Abstract
Rationale
Patients with anxious major depressive disorder (AMDD) have more severe symptoms and poorer treatment response than patients with non-AMDD. Increasing evidence implicates the endogenous opioid system in the pathophysiology of depression. AZD2327 is a selective delta opioid receptor (DOR) agonist with anxiolytic and antidepressant activity in animal models.
Objective
This double-blind, parallel group design, placebo-controlled pilot study evaluated the safety and efficacy of AZD2327 in a preclinical model and in patients with AMDD.
Methods
We initially tested the effects of AZD2327 in an animal model of AMDD. Subsequently, 22 subjects with AMDD were randomized to receive AZD2327 (3 mg BID) or placebo for 4 weeks. Primary outcome measures included the Hamilton Depression Rating Scale (HAM-D) and the Hamilton Anxiety Rating Scale (HAM-A). We also evaluated neurobiological markers implicated in mood and anxiety disorders, including vascular endothelial growth factor (VEGF) and electroencephalogram (EEG).
Results
Seven (54 %) patients responded to active drug and three (33 %) responded to placebo. No significant main drug effect was found on either the HAM-D (p = 0.39) or the HAM-A (p = 0.15), but the HAM-A had a larger effect size. Levels of AZ12311418, a major metabolite of AZD2327, were higher in patients with an anti-anxiety response to treatment compared to nonresponders (p = 0.03). AZD2327 treatment decreased VEGF levels (p = 0.02). There was a trend (p < 0.06) for those with an anti-anxiety response to have higher EEG gamma power than nonresponders.
Conclusion
These results suggest that AZD2327 has larger potential anxiolytic than antidepressant efficacy. Additional research with DOR agonists should be considered.
Keywords: Anxiety, Anxiousdepression, Anxiolytic, Major depressive disorder, Opiate, BDNF, EEG, Preclinical, Biomarkers, AZD2327
Introduction
Despite the array of antidepressants on the market, many patients continue to struggle with major depressive disorder (MDD). Comorbid anxiety disorders and/or co-occurring syndromal anxiety symptoms contribute to reduced remission rates in MDD (Ionescu et al. 2014); indeed, studies have reported rates of comorbid anxiety between 40–53 % in outpatients with MDD (Fava et al. 2000; Sanderson et al. 1990). Patients with anxious depression (AMDD)—defined as MDD with high levels of anxiety symptoms—often have poorer treatment outcomes, greater chronicity, and significantly lower antidepressant response and remission rates than those with non-AMDD (Andreescu et al. 2007; Domschke et al. 2010; Farabaugh et al. 2012; Fava et al. 2008; Greenlee et al. 2010; Ionescu et al. 2013; Ionescu et al. 2014), underscoring the urgent need to better identify patient characteristics and develop improved therapeutics for this population.
Although opioids have traditionally been associated with analgesia, increasing evidence links the endogenous opioid system to the development of MDD and to its treatment. There are three well-defined classes of opioid receptors—mu, kappa, and delta—each of which has been implicated, to varying degrees, in the pathophysiology of depression (Lutz and Kieffer 2013). In preclinical models, antidepressant treatment increased mu-opioid receptor (MOR) immunoreactivity in brain regions related to pain and affective state (de Gandarias et al. 1999; Ortega-Alvaro et al. 2004). Increased MOR density was also found in the brains of suicide victims (Escriba et al. 2004; Gross-Isseroff et al. 1990). Kappa opioid receptor (KOR) antagonists were found to have antidepressant- and anti-anxiety like effects in preclinical models (Knoll et al. 2011; Reindl et al. 2008).
The delta opioid receptor (DOR) has also been implicated in circuitry related to mood and anxiety (Jutkiewicz 2006; Kennedy et al. 2006). Receptor localization studies have shown that DORs reside in areas of the brain implicated in mood regulation (Blackburn et al. 1988; Cahill et al. 2001; Goodman et al. 1980; Mansour et al. 1993; Quirion et al. 1983). For example, localization of the DOR in the amygdala is consistent with modulating fear and anxiety states (Knoll et al. 2011), whereas localization in the cortex and hippocampus is consistent with potential antidepressant action (Torregrossa et al. 2004). In addition, DOR agonist compounds have shown antidepressant properties comparable to those of prototypic antidepressants in several preclinical models (Broom et al. 2002; Naidu et al. 2007; Saitoh et al. 2004; Tejedor-Real et al. 1998; Torregrossa et al. 2006). DOR knockout mice also demonstrated increased levels of anxiety and depressive-like behaviors, while KOR and MOR knockout mice did not (Filliol et al. 2000), suggesting that DORs are a comparatively more interesting target for studies focusing on the treatment of AMDD.
Although the mechanistic underpinnings of the antidepressant and anxiolytic profiles of DOR agonists are not well understood, earlier studies found that they do not affect basal norepinephrine, dopamine, or serotonin levels (Smagin et al. 2008). These observations suggest a different mechanism than most currently marketed antidepressants. For example, the neurotrophic hypothesis of depression posits that alterations in neuroplasticity involved in emotional and cognitive processing contribute to the development of MDD (Duman 2004). In addition, both brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) are widely referred to as potential biomarkers of pathophysiology and treatment response in mood disorders. Specifically, serum BDNF levels have generally been found to be decreased in patients with MDD compared to healthy controls (Bocchio-Chiavetto et al. 2010), and results are mixed regarding differences in VEGF levels (Elfving et al. 2014; Ventriglia et al. 2009). Notably, DOR agonists have been shown to increase levels of BDNF (Torregrossa et al. 2005; Torregrossa et al. 2004), which is involved in neuroplasticity and in the putative mechanism of action of current antidepressant drugs (Duman 2004). However, the effects of DOR agonists on other biomarkers linked to the mechanism of antidepressant response, including VEGF, remain unknown. Monitoring changes in these neurotrophic factors may help elucidate biomarkers of AMDD and predict which patients are most likely to respond to specific treatments.
AZD2327 is a highly potent and selective DOR agonist. It binds with sub-nanomolar affinity to the human opioid receptor and is highly selective over the MOR and KOR subtypes (Hudzik et al. 2011). AZD2337 readily crosses the blood-brain barrier, and demonstrated anxiolytic and antidepressant activities (comparable to diazepam and imipramine, respectively) in rodent models of anxiety and depression (Hudzik et al. 2011). The anxiolytic activity of AZD2327 was fully reversible by pretreatment with a selective DOR antagonist (naltrindole), further confirming relevance to this target mechanism. Compared to other opioid agonist subtypes, DOR agonists produce minimal effects on respiratory and gastrointestinal systems and have lower abuse potential (Hudzik et al. 2014; Porreca et al. 1984). Although DOR agonists may be associated with a higher risk of producing convulsions than agonists at the other receptor subtypes (Filliol et al. 2000), and AZD2327 has produced seizures in animals, preclinical and phase I studies suggest that keeping plasma levels below 15 ng/ml (corresponding to oral doses of AZD2327 <10 mg) minimizes these events (Hudzik et al. 2011; Hudzik et al. 2014).
