Abstract
Objective
To examine whether genetic, environmental, and serologic rheumatoid arthritis (RA) risk factors are associated with inflammatory joint signs (IJS) in a cohort of RA first-degree relatives (FDRs).
Methods
We evaluated RA risk factors and IJS in a prospective cohort of FDRs without RA in the Studies of the Etiology of RA. Genetic factors included five HLA-DRB1 shared epitope alleles and 45 RA-associated single nucleotide polymorphisms; loci were combined using genetic risk scores (GRS) weighted by RA risk. Environmental factors (smoking, body mass index, education, and parity) and RA-related autoantibodies were assessed at baseline. Physical examination at baseline and two-year follow-up by observers blinded to autoantibody status assessed IJS as tender or swollen joints at sites typical for RA. Logistic regression was performed to evaluate associations of genetic, environmental, and serologic factors with IJS.
Results
We analyzed 966 non-Hispanic white FDRs at baseline and 262 at two-year follow-up after excluding those with IJS at baseline. Mean age was 47.2 years (SD 15.5), 71% were female, and 55% were shared epitope-positive. Smoking >10 pack-years was associated with IJS at baseline (OR 1.59, 95%CI 1.09–2.32) and at 2 years (OR 2.66, 95%CI 1.01–7.03), compared to never smokers. Smoking and age significantly interacted for risk of IJS (p=0.02). FDRs aged <50 years with >10 pack-years had the highest risk of IJS (OR 4.39, 95%CI 2.22–8.66) compared to never smokers aged <50 years).
Conclusion
In a high-risk cohort of FDRs, smoking and age were associated with both prevalent and incident IJS at sites typical for RA. Further prospective investigations of the factors affecting the transitions between pre-clinical RA phases are warranted.
Keywords: rheumatoid arthritis, pathogenesis, smoking, genetics, autoantibodies
Genetic, environmental, and serologic factors have been associated with RA risk (1–3). The current paradigm for RA pathogenesis is that genetically susceptible individuals progress through preclinical stages prior to the development of classifiable RA (4–6). These stages include genetic risk, immune activation without symptoms, arthralgias and non-specific symptoms, and inflammatory arthritis prior to classifiable RA. Presence of RA-related autoantibodies, rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP), is strongly associated with development of RA (2, 7–9). Epitope spreading has been shown to occur years prior to clinically apparent RA (7, 10, 11). Determining the factors that affect transitions through preclinical RA stages may help elucidate the pathogenesis of RA and may aid in the identification of high-risk individuals for RA prevention strategies (5).
Prior studies of preclinical RA have evaluated progression to inflammatory arthritis or classifiable RA among symptomatic subjects in the arthralgia phase (12). Environmental factors such as smoking, excess body mass, and low alcohol intake, in addition to serologic factors such as anti-CCP status, have been associated with the development of RA in these arthralgia cohorts (13–17).
Family history of RA in a first-degree relative (FDR) increases the risk of RA by nearly four-fold independent of known genetic and environmental RA risk factors (18, 19). Family history of RA is a risk factor for developing RA among seropositive patients with arthralgias (17). Even among those with a family history of RA, up to 46% of the attributable risk for RA may be due to known environmental factors (18). While a prior cross-sectional study evaluated human leukocyte antigen (HLA) shared epitope alleles and risk of RA among unaffected family members, no prospective studies have evaluated the shared epitope and non-HLA genetic loci for risk of inflammatory joint signs (IJS) (20). Moreover, limited data exist investigating environmental and serologic risk factors for pre-clinical RA progression in unaffected FDRs.
We therefore aimed to determine whether known environmental, genetic, and serologic RA risk factors are associated with IJS in a prospective FDR cohort, the Studies of the Etiology of RA (SERA).
SUBJECTS AND METHODS
Studies of the Etiology of Rheumatoid Arthritis (SERA)
The study sample was derived from SERA, a multicenter, prospective FDR cohort study (21). Subjects in this analysis included parents, offspring, or siblings of patients with established RA and were enrolled in Denver, Los Angeles, Chicago, New York, Nebraska (Rheumatology and Arthritis Investigational Network), and Seattle from 2002–2012. At enrollment, FDRs were surveyed regarding signs and symptoms of RA and underwent a 68-joint physical examination confirming no RA according to the 1987 American College of Rheumatology (ACR) classification criteria (22). All studies were approved by institutional review boards at all participating institutions.
Study visits consisted of questionnaires regarding environmental risk factors, a physical examination by a trained study rheumatologist or nurse, and collection of blood samples for biomarker/genetic studies. Since we genotyped single nucleotide polymorphisms (SNPs) associated with RA in Caucasian/European populations, we only included subjects who reported race as non-Hispanic white for this analysis to protect against possible erroneous genetic associations due to population stratification.
Assessment of inflammatory joint signs (IJS)
We defined IJS as presence of tender or swollen joints on a 68-count joint examination administered by a trained study rheumatologist or nurse blinded to autoantibody status (23). We only considered IJS at small joints at RA-specific sites according to the 2010 ACR/European League Against Rheumatism (EULAR) classification criteria (24). Joints included were metacarpophalangeal (MCP) joints, proximal interphalangeal joints, wrists, and metatarsophalangeal (MTP) joints. Given its relative specificity for RA, elbows were also included in these analyses despite its classification as a large joint in the 2010 ACR/EULAR criteria (24). Swollen joints determined by the examiner to be due to degenerative joint disease or injury according to physical examination findings or by history were not included in the IJS outcomes. First MCP or MTP joints and swollen joints deemed to be due to trauma or degenerative disease were not included. To optimize sensitivity, we considered IJS to be present if there was at least one tender or one swollen joint.
Genetic factors and genotyping
Shared epitope
Complete subtyping of HLA-DR4 alleles was performed using polymerase chain reaction with sequence-specific primers (SSP) (Benaroya Research Institute, Seattle, Washington, USA), as previously described (21, 25). Shared epitope positivity was considered as positive in the presence of DRB1*01 or *04 by SSP (26). For samples with positive two-digit HLA signals at DRB1*04, SSP were used for high-resolution four-digit allele detection of *0401, *0404, *0405, and *0408.
