phosphonate bond cleavage may occur in a variety of freshwater environments, including lakes, lake sediments, bogs, rivers, and streams.
TABLE 3.
Distribution of phnJ in freshwater metagenomic data sets deposited at IMG or ENAa
| Sample source | Environment type | phnJ | Study (reference[s]) |
|---|---|---|---|
| Lake Damariscotta, Maine, USA | Lake | + | ENA-PRJEB4844 (43) |
| Lake Ekoln, Sweden | Lake | + | ENA-PRJEB4844 (43) |
| Lake Erken, Sweden | Lake | − | ENA-PRJEB4844 (43) |
| Lake Mendota, Wisconsin, USA | Lake | + | ENA-PRJEB4844 (43) |
| Lake Vättern, Sweden | Lake | + | ENA-PRJEB4844 (43) |
| Sparkling Lake, Wisconsin, USA | Lake | + | ENA-PRJEB4844 (43) |
| Trout Bog Lake, Wisconsin, USA | Lake | + | ENA-PRJEB4844 (43) |
| Lake Erie, Canada/USA | Lake | + | GOLD project ID Gp0111910 |
| Lake Sakinaw, British Columbia, Canada | Lake | + | GOLD project ID Gp0052015 (68, 69) |
| Lake Grosse Fuchskuhle, Germany | Mixed culture from lake | + | GOLD project ID: Gp0057572 (70) |
| Lake Superior, Canada/USA | Lake | + | GOLD study ID Gs0053068 |
| Sandusky Bay, Ohio, USA | Lake | − | GOLD study ID Gs0053068 (71) |
| Lake Ontario, Canada/USA | Lake sediment | − | GOLD study ID Gs0053068 |
| Lake Washington, Washington, USA | Lake sediment | + | GOLD study ID Gs0060820 (72) |
| Laguna de Carrizo, Spain | Lake sediment | − | GOLD study ID Gs0063259 (73) |
| Delaware River, Delaware/New Jersey, USA | River | + | GOLD study ID Gs0063440 |
| Lake Michigan, Canada/USA | Lake | + | GOLD study ID Gs0110155 (74) |
| Crystal Bog, Wisconsin, USA | Bog | + | GOLD study ID Gs0110170 |
| Loktak Lake, India | Lake | + | GOLD study ID Gs0111445 (75) |
| Notre Dame University, Indiana, USA | Anoxic pond sediments | + | GOLD study ID Gs011357 |
| University of Edinburgh, Edinburgh, UK | Pond | + | GOLD study ID Gs0114295 |
| Streams affected by fracking, Pennsylvania, USA | Stream | + | GOLD study ID Gs0114818 (76) |
The nucleotide sequence of phnJ from Pseudomonas stutzeri (accession no. AAR91738.1) or E. coli (accession no. AAC77059.1) was used to query all of these data sets; a data set was scored as positive if it had at least one hit with an E value of <10−10. Approximately 25% of samples from Yellowstone National Park also had phnJ homologs. IMG, Integrated Microbial Genomes & Microbiomes.
Methanogenesis has been observed in oxic surface waters of lakes (4–6, 8, 10). However, it has not been directly observed in Lake Matano surface waters, so we assessed the metagenomic data set from 10 m to determine whether surface water methanogenesis seemed likely. No reads mapping to archaeal rRNA sequences (5S, 16S, or 23S) are present in this data set. Ninety-five reads (of a total of 798,463 reads) mapped to archaeal genomes. Most of these (75 of 95 reads) mapped to the class Halobacteria, the members of which are not methanogenic. The remaining 20 reads were initially mapped to the methanogen genera Methanococcus and Methanomicrobium; however, when these reads were used as blastn queries against the NBCI nonredundant database, only 4 were identified as similar to sequences from methanogen genomes (Methanoculleus and Methanococcus). After the metagenomic reads were used as queries in a blast search against the COG database, the results were screened for reads mapping to methanogenesis pathways, but none were identified. Additionally, the amino acid sequence of McrA from Methanosarcina barkeri DSM804 (RefSeq: WP_011305916.1) was used as a query against the metagenomic data set, and no reads with homology to McrA were identified. We thus conclude that if water column methanogenesis occurs in the epilimnion of Lake Matano, it is either localized deeper than 10 m or carried out by a very small number of organisms not detected in our metagenomic survey.
Utilization of MPn as a P source by Lake Matano isolates.
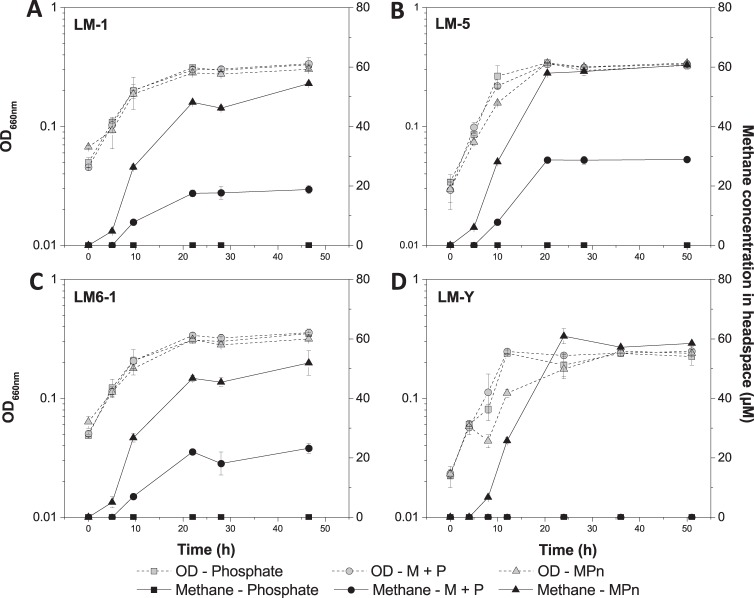
Seven isolates were screened for growth on MPn. Four were capable of growth on MPn as the sole P source and were selected for further analysis (Table 1). All four strains grow at the same rates on MPn, K2HPO4, or both and reach stationary phase at approximately the same time (Fig. 3, dashed lines). These isolates do not appear to be capable of consuming the methane released as a carbon or energy source, since they do not grow when MPn is provided as the sole P, C, and energy source (data not shown).