The preclinical signals for efficacy and the acceptable safety profile observed in phase I studies (AstraZeneca, unpublished data on file) suggest that AZD2327 may be a novel and effective therapy for anxiety and depression. Because AMDD has not been adequately modeled preclinically, this study initially tested the effects of AZD2327 in a novel animal model of AMDD. We subsequently performed a 4-week pilot clinical study in subjects with AMDD to evaluate the antidepressant and anxiolytic efficacy, safety profile, and pharmacokinetic and pharmacodynamic effects of AZD2327 compared to placebo. We postulated that directly targeting the DOR with an agonist would bring about antidepressant and anxiolytic effects in patients with AMDD. We also evaluated the aforementioned neurobiological markers that have been implicated in mood and anxiety disorders, including VEGF and BDNF, in relationship to drug effects and response.
Materials and methods
Preclinical studies
Animal experiments were carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Detailed methods of the novel technique to evaluate preclinical models of anxiety can be found in the Online Resources. Briefly, pregnant female Sprague Dawley (SD) rats (Charles River Laboratories, Wilmington, MA) were single-housed in standard rat cages. They were assigned to either the control group or the prenatal stress (PNS) group in which the dams were exposed to a novel, variable stress paradigm. Behavioral measures, including the elevated plus maze (EPM) and forced swim test, were assessed in adult offspring after postnatal day 70 to determine the anxiolytic and antidepressant-like effects of AZD2327 (3.0 mg/kg orally for 7 days). In addition, BDNF levels were analyzed from samples of prefrontal cortex, hippocampus, and plasma of SD rats treated with AZD2327 (see Online Resources for detailed methods).
Human participants
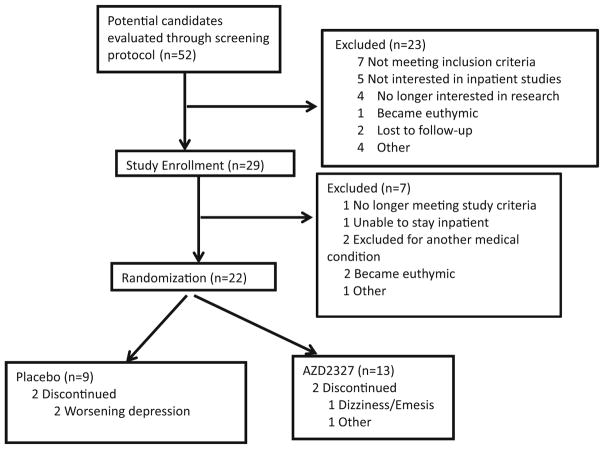
Subjects were recruited from local inpatient psychiatric units, the Internet, and local and national physician referrals. Eligible participants were male and female, 18 to 65 years old, with a diagnosis of MDD, currently depressed without psychotic features as diagnosed by the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Disorders (DSM-IV)-Patient Version (SCID-P) (First et al. 2001). The original protocol called for randomizing 80 patients into the study; however, the trial was terminated for strategic reasons and only 22 subjects completed the study. The National Institute of Mental Health (NIMH) was the only site to participate, and 13 subjects (59 %) received active drug (Fig. 1).
Fig. 1.
Consort diagram for overall study design and total number of subjects screened
Participants were required to be currently experiencing a major depressive episode lasting at least 8 weeks and less than 24 months. This cutoff was chosen to delineate patients experiencing an acute major depressive episode vs. those with more chronic symptoms, given that outcomes may differ between these two populations (Rush et al. 2012). In addition to a diagnosis of MDD, participants were required to have a 17-item Hamilton Depression Rating Scale (HAM-D) total score ≥20, a Hamilton Anxiety Rating Scale (HAM-A) total score ≥16, and a Clinical Global Impression–Severity (CGI-S) score ≥4 at both screening and randomization (an interval of at least 2 weeks). Women had to be either of non-childbearing potential or using a highly effective form of birth control as well as double barrier method contraception. All subjects were studied at the NIMH Clinical Research Center in Bethesda, Maryland from November 2008 to October 2011.
Subjects were in good physical health as determined by medical history, physical exam with vital signs, blood screening labs, baseline electrocardiogram (ECG), electroencephalogram (EEG), urinalysis, and toxicology. They were free from comorbid substance abuse for at least 6 months and judged clinically not to be at serious risk for suicide prior to enrollment. Comorbid Axis II disorders were excluded if they had a major impact on the subject’s current psychiatric status. Subjects with any history of seizure, a family history of epilepsy, or an EEG with evidence of epileptiform activity on initial baseline screening or after medication washout were also excluded. Additional exclusion criteria included any serious unstable medical disorder or condition, treatment with electroconvulsive therapy (ECT) within the past 3 months, or treatment with psychotropic medications 2 weeks before randomization (28 days for fluoxetine).
The study was approved by the Combined Neuroscience Institutional Review Board (IRB) at the NIH. All subjects provided written informed consent before entry into the study and were assigned a Clinical Research Advocate from the NIMH Human Subjects Protection Unit to monitor the consent process and research participation throughout the study.
Study design
This was a single center, double-blind, randomized, parallel group design, placebo-controlled phase II pilot study to assess the clinical effects and safety of AZD2327 for use as mono-therapy treatment of AMDD. The dose selected for the study was based on preclinical findings and greatest tolerability during phase I studies. AZD2327 appeared to be safe and well tolerated up to a daily oral dose of 15 mg; however, because syncope (n = 1, 15 mg) and a brief (<30 s) convulsion (n = 1, 25 mg) had been noted in earlier trials (AstraZeneca, data on file), we elected to study a maximum dose of 6 mg/day. The total duration of participation was up to 12 weeks (including an initial screening period). All primary and secondary analyses were evaluated during the 4-week treatment period. In order to ensure that approximately equal numbers of men and women were represented in both treatment conditions, a stratified randomization of participants by sex was employed. After meeting eligibility criteria and obtaining consent, subjects on any prior medications were tapered off and then remained medication-free for at least 14 days before randomization. Subjects remained inpatients until all study procedures were completed on day 4, as this time frame was thought to be associated with the greatest risk of serious adverse events and allowed for careful monitoring with regard to cardiovascular safety (blood pressure monitoring and ECG), risk for seizures (EEG), and other laboratory safety tests.
On day 1 of the treatment period, participants were randomized in a 2:1 ratio to receive either a 3 mg dose of AZD2327 or identically appearing placebo capsules based on the randomization schedule. Starting on day 2, the dose of AZD2327 or placebo was then increased to twice a day for a total of 4 weeks. Subjects were discontinued from the study if they developed significant side effects or could not tolerate the dose of 6 mg/day.
Outcome measures
The HAM-D and HAM-A were the primary outcome measures. Secondary outcome measures included the HAM-D item 10 score (psychic anxiety), analysis with the HAM-D anxiety/somatization (A/S) factor scores for subgroup effects, and the Clinical Global Impression–Improvement scale (CGI-I). The A/S factor, derived from Cleary and Guy’s factor analysis of the HAM-D scale, includes six items: psychic anxiety, somatic anxiety, gastrointestinal somatic symptoms, general somatic symptoms, hypochondriasis, and insight (Cleary and Guy 1977).
Pharmacokinetic, pharmacodynamic, and safety evaluation
Pharmacokinetic samples to determine plasma levels of AZD2327 and AZ12311418, one of its major metabolites, were collected before the first dose on day 1 of treatment, and at 1, 3, 7, and 12 h following administration of the first dose. Pharmacokinetic samples were also collected before the dose given on day 7 and at each subsequent outpatient visit. Given the potential adverse events of AZD2327 observed in phase I studies, additional precautions for convulsions and syncope were implemented (see Online Resources).