Non-HLA loci
SNPs that were previously associated with RA among those with Caucasian ancestry were included for genotyping (1). Therefore, we genotyped 65 SNPs that were associated with RA among Caucasians in a large meta-genome-wide association study (1). Genotyping was performed on a custom Sequenom platform at the Broad Institute (Cambridge, Massachusetts, USA). We used proxy SNPs for some RA-associated SNPs if they were unable to be genotyped on our platform. We used proxy SNPs in linkage disequilibrium (LD, r2>0.9) with the RA-associated SNP for six RA-associated loci. We required a call rate >95% and Hardy-Weinberg equilibrium P value >0.01. All genotyped SNPs had minor allele frequency >0.01. Thirteen RA-associated SNPs were genotyped but failed quality control due to low call rates. Five genotyped SNPs failed quality control by violating Hardy-Weinberg equilibrium. Two pairs of proxy SNPs were in LD with each other. For both pairs of SNPs, we included the SNP with higher LD with the RA-associated SNP for these analyses. Our analyses therefore included a total of 45 non-HLA SNPs and five shared epitope alleles.
Genetic risk scores (GRS)
RA risk alleles were combined to form weighted GRS for RA. Weighted GRS have been validated to predict RA in several case-control studies (3, 27, 28). GRS were weighted by the natural logarithm of published odds ratios (ORs) for RA in previous studies (1, 26). For proxy SNPs, we utilized the OR for RA risk of the original SNP associated with RA. For missing SNPs in a subject, we assigned a value of twice the minor allele frequency for that SNP. We studied three GRS: 1) HLA GRS5 composed of the five shared epitope alleles genotyped, 2) non-HLA GRS45 composed of 45 non-HLA SNPs associated with RA, and 3) full GRS50 composed of all 50 genotyped RA genetic risk factors. Supplemental Table 1 lists the HLA alleles and non-HLA SNPs genotypes, odds ratio for RA risk for each allele, and weights used for calculation of each GRS.
Environmental factors
We considered epidemiologic and environmental factors associated with RA in previous studies: age, cigarette smoking, alcohol intake, body mass index (BMI), education, and parity (29–33). RA risk models using these factors among FDRs were validated to discriminate RA from controls (3). Data on alcohol were not available for most subjects, so was not considered for these analyses. All covariates were self-reported at baseline study visit, except for BMI which was calculated from measured weight and height at baseline.
Cigarette smoking was assessed using cumulative pack-years or categorized as never, past, or current. We categorized cumulative smoking pack-years as never, >0 to ≤10 pack-years, or >10 pack-years since the latter category has been associated with RA (29, 34).
BMI was dichotomized as normal/underweight (<25 kg/m2) or overweight/obese (≥25 kg/m2). Education was dichotomized as high school graduate or less/some college or greater. Parity was considered as parous or nulliparous for females. Age was considered as a continuous variable in years at baseline.
Serologic factors
Serologic factors were RA-related autoantibodies tested at baseline. These included second-generation anti-CCP (Axis-Shield, Dundee, Scotland, UK), RF by nephelometry (BN II, Dade Behring, Westwood, Massachusetts, USA), and RF IgA and IgM isotypes (Inova Diagnostics, Inc., San Diego, California, USA) by enzyme-linked immunosorbent assay (ELISA). Methods for testing of anti-CCP2 and RF were described previously (21, 35). We studied two definitions for positive RA-related autoantibodies: 1) clinical cutoffs (anti-CCP >5 units or positive RF by nephelometry), and 2) highly sensitive cutoffs (anti-CCP >2 units or positive RF by nephelometry or IgA/IgM ELISA) (36).
Statistical analysis
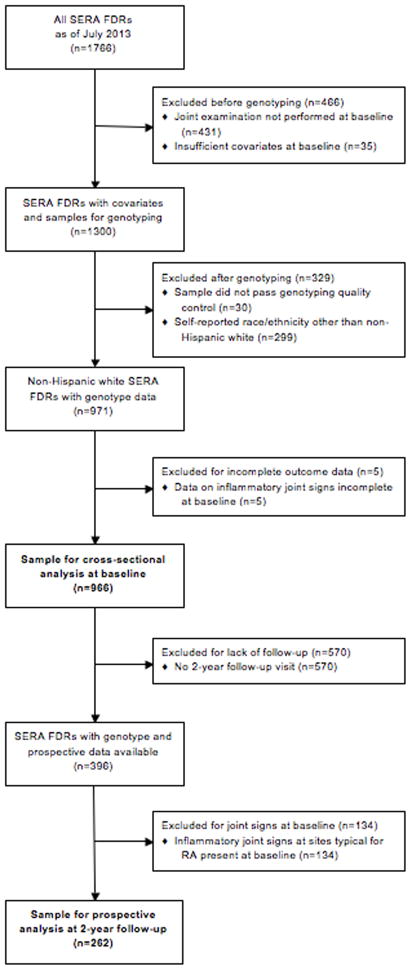
We performed cross-sectional analyses of the study sample at baseline (see Figure 1 for flow diagram). We used logistic regression models to estimate ORs and 95% confidence intervals (CI), adjusted for age in continuous years, for the association of each RA risk factor with IJS. We evaluated four definitions of IJS outcomes for the baseline analysis: 1) any swollen joint, 2) any tender joint, 3) any swollen or tender joint, and 4) any swollen MCP or wrist. For genetic analyses, we dichotomized GRS as high or low with the median value as the cutoff. We evaluated sex and parity as a single model: female/parous (reference), female/nulliparous, and male.
Figure 1.
Flow diagram illustrating the analyzed study samples (SERA, Studies of the Etiology of Rheumatoid Arthritis; FDRs, first-degree relatives).
We evaluated the prospective association between RA risk factors at baseline and IJS at the two-year study visit (see Figure 1). We only included subjects that had no tender or swollen RA-specific joints at baseline and had follow-up data. We used logistic regression to estimate ORs and 95% CIs for IJS. Given the limited number of outcomes in this subset, we only studied the IJS outcome of any swollen/tender joint in this analysis. For cigarette smoking, we dichotomized pack-years at baseline as never to ≤10 pack-years (reference) or >10 pack-years given limited sample size.
We examined multivariable models using forward stepwise variable selection for covariates with p values ≤0.2 in univariate models. We tested for multiplicative interactions for covariates that were previously demonstrated to interact for RA risk (RA-related autoantibodies and smoking as well as HLA shared epitope and smoking) (7, 37). Given a priori hypotheses in these analyses, we did not correct for multiple comparisons. For interaction analyses, we created multiplicative terms between the two variables being considered. Wald tests were used for binary covariates and likelihood ratio tests were used for categorical variables. If p for interaction was <0.05, we evaluated for stratum-specific effects of these variables and the combined effect of these covariates.
To address the possible effect of relatedness of study participants on our results, we performed secondary analyses by randomly selecting one participant per family and repeating the analyses, with identical methods as described above.