FIG 3.
Growth and methane production of isolates grown with phosphate, MPn, or both. Cells were grown at 30°C in serum bottles in NBRIP medium (44) with MPn (0.2 mM), K2HPO4 (0.2 mM), or both (0.2 mM each) supplied as P sources. Prior to measuring growth curves, cells were grown in NBRIP with K2HPO4 as the only P source. Growth was measured by monitoring the optical density at 660 nm, and methane in the headspace was measured by gas chromatography coupled with a flame ionization detector (GC-FID), Agilent 7890A (G3440A). All isolates grew at similar rates on all P sources (dashed lines). Methane was produced by LM-1, LM-5, and LM6-1 when MPn was present (A, B, C, solid lines). LM-Y produced methane only when MPn was present and phosphate was absent (D, solid lines).
Methane was produced only in the presence of MPn (Fig. 3, solid lines). In the presence of both MPn and 200 μM K2HPO4, strain LM-Y did not produce any methane (Fig. 3D). However, the other isolates produced some methane in the presence of K2HPO4 when MPn was also present (Fig. 3A to C). No methane was produced by any strain when MPn was not provided, indicating that the methane is a product of MPn metabolism.
To further investigate the relationship between MPn concentration and methane production, strain LM-Y was grown in NBRIP with different concentrations of MPn (Fig. 4). This strain has been shown to decrease RNA content and replace its phospholipids with amino- and glycolipids when P is limited, so the cells may increase their P content without necessarily altering the growth yield (38). As the MPn concentration increases, the cell yield and total methane production also increase, suggesting that availability of additional P promotes additional growth, as expected (Fig. 4). Both yield and methane production are similar when 100 or 200 μM MPn is added, suggesting that at concentrations of >100 μM MPn, sufficient P is available to maximize growth yield in NBRIP and the excess Pi in solution may even inhibit further degradation of MPn (54).
FIG 4.
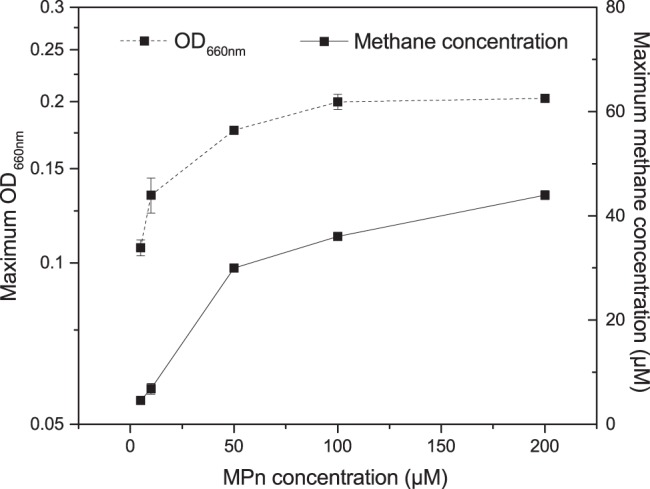
Growth and methane production of LM-Y grown on different MPn concentrations. MPn was added to final concentrations of 5 to 200 μM. Cells were grown ∼36 h, and OD660 and methane concentrations in the headspace were measured at different times during incubation. The maximum methane concentration and OD660 were reached after about 24 h of incubation.
Effect of phosphate on methane release from MPn.
Addition of 200 μM phosphate to the cultures with initial MPn concentrations of 200 μM reduced the amount of methane produced by strains LM-1, LM-5, and LM6-1 by 40 to 60% but completely repressed methane production in the culture of LM-Y. To better understand the effect of phosphate on phosphonate consumption by this strain, LM-Y was grown with 50 μM MPn and 0 to 100 μM KH2PO4 (Fig. 5). Methane production was completely inhibited in cultures grown with 50 μM MPn and 50 or 100 μM KH2PO4 (Fig. 5A). In cultures grown with 50 μM MPn and 0 μM, 4 μM, or 10 μM Pi, the amount of methane produced decreased as KH2PO4 increased (Fig. 5A). The relationship between added KH2PO4 concentration and percent repression of methane production is linear (Fig. 5B), and we extrapolate that methane production would be completely repressed at Pi concentrations above 30 μM.
FIG 5.
