Abstract
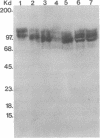
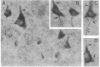
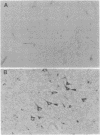
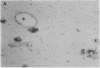
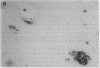
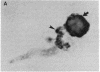
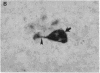
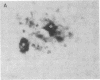
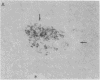
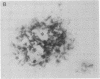
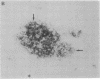
In individuals with Alzheimer disease and in aged nonhuman primates, deposits of amyloid occur in senile plaques in brain parenchyma and in the walls of some meningeal and cortical vessels. Amyloid is primarily composed of beta/A4, a 4-kDa peptide derived from the transmembrane form of an amyloid precursor protein (APP). We examined the distribution of beta/A4 and APP (outside the beta/A4 domain) in cerebral cortices of monkeys ranging in age from 4 to 41 years. In all animals, APP immunoreactivity was present in cell bodies, proximal dendrites, and axons of cortical neurons. In aged animals, all of which showed senile plaques, large APP-positive axons were conspicuous, and APP immunoreactivity was present in neurites around beta/A4-immunoreactive plaques. In some plaques, APP-immunoreactive elements were located in proximity to deposits of beta/A4. The presence of APP immunoreactivity in neuronal perikarya, dendrites, axons, and in neurites within beta/A4-containing plaques supports the hypothesis that neurons can serve as one source of amyloid deposited in brain parenchyma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H., Price D. L., Cork L. C. Alpha 1-antichymotrypsin is present together with the beta-protein in monkey brain amyloid deposits. Neuroscience. 1989;32(3):715–720. doi: 10.1016/0306-4522(89)90292-3. [DOI] [PubMed] [Google Scholar]
- Allsop D., Wong C. W., Ikeda S., Landon M., Kidd M., Glenner G. G. Immunohistochemical evidence for the derivation of a peptide ligand from the amyloid beta-protein precursor of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2790–2794. doi: 10.1073/pnas.85.8.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmanyar S., Higgins G. A., Goldgaber D., Lewis D. A., Morrison J. H., Wilson M. C., Shankar S. K., Gajdusek D. C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer's disease. Science. 1987 Jul 3;237(4810):77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- Card J. P., Meade R. P., Davis L. G. Immunocytochemical localization of the precursor protein for beta-amyloid in the rat central nervous system. Neuron. 1988 Nov;1(9):835–846. doi: 10.1016/0896-6273(88)90131-6. [DOI] [PubMed] [Google Scholar]
- Castaño E. M., Frangione B. Human amyloidosis, Alzheimer disease and related disorders. Lab Invest. 1988 Feb;58(2):122–132. [PubMed] [Google Scholar]
- Cohen M. L., Golde T. E., Usiak M. F., Younkin L. H., Younkin S. G. In situ hybridization of nucleus basalis neurons shows increased beta-amyloid mRNA in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1227–1231. doi: 10.1073/pnas.85.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Goedert M. Neuronal localization of amyloid beta protein precursor mRNA in normal human brain and in Alzheimer's disease. EMBO J. 1987 Dec 1;6(12):3627–3632. doi: 10.1002/j.1460-2075.1987.tb02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. N., Merz P., Bennett-Gray J., Menezes A. H., Goeken J. A., Schelper R. L., Wisniewski H. M. beta-amyloid protein of Alzheimer's disease is found in cerebral and spinal cord vascular malformations. Am J Pathol. 1988 Jul;132(1):167–172. [PMC free article] [PubMed] [Google Scholar]
- Higgins G. A., Lewis D. A., Bahmanyar S., Goldgaber D., Gajdusek D. C., Young W. G., Morrison J. H., Wilson M. C. Differential regulation of amyloid-beta-protein mRNA expression within hippocampal neuronal subpopulations in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1297–1301. doi: 10.1073/pnas.85.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Kametani F., Haga S., Sato M. The immunohistochemical demonstration of subsequences of the precursor of the amyloid A4 protein in senile plaques in Alzheimer's disease. Neuropathol Appl Neurobiol. 1989 Mar-Apr;15(2):135–147. doi: 10.1111/j.1365-2990.1989.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Kitt C. A., Price D. L., Struble R. G., Cork L. C., Wainer B. H., Becher M. W., Mobley W. C. Evidence for cholinergic neurites in senile plaques. Science. 1984 Dec 21;226(4681):1443–1445. doi: 10.1126/science.6505701. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Cork L. C., Unterbeck A., Bayney R. M., Price D. L. Differential expression of amyloid precursor protein mRNAs in cases of Alzheimer's disease and in aged nonhuman primates. Neuron. 1990 Jan;4(1):97–104. doi: 10.1016/0896-6273(90)90446-m. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hill B., Beyreuther K. Molecular biology of Alzheimer's disease. Annu Rev Biochem. 1989;58:287–307. doi: 10.1146/annurev.bi.58.070189.001443. [DOI] [PubMed] [Google Scholar]