We performed sensitivity analyses examining whether other cutpoints of each GRS and anti-CCP2 were associated with IJS outcomes both at baseline and during follow-up. We examined these predictors as continuous values (log-transformed for anti-CCP2 due to non-normal distribution), in tertiles, and dichotomized at the 75th percentile.
Two-sided p values <0.05 were considered statistically significant. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
We analyzed a total of 966 non-Hispanic white unaffected FDRs of RA probands at baseline. Characteristics of study participants at baseline are shown in Table 1. Mean age was 47.2 years (SD 15.5), 70.8% were female, mean BMI was 27.4 kg/m2 (SD 6.1), and 43.7% ever smoked. Most study participants (54.9%) had at least one HLA shared epitope allele. Thirty-five subjects were positive for anti-CCP2 at >2 units (3.6%) and 48 were positive for RF by nephelometry (5.0%). Also considering RF by ELISA, 137 (14.2%) subjects had at least one RA-related autoantibody. At baseline, 222 (23.0%) subjects had at least one swollen/tender joint present at RA-specific sites by physical examination. Eighty subjects (8.3%) had ≥1 swollen joint and 195 (20.2%) had ≥1 tender joint at baseline.
Table 1.
Characteristics of analyzed subjects in the Studies for the Etiology of RA at baseline (n=966).
| Characteristics | N (%), Mean (SD), or Median (IQR) |
|---|---|
| Age, years, mean (SD) | 47.2 (15.5) |
| Age ≥50 years, N (%) | 406 (42.0%) |
| Female, N (%) | 684 (70.8%) |
| Non-Hispanic white race, N (%) | 966 (100%) |
| Cumulative cigarette smoking pack-years, N (%) | |
| Never | 542 (56.3%) |
| >0 to ≤10 | 249 (25.9%) |
| >10 | 171 (17.8%) |
| Cigarette smoking status, N (%) | |
| Never | 542 (56.1%) |
| Past | 308 (31.9%) |
| Current | 116 (12.0%) |
| Body mass index, kg/m2, mean (SD) | 27.4 (6.1%) |
| Body mass index category, N (%) | |
| Underweight (BMI <18.5 kg/m2) | 12 (1.3%) |
| Normal (BMI 18.5 to <25 kg/m2) | 381 (39.9%) |
| Overweight (BMI 25 to <30 kg/m2) | 301 (31.5%) |
| Obese (BMI ≥30 kg/m2) | 261 (27.3%) |
| Education, N (%) | |
| ≤High school graduate | 187 (19.5%) |
| Some college or greater | 770 (80.5%) |
| Parity, N (%)* | |
| Parous | 161 (23.5%) |
| Nulliparous | 523 (76.5%) |
| Full GRS50, median (IQR) | 2.9 (2.5–3.6) |
| HLA GRS5, median (IQR) | 0.47 (0.0–1.2) |
| Non-HLA GRS45, median (IQR) | 2.5 (2.3–2.7) |
| Shared epitope alleles present, N (%) | |
| 0 | 436 (45.1%) |
| 1 | 443 (45.9%) |
| 2 | 87 (9.0%) |
| RA-related autoantibody positivity, N (%) | |
| RF by nephelometry | 48 (5.0%) |
| RF-IgA by ELISA | 33 (3.4%) |
| RF-IgM by ELISA | 52 (5.4%) |
| Any positive RF | 109 (11.3%) |
| Anti-CCP2 >5 units | 14 (1.5%) |
| Anti-CCP2 >2 units | 35 (3.6%) |
| Anti-CCP2 >5 units or RF by nephelometry | 59 (6.1%) |
| Anti-CCP2 >2 units or any positive RF | 137 (14.2%) |
| Inflammatory joint signs at RA-specific joints**, N (%) | |
| Any swollen joint | 80 (8.3%) |
| Any tender joint | 195 (20.2%) |
| Any swollen or tender joint | 222 (23.0%) |
| Any swollen MCP or wrist | 34 (3.5%) |
Parity was only considered for females.
RA-specific joints considered were metacarpophalangeal joints, proximal interphalangeal joints, wrists, elbows, and metatarsophalangeal joints. First metacarpophalangeal or metatarsophalangeal joints as well as swollen joints deemed to be due to trauma or degenerative disease were not included.
BMI, body mass index; anti-CCP2, anti-cyclic citrullinated peptide (2nd generation), ELISA, enzyme-linked immunosorbent assay, GRS, genetic risk score; HLA, human leukocyte antigen; IQR, interquartile range; MCP, metacarpophalangeal; RA, rheumatoid arthritis; RF, rheumatoid factor; SD, standard deviation.
Numbers of subjects with missing covariates: 4 for cumulative smoking, 11 for body mass index, and 9 for education. All subjects had complete data available on other covariates, including smoking status.
Cross-sectional analyses at baseline
Age-adjusted ORs for IJS at baseline are shown in Table 2. Increasing age was significantly associated with any swollen joint (OR 1.04, 95%CI 1.02–1.05), any tender joint (OR 1.03, 95%CI 1.02–1.04), and any swollen/tender joint (OR 1.03, 95%CI 1.02–1.04). Compared to never smoking, >10 pack-years of smoking was associated with any swollen joint (OR 1.89, 95%CI 1.05–3.41), any tender joint (OR 1.87, 95%CI 1.23–2.83), and any swollen/tender joint (OR 1.89, 95%CI 1.26–2.82). Compared to never smoking, current smoking was associated with swollen joints (OR 2.51, 95%CI 1.28–4.94) and swollen MCP/wrist (OR 1.96, 95%CI 1.14–7.72). Other RA environmental risk factors including BMI, education, and sex/parity, were not associated with any IJS at baseline. Only age and cigarette smoking remained in the final multivariable models for each IJS outcome considered.
Table 2.