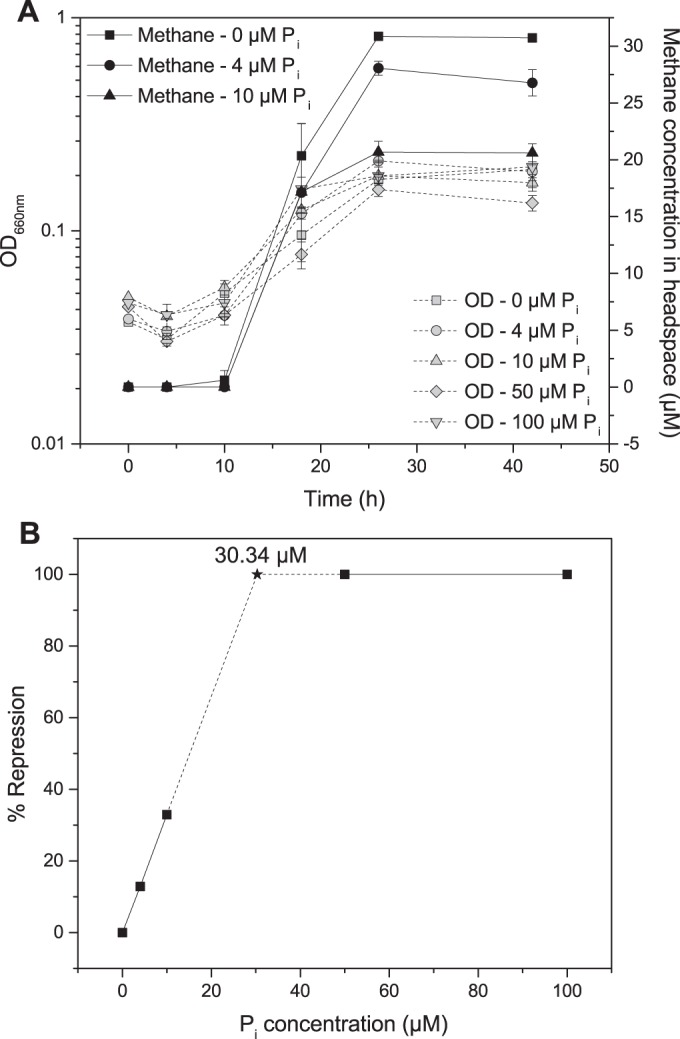
Phosphate represses methane production from MPn in LM-Y. All cultures were grown with 50 μM MPn and the indicated concentration of KH2PO4. Since no CH4 production was observed when KH2PO4 was used, we assume that there is no inhibition in the absence of KH2PO4, and 100% inhibition in the presence of ≥50 μM KH2PO4. Percent repression was calculated as described in equation 2.
Expression of phnJ during growth on different P sources.
The phnJ gene encodes the enzyme that catalyzes release of methane from the intermediate α-d-ribose-1-methylphosphonate-5-phosphate (53). We examined the expression of this gene in all four bacterial strains grown with 0.2 mM phosphate, 0.2 mM MPn, or both in the medium. LM-Y, the only strain in which methane production from MPn is abolished in the presence of phosphate (Fig. 3), appears to express phnJ only when MPn is present and phosphate is absent (Fig. 6). The other three strains, which produce methane when MPn is provided, whether or not it is the only P source (Fig. 6), express phnJ under both conditions. However, the phnJ transcript is less abundant in the presence of phosphate (Fig. 6 and Table 4).
FIG 6.
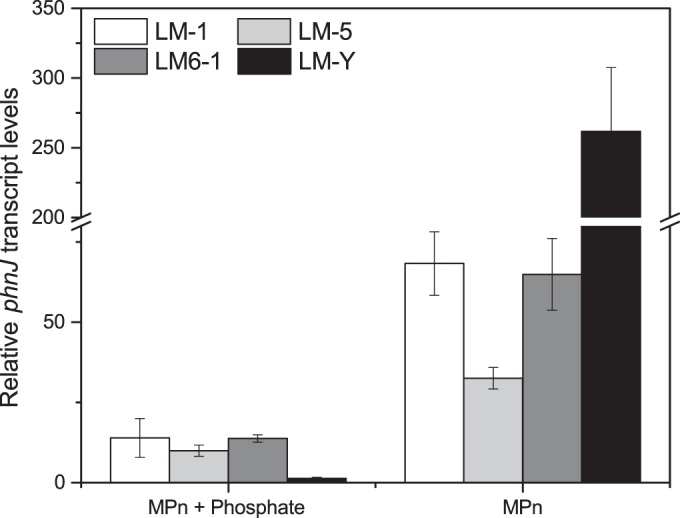
Transcript abundance of phnJ in isolates grown with phosphate, MPn, or both. Liquid subsamples (1.5 or 3.0 ml) were removed from all cultures during mid-exponential and stationary phases of growth on each P source (MPn, K2HPO4, or both). Transcript levels were calculated relative to phnJ in cells grown with phosphate and harvested during the exponential growth phase. Expression of phnJ was highest in cells grown with MPn as the sole P source; in strains LM-1, LM-5, and LM6-1, expression was lower but still detectable when both MPn and phosphate were supplied as P sources. Expression of phnJ in LM-Y in the presence of both MPn and phosphate is indistinguishable from its expression in the presence of phosphate only.
TABLE 4.
Relative expression levels of phnJ expression in isolates grown under different conditions
| Phase | P sourcea | Avg ± SD of relative levels of phnJ transcripts |
|||
|---|---|---|---|---|---|
| LM-1 | LM-5 | LM6-1 | LM-Y | ||
| Exponential growth | Phosphate | 1.00 | 1.00 | 1.00 | 1.00 |
| MPn + phosphate | 13.92 ± 6.05 | 9.97 ± 1.77 | 13.75 ± 1.09 | 1.35 ± 0.32 | |
| MPn | 68.26 ± 9.94 | 32.50 ± 3.33 | 64.90 ± 11.16 | 261.81 ± 45.67 | |
| Stationary | Phosphate | 0.35 ± 0.06 | 0.06 ± 0.01 | 0.19 ± 0.06 | 0.17 ± 0.01 |
| MPn + phosphate | 0.6 ± 0.01 | 0.54 ± 0.21 | 0.52 ± 0.04 | 0.17 ± 0.03 | |
| MPn | 0.94 ± 0.32 | 1.60 ± 0.28 | 0.70 ± 0.04 | 1.36 ± 0.06 | |
Relative levels of phnJ transcripts in cells grown with phosphate (0.2 mM), phosphate + MPn (0.2 mM each), or MPn alone (0.2 mM) were determined, normalized based on total RNA, and compared to levels in cells grown on phosphate during exponential growth phase. For each sample, two biological replicates and two technical replicates were analyzed.