- Navone F., Jahn R., Di Gioia G., Stukenbrok H., Greengard P., De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T., Fritz L. C., Schenk D. B., Lieberburg I., Johnson-Wood K. L., Beattie E. C., Ward P. J., Blacher R. W., Dovey H. F., Sinha S. The secreted form of the Alzheimer's amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989 Sep 14;341(6238):144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Golde T. E., Cohen M. L., Kovacs D. M., Tanzi R. E., Gusella J. F., Usiak M. F., Younkin L. H., Younkin S. G. Amyloid protein precursor messenger RNAs: differential expression in Alzheimer's disease. Science. 1988 Aug 26;241(4869):1080–1084. doi: 10.1126/science.2457949. [DOI] [PubMed] [Google Scholar]
- Perry G., Lipphardt S., Mulvihill P., Kancherla M., Mijares M., Gambetti P., Sharma S., Maggiora L., Cornette J., Lobl T. Amyloid precursor protein in senile plaques of Alzheimer disease. Lancet. 1988 Sep 24;2(8613):746–746. doi: 10.1016/s0140-6736(88)90219-x. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Price D. L. New perspectives on Alzheimer's disease. Annu Rev Neurosci. 1986;9:489–512. doi: 10.1146/annurev.ne.09.030186.002421. [DOI] [PubMed] [Google Scholar]
- Schmechel D. E., Goldgaber D., Burkhart D. S., Gilbert J. R., Gajdusek D. C., Roses A. D. Cellular localization of messenger RNA encoding amyloid-beta-protein in normal tissue and in Alzheimer disease. Alzheimer Dis Assoc Disord. 1988;2(2):96–111. doi: 10.1097/00002093-198802020-00002. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Saitoh T., Cole G. Characterization of an amyloid beta precursor protein that binds heparin and contains tyrosine sulfate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2066–2069. doi: 10.1073/pnas.86.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Bell D. S., Podlisny M. B., Price D. L., Cork L. C. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987 Feb 20;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Biochemistry of altered brain proteins in Alzheimer's disease. Annu Rev Neurosci. 1989;12:463–490. doi: 10.1146/annurev.ne.12.030189.002335. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers B. D., Hilbich C., Multhaup G., Salbaum M., Beyreuther K., Seeburg P. H. Alzheimer's disease amyloidogenic glycoprotein: expression pattern in rat brain suggests a role in cell contact. EMBO J. 1988 May;7(5):1365–1370. doi: 10.1002/j.1460-2075.1988.tb02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Hirai S., Yamaguchi H., Harigaya Y., Kawarabayashi T. Amyloid beta-protein precursor accumulates in dystrophic neurites of senile plaques in Alzheimer-type dementia. Brain Res. 1990 Mar 26;512(1):164–168. doi: 10.1016/0006-8993(90)91187-l. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struble R. G., Price D. L., Jr, Cork L. C., Price D. L. Senile plaques in cortex of aged normal monkeys. Brain Res. 1985 Dec 30;361(1-2):267–275. doi: 10.1016/0006-8993(85)91298-3. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Tate-Ostroff B., Majocha R. E., Marotta C. A. Identification of cellular and extracellular sites of amyloid precursor protein extracytoplasmic domain in normal and Alzheimer disease brains. Proc Natl Acad Sci U S A. 1989 Jan;86(2):745–749. doi: 10.1073/pnas.86.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand W. E., Wagner S. L., Suzuki M., Choi B. H., Farrow J. S., Geddes J. W., Cotman C. W., Cunningham D. D. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature. 1989 Oct 12;341(6242):546–549. doi: 10.1038/341546a0. [DOI] [PubMed] [Google Scholar]
- Walker L. C., Kitt C. A., Struble R. G., Schmechel D. E., Oertel W. H., Cork L. C., Price D. L. Glutamic acid decarboxylase-like immunoreactive neurites in senile plaques. Neurosci Lett. 1985 Aug 30;59(2):165–169. doi: 10.1016/0304-3940(85)90194-6. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K., Herget T., Salbaum J. M., Schubert W., Hilbich C., Cramer M., Masters C. L., Multhaup G., Kang J., Lemaire H. G. Localization of the putative precursor of Alzheimer's disease-specific amyloid at nuclear envelopes of adult human muscle. EMBO J. 1988 Feb;7(2):367–372. doi: 10.1002/j.1460-2075.1988.tb02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]