Age-adjusted odds ratios for inflammatory joint signs at sites typical for RA* among first-degree relatives without RA in the Studies of the Etiology of RA at baseline (n=966).
| Model | Covariates | OR (95% CI) for any swollen joint** | OR (95% CI) for any tender joint** | OR (95% CI) for any swollen or tender joint** | OR (95% CI) for any swollen MCP or wrist** |
|---|---|---|---|---|---|
|
| |||||
| Age | Per year | 1.04 (1.02–1.05) | 1.03 (1.02–1.04) | 1.03 (1.02–1.04) | 1.02 (1.00–1.04) |
|
| |||||
| Dichotomized age | <50 years | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| ≥50 years | 2.21 (1.39–3.53) | 2.13 (1.55–2.93) | 2.18 (1.61–2.95) | 1.78 (0.90–3.55) | |
|
| |||||
| Cumulative cigarette smoking | Never | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| >0 to ≤10 pack-years | 1.68 (0.96–2.95) | 1.48 (1.01–2.17) | 1.62 (1.12–2.33) | 2.18 (0.96–1.94) | |
| >10 pack-years | 1.89 (1.05–3.41) | 1.87 (1.23–2.83) | 1.89 (1.26–2.82) | 2.01 (0.80–5.04) | |
|
| |||||
| Cigarette smoking status | Never | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Past | 1.59 (0.94–2.69) | 1.52 (1.06–2.18) | 1.61 (1.14–2.27) | 1.94 (0.88–4.32) | |
| Current | 2.51 (1.28–4.94) | 1.94 (1.19–3.16) | 2.12 (1.33–3.38) | 1.96 (1.14–7.72) | |
|
| |||||
| Body mass index category | Normal/underweight | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Overweight/obese | 0.95 (0.59–1.54) | 0.89 (0.64–1.24) | 0.93 (0.68–1.28) | 0.91 (0.45–1.84) | |
|
| |||||
| Education | ≤High school graduate | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Some college or greater | 0.77 (0.45–1.32) | 0.88 (0.60–1.31) | 0.76 (0.53–1.10) | 0.56 (0.26–1.21) | |
|
| |||||
| Sex/Parity | Female/parous | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Female/nulliparous | 1.26 (0.61–2.63) | 1.28 (0.78–2.09) | 1.21 (0.76–1.95) | 2.05 (0.81–5.21) | |
| Male | 0.72 (0.41–1.45) | 0.79 (0.54–1.15) | 0.77 (0.54–1.11) | 0.57 (0.23–1.44) | |
|
| |||||
| Non-HLA GRS45 | Low (≤Median) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| High (>Median) | 1.02 (0.64–1.62) | 1.09 (0.79–1.50) | 1.09 (0.81–1.49) | 1.11 (0.56–2.20) | |
|
| |||||
| HLA GRS5 | Low (≤Median) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| High (>Median) | 0.80 (0.49–1.23) | 1.01 (0.74–1.40) | 0.96 (0.70–1.30) | 1.25 (0.62–2.51) | |
|
| |||||
| Full GRS50 | Low (≤Median) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| High (>Median) | 0.77 (0.49–1.23) | 1.01 (0.74–1.39) | 1.00 (0.73–1.35) | 0.77 (0.39–1.53) | |
|
| |||||
| RA-related autoantibodies at clinical cutoffs | Anti-CCP2 ≤5 units and negative RF by nephelometry | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Anti-CCP2 >5 units or positive RF by nephelometry | 1.28 (0.55–2.97) | 1.45 (0.79–2.65) | 1.20 (0.66–2.19) | 2.56 (0.94–6.95) | |
|
| |||||
| Highly sensitive RA-related autoantibodies | Anti-CCP2 ≤2 units and negative RF*** | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Anti-CCP2 >2 units or any positive RF*** | 1.38 (0.76–2.49) | 1.29 (0.84–1.98) | 1.32 (0.87–1.99) | 1.78 (0.78–4.05) | |
RA-specific joints considered were metacarpophalangeal joints, proximal interphalangeal joints, wrists, elbows, and metatarsophalangeal joints. First metacarpophalangeal or metatarsophalangeal joints as well as swollen joints deemed to be due to trauma or degenerative disease were not included.
There were 80 subjects with swollen joint(s), 195 with tender joint(s), 222 with swollen or tender joint(s), and 34 with swollen metacarpophalangeal joint(s) or wrist(s) at the baseline visit.
Rheumatoid factor positivity was considered as positive nephelometry or enzyme-linked immunosorbent assay (IgA or IgM).
Anti-CCP2, anti-cyclic citrullinated peptide (2nd generation); CI, confidence interval; GRS, genetic risk score; MCP, metacarpophalangeal; OR, odds ratio; RA, rheumatoid arthritis; RF, rheumatoid factor.
Bolding indicates p<0.05. All models were additionally adjusted for age in continuous years except for the age model.
None of the GRS (dichotomized at the median) and RA-related autoantibodies were associated with any IJS at baseline. Elevated full GRS50 had an OR of 1.01 (95%CI 0.74–1.39) for any tender/swollen joint. Positive RA-related autoantibodies at clinical cutoffs had increased odds for swollen MCP/wrist that was not statistically significant (OR 2.56, 95%CI 0.94–6.95). Using a highly sensitive definition for RA-related autoantibodies (with a lower cutoff for anti-CCP2 and also considering RF by ELISA) did not detect associations with any IJS.
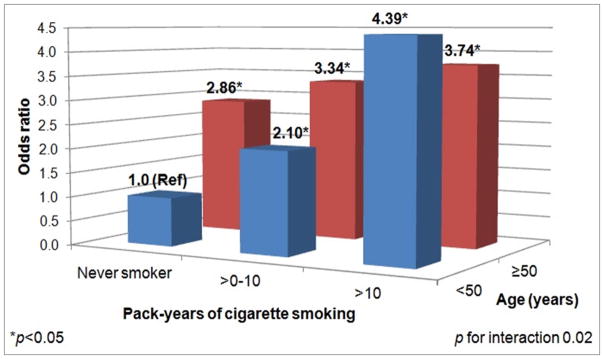
We evaluated interactions for variables significant in univariate analyses and those that were pre-specified. Age and cigarette smoking pack-years had a statistically significant multiplicative interaction (p=0.02) for IJS. Among those who were <50 years old, those who smoked >10 pack-years had an OR of 4.39 (95%CI 2.23–8.66) for swollen/tender joints compared to never smokers (Table 3). Among those aged ≥50 years, there was no significant association of smoking pack-years with IJS. We stratified by cumulative smoking pack-year (by never, ≤10, or >10 pack-years). Among never smokers, age of ≥50 years was significantly associated with increased odds of IJS at baseline (OR 2.86, 95%CI 1.82–4.49). Figure 2 shows the combined effect of smoking and younger age for IJS. While older individuals had significantly increased odds of IJS compared to younger/never smokers, younger subjects aged <50 years and smokers of >10 pack-years had the highest odds for IJS at baseline (OR 4.39, 95%CI 2.22–8.66; reference: never smokers aged <50 years, p for interaction 0.02). There was no interaction for HLA shared epitope and smoking (p=0.34) or RA-related autoantibodies and smoking (p=0.90) for IJS.
Table 3.