Environmental significance.
Here, we show that freshwater heterotrophic bacteria are capable of producing methane from MPn and that the pathway for phosphonate degradation is widespread in freshwater lakes. We further demonstrate that in Lake Matano, Alphaproteobacteria, Gammaproteobacteria, and likely Actinobacteria can take up phosphonate compounds and cleave the C-P bond to acquire P and that this activity is repressed in the presence of Pi. Similarly, the phnJ gene is expressed in these strains only in the presence of MPn, and its expression level is modulated by addition of Pi. In sum, this work shows that methane production in freshwater systems may occur as a result of phosphate limitation, as microbes acquire P from phosphonates.
Phosphonates, including MPn, may comprise up to 10% of dissolved organic phosphorus (DOP) in some lakes (55–57), though many freshwaters have no detectable phosphonates (58, 59) or only very small quantities thereof (60, 61). However, homologs of phnJ are present in 18 of 23 metagenomic data sets from freshwater environments (water and sediment), including bogs, lakes, ponds, rivers, and streams (Table 3). The number of data sets in which phnJ is found and the diversity of environments indicate that the ability to degrade phosphonates is widespread in freshwater environments. Additionally, based on the abundance of phnD, which encodes one subunit of the phosphonate transporter, picocyanobacteria in the Great Lakes are predicted to be capable of phosphonate uptake (62). Given the broad distribution of phosphonate uptake and degradation pathways, phosphonates may be undetectable in freshwater systems not because they are not present but because they are rapidly broken down. In fact, phosphonates in marine systems have been shown to be highly reactive (63), and the same may be true in freshwater environments, which are typically P limited (64). This P limitation may result in microbial acquisition of P from a wide variety of sources (38), ultimately leading to release of organic by-products such as methane.
The steady-state concentration of methane at 40 m in Lake Matano is ∼3 μM, 3 orders of magnitude greater than the saturation concentration (7). Many freshwater lakes are supersaturated with regard to methane, often more than can be explained by methanogenesis in anoxic sediments or bottom waters (5, 6, 10). In some of these systems, surface water methane is produced by methanogenesis in oxic surface waters (5, 10). However, in severely P-limited systems, demethylation of P-containing compounds may also contribute to surface water methane production. Because Lake Matano has less than 50 nM total Pi in the surface water (37), planktonic organisms there must be capable of immediate uptake and utilization of P in any available form (38). Both physiological experiments with isolates and bioinformatics analysis of metagenomic data indicate that heterotrophic bacteria in Lake Matano surface water are capable of producing methane as a by-product of acquisition of P from phosphonate compounds. Given the prevalence of genes encoding phosphonate biosynthesis and degradation in freshwater metagenomic data sets, P metabolism in freshwater systems may have unexpectedly large effects not only on P cycling but also on release of greenhouse gases. The current models for methane cycling in fresh waters use lake size, shape, nutrient status, temperature, and primary production to predict rates of methane production (19, 21, 65) but do not include methane production by any pathway in oxic water columns, even though this phenomenon has been observed in many lakes (4–6, 8, 10) and is known to be important in the ocean (12, 13). Because lakes may release as much as ∼100 Tg methane globally each year, or ∼20% of total annual natural methane emissions, which is more than the emissions from the world's oceans (1, 2), understanding the environmental and biological factors contributing to methane emissions from freshwater systems is critical to making more-accurate predictions of both freshwater and global methane emissions (9, 18–21).
ACKNOWLEDGMENTS
We gratefully acknowledge Thomas Hanson at the Delaware Biotechnology Institute for the use of the GC-FID, Brewster Kingham at the University of Delaware Sequencing and Genotyping Center for sequencing the phnJ products, and Nicole Donofrio in the University of Delaware Plant & Soil Sciences Department for technical assistance with the qRT-PCR.
We declare that we have no conflicts of interest.
Author contributions: M.Y. analyzed metagenomic data, monitored growth and methane production, quantified gene expression rates using qPCR, made the figures, and contributed to the manuscript writing. C.H. provided essential logistical support for the field expedition and critical commentary on the manuscript. J.A.M. extracted DNA from field samples, analyzed metagenomic and biochemical data, supervised experiments, and coordinated the manuscript writing and editing.