EEG, BDNF, and VEGF
EEG and serum samples for BDNF and VEGF analysis were collected as putative biomarkers of drug effects and response. While EEGs were primarily collected for safety reasons (as noted above), they were also used to measure gamma (30–50Hz) power vs. control band (2–30 Hz) power for pharmacodynamic effects. VEGF and BDNF samples were collected using the vacutainer system before initial administration of AZD2327 and at days 1, 4, 7, 14, 21, and 28 post-administration. Anti-VEGF and anti-BDNF sandwich ELISA was performed blind to clinical information.
Statistical analysis
The primary aim of the study was to assess the efficacy of 28 days of AZD2327 compared with placebo in improving overall depressive symptomatology in participants with AMDD. Although HAM-D and HAM-A scores served as the primary outcome measures, other individual items from the HAM-D and HAM-A were examined in an exploratory analysis to understand the specific effects of the drug. Comparing drugs, change from baseline in the intent-to-treat sample was examined using repeated measures ANCOVA where post-treatment ratings were examined every week for 4 weeks and the baseline rating was a covariate. Missing data were handled with the last observation carried forward. Cohen’s d was calculated to estimate the effect size for drug effects.
Additional secondary analyses included a logistic regression that compared the proportion of responders on each drug. Response was defined as a ≥50 % reduction in HAM-D and HAM-A scores and a CGI-I score of 1 or 2 at week 4. Subgroup analyses with the HAM-D A/S factor evaluated subjects with a factor score ≥7. Exploratory analyses also examined changes in responders vs. nonresponders based on HAM-D and HAM-A criteria. The significance criterion was set at 0.05, two-tailed. IBM SPSS Statistics version 21 was used to conduct the analyses.
A linear mixed model with restricted maximum likelihood estimation and a compound symmetry covariance structure was used to examine EEG, VEGF, BDNF, and drug levels over time with drug and time as fixed factors. VEGF and BDNF values were transformed using a base 10 log prior to analysis.
Results
Preclinical studies
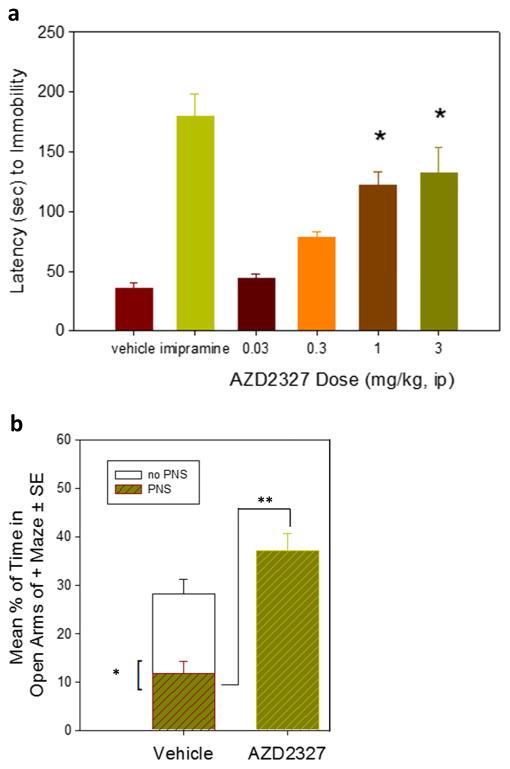
PNS rats exhibited an anxious phenotype as evidenced by a markedly reduced amount of time spent in the open arms of the EPM compared to non-PNS animals (Fig. 2). At 1 mg/kg and 3 mg/kg doses, AZD2327 increased latency to immobility in PNS rats to levels similar to animals treated with imipramine (Fig. 1a). Pretreatment with a single dose of AZD2327 (3 mg/kg ip) 30 min prior to testing increased the percentage of time spent in the open arms of the EPM (Fig. 1b). BDNF expression was significantly elevated in the hippocampus of animals treated with AZD2327 but not in the frontal cortex (Online Resource Figure S1). Plasma BDNF levels in animals treated with AZD2327 remained unchanged compared to PNS controls (Online Resource Figure S1).
Fig. 2.
Preclinical behavioral analysis of prenatally stressed (PNS) rats treated with AZD2327. a PNS rats treated with AZD2327 demonstrated increased latency to immobility at 1 and 3 mg/kg doses. This increase was similar to that seen when PNS rats were treated with imipramine (15 mg/kg). b PNS rats exhibited a phenotype consistent with anxiety as indicated by decreased time spent in the open arms of the elevated plus maze (EPM). This decrease was reversed when PNS rats were treated with AZD2327 (3 mg/kg) 30 min before testing t(49) = 6/120, p < 0.0001
Clinical study
Demographic characteristics for the participants appear in Table 1. Completion rates did not differ between groups: 85 % (11/13) for active drug and 77 % (7/9) for placebo (χ2 = 0.17, df = 1, p = 0.68). Two patients receiving active drug left the study. The first patient dropped out on day 1 because of dizziness, orthostatic hypotension, and emesis. The second patient dropped out on day 28 after expressing concern over the transition to standard therapy following the study. Two participants receiving placebo left the study after 2 weeks due to worsening depression, and one also had worsening anxiety.
Table 1.
Demographics and clinical characteristics of the patient sample (n = 22)
| Placebo | AZD2327 | ||||
|---|---|---|---|---|---|
|
| |||||
| N | % | N | % | p value | |
| Gender (male) | 5 | 56 | 7 | 54 | 0.94 |
| Education (college graduate) | 6 | 67 | 7 | 54 | 0.55 |
| Family history of mood disorder | 6 | 67 | 9 | 69 | 0.90 |
| Race (Caucasian) | 7 | 78 | 6 | 46 | 0.14 |
| Ethnicity (Hispanic) | 2 | 22 | 7 | 54 | 0.14 |
| Mean | SD | Mean | SD | p value | |
| Age (years) | 48.7 | 11.3 | 40.0 | 12.2 | 0.11 |
| Age of Onset (Years) | 17.3 | 7.0 | 25.5 | 12.2 | 0.09 |
| Length of illness (years) | 29.1 | 11.4 | 13.3 | 8.6 | 0.001 |
| Length of current episode (months) | 10.2 | 8.4 | 9.8 | 8.1 | 0.91 |
| BMI | 28.2 | 4.3 | 28.6 | 4.2 | 0.83 |
| Weight (kg) | 82.3 | 20.6 | 81.3 | 17.5 | 0.90 |
| HAM-D (17 item) | 23.0 | 2.6 | 24.3 | 2.3 | 0.23 |
| HAM-A | 24.7 | 3.1 | 24.9 | 4.8 | 0.91 |
| CGI | 4.7 | 0.7 | 4.6 | 0.5 | 0.76 |
BMI body mass index, CGI Clinical Global Impression rating scale, HAM-A Hamilton Anxiety Rating Scale, HAM-D Hamilton Depression Rating Scale
Efficacy
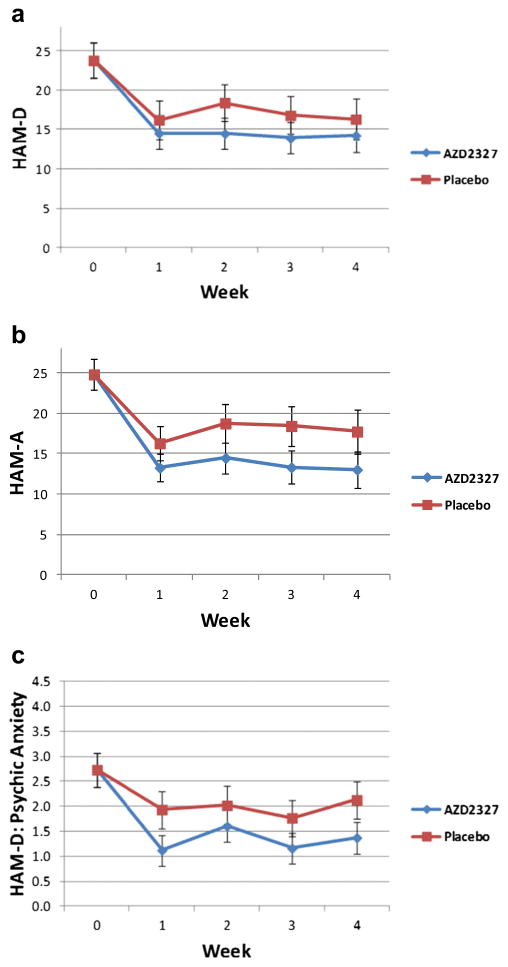
For all primary and secondary efficacy measures, no significant drug effects were found. Repeated measures ANCOVA found no significant drug effect as assessed by the HAM-D (F = 0.77, df = 1,19, p = 0.39; d = 0.40; Fig. 3a), the HAM-A (F = 2.30, df = 1,19, p = 0.15; d = 0.70; Fig. 3b), the psychic anxiety item of the HAM-D (F = 2.32, df = 1,19, p = 0.14; d = 0.70; Fig. 3c), or the CGI-I (F = 1.63, df = 1,18, p = 0.22; d = 0.60). However, effect sizes for AZD2327 on the HAM-A were higher than on the HAM-D (d = 0.7 vs. 0.4).