Odds ratios for swollen or tender joints at sites typical for RA* stratified by age and cumulative smoking at baseline (n=962).
| Stratum | Covariates | Outcomes/Total N | OR (95% CI) |
|---|---|---|---|
| Age <50 years | Never smoker | 44/362 | 1.0 (Ref) |
| >0 to ≤10 pack-years | 34/151 | 2.10 (1.28–3.45) | |
| >10 pack-years | 17/45 | 4.39 (2.23–8.66) | |
|
| |||
| Age ≥50 years | Never smoker | 51/180 | 1.0 (Ref) |
| >0 to ≤10 pack-years | 31/98 | 1.17 (0.69–2.00) | |
| >10 pack-years | 43/126 | 1.31 (0.80–2.14) | |
|
| |||
| Never smoker | Age <50 years | 44/362 | 1.0 (Ref) |
| Age ≥50 years | 51/180 | 2.86 (1.82–4.49) | |
|
| |||
| >0 to ≤10 pack-years | Age <50 years | 34/151 | 1.0 (Ref) |
| Age ≥50 years | 31/98 | 1.59 (0.90–2.82) | |
|
| |||
| >10 pack-years | Age <50 years | 17/45 | 1.0 (Ref) |
| Age ≥50 years | 43/126 | 0.85 (0.42–1.73) | |
RA-specific joints considered were metacarpophalangeal joints, proximal interphalangeal joints, wrists, elbows, and metatarsophalangeal joints. First metacarpophalangeal or metatarsophalangeal joints as well as swollen joints deemed to be due to trauma or degenerative disease were not included.
Four subjects did not have data on cumulative smoking at baseline.
CI, confidence interval; OR, odds ratio; RA, rheumatoid arthritis.
Bolding indicates p<0.05.
Figure 2.
Combined effect of age and cigarette smoking on swollen or tender joints at sites typical for RA among first-degree relatives without RA at baseline in the Studies of the Etiology of RA (n=962).
Prospective analyses for incident IJS
Among those with follow-up data available (n=396), we evaluated the prospective development of IJS at two-year follow-up for subjects without IJS at baseline (n=262). No subjects progressed to classifiable RA within two years of follow-up. We considered the presence of any swollen/tender joint as IJS due to limited sample size (27 had incident tender/swollen joints). Age (OR 1.05, 95%CI 1.02–1.08) and smoking >10 pack-years (OR 2.66, 95%CI 1.01–7.03) were associated with IJS (Table 4). Other environmental RA risk factors, GRS, and RA-related autoantibodies were not associated with IJS after two years of prospective follow-up. Age and cigarette smoking remained in the final multivariable model for incident IJS at two-year follow-up. The associations between autoantibody positivity and incident swollen MCP or wrist could not be calculated because there were no subjects had these outcomes at the two-year follow-up visit.
Table 4.
Age-adjusted odds ratios for inflammatory joint signs at sites typical for RA* among first-degree relatives without RA in the Studies of the Etiology of RA at two-year follow-up (n=262).
| Model | Covariates | OR (95% CI)** |
|---|---|---|
|
| ||
| Age | Per year | 1.05 (1.02–1.08) |
|
| ||
| Dichotomized age | <50 years | 1.0 (Ref) |
| ≥50 years | 3.04 (1.35–6.87) | |
|
| ||
| Cumulative cigarette smoking | None to ≤10 pack-years | 1.0 (Ref) |
| >10 pack-years | 2.66 (1.01–7.03) | |
|
| ||
| Body mass index category | Normal/underweight | 1.0 (Ref) |
| Overweight/obese | 1.49 (0.61–3.64) | |
|
| ||
| Education | ≤High school graduate | 1.0 (Ref) |
| Some college or greater | 1.37 (0.42–4.46) | |
|
| ||
| Sex/Parity | Female/parous | 1.0 (Ref) |
| Female/nulliparous | 2.52 (0.76–8.35) | |
| Male | 0.91 (0.34–2.44) | |
|
| ||
| Non-HLA GRS45 | Low (≤Median) | 1.0 (Ref) |
| High (>Median) | 1.89 (0.82–4.34) | |
|
| ||
| HLA GRS5 | Low (≤Median) | 1.0 (Ref) |
| High (>Median) | 0.73 (0.32–1.67) | |
|
| ||
| Full GRS50 | Low (≤Median) | 1.0 (Ref) |
| High (>Median) | 0.95 (0.42–2.16) | |
|
| ||
| RA-related autoantibodies at clinical cutoffs | Anti-CCP2 <5 units and negative RF by nephelometry | 1.0 (Ref) |
| Anti-CCP2 ≥5 units or positive RF by nephelometry | 1.90 (0.46–7.84) | |
|
| ||
| Highly sensitive RA-related autoantibodies | Anti-CCP2 ≤2 units and negative RF*** | 1.0 (Ref) |
| Anti-CCP2 >2 units or any positive RF*** | 1.22 (0.42–3.58) | |
RA-specific joints considered were metacarpophalangeal joints, proximal interphalangeal joints, wrists, elbows, and metatarsophalangeal joints. First metacarpophalangeal or metatarsophalangeal joints as well as swollen joints deemed to be due to trauma or degenerative disease were not included.
There were 27 subjects with tender or swollen joint(s) at the two-year follow-up visit.
Rheumatoid factor positivity was considered as positive nephelometry or enzyme-linked immunosorbent assay (IgA or IgM).
Anti-CCP2, anti-cyclic citrullinated peptide (2nd generation); CI, confidence interval; GRS, genetic risk score; OR, odds ratio; RA, rheumatoid arthritis; RF, rheumatoid factor.
Bolding indicates p<0.05. All models were additionally adjusted for age in continuous years except for the age model.
Single FDR per family analyses
Since some participants were from the same family, we performed sensitivity analyses restricting the sample for analysis to one randomly chosen FDR per family at baseline (Supplementary Table 2) and after two-years of prospective follow-up (Supplementary Table 3) with similar results. Smoking was significantly associated with IJS at baseline (OR 2.10, 95%CI 1.36–4.14 for >10 pack-years compared to never smokers) and at two years (OR 3.19, 95%CI 1.03–9.88). Other environmental, genetic, and serologic RA risk factors were not associated with IJS in these analyses.
Sensitivity analyses
When analyzing other cutpoints of anti-CCP2 (as a log-transformed continuous predictor, in tertiles, and dichotomized at the 75th percentile) as well as non-HLA GRS45, HLA GRS5, and the full GRS50 (continuous, in tertiles, and dichotomized at the 75th percentile), there were no significant associations with any of the IJS outcomes in the baseline or prospective analyses.
