Abstract
Introduction
While women and girls are disproportionately at risk of HIV acquisition, particularly in low- and middle-income countries (LMIC), globally men and women comprise similar proportions of people living with HIV who are eligible for antiretroviral therapy. However, men represent only approximately 41% of those receiving antiretroviral therapy globally. There has been limited study of men’s outcomes in treatment programmes, despite data suggesting that men living with HIV and engaged in treatment programmes have higher mortality rates. This systematic review (SR) and meta-analysis (MA) aims to assess differential all-cause mortality between men and women living with HIV and on antiretroviral therapy in LMIC.
Methods
A SR was conducted through searching PubMed, Ovid Global Health and EMBASE for peer-reviewed, published observational studies reporting differential outcomes by sex of adults (≥15 years) living with HIV, in treatment programmes and on antiretroviral medications in LMIC. For studies reporting hazard ratios (HRs) of mortality by sex, quality assessment using Newcastle–Ottawa Scale (cohort studies) and an MA using a random-effects model (Stata 14.0) were conducted.
Results
A total of 11,889 records were screened, and 6726 full-text articles were assessed for eligibility. There were 31 included studies in the final MA reporting 42 HRs, with a total sample size of 86,233 men and 117,719 women, and total time on antiretroviral therapy of 1555 months. The pooled hazard ratio (pHR) showed a 46% increased hazard of death for men while on antiretroviral treatment (1.35–1.59). Increased hazard was significant across geographic regions (sub-Saharan Africa: pHR 1.41 (1.28–1.56); Asia: 1.77 (1.42–2.21)) and persisted over time on treatment (≤12 months: 1.42 (1.21–1.67); 13–35 months: 1.48 (1.23–1.78); 36–59 months: 1.50 (1.18–1.91); 61 to 108 months: 1.49 (1.29–1.71)).
Conclusions
Men living with HIV have consistently and significantly greater hazards of all-cause mortality compared with women while on antiretroviral therapy in LMIC. This effect persists over time on treatment. The clinical and population-level prevention benefits of antiretroviral therapy will only be realized if programmes can improve male engagement, diagnosis, earlier initiation of therapy, clinical outcomes and can support long-term adherence and retention.
Keywords: treatment, retention, all-cause mortality, men, males, gender, HIV/AIDS, developing countries
Introduction
In 2015, some 37 million people worldwide were living with HIV. The past decade has seen a dramatic scale-up in antiretroviral treatment (ART) in low- and middle-income countries (LMIC). Nearly 12 million people in LMIC were on ART in 2013, a 30-fold increase from the 400,000 people on treatment in 2003 [1–3]. Recent trials have clearly demonstrated both the clinical and preventive benefits of early and sustained treatment [4,5], yet 22 million men, women and children living with HIV remain untreated, and more than 2 million HIV-associated deaths are estimated to occur annually [6]. Undiagnosed, untreated or insufficiently treated HIV infection remains an enormous global health challenge, and one for which tailored interventions are likely to be required, including interventions relevant for adult men living with HIV.
In the aggregate of all LMIC, men and women comprise similar proportions of adults living with HIV who are eligible for ART (49 and 51%, respectively). However, adult men represent only 41% of those receiving ART [2]. This trend is observed in most regions of the world and is most evident in the WHO African Region where men make up 44% of those living with HIV but only 36% of those receiving ART [2].
Attention towards sex differences in the global HIV pandemic has predominantly focused on the vulnerabilities – behavioural, biomedical and structural [7] – experienced by women and girls. In contrast, there has been relatively limited investigation on why men are less likely to enrol and to be retained in ART programmes, and why they have had higher HIV-associated mortality in many reports [8]. Although there is growing and essential attention to men in research on vulnerable populations (who have sex with men, transgender populations and people who use drugs), there remains a policy and programme “blind spot” regarding men and HIV outcomes in sub-Saharan Africa [9] and globally. A number of studies have suggested that male enrolees have higher mortality rates than females in HIV treatment studies [10]. Differential treatment outcomes by sex need to be understood to better design and specify interventions for men living with HIV.
The aim of this review is to elucidate sex differentials in mortality between men and women living with HIV and on ART. This review, in particular, focuses on all-cause mortality as an important treatment outcome.
Methods
A systematic review (SR) and meta-analysis (MA) were conducted to assess differences in all-cause mortality between adult men and women living with HIV and on ART in LMIC. We used the Cochrane Group approach [11], following PRISMA guidelines [12].
Inclusion and exclusion criteria
Study design
Any observational study design.
Populations
Male and female adults (age ≥15 years); in LMIC (World Bank 2012 definition) [13].
Exposures
On or initiated ART at the beginning of follow-up.
Outcomes
All-cause mortality, with data disaggregated by sex, even if sex differences and mortality were not the primary outcome.
Publication
Published in peer-reviewed journals from 1 January 2008 to 13 December 2013.
Language
Any language.
Exclusion
Total sample size <50 men or <50 women; or article type was a cost-effectiveness study, modelling, grey literature or literature review.
Search strategy
Electronic searches of PubMed, EMBASE and Ovid Global Health were performed on 5 December 2013. With the assistance of an information specialist, the following medical subject heading (MeSH) terms were developed along with the LMIC terms for the PubMed search query, and similar terms were used as keywords for EMBASE and Ovid Global Health: “Anti-Retroviral Agents” OR “Antiretroviral Therapy, Highly Active,” which were together cross-referenced with the keywords “HIV” OR “AIDS” OR “HIV Infections” along with NOT queries for “Pediatrics” and “Children.” See the Supplementary material for the complete PubMed search term.
Study selection and management of results
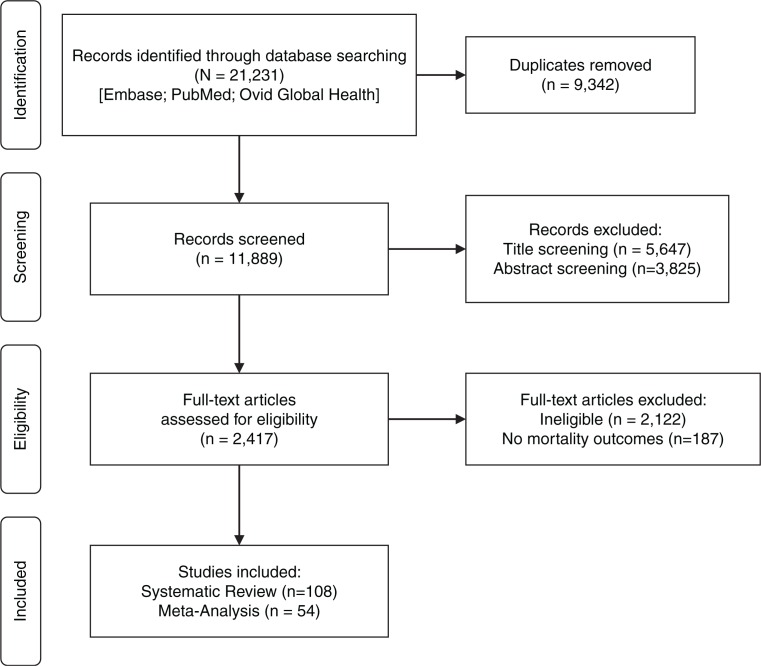
References obtained from three databases (n=21,231) were exported and duplicates were removed, leaving 11,889 citations. Reviewers and several research assistants independently conducted title screening (5604 records excluded), abstract screening (3825 excluded) and full-text review (6285 reviewed and 2352 excluded). Data were abstracted and collated through a Microsoft Access database that captured study information and mortality outcomes differentiated by sex. Title/abstract screening, full-text review and data abstraction were conducted in duplicate (in pairs by PL, SWB, KD, SP, EC, AG, WE and RM) and a third reviewer (SWB) resolved conflicts. This was conducted as part of a larger SR that abstracted data on multiple baseline factors (age, CD4 count and WHO stage) and outcomes (e.g. mortality, viral load (VL), CD4 count and opportunistic infections) (n=295 articles with such outcomes disaggregated by sex). For this analysis, all records with any mortality data disaggregated by sex were included (n=108 records) (see Figure 1).
Figure 1.
PRISMA flow diagram.
Statistical analyses
Using Stata 14.0 (College Station, TX, USA), a random-effects MA was conducted on reported hazard ratios (HRs) between men and women for all-cause mortality, using the metan command. Reciprocals of HRs were calculated as necessary such that all HRs used women as the reference group. I2 test statistics were calculated to assess heterogeneity, and an a priori cutoff of I2 values above 80% was chosen [11]. To assess the potential for publication bias, a funnel plot and p-value were generated using Stata (metafunnel and metabias commands with the Egger option and using standard error).
Sensitivity and subgroup analyses
To address possible methodological and clinical heterogeneity [11], several decisions were made. Cohort and case–control studies are systematically different and not recommended to pool in MA; thus, case–control studies were excluded (n=1), as were cross-sectional studies (n=1). Studies reporting only crude mortality (n=17), odds ratios (n=8) or relative risk ratios (n=4) rather than HRs were likewise excluded [11]. Some studies reported HRs, but combined mortality with losses to follow-up in one measure, so were excluded from analysis (n=4). Articles were assessed for use of data from the same cohorts. Several potential overlaps were identified and the studies with larger sample sizes and longer follow-up times were retained, while 16 studies were dropped from the MA. See Supplementary Table 2 for a complete list of the original 108 studies, which were included in or excluded from the final MA, with reasons.
Subgroup analysis
Several subgroups were predetermined and reported separately (geography, time on ART and population characteristics).
Geography
The studies were grouped by geographic regions (sub-Saharan Africa (SSA), Asia, Latin American and the Caribbean (LAC), and studies that cover multiple regions). The one study from Eastern Europe/central Asia (Georgia) [14] was grouped with Asia. Pooled HRs (pHRs) were calculated overall and for each subgroup. Because there was only one study from LAC, it was not reported as a separate subgroup, but did contribute to the overall pooled effect size. Because there were a large number of studies from SSA, these were further subgrouped into geographic regions [15]: East (Ethiopia, Kenya, Malawi, Rwanda, Tanzania and Uganda); West/Central (Burkina Faso, Cameroon, Côte d’Ivoire, Gambia, Nigeria and Senegal); Southern (Botswana, Lesotho, Mozambique, South Africa, Zambia and Zimbabwe); and studies that cross multiple subregions (e.g. one study covering Central African Republic, Côte d’Ivoire, Democratic Republic of the Congo, Ethiopia and Niger).
Time on ART
Time on ART was divided into quartiles of months since initiation of ART (0–12, 13–35, 36–59 and 60–108 months). For studies that reported more than one HR with time overlaps (e.g. 0–3 and 0–12 months), the shorter time period was dropped. For studies that reported more than one HR without time overlaps (e.g. 0–12 and 13–24 months), all HRs were used.
Population characteristics
Certain features of the populations that may affect analyses and interpretation or increase heterogeneity were noted (e.g. tuberculosis co-infection, ART naïve or experienced and people who inject drugs (PWID)). The one study of tuberculosis co-infected patients [16] and one with the most immunosuppressed patients (CD4 count <50/mL) [17] were excluded. The proportion of HIV infected who were PWID were noted, when reported. Some mortality among PWID may be attributable to drug overdose, and men are more likely to inject drugs than women [18], thus possibly confounding the results. There were four studies in Asia where the proportion of reported PWID was ≥20%. These are subgrouped in MA and reported separately from the rest of the countries in Asia.
Quality assessment
To assess the risk of bias, the Newcastle–Ottawa Quality Assessment Scale (NOS) was used [19]. This scale is used to assess the quality of observational studies in meta-analyses. For this article, the scale was used to rate all studies that reported HRs of mortality comparing men and women. As all studies reporting HRs were cohort studies, the NOS Coding Manual for Cohort Studies was employed. For each study, this scale addressed selection bias, comparability bias and outcome bias. Studies were awarded stars when they satisfied a requirement on the scale, with a maximum of nine stars. To calibrate scoring and minimize rating bias, two reviewers (including the first author) independently and dually applied the NOS to six randomly selected studies (10%). Once we aligned the scores, one reviewer applied the scale to the remaining studies, which were then checked by the first author. Studies that earned 1 to 3 stars were considered “low” quality; 4 to 6 stars “moderate”; and 7 to 9 stars were considered “high” quality.
The NOS requires that decisions be made to fit the particular analysis. The following decisions were discussed by authors and applied. For selection, (1) the exposure is sex, for example, males are exposed and females are non-exposed; (2) the community is people living with HIV and on ART. Thus, studies received a star for representativeness if the exposed cohort was a random sample of those in care and on ART, and another star if the females came from the same care centres as the males. Ascertainment of exposure (sex) received a star if the study drew this information from medical records. The NOS provides guidance for defining the outcome of interest in mortality studies and suggests using the presence of disease/incident, rather than death, as the outcome of interest. In this case, “known to be living with HIV” was used as the baseline disease and earned a star. As being on ART was an inclusion criterion, it is assumed that all were seropositive by biological measures (e.g. not self-reported living with HIV).
To assess comparability of cohorts, adjustment for factors that could be related to both the exposure (sex) and the outcome (all-cause mortality) were considered important. One star was awarded if the study controlled for age (first important factor) and a second star if the study also controlled for two or more variables from at least two distinct categories (see Supplementary Table 1 for a complete list of variables adjusted for in each study):
Category 1: CD4 count, VL and WHO stage
Category 2: Weight, body mass index (BMI) and mid-upper arm circumference (MUAC)
Category 3: Comorbidities (including previous or current tuberculosis)
Category 4: Adherence
Category 5: Risk behaviours (i.e. drug use)
To assess outcome bias, the method of assessing the outcome (mortality) earned a star if the information came from medical records and/or from confirmation by a health worker. The appropriateness of the follow-up time was defined as at least three months of follow-up. The adequacy of follow-up of cohorts was awarded a star if there was <15% loss to follow-up (LTFU); a higher percentage could be awarded a star if a description and comparison of those LTFU was included, for example, it was evaluated as an outcome.
To test for effects of study quality on pooled effect size, sensitivity analyses were run (see Table 4) on six models: (1) all studies that reported HRs of mortality, (2) only studies that earned a high-quality rating (7–9 stars), (3) only studies that had LTFU rates of <18% and (4) only studies that LTFU rates of <15%. Additional sensitivity analyses were run further on Model 4 to exclude studies that did not adjust for age (n=2) (Model 5) and exclude studies that were outliers in the funnel plot (see Supplementary Figure 1) and thus presented potential publication bias of extreme results (n=2) (Model 6).
Table 4.