Fig. 3.
No significant differences were noted in several mood and anxiety rating scales over 4 weeks of treatment with AZD2327. a Hamilton Depression Rating Scale (HAM-D), b Hamilton Anxiety Rating Scale (HAM-A), and c HAM-D psychic anxiety item all showed no significant differences in treatment with active drug (6 mg/day) vs. placebo (N = 22)
Repeated measures ANCOVA similarly found no interaction between drug and time for the HAM-D (F = 0.51, df = 3, 57, p = 0.68; Fig. 3a), the HAM-A (F = 0.32, df = 3,57, p = 0.81; Fig. 3b), the psychic anxiety item of the HAM-D (F = 0.35, df = 3,57, p = 0.79; Fig. 3c), or the CGI-I (F = 0.68, df = 3,54, p = 0.57).
An examination of the endpoint (day 28) as the sole time point with baseline as the covariate also showed no significant drug difference on the HAM-D (F=0.36, df=1,19, p=0.56; d=0.28), the HAM-A (F=1.78, df=1,19, p=0.20; d=0.61), the psychic anxiety item of the HAM-D (F = 2.25, df = 1,19, p = 0.15; d=0.69), or the CGI-I (F=0.79, df=1,18, p=0.39; d=0.42).
For all four measures, using length of illness as a covariate also did not alter the significance of the drug effect or its interaction with time. Participants receiving AZD2327 did not have significantly lower scores than the placebo group. A linear mixed model with drug and time as factors, baseline as a covariate, and restricted maximum likelihood estimation instead of last observation carried forward showed similar results. Finally, a logistic regression showed no difference in response rates at endpoint (day 28). For the HAM-D, five (39 %) participants responded to active drug and four (44 %) responded to placebo (χ2 = 0.08, p = 0.78; OR = 0.78). For the HAM-A, seven (54 %) participants responded to active drug and three (33 %) to placebo (χ2 = 0.89, p = 0.35; OR = 2.33).
Exploratory analyses with the remaining HAM-D items as well as the anxiety subscale from Cleary and Guy similarly found no significant changes. However, for the HAM-A, participants on active drug had significantly lower scores for autonomic symptoms (F = 5.07, df = 1,19, p = 0.04; d = 1.03). This finding did not remain significant after correction for multiple comparisons.
AZD2327 plasma levels
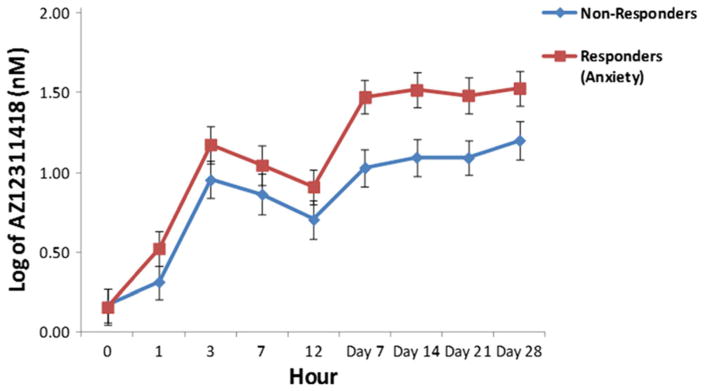
Analyses were performed separately for AZD2327 and AZ12311418, one of its major metabolites (see Online Resources for additional analyses). HAM-D responders did not have significantly higher levels of either AZ12311418 or AZD2327 than nonresponders (response: F = 3.36, df = 1,11, p = 0.09, d = 1.09; response by time: F = 1.38, df = 8,71, p = 0.22). However, responders as assessed by the HAM-A had significantly higher AZ12311418 levels than nonresponders (response: F = 5.99, df = 1,11, p = 0.03, d = 1.49; response by time: F = 1.42, df = 8,71, p = 0.20) (Fig. 4). The difference between responders and nonresponders began at day 7 and persisted through the end of the study.
Fig. 4.
Log transformed AZ12311418 (major metabolite) levels by anxiety response over 4 weeks. Responders had significantly higher levels of AZ12311418 than nonresponders (F = 5.99, df = 1,11, p = 0.03, d = 1.49). No response by time interaction was observed (F = 1.42, df = 8,71, p = 0.20)
VEGF and BDNF plasma levels
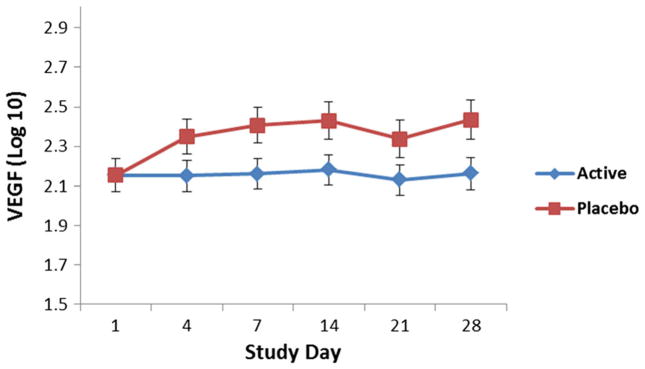
VEGF levels were significantly higher in participants who received placebo compared to those who received AZD2327 (F = 6.23, df = 1,19, p = 0.02; Fig. 5). Elevated VEGF levels were noted at all time points (no drug by time effect was noted). BDNF levels did not significantly differ between participants receiving AZD2327 and those receiving placebo (F = 0.68, df = 1,19, p = 0.42).