DISCUSSION
We demonstrated that age and cigarette smoking were associated with IJS among in a high-risk cohort of RA FDRs in SERA. We considered joint swelling/tenderness on examination at RA-specific joints as IJS, an intermediate outcome towards the progression to classifiable RA (22, 24). We found that cumulative cigarette smoking was associated with IJS both at baseline and at two years of follow-up. We found an interaction between smoking and age, where smoking at younger ages increased risk of IJS more than four-fold. These findings provide evidence that cigarette smoking is important in the transition between genetic susceptibility to objective signs of inflammatory arthritis. We did not find significant associations for other RA risk factors, including GRS with 5 shared epitope alleles and 50 non-HLA alleles, RA-related autoantibodies, and other environmental factors.
Most previous studies evaluating the transitions between pre-clinical RA phases have focused on the transition from a state of arthralgias or inflammatory arthritis to classifiable RA. In these previous studies, smoking has been shown to increase risk of, and hasten the transition, to classifiable RA (4, 38). In addition, excess BMI, lack of alcohol intake, dyslipidemia, and morning stiffness have been associated with RA development in arthralgia and early arthritis cohorts (15–17). However, many of these studies were performed exclusively among patients with positive RA-related autoantibodies, a group already at very elevated RA risk (13, 17). Our study is the first to prospectively evaluate genetic, environmental, and serologic risk factors among unaffected FDRs. This group is at increased risk for RA based on positive family history, but many were asymptomatic and seronegative, so are likely further away from developing classifiable RA than these previous studies.
Our findings add to the literature that smoking is an important, and potentially modifiable, risk factor during pre-clinical transitional phases of RA pathogenesis. In our study, those who smoked >10 pack-years as well as current smokers had significantly increased odds for tender and swollen joints both at baseline and after two years of follow-up. Smoking has a clear association with RA risk and may contribute up to 35% of the attributable risk for seropositive RA (29, 34). In addition to shared genetics, prior studies suggested that modifiable behaviors, particularly smoking, contribute to the increased risk of those with family history of RA (18, 19). Smoking also increases the risk of transition from arthralgias to classifiable RA (17). Potential mechanisms include that smoking interacts with the HLA shared epitope for RA risk and may also affect transcription of heat shock proteins specifically in the joints (37, 39, 40). When evaluating age alone, without accounting for smoking, increased age was associated with increased risk of IJS. Since RA is an age-related disease, the association between age and IJS was expected. Risk of IJS increased with heavier smoking in all age groups. However, we found that IJS risk was especially high among those with >10 pack-years who were <50 years old. Since both current and cumulative smoking were strongly associated with IJS, younger smokers with more intensive smoking behaviors were at highest risk of IJS. Our findings provide evidence that smoking is important in the transition from genetic risk to symptomatic and objective inflammatory joint findings prior to RA onset and that this risk is highest for heavy smokers at younger ages. These findings emphasize the public health importance of smoking cessation, particularly among younger family members of patients with RA.
Seropositivity, particularly anti-CCP positivity, has been shown to be strongly associated with the future development of RA in a variety of study designs (2, 7, 9, 14, 41). While there was a trend toward an increased risk of IJS at the MCP/wrist for RA-related antibodies at baseline, this was not statistically significant and other definitions for autoantibodies showed no association with IJS. This lack of association may be due to the low numbers of individuals with RA-related autoantibodies as well as relatively low number of individuals with incident IJS. In particular, only 14 subjects were anti-CCP positive, and RF may have limited specificity for IJS. The relatively short period of follow-up also limits conclusions about the temporal relationship of autoantibodies with IJS.
Smoking may induce the citrullination of self-antigens at mucosal surfaces resulting in the production of anti-citrullinated protein antibodies (ACPA) (42). In addition, smoking may contribute to propagation of ACPA-related arthritis in individuals where autoimmunity has already been induced (43, 44). Smoking may therefore play a dual role in the pathogenesis of RA by inducing autoantibody production while also propagating joint inflammation. Since smoking was associated with IJS even in the absence of autoantibodies, IJS may develop before detectable autoantibodies (45). We found no statistical interaction between the shared epitope and smoking for risk of IJS. The shared epitope and smoking strongly interact for the risk of seropositive RA (38). Since few participants in our study were seropositive, our findings provide evidence that smoking promotes inflammatory arthritis independent of the presence of serologic RA-related autoantibodies and independent of the shared epitope. We have previously identified a portion of FDRs who demonstrate increases in RA-related autoantibodies in the lung in the absence of concomitant serum elevations, suggesting that some seronegative FDRs have RA-related autoimmunity (46). Since we defined seropositivity as positive anti-CCP2 and RF by nephelometry or ELISA, it is possible that other biomarkers may be important in the development of IJS (7, 8, 10, 15, 47). Future studies are needed to understand the role, timing, and site of influence of smoking throughout RA pathogenesis.
It is possible that physical examination may misclassify inflammatory arthritis. To minimize this, we assessed joint-related outcomes by standardized physical examination by rheumatologists and trained staff, and restricted the joints evaluated to those deemed most specific for RA by the 2010 ACR/EULAR classification criteria (24). Since joint signs at the first MCPs and MTPs are often due to degenerative disease, we did not include findings at these joints in the IJS outcomes. We also excluded IJS due to degenerative disease and trauma based on history obtained by the examiner trained to detect inflammatory arthritis. However, it is still possible that the joint signs identified, especially in joints outside of the MCPs and wrists were not truly due to inflammation or synovitis. Plain films, magnetic resonance imaging, and ultrasonography may have improved the classification of IJS but were not systematically available. Future studies using advanced imaging or synovial biopsy may provide more sensitive assessments of joint inflammation in susceptible individuals.
We did not find associations of other established RA risk factors with IJS. These include GRS, excess BMI, gender/parity, and education (3, 31–33). While our study had a large sample size, there were relatively few outcomes, especially in the prospective analyses, so we may have been underpowered to find true associations. Since FDRs who had been diagnosed with RA were not enrolled in SERA, we were unable to address whether FDRs who developed RA at younger ages had a greater contribution of genetic and environmental factors than the FDRs in this cohort. Since we considered genetic factors associated with Caucasians for this study, we limited the analysis to subjects who were non-Hispanic whites. The majority of study participants were female. Since RA affects more women than men, female relatives may have been more willing to participate in SERA than male relatives. While SERA is enriched with diverse a diverse population recruited from several US sites, study participants were relatively well-educated and agreed to be enrolled in this prospective observational study so our findings may not be generalizable to all FDRs. We were unable to assess other environmental factors, such as silica exposure or dietary factors (such as alcohol, soda, and fish consumption), that may be important in RA pathogenesis (30, 48–50).