Funding Statement
This research was supported by the Department of Civil and Environmental Engineering at the University of Delaware but received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Bastviken D, Cole J, Pace M, Tranvik L. 2004. Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochem Cycles 18:GB4009.1–GB4009.12. [Google Scholar]
- 2.Bastviken D, Tranvik LJ, Downing JA, Crill PM, Enrich-Prast A. 2011. Freshwater methane emissions offset the continental carbon sink. Science 331:50. doi: 10.1126/science.1196808. [DOI] [PubMed] [Google Scholar]
- 3.Kirschke S, Bousquet P, Ciais P, Saunois M, Canadell JG, Dlugokencky EJ, Bergamaschi P, Bergmann D, Blake DR, Bruhwiler L, Cameron-Smith P, Castaldi S, Chevallier F, Feng L, Fraser A, Heimann M, Hodson EL, Houweling S, Josse B, Fraser PJ, Krummel PB, Lamarque J-F, Langenfelds RL, Le Quéré C, Naik V, O'Doherty S, Palmer PI, Pison I, Plummer D, Poulter B, Prinn RG, Rigby M, Ringeval B, Santini M, Schmidt M, Shindell DT, Simpson IJ, Spahni R, Steele LP, Strode SA, Sudo K, Szopa S, van der Werf GR, Voulgarakis A, van Weele M, Weiss RF, Williams JE, Zeng G. 2013. Three decades of global methane sources and sinks. Nat Geosci 6:813–823. doi: 10.1038/ngeo1955. [DOI] [Google Scholar]
- 4.Blees J, Niemann H, Erne M, Zopfi J, Schubert CJ, Lehmann MF. 2015. Spatial variations in surface water methane super-saturation and emission in Lake Lugano, southern Switzerland. Aquat Sci 77:535–545. doi: 10.1007/s00027-015-0401-z. [DOI] [Google Scholar]
- 5.Grossart H-P, Frindte K, Dziallas C, Eckert W, Tang KW. 2011. Microbial methane production in oxygenated water column of an oligotrophic lake. Proc Natl Acad Sci U S A 108:19657–19661. doi: 10.1073/pnas.1110716108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang KW, McGinnis DF, Frindte K, Brüchert V, Grossart H-P. 2014. Paradox reconsidered: methane oversaturation in well-oxygenated lake waters. Limnol Oceanogr 59:275–284. doi: 10.4319/lo.2014.59.1.0275. [DOI] [Google Scholar]
- 7.Kuntz LB, Laakso TA, Schrag DP, Crowe SA. 2015. Modeling the carbon cycle in Lake Matano. Geobiology 13:454–461. doi: 10.1111/gbi.12141. [DOI] [PubMed] [Google Scholar]
- 8.Tang KW, McGinnis DF, Ionescu D, Grossart H-P. 2016. Methane production in oxic lake waters potentially increases aquatic methane flux to air. Environ Sci Technol Lett 3:227–233. [Google Scholar]
- 9.Wik M, Varner RK, Anthony KW, MacIntyre S, Bastviken D. 2016. Climate-sensitive northern lakes and ponds are critical components of methane release. Nat Geosci 9:99–105. doi: 10.1038/ngeo2578. [DOI] [Google Scholar]
- 10.Bogard MJ, del Giorgio PA, Boutet L, Chaves MCG, Prairie YT, Merante A, Derry AM. 2014. Oxic water column methanogenesis as a major component of aquatic CH4 fluxes. Nat Commun 5:5350. doi: 10.1038/ncomms6350. [DOI] [PubMed] [Google Scholar]
- 11.Robertson AM, Wolfe RS. 1970. Adenosine triphosphate pools in Methanobacterium. J Bacteriol 102:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karl DM, Beversdorf L, Björkman KM, Church MJ, Martinez A, Delong EF. 2008. Aerobic production of methane in the sea. Nat Geosci 1:473–478. doi: 10.1038/ngeo234. [DOI] [Google Scholar]
- 13.Martinez A, Tyson GW, Delong EF. 2010. Widespread known and novel phosphonate utilization pathways in marine bacteria revealed by functional screening and metagenomic analyses. Environ Microbiol 12:222–238. doi: 10.1111/j.1462-2920.2009.02062.x. [DOI] [PubMed] [Google Scholar]
- 14.Martínez A, Ventouras L-A, Wilson ST, Karl DM, Delong EF. 2013. Metatranscriptomic and functional metagenomic analysis of methylphosphonate utilization by marine bacteria. Front Microbiol 4:340. doi: 10.3389/fmicb.2013.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carini P, White AE, Campbell EO, Giovannoni SJ. 2014. Methane production by phosphate-starved SAR11 chemoheterotrophic marine bacteria. Nat Commun 5:4346. [DOI] [PubMed] [Google Scholar]
- 16.Dyhrman ST, Chappell PD, Haley ST, Moffett JW, Orchard ED, Waterbury JB, Webb EA. 2006. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439:68–71. doi: 10.1038/nature04203. [DOI] [PubMed] [Google Scholar]
- 17.Conrad R. 2009. The global methane cycle: recent advances in understanding the microbial processes involved. Environ Microbiol Rep 1:285–292. doi: 10.1111/j.1758-2229.2009.00038.x. [DOI] [PubMed] [Google Scholar]
- 18.Lundin EJ, Klaminder J, Bastviken D, Olid C, Hansson SV, Karlsson J. 2015. Large difference in carbon emission—burial balances between boreal and arctic lakes. Sci Rep 5:14248. doi: 10.1038/srep14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasilo T, Prairie YT, Del Giorgio PA. 2015. Large-scale patterns in summer diffusive CH4 fluxes across boreal lakes, and contribution to diffusive C emissions. Glob Chang Biol 21:1124–1139. doi: 10.1111/gcb.12741. [DOI] [PubMed] [Google Scholar]