Fig. 5.
Vascular endothelial growth factor (VEGF) levels were significantly lower across all time points in patients who received AZD2327. Compared to placebo (p = 0.02), no time by drug effect was observed
EEG analysis
Participants receiving AZD2327 had significantly larger gamma EEG power (F = 6.34, df = 1,28, p = 0.02) than those receiving placebo, both at day 1 post-drug and at day 28. However, in control band (2–30Hz), EEG power did not significantly differ from placebo at either time point. While no significant difference in gamma EEG power was observed between HAM-D or HAM-A responders and nonresponders, there was a trend (p < 0.06) for HAM-A responders to have higher EEG gamma power than HAM-A nonresponders (Online Resource Figure S2).
Correlations for all participants between day 1 serum AZ12311418 levels and gamma power, both elevated in HAM-A responders, were not significant.
Adverse events
Adverse events were recorded daily for the first week then weekly for the following 3 weeks (see Table 2). One patient dropped out of study after experiencing orthostatic hypotension followed by nausea and vomiting. These reactions were thought to be secondary to receiving active drug (see Online Resources for further details). No seizures occurred in either group, and no significant changes were noted in ECG, EEG, or laboratory values. The most commonly observed adverse events were headache (69 % active vs. 67 % placebo, p = 0.90), dry mouth (69 % active vs. 44 % placebo, p = 0.25), and weight gain (62 % active vs. 22 % placebo, p = 0.07). Difficulty falling asleep occurred significantly more often on active drug than placebo (54 vs. 11 %, p = 0.04). Eye irritation occurred significantly less often on active drug than placebo (15 vs. 56 %, p = 0.047). The frequency of other treatment-related side effects did not differ significantly between the active drug and placebo groups.
Table 2.
Side effects for patients receiving placebo and AZD2327
| System | Placebo
|
AZD2327
|
χ2 | p value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Cardiovasculara | Hypertension | 0 | 0 | 2 | 15 | 1.52 | 0.22 |
| Hypotension | 2 | 22 | 2 | 15 | 0.17 | 0.68 | |
| Tachycardia | 1 | 11 | 2 | 15 | 0.08 | 0.77 | |
| Chest | Breast pain/swelling | 0 | 0 | 1 | 8 | 0.73 | 0.39 |
| Chest pain | 1 | 11 | 6 | 46 | 3.01 | 0.08 | |
| Coughing | 0 | 0 | 2 | 15 | 1.52 | 0.22 | |
| Shortness of breath | 1 | 11 | 3 | 23 | 0.51 | 0.47 | |
| Wheezing | 0 | 0 | 3 | 23 | 2.40 | 0.12 | |
| Ear | Ear ache | 1 | 11 | 2 | 15 | 0.08 | 0.77 |
| Tinnitus | 2 | 22 | 5 | 38 | 0.65 | 0.42 | |
| Eye | Blurred vision | 1 | 11 | 1 | 8 | 0.08 | 0.78 |
| Eye irritation | 5 | 56 | 2 | 15 | 3.96 | 0.047 | |
| Gastrointestinal | Appetite decrease | 4 | 44 | 5 | 38 | 0.08 | 0.78 |
| Appetite increase | 2 | 22 | 5 | 38 | 0.65 | 0.42 | |
| Constipation | 4 | 44 | 6 | 46 | 0.01 | 0.94 | |
| Diarrhea | 2 | 22 | 3 | 23 | 0.00 | 0.96 | |
| Flatulence | 2 | 22 | 5 | 38 | 0.65 | 0.42 | |
| Increased thirst | 1 | 11 | 6 | 46 | 3.01 | 0.08 | |
| Increased urination | 0 | 0 | 4 | 31 | 3.38 | 0.07 | |
| Nausea | 2 | 22 | 5 | 38 | 0.65 | 0.42 | |
| Stomach or abdominal discomfort | 2 | 22 | 6 | 46 | 1.32 | 0.25 | |
| Stool discoloration | 0 | 0 | 1 | 8 | 0.73 | 0.39 | |
| Taste abnormality | 1 | 11 | 4 | 31 | 1.17 | 0.28 | |
| Vomiting | 1 | 11 | 1 | 8 | 0.08 | 0.78 | |
| Weight gain | 2 | 22 | 8 | 62 | 3.32 | 0.07 | |
| Weight loss | 4 | 44 | 7 | 54 | 0.19 | 0.66 | |
| Genitourinary | Cramps | 0 | 0 | 2 | 33 | 1.67 | 0.20 |
| Decreased libido | 0 | 0 | 3 | 23 | 2.40 | 0.12 | |
| Genital discomfort | 1 | 11 | 1 | 8 | 0.08 | 0.78 | |
| Menstrual irregularity | 1 | 25 | 0 | 0 | 1.67 | 0.20 | |
| Painful urination | 1 | 11 | 0 | 0 | 1.51 | 0.22 | |
| Premenstrual tension | 0 | 0 | 2 | 33 | 1.67 | 0.20 | |
| Head | Dizziness/faintness | 3 | 33 | 7 | 54 | 0.90 | 0.34 |
| Headache | 6 | 67 | 9 | 69 | 0.02 | 0.90 | |
| Mouth | Dental problems | 2 | 22 | 0 | 0 | 3.18 | 0.07 |
| Dry mouth | 4 | 44 | 9 | 69 | 1.35 | 0.25 | |
| Gum problems | 1 | 11 | 1 | 8 | 0.08 | 0.78 | |
| Hypersalivation | 1 | 11 | 1 | 8 | 0.08 | 0.78 | |
| Mouth ulcer | 2 | 22 | 2 | 15 | 0.17 | 0.68 | |
| Sore tongue | 0 | 0 | 0 | 0 | 0.00 | 1.00 | |
| Musculoskeletal | Akathisia | 1 | 11 | 0 | 0 | 1.51 | 0.22 |
| Edema | 1 | 11 | 1 | 8 | 0.08 | 0.78 | |
| Muscle rigidity | 1 | 11 | 3 | 23 | 0.51 | 0.47 | |
| Muscle/bone/joint pain | 3 | 33 | 5 | 38 | 0.06 | 0.81 | |
| Tic movements | 0 | 0 | 1 | 8 | 0.73 | 0.39 | |
| Tremor | 0 | 0 | 3 | 23 | 2.40 | 0.12 | |
| Nose/throat | Difficulty swallowing | 0 | 0 | 3 | 23 | 2.40 | 0.12 |
| Flu/upper respiratory | 1 | 11 | 4 | 31 | 1.17 | 0.28 | |
| Nasal congestion | 4 | 44 | 5 | 38 | 0.08 | 0.78 | |
| Sore throat | 2 | 22 | 4 | 31 | 0.20 | 0.66 | |
| Psychological/behavioral | Anxiety | 0 | 0 | 0 | 0 | 0.00 | 1.00 |
| Difficulty concentrating | 1 | 11 | 2 | 15 | 0.08 | 0.77 | |
| Depression | 1 | 11 | 2 | 15 | 0.08 | 0.77 | |
| Difficulty falling asleep | 1 | 11 | 7 | 54 | 4.20 | 0.040 | |
| Drowsiness/sedation | 3 | 33 | 7 | 54 | 0.90 | 0.34 | |
| Early morning awakening | 2 | 22 | 6 | 46 | 1.32 | 0.25 | |
| Interrupted sleep | 3 | 33 | 5 | 38 | 0.06 | 0.81 | |
| Irritability | 3 | 33 | 6 | 46 | 0.36 | 0.55 | |
| Memory problems | 2 | 22 | 3 | 23 | 0.00 | 0.96 | |
| Motor activity: decreased | 0 | 0 | 3 | 23 | 2.40 | 0.12 | |
| Motor activity: increased | 2 | 22 | 5 | 38 | 0.65 | 0.42 | |
| Slurred speech | 0 | 0 | 1 | 8 | 0.73 | 0.39 | |
| Suicidal ideas | 1 | 11 | 2 | 15 | 0.08 | 0.77 | |
| Tiredness/fatigue | 2 | 22 | 4 | 31 | 0.20 | 0.66 | |
| Skin | Dermatological irritation | 3 | 33 | 4 | 31 | 0.02 | 0.90 |
| Hair problems | 0 | 0 | 0 | 0 | 0.00 | 1.00 | |
| Sweating | 1 | 11 | 0 | 0 | 1.51 | 0.22 | |
| Other | Accidental injury | 1 | 11 | 1 | 8 | 0.08 | 0.78 |
| Fever | 1 | 11 | 2 | 15 | 0.08 | 0.77 | |
A single patient receiving AZD2327 had elevated systolic (160, normal range 90–150) and diastolic blood pressure (98, normal range 55–85). On placebo, one patient had low systolic (86) and another had low diastolic (46) blood pressure. In addition, one patient on AZD2327 had a low pulse (50, normal range 60–120)
Discussion
This study investigated the effects of AZD2327, a DOR agonist, in rodent models of anxiety and depression and in participants with AMDD. The preclinical component of this study found that AZD2327 had anxiolytic-like properties in PNS rodents. Specifically, AZD2327 increased the percentage of open-arm entries and time spent in open arms in the EPM. These anxiolytic-like properties are similar to those previously demonstrated in other preclinical models (Hudzik et al. 2011). In addition, a single dose of AZD2327 in rodents significantly increased BDNF expression in the hippocampus, but not in the prefrontal cortex or in plasma.