We found no association of GRS with baseline or incident IJS among FDRs in our study. Since original genetic associations were drawn from the general population, established RA genetic risk factors have an unclear association with intermediate RA outcomes among a population of FDRs (1). While many genetic risk factors have been described, much of the genetic heritability of RA remains unexplained in the general population. Our study population of FDRs was enriched with RA-related genes compared to the general population. Differences between genetic profiles would therefore likely be more difficult to distinguish in FDRs, especially considering that genetic factors typically have small effect sizes. Two previous studies evaluated genetic factors and risk of RA among FDRs with conflicting results (20, 51). These studies showed associations of IL10, MMEL1-TNFRSF14, and TRAF1-C5 with RA risk, the latter of which was included in our GRS (20, 51). Finally, our analyses included some related family members so genetic factors may not have been independent in these families. When restricting the analysis to unrelated family members, there was no association of GRS with IJS.
In conclusion, we demonstrated that smoking and age were associated with IJS in this study of unaffected RA FDRs. We found no evidence of association of other RA risk factors, including GRS, excess BMI, sex, parity, education, and RA-related autoantibodies. Cigarette smoking may have an important role throughout the phases of pre-clinical RA pathogenesis, including this group at high genetic risk due to having an affected first-degree relative with RA. Larger studies with longer follow-up are needed to investigate the transitions of pre-clinical RA phases.
Acknowledgments
Financial disclosures: This work was supported by the National Institutes of Health (NIH) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award numbers R01 AR049880, K24 AR052403, P60 AR047782, R01 AR051394, M01 RR00069, M01 RR00425, K23 AR051461, T32 AR007534, U19 AI050864 and U01 AI101981, UL1 RR033176, R56 AI103023, and UL1 TR000124), the Walter S. and Lucienne Driskill Foundation, the Rheumatology Research Foundation, the Research Support Fund grant from the Nebraska Medical Center, and the University of Nebraska Medical Center. Dr. Sparks was supported by the Rheumatology Research Foundation Scientist Development Award and the NIH Loan Repayment Award (L30 AR066953). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank the SERA study participants for their important contributions. We also thank the staffs of the Section of Clinical Sciences at Brigham and Women’s Hospital and the Rheumatology Division at the University of Colorado for helpful feedback and support.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Sparks had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sparks, Deane, Holers, Norris, Karlson.
Acquisition of data. Sparks, Deane, Buckner, Keating, Gregersen, Weisman, Mikuls, O’Dell, Holers, Norris, Karlson.
Analysis and interpretation of data. Sparks, Chang, Deane, Gan, Demoruelle, Feser, Moss, Buckner, Keating, Costenbader, Gregersen, Weisman, Mikuls, O’Dell, Holers, Norris, Karlson.
References
- 1.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506(7488):376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 3.Sparks JA, Chen CY, Jiang X, Askling J, Hiraki LT, Malspeis S, et al. Improved performance of epidemiologic and genetic risk models for rheumatoid arthritis serologic phenotypes using family history. Ann Rheum Dis. 2015;74(8):1522–9. doi: 10.1136/annrheumdis-2013-205009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Steenbergen HW, Huizinga TW, van der Helm-van Mil AH. The preclinical phase of rheumatoid arthritis: what is acknowledged and what needs to be assessed? Arthritis Rheum. 2013;65(9):2219–32. doi: 10.1002/art.38013. [DOI] [PubMed] [Google Scholar]
- 5.Karlson EW, van Schaaardenburg D, van der Helm-van Mil AH. Strategies to predict rheumatoid arthritis development in at-risk populations. Rheumatology (Oxford) 2014 doi: 10.1093/rheumatology/keu287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlag DM, Raza K, van Baarsen LG, Brouwer E, Buckley CD, Burmester GR, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis. 2012;71(5):638–41. doi: 10.1136/annrheumdis-2011-200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arkema EV, Goldstein BL, Robinson W, Sokolove J, Wagner CA, Malspeis S, et al. Anti-citrullinated peptide autoantibodies, human leukocyte antigen shared epitope and risk of future rheumatoid arthritis: a nested case-control study. Arthritis Res Ther. 2013;15(5):R159. doi: 10.1186/ar4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010;62(11):3161–72. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 10.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos WH, van de Stadt LA, Sohrabian A, Ronnelid J, van Schaardenburg D. Development of anti-citrullinated protein antibody and rheumatoid factor isotypes prior to the onset of rheumatoid arthritis. Arthritis Res Ther. 2014;16(2):405. doi: 10.1186/ar4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Steenbergen HW, Mangnus L, Reijnierse M, Huizinga TW, van der Helm-van Mil AH. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2015-208138. [DOI] [PubMed] [Google Scholar]
- 13.de Hair MJ, Landewe RB, van de Sande MG, van Schaardenburg D, van Baarsen LG, Gerlag DM, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis. 2013;72(10):1654–8. doi: 10.1136/annrheumdis-2012-202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bos WH, Wolbink GJ, Boers M, Tijhuis GJ, de Vries N, van der Horst-Bruinsma IE, et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis. 2010;69(3):490–4. doi: 10.1136/ard.2008.105759. [DOI] [PubMed] [Google Scholar]
- 15.van de Stadt LA, van Sijl AM, van Schaardenburg D, Nurmohamed MT. Dyslipidaemia in patients with seropositive arthralgia predicts the development of arthritis. Ann Rheum Dis. 2012;71(11):1915–6. doi: 10.1136/annrheumdis-2012-201709. [DOI] [PubMed] [Google Scholar]
- 16.van de Stadt LA, van Schaardenburg D. Alcohol consumption protects against arthritis development in seropositive arthralgia patients. Ann Rheum Dis. 2012;71(8):1431–2. doi: 10.1136/annrheumdis-2011-201418. [DOI] [PubMed] [Google Scholar]
- 17.van de Stadt LA, Witte BI, Bos WH, van Schaardenburg D. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis. 2013;72(12):1920–6. doi: 10.1136/annrheumdis-2012-202127. [DOI] [PubMed] [Google Scholar]
- 18.Sparks JA, Chen CY, Hiraki LT, Malspeis S, Costenbader KH, Karlson EW. Contributions of familial rheumatoid arthritis or lupus and environmental factors to risk of rheumatoid arthritis in women: a prospective cohort study. Arthritis Care Res (Hoboken) 2014;66(10):1438–46. doi: 10.1002/acr.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X, Frisell T, Askling J, Karlson EW, Klareskog L, Alfredsson L, et al. To what extent is the familial risk of rheumatoid arthritis explained by established rheumatoid arthritis risk factors? Arthritis Rheumatol. 2015;67(2):352–62. doi: 10.1002/art.38927. [DOI] [PubMed] [Google Scholar]