Sensitivity analyses results
| All LMIC | Male (n) | Female (n) | ≤12a | df | I 2 (%) | 13–35a | df | I 2 (%) | 36–59a | df | I 2 (%) | 60–108a | df | I 2 (%) | Overall | df | I 2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | 186,452 | 283,811 |
1.42 (1.25–1.63)
p=0.002 |
15 | 57.5 |
1.39 (1.23–1.57)
p=0.000 |
13 | 70.1 |
1.36 (1.22–1.52)
p=0.000 |
14 | 66.7 |
1.62 (1.46–1.80)
p=0.000 |
21 | 74.8 |
1.46 (1.38–1.56)
p=0.000 |
66 | 75.2 |
| Model 2 | 175,554 | 266,621 |
1.42 (1.25–1.63)
p=0.002 |
15 | 57.5 |
1.40 (1.38–1.56)
p=0.000 |
12 | 72.3 |
1.37 (1.22–1.53)
p=0.000 |
13 | 69.1 |
1.62 (1.46–1.80)
p=0.000 |
21 | 74.8 |
1.47 (1.38–1.56)
p=0.000 |
64 | 75.9 |
| Model 3 | 119,470 | 168,465 |
1.42 (1.25–1.63)
p=0.002 |
15 | 57.5 |
1.43 (1.24–1.64)
p=0.000 |
11 | 70.8 |
1.46 (1.21–1.75)
p=0.000 |
10 | 72.3 |
1.49 (1.35–1.54)
p=0.006 |
14 | 54.2 |
1.44 (1.35–1.54)
p=0.000 |
53 | 62.7 |
| Model 4 | 86,233 | 117,719 |
1.42 (1.21–1.67)
p=0.002 |
12 | 60.7 |
1.48 (1.23–1.78)
p=0.027 |
5 | 60.4 |
1.50 (1.18–1.91)
p=0.000 |
8 | 77.8 |
1.49 (1.29–1.71)
p=0.005 |
13 | 56.8 |
1.46 (1.35–1.59)
p=0.000 |
41 | 63.4 |
| Model 5 | 85,552 | 116,994 |
1.44 (1.22–1.71)
p=0.000 |
11 | 63.0 |
1.48 (1.23–1.78)
p=0.000 |
5 | 60.4 |
1.54 (1.18–2.02)
p=0.002 |
7 | 80.6 |
1.49 (1.29–1.71)
p=0.000 |
13 | 56.8 |
1.47 (1.35–1.60)
p=0.000 |
39 | 64.8 |
| Model 6 | 85,431 | 116,756 |
1.37 (1.20–1.56)
p=0.000 |
10 | 43.9 |
1.48 (1.23–1.78)
p=0.000 |
5 | 60.4 |
1.54 (1.18–2.02)
p=0.002 |
7 | 80.6 |
1.43 (1.27–1.61)
p=0.000 |
12 | 42.2 |
1.43 (1.33–1.55)
p=0.000 |
37 | 58.9 |
df, degrees of freedom; LTFU, lost to follow-up; HR, hazard ratio.
Model 1: all HRs.
Model 2: high-quality studies only (7–9 stars).
Model 3: HRs with LTFU <18%.
Model 4: HRs with LTFU <15% (all also scored high on the NOS).
Model 5: HRs with LTFU <15% and adjusted for age (excludes n=2 HRs).
Model 6: HRs with LTFU <15%, adjusted for age, and accounts for potential publication extremes on the funnel plot (excludes two additional HRs beyond Model 5).
Months since initiation of ART
Results
There were 108 studies included in the SR of sex differences in mortality on ART in LMIC. The characteristics of the included studies are in Table 1. Most studies were from sub-Saharan Africa, were cohort studies of ART-naïve patients followed from initiation of ART and were among general population, reproductive age adults. The median follow-up time was 36 months (mean=42 months). The studies included a total of 319,677 men and 466,822 women.
Table 1.
Characteristics of included studies
| First author, year; country; study designa | Population (adults (age ≥15 years) on ART) and setting | Timeb | Male (n) | Female (n) | References |
|---|---|---|---|---|---|
| Africa | |||||
| Abaasa, 2008; Uganda; R | ART naïve; urban clinic | 31.2 | 222 | 675 | [20] |
| Ahonkhai, 2012; South Africa; R | ART naïve; urban and rural clinics and hospitals | 60 | 3818 | 7579 | [21] |
| Alamo, 2012; Uganda; R | ART experienced and naïve; urban clinics and home-based care | 120 | 237 | 342 | [22] |
| Alemu, 2010; Ethiopia; R | ART naïve; rural hospital | 24 | 117 | 155 | [23] |
| Alibhai, 2010; Uganda; P | ART naïve; rural community- and hospital-based clinics | 5.5 | 163 | 222 | [24] |
| Balcha, 2010; Ethiopia; R | ART naïve; urban and rural clinics and hospitals | 24 | 703 | 1006 | [25] |
| Bastard, 2011; Senegal; P | ART naïve; urban hospital | 108 | 146 | 184 | [26] |
| Biadgilign, 2012; Ethiopia; R | ART naïve; hospitals | 60 | 574 | 963 | [27] |
| Birbeck, 2011; Zambia; P | ART naïve; rural clinics | 24 | 205 | 291 | [28] |
| Bisson, 2008; Botswana; R | ART naïve; urban clinic | 12 | 166 | 244 | [29] |
| Boyles, 2011; South Africa; P | ART naïve; rural hospital and clinics | 48 | 563 | 1231 | [30] |
| Brennan, 2013; South Africa; R | ART naïve, non-pregnant; urban clinic | 48 | 5770 | 9162 | [31] |
| Brinkhof, 2009, 4 countriesc; R | ART naïve; urban clinics | 24 | 4418 | 8831 | [32] |
| Chalamilla, 2012; Tanzania; P | ART naïve; urban clinics | 36 | 4383 | 8459 | [33] |
| Chen, 2008; Malawi; R | ART naïve, urban hospital | 30 | 1122 | 1716 | [34] |
| Chi, 2009; Zambia; P | ART ≥12 months; urban clinics | 36 | 10,226 | 16,889 | [35] |
| Chi, 2010; Zambia; R | ART naïve; urban clinics | 30 | 4618 | 5867 | [36] |
| Chu, 2010; Malawi; R | ART naïve; rural hospital | 13 | 4369 | 6753 | [37] |
| Cornell, 2009; South Africa; P | ART naïve; urban clinic | 12 | 717 | 1479 | [38] |
| Cornell, 2012; South Africa; R | ART naïve; urban and rural clinics and hospitals | 84 | 16,108 | 30,093 | [39] |
| Dalal, 2008; South Africa; R | Patients LTFU ≥6 weeks; urban clinic | 15 | 554 | 1077 | [40] |
| De Beaudrap, 2008; Senegal; P | 95% ART naïve, 5% experienced; urban multiple settings (ANRS-1215) | 84 | 183 | 221 | [41] |
| De Luca, 2012; Guinea, Malawi, Mozambique; P | ART naïve; urban and rural clinics | 40 | 954 | 1558 | [42] |
| Deribe, 2013; Ethiopia; CC | ART experienced, TB co-infected; urban hospital | 48 | 124 | 149 | [43] |
| Desilva, 2009; Nigeria; R | ART naïve; urban clinic | 24 | 452 | 1100 | [44] |
| Ekouevi, 2010; 5 countriesd; P | ART naïve; urban clinics | 12 | 5542 | 8810 | [45] |
| Evans, 2012; South Africa; R | ART naïve; urban clinic | 12 | 3205 | 5204 | [46] |
| Fatti, 2010; South Africa; R | ART naïve urban and rural clinics and hospitals | 36 | 9317 | 19,886 | [47] |
| Ford, 2010; Lesotho; P | ART naïve; urban and rural clinics and hospitals | 24 | 400 | 801 | [48] |
| Fox, 2010; South Africa; R | ART naïve; urban clinic | 48 | 2229 | 3976 | [49] |
| Fox, 2012; South Africa; R | ART naïve, ≥6 months follow-up; urban/rural hospitals/clinics | 96 | 6665 | 12,980 | [50] |
| Franke, 2011; Rwanda; R | ART-naïve CD4 ≥350, with TB; urban/rural hospitals/clinics | 24 | 121 | 187 | [16] |
| Geng, 2010; Uganda; P | ART naïve, plus LTFU; rural clinic | 45 | 1415 | 2213 | [51] |
| Geng, 2010; Uganda; P | ART naïve, plus LTFU; rural clinic | 45 | 1415 | 2213 | [52] |
| Greig, 2012; 9 countriese; R | ART naïve; urban and rural clinics and hospitals | 24 | 6201 | 11,360 | [53] |
| Hawkins, 2011; Tanzania; P | ART naïve; urban clinics | 33 | 4383 | 8459 | [54] |
| Hermans, 2012; Uganda; R | ART naïve; urban university-based clinic | 12 | 2730 | 4929 | [55] |
| Hoffmann, 2010; South Africa; P | ART naïve; community- and workplace-based clinics | 12 | 8696 | 5401 | [56] |
| Hoffmann, 2011; South Africa; P | ART naïve with ≥4 years follow-up; national clinics | 48 | 9605 | 5455 | [57] |
| Johannessen, 2008; Tanzania; P | ART naïve; rural hospital | 36 | 97 | 223 | [58] |
| Karstaedt, 2012; South Africa; R | ART naïve; urban hospital | 56 | 1030 | 1608 | [59] |
| Kassa, 2012; Ethiopia; R | ART naïve; urban hospital | 60 | 1737 | 2473 | [60] |
| Kebebew, 2012; Ethiopia; R | ART naïve; military personnel; urban hospital | 12 | 548 | 186 | [61] |
| Kipp, 2012; Uganda; P | ART naïve; community- and hospital-based, urban and rural patients | 24 | 163 | 222 | [62] |
| Kouanda, 2012; Burkina Faso; R | ART naïve; urban and rural clinics | 70 | 1682 | 3926 | [63] |
| Lowrance, 2009; Rwanda; R | ART naïve; urban and rural clinics and hospitals | 12 | 1123 | 2071 | [64] |
| MacPherson, 2009; South Africa; R | ART naïve; rural clinics | 24 | 446 | 907 | [65] |
| Mageda, 2012; Tanzania; R | ART naïve; ≥12 months follow-up; urban and rural clinics and hospitals | 60 | 226 | 320 | [66] |
| Maman, 2012a; Malawi, Uganda, Kenya; R | ART ≥9 months and >1 CD4 count thereafter; urban/rural clinics | 80 | 4068 | 8878 | [67] |
| Maman, 2012b; Malawi, Uganda, Kenya; R | ART ≥9 months; urban/rural clinics | 60 | 7682 | 16,355 | [68] |
| Maman, 2012c; Malawi; P | ART naïve; rural clinic | 12 | 235 | 338 | [69] |
| Maskew, 2012; South Africa; R | ART naïve; urban clinic | 24 | 3491 | 5648 | [70] |
| Maskew, 2013; South Africa; R | ART naïve, non-pregnant; urban clinic | 36 | 2733 | 4621 | [71] |
| Massaquoi, 2009; Malawi; R | ART naïve; rural clinics/hospital | 14 | 1449 | 2625 | [72] |
| Moore, 2011; Uganda; P | ART naïve in home-based care; rural clinic/home-based care | 60 | 306 | 826 | [73] |
| Mossdorf, 2011; Tanzania; P | ART naïve; rural hospital; 7.6% with TB | 12 | 518 | 945 | [74] |
| Mosha, 2013; Tanzania; P | ART naïve; urban hospital | 12 | 70 | 164 | [75] |
| Mujugira, 2009; Botswana; R | ART naïve, CD4 <50; urban hospital | 12 | 144 | 205 | [17] |
| Murphy, 2010; South Africa; P | ART experienced patients with virologic failure; urban clinics | 5.5 | 70 | 71 | [76] |
| Mutevedzi, 2010; South Africa; P | ART naïve; urban/rural hospital/clinics | 12 | 1836 | 3883 | [77] |
| Mutevedzi, 2011; South Africa; R | ART naïve; urban/rural hospital/clinics | 24 | 3011 | 5835 | [78] |
| Mzileni, 2008; South Africa; P | ART naïve; urban hospital | 18 | 1002 | 2071 | [79] |
| Negin, 2011; Malawi; R | ART naïve; ≥25 years old; mixed settings | 60 | 4099 | 6789 | [80] |
| Nglazi, 2011; South Africa; P | ART naïve; semi-urban clinics | 84 | 1046 | 2116 | [81] |
| Odafe, 2012; Nigeria; R | ART naïve; non-pregnant; hospitals | 36 | 1945 | 2840 | [82] |
| Ojikutu, 2008; South Africa; R | ART naïve and experienced; urban clinic | 62 | 132 | 174 | [83] |
| Palombi, 2009; Guinea, Malawi, Mozambique; R | ART naïve; urban clinics | 42 | 1800 | 2325 | [84] |
| Palombi, 2010; Mozambique; P | ART naïve with ≥2 CD4 counts; urban and rural clinics | 3 | 312 | 441 | [85] |
| Peltzer, 2011; South Africa; P | ART naïve; urban and rural hospitals | 12 | 217 | 518 | [86] |
| Peterson, 2011, Gambia; P | ART naïve; urban hospital | 36 | 121 | 238 | [87] |
| Poka-Mayap, 2013; Cameroon; R | ART naïve; urban clinic | 60 | 617 | 827 | [88] |
| Rougemont, 2009; Cameroon; P | ART naïve; urban hospital | 6 | 106 | 198 | [89] |
| Russell, 2010; South Africa; P | ART naïve; urban clinics | 9 | 542 | 808 | [90] |
| Schoni-Affolter, 2011; Zambia; P | ART naïve; multiple sites; these data restricted to Zambia cohort | 41 | 34,907 | 54,432 | [91] |
| Sieleunou, 2009; Cameroon; R | ART naïve; rural hospital | 60 | 660 | 527 | [92] |