Despite these promising preclinical results, this first published report of a DOR agonist evaluated in a proof-of-concept, double-blind, randomized, parallel group design, placebo-controlled study in the treatment of AMDD observed no statistically significant differences between drug and placebo groups on change in rating scale scores for either anxiety or depression. Nevertheless, we did find a number of interesting observations that warrant further investigation in a clinical setting.
The effect sizes for AZD2327 on the HAM-A were higher than on the HAM-D (d = 0.7 vs. 0.4), suggesting that AZD2327 may have more of an anxiolytic than antidepressant profile. This observation further supports the findings of earlier preclinical studies (Hudzik et al. 2011; Hudzik et al. 2014). Although the present findings did not reach clinical significance, a larger sample size might have detected a significant difference with this outcome measure. Similarly, increasing the dose of AZD2327 may also have resulted in a greater effect; however, since earlier studies observed significant adverse event profiles with doses as low as 15 mg (Cmax concentrations <15 ng/ml), participants should be monitored closely at higher doses in future studies.
In the present study, we also found that VEGF levels were significantly higher in participants who received placebo compared to AZD2327. Other studies have similarly observed elevated baseline levels of VEGF in mRNA and plasma from depressed individuals compared to controls (Iga et al. 2007; Kahl et al. 2009). It has been hypothesized that elevated VEGF levels in depression could be a compensatory mechanism to promote neuroprotection (Lee and Kim 2012). However, additional studies focusing on VEGF are warranted since results from prior studies have been variable; increases, decreases, and no change in VEGF concentrations have all been linked to depression and to potential antidepressant response (Clark-Raymond and Halaris 2013; Fornaro et al. 2013). Both our clinical and preclinical studies found no significant changes in plasma BDNF associated with active drug.
As no positron emission tomography (PET) ligands are available to measure drug occupancy at the DOR, EEGs were used as surrogate markers of CNS involvement. The 6 mg dose used in this study suggested likely CNS pharmacodynamics as evidenced by the fact that those participants receiving AZD2327 had significantly larger frontal gamma EEG power than those receiving placebo, both at treatment days 1 and 28.
Changes in EEG gamma band power have previously been thought to suggest enhanced cognitive processes in other neuropsychiatric disorders (Herrmann and Demiralp 2005) and were also found to be markers of clinical response in individuals with treatment-resistant MDD receiving the N-methyl-D-aspartate (NMDA) antagonist ketamine (Cornwell et al. 2012). The results of the present study may therefore support reported findings of complex interactions between DORs and the NMDA systems that include nociception, signal transduction, and dopamine efflux (Baker et al. 2002; Bosse et al. 2014; Cai et al. 1997; Fusa et al. 2005) and that may ultimately affect mood response.
Anxiety responders (as assessed by the HAM-A) had significantly higher levels of AZ12311418 (an active metabolite of AZD2327) than nonresponders; the difference appeared at day 7 and lasted through the end of the study. This finding has interesting implications with regard to individual patient metabolism or the potential off-site target action of AZ12311418. While no significant effects were observed on any of the 142 secondary targets (G-protein coupled receptors (GPCRs), ion channels, nuclear hormone receptors, and enzymes) for AZD2327, investigating the possible off-site target action of AZ1231148 may be valuable in future research. It should be noted that the elimination t1/2λz for AZ1231148 was longer than for the parent compound. This is particularly important because, in addition to being pharmacologically active, metabolites of some drugs can be a source of new drug candidates (Lin and Lu 1997). Because metabolites are subject to phase II conjugation reactions, they can also have better safety profiles.
Other areas to explore include AZD2327’s potential role as an augmentation agent in AMDD. Medication augmentation after failure with primary antidepressants has shown clear benefit in outpatient populations (Trivedi et al. 2006). Furthermore, current treatments are often associated with CNS side effects, risk of addiction (eg, benzodiazepines), or slow onset of action (as with selective serotonin reuptake inhibitors (SSRIs)). These observations are compounded by the overall lower rates of antidepressant response and remission seen in AMDD and worse response to other treatment strategies, such as cognitive behavioral therapy (Domschke et al. 2010; Farabaugh et al. 2012; Fava et al. 2008). Thus, the development of new treatments for individuals with AMDD is vital, particularly given the likelihood of increased melancholic features, suicidal ideation, suicidal attempts, and added medical comorbidities (Fava et al. 2008). Lastly, AZD2327 may be beneficial as an adjunctive treatment in other disorders, such as generalized anxiety disorder (GAD), social anxiety disorder (SAD), and post-traumatic stress disorder (PTSD), particularly given their low remission rates (Taylor et al. 2012).
It should also be noted that although the DOR was targeted in this study, it may be useful to target two or more different opioid receptors at the same time, as modeled by other medications used in combination for clinical trials; one example would be combining buprenorphine (a moderate partial MOR agonist and KOR antagonist) and samidorphan (a selective MOR antagonist). This combination was found to improve mood in patients with treatment-resistant MDD while minimizing risk, including abuse liability (Alkermes 2015). Similar combinations that target the DOR may prove useful in treating AMDD.