- 20.Hershey AE, Northington RM, Whalen SC. 2013. Substrate limitation of sediment methane flux, methane oxidation and use of stable isotopes for assessing methanogenesis pathways in a small arctic lake. Biogeochemistry 117:325–336. [Google Scholar]
- 21.West WE, Creamer KP, Jones SE. 2015. Productivity and depth regulate lake contributions to atmospheric methane. Limnol Oceanogr doi: 10.1002/lno.10247. [DOI] [Google Scholar]
- 22.Metcalf WW, Griffin BM, Cicchillo RM, Gao J, Janga SC, Cooke HA, Circello BT, Evans BS, Martens-Habbena W, Stahl DA, van der Donk WA. 2012. Synthesis of methylphosphonic acid by marine microbes: a source for methane in the aerobic ocean. Science 337:1104–1107. doi: 10.1126/science.1219875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju K, Doroghazi JR, Metcalf WW. 2014. Genomics-enabled discovery of phosphonate natural products and their biosynthetic pathways. J Ind Microbiol Biotechnol 41:345–356. doi: 10.1007/s10295-013-1375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu X, Doroghazi JR, Janga SC, Zhang JK, Circello B, Griffin BM, Labeda DP, Metcalf WW. 2013. Diversity and abundance of phosphonate biosynthetic genes in nature. Proc Natl Acad Sci U S A 110:20759–20764. doi: 10.1073/pnas.1315107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath JW, Chin JP, Quinn JP. 2013. Organophosphonates revealed: new insights into the microbial metabolism of ancient molecules. Nat Rev Microbiol 11:412–419. doi: 10.1038/nrmicro3011. [DOI] [PubMed] [Google Scholar]
- 26.Quinn JP, Kulakova AN, Cooley NA, McGrath JW. 2007. New ways to break an old bond: the bacterial carbon-phosphorus hydrolases and their role in biogeochemical phosphorus cycling. Environ Microbiol 9:2392–2400. doi: 10.1111/j.1462-2920.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- 27.White AK, Metcalf WW. 2007. Microbial metabolism of reduced phosphorus compounds. Annu Rev Microbiol 61:379–400. doi: 10.1146/annurev.micro.61.080706.093357. [DOI] [PubMed] [Google Scholar]
- 28.Metcalf WW, Wanner BL. 1993. Mutational analysis of an Escherichia coli fourteen-gene operon for phosphonate degradation, using TnphoA' elements. J Bacteriol 175:3430–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumora C, Lacoste AM, Cassaigne A. 1989. Phosphonoacetaldehyde hydrolase from Pseudomonas aeruginosa: purification properties and comparison with Bacillus cereus enzyme. Biochim Biophys Acta 997:193–198. doi: 10.1016/0167-4838(89)90186-6. [DOI] [PubMed] [Google Scholar]
- 30.McGrath JW, Ternan NG, Quinn JP. 1997. Utilization of organophosphonates by environmental micro-organisms. Lett Appl Microbiol 24:69–73. doi: 10.1046/j.1472-765X.1997.00350.x. [DOI] [Google Scholar]
- 31.Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 32.Panas P, Ternan NG, Dooley JSG, McMullan G. 2006. Detection of phosphonoacetate degradation and phnA genes in soil bacteria from distinct geographical origins suggest its possible biogenic origin. Environ Microbiol 8:939–945. doi: 10.1111/j.1462-2920.2005.00974.x. [DOI] [PubMed] [Google Scholar]
- 33.Beversdorf LJ, White AE, Björkman KM, Letelier RM, Karl DM. 2010. Phosphonate metabolism by Trichodesmium IMS101 and the production of greenhouse gases. Limnol Oceanogr 55:1768–1778. doi: 10.4319/lo.2010.55.4.1768. [DOI] [Google Scholar]
- 34.Bastviken D, Ejlertsson J, Tranvik L. 2002. Measurement of methane oxidation in lakes: a comparison of methods. Environ Sci Technol 36:3354–3361. doi: 10.1021/es010311p. [DOI] [PubMed] [Google Scholar]
- 35.Crowe SAA, Katsev S, Leslie K, Sturm A, Magen C, Nomosatryo S, Douglas G, Pack MAA, Kessler JDD, Reeburgh WSS, Roberts JAA, Gonza L, Gonzalez L, Haffner GD, Mucci A, Sundby B, Fowle DA. 2011. The methane cycle in ferruginous Lake Matano. Geobiology 9:61–78. doi: 10.1111/j.1472-4669.2010.00257.x. [DOI] [PubMed] [Google Scholar]
- 36.Zegeye A, Bonneville S, Benning LG, Sturm A, Fowle DA, Jones C, Canfield DE, Ruby C, MacLean LC, Nomosatryo S, Crowe SA, Poulton SW. 2012. Green rust formation controls nutrient availability in a ferruginous water column. Geology 40:599–602. doi: 10.1130/G32959.1. [DOI] [Google Scholar]
- 37.Crowe SA, O'Neill AH, Katsev S, Hehanussa P, Haffner GD, Sundby B, Mucci A, Fowle DA. 2008. The biogeochemistry of tropical lakes: a case study from Lake Matano, Indonesia. Limnol Oceanogr 53:319–331. doi: 10.4319/lo.2008.53.1.0319. [DOI] [Google Scholar]
- 38.Yao M, Elling FJ, Jones C, Nomosatryo S, Long CP, Crowe SA, Antoniewicz MR, Hinrichs K-U, Maresca JA. 2016. Heterotrophic bacteria from an extremely phosphate-poor lake have conditionally reduced phosphorus demand and utilize diverse sources of phosphorus. Environ Microbiol 18:656–667. doi: 10.1111/1462-2920.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seweryn P, Van LB, Kjeldgaard M, Russo CJ, Passmore LA, Hove-Jensen B, Jochimsen B, Brodersen DE. 2015. Structural insights into the bacterial carbon-phosphorus lyase machinery. Nature 525:68–72. doi: 10.1038/nature14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowe SA, Maresca JA, Jones C, Sturm A, Henny C, Fowle DA, Cox RP, Delong EF, Canfield DE. 2014. Deep-water anoxygenic photosythesis in a ferruginous chemocline. Geobiology 12:322–339. doi: 10.1111/gbi.12089. [DOI] [PubMed] [Google Scholar]