| Siika, 2010; Kenya; CC | ART experienced; randomly selected deceased patients matched 1:2 to living; urban/rural clinics/hospitals | 69 | 613 | 968 | [93] |
| Somi, 2012; Tanzania; R | ART naïve; national clinics | 60 | 29,869 | 59,006 | [94] |
| Steele, 2011; Botswana; P | ART naïve; urban clinic | 6 | 152 | 250 | [95] |
| Sunpath, 2012; South Africa; P | ART naïve patients with TB or other OIs; urban hospital | 5.5 | 198 | 184 | [96] |
| Taylor-Smith, 2010; Malawi; R | ART experienced, some naïve; HCWs, teachers, policy/army personnel; urban/rural hospitals/clinics | 36 | 2346 | 2324 | [97] |
| Toure, 2008; Côte d’Ivoire; P | ART naïve; mixed sites | 32 | 3024 | 7187 | [98] |
| Van Cutsem, 2011; South Africa; P | ART naïve; semi-urban clinics | 24 | 2067 | 4344 | [99] |
| Wandeler, 2012; Lesotho, Mozambique, Zimbabwe; P | ART ≥3 months; rural hospitals and clinics | 36 | 2707 | 5018 | [100] |
| Weigel, 2011; Malawi; CS | Patients LTFU ≥2 weeks; urban clinic | na | 308 | 351 | [101] |
| Wubshet, 2012; Ethiopia; R | ART naïve, non-pregnant; urban hospital | 66 | 1349 | 1663 | [102] |
| Zachariah, 2009; Malawi; R | ART naïve; rural clinics | 3 | 706 | 1610 | [103] |
| Eastern Europe/central Asia | |||||
| Tsertsvadze, 2011; Georgia; R | ART naïve; 60% drug users; national mixed settings | 60 | 570 | 182 | [14] |
| Latin American and Caribbean countries | |||||
| Wolff, 2010; Chile; P | ART naïve; transmission 2% drug use, >50% homosexual; mixed sites | 84 | 4297 | 818 | [104] |
| Asia | |||||
| Alvarez-Uria, 2013; India; R | ART experience unclear; drug use NR; rural hospitals | 60 | 1876 | 1283 | [105] |
| Argemi, 2012; Cambodia; R | ART naïve; drug use NR; rural clinic | 56 | 498 | 504 | [106] |
| Bastard, 2013; Laos; R | ART naïve; non-pregnant; urban hospital; drug use NR | 60 | 507 | 405 | [107] |
| Bhowmik, 2012; India; R | ART naïve; drug use NR; urban tertiary hospital | 12 | 502 | 254 | [108] |
| Chen, 2013; China; R | ART ≥3 months, 40% infected through drug use; rural/semirural clinics | 14 | 1211 | 756 | [109] |
| Dou, 2011; China; R | ART naïve, 20% infected through drug use; national ART database | 24 | 2047 | 1410 | [110] |
| Fregonese, 2012; Thailand; P | ART ≥3 months, past-PMTCT included; drug use NR; urban/rural hospitals | 60 | 408 | 1170 | [111] |
| Kumarasamy, 2008; India; P | ART ≥12 months; drug use NR; urban tertiary HIV centre | 12 | 1512 | 460 | [112] |
| Limmahakhun, 2012; Thailand; R | ART naïve and experienced with TB; drug use NR; urban hospital | 120 | 100 | 71 | [113] |
| Rai, 2013; India; R | ART naïve; drug use NR; urban clinic | 36 | 182 | 57 | [114] |
| Sabapathy, 2012; Burma; P | ART naïve; drug use NR; rural clinics | 60 | 3656 | 2307 | [115] |
| Susaengrat, 2011; Thailand; R | ART naïve; 88% heterosexual transmission (drug use NR); urban hospital | 45 | 548 | 438 | [116] |
| Thai, 2009, Cambodia; P | ART naïve; drug use NR; urban hospital | 57 | 824 | 843 | [117] |
| Tran, 2013; Vietnam; P | ART naïve; 65% infected through drug use; urban and rural clinics | 6 | 2573 | 867 | [118] |
| van Griensven, 2011; Cambodia; R | ART naïve; drug use NR; urban tertiary hospital | 60 | 1333 | 1507 | [119] |
| Zhang, 2008; China; R | ART naïve former plasma donors; data here are restricted to those on ART; mixed sites, national database | 144 | 1302 | 1402 | [120] |
| Zhang, 2009; China; P | ART naïve; 37.8% history of drug use; national database | 60 | 28,320 | 20,441 | [121] |
| Zhang, 2012; China; R | ART ≥7.5 months; 22% infected through drug use; national database | 42 | 16,821 | 10,669 | [122] |
| Multi-region studies | |||||
| Brinkhof, 2008; 11 countriesf; P | ART naïve, drug use NR; multiple sites | 12 | 2972 | 2519 | [123] |
| Wandel, 2008; 3 countriesg; R | ART naive; drug use NR; multiple sites | 330 | 1272 | 800 | [124] |
ART, antiretroviral therapy; NR, not reported; na, not applicable; TB, tuberculosis; CD4, CD4+ cells/mL; OIs, opportunistic infections; LTFU, lost to follow-up; HCW, healthcare worker; PMTCT, prevention of mother-to-child transmission
study design: P, prospective cohort; R, retrospective cohort; CS, cross-sectional; CC, case–control
time in months since initiation of ART
Côte d’Ivoire, Malawi, South Africa, Zimbabwe
Benin, Côte d’Ivoire, Gambia, Mali, Senegal
Central African Republic, Côte d’Ivoire, Democratic Republic of the Congo, Ethiopia, Nigeria, Republic of Congo, Uganda, Zambia, Zimbabwe
Morocco, Botswana, Malawi, South Africa, Kenya, Côte d’Ivoire, Nigeria, Senegal, Brazil, India, Thailand
Uganda, Côte d’Ivoire, Thailand.
Table 2 shows the mortality outcomes reported by the individual studies. Some studies reported only crude mortality, while others reported mortality per person-year and a majority reported a mortality ratio (odds ratio, relative risk or HR) comparing men and women. Significant findings are indicated in bold. Without exception, significant findings show worse mortality outcomes for men compared with women.
Table 2.
All-cause mortality among adults on ART by sex
| Crude n (%) | Rate/100 py | cHR (95% CI), females | aHR (95% CI), females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| First author, date, country [Ref.] | Males | Females | Males | Females | Males | Females | Males | Females | Timeb |
| Africa | |||||||||
| Abaasa, 2008, Uganda [20] | 34 (15.3) | 130 (19.3) | 10.2 | 12.6 | 1.26 (0.86–1.83) | Ref | 1.48 (0.98–2.17) | Ref | 31.2 |
| Ahonkhai, 2012, South Africa [21] | 372 (9.7) | 616 (8.1) | – | – | Ref | OR 0.86 (0.75–0.99) | – | – | 6.9 |
| Alamo, 2012, Uganda [22] | 29 (12.2) | 37 (10.8) | – | – | – | – | – | – | 10 |
| Alemu, 2010, Ethiopia [23] | – | – | – | – | 1.22 (0.58–2.56) | Ref | – | – | 24 |
| Alibhai, 2010, Uganda [24] | 22 (13.5) | 20 (9.0) | – | – | – | – | – | – | 5.6 |
| Balcha, 2010, Ethiopia [25] | 78 (11.1) | 97 (9.6) | – | – | – | – | – | – | 24 |
| Bastard, 2011, Senegal [26] | – | – | – | – | – | – | Ref | 0.47 (0.26–0.84) | 108 |
| Biadgilign, 2012, Ethiopia [27] | – | – | – | – | Ref | 1.05 (0.68–1.64) | Ref | 1.16 (0.68–2.00) | 60 |
| Birbeck, 2011, Zambia [28] | 36 (17.6) | 65 (22.5) | – | – | OR 0.72 (0.47–1.15) | Ref | – | – | 24 |
| Bisson, 2008, Botswana [29] | 36 (21.7), p =0.03 | 33 (15.0) | – | – | – | – | 1.74 (1.05–2.87)c | Ref | 12 |
| Boyles, 2011, South Africa [30] | – | – | – | – | – | – | Ref | 1.20 (0.87–1.66) | 48 |
| Brennan, 2013, South Africa [31] | 906 (9.9) | 1079 (18.7) | – | – | RR 1.40 (1.30–1.60) | Ref | RR 1.0 (0.90–1.10) | Ref | 84 |
| Brinkhof, 2009, 4 countries [32] | – | – | 10.24 (9.66)d | 6.98 (6.69)d | – | – | Ref | 0.84 (0.71–0.99) | 24 |
| Chalamilla, 2012, Tanzania [33] | – | – | – | – | – | – |
1.19 (1.06–1.34),
p
<0.001
1.19 (1.07–1.32), p <0.001 |
Ref Ref |
<3 36 |
| Chen, 2008, Malawi [34] | 188 (16.8), p <0.0001 | 188 (11.0) | – | – | – | – | 1.70 (1.35–2.15) | Ref | 30 |
| Chi, 2009, Zambia [35] | – | – | – | – | 1.40 (1.20–1.60) | Ref | 1.30 (1.10–1.50) | Ref | 12–36 |
| Chi, 2010, Zambia [36] | – | – | – | – | – | – | 1.82 (1.47–2.26) | Ref | 30 |
| Chu, 2010, Malawi [37] | 175 (0.13), KS | 77 (22.3), KS | – | – | 0.98 (0.73–1.31), KS | Ref | – | – | 13 |
| Cornell, 2009, South Africa [38] | 51 (7.0) 14 (3.0) |
59 (4.0) 32 (3.0) |
22.8,
p
=0.002
3.5 |
12.5
3.8 |
1.83 (1.26–2.26),
p
=0.02
0.98 (0.52–1.84) |
Ref Ref |
1.46 (0.96–2.22) 0.70 (0.37–1.34) |
Ref Ref |
0–4 4–12 |
| Cornell, 2012, South Africa [39] | – | – | – | – |
1.28 (1.09–1.51) 1.63 (1.37–1.94) 1.62 (1.22–2.14) 1.71 (1.08–2.70) 1.46 (1.37–1.56) |
Ref Ref Ref Ref Ref |
1.10 (0.93–1.31) 1.36 (1.05–1.78) 1.39 (0.94–2.06) 1.35 (0.76–2.38) 1.31 (1.22–1.41) |
Ref Ref Ref Ref Ref |
0–12 12–24 24–36 36–84 0–84 |
| Dalal, 2008, South Africa [40] | 31 (5.6)c | 52 (4.8)c | – | – | – | – | – | – | 15 |
| De Beaudrap, 2008, Senegal [41] | – | – | – | – | Ref | 1.03 (0.50–2.15) 0.69 (0.42–1.16) |
– | – | 0–6 6–84 |
| De Luca, 2012, 3 countries [42] | – | – | – | – | Ref | 0.60 (0.40–0.90), p =0.015 | Ref | 0.57 (0.36–0.90), p =0.017 | 6–48 |
| Deribe, 2013, Ethiopia [43] | 40 (32.3), TB | 28 (18.8), TB | – | – | OR 2.06 (1.18–3.59), TB | Ref | OR 2.04 (1.04–4.02), p =0.039, TB | Ref | 48 |
| Desilva, 2009, Nigeria [44] | – | – | – | – | – | – | 1.76 (1.14–2.72), p =0.0114 | Ref | 24 |
| Ekouevi, 2010, 5 countries [45] | 1.14 (1.08–1.21), p =0.0004e | Ref | 1.16 (1.10–1.24), p =0.0002e | Ref | 12 | ||||
| Evans, 2012, South Africa [46] | – | – | – | – | 1.17 (0.99–1.37) | Ref | – | – | 12 |
| Fatti, 2010, South Africa [47] | – | – | – | – | – | – | 1.14 (1.00–1.30), p =0.047 | Ref | 36 |
| Ford, 2010, Lesotho [48] | – | – | – | – | – | – | 0.90 (0.34–2.38) | Ref | 24 |
| Fox, 2010, South Africa [49] | 278 (12.5) | 398 (10.0) | – | – | 1.25 (1.08–1.44) | Ref | – | – | 48 |
| Fox, 2012, South Africa [50] | – | – | 3.34 | 3.46 | Ref | 1.02 (0.91–1.14) | Ref | 0.95 (0.81–1.12); 0.86 (0.76–0.97)d | 6–96 |
| Franke, 2011, Rwanda [16] | – | – | – | – | – | – | Ref | 1.6 (0.8–3.2), TB | 24 |
| Geng, 2010a, Uganda [51] | 46 (41.0)f | 65 (59.0)f | – | – | 0.85 (0.42–1.75)f | Ref | 1.54 (0.53–4.12)f | Ref | 45 |
| Geng, 2010b, Uganda [52] | – | – | – | – |
2.19 (1.30–3.72),
p
<0.01
1.11 (0.65–1.92)c |
Ref Ref |
1.86 (1.06–3.26),
p
=0.03
1.02 (0.57–1.83)c |
Ref Ref |
45 |
| Greig, 2012, 9 countries [53] | – | – | – | – |
1.33 (1.14–1.54),
p
<0.001
1.34 (1.13–1.59), p =0.001 |
Ref Ref |
1.20 (1.01–1.41),
p
=0.035
1.02 (0.84–1.22) |
Ref Ref |
0–3 3–24 |
| Hawkins, 2011, Tanzania [54] | 643 (14.6) | 1039 (12.3) | – | – | 1.23 (1.12–1.36), p <0.01 | Ref | 1.19 (1.05–1.30), p =0.001 | Ref | 33 |
| Hermans, 2012, Uganda [55] | – | – | – | – | 1.82 (1.47–2.25), p <0.001 | Ref | 1.41 (1.12–1.77), p =0.004 | Ref | 12 |
| Hoffmann, 2010, South Africa [56] | 1044 (12.0) | 432 (8.0) | – | – | Ref | 0.75 (0.66–0.86), p <0.001 | Ref | 0.89 (0.77–1.0) | 12 |
| Hoffmann, 2011, South Africa [57] | 1706 (17.8) | 952 (17.5) | 9.85 | 9.03 |
1.30 (1.20–1.50)
1.20 (0.99–1.40) 1.30 (1.20–1.40), p <0.001 |
Ref Ref Ref |