- 20.Oen K, Robinson DB, Nickerson P, Katz SJ, Cheang M, Peschken CA, et al. Familial seropositive rheumatoid arthritis in North American Native families: effects of shared epitope and cytokine genotypes. J Rheumatol. 2005;32(6):983–91. [PubMed] [Google Scholar]
- 21.Kolfenbach JR, Deane KD, Derber LA, O’Donnell C, Weisman MH, Buckner JH, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61(12):1735–42. doi: 10.1002/art.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 23.Gan RW, Deane KD, Zerbe GO, Demoruelle MK, Weisman MH, Buckner JH, et al. Relationship between air pollution and positivity of RA-related autoantibodies in individuals without established RA: a report on SERA. Ann Rheum Dis. 2013;72(12):2002–5. doi: 10.1136/annrheumdis-2012-202949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 25.Gersuk VH, Nepom GT. A real-time PCR approach for rapid high resolution subtyping of HLA-DRB1*04. J Immunol Methods. 2006;317(1–2):64–70. doi: 10.1016/j.jim.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernando MM, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4(4):e1000024. doi: 10.1371/journal.pgen.1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlson EW, Chibnik LB, Kraft P, Cui J, Keenan BT, Ding B, et al. Cumulative association of 22 genetic variants with seropositive rheumatoid arthritis risk. Ann Rheum Dis. 2010;69(6):1077–85. doi: 10.1136/ard.2009.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlson EW, Ding B, Keenan BT, Liao K, Costenbader KH, Klareskog L, et al. Association of environmental and genetic factors and gene-environment interactions with risk of developing rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65(7):1147–56. doi: 10.1002/acr.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 30.Lu B, Solomon DH, Costenbader KH, Karlson EW. Alcohol consumption and risk of incident rheumatoid arthritis in women: a prospective study. Arthritis Rheumatol. 2014;66(8):1998–2005. doi: 10.1002/art.38634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015;17(1):86. doi: 10.1186/s13075-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bengtsson C, Nordmark B, Klareskog L, Lundberg I, Alfredsson L. Socioeconomic status and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2005;64(11):1588–94. doi: 10.1136/ard.2004.031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orellana C, Wedren S, Kallberg H, Holmqvist M, Karlson EW, Alfredsson L, et al. Parity and the risk of developing rheumatoid arthritis: results from the Swedish Epidemiological Investigation of Rheumatoid Arthritis study. Ann Rheum Dis. 2014;73(4):752–5. doi: 10.1136/annrheumdis-2013-203567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kallberg H, Ding B, Padyukov L, Bengtsson C, Ronnelid J, Klareskog L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70(3):508–11. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demoruelle MK, Parish MC, Derber LA, Kolfenbach JR, Hughes-Austin JM, Weisman MH, et al. Performance of anti-cyclic citrullinated Peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease. Arthritis Rheum. 2013;65(9):2243–52. doi: 10.1002/art.38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chibnik LB, Mandl LA, Costenbader KH, Schur PH, Karlson EW. Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol. 2009;36(4):706–11. doi: 10.3899/jrheum.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):54–60. doi: 10.1136/ard.2008.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. 2007;56(2):433–40. doi: 10.1002/art.22380. [DOI] [PubMed] [Google Scholar]
- 39.Kim K, Jiang X, Cui J, Lu B, Costenbader KH, Sparks JA, et al. Interactions between amino acid-defined major histocompatibility complex class II variants and smoking in seropositive rheumatoid arthritis. Arthritis Rheumatol. 2015;67(10):2611–23. doi: 10.1002/art.39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ospelt C, Camici GG, Engler A, Kolling C, Vogetseder A, Gay RE, et al. Smoking induces transcription of the heat shock protein system in the joints. Ann Rheum Dis. 2014;73(7):1423–6. doi: 10.1136/annrheumdis-2013-204486. [DOI] [PubMed] [Google Scholar]
- 41.Ramos-Remus C, Castillo-Ortiz JD, Aguilar-Lozano L, Padilla-Ibarra J, Sandoval-Castro C, Vargas-Serafin CO, et al. Autoantibodies in Predicting Rheumatoid Arthritis in Healthy Relatives of Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2015 doi: 10.1002/art.39297. [DOI] [PubMed] [Google Scholar]
- 42.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 43.Hyun Sohn D, Rhodes C, Onuma K, Zhao X, Sharpe O, Gazitt T, et al. Local joint inflammation and histone citrullination provides a murine model for the transition from preclinical autoimmunity to inflammatory arthritis. Arthritis Rheumatol. 2015 doi: 10.1002/art.39283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116(4):961–73. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barra L, Bykerk V, Pope JE, Haraoui BP, Hitchon CA, Thorne JC, et al. Anticitrullinated protein antibodies and rheumatoid factor fluctuate in early inflammatory arthritis and do not predict clinical outcomes. J Rheumatol. 2013;40(8):1259–67. doi: 10.3899/jrheum.120736. [DOI] [PubMed] [Google Scholar]
- 46.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–61. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gan RW, Trouw LA, Shi J, Toes RE, Huizinga TW, Demoruelle MK, et al. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J Rheumatol. 2015;42(4):572–9. doi: 10.3899/jrheum.140767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y, Costenbader KH, Gao X, Al-Daabil M, Sparks JA, Solomon DH, et al. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am J Clin Nutr. 2014;100(3):959–67. doi: 10.3945/ajcn.114.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Giuseppe D, Crippa A, Orsini N, Wolk A. Fish consumption and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther. 2014;16(5):446. doi: 10.1186/s13075-014-0446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stolt P, Kallberg H, Lundberg I, Sjogren B, Klareskog L, Alfredsson L. Silica exposure is associated with increased risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2005;64(4):582–6. doi: 10.1136/ard.2004.022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Gabalawy HS, Robinson DB, Daha NA, Oen KG, Smolik I, Elias B, et al. Non-HLA genes modulate the risk of rheumatoid arthritis associated with HLA-DRB1 in a susceptible North American Native population. Genes Immun. 2011;12(7):568–74. doi: 10.1038/gene.2011.30. [DOI] [PubMed] [Google Scholar]