- 41.Galperin MY, Makarova KS, Wolf YI, Koonin EV. 2014. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res 43:D261–D269. doi: 10.1093/nar/gku1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. 2006. Metagenomic analysis of the human distal gut microbiome. Science 312:1355–1360. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eiler A, Zaremba-Niedzwiedzka K, Martínez-García M, McMahon KD, Stepanauskas R, Andersson SGE, Bertilsson S. 2014. Productivity and salinity structuring of the microplankton revealed by comparative freshwater metagenomics. Environ Microbiol 16:2682–2698. doi: 10.1111/1462-2920.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nautiyal CS. 1999. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 45.Stumm W, Morgan JJ. 1996. Aquatic chemistry: chemical equilibrium and rates in natural waters. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- 46.Fox A, Kwapinski W, Griffiths BS, Schmalenberger A. 2014. The role of sulfur- and phosphorus-mobilizing bacteria in biochar-induced growth promotion of Lolium perenne. FEMS Microbiol Ecol 90:78–91. doi: 10.1111/1574-6941.12374. [DOI] [PubMed] [Google Scholar]
- 47.Karl H. 2007. Charakterisierung von mikrobiellen, C-P-Lyasen kodierenden Genen in zwei unterschiedlichen Ackerböden. Dissertation. Technische Universität München, Munich, Germany. [Google Scholar]
- 48.Kamat SS, Williams HJ, Raushel FM. 2011. Intermediates in the transformation of phosphonates to phosphate by bacteria. Nature 480:570–573. doi: 10.1038/nature10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crowe SA, Jones C, Katsev S, Magen C, O'Neill AH, Sturm A, Canfield DE, Haffner GD, Mucci A, Sundby B, Fowle DA. 2008. Photoferrotrophs thrive in an Archean Ocean analogue. Proc Natl Acad Sci U S A 105:15938–15943. doi: 10.1073/pnas.0805313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metcalf WW, Wanner BL. 1993. Evidence for a fourteen-gene, phnC to phnP locus for phosphonate metabolism in Escherichia coli. Gene 129:27–32. doi: 10.1016/0378-1119(93)90692-V. [DOI] [PubMed] [Google Scholar]
- 51.Hove-Jensen B, Zechel DL, Jochimsen B. 2014. Utilization of glyphosate as phosphate source: biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol Mol Biol Rev 78:176–197. doi: 10.1128/MMBR.00040-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poehlein A, Daniel R, Schink B, Simeonova DD. 2013. Life based on phosphite: a genome-guided analysis of Desulfotignum phosphitoxidans. BMC Genomics 14:753. doi: 10.1186/1471-2164-14-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamat SS, Raushel FM. 2013. The enzymatic conversion of phosphonates to phosphate by bacteria. Curr Opin Chem Biol 17:589–596. doi: 10.1016/j.cbpa.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Imazu K, Tanaka S, Kuroda A, Anbe Y, Kato J, Ohtake H. 1998. Enhanced utilization of phosphonate and phosphite by Klebsiella aerogenes. Appl Environ Microbiol 64:3754–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheesman AW, Turner BL, Ramesh Reddy K. 2012. Soil phosphorus forms along a strong nutrient gradient in a tropical ombrotrophic wetland. Soil Sci Soc Am J 76:1496. doi: 10.2136/sssaj2011.0365. [DOI] [Google Scholar]
- 56.Nanny MA, Minear RA. 1997. Characterization of soluble unreactive phosphorus using 31P nuclear magnetic resonance spectroscopy. Mar Geol 139:77–94. doi: 10.1016/S0025-3227(96)00098-9. [DOI] [Google Scholar]
- 57.Coupe RH, Kalkhoff SJ, Capel PD, Gregoire C. 2012. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag Sci 68:16–30. doi: 10.1002/ps.2212. [DOI] [PubMed] [Google Scholar]
- 58.Dong L, Yang Z, Liu X, Liu G. 2012. Investigation into organic phosphorus species in sediments of Baiyangdian Lake in China measured by fractionation and 31P NMR. Environ Monit Assess 184:5829–5839. doi: 10.1007/s10661-012-2550-z. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Wang H, Yang H, Ma Y, Cai O. 2009. Detection of phosphorus species in sediments of artificial landscape lakes in China by fractionation and phosphorus-31 nuclear magnetic resonance spectroscopy. Environ Pollut 157:49–56. doi: 10.1016/j.envpol.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Zhang R, Wang L, Wu F, Song B. 2013. Phosphorus speciation in the sediment profile of Lake Erhai, southwestern China: fractionation and 31P NMR. J Environ Sci 25:1124–1130. doi: 10.1016/S1001-0742(12)60163-6. [DOI] [PubMed] [Google Scholar]