– – 1.20 (1.00–1.30), p =0.004 |
– – Ref |
0–12 13–48 >48 |
| Johannessen, 2008, Tanzania [58] | 38 (39.2) | 57 (25.6) | – | – | 1.73 (1.15–2.61), p =0.009 | Ref | 1.60 (1.00–2.57), p=0.053 | Ref | 36 |
| Karstaedt, 2012, South Africa [59] | 118 (11.5) | 187 (11.6) | – | – | – | – | – | – | 60 |
| Kassa, 2012, Ethiopia [60] | – | – | – | 1.19 (0.95–1.50) | Ref | 1.07 (0.84–1.36) | Ref | 60 | |
| Kebebew, 2012, Ethiopia [61] | 64 (11.7) | 22 (11.8) | – | – | – | – | – | – | 48 |
| Kipp, 2012, Uganda [62] | 56 (31.8)e | 58 (26.1)e | – | – | – | – | – | – | 24 |
| Kouanda, 2012, Burkina Faso [63] | – | – | – | – | 1.73 (1.49–2.02), p <0.001 | Ref | 1.33 (1.05–1.68), p =0.02 | Ref | 70 |
| Lowrance, 2009, Rwanda [64] | – | – | – | – | Ref Ref |
OR 0.67
(0.46–0.98) OR 0.88 (0.62–1.26) |
Ref Ref |
OR 0.56
(0.37–0.84) 0.83 (0.56–1.12) |
6 12 |
| MacPherson, 2009, South Africa [65] | – | – | – | – | 1.55 (1.09–2.21) | Ref | 1.63 (1.12–2.36) | Ref | 24 |
| Mageda, 2012, Tanzania [66] | 31 (13.7) | 16 (5.0) |
7.83
(5.51–11.14), p =0.001 |
2.30
(1.41–3.76) |
3.19 (1.74–5.84) | Ref | 4.71 (2.00–11.05), p =0.001 | Ref | 12–60 |
| Maman, 2012a, 3 countries [67] | 84 (2) | 119 (1) | – | – | – | – | – | – | 72 |
| Maman, 2012b, 3 countries [68] | 246 | 322 | 1.15 | 0.67 | – | – | 1.33 (1.10–1.61) | Ref | 9–60 |
| Maman, 2012c, Malawi [69] | 60 (25.5) | 58 (17.2) | 85.3 (60/70.3 py) | 55.6 (58/104.3 py) | 1.53 (1.07–2.19), p =0.021 | Ref | 1.36 (0.93–2.00) | Ref | 12 |
| Maskew, 2012, South Africa [70] | 364 (10.4) 446 (12.8) |
446 (7.9) 546 (9.7) |
– | – |
1.35 (1.17–1.55)
1.36 (1.20–1.54) |
Ref Ref |
1.23 (1.06–1.42)
1.23 (1.08–1.41) |
Ref Ref |
0–12 12–24 |
| Maskew, 2013, South Africa [71] | 143 (5.2) | 190 (4.1) | – | – | 1.00 (0.70–1.50) 1.20 (0.9–1.60) 1.30 (1.0–1.60) |
Ref Ref Ref |
1.00 (0.70–1.50) 1.20 (0.90–1.60) 1.20 (0.90–1.60) |
Ref Ref Ref |
0–12 12–24 24–36 |
| Massaquoi, 2009, Malawi [72] | 63 (4.3) | 85 (3.2) | – | – | – | – | – | – | 14 |
| Moore, 2011, Uganda [73] | 38 (10.8), p=0.093 | 74 (9.0) | – | – | – | – | – | – | 60 |
| Mossdorf, 2011, Tanzania [74] | 130 (25.1)e | 188 (19.9)e | – | – | Ref | 0.77 (0.62–0.97) e | Ref | 0.77 (0.52–1.15)e | 12 |
| Mosha, 2013, Tanzania [75] | 14 (20.0) | 21 (12.8) | – | – | – | – | – | – | 12 |
| Mujugira, 2009, Botswana [17] | – | – | – | – | Ref | 0.83 (0.52–1.33) (CD4 <50) |
Ref | 0.68 (0.33–1.38) (CD4 <50) |
12 |
| Murphy, 2010, South Africa [76] | 4 (6.0) | 4 (6.0) | – | – | – | – | OR 1.50 (0.20–12.30) | Ref | 5.3 |
| Mutevedzi, 2010, South Africa [77] | – | – | – | – | 1.62 (1.49–1.77) | Ref | 1.33 (1.06–1.67) | Ref | 12 |
| Mutevedzi, 2011, South Africa [78] | – | – | – | – | – | – | <50 yo, 1.64 (1.32–2.03) | Ref | 0–3 |
| ≥50 yo, 1.84 (1.06–3.17) | Ref | 0–3 | |||||||
| <50 yo, 1.40 (1.09–1.80) | Ref | 3–12 | |||||||
| >50 yo, 1.33 (0.73–2.41) | Ref | 3–12 | |||||||
| 1.95 (1.46–2.57) | Ref | 12–24 | |||||||
| Mzileni, 2008, South Africa [79] | 193 (28.0), p <0.0001 | 119 (8.0) | – | – | – | – | – | – | 18 |
| Negin, 2011, Malawi [80] | – | – | – | – | – | – | Ref | 0.52 (0.46–0.60), p <0.0001 | 60 |
| Nglazi, 2011, South Africa [81] | – | – | – | – | – | – | 1.48 (1.17–1.88), p =0.001 | Ref | 84 |
| Odafe, 2012, Nigeria [82] | – | – | – | – | 1.70 (1.22–2.39), p =0.002 | Ref | 1.29 (0.89–1.86) | Ref | 36 |
| Ojikutu, 2008, South Africa [83] | 26 (20.0) | 23 (13.0) | – | – | Ref | 0.69 (0.38–1.25) | Ref | 0.75 (0.40–1.38) | 62 |
| Palombi, 2009, 3 countries [84] | – | – | – | – | Ref | 0.58 (0.48–0.71), p <0.001 | Ref |
Model 1:
0.51 (0.40–0.63),
p
<0.001
Model 2: 0.45 (0.34–0.59), p <0.001 |
42 |
| Palombi, 2010, Mozambique [85] | – | – | – | – | 1.49 (0.96–2.31) | Ref | 1.81 (1.08–3.02) | Ref | 3 |
| Peltzer, 2011, South Africa [86] | – | – | – | – | RR 1.21 (0.70–2.09) | Ref | – | – | 12 |
| Peterson, 2011, Gambia [87] | – | – | – | – |
2.00 (1.00–3.90),
p
=0.0365
2.20 (0.90–5.80) |
Ref Ref |
4.90 (2.50–10.80),
p
<0.0001
2.50 (1.20–5.60), p =0.0184 |
Ref Ref |
0–6 6–36 |
| Poka–Mayap, 2013, Cameroon [88] | – | – | – | – | 1.44 (0.94–2.11) | Ref | 2.15 (1.34–3.45), p =0.002 | Ref | 60 |
| Rougemont, 2009, Cameroon [89] | – | – | – | – | Ref | OR 0.68 (0.30–1.55) | – | – | 6 |
| Russell, 2010, South Africa [90] | – | – | – | – | Ref | 0.89 (0.65–1.23) | Ref | 0.87 (0.63–1.20) | 9 |
| Schoni–Affolter, 2011, Zambia [91] | 3986 (11.5) | 4512 (8.3) | – | – | – | – | – | – | 41 |
| Sieleunou, 2009, Cameroon [92] | – | – | – | – | – | – | 1.73 (1.37–2.19), p <0.001 | Ref | 60 |
| Siika, 2010, Kenya [93] | 241 (39.3) | 286 (29.5) | – | – | – | – | Ref | 0.71 (0.55–0.99) | 5.75 |
| Somi, 2010, Tanzania [94] | 3580 (12.0) | 4746 (8.0) | – | – | Ref | Min. estimate: 0.65 (0.62–0.68); Max. estimate: 0.95 (0.93–0.97) | Ref | Min. estimate 0.59 (0.56–0.62); Max. estimate: 0.88 (0.86–0.91) | 60 |
| Steele, 2011, Botswana [95] | 19 (12.5) | 18 (7.2) | – | – | RR 1.74 (0.94–3.20) | Ref | – | – | 6 |
| Sunpath, 2012, South Africa [96] | 49 (25.0) | 49 (26.0) | – | – | 0.90 (0.60–1.50) | Ref | – | – | 5.5 |
| Taylor–Smith, 2010, Malawi [97] | 341 (14.5) | 206 (8.8) | 15.71 (14.13–17.47) | 9.80 (7.93–10.41) | RR 1.73 (1.45–2.06) | Ref | RR 1.90 (1.57–2.29) | Ref | 36 |
| Toure, 2008, Côte d’Ivoire [98] | – | – | – | – | – | – | 1.52 (1.29–1.80), p <0.0001 | Ref | 32 |
| Van Cutsem, 2011, South Africa [99] | – | – | – | – | 1.69 (1.16–2.47), p =0.007 e | Ref | 1.14 (0.74–1.76)e | Ref | 24 |
| Wandeler, 2012, 3 countries [100] | – | – | – | – | Ref | Sub-HR 0.69 (0.59–0.80), p <0.001 | – | – | 36 |
| Weigel, 2011, Malawi [101] | – | – | – | – | – | – | 1.0 (Ref) | OR 0.85 (0.56–1.29), p=0.45 | na |
| Wubshet, 2012, Ethiopia [102] | – | – | – | – | – | – | 3.26 (2.19–4.88), p <0.001 | Ref | 66 |
| Zachariah, 2009, Malawi [103] | 84 (12.1), p=0.001 | 122 (7.7) | – | – | OR 1.60 (1.20–2.20) | Ref | OR 1.60 (1.20–2.10), p =0.03 | Ref | 3 |
| Eastern Europe/central Asia | |||||||||
| Tsertsvadze, 2011, Georgia [14] | – | – | – | – | 2.14 (1.38–2.32) | Ref | 1.96 (1.19–3.24) | Ref | 60 |
| Latin America/Caribbean | |||||||||
| Wolff, 2010, Chile [104] | – | – | – | – | 1.23 (0.95–1.62) | Ref | 0.81 (0.61–1.08) | Ref | 84 |
| Asia | |||||||||
| Alvarez-Uria, 2013, India [105] | – | – | – | – | – | – | Ref | 0.65 (0.52–0.83) | 60 |
| Argemi, 2012, Cambodia [106] | – | – | – | – | Ref | 0.78 (0.53–1.14) | – | – | 56 |
| Bastard, 2013, Laos [107] | – | – | – | – | – | – | Ref Ref |
1.19 (0.69–2.05) 0.17 (0.07–0.44), p <0.001 |
0–9 10–60 |
| Bhowmik, 2012, India [108] | 43 (8.6) | 13 (5.4) | – | – | – | – | – | – | 12 |
| Chen, 2013, China [109] | 88 (7.3) | – | – | 2.70 (1.70–4.40) | Ref | 2.10 (1.20–3.50) | Ref | 14 | |
| Dou, 2011, China [110] | 292 (14.3) | 151 (10.7) | – | – |
1.38 (1.13–1.68)
1.12 (0.86–1.45) 1.56 (1.15–2.11) |
Ref Ref Ref |
– 1.31 (0.95–1.81) 1.46 (1.04–2.06) |
– Ref Ref |
24 <3 3–24 |
| Fregonese, 2012, Thailand [111] | 14 20 |
23 32 |
7.2 (4.2–12.1) 1.5 (1.0–2.3) |
4.2 (2.8–6.3) 0.80 (0.6–1.2) |
1.70 (0.90–3.40) 1.80 (1.00–3.01) |
Ref Ref |
1.50 (0.70–3.10) 2.40 (1.20–4.80), p =0.01 |
Ref Ref |
3–6 7–60 |
| Kumarasamy, 2008, India [112] | 6.2%, p =0.033 | 4.0% | – | – | – | – | – | – | 12 |
| Limmahakhun, 2012, Thailand [113] | 4 (4.0), TB | 2 (2.8), TB | – | – | – | – | – | – | 120 |
| Rai, 2013, India [114] | 86 (47.3) | 18 (31.6) | 34.4 (27.9–42.5) | 16.6 (10.4–26.3) | – | – | 2.80 (1.60–4.90) | Ref | 36 |
| Sabapathy, 2012, Burma [115] | – | – | 19.6 (17.6–21.8) 4.9 (4.4–5.5) |
14.9 (12.8–17.3) 4.3 (3.7–4.9) |
1.31 (1.09–1.59)
1.16 (0.87–1.38), p=0.09e |
Ref Ref |
1.29 (0.94–1.78) 1.63 (1.23–2.15), p <0.001 e |
Ref Ref |
0–6 7–36 |
| Susaengrat, 2011, Thailand [116] | 75 (13.7) | 50 (11.4) | – | – | – | – | – | – | 45 |
| Thai, 2009, Cambodia [117] | – | – | – | – | 1.55 (1.18–2.05), p =0.002 e | Ref | 1.73 (1.29–2.32), p <0.001 e | Ref | 57 |
| Tran, 2013, Vietnam [118] | 141 (5.5) | 57 (6.6) | – | – | Ref | 0.40 (0.29–0.57) | Ref | 0.54 (0.34–0.85) | 0–6 |
| Van Griensven, 2011, Cambodia [119] | 60 (4.5) 38 |
37 (2.5) 26 |
10.45 1.27 |
5.44 0.77 |
Ref Ref |
0.52 (0.35–0.70),
p
<0.01
0.60 (0.36–0.99), p =0.04 |
Ref Ref |
0.48 (0.31–0.74),
p
<0.01
0.58 (0.34–0.99), p =0.05 |
0–6 6–60 |
| Zhang, 2008, China [120] | 208 (16) | 144 (10.3) | – | – | 1.60 (1.30–2.00), p <0.001 | Ref | 1.90 (1.20–2.90), p =0.004 | Ref | 144 |
| Zhang, 2009, China [121] | 4136, (14.6) p<0.001 | 2354 (11.5) | 9 | 5.7 | 1.50 (1.40–1.50) | Ref | 1.40 (1.03–1.50) | Ref | 60 |
| Zhang, 2012, China [122] | – | – | – | – | 1.50 (1.40–1.70) | Ref | 1.50 (1.20–1.70) | Ref | 6–48 |
| Multi-regional | |||||||||
| Brinkhof, 2008, 11 countries [123] | – | – | – | – | – | – | Ref | 0.83 (0.58–1.18) | 6 |
| Wandel, 2008, 3 countries [124] | – | – | – | – | Ref | Ranged 0.91–1.09, none significant | – | – | 330 |
Bold indicates significant outcomes; cHR, crude, unadjusted hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval; OR, odds ratios; RR, relative risk ratio; Ref, referent; sub-HR, subdistribution hazard ratio; TB, tuberculosis co-infection; py, person-years; KS, patients with Kaposi’s sarcoma; yo, years old
Cox proportional hazard ratio, unless otherwise noted
Time in months since initiation of ART
Includes deaths among patients who were lost to follow-up and then traced (included in MA)
with multiple imputations
both known deaths and patients lost to follow-up (LTFU) in one measure (excluded from MA)
exclusively patients who were LTFU and traced.
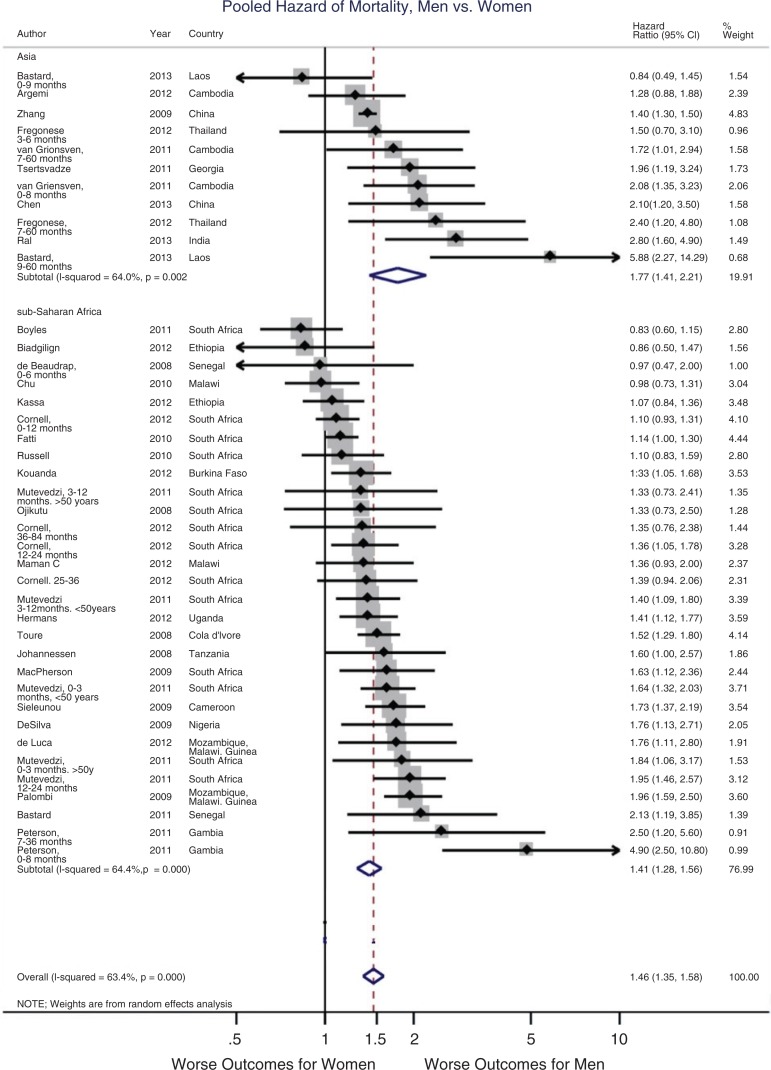
The results of an MA of HRs of mortality, comparing men with women, are presented in the forest plot in Figure 2 and Table 3. The final model of pHR of the included studies from LMIC was 1.46 (95% CI: 1.53–1.59), indicating men had a 46% increased hazard of death while on ART. The total sample size for this analysis was 203,952 (86,233 men and 117,719 women), and the total follow-up time was 1555 months (range=3–108 months). See Supplementary Table 2 for which studies that reported mortality were included and excluded and why.