This study has several limitations. The main limitation is the small sample size, given that only one site participated in the trial. Although this was originally proposed as a multi-site trial, the NIMH was the only site that participated because the study was terminated early by the company for strategic reasons. Consequently, only 22 patients (out of a projected 80) were recruited for the study. Therefore, while we were able to identify the signal of an effect, and to explore both safety and target engagement, the study was underpowered and thus unable to detect small to moderate differences in efficacy between active drug and placebo. The study was also limited to 4 weeks, and potentially significant antidepressant/anxiolytic effects might have separated from placebo given more time (i.e., >6 weeks), as is commonly observed with traditional antidepressants (National Institute for Clinical Excellence (NICE) 2004). Nevertheless, the study was also associated with several study design strengths that minimized confounding factors. Foremost among these was that AZD2327 was relatively well-tolerated, with higher completion rates than placebo. Common side effects were similar to those experienced with current antidepressants, and no epileptiform activity or seizures were observed in subjects, despite concerns in preclinical models.
As previously noted, novel, effective treatments for AMDD are urgently needed. Overall, these preliminary results suggest that AZD2327 may have greater potential anxiolytic vs. antidepressant signals; HAM-A responders had increased concentrations of the active metabolite AZ12311418. Future studies with this compound or its metabolites may be warranted on the basis of the signals noted earlier, particularly those exploring efficacy and tolerability with longer study duration or in combination with current antidepressants. In addition, enriching patient subgroups with more AMDD features in future trials with similar agents may contribute to more robust treatment effects. In conclusion, the potential utility of DOR agonist compounds as novel treatments of AMDD and/or primary anxiety disorders warrants further exploration.
Supplementary Material
Acknowledgments
Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; NCT00759395, Protocol 08-M-0196), by a NARSAD Independent Investigator to Dr. Zarate, and by a Brain & Behavior Mood Disorders Research Award to Dr. Zarate. Dr. Zarate had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank the 7SE research unit and staff for their support, and Mercy Uwakwe for assistance in performing the clinical BDNF and VEGF ELISA assays. Ioline Henter (NIMH) provided invaluable editorial assistance.
Funding
Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; NCT00759395, protocol 08-M-0196), by a NARSAD Independent Investigator to Dr. Zarate, and by a Brain & Behavior Mood Disorders Research Award to Dr. Zarate. These funding sources had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. A patent for the use of ketamine in depression has been awarded that lists Dr. Zarate among the inventors; he has assigned his rights on the patent to the US government, but will share a percentage of any royalties that may be received by the government. Carla Maciag and Drs. Mark Smith, Thomas Hudzik, and Alan Cross were full-time employees of AstraZeneca Neuroscience Innovative Medicines at the time the study was conducted. AstraZeneca Pharmaceuticals provided the study compound.
Footnotes
ClinicalTrials.gov Identifier: NCT00759395, Protocol 08-M-0196
Electronic supplementary material
The online version of this article (doi:10.1007/s00213-015-4195-4) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
The study was approved by the Combined Neuroscience Institutional Review Board (IRB) at the NIH. All subjects provided written informed consent before entry into the study and were assigned a Clinical Research Advocate from the NIMH Human Subjects Protection Unit to monitor the consent process and research participation throughout the study.
Conflict of interest
The authors declare that they have no competing interests.
References
- Alkermes PLC. Alkermes announces positive results from study of ALKS 5461 for treatment of major depressive disorder. 2015. [Google Scholar]
- Andreescu C, Lenze EJ, Dew MA, Begley AE, Mulsant BH, Dombrovski AY, Pollock BG, Stack J, Miller MD, Reynolds CF. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry. 2007;190:344–349. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- Baker AK, Hoffman VL, Meert TF. Interactions of NMDA antagonists and an alpha 2 agonist with mu, delta and kappa opioids in an acute nociception assay. Acta Anaesthesiol Belg. 2002;53:203–212. [PubMed] [Google Scholar]
- Blackburn TP, Cross AJ, Hille C, Slater P. Autoradiographic localization of delta opiate receptors in rat and human brain. Neuroscience. 1988;27:497–506. doi: 10.1016/0306-4522(88)90283-7. [DOI] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A, Giovannini C, Rillosi L, Ventriglia M, Riva MA, Gennarelli M. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry. 2010;11:763–773. doi: 10.3109/15622971003611319. [DOI] [PubMed] [Google Scholar]
- Bosse KE, Jutkiewicz EM, Schultz-Kuszak KN, Mabrouk OS, Kennedy RT, Gnegy ME, Traynor JR. Synergistic activity between the delta-opioid agonist SNC80 and amphetamine occurs via a glutamatergic NMDA-receptor dependent mechanism. Neuropharmacology. 2014;77:19–27. doi: 10.1016/j.neuropharm.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O’Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Cai YC, Ma L, Fan GH, Zhao J, Jiang LZ, Pei G. Activation of N-methyl-D-aspartate receptor attenuates acute responsiveness of delta-opioid receptors. Mol Pharmacol. 1997;51:583–587. doi: 10.1124/mol.51.4.583. [DOI] [PubMed] [Google Scholar]
- Clark-Raymond A, Halaris A. VEGF and depression: a comprehensive assessment of clinical data. J Psychiatr Res. 2013;47:1080–1087. doi: 10.1016/j.jpsychires.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Cleary P, Guy W. Factor analysis of the Hamilton depression scale. Drugs Exp Clin Res. 1977;1:115–120. [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CAJ. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gandarias JM, Echevarria E, Acebes I, Abecia LC, Casis O, Casis L. Effects of fluoxetine administration on mu-opoid receptor immunostaining in the rat forebrain. Brain Res. 1999;817:236–240. doi: 10.1016/s0006-8993(98)01256-6. [DOI] [PubMed] [Google Scholar]
- Domschke K, Deckert J, Arolt V, Baune BT. Anxious versus non-anxious depression: difference in treatment outcome. J Psychopharmacol. 2010;24:621–622. doi: 10.1177/0269881108097723. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Elfving B, Buttenschon HN, Foldager L, Poulsen PH, Grynderup MB, Hansen AM, Kolstad HA, Kaerlev L, Mikkelsen S, Borglum AD, Wegener G, Mors O. Depression and BMI influences the serum vascular endothelial growth factor level. Int J Neuropsychopharmacol. 2014;17:1409–1417. doi: 10.1017/S1461145714000273. [DOI] [PubMed] [Google Scholar]
- Escriba PV, Ozaita A, Garcia-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29:1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- Farabaugh A, Alpert J, Wisniewski SR, Otto MW, Fava M, Baer L, Perlis R, Friedman E, Nyer M, Bitran S, Balasubramani GK, Inamori A, Trivedi M, Thase ME. Cognitive therapy for anxious depression in STAR(*) D: what have we learned? J Affect Disord. 2012;142:213–218. doi: 10.1016/j.jad.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Rankin MA, Wright EC, Alpert JE, Nierenberg AA, Pava J, Rosenbaum JF. Anxiety disorders in major depression. Compr Psychiatry. 2000;41:97–102. doi: 10.1016/s0010-440x(00)90140-8. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research; New York: 2001. [Google Scholar]