- 61.Bai X, Ding S, Fan C, Liu T, Shi D, Zhang L. 2009. Organic phosphorus species in surface sediments of a large, shallow, eutrophic lake, Lake Taihu, China. Environ Pollut 157:2507–2513. doi: 10.1016/j.envpol.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 62.Kutovaya OA, McKay RML, Bullerjahn GS. 2013. Detection and expression of genes for phosphorus metabolism in picocyanobacteria from the Laurentian Great Lakes. J Great Lakes Res 39:612–621. doi: 10.1016/j.jglr.2013.09.009. [DOI] [Google Scholar]
- 63.Young CL, Ingall ED. 2010. Marine dissolved organic phosphorus composition: insights from samples recovered using combined electrodialysis/reverse osmosis. Aquat Geochem 16:563–574. doi: 10.1007/s10498-009-9087-y. [DOI] [Google Scholar]
- 64.Hecky R, Campbell P, Hendzel L. 1993. The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol Oceanogr 38:709–724. doi: 10.4319/lo.1993.38.4.0709. [DOI] [Google Scholar]
- 65.Juutinen S, Rantakari M, Kortelainen P, Huttunen JT, Larmola T, Alm J, Silvola J, Martikainen PJ. 2009. Methane dynamics in different boreal lake types. Biogeosciences 6:209–223. doi: 10.5194/bg-6-209-2009. [DOI] [Google Scholar]
- 66.Peck SC, van der Donk WA. 2013. Phosphonate biosynthesis and catabolism: a treasure trove of unusual enzymology. Curr Opin Chem Biol 17:580–588. doi: 10.1016/j.cbpa.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wanner BL, Metcalf WW. 1992. Molecular genetic studies of a 10.9-kb operon in Escherichia coli for phosphonate uptake and biodegradation. FEMS Microbiol Lett 100:133–139. [DOI] [PubMed] [Google Scholar]
- 68.Nobu MK, Dodsworth JA, Murugapiran SK, Rinke C, Gies EA, Webster G, Schwientek P, Kille P, Parkes RJ, Sass H, Jørgensen BB, Weightman AJ, Liu W-T, Hallam SJ, Tsiamis G, Woyke T, Hedlund BP. 2016. Phylogeny and physiology of candidate phylum “Atribacteria” (OP9/JS1) inferred from cultivation-independent genomics. ISME J 10:273–286. doi: 10.1038/ismej.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rinke C, Schwientek P, Sczyrba A, Ivanova NN, Anderson IJ, Cheng J-F, Darling A, Malfatti S, Swan BK, Gies EA, Dodsworth JA, Hedlund BP, Tsiamis G, Sievert SM, Liu W-T, Eisen JA, Hallam SJ, Kyrpides NC, Stepanauskas R, Rubin EM, Hugenholtz P, Woyke T. 2013. Insights into the phylogeny and coding potential of microbial dark matter. Nature 499:431–437. doi: 10.1038/nature12352. [DOI] [PubMed] [Google Scholar]
- 70.Garcia SL, Buck M, McMahon KD, Grossart H-P, Eiler A, Warnecke F. 2015. Auxotrophy and intrapopulation complementary in the “interactome” of a cultivated freshwater model community. Mol Ecol 24:4449–4459. doi: 10.1111/mec.13319. [DOI] [PubMed] [Google Scholar]
- 71.Davis TW, Bullerjahn GS, Tuttle T, McKay RM, Watson SB. 2015. Effects of increasing nitrogen and phosphorus concentrations on phytoplankton community growth and toxicity during Planktothrix blooms in Sandusky Bay, Lake Erie. Environ Sci Technol 49:7197–7207. doi: 10.1021/acs.est.5b00799. [DOI] [PubMed] [Google Scholar]
- 72.Beck DAC, Kalyuzhnaya MG, Malfatti S, Tringe SG, Glavina del Rio T, Ivanova N, Lidstrom ME, Chistoserdova L. 2013. A metagenomic insight into freshwater methane-utilizing communities and evidence for cooperation between the Methylococcaceae and the Methylophilaceae. PeerJ 1:e23. doi: 10.7717/peerj.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrer M, Guazzaroni M-E, Richter M, García-Salamanca A, Yarza P, Suárez-Suárez A, Solano J, Alcaide M, Dillewijn P, Molina-Henares MA, López-Cortés N, Al-Ramahi Y, Guerrero C, Acosta A, Eugenio LI, Martínez V, Marques S, Rojo F, Santero E, Genilloud O, Pérez-Pérez J, Rosselló-Móra R, Ramos JL. 2011. Taxonomic and functional metagenomic profiling of the microbial community in the anoxic sediment of a sub-saline shallow lake (Laguna de Carrizo, Central Spain). Microb Ecol 62:824–837. doi: 10.1007/s00248-011-9903-y. [DOI] [PubMed] [Google Scholar]
- 74.Denef VJ, Mueller RS, Chiang E, Liebig JR, Vanderploeg HA. 2016. Chloroflexi CL500-11 populations that predominate deep-lake hypolimnion bacterioplankton rely on nitrogen-rich dissolved organic matter metabolism and C1 compound oxidation. Appl Environ Microbiol 82:1423–1432. doi: 10.1128/AEM.03014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puranik S, Ramavadh Pal R, Prabhakar More R, Purohit HJ. 9 August 2016. Metagenomic approach to characterize soil microbial diversity of Phumdi at Loktak Lake. Water Sci Technol doi: 10.2166/wst.2016.370. [DOI] [PubMed] [Google Scholar]
- 76.Trexler R, Solomon C, Brislawn CJ, Wright JR, Rosenberger A, McClure EE, Grube AM, Peterson MP, Keddache M, Mason OU, Hazen TC, Grant CJ, Lamendella R. 2014. Assessing impacts of unconventional natural gas extraction on microbial communities in headwater stream ecosystems in Northwestern Pennsylvania. Front Microbiol 5:522. doi: 10.3389/fmicb.2014.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]