Figure 2.
Forest plot of pooled hazard ratio of mortality on ART, men vs. women.
Table 3.
Pooled hazard ratios for mortality by sex and time on ART in lower- and middle-income countries
| Male (n) | Female (n) | ≤12a | df | I2 (%) | 13–35a | df | I2 (%) | 36–59a | df | I2 (%) | 60–108a | df | I2 (%) | Overall | df | I2 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All LMIC | 86,233 | 117,719 |
1.42
(1.21–1.67) p=0.002 |
12 | 60.7 |
1.48
(1.23–1.78) p=0.027 |
5 | 60.4 |
1.50
(1.18–1.91) p=0.000 |
8 | 77.8 |
1.49
(1.29–1.71) p=0.005 |
13 | 56.8 |
1.46
(1.35–1.59) p=0.000 |
41 | 63.4 |
Months since initiation of ART; df=degrees of freedom; Model 4.
After sensitivity analysis comparing six models (see Table 4), the final analysis included only studies with high-quality ratings on the NOS (7–9 stars) and had losses to follow-up of <15%. This resulted in 31 studies and 42 individual HRs in this analysis. The effect size of this final model compared with Model 1 (all 54 studies with 67 HRs, regardless of study quality and LTFU rates) is identical (1.46), but with a slightly wider confidence interval. However, the final model has a much lower I 2 (63.4% vs. 75.2%), indicating less heterogeneity between studies.
Subgroup analyses
Analyses were run separately by geographic region, time since initiation of ART and injection drug use rates, including only studies eligible for the final model. Analyses were temporally stratified by quartiles of time since initiation of ART (0–12, 13–35, 36–59 and 60–108 months) (see Table 3). The overall significant effect of increased hazard of mortality for men persisted over time. In all LMIC, the pHR in the first year on ART was slightly ameliorated but still significant, showing a 42% increased hazard of death for men (95% CI: 1.21–1.67). This increased to 48% in months 13 to 35 (95% CI: 1.23–1.78), 50% in months 36 to 59 (95% CI: 1.18–1.91) and 49% in months 60 to 108 (95% CI: 1.29–1.71) since initiation.
For only SSA studies (pHR, n=30), the effect was slightly lower but still significant at 1.41 (1.28–1.56). In Asian countries (pHR, n=11), the effect was greater, with a 77% increased hazard of death for men (95% CI: 1.43–2.21, df=10, I 2=64.0%) (see Figure 2). pHRs for East, Southern and West/Central Africa are also calculated separately to explore differences by region given heterogeneity. The West/Central Africa subregion showed the worst outcomes, with all HRs above 70% higher for men (95% CI: 1.39–2.08, df=7, I 2=57.1%). East African studies showed a 19% increased hazard of death (95% CI: 1.01–1.41, df=5, I 2=36.7%), while Southern African studies showed a 33% increased hazard (95% CI: 1.18–1.51, df=13, I 2=58.1%).
Since the effect of the HR for mortality may be partly attributed to drug use deaths, particularly across Asian countries, we also calculated HRs for the studies that reported a proportion of their participants were PWID (reported between 20 and 60%). The pHR for studies with reported PWID was decreased (1.62 (95% CI: 1.23–2.14, df=1, I 2=47.3%)) compared with the overall effect, while the pHR for studies with no reported drug users was increased (1.85 (95% CI: 1.32–2.61, df=7, I 2=65.9%)).
Quality assessment
Table 5 shows the results of risk of bias assessment using the NOS for observational cohort studies. Only studies that had the potential of being included in the final MA were assessed, for example, those that reported an HR for mortality. All but two studies scored a high rating (7–9 stars); lower scores generally reflected a lack of adjustment for key factors such as age and/or had high or unreported LTFU rates. Supplementary Table 1 indicates key baseline variables adjusted for in the mortality analyses: age; CD4 count, WHO stage and VL; weight (e.g. BMI); haemoglobin status; current or previous tuberculosis; ART start year and ART regimen; as well as other variables (listed in the table). In the final MA, only studies with <15% LTFU were included. All studies with <15% LTFU also earned high ratings.
Table 5.
Quality assessment of studies included in the meta-analysis (Newcastle–Ottawa Scale for cohort studies)
| Selection | Comparability | Outcome | Total | Rating | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, year [Reference] | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on basis of design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | Number of stars (max. 9) | 7–9=high; 4–6=moderate; 1–3=low |
| Africa | ||||||||||
| Abaasa, 2008 [20] | * | * | * | * | * | * | * | 7 | High | |
| Alemu, 2010 [23] | * | * | * | * | * | * | 6 | Moderate | ||
| Bastard, 2011 [26] | * | * | * | * | * | * | * | * | 8 | High |
| Biadgilign, 2012 [27] | * | * | * | * | ** | * | * | * | 9 | High |
| Bisson, 2008 [29] | * | * | * | * | * | * | * | * | 8 | High |
| Boyles, 2011 [30] | * | * | * | * | ** | * | * | * | 9 | High |
| Brinkhof, 2009 [32] | * | * | * | * | * | * | * | 7 | High | |
| Chalamilla, 2012 [33] | * | * | * | * | ** | * | * | 8 | High | |
| Chen, 2008 [34] | * | * | * | * | ** | * | * | 8 | High | |
| Chi, 2009 [35] | * | * | * | * | ** | * | * | 8 | High | |
| Chi, 2010 [36] | * | * | * | * | ** | * | * | * | 9 | High |
| Chu, 2010 [37] | * | * | * | * | * | * | * | * | 8 | High |
| Cornell, 2012 [39] | * | * | * | * | ** | * | * | * | 9 | High |
| De Beaudrap, 2008 [41] | * | * | * | * | * | * | * | 7 | High | |
| De Luca, 2012 [42] | * | * | * | * | ** | * | * | * | 9 | High |
| Desilva, 2009 [44] | * | * | * | * | ** | * | * | * | 9 | High |
| Fatti, 2010 [47] | * | * | * | * | * | * | * | * | 8 | High |
| Ford, 2010 [48] | * | * | * | * | * | * | * | * | 8 | High |
| Geng, 2010a [51] | * | * | * | * | * | * | * | * | 8 | High |
| Greig, 2012 [53] | * | * | * | * | ** | * | * | * | 9 | High |
| Hawkins, 2011 [54] | * | * | * | * | ** | * | * | 8 | High | |
| Hermans, 2012 [55] | * | * | * | * | * | * | * | * | 8 | High |
| Hoffman, 2011 [57] | * | * | * | * | ** | * | * | 8 | High | |
| Johannessen, 2008 [58] | * | * | * | * | * | * | * | * | 8 | High |
| Kassa, 2012 [60] | * | * | * | * | ** | * | * | * | 9 | High |
| Kouanda, 2012 [63] | * | * | * | * | ** | * | * | * | 9 | High |
| MacPherson, 2009 [65] | * | * | * | * | ** | * | * | * | 9 | High |
| Mageda, 2012 [66] | * | * | * | * | ** | * | * | 8 | High | |
| Maman, 2012b [68] | * | * | * | * | ** | * | * | * | 9 | High |
| Maman, 2012c [69] | * | * | * | * | ** | * | * | 8 | High | |
| Mutevedzi, 2011 [78] | * | * | * | * | ** | * | * | * | 9 | High |
| Negin, 2011 [80] | * | * | * | * | * | * | * | 7 | High | |
| Odafe, 2012 [82] | * | * | * | * | * | * | 6 | Moderate | ||
| Ojikutu, 2008 [83] | * | * | * | * | ** | * | * | * | 9 | High |
| Palombi, 2009 [84] | * | * | * | * | ** | * | * | * | 9 | High |
| Peterson, 2011 [87] | * | * | * | * | * | * | * | * | 8 | High |
| Poka-Mayap, 2013 [88] | * | * | * | * | ** | * | * | 8 | High | |
| Russell, 2010 [90] | * | * | * | * | * | * | * | * | 8 | High |
| Sieleunou, 2009 [92] | * | * | * | * | * | * | * | * | 8 | High |
| Somi, 2012 [94] | * | * | * | * | ** | * | * | 8 | High | |
| Toure, 2008 [98] | * | * | * | * | ** | * | * | * | 9 | High |
| Wandeler, 2012 [100] | * | * | * | * | * | * | * | 7 | High | |
| Wubshet, 2012 [102] | * | * | * | * | * | * | * | 7 | High | |
| Central Europe/East Europe | ||||||||||
| Tsertsvadze, 2011 [14] | * | * | * | * | ** | * | * | * | 9 | High |
| Latin America | ||||||||||
| Wolff, 2010 [104] | * | * | * | * | ** | * | * | * | 9 | High |
| Asia | ||||||||||
| Alvarez-Uria, 2013 [105] | * | * | * | * | ** | * | * | 8 | High | |
| Argemi, 2012 [106] | * | * | * | * | * | * | * | 7 | High | |
| Bastard, 2013 [107] | * | * | * | * | * | * | * | * | 8 | High |
| Chen, 2013 [109] | * | * | * | * | ** | * | * | * | 9 | High |
| Fregonese, 2012 [111] | * | * | * | * | ** | * | * | * | 9 | High |
| Rai, 2013 [114] | * | * | * | * | ** | * | * | * | 9 | High |
| Tran, 2013, Vietnam [118] | * | * | * | * | ** | * | * | 8 | High | |
| Van Griensven, 2011 [119] | * | * | * | * | * | * | * | * | 8 | High |
| Zhang, 2009 [121] | * | * | * | * | * | * | * | * | 8 | High |
*The study adequately met the criteria; 0–3 stars=low-quality rating, 4–6 moderate quality rating and 7–9 high-quality rating.
To assess the potential for publication bias, a funnel plot was generated and Egger’s test for small study effects was generated. A visual analysis of the funnel plot (see Supplementary Figure 1) indicates that there may be two extreme HRs. However, the confidence interval for Egger’s test overlaps the null, and the p-value is marginally insignificant (0.057), indicating no small study effects. A sensitivity analysis was conducted (see Table 4) excluding these two HRs (Model 6), which did not significantly change the outcome, however, so they were retained in the final (Model 4) analysis.
Discussion
These analyses identified a consistent, significant and sustained sex differential in all-cause mortality between adult men and women living with HIV and on ART in LMIC. The data are consistent with previous studies suggesting higher mortality among men living with HIV on ART in sub-Saharan Africa [125]. However, the trend of increased mortality transcends SSA and is consistent across all LMIC with persistent sex disparities in mortality over time on treatment.
The differences between men’s and women’s mortality within the first 12 months are smaller, though still significant, showing worse outcomes for men as compared with women. For time on ART >12 months, the HRs retained significant and persisted over time. The sustained differential mortality suggests that even after the initial period on ART when there is typically a spike in mortality, there is a persistent and stronger hazard for men. This echoes data from South Africa, where the relationship between gender and increased mortality persists with increased duration on ART for those living with HIV [39]. Some of these effects may be attributable to baseline factors; men tended to be older when they initiated ART, for example, and presented at lower CD4 counts and higher VL in multiple reports [9,125,126]. Many of the studies in the MA adjusted for clinically relevant factors at baseline and ART initiation. Supplementary Table 1 shows which baseline factors were controlled for in all included studies, and adjusted HRs were used in the MA whenever they were available. Only two studies in the final model were not adjusted, and sensitivity analysis excluding those two did not change the results. Thus, baseline differences do not account for the differential mortality observed, though some confounding may remain and bias the results. Besides such baseline factors, the consistent and significant increased mortality among men can also be reflection of the higher background mortality rate from all other causes among men compared with women [39,127]. Men have been consistently shown to have higher mortality, which is multifactorial in nature, but in part related to limited access to or uptake of healthcare services. Since the men in this analysis were living with HIV and engaged in ART, there is an expectation that differential mortality is attenuated through engagement in care. In Cornell’s analysis from South Africa, the authors compared the increased hazard for mortality among men on ART (adjusted HR 1.31 (1.22–1.41)) to the background differential mortality and found that HIV-positive men in care were indeed protected from mortality – HIV-negative men had twice the hazard of death as men on ART [39]. This requires more investigation; despite being on treatment, men living with HIV are still dying significantly more than women.
These findings suggest that tailored interventions to improve early treatment initiation as well as treatment outcomes and reduce mortality on ART for men are urgently needed across Asia and Africa. Such interventions may likely be required across the HIV treatment cascade, but may be particularly important in several key domains. First, earlier diagnosis of HIV infection is likely to be critical, particularly given the individual clinical benefits of early initiation of ART as formally demonstrated in the START trial and currently recommended by WHO [4,128]. Since women, but not men, are much more frequent users of reproductive and other healthcare services, diagnoses and linkage to treatment may happen earlier especially through implementation of B+ programmes [129]. Improved outreach to men at risk will likely need to go beyond the clinic to where men work, socialize and engage in risk, and include risk-leveraging approaches such as HIV self-testing. In addition, clinic hours and wait times that are fit to the working hours and demands of men are likely critical, as are gender-transformative interventions [130]. The findings of durable differentials in mortality out to five years post-ART initiation call for greater understanding of underlying causes, including challenges related to treatment adherence and retention on ART for men specifically. There is relatively little research on adherence and retention differentials by sex [8,9], and throughout completion of this SR, randomized evaluations which reported treatment outcomes by sex were rarely found. There is also a need for greater attention to and interventions for conditions which tend to affect men more and exacerbate HIV/AIDS prevention and treatment, and increase men’s morbidity and mortality, such as tuberculosis [131] and substance use [18].