- Fornaro M, Rocchi G, Escelsior A, Contini P, Ghio M, Colicchio S, De Berardis D, Amore M, Fornaro P, Martino M. VEGF plasma level variations in duloxetine-treated patients with major depression. J Affect Disord. 2013;151:590–595. doi: 10.1016/j.jad.2013.06.055. [DOI] [PubMed] [Google Scholar]
- Fusa K, Takahashi I, Watanabe S, Aono Y, Ikeda H, Saigusa T, Nagase H, Suzuki T, Koshikawa N, Cools AR. The non-peptidic delta opioid receptor agonist TAN-67 enhances dopamine efflux in the nucleus accumbens of freely moving rats via a mechanism that involves both glutamate and free radicals. Neuroscience. 2005;130:745–755. doi: 10.1016/j.neuroscience.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Goodman RR, Snyder SH, Kuhar MJ, Young WS., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980;77:6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee A, Karp JF, Dew MA, Houck P, Andreescu C, Reynolds CF., 3rd Anxiety impairs depression remission in partial responders during extended treatment in late-life. Depress Anxiety. 2010;27:451–456. doi: 10.1002/da.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Regionally selective increases in mu opioid receptor density in the brains of suicide victims. Brain Res. 1990;530:312–316. doi: 10.1016/0006-8993(90)91301-v. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hudzik TJ, Maciag C, Smith MA, Caccese R, Pietras MR, Bui KH, Coupal M, Adam L, Payza K, Griffin A, Smagin G, Song D, Swedberg MD, Brown W. Preclinical pharmacology of AZD2327: a highly selective agonist of the delta-opioid receptor. J Pharmacol Exp Ther. 2011;338:195–204. doi: 10.1124/jpet.111.179432. [DOI] [PubMed] [Google Scholar]
- Hudzik TJ, Pietras MR, Caccese R, Bui KH, Yocca F, Paronis CA, Swedberg MD. Effects of the delta opioid agonist AZD2327 upon operant behaviors and assessment of its potential for abuse. Pharmacol Biochem Behav. 2014;124:48–57. doi: 10.1016/j.pbb.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Iga J, Ueno S, Yamauchi K, Numata S, Tayoshi-Shibuya S, Kinouchi S, Nakataki M, Song H, Hokoishi K, Tanabe H, Sano A, Ohmori T. Gene expression and association analysis of vascular endothelial growth factor in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:658–663. doi: 10.1016/j.pnpbp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Niciu MJ, Henter ID, Zarate CA. Defining anxious depression: a review of the literature. CNS Spectr. 2013:1–9. doi: 10.1017/S1092852913000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Niciu MJ, Richards EM, Zarate CA., Jr Pharmacologic treatment of dimensional anxious depression: a review. Prim Care Companion CNS Disord. 2014:16. doi: 10.4088/PCC.13r01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM. The antidepressant-like effects of delta-opioid receptor agonists. Mol Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Bens S, Ziegler K, Rudolf S, Kordon A, Dibbelt L, Schweiger U. Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrinology. 2009;34:353–357. doi: 10.1016/j.psyneuen.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, Carroll FI, Nestler EJ, Konradi C, Carlezon WA., Jr Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Kim YK. Increased plasma VEGF levels in major depressive or manic episodes in patients with mood disorders. J Affect Disord. 2012;136:181–184. doi: 10.1016/j.jad.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Lin JH, Lu AY. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev. 1997;49:403–449. [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Thompson RC, Akil H, Watson SJ. Delta opioid receptor mRNA distribution in the brain: comparison to delta receptor binding and proenkephalin mRNA. J Chem Neuroanat. 1993;6:351–362. doi: 10.1016/0891-0618(93)90010-2. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Lichtman AH, Archer CC, May EL, Harris LS, Aceto MD. NIH 11082 produces anti-depressant-like activity in the mouse tail-suspension test through a delta-opioid receptor mechanism of action. Eur J Pharmacol. 2007;566:132–136. doi: 10.1016/j.ejphar.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence (NICE) Clinical Guideline 23. NICE; London: 2004. Depression: management of depression in primary and secondary care. [Google Scholar]
- Ortega-Alvaro A, Acebes A, Saracibar G, Echevarria E, Casis L, Mico JA. Effect of the antidepressant nefazodone on the density of cells expressing mu-opioid receptors in discrete brain areas processing sensory and affective dimensions of pain. Psychopharmacology Berl. 2004;176:305–311. doi: 10.1007/s00213-004-1894-7. [DOI] [PubMed] [Google Scholar]
- Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF. Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther. 1984;230:341–348. [PubMed] [Google Scholar]
- Quirion R, Zajac JM, Morgat JL, Roques BP. Autoradiographic distribution of mu and delta opiate receptors in rat brain using highly selective ligands. Life Sci. 1983;33(Suppl 1):227–230. doi: 10.1016/0024-3205(83)90484-8. [DOI] [PubMed] [Google Scholar]
- Reindl JD, Rowan K, Carey AN, Peng X, Neumeyer JL, McLaughlin JP. Antidepressant-like effects of the novel kappa opioid antagonist MCL-144B in the forced-swim test. Pharmacology. 2008;81:229–235. doi: 10.1159/000112867. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Wisniewski SR, Zisook S, Fava M, Sung SC, Haley CL, Chan HN, Gilmer WS, Warden D, Nierenberg AA, Balasubramani GK, Gaynes BN, Trivedi MH, Hollon SD. Is prior course of illness relevant to acute or longer-term outcomes in depressed out-patients? A STAR*D report. Psychol Med. 2012;42:1131–1149. doi: 10.1017/S0033291711002170. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, Beck AT, Beck J. Syndrome comorbidity in patients with major depression or dysthymia: prevalence and temporal relationships. Am J Psychiatry. 1990;147:1025–1028. doi: 10.1176/ajp.147.8.1025. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Song D, Medd A, Cross AJ, Hudzik T, Mrzljak L. Neurochemical validation of centrally active delta-opioid receptor agonists as novel treatment of anxiety disorders. Eur Neuropsychopharmacol. 2008;18:S496. [Google Scholar]
- Taylor S, Abramowitz JS, McKay D. Non-adherence and non-response in the treatment of anxiety disorders. J Anxiety Disord. 2012;26:583–589. doi: 10.1016/j.janxdis.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Tejedor-Real P, Mico JA, Smadja C, Maldonado R, Roques BP, Gilbert-Rahola J. Involvement of delta-opioid receptors in the effects induced by endogenous enkephalins on learned helplessness model. Eur J Pharmacol. 1998;354:1–7. doi: 10.1016/s0014-2999(98)00423-3. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Isgor C, Folk JE, Rice KC, Watson SJ, Woods JH. The delta-opioid receptor agonist (+)BW373U86 regulates BDNF mRNA expression in rats. Neuropsychopharmacology. 2004;29:649–659. doi: 10.1038/sj.npp.1300345. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Folk JE, Rice KC, Watson SJ, Woods JH. Chronic administration of the delta opioid receptor agonist (+)BW373U86 and antidepressants on behavior in the forced swim test and BDNF mRNA expression in rats. Psychopharmacology Berl. 2005;183:31–40. doi: 10.1007/s00213-005-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Jutkiewicz EM, Mosberg HI, Balboni G, Watson SJ, Woods JH. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res. 2006;1069:172–181. doi: 10.1016/j.brainres.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- Ventriglia M, Zanardini R, Pedrini L, Placentino A, Nielsen MG, Gennarelli M, Bocchio-Chiavetto L. VEGF serum levels in depressed patients during SSRI antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:146–149. doi: 10.1016/j.pnpbp.2008.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.