This study has several limitations. Due to the nature of the available data, the main outcome of these analyses was all-cause mortality, rather than HIV-related mortality. While HIV-related mortality would be a more precise outcome, these data were rarely reported in studies found in this review, likely due to limitations in mortality reporting in LMIC. Thus, these findings likely include deaths from causes other than HIV/AIDS that differentially affect men, such as substance use and intentional and unintentional violence, as well as AIDS-related causes such as tuberculosis. Furthermore, only a small proportion of outcome data found in the SR was disaggregated by sex; hence, this review was limited to those that completed stratified analyses and/or reported sex-stratified data.
An additional caution in LMIC settings is the paucity of data on treatment outcomes among patients who are LTFU. In several settings, this group includes unascertained death, self-referral to other clinics and care discontinuation or “true lost to follow ups.” To address this issue, the sensitivity analysis ran multiple models allowing for different LTFU rates (all studies, LTFU <18% only and LTFU <15% only), and the final reported pHR includes only studies with <15% LTFU. However, there was no difference in the pHRs between the full and restricted models (both 1.46 with only a slightly widened 95% CI in the restricted model; see Table 4). This review did not include studies that exclusively reported on men’s treatment outcomes, which may give more insight into specific treatment outcomes. In particular, significant data on predominantly male populations living with HIV would be harnessed in studies on vulnerable and key populations including men who have sex with men (MSM) and men who inject drugs. The eligibility criterion of including both men and women necessarily excluded those studies if they did not compare men’s and women’s outcomes. Interventions for these populations may provide insight into interventions to better tailor services for men living with HIV whose acquisition risks have not been defined as well. Finally, this review also only included quantitative outcomes. Qualitative studies would illuminate some of the reasons for these sex differentials, providing testable hypotheses and representing an important next step in informing interventions to address this disparity.
Taken together, these findings call for greater attention to sex and gender as a factor in the analyses of outcomes, given the importance of sex as a determinant of mortality reported here. It is also important to understand how much of the differential mortality among people living with HIV and on ART is due to background mortality, for example, a decreased life expectancy of men in the general population. Additionally, we could not assess risk factors for HIV acquisition, largely because such data are rarely collected in treatment programmes. However, some proportion of adult men across sub-Saharan Africa and Asia also belong to stigmatized and harder to reach subgroups, including PWID, MSM, male sex workers, prisoners, men in uniform, and transnational migrants and seasonal and migrant workers. All of these groups may face additional determinants of risk including lower access to health services, greater likelihood of treatment interruptions, discrimination in healthcare services and other social and structural barriers to continuity of treatment [132,133]. An implementation research agenda is called for to assess the optimal strategies to link and retain in treatment these men who are living with HIV, given additional stigma that can exclude or cause men to self-exclude from diagnostic and treatment services.
Conclusions
Consistent differentials in HIV outcomes for men pose an additional challenge: control of HIV incidence among their sexual partners. As long as men living with HIV are significantly less likely to be virally suppressed, suboptimal clinical outcomes will manifest, combined with ongoing risks of onward HIV transmission to all within their sexual networks. This is perhaps most true across SSA, where sexual transmission of HIV predominates and improved HIV prevention for women and girls is vital. To realize HIV prevention gains, the higher proportion of untreated men in these settings must be addressed. In short, improving HIV clinical outcomes for men is an urgent public health priority.
Supplementary Material
Acknowledgements
The authors kindly thank Emily Clouse, Kim Dam, Whitney Ewing, Amanda Gatewood, Ryan Max and Sarah Peitzmeier for their assistance in the screening and abstraction process, and Kashmira Kale for her assistance in the quality assessment.
Competing interests
The authors have no conflicts of interest to declare.
Authors’ contributions
All authors contributed to the design of the work and interpretation of data, contributed to the revisions of the manuscript, gave final approval for publication and agreed to be accountable for the work. SWB, SDB, CB and PL drafted the manuscript. SDB, CB and PL planned the systematic review. SWB, SDB and CB planned the meta-analyses, and SWB ran the analyses and created the tables and figures. All authors have read and approved the final version.
Funding
Funding was from The World Health Organization, the Center for Public Health and Human Rights at the Johns Hopkins Bloomberg School of Public Health, the Johns Hopkins University Center for AIDS Research (P30AI094189), the Foundation for AIDS Research (amfAR) and NIAID of the NIH under Award Number T32AI102623.
References
- 1.World Health Organization, UNAIDS, UNICEF. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report, April 2007. Geneva: WHO; 2007. [Google Scholar]
- 2.UNAIDS. Antiretroviral therapy (ART) coverage among all age groups. Geneva: UNAIDS; 2013. [Google Scholar]
- 3.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS; 2013. [Google Scholar]
- 4.NIH. Starting antiretroviral treatment early improves outcomes for HIV-infected individuals. Bethesda, MD: NIAID, National Institutes of Health; 2015. [Google Scholar]
- 5.HPTN. HPTN 052 HIV prevention study demonstrates sustained benefit of early antiretroviral therapy. Bethesda, MD: NIAID, National Institutes of Health; 2015. [Google Scholar]
- 6.UNAIDS. How AIDS changed everything: MDG6: 15 years, 15 lessons of hope from the AIDS response. Geneva, Switzerland: UNAIDS Joint Program on AIDS; 2015. [Google Scholar]
- 7.Hankins C, de Zalduondo B. Combination prevention: a deeper understanding of effective HIV prevention. AIDS. 2010;24(Suppl 4):S70–80. doi: 10.1097/01.aids.0000390709.04255.fd. doi: http://dx.doi.org/10.1097/01.aids.0000390709.04255.fd. [DOI] [PubMed] [Google Scholar]
- 8.Mills EJ, Beyrer C, Birungi J, Dybul MR. Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med. 2012;9(2):e1001167. doi: 10.1371/journal.pmed.1001167. doi: http://dx.doi.org/10.1371/journal.pmed.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornell M, McIntyre J, Myer L. Men and antiretroviral therapy in Africa: our blind spot. Trop Med Int Health. 2011;16(7):828–9. doi: 10.1111/j.1365-3156.2011.02767.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2011.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Global update on HIV treatment 2013: results, impact, and opportunities: WHO report in partnership with UNICEF and UNAIDS. Geneva: WHO; 2013. [Google Scholar]
- 11.Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. 5.1.0 ed. West Sussex, England: Wiley; 2011. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRIMSA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. doi: http://dx.doi.org/10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Bank. Country and lending groups. Washington, DC: World Bank; 2012. [Google Scholar]
- 14.Tsertsvadze T, Chkhartishvili N, Sharvadze L, Dvali N, Chokoshvili O, Gabunia P, et al. Outcomes of universal access to antiretroviral therapy (ART) in Georgia. AIDS Res Treat. 2011;2011:621078. doi: 10.1155/2011/621078. doi: http://dx.doi.org/10.1155/2011/621078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United Nations Economic Commission for Africa. Subregional offices UNCA. Geneva, Switzerland: United Nations; 2015. [Google Scholar]
- 16.Franke MF, Robins JM, Mugabo J, Kaigamba F, Cain LE, Fleming JG, et al. Effectiveness of early antiretroviral therapy initiation to improve survival among HIV-infected adults with tuberculosis: a retrospective cohort study. PLoS Med. 2011;8(5):e1001029. doi: 10.1371/journal.pmed.1001029. doi: http://dx.doi.org/10.1097/FTD.0b013e3182526e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mujugira A, Wester CW, Kim S, Bussmann H, Gaolathe R. Patients with advanced HIV type 1 infection initiating antiretroviral therapy in Botswana: treatment response and mortality. AIDS Res Hum Retroviruses. 2009;25(2):127–33. doi: 10.1089/aid.2008.0172. doi: http://dx.doi.org/10.1089/aid.2008.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376(9737):268–84. doi: 10.1016/S0140-6736(10)60743-X. doi: http://dx.doi.org/10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. 2000. [cited 2016 Aug 19]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 20.Abaasa AM, Todd J, Ekoru K, Kalyango JN, Levin J, Odeke E, et al. Good adherence to HAART and improved survival in a community HIV/AIDS treatment and care programme: the experience of The AIDS Support Organization (TASO), Kampala, Uganda. BMC Health Serv Res. 2008;8:241. doi: 10.1186/1472-6963-8-241. doi: http://dx.doi.org/10.1186/1472-6963-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahonkhai AA, Noubary F, Munro A, Stark R, Wilke M, Freedberg KA, et al. Not all are lost: interrupted laboratory monitoring, early death, and loss to follow-up (LTFU) in a large South African treatment program. PLoS One. 2012;7(3):e32993. doi: 10.1371/journal.pone.0032993. doi: http://dx.doi.org/10.1371/journal.pone.0032993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alamo ST, Colebunders R, Ouma J, Sunday P, Wagner G, Wabwire-Mangen F, et al. Return to normal life after AIDS as a reason for lost to follow-up in a community-based antiretroviral treatment program. J Acquir Immune Defic Syndr. 2012;60(2):e36–45. doi: 10.1097/FTD.0b013e3182526e6a. doi: http://dx.doi.org/10.1097/FTD.0b013e3182526e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alemu AW, Sebastian MS. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob Health Action. 2010;3:5398. doi: 10.3402/gha.v3i0.5398. doi: http://dx.doi.org/10.3402/gha.v3i0.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alibhai A, Kipp W, Saunders LD, Kaler ASA, Houston S, Konde-Lule J, et al. Gender-related mortality for HIV-infected patients on highly active antiretroviral therapy (HAART) in rural Uganda. Int J Womens Health. 2010;2:45–52. doi: 10.2147/ijwh.s9408. doi: http://dx.doi.org/10.2147/IJWH.S9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balcha TT, Jeppsson A. Outcomes of antiretroviral treatment: a comparison between hospitals and health centers in Ethiopia. J Int Assoc Physicians AIDS Care (Chic) 2010;9(5):318–24. doi: 10.1177/1545109710367518. doi: http://dx.doi.org/10.1177/1545109710367518. [DOI] [PubMed] [Google Scholar]
- 26.Bastard M, Fall MB, Laniece I, Taverne B, Desclaux A, Ecochard R, et al. Revisiting long-term adherence to highly active antiretroviral therapy in Senegal using latent class analysis. J Acquir Immune Defic Syndr. 2011;57:55–61. doi: 10.1097/QAI.0b013e318211b43b. doi: http://dx.doi.org/10.1097/QAI.0b013e318211b43b. [DOI] [PubMed] [Google Scholar]
- 27.Biadgilign S, Reda AA, Digaffe T. Predictors of mortality among HIV infected patients taking antiretroviral treatment in Ethiopia: a retrospective cohort study. AIDS Res Ther. 2012;9(1):15. doi: 10.1186/1742-6405-9-15. doi: http://dx.doi.org/10.1186/1742-6405-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birbeck GL, Kvalsund MP, Byers PA, Bradbury R, Mang’ombe C, Organek N, et al. Neuropsychiatric and socioeconomic status impact antiretroviral adherence and mortality in rural Zambia. Am J Trop Med Hyg. 2011;85(4):782–9. doi: 10.4269/ajtmh.2011.11-0187. doi: http://dx.doi.org/10.4269/ajtmh.2011.11-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisson GP, Gaolathe T, Gross R, Rollins C, Bellamy S, Mogorosi M, et al. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS One. 2008;3(3):e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyles TH, Wilkinson LS, Leisegang R, Maartens G. Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PLoS One. 2011;6(5):e19201. doi: 10.1371/journal.pone.0019201. doi: http://dx.doi.org/10.1371/journal.pone.0019201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennan AT, Maskew M, Sanne I, Fox MP. The interplay between CD4 cell count, viral load suppression and duration of antiretroviral therapy on mortality in a resource-limited setting. Trop Med Int Health. 2013;18(5):619–31. doi: 10.1111/tmi.12079. doi: http://dx.doi.org/10.1111/tmi.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinkhof MW, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6(4):e1000066. doi: 10.1371/journal.pmed.1000066. doi: http://dx.doi.org/10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalamilla G, Hawkins C, Okuma J, Spiegelman D, Aveika A, Christian B, et al. Mortality and treatment failure among HIV-infected adults in Dar Es Salaam, Tanzania. J Int Assoc Physicians AIDS Care (Chic) 2012;11(5):296–304. doi: 10.1177/1545109711406733. doi: http://dx.doi.org/10.1177/1545109711406733. [DOI] [PubMed] [Google Scholar]
- 34.Chen SC, Yu JK, Harries AD, Bong CN, Kolola-Dzimadzi R, Tok TS, et al. Increased mortality of male adults with AIDS related to poor compliance to antiretroviral therapy in Malawi. Trop Med Int Health. 2008;13(4):513–9. doi: 10.1111/j.1365-3156.2008.02029.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 35.Chi BH, Cantrell RA, Zulu I, Mulenga LB, Levy JW, Tambatamba BC, et al. Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. Int J Epidemiol. 2009;38(3):746–56. doi: 10.1093/ije/dyp004. doi: http://dx.doi.org/10.1093/ije/dyp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chi BH, Mwango A, Giganti M, Mulenga L, Tambatamba-Chapula B, Reid S, et al. Early clinical and programmatic outcomes with tenofovir-based antiretroviral therapy in Zambia. J Acquir Immune Defic Syndr. 2010;54:63–70. doi: 10.1097/QAI.0b013e3181c6c65c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu K, Misinde D, Massaquoi M, Pasulani O, Mwagomba B, Ford N, et al. Risk factors for mortality in AIDS-associated Kaposi sarcoma in a primary care antiretroviral treatment program in Malawi. Int Health. 2010;2(2):99–102. doi: 10.1016/j.inhe.2010.04.001. doi: http://dx.doi.org/10.1016/j.inhe.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;14(7):722–31. doi: 10.1111/j.1365-3156.2009.02290.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9(9):e1001304. doi: 10.1371/journal.pmed.1001304. doi: http://dx.doi.org/10.1371/journal.pmed.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalal RP, MacPhail C, Mqhayi M, Wing J, Feldman C, Chersich MF, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–7. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 41.De Beaudrap P, Etard JF, Ecochard R, Diouf A, Dieng AB, Cilote V, et al. Change over time of mortality predictors after HAART initiation in a Senegalese cohort. Eur J Epidemiol. 2008;23(3):227–34. doi: 10.1007/s10654-007-9221-3. doi: http://dx.doi.org/10.1007/s10654-007-9221-3. [DOI] [PubMed] [Google Scholar]
- 42.De Luca A, Marazzi MC, Mancinelli S, Ceffa S, Altan AM, Buonomo E, et al. Prognostic value of virological and immunological responses after 6 months of antiretroviral treatment in adults with HIV-1 infection in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2012;59(3):236–44. doi: 10.1097/QAI.0b013e31824276e9. doi: http://dx.doi.org/10.1097/QAI.0b013e31824276e9. [DOI] [PubMed] [Google Scholar]
- 43.Deribe K, Yami A, Deribew A, Mesfin N, Colebunders R, Van Geertruyden JP, et al. Predictors of mortality among tuberculosis-HIV-coinfected persons in Southwest Ethiopia: a case-control study. J Int Assoc Provid AIDS Care. 2013;14(3):269–73. doi: 10.1177/2325957413500528. doi: http://dx.doi.org/10.1177/2325957413500528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desilva MB, Merry SP, Fischer PR, Rohrer JE, Isichei CO, Cha SS. Youth, unemployment, and male gender predict mortality in AIDS patients started on HAART in Nigeria. AIDS Care. 2009;21(1):70–7. doi: 10.1080/09540120802017636. doi: http://dx.doi.org/10.1080/09540120802017636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekouevi DK, Balestre E, Ba-Gomis FO, Eholie SP, Maiga M, Amani-Bosse C, et al. Low retention of HIV-infected patients on antiretroviral therapy in 11 clinical centres in West Africa. Trop Med Int Health. 2010;15(Suppl 1):34–42. doi: 10.1111/j.1365-3156.2010.02505.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans D, Maskew M, Sanne I. Increased risk of mortality and loss to follow-up among HIV-positive patients with oropharyngeal candidiasis and malnutrition before antiretroviral therapy initiation: a retrospective analysis from a large urban cohort in Johannesburg, South Africa. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(3):362–72. doi: 10.1016/j.oooo.2011.09.004. doi: http://dx.doi.org/10.1016/j.oooo.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fatti G, Grimwood A, Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS One. 2010;5(9):e12888. doi: 10.1371/journal.pone.0012888. doi: http://dx.doi.org/10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford N, Kranzer K, Hilderbrand K, Jouquet G, Goemaere E, Vlahakis N, et al. Early initiation of antiretroviral therapy and associated reduction in mortality, morbidity and defaulting in a nurse-managed, community cohort in Lesotho. AIDS. 2010;24(17):2645–50. doi: 10.1097/QAD.0b013e32833ec5b2. doi: http://dx.doi.org/10.1097/QAD.0b013e32833ec5b2. [DOI] [PubMed] [Google Scholar]
- 49.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15(4):405–13. doi: 10.1111/j.1365-3156.2010.02473.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox MP, Van Cutsem G, Giddy J, Maskew M, Keiser O, Prozesky H, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr. 2012;60(4):428–37. doi: 10.1097/QAI.0b013e3182557785. doi: http://dx.doi.org/10.1097/QAI.0b013e3182557785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geng E, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53(3):405–11. doi: 10.1097/QAI.0b013e3181b843f0. doi: http://dx.doi.org/10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geng EH, Glidden DV, Emenyonu N, Musinguzi N, Bwana MB, Neilands TB, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health. 2010;15(Suppl 1):63–9. doi: 10.1111/j.1365-3156.2010.02507.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2010.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greig J, Casas EC, O’Brien DP, Mills EJ, Ford N. Association between older age and adverse outcomes on antiretroviral therapy: a cohort analysis of programme data from nine countries. AIDS. 2012;26(Suppl 1):S31–7. doi: 10.1097/QAD.0b013e3283558446. doi: http://dx.doi.org/10.1097/QAD.0b013e3283558446. [DOI] [PubMed] [Google Scholar]
- 54.Hawkins C, Chalamilla G, Okuma J, Spiegelman D, Hertzmark E, Aris E, et al. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. AIDS. 2011;25(9):1189–97. doi: 10.1097/QAD.0b013e3283471deb. doi: http://dx.doi.org/10.1097/QAD.0b013e3283471deb. [DOI] [PubMed] [Google Scholar]
- 55.Hermans SM, Van Leth F, Manabe YC, Hoepelman AI, Lange JM, Kambugu A. Earlier initiation of antiretroviral therapy, increased tuberculosis case finding and reduced mortality in a setting of improved HIV care: a retrospective cohort study. HIV Med. 2012;13(6):337–44. doi: 10.1111/j.1468-1293.2011.00980.x. doi: http://dx.doi.org/10.1111/j.1468-1293.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann CJ, Fielding KL, Charalambous S, Innes C, Chaisson RE, Grant AD, et al. Reducing mortality with cotrimoxazole preventive therapy at initiation of antiretroviral therapy in South Africa. AIDS. 2010;24(11):1709–16. doi: 10.1097/QAD.0b013e32833ac6bc. doi: http://dx.doi.org/10.1097/QAD.0b013e32833ac6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmann CJ, Fielding K, Johnston V, Charalambous S, Innes C, Moore RD, et al. Changing predictors of mortality over time from cART start: implications for care. J Acquir Immune Defic Syndr. 2011;58(3):269–76. doi: 10.1097/QAI.0b013e31823219d1. doi: http://dx.doi.org/10.1097/QAI.0b013e31823219d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI, Aglen HE, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. doi: http://dx.doi.org/10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karstaedt AS. Profile of cause of death assigned to adults on antiretroviral therapy in Soweto. S Afr Med J. 2012;102(8):680–2. doi: 10.7196/samj.5369. doi: http://dx.doi.org/10.7196/SAMJ.5369. [DOI] [PubMed] [Google Scholar]
- 60.Kassa A, Teka A, Shewaamare A, Jerene D. Incidence of tuberculosis and early mortality in a large cohort of HIV infected patients receiving antiretroviral therapy in a tertiary hospital in Addis Ababa, Ethiopia. Trans R Soc Trop Med Hyg. 2012;106(6):363–70. doi: 10.1016/j.trstmh.2012.03.002. doi: http://dx.doi.org/10.1016/j.trstmh.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Kebebew K, Wencheko E. Survival analysis of HIV-infected patients under antiretroviral treatment at the Armed Forced General Teaching Hospital, Addis Ababa, Ethiopia. Ethiop J Health Dev. 2012;26(3):187–92. [Google Scholar]
- 62.Kipp W, Konde-Lule J, Saunders LD, Alibhai A, Houston S, Rubaale T, et al. Antiretroviral treatment for HIV in rural Uganda: two-year treatment outcomes of a prospective health centre/community-based and hospital-based cohort. PLoS One. 2012;7(7):e40902. doi: 10.1371/journal.pone.0040902. doi: http://dx.doi.org/10.1371/journal.pone.0040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kouanda S, Meda IB, Nikiema L, Tiendrebeogo S, Doulougou B, Kabore I, et al. Determinants and causes of mortality in HIV-infected patients receiving antiretroviral therapy in Burkina Faso: a five-year retrospective cohort study. AIDS Care. 2012;24(4):478–90. doi: 10.1080/09540121.2011.630353. [DOI] [PubMed] [Google Scholar]
- 64.Lowrance DW, Ndamage F, Kayirangwa E, Ndagije F, Lo W, Hoover DR, et al. Adult clinical and immunologic outcomes of the national antiretroviral treatment program in Rwanda during 2004–2005. J Acquir Immune Defic Syndr. 2009;52(1):49–55. doi: 10.1097/QAI.0b013e3181b03316. doi: http://dx.doi.org/10.1097/QAI.0b013e3181b03316. [DOI] [PubMed] [Google Scholar]
- 65.MacPherson P, Moshabela M, Martinson N, Pronyk P. Mortality and loss to follow-up among HAART initiators in rural South Africa. Trans R Soc Trop Med Hyg. 2009;103(6):588–93. doi: 10.1016/j.trstmh.2008.10.001. doi: http://dx.doi.org/10.1016/j.trstmh.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Mageda K, Leyna GH, Mmbaga EJ. High initial HIV/AIDS-related mortality and its predictors among patients on antiretroviral therapy in the Kagera region of Tanzania: a five-year retrospective cohort study. AIDS Res Treat. 2012;2012:843598. doi: 10.1155/2012/843598. doi: http://dx.doi.org/10.1155/2012/843598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maman D, Pujades-Rodriguez M, Subtil F, Pinoges L, McGuire M, Ecochard R, et al. Gender differences in immune reconstitution: a multicentric cohort analysis in sub-Saharan Africa. PLoS One. 2012;7(2):e31078. doi: 10.1371/journal.pone.0031078. doi: http://dx.doi.org/10.1371/journal.pone.0031078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maman D, Pujades-Rodriguez M, Nicholas S, McGuire M, Szumilin E, Ecochard R, et al. Response to antiretroviral therapy: improved survival associated with CD4 above 500 cells/mul. AIDS. 2012;26(11):1393–8. doi: 10.1097/QAD.0b013e328352d054. doi: http://dx.doi.org/10.1097/QAD.0b013e328352d054. [DOI] [PubMed] [Google Scholar]
- 69.Maman D, Glynn JR, Crampin AC, Kranzer K, Saul J, Jahn A, et al. Very early anthropometric changes after antiretroviral therapy predict subsequent survival, in Karonga, Malawi. Open AIDS J. 2012;6:36–44. doi: 10.2174/1874613601206010036. doi: http://dx.doi.org/10.2174/1874613601206010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maskew M, Brennan AT, MacPhail AP, Sanne IM, Fox MP. Poorer ART outcomes with increasing age at a large public sector HIV clinic in Johannesburg, South Africa. J Int Assoc Physicians AIDS Care (Chic) 2012;11(1):57–65. doi: 10.1177/1545109711421641. doi: http://dx.doi.org/10.1177/1545109711421641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maskew M, Brennan AT, Westreich D, McNamara L, MacPhail AP, Fox MP. Gender differences in mortality and CD4 count response among virally suppressed HIV-positive patients. J Womens Health. 2013;22(2):113–20. doi: 10.1089/jwh.2012.3585. doi: http://dx.doi.org/10.1089/jwh.2012.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Massaquoi M, Zachariah R, Manzi M, Pasulani O, Misindi D, Mwagomba B, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Trans R Soc Trop Med Hyg. 2009;103(6):594–600. doi: 10.1016/j.trstmh.2009.02.012. doi: http://dx.doi.org/10.1016/j.trstmh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 73.Moore D, Yiannoutsos CT, Musick BS, Tappero J, Degerman R, Campbell J, et al. Determinants of early and late mortality among HIV-infected individuals receiving home-based antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr. 2011;58(3):289–96. doi: 10.1097/QAI.0b013e3182303716. doi: http://dx.doi.org/10.1097/QAI.0b013e3182303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mossdorf E, Stoeckle M, Mwaigomole EG, Chiweka E, Kibatala PL, Geubbels E, et al. Improved antiretroviral treatment outcome in a rural African setting is associated with cART initiation at higher CD4 cell counts and better general health condition. BMC Infect Dis. 2011;11:98. doi: 10.1186/1471-2334-11-98. doi: http://dx.doi.org/10.1186/1471-2334-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mosha F, Muchunguzi V, Matee M, Sangeda RZ, Vercauteren J, Nsubuga P, et al. Gender differences in HIV disease progression and treatment outcomes among HIV patients one year after starting antiretroviral treatment (ART) in Dar es Salaam, Tanzania. BMC Public Health. 2013;13:38. doi: 10.1186/1471-2458-13-38. doi: http://dx.doi.org/10.1186/1471-2458-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy RA, Sunpath H, Lu Z, Chelin N, Losina E, Gordon M, et al. Outcomes after virologic failure of first-line ART in South Africa. AIDS. 2010;24(7):1007–12. doi: 10.1097/QAD.0b013e3283333639. doi: http://dx.doi.org/10.1097/QAD.0b013e3283333639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mutevedzi PC, Lessells RJ, Heller T, Barnighausen T, Cooke GS, Newell ML. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bull World Health Organ. 2010;88(8):593–600. doi: 10.2471/BLT.09.069419. doi: http://dx.doi.org/10.2471/BLT.09.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mutevedzi PC, Lessells RJ, Rodger AJ, Newell ML. Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PLoS One. 2011;6(7):e21795. doi: 10.1371/journal.pone.0021795. doi: http://dx.doi.org/10.1371/journal.pone.0021795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mzileni MO, Longo-Mbenza B, Chephe TJ. Mortality and causes of death in HIV-positive patients receiving antiretroviral therapy at Tshepang Clinic in Doctor George Mukhari Hospital. Pol Arch Med Wewn. 2008;118(10):548–54. [PubMed] [Google Scholar]
- 80.Negin J, Van Lettow M, Semba M, Martiniuk A, Chan A, Cumming RG. Anti-retroviral treatment outcomes among older adults in Zomba district, Malawi. PLoS One. 2011;6(10):e26546. doi: 10.1371/journal.pone.0026546. doi: http://dx.doi.org/10.1371/journal.pone.0026546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nglazi M, Lawn SD, Kaplan R, Kranzer K, Orrell C, Wood R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr. 2011;56:e1–8. doi: 10.1097/QAI.0b013e3181ff0bdc. doi: http://dx.doi.org/10.1097/QAI.0b013e3181ff0bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Odafe S, Idoko O, Badru T, Aiyenigba B, Suzuki C, Khamofu H, et al. Patients’ demographic and clinical characteristics and level of care associated with lost to follow-up and mortality in adult patients on first-line ART in Nigerian hospitals. J Int AIDS Soc. 2012;15(2):17424. doi: 10.7448/IAS.15.2.17424. doi: http://dx.doi.org/10.7448/IAS.15.2.17424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ojikutu BO, Zheng H, Walensky RP, Zhigang L, Losina E, Giddy J, et al. Predictors of mortality in patients initiating antiretroviral therapy in Durban, South Africa. S Afr Med J. 2008;98(3):204–8. [PMC free article] [PubMed] [Google Scholar]
- 84.Palombi L, Marazzi MC, Guidotti G, Germano P, Buonomo E, Scarcella P, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral-treated patients in sub-Saharan African sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48(1):115–22. doi: 10.1086/593312. doi: http://dx.doi.org/10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 85.Palombi L, Dorrucci M, Zimba I, Scarcella P, Mancinelli S, Buonomo E, et al. Immunologic response to highly active antiretroviral therapy and mortality reduction in a cohort of human immunodeficiency virus-positive persons in Mozambique. Am J Trop Med Hyg. 2010;83(5):1128–32. doi: 10.4269/ajtmh.2010.09-0705. doi: http://dx.doi.org/10.4269/ajtmh.2010.09-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peltzer K, Ramlagan S, Khan MS, Gaede B. The social and clinical characteristics of patients on antiretroviral therapy who are “lost to follow-up” in KwaZulu-Natal, South Africa: a prospective study. SAHARA J. 2011;8(4):179–86. doi: 10.1080/17290376.2011.9725002. doi: http://dx.doi.org/10.1080/17290376.2011.9725002. [DOI] [PubMed] [Google Scholar]
- 87.Peterson I, Togun O, de Silva T, Oko F, Rowland-Jones S, Jaye A, et al. Mortality and immunovirological outcomes on antiretroviral therapy in HIV-1 and HIV-2-infected individuals in the Gambia. AIDS. 2011;25(17):2167–75. doi: 10.1097/QAD.0b013e32834c4adb. doi: http://dx.doi.org/10.1097/QAD.0b013e32834c4adb. [DOI] [PubMed] [Google Scholar]
- 88.Poka-Mayap V, Pefura-Yone EW, Kengne AP, Kuaban C. Mortality and its determinants among patients infected with HIV-1 on antiretroviral therapy in a referral centre in Yaounde, Cameroon: a retrospective cohort study. BMJ Open. 2013;3(7):e003210. doi: 10.1136/bmjopen-2013-003210. doi: http://dx.doi.org/10.1136/bmjopen-2013-003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rougemont M, Stoll BE, Elia N, Ngang P. Antiretroviral treatment adherence and its determinants in Sub-Saharan Africa: a prospective study at Yaounde Central Hospital, Cameroon. AIDS Res Ther. 2009;6:21. doi: 10.1186/1742-6405-6-21. doi: http://dx.doi.org/10.1186/1742-6405-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Russell EC, Charalambous S, Pemba L, Churchyard GJ, Grant AD, Fielding K. Low haemoglobin predicts early mortality among adults starting antiretroviral therapy in an HIV care programme in South Africa: a cohort study. BMC Public Health. 2010;10:433. doi: 10.1186/1471-2458-10-433. doi: http://dx.doi.org/10.1186/1471-2458-10-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schoni-Affolter F, Keiser O, Mwango A, Stringer J, Ledergerber B, Mulenga L, et al. Estimating loss to follow-up in HIV-infected patients on antiretroviral therapy: the effect of the competing risk of death in Zambia and Switzerland. PLoS One. 2011;6(12):e27919. doi: 10.1371/journal.pone.0027919. doi: http://dx.doi.org/10.1371/journal.pone.0027919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sieleunou I, Souleymanou M, Schonenberger AM, Menten J, Boelaert M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009;14(1):36–43. doi: 10.1111/j.1365-3156.2008.02183.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 93.Siika AM, Wools-Kaloustian K, Mwangi AW, Kimaiyo SN, Diero LO, Ayuo PO, et al. Risk factors for death in HIV-infected adult African patients receiving anti-retroviral therapy. East Afr Med J. 2010;87(11):443–51. [PubMed] [Google Scholar]
- 94.Somi G, Keogh SC, Todd J, Kilama B, Wringe A, Van den Hombergh J, et al. Low mortality risk but high loss to follow-up among patients in the Tanzanian national HIV care and treatment programme. Trop Med Int Health. 2012;17(4):497–506. doi: 10.1111/j.1365-3156.2011.02952.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2011.02952.x. [DOI] [PubMed] [Google Scholar]
- 95.Steele KT, Steenhoff AP, Newcomb CW, Rantleru T, Nthobatsang R, Lesetedi G, et al. Early mortality and AIDS progression despite high initial antiretroviral therapy adherence and virologic suppression in Botswana. PLoS One. 2011;6(6):e20010. doi: 10.1371/journal.pone.0020010. doi: http://dx.doi.org/10.1371/journal.pone.0020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sunpath H, Edwin C, Chelin N, Nadesan S, Maharaj R, Moosa Y, et al. Operationalizing early antiretroviral therapy in HIV-infected in-patients with opportunistic infections including tuberculosis. Int J Tuberc Lung Dis. 2012;16(7):917–23. doi: 10.5588/ijtld.11.0651. doi: http://dx.doi.org/10.5588/ijtld.11.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taylor-Smith K, Tweya H, Harries A, Schoutene E, Jahn A. Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J. 2010;22(2):49–56. doi: 10.4314/mmj.v22i2.58794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Toure S, Kouadio B, Seylerb C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–82. doi: 10.1097/QAD.0b013e3282f768f8. doi: http://dx.doi.org/10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Cutsem G, Ford N, Hildebrand K, Goemaere E, Mathee S, Abrahams M, et al. Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS One. 2011;6(2):e14684. doi: 10.1371/journal.pone.0014684. doi: http://dx.doi.org/10.1371/journal.pone.0014684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wandeler G, Keiser O, Pfeiffer K, Pestili S, Fritz C, Labhardt N, et al. Outcomes of antiretroviral treatment programs in rural Southern Africa. J Acquir Immune Defic Syndr. 2012;59:e9–16. doi: 10.1097/QAI.0b013e31823edb6a. doi: http://dx.doi.org/10.1097/QAI.0b013e31823edb6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weigel R, Hochgesang M, Brinkhof MW, Hosseinipour MC, Boxshall M, Mhango E, et al. Outcomes and associated risk factors of patients traced after being lost to follow-up from antiretroviral treatment in Lilongwe, Malawi. BMC Infect Dis. 2011;11:31. doi: 10.1186/1471-2334-11-31. doi: http://dx.doi.org/10.1186/1471-2334-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wubshet M, Berhane Y, Worku A, Kebede Y, Diro E. High loss to followup and early mortality create substantial reduction in patient retention at antiretroviral treatment program in north-west Ethiopia. ISRN AIDS. 2012;2012:721720. doi: 10.5402/2012/721720. doi: http://dx.doi.org/10.5402/2012/721720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zachariah R, Harries K, Moses M, Manzi M, Line A, Mwagomba B, et al. Very early mortality in patients starting antiretroviral treatment at primary health centres in rural Malawi. Tropical Med Int Health. 2009;14(7):713–21. doi: 10.1111/j.1365-3156.2009.02291.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2009.02291.x. [DOI] [PubMed] [Google Scholar]
- 104.Wolff MJ, Cortés CP, Shepherd BE, Beltrán CJ, Chilean AIDS Cohort Study Group. Long-term outcomes of a national expanded access program to antiretroviral therapy: the Chilean AIDS cohort. J Acquir Immune Defic Syndr. 2010;55(3):368–74. doi: 10.1097/QAI.0b013e3181eb4fb9. doi: http://dx.doi.org/10.1097/QAI.0b013e3181eb4fb9. [DOI] [PubMed] [Google Scholar]
- 105.Alvarez-Uria G, Naik PK, Pakam R, Midde M. Factors associated with attrition, mortality, and loss to follow up after antiretroviral therapy initiation: data from an HIV cohort study in India. Glob Health Action. 2013;6:21682. doi: 10.3402/gha.v6i0.21682. doi: http://dx.doi.org/10.3402/gha.v6i0.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Argemi X, Dara S, You S, Mattei JF, Courpotin C, Simon B, et al. Impact of malnutrition and social determinants on survival of HIV-infected adults starting antiretroviral therapy in resource-limited settings. AIDS. 2012;26(9):1161–6. doi: 10.1097/QAD.0b013e328353f363. doi: http://dx.doi.org/10.1097/QAD.0b013e328353f363. [DOI] [PubMed] [Google Scholar]
- 107.Bastard M, Soulinphumy K, Phimmasone P, Saadani AH, Ciaffi L, Communier A, et al. Women experience a better long-term immune recovery and a better survival on HAART in Lao People’s Democratic Republic. BMC Infect Dis. 2013;13:27. doi: 10.1186/1471-2334-13-27. doi: http://dx.doi.org/10.1186/1471-2334-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhowmik A, Bhandari S, De R, Guha SK. Predictors of mortality among HIV–infected patients initiating anti retroviral therapy at a tertiary care hospital in Eastern India. Asian Pac J Trop Med. 2012;5(12):986–90. doi: 10.1016/S1995-7645(12)60187-4. doi: http://dx.doi.org/10.1016/S1995-7645(12)60187-4. [DOI] [PubMed] [Google Scholar]
- 109.Chen J, Yu B, Wang Y, Tang M, Hu Y, Cai T, et al. Expansion of HIV care and treatment in Yunnan Province, China: treatment outcomes with scale up of combination antiretroviral therapy. AIDS Care. 2014;26(5):633–41. doi: 10.1080/09540121.2013.845281. doi: http://dx.doi.org/10.1080/09540121.2013.845281. [DOI] [PubMed] [Google Scholar]
- 110.Dou Z, Xu J, Jiao JH, Ma Y, Durako S, Yu L, et al. Gender difference in 2-year mortality and immunological response to ART in an HIV-infected Chinese population, 2006–2008. PLoS One. 2011;6(8):e22707. doi: 10.1371/journal.pone.0022707. doi: http://dx.doi.org/10.1371/journal.pone.0022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fregonese F, Collins IJ, Jourdain G, LeCoeur S, Cressey TR, Ngo-Giang-Houng N, et al. Predictors of 5-year mortality in HIV-infected adults starting highly active antiretroviral therapy in Thailand. J Acquir Immune Defic Syndr. 2012;60(1):91–8. doi: 10.1097/QAI.0b013e31824bd33f. doi: http://dx.doi.org/10.1097/QAI.0b013e31824bd33f. [DOI] [PubMed] [Google Scholar]
- 112.Kumarasamy N, Venkatesh KK, Cecelia AJ, Devaleenol B, Saghayam S, Yepthomi T, et al. Gender-based differences in treatment and outcome among HIV patients in South India. J Womens Health. 2008;17(9):1471–5. doi: 10.1089/jwh.2007.0670. doi: http://dx.doi.org/10.1089/jwh.2007.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Limmahakhun S, Chaiwarith R, Nuntachit N, Sirisanthana T, Supparatpinyo K. Treatment outcomes of patients co-infected with tuberculosis and HIV at Chiang Mai University Hospital, Thailand. Int J STD AIDS. 2012;23(6):414–8. doi: 10.1258/ijsa.2012.011291. doi: http://dx.doi.org/10.1258/ijsa.2012.011291. [DOI] [PubMed] [Google Scholar]
- 114.Rai S, Mahapatra B, Sircar S, Raj PY, Venkatesh S, Shaukat M, et al. Adherence to antiretroviral therapy and its effect on survival of HIV-infected individuals in Jharkhand, India. PLoS One. 2013;8(6):e66860. doi: 10.1371/journal.pone.0066860. doi: http://dx.doi.org/10.1371/journal.pone.0066860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sabapathy K, Ford N, Chan KN, Kyaw MK, Elema R, Smithuis F, et al. Treatment outcomes from the largest antiretroviral treatment program in Myanmar (Burma): a cohort analysis of retention after scale-up. J Acquir Immune Defic Syndr. 2012;60(2):e53–62. doi: 10.1097/QAI.0b013e31824d5689. doi: http://dx.doi.org/10.1097/QAI.0b013e31824d5689. [DOI] [PubMed] [Google Scholar]
- 116.Susaengrat W, Leeratanapetch N, Panaput T. Association of mortality and immunological response after initiating antiretroviral therapy among HIV-infected patients in Khon Kaen: a cohort study. J Infect Dis Antimicrob Agents. 2011;28:87–97. [Google Scholar]
- 117.Thai S, Koole O, Un P, Ros S, De Munter P, Van Damme W, et al. Five-year experience with scaling-up access to antiretroviral treatment in an HIV care programme in Cambodia. Trop Med Int Health. 2009;14(9):1048–58. doi: 10.1111/j.1365-3156.2009.02334.x. doi: http://dx.doi.org/10.1111/j.1365-3156.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 118.Tran DA, Ngo AD, Shakeshaft A, Wilson DP, Doran C, Zhang L. Trends in and determinants of loss to follow up and early mortality in a rapid expansion of the antiretroviral treatment program in Vietnam: findings from 13 outpatient clinics. PLoS One. 2013;8(9):e73181. doi: 10.1371/journal.pone.0073181. doi: http://dx.doi.org/10.1371/journal.pone.0073181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Griensven J, Thai S. Predictors of immune recovery and the association with late mortality while on antiretroviral treatment in Cambodia. Trans R Soc Trop Med Hyg. 2011;105(12):694–703. doi: 10.1016/j.trstmh.2011.08.007. doi: http://dx.doi.org/10.1016/j.trstmh.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 120.Zhang F, Dou Z, Yu L, Xu J, Jiao JH, Wang N, et al. The effect of highly active antiretroviral therapy on mortality among HIV-infected former plasma donors in China. Clin Infect Dis. 2008;47(6):825–33. doi: 10.1086/590945. doi: http://dx.doi.org/10.1086/590945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151:241–51. doi: 10.7326/0003-4819-151-4-200908180-00006. doi: http://dx.doi.org/10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y, Dou Z, Sun K, Ma Y, Chen RY, Bulterys M, et al. Association between missed early visits and mortality among patients of China National Free Antiretroviral Treatment cohort. J Acquir Immune Defic Syndr. 2012;60(1):59–67. doi: 10.1097/QAI.0b013e31824c3d9f. doi: http://dx.doi.org/10.1097/QAI.0b013e31824c3d9f. [DOI] [PubMed] [Google Scholar]
- 123.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86(7):559–67. doi: 10.2471/BLT.07.044248. doi: http://dx.doi.org/10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wandel S, Egger M, Rangsin R, Nelson KE, Costello C, Lewden C, et al. Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sex Transm Infect. 2008;84(Suppl 1):i31–6. doi: 10.1136/sti.2008.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Druyts E, Dybul M, Kanters S, Nachega J, Birungi J, Ford N, et al. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. AIDS. 2013;27(3):417–25. doi: 10.1097/QAD.0b013e328359b89b. doi: http://dx.doi.org/10.1097/QAD.0b013e328359b89b. [DOI] [PubMed] [Google Scholar]
- 126.Doyal L. What do we know about men living with HIV and dying with AIDS? J Men’s Health. 2009;6(3):155–7. doi: http://dx.doi.org/10.1016/j.jomh.2009.06.002. [Google Scholar]
- 127.Global Burden of Diseases 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.World Health Organization. Geneva: World Health Organization; 2015. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV; p. 78. [PubMed] [Google Scholar]
- 129.WHO HIV/AIDS Programme. Programmatic Update: Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva, Switzerland: World Health Organization; 2012. Apr, [PubMed] [Google Scholar]
- 130.Dunkle KL, Jewkes R. Effective HIV prevention requires gender-transformative work with men. Sex Transm Infect. 2007;83(3):173–4. doi: 10.1136/sti.2007.024950. doi: http://dx.doi.org/10.1136/sti.2007.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Horton K, MacPherson P, Houben RM, White R, Corbett EL. Sex differences in tuberculosis burden and notifications in now- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2016;13(9):e1002119. doi: 10.1371/journal.pmed.1002119. doi: http://dx.doi.org/10.1371/journal.pmed.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Beyrer C, Malinowska-Sempruch K, Kamarulzaman A, Kazatchine M, Sidibe M, Strathdee SA. Time to act: a call for comprehensive responses to HIV in people who use drugs. Lancet. 2010;376(9740):551–63. doi: 10.1016/S0140-6736(10)60928-2. doi: http://dx.doi.org/10.1016/S0140-6736(10)60928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beyrer C, Sullivan PS, Sanchez J, Dowdy D, Altman D, Trapence G, et al. A call to action for comprehensive HIV services for men who have sex with men. Lancet. 2012;280(9839):424–38. doi: 10.1016/S0140-6736(12)61022-8. doi: http://dx.doi.org/10.1016/S0140-6736(12)